Abstract
Background
The purpose of this study was to evaluate the relationship between serum sodium at the time of diagnosis and long term clinical outcomes in a large national cohort of patients with heart failure with preserved ejection fraction.
Methods and Results
We studied 25 440 patients with heart failure with preserved ejection fraction treated at Veterans Affairs medical centers across the United States between 2002 and 2012. Serum sodium at the time of heart failure diagnosis was analyzed as a continuous variable and in categories as follows: low (115.00–134.99 mmol/L), low‐normal (135.00–137.99 mmol/L), referent group (138.00–140.99 mmol/L), high normal (141.00–143.99 mmol/L), and high (144.00–160.00 mmol/L). Multivariable Cox regression and negative binomial regression were performed to estimate hazard ratios (95% confidence interval [CI]) and incidence density ratios (95% CI) for the associations of serum sodium with mortality and hospitalizations (heart failure and all‐cause), respectively. The average age of patients was 70.8 years, 96.2% were male, and 14% were black. Compared with the referent group, low, low‐normal, and high sodium values were associated with 36% (95% CI, 28%–44%), 6% (95% CI, 1%–12%), and 9% (95% CI, 1%–17%) higher risk of all‐cause mortality, respectively. Low and low‐normal serum sodium were associated with 48% (95% CI, 10%–100%) and 38% (95% CI, 8%–77%) higher risk of number of days of heart failure hospitalizations per year, and with 44% (95% CI, 32%–56%) and 18% (95% CI, 10%–27%) higher risk of number of days of all‐cause hospitalizations per year, respectively.
Conclusions
Both elevated and reduced serum sodium, including values currently considered within normal range, are associated with adverse outcomes in patients with heart failure with preserved ejection fraction.
Keywords: heart failure, hospitalization, hyponatremia, mortality, preserved left ventricular function
Subject Categories: Heart Failure
Clinical Perspective
What Is New?
A J‐shaped relationship is observed between serum sodium levels at time of diagnosis and long‐term clinical outcomes in heart failure with preserved ejection fraction.
What Are the Clinical Implications?
Serum sodium could be an important clinical variable for identifying heart failure with preserved ejection fraction patients at increased risk of adverse events.
Neurohormonal activation, a major determinant of serum sodium levels in heart failure, may play a role in the natural history of heart failure with preserved ejection fraction.
Epidemiologic studies estimate that the prevalence of heart failure with preserved ejection fraction (HFpEF), previously termed diastolic heart failure (HF), is 1.1% to 5.5% of the general population,1, 2 and ranges from 40% to 71% among HF patients.1, 3, 4 Over the past 3 decades, while the prevalence of HF with reduced ejection fraction (EF) or systolic HF has remained stable, HFpEF has increased commensurately with the aging of the population at a rate of 1% per year.3 Hospitalizations for HFpEF are increasing relative to HF with reduced ejection fraction,1 and the mortality rates from HFpEF have ranged from 10% to 30% per year in epidemiologic studies.5 The cost associated with this condition is high and poses a significant burden on the healthcare system.6 A major gap in treatment of HFpEF is that no evidence‐based therapy has yet been identified for HFpEF in spite of multiple clinical trials testing the efficacy of angiotensin‐converting enzyme inhibitors,7 angiotensin receptor blockers,8 β‐blockers,9 digoxin,10 and spironolactone.11 The major limiting factors in previously reported clinical trials and prospective observational studies include lack of uniformity in accurately defining the HFpEF cohort, and failure in distinguishing HFpEF from HF with intermediate EF and HF with recovered EF, as advocated in the clinical guidelines of the European Society of Cardiology and the American College of Cardiology Foundation/American Heart Association.4 HFpEF is extremely difficult to curate in a large national database given its lack of consistency in identifying and reporting by healthcare providers and the difficulty of extracting all known left ventricular ejection fraction (LVEF) values to ensure accurate diagnosis.
Low serum sodium, defined as serum sodium <135 mmol/L (hyponatremia), is a powerful prognostic marker in HF with reduced EF and indicates neurohormonal activation.12 However, studies on the relation between hyponatremia and long‐term clinical outcomes in HFpEF patients have yielded inconsistent results.13, 14, 15, 16 To our knowledge, previous studies have not addressed whether values of serum sodium >135 mmol/L are associated with adverse clinical outcomes in HFpEF patients in a large cohort. Characterizing the relation between the whole spectrum of sodium values and outcomes in HFpEF would be important to understand the role of sodium homeostasis on the prognosis of patients with HFpEF. Accordingly, we curated a large cohort of HFpEF subjects from the national Veterans Affairs (VA) patient database following published European Society of Cardiology/American College of Cardiology Foundation/American Heart Association guidelines and tested the hypothesis that there is a J‐shaped relationship between serum sodium measured at the time of diagnosis of HFpEF and all‐cause mortality, heart failure hospitalizations, and all‐cause hospitalizations in subjects with HFpEF.
Methods
In accordance with current VA policies, we are not able to make our data publicly available, but we can provide the methods used in analysis to any researcher for purposes of replicating the procedure upon request from the senior author. All study procedures were approved by the Institutional Review Board of the Veterans Affairs Boston Healthcare System. Because this was a database study, the informed consent requirement was waived.
Development of the HFpEF Algorithm
Using an iterative process, we arrived at the following algorithm to curate HFpEF cases from the national VA patient care databases. The inclusion criteria in the algorithm consisted of (1) International Classification of Diseases, Ninth Revision (ICD‐9) codes diagnosis of HF (any 428.xx); (2) presence of B‐type natriuretic peptide (≥100 pg/mL) or aminoterminal pro–B‐type natriuretic peptide (≥300 pg/mL), or diuretic usage within 1 month of HF diagnosis; (3) availability of echocardiogram in our system; (4) all recorded LVEF values ≥50% (during the entire study follow‐up); and (5) at least 1 serum sodium measurement within 30 days after the HF diagnosis. As will be described below, criterion 2 was arrived at by an iterative process to ensure selection of cases with definite clinical heart failure. We included subjects diagnosed with HF in either an inpatient or outpatient setting between January 1, 2002, and December 31, 2012. We defined the index date as this initial HF diagnosis date around which patients also displayed elevated B‐type natriuretic peptide/pro–B‐type natriuretic peptide laboratory values or diuretic usage. For outpatient diagnoses, the diagnosis date was used as the index date. If an outpatient HF diagnosis was followed by hospitalization within 48 hours or if the diagnosis was made as an inpatient, the discharge date was used as the index date. As validated before,17, 18, 19 a natural language processing tool was used to extract EF values from text integration utilities documents, including history and physical examination notes, progress notes, discharge summary notes, echocardiography reports, nuclear medicine reports, cardiac catheterization reports, and other cardiology notes. All recorded LVEF values were used for classifying cases as HFpEF. Patients with any diagnosis code for constrictive pericarditis (423.2), hypertrophic cardiomyopathy (425.1), restrictive cardiomyopathy (425.4), or amyloidosis (277.30, 277.39, 425.7) were excluded. To be included in the analysis cohort, patients were required to have a loop diuretic or thiazide diuretic prescription within 1 year before or after the index date.
Validation of HFpEF Cohort
Three independent reviewers (Y.R.P., T.F.I., A.R.O., all physicians) completed chart review of 100 algorithm‐identified cases and 100 controls in a blinded fashion, with each chart reviewed by 2 reviewers. Validation was based on ascertaining the presence of definite HF (at least 1 symptom, at least 2 signs, and definite treatment for HF), and confirming that all recorded LVEF values were ≥50%.20 Chart review validation of the HFpEF algorithm demonstrated a positive predictive value of 96% and a negative predictive value of 87%.
Exposure
The primary exposure was serum sodium concentration measured within 30 days after the index date. For subjects who were hospitalized (inpatient diagnosis), we used the last serum sodium measurement in the series as the baseline value under the assumption that the most stable serum sodium value would be on the day of hospital discharge. By doing this, we reduced variability of serum sodium values that could occur with diuretic use and clinical status of subjects during their hospitalization. Outpatient diagnoses followed by hospitalization within 48 hours were counted as inpatient diagnoses, and sodium values were taken at discharge or later. We excluded subjects with any history of HF before the index date in order to ensure that we were analyzing sodium values obtained at the time of the initial diagnosis.
Outcomes
Our primary outcome was all‐cause mortality. Our secondary outcomes were the number of days hospitalized for HF and for all‐cause hospitalizations per year. HF hospitalizations were defined as having an associated primary diagnosis for heart failure (ICD‐9 code of 428.xx). The outcomes were captured from the VA health system as well as from Centers for Medicare and Medicaid Services data to include care outside the VA system. We excluded people who stayed in the hospital for more than 180 days so as to accurately capture the hospitalization outcomes.
Covariates
We obtained baseline anthropometric and laboratory variables falling on or within 30 days before the baseline sodium date, closest to the baseline sodium date. This was primarily done to extract information from when subjects were most stable after their acute HF event.
Demographic and anthropometric variables including age, body mass index, heart rate, and race were obtained from electronic medical health records. Estimated glomerular filtration rate was calculated using the chronic kidney disease epidemiology collaboration formula.21 Two independent physicians adjudicated laboratory values of serum creatinine (used for estimated glomerular filtration rate calculation) and serum potassium, and any discrepancy was resolved by further discussion. We obtained medical comorbidities falling anytime before baseline sodium measurement using ICD‐9 codes from inpatient and outpatient medical records. These include history of type 2 diabetes mellitus (250.x, 362.0, 357.2, 366.41, 249, 790.2–791.6, V45.85, V53.91, V65.46), coronary artery disease (including myocardial infarction and history of percutaneous coronary intervention or coronary artery bypass graft; 410, 411, 414.0x, 429.2x, 429.5x, 440.x, 429.7x, V45.82), hypertension (401–405.99, 437.2), hyperlipidemia (272.x), atrial fibrillation (427.31), chronic obstructive pulmonary disease (490–496, 510, 781.5), and anemia (280–285). Beta‐blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, and calcium channel blockers that were dispensed within 6 months before the baseline sodium date were identified from pharmacy records in the VA system and adjudicated by 2 independent physicians. Medications that were prescribed but not dispensed by the pharmacy and clinical trial medications were excluded from our analyses.
Statistical Analysis
We analyzed serum sodium both as a continuous and then as a categorical variable. To examine the nonlinear relationship between baseline sodium as a continuous variable and mortality and hospitalization outcomes, we developed macros to fit cubic splines with 6 equally distributed knots to crude and multivariable proportional hazards regression and generalized estimating equation–based negative binomial regression models with a sodium value of 139.00 mmol/dL as the reference. Because of instability and low frequency among extreme values, all sodium values <125.00 mmol/dL and >150.00 mmol/dL were winsorized. Five baseline serum sodium categories were determined based on the shape and inflection points of the cubic spline plots, the distribution and quantiles of serum sodium values, and generally established clinical ranges. These categories were used for all proceeding regression analyses. We also compared these results to an a priori decile‐based analysis. We built our models based on a priori knowledge of risk factors and examination of confounders, starting with crude models, then age‐adjusted, parsimonious (age, sex, race), and finally multivariable adjusted. Given that the results of the parsimonious model were similar to the age‐adjusted and multivariable models, we will not present those results. No automated variable selection process was used (eg, stepwise). Subjects entered the analysis at the time of their first sodium measurement, using a counting process approach22, 23 and person‐time accrued from baseline sodium measurement until the occurrence of death, last VA visit, or the end of follow‐up (December 31, 2014). As expected, the association between sodium and mortality did not meet the proportional hazards assumption, leading us to do a piecewise Cox proportional hazard analysis, with the first time interval covering the first 30 days and the second covering the post–30‐day period. Subjects entered the model at different times within the initial 30‐day period, and we expected them to be less clinically stable during that period, thus having different hazard rates than at later time points. Further assessment of nonproportionality of hazards showed no meaningful deviations. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated for each period for serum sodium categories, relative to the referent group. We also examined whether the relationship between baseline serum sodium and mortality varied by age. We stratified the analysis by age, categorized as <65, 65 to 75, and >75 years.
Generalized estimating equation–based negative binomial regression analyses were used to estimate the incidence density ratios for number of days of HF and all‐cause hospitalizations per year for sodium categories relative to the referent group. Incidence density was defined as the total number of days hospitalized per year divided by the total number of days observed per year. The number of days observed per year (on the log scale) was included in these models as an offset. Crude Poisson hospitalization rates were also estimated. Patients who had any hospital stay >180 days were excluded from these analyses, as they were likely part of long‐term care.
The multivariable models were adjusted for age; sex; race; body mass index; heart rate; estimated glomerular filtration rate; serum potassium; average LVEF; history of coronary artery disease, hypertension, hyperlipidemia, atrial fibrillation, chronic obstructive pulmonary disease, diabetes mellitus, and anemia; and history of medications including calcium channel blockers, β‐blockers, and angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers. Diuretics were not adjusted for in the multivariate analysis, as everyone in the analysis cohort was required to have a loop diuretic or thiazide diuretic prescription within 1 year before or after the diagnosis of HF. Medications including aldosterone antagonists and statins were not included in multivariate analysis, as they were not only not associated with baseline serum sodium measurements but also not clearly associated with clinical outcomes in HFpEF (P>0.25). All analyses were completed using SAS Enterprise Guide 7.1 (SAS Institute Inc, Cary, NC). All P values were 2‐tailed, and significance level was set at an alpha of 0.05.
Results
For the whole cohort, mean age±SD was 70.8±11.5 years. During a median follow‐up of 3.6 (interquartile range, 1.7–6.4) years there were 4983 deaths. There were 107 397.5 person‐years of follow‐up to estimate incidence density ratio for number of days of HF and all‐cause hospitalizations per year. Table 1 demonstrates baseline characteristics across serum sodium categories. Lower sodium categories tended to have younger and thinner subjects, fewer black subjects, higher average heart rate, and estimated glomerular filtration rate values, and among comorbidities a higher incidence of atrial fibrillation, anemia, diabetes mellitus, coronary artery disease, and chronic obstructive pulmonary disease and a lower incidence of hypertension. Mean LVEF was slightly but significantly higher in the lower sodium categories. Prescriptions for calcium channel blockers were less frequent in lower sodium categories.
Table 1.
Baseline Characteristics of Patients With Heart Failure With Preserved Ejection Fraction (n=27 975) According to Serum Sodium Categories
| Characteristics | Serum Sodium Measurements (mmol/L) | P Valuea | ||||
|---|---|---|---|---|---|---|
| 115.00–134.99 (N=2928) | 135.00–137.99 (N=5239) | 138.00–140.99 (N=9272) | 141.00–143.99 (N=6192) | 144.00–160.00 (N=1809) | ||
| Demographics | ||||||
| Sex (% male) | 96.3 | 96.1 | 96.2 | 96.0 | 96.6 | 0.7922 |
| Black, % | 10.3 | 12.7 | 13.7 | 15.0 | 17.8 | <0.0001 |
| Age, y | 70.0±11.6 | 69.9±11.6 | 70.6±11.5 | 71.7±11.2 | 72.5±11.0 | <0.0001 |
| BMI (kg/m2) | ||||||
| <18.5, % | 11.7 | 11.2 | 11.5 | 11.2 | 12.3 | <0.0001 |
| 18.5–24.9, % | 22.3 | 15.2 | 12.2 | 12.1 | 14.2 | |
| 25–29.9, % | 25.1 | 23.5 | 23.8 | 22.5 | 23.3 | |
| 30–34.9, % | 18.3 | 21.4 | 21.7 | 22.7 | 21.8 | |
| ≥35.0, % | 22.6 | 28.7 | 30.9 | 31.6 | 28.5 | |
| Clinical | ||||||
| Heart rate, bpm | 79.7±15.6 | 77.7±15.0 | 75.9±14.8 | 75.0±14.9 | 75.8±15.2 | <0.0001 |
| Average LVEF, % | 60.6±6.1 | 60.3±5.8 | 60.2±5.8 | 60.1±5.7 | 60.1±5.9 | 0.0013 |
| Comorbidities | ||||||
| Coronary artery disease, % | 42.0 | 40.5 | 41.8 | 41.7 | 40.7 | 0.4708 |
| Hypertension, % | 86.3 | 87.2 | 88.1 | 89.3 | 90.1 | <0.0001 |
| Hyperlipidemia, % | 59.2 | 65.1 | 67.1 | 67.2 | 63.6 | <0.0001 |
| Atrial fibrillation, % | 24.1 | 21.0 | 22.1 | 21.7 | 21.4 | 0.0286 |
| Chronic obstructive pulmonary disease, % | 50.6 | 47.0 | 44.2 | 42.3 | 41.8 | <0.0001 |
| Diabetes mellitus type 2, % | 54.0 | 55.5 | 53.0 | 52.2 | 52.6 | 0.0065 |
| Anemia, % | 42.4 | 35.8 | 32.0 | 31.6 | 35.2 | <0.0001 |
| Laboratory | ||||||
| eGFR (mL/min per 1.73 m2) | 67.1±26.8 | 64.7±25.1 | 63.9±23.9 | 61.5±23.6 | 57.2±23.8 | <0.0001 |
| Serum potassium (mmol/L) | 4.27±0.59 | 4.23±0.53 | 4.20±0.51 | 4.20±0.51 | 4.17±0.54 | <0.0001 |
| Medications | ||||||
| Calcium channel blocker, % | 40.3 | 38.5 | 40.4 | 41.9 | 42.5 | 0.0027 |
| ACE/ARB, % | 60.9 | 60.9 | 61.1 | 62.2 | 61.3 | 0.5696 |
| β‐Blocker, % | 60.7 | 59.7 | 59.6 | 59.7 | 61.0 | 0.7754 |
Missing values: race=1378; heart rate=780; eGFR=440; serum postassium=36. ACE‐I/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction.
P value for general association (ANOVA or chi‐square).
The 147 patients who died or were censored on the same day as their baseline sodium measurement were excluded from all analyses. An additional 2662 patients were excluded from the mortality multivariable analyses because of missing covariates.
Examining Sodium as a Continuous Measure
As shown in Figure 1, the association between serum sodium and all‐cause mortality follows a J‐shaped hazard curve both during the first 30 days (test for nonlinear trend, P<0.0001) and after 30 days of follow‐up (P<0.0001). Considering the obvious J shape of these cubic spline plots, the distribution of sodium values, and established sodium clinical ranges, baseline serum sodium was categorized as 115.00 to 134.99 (low serum sodium), 135.00 to 137.99 (low‐normal serum sodium), 138.00 to 140.99 (referent group), 141.00 to 143.99 (high‐normal serum sodium), and 144.00 to 160.00 (high serum sodium) mmol/dL. Sodium values between 138.00 and 140.99 mmol/L were selected as the referent group for all categorical analyses because they were considered to be a clinically normal and the center of the distribution fell within this range. A similar pattern was observed for the all‐cause hospitalization and HF hospitalization outcomes, leading us to use the same sodium categories (Figure 2). We also looked at the association of serum sodium deciles and mortality and hospitalization outcomes, obtaining similar results.
Figure 1.
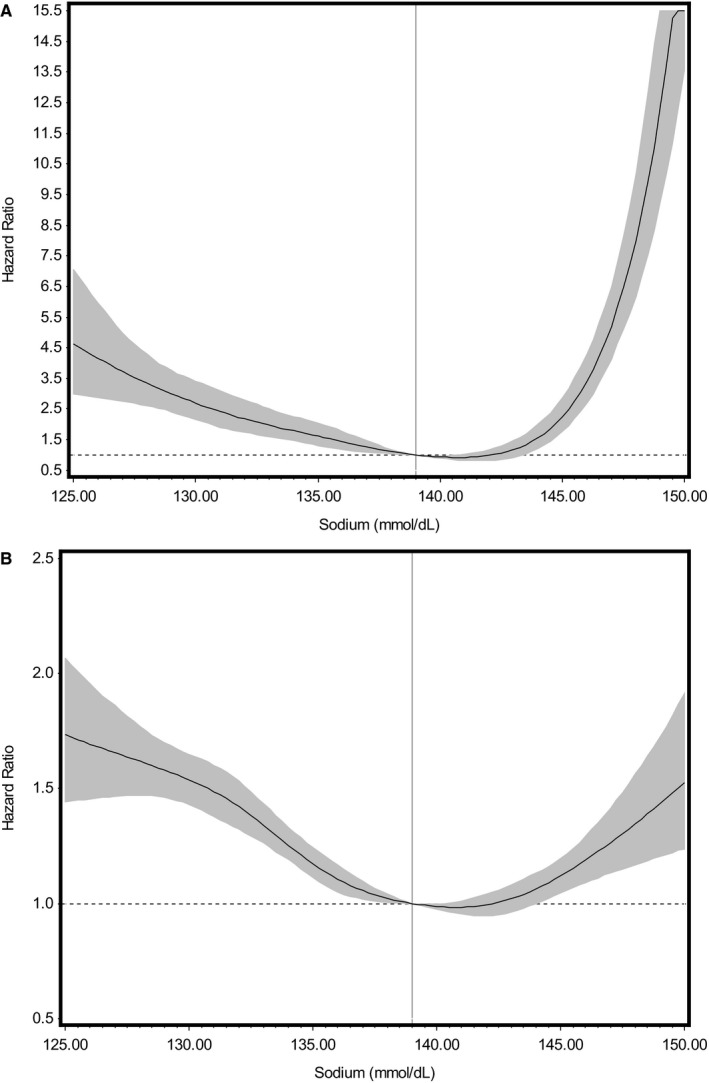
Cubic spline plots showing the multivariable adjusted Cox proportional hazards models for the relationship of baseline serum sodium to risk of death. These models were adjusted for age; sex; race; body mass index; heart rate; estimated glomerular filtration rate; serum potassium; average left ventricular ejection fraction; use of calcium channel blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, and β‐blockers; and history of coronary artery disease, hypertension, hyperlipidemia, atrial fibrillation, chronic obstructive pulmonary disease, diabetes mellitus, and anemia. A demonstrates the relationship between serum sodium as a continuous variable at baseline and mortality for the first 30 days of follow‐up (test for nonlinear trend, P<0.0001). B demonstrates the relationship between serum sodium as a continuous variable at baseline and mortality 30 days after diagnosis (P<0.0001). Shaded areas denote 95% confidence intervals for hazard ratios, and the vertical line denotes the reference value (139.00 mmol/dL).
Figure 2.
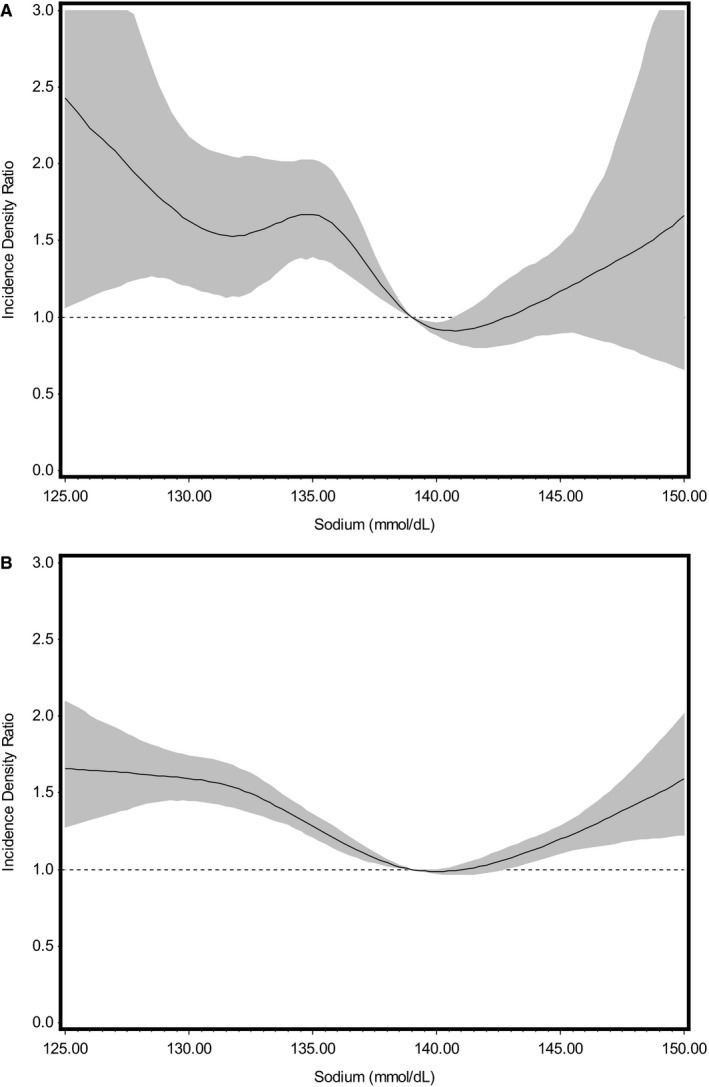
Cubic spline plot showing the multivariable adjusted negative binomial models for the relationship of baseline serum sodium to number of days hospitalized per year (heart failure and any cause). These models were adjusted for age; sex; race; body mass index; heart rate; estimated glomerular filtration rate; serum potassium; average left ventricular ejection fraction; use of calcium channel blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, and β‐blockers; and history of coronary artery disease, hypertension, hyperlipidemia, atrial fibrillation, chronic obstructive pulmonary disease, diabetes mellitus, and anemia. A demonstrates the relationship between serum sodium as a continuous variable at baseline and number of days heart failure hospitalization per year (test for nonlinear trend, P=0.0006). B demonstrates the relationship between serum sodium as a continuous variable at baseline and number of days of all‐cause hospitalization per year (P<0.0001). Shaded areas denote 95% confidence intervals for hazard ratios, and the vertical line denotes the reference value (139.00 mmol/dL).
All‐Cause Mortality Within 30 Days of Index Diagnosis
Compared with the referent group, low serum sodium and low‐normal serum sodium were associated with a 2.5‐fold (HR, 2.46; 95% CI, 1.99–3.05) and 56% (HR, 1.56; 95% CI, 1.26–1.93) higher risk of all‐cause mortality, respectively, during the first 30 days after the index diagnosis, in a multivariable model controlling for age; sex; race; body mass index; heart rate; estimated glomerular filtration rate; serum potassium; LVEF; history of coronary artery disease, hypertension, hyperlipidemia, atrial fibrillation, chronic obstructive pulmonary disease, diabetes mellitus, or anemia; and history of medications including calcium channel blockers, β‐blockers, and angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (not shown in tables). Similarly, in this multivariable model, high serum sodium was associated with an almost 3‐fold (HR, 2.81; 95% CI, 2.19–3.59) higher risk of all‐cause mortality during the first 30 days of follow‐up compared with the referent group. The 30‐day predicted mortality rate for a patient with a baseline sodium value of 139.00 mmol/dL is 2.4%.
All‐Cause Mortality After 30 Days of Index Diagnosis
Multivariable analyses also demonstrated that compared with the referent group, low, low‐normal, and high serum sodium were associated with 36% (HR, 1.36; 95% CI, 1.28–1.44), 6% (HR, 1.06; 95% CI, 1.01–1.12), and 9% (HR, 1.09; 95% CI, 1.01–1.17) higher risk of all‐cause mortality, respectively (Table 2), after 30 days following the index date. Median survival time (95% CI) was 3.13 (2.86–3.42) years, 4.90 (4.70–5.10) years, 5.68 (5.49–5.88) years, 5.72 (5.49–5.89) years, and 4.80 (4.34–5.25) years for the baseline serum sodium categories 115.00 to 134.99, 135.00 to 137.99, 138.00 to 140.99, 141.00 to 143.99, and 144.00 to 160.00 mmol/dL, respectively. The 5‐year predicted mortality rate for a patient who survived the first 30 days after initial heart failure diagnosis and had a baseline sodium value of 139.00 mmol/dL is 44.4%.
Table 2.
Hazard Ratios for All‐Cause Mortality (Beginning 30 days After Initial Heart Failure Diagnosis) by Baseline Serum Sodium Category in 24 517 Subjects With Heart Failure With Preserved Ejection Fraction Who Survived Past Day 30
| Serum Sodium (mmol/L) | Number of Deaths/Total Number of Subjects in Group | Person‐Time (y) | Crude HR (95% CI) | Age‐Adjusted HR (95% CI) | Multivariable HR (95% CI) |
|---|---|---|---|---|---|
| 115.00–134.99 | 1797/2717 | 9655.95 | 1.55 (1.47–1.63) | 1.59 (1.51–1.68) | 1.36 (1.28–1.44) |
| 135.00–137.99 | 2906/5046 | 21 831.45 | 1.12 (1.07–1.17) | 1.14 (1.09–1.20) | 1.06 (1.01–1.12) |
| 138.00–140.99 | 4936/9053 | 41 477.88 | REF | REF | REF |
| 141.00–143.99 | 3362/6041 | 28 482.09 | 0.99 (0.95–1.04) | 0.95 (0.91–0.99) | 0.99 (0.95–1.04) |
| 144.00–157.00 | 1009/1660 | 7535.83 | 1.13 (1.05–1.21) | 1.07 (1.00–1.14) | 1.09 (1.01–1.17) |
Multivariable model was adjusted for age; sex; race; body mass index; heart rate; estimated glomerular filtration rate; serum potassium; average LVEF; use of calcium channel blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, and β‐blockers; and history of coronary artery disease, hypertension, hyperlipidemia, atrial fibrillation, chronic obstructive pulmonary disease, diabetes mellitus, and anemia. CI indicates confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction; REF, referent group.
Number of Days of HF Hospitalizations per Year
Low serum sodium and low‐normal serum sodium were associated with 48% (incidence density ratio [IDR], 1.48; 95% CI, 1.10–2.00) and 38% (IDR, 1.38; 95% CI, 1.08–1.77) increases in the average number of days hospitalized for HF per year, respectively, compared with the referent group (Table 3) in the multivariable model. High serum sodium was not significantly associated with an increased risk of HF hospitalizations.
Table 3.
Incidence Density Ratios for Number of Days Hospitalized for Heart Failure per Year by Baseline Serum Sodium Category in 25 095a Subjects With Heart Failure With Preserved Ejection Fraction
| Serum Sodium (mmol/L) | Average Number of Days Hospitalized for HF Per 100 Person‐Years (95% CI) | Crude IDR (95% CI) | Age‐Adjusted IDR (95% CI) | Multivariable IDR (95% CI) |
|---|---|---|---|---|
| 115.00–134.99 | 35.67 (34.49–36.89) | 1.29 (1.00–1.66) | 1.44 (1.08–1.92) | 1.48 (1.10–2.00) |
| 135.00–137.99 | 32.85 (32.09–33.62) | 1.32 (1.08–1.63) | 1.35 (1.09–1.66) | 1.38 (1.08–1.77) |
| 138.00–140.99 | 26.12 (25.63–26.62) | REF | REF | REF |
| 141.00–143.99 | 25.49 (24.90–26.08) | 0.93 (0.77–1.12) | 0.86 (0.72–1.03) | 0.84 (0.70–1.01) |
| 144.00–160.00 | 28.66 (27.47–29.90) | 1.24 (0.94–1.63) | 1.19 (0.91–1.54) | 1.24 (0.94–1.64) |
Multivariable model was adjusted for age; sex; race; body mass index; heart rate; estimated glomerular filtration rate; serum potassium; average LVEF; use of calcium channel blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, and β‐blockers; and history of coronary artery disease, hypertension, hyperlipidemia, atrial fibrillation, chronic obstructive pulmonary disease, diabetes mellitus, and anemia. CI indicates confidence interval; HF, heart failure; IDR, incidence density ratio; LVEF, left ventricular ejection fraction; REF, referent group.
Excludes people who had a hospital stay >180 days.
Number of Days of All‐Cause Hospitalizations per Year
Low serum sodium and low‐normal serum sodium were associated with 44% (IDR, 1.43; 95% CI, 1.32–1.56) and 18% (IDR, 1.18; 95% CI, 1.10–1.27) increases in the average number of days of all‐cause hospitalizations per year, respectively (Table 4), in the multivariable model. In this analysis, higher serum sodium was associated with 19% (IDR, 1.19; 95% CI, 1.06–1.33) higher risk of number of days for all‐cause hospitalizations in the multivariable mode.
Table 4.
Incidence Density Ratios for Number of Days Hospitalized for Any Cause per Year by Baseline Serum Sodium Category in 25 095a Subjects With Heart Failure With Preserved Ejection Fraction
| Serum Sodium (mmol/L) | Average Number of Days Hospitalized for Any Cause per Person‐Year (95% CI) | Crude IDR (95% CI) | Age‐Adjusted IDR (95% CI) | Multivariable IDR (95% CI) |
|---|---|---|---|---|
| 115.0–134.9 | 7.59 (6.98–8.26) | 1.55 (1.44–1.67) | 1.61 (1.50–1.74) | 1.44 (1.32–1.56) |
| 135.0–137.9 | 5.95 (5.59–6.34) | 1.20 (1.13–1.28) | 1.22 (1.14–1.31) | 1.18 (1.10–1.27) |
| 138.0–140.9 | 5.06 (4.82–5.32) | REF | REF | REF |
| 141.0–143.9 | 5.02 (4.73–5.33) | 1.03 (0.96–1.09) | 1.00 (0.94–1.07) | 1.03 (0.96–1.10) |
| 144.0–160.0 | 5.66 (5.08–6.32) | 1.19 (1.08–1.31) | 1.16 (1.05–1.28) | 1.19 (1.06–1.33) |
Multivariable model was adjusted for age; sex; race; body mass index; heart rate; estimated glomerular filtration rate; serum potassium; average LVEF; use of calcium channel blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers; and β‐blockers; and history of coronary artery disease, hypertension, hyperlipidemia, atrial fibrillation, chronic obstructive pulmonary disease, diabetes mellitus, and anemia. CI indicates confidence interval; HF, heart failure; IDR, incidence density ratio; LVEF, left ventricular ejection fraction; REF, referent group.
Excludes people who had a hospital stay >180 days.
Age‐Stratified Analysis
There were 8750 patients <65 years old, 6183 patients 65 to 75, and 10 507 people >75 years old. Among patients <65 years of age, compared with the referent group, low‐normal and low serum sodium were associated with 13% (HR, 1.13; 95% CI, 1.03–1.24) and 50% (HR, 1.50; 95% CI, 1.35–1.67) (not shown in tables) higher risk of all‐cause mortality, respectively, in the multivariable model. Similarly, low serum sodium was associated with 39% (HR, 1.39; 95% CI, 1.23–1.56) and 23% (HR, 1.23; 95% CI, 1.13–1.34) higher risk for all‐cause mortality among patients between 65 and 75 years and >75 years of age, respectively (not shown in tables).
Discussion
In a well‐curated national cohort of HFpEF subjects, we found a J‐shaped relationship between serum sodium and all‐cause mortality and number of days of all‐cause hospitalizations per year. We also found that low sodium levels were associated with an increased number of days of hospitalizations for HF per year. In addition to low sodium values, values in the low‐normal range were also significantly associated with worse outcomes. Our study, the largest to our knowledge that has examined the relation of serum sodium to outcomes in HFpEF, suggests the utility of serum sodium to identify HFpEF patients who are at increased risk of adverse outcomes. In addition, our study also suggests that further investigations into the mechanisms of the relation of serum sodium to the natural history of HFpEF are warranted, especially whether there is a causative role for neurohormonal activation in the association between low serum sodium and adverse outcomes in HFpEF.
In our study, we demonstrated that low serum sodium is associated with worse immediate (30‐day follow up) and long‐term adverse outcomes in HFpEF. Similar associations were reported by previous smaller studies.13, 15, 16 For example, Rosinaru and colleagues15 showed that among 358 patients with HFpEF who survived a first hospitalization for HF during 7‐year follow‐up, hyponatremia (defined as serum sodium <136 mEq/L) was associated with an 87% higher risk of mortality (HR, 1.87; 95% CI, 1.40–2.52) compared with serum sodium >136 mEq/L. A meta‐analysis done by the Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) showed that hyponatremia (serum sodium <135 mEq/L) at baseline is associated with a 35% higher risk of mortality during 3 years of follow‐up among 3737 patients with HFpEF (HR, 1.35; 95% CI, 1.06–1.72), compared with serum sodium >135 mEq/L.16 On analyzing serum sodium as a continuous variable, the MAGGIC meta‐analysis found a linear increase in the risk of all‐cause mortality at 3 years at concentrations <140 mEq/L, which plateau at concentrations ≥140 mEq/L.16 These studies examined the end point of all‐cause mortality alone and included patients with a known history of HF; hence, the relation of serum sodium to time after diagnosis of HF was variable. In our study, low serum sodium was independently associated with not only a higher risk of mortality but also a higher risk of recurrent all‐cause hospitalizations and HF hospitalizations in patients without any previous history of HF before assessment of serum sodium included in the study. By excluding previous diagnosis of HF, our study enabled us to examine the association of serum sodium at the time of initial diagnosis with long‐term outcomes and avoid confounding effects of previous therapy for HF on serum sodium levels. It also allows us to examine how these associations might differ in the immediate aftermath of a diagnosis as well as longer‐term effects. In addition, our study incorporated a comprehensive list of clinical and laboratory variables, as well as medications, as covariates in our analyses. Another study addressed the association including only outpatients identified by ICD‐9 codes.13 Among 2704 veterans with HFpEF treated in VA ambulatory clinics, Bavishi et al13 showed that at 2‐year follow‐up, hyponatremia (defined as serum sodium ≤135 mEq/L) was associated with all‐cause mortality (HR, 1.40; 95% CI, 1.12–1.75) but not with all‐cause hospitalization. In contrast to this, in our study, which also included subjects identified during an index admission to ensure that serum sodium was captured at baseline, low serum sodium was associated with an increased number of days of all‐cause hospitalizations and HF hospitalizations per year. Our study was able to capture outcomes more accurately by not only including readmissions within the VA health system but also including outcomes from Centers for Medicare and Medicaid Services in case patients were admitted to hospitals outside of the VA health system. In another study that analyzed the relation of serum sodium as a continuous variable with outcomes in 407 patients with HFpEF, Kusaka et al14 showed that serum sodium predicted combined cardiovascular death and rehospitalization for HF. In this study, both hyponatremia (serum sodium <135 mmol/L) and low‐normal serum sodium (between 135 and 140 mmol/L) were independently predictive of future combined cardiovascular death and rehospitalization for HF. This study had various limitations, including being a single‐center study with a small sample size of subjects included after the initiation of medical therapy, with only one third of enrolled subjects treated with diuretics.
In previous studies, the prevalence of hyponatremia (serum sodium <135 mmol/L) among patients with HFpEF ranged from 11% to 14%.13, 16 The exact prevalence of low‐normal serum sodium is unknown, but in a study conducted by Kusaka and colleagues14 the prevalence of low‐normal serum sodium between 135 and 140 mmol/L was 47%, which was associated with higher HF‐related events compared with serum sodium more than 140 mmol/L. In our study, the prevalence of low‐normal serum sodium (between 135 and 137.99 mmol/L) was 21% and was associated with worse 30‐day and long‐term outcomes in patients with HFpEF. This shows that the prevalence of low‐normal serum sodium is high in patients with HFpEF and is associated with a worse prognosis.
There are several potential mechanisms that could explain the observed relation between low serum sodium and adverse clinical outcomes. Hyponatremia is associated with activation of vasoactive neurohormonal systems such as the sympathetic nervous system, renin‐angiotensin‐aldosterone system, and arginine vasopressin.24 Activation of the sympathetic nervous system leads to systemic vasoconstriction and an increase in heart rate and cardiac contractility, whereas activation of the renin‐angiotensin‐aldosterone system leads to direct systemic vasoconstriction and activation of secretion of aldosterone and arginine vasopressin.25 Arginine vasopressin activation leads to an increase in free‐water reabsorption in the renal collecting duct, thereby increasing the blood volume.25 These defense mechanisms are important compensatory mechanisms in emergent conditions including blood loss and low cardiac output; however, sustained activation of these systems can lead to worsening HF by multiple biologic mechanisms, including adverse effects on afterload and myocardial oxygen consumption, as well as promotion of myocyte loss and fibrosis.26, 27
In our analysis, we also found that higher serum sodium measurements are associated with a 19% increase in average number days of all‐cause hospitalizations per year in patients with HFpEF. Our inability to find this for heart failure hospitalizations is likely related to a paucity of events. This is consistent with the study by Kovesdy et al28 showing a U‐shaped relationship between serum sodium level and mortality in patients with chronic kidney disease with and without HF. Hypernatremia has also been shown to be associated with increased mortality in the general population29 and patients with HFpEF.30 The mean age in this analysis is 70 years; elderly patients are at higher risk for developing hypernatremia because of an inability to replace water losses from physical and mental limitations to access water and decreased thirst perception. Hypernatremia can have direct adverse effects on multiple organ systems, most notably on the central nervous system.31 Because high sodium values were significantly associated only with all‐cause hospitalization in our study, it is also possible that hypernatremia is merely a surrogate marker of the more severe multimorbid state.
Limitations and Strengths
There were a few limitations in this analysis of a large cohort. Care received outside of VA medical centers may not have been completely captured in this database, although the inclusion of data derived from the Centers for Medicare and Medicaid Services enabled us to capture the vast majority of events in those >65 years old. Events in veterans who were <65 years of age and ineligible for Medicaid could have been missed. There is no expectation that serum sodium would be associated with the probability of having missing events, resulting in minimal bias. In this study, we were not able to determine cause‐specific mortality. The population is predominantly white males; however, because there are no sex differences reported in the association of serum sodium with mortality in HF with reduced EF, we do not consider our findings to be affected by sex. As with any observational study, residual confounding or confounding due to unmeasured factors cannot be entirely excluded.
The current study has several strengths, this being the largest cohort of HFpEF with a long period of follow‐up compared with previously published studies. By limiting our cohort to incident heart failure, we were able to truly assess the influence of baseline sodium close to time of diagnosis. Finally, our algorithm, by ensuring that all recorded EFs were ≥50% at any time period in the VA system, prevented inclusion of heart failure with reduced, recovered, and intermediate EF in the cohort.
Conclusions
Our study, in a large cohort of patients with HFpEF rigorously curated from a large national database, demonstrates a J‐shaped relationship between serum sodium levels and long‐term clinical outcomes. Our data suggest that serum sodium could be an important clinical variable for identifying patients with HFpEF at increased risk of adverse events, healthcare utilization, and increased costs. Further investigation is needed to understand the role of neurohormonal activation in the relation between sodium levels and the natural history of HFpEF.
Author Contributions
The authors responsibilities were as follows—research design: Patel, Joseph, Gagnon, Kurgansky, Djousse, McLean; data analysis: Gagnon, Kurgansky; Article draft: Patel, Joseph, Kurgansky; article revisions: Patel, Joseph, Gagnon, Kurgansky, Djousse, McLean, Imran, Orkaby, Ho, Cho, Gaziano. The authors thank Ms Constance Nelson for her assistance with management of this project.
Sources of Funding
This project was funded by a grant from Otsuka Pharmaceuticals to Dr Joseph.
Disclosures
Joseph reports research grant support from the National Heart, Lung, and Blood Institute; Novartis; Otsuka; Amgen; and consulting for Amgen. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2018;7:e007529 DOI: 10.1161/JAHA.117.007529.)
References
- 1. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- 2. Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–332. [DOI] [PubMed] [Google Scholar]
- 3. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 5. Chan MM, Lam CS. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail. 2013;15:604–613. [DOI] [PubMed] [Google Scholar]
- 6. Liao L, Jollis JG, Anstrom KJ, Whellan DJ, Kitzman DW, Aurigemma GP, Mark DB, Schulman KA, Gottdiener JS. Costs for heart failure with normal vs reduced ejection fraction. Arch Intern Med. 2006;166:112–118. [DOI] [PubMed] [Google Scholar]
- 7. Fu M, Zhou J, Sun A, Zhang S, Zhang C, Zou Y, Fu M, Ge J. Efficacy of ACE inhibitors in chronic heart failure with preserved ejection fraction—a meta analysis of 7 prospective clinical studies. Int J Cardiol. 2012;155:33–38. [DOI] [PubMed] [Google Scholar]
- 8. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J; Investigators C Committees . Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the charm‐preserved trial. Lancet. 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 9. Yamamoto K, Origasa H, Hori M; Investigators JD . Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J‐DHF). Eur J Heart Fail. 2013;15:110–118. [DOI] [PubMed] [Google Scholar]
- 10. Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF Jr, Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; Investigators T . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 12. Ghali JK, Tam SW. The critical link of hypervolemia and hyponatremia in heart failure and the potential role of arginine vasopressin antagonists. J Card Fail. 2010;16:419–431. [DOI] [PubMed] [Google Scholar]
- 13. Bavishi C, Ather S, Bambhroliya A, Jneid H, Virani SS, Bozkurt B, Deswal A. Prognostic significance of hyponatremia among ambulatory patients with heart failure and preserved and reduced ejection fractions. Am J Cardiol. 2014;113:1834–1838. [DOI] [PubMed] [Google Scholar]
- 14. Kusaka H, Sugiyama S, Yamamoto E, Akiyama E, Matsuzawa Y, Hirata Y, Fujisue K, Kurokawa H, Matsubara J, Sugamura K, Maeda H, Jinnouchi H, Matsui K, Ogawa H. Low‐normal serum sodium and heart failure‐related events in patients with heart failure with preserved left ventricular ejection fraction. Circ J. 2016;80:411–417. [DOI] [PubMed] [Google Scholar]
- 15. Rusinaru D, Buiciuc O, Leborgne L, Slama M, Massy Z, Tribouilloy C. Relation of serum sodium level to long‐term outcome after a first hospitalization for heart failure with preserved ejection fraction. Am J Cardiol. 2009;103:405–410. [DOI] [PubMed] [Google Scholar]
- 16. Rusinaru D, Tribouilloy C, Berry C, Richards AM, Whalley GA, Earle N, Poppe KK, Guazzi M, Macin SM, Komajda M, Doughty RN; Investigators M . Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction: an individual patient data meta‐analysis(dagger): Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC). Eur J Heart Fail. 2012;14:1139–1146. [DOI] [PubMed] [Google Scholar]
- 17. Garvin JH, DuVall SL, South BR, Bray BE, Bolton D, Heavirland J, Pickard S, Heidenreich P, Shen S, Weir C, Samore M, Goldstein MK. Automated extraction of ejection fraction for quality measurement using regular expressions in unstructured information management architecture (UIMA) for heart failure. J Am Med Inform Assoc. 2012;19:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So‐Armah KA, Vasan RS, Oursler KA, Gottdiener J, Gottlieb S, Leaf D, Rodriguez‐Barradas M, Tracy RP, Gibert CL, Rimland D, Bedimo RJ, Brown ST, Goetz MB, Warner A, Crothers K, Tindle HA, Alcorn C, Bachmann JM, Justice AC, Butt AA. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the veterans aging cohort study. JAMA Cardiol. 2017;2:536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patterson OV, Freiberg MS, Skanderson M, J Fodeh S, Brandt CA, DuVall SL. Unlocking echocardiogram measurements for heart disease research through natural language processing. BMC Cardiovasc Disord. 2017;17:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (writing committee to develop cardiovascular endpoints data standards). J Am Coll Cardiol. 2015;66:403–469. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; Ckd EPI . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. The sas lgtphcurv9 macro. 2017. Available at: https://cdn1.sph.harvard.edu/wp-content/uploads/sites/271/2012/09/lgtphcurv9_7-3-2011.pdf. Accessed June 9, 2018.
- 23. Sas/stat(r) 14.1 user's guide. The phreg procedure. Available at: http://support.sas.com/documentation/cdl/en/statug/68162/HTML/default/viewer.htm#statug_phreg_gettingstarted.htm. Accessed June 9, 2018.
- 24. Dzau VJ, Colucci WS, Hollenberg NK, Williams GH. Relation of the renin‐angiotensin‐aldosterone system to clinical state in congestive heart failure. Circulation. 1981;63:645–651. [DOI] [PubMed] [Google Scholar]
- 25. Chatterjee K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol. 2005;95:8B–13B. [DOI] [PubMed] [Google Scholar]
- 26. Goldsmith SR, Francis GS, Cowley AW Jr, Goldenberg IF, Cohn JN. Hemodynamic effects of infused arginine vasopressin in congestive heart failure. J Am Coll Cardiol. 1986;8:779–783. [DOI] [PubMed] [Google Scholar]
- 27. Fan YH, Zhao LY, Zheng QS, Dong H, Wang HC, Yang XD. Arginine vasopressin increases iNOS‐NO system activity in cardiac fibroblasts through NF‐kappaB activation and its relation with myocardial fibrosis. Life Sci. 2007;81:327–335. [DOI] [PubMed] [Google Scholar]
- 28. Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, Kalantar‐Zadeh K. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wannamethee SG, Shaper AG, Lennon L, Papacosta O, Whincup P. Mild hyponatremia, hypernatremia and incident cardiovascular disease and mortality in older men: a population‐based cohort study. Nutr Metab Cardiovasc Dis. 2016;26:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deubner N, Berliner D, Frey A, Guder G, Brenner S, Fenske W, Allolio B, Ertl G, Angermann CE, Stork S. Dysnatraemia in heart failure. Eur J Heart Fail. 2012;14:1147–1154. [DOI] [PubMed] [Google Scholar]
- 31. Arieff AI, Guisado R. Effects on the central nervous system of hypernatremic and hyponatremic states. Kidney Int. 1976;10:104–116. [DOI] [PubMed] [Google Scholar]


