Abstract
Background
There is a paucity of contemporary data on the characteristics and outcomes of acute ischemic stroke (AIS) in patients on maintenance dialysis.
Methods and Results
We used the nationwide inpatient sample to examine contemporary trends in the incidence, management patterns, and outcomes of AIS in dialysis patients. A total of 930 010 patients were admitted with AIS between 2003 and 2014, of whom 13 642 (1.5%) were on dialysis. Overall, the incidence of AIS among dialysis patients decreased significantly (P trend<0.001), while it remained stable in non‐dialysis patients (P trend=0.78). Compared with non‐dialysis patients, those on dialysis were younger (67±13 years versus 71±15 years, P<0.001), and had higher prevalence of major comorbidities. Black patients constituted 35.2% of dialysis patients admitted with AIS compared with 16.7% of patients in the non‐dialysis group (P<0.001). After propensity score matching, in‐hospital mortality was higher in the dialysis group (7.6% versus 5.2%, P<0.001), but this mortality gap narrowed overtime (P trend<0.001). Hemorrhagic conversion and gastrointestinal bleeding rates were similar, but blood transfusion was more common in the dialysis group. Rates of severe disability surrogates (tracheostomy, gastrostomy, mechanical ventilation and non‐home discharge) were also similar in both groups. However, dialysis patients had longer hospitalizations, and accrued a 25% higher total cost of acute care.
Conclusions
Dialysis patients have 8‐folds higher incidence of AIS compared withnon‐dialysis patients. They also have higher risk‐adjusted in‐hospital mortality, sepsis and blood transfusion, longer hospitalizations, and higher cost. There is a need to identify preventative strategies to reduce the risk of AIS in the dialysis population.
Keywords: dialysis, end stage renal disease, ischemic stroke
Subject Categories: Ischemic Stroke
Clinical Perspective
What Is New?
Dialysis patients have 8‐fold higher incidence of acute ischemic stroke than non‐dialysis patients.
Patients of black race constituted 35% of all dialysis patients admitted with ischemic stroke.
Acute ischemic strokes in dialysis patients are associated with higher in‐hospital morbidity, mortality, resource utilization, and cost compared with acute ischemic patients not on dialysis, although the mortality difference between the 2 groups narrowed overtime.
What Are the Clinical Implications?
Further research is needed to identify effective stroke prevention strategies in dialysis patients, given the persistent high incidence of ischemic stroke among them.
Stroke is a leading cause of morbidity and mortality worldwide.1 Patients with end‐stage renal disease receiving maintenance dialysis have higher incidences of both ischemic and hemorrhagic strokes compared with the general population.2, 3, 4, 5 In addition, distinctive risk profiles, mechanisms of stroke, and racial disparities have been suggested in several population studies of ischemic stroke in the dialysis population.2, 3, 4, 5, 6, 7, 8, 9 Dialysis status has also been shown to be an independent predictor of in‐hospital mortality following acute stroke.2, 5, 10, 11, 12 However, contemporary studies focusing on the incidence, characteristics, and outcomes of acute ischemic stroke (AIS) in patients on maintenance dialysis are sparse. This study uses a large nationwide representative sample and aims to assess (1) temporal trends in the incidence of AIS among patients on maintenance dialysis, (2) the characteristics and clinical risk profiles of dialysis patients admitted with AIS, and (3) temporal trends in in‐hospital morbidity and mortality, cost, and resource utilization among dialysis and non‐dialysis patients.
Methods
The data, analytic methods, and study materials will be made available upon request to other researchers for purposes of reproducing the results.
Study Data
The Nationwide Inpatient Sample (NIS) was used to derive patient relevant information between January 1, 2003 and December 31, 2014. NIS is the largest publicly available all‐payer administrative claims‐based database and contains information about patient discharges from 1000 hospitals in 45 states. It contains clinical and resource utilization information on 5 to 8 million discharges annually, with safeguards to protect the privacy of individual patients, physicians, and hospitals. These data are stratified to represent ≈20% of US inpatient hospitalizations across different hospital and geographic regions (random sample). National estimates (NE) of the entire US hospitalized population were calculated using the Agency for Healthcare Research and Quality sampling and weighting method. The Institutional Review Board at West Virginia University exempted the study from board approval and waived the requirement for informed consent because the NIS is a publicly available deidentified database.
Study Population
Patients with a principle discharge diagnosis of AIS (International Classification of Diseases‐Ninth Revision‐Clinical Modification [ICD‐9‐CM] codes 433–437.1) during the study period were identified. The study population was then further divided into 2 groups based on maintenance dialysis status. Maintenance dialysis was defined as patients with ICD‐9‐CM code for end stage renal disease 585.6, procedure code for hemodialysis 39.95 or peritoneal dialysis 54.98 and absence of ICD‐9‐CM code for acute kidney injury 584.X. ICD‐9‐CM codes 584.X have >90% sensitivity and negative predictive value for acute kidney injury.13 Patients admitted with AIS with or without maintenance dialysis status were entered into a nearest neighbor 1:1 variable ratio, parallel, balanced propensity‐matching model using a caliper of 0.01 without replacement to derive 2 propensity matched groups of patients for comparative analyses. A flow diagram of the study population is shown in Figure 1. The variables included in the propensity match model are listed in Table S1.
Figure 1.
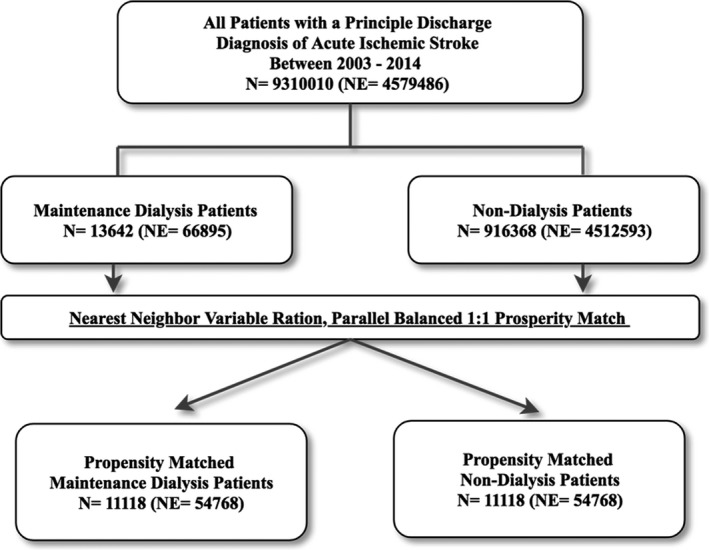
Flow diagram of the study's population. NE indicates national estimate.
Study End Points
The primary end points of the study were (1) Incidence rate of AIS among dialysis and non‐dialysis patients, and (2) in‐hospital mortality. Secondary end points were (1) in‐hospital morbidities (bleeding and infectious complications), (2) surrogates of severe disability (non‐home discharges, gastrostomy, mechanical ventilation, and tracheostomy), and (3) cost of hospitalization and length of stay. These end points were compared between 2 study groups before and after propensity score matching. The ICD‐9 codes used to identify secondary end points are listed in Table S2.
Statistical Analysis
Outcomes analysis was performed using the actual 20% sample available in NIS, while the trend analysis was performed using the national estimate. This is a standard methodology in other research involving the NIS. Descriptive statistics presented as frequencies with percentages for categorical variables. Mean, SD, median, and interquartile range were reported for continuous measures. Baseline characteristics were compared using a Pearson chi‐squared test and Fisher's exact test for categorical variables and an independent‐samples t test for continuous variables. Trend weights accounting for changes in the NIS sampling design are only available for data between 1998 and 2011. For 2012 and 2013, trend weights were not available, and the standard survey weights were used. Incidence was calculated based on total number of patients in respective years from the official US census data (https://census.gov) and the USRDS Annual Data Report (https://usrds.org) (Table S3).14, 15 Matched categorical variables were presented as frequencies with percentages and compared using McNamara's test. Matched continuous variables were presented as means with SDs and compared using a paired‐samples t test. Tends over time were examined using a Mann–Kendall test for trend. A Type‐I error of <0.05 was considered statistically significant. Given the high incidence of blood transfusion and sepsis in the dialysis population, 2 multiple logistic regression models were developed using unmatched sample to assess the association between blood transfusion or sepsis with in‐hospital mortality: model‐1 adjusted for patients’ characteristics and model‐2 adjusted for patients and hospital characteristics. All statistical analyses were performed with SPSS version 24 (IBM Corporation, Armonk, NY) and R, version 3.3.1.
Results
A total of 930 010 patients (representing a national estimate of 4 579 486 patients) were admitted with AIS between 2003 and 2014, of whom 13 642 (1.5%) were on maintenance hemodialysis. Averaged over the study years, the incidence rate of AIS in patients on maintenance dialysis was 8‐folds higher than in non‐dialysis patients (1019 per 100 000 versus 123 per 100 000, P<0.001) (Figure 2). However, there were significant differences in the trends of AIS incidence between the 2 groups: While the incidence of AIS decreased significantly overtime in dialysis patients (from 1390 per 100 000 in 2003 to 783 per 100 000 in 2014, P trend<0.001), it remained stable in non‐dialysis patients (133 per 100 000 in 2003 and 124 per 100 000 in 2014, P trend=0.78).
Figure 2.
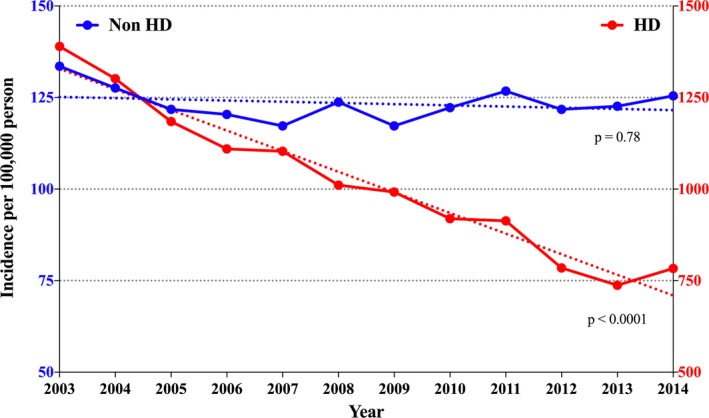
Incidence of acute ischemic stroke in dialysis and non‐dialysis patients in the United States between 2003 and 2014. The x axis shows the calendar years (2003–2014). The y axis shows the incidence of acute ischemic stroke in dialysis patient on the right (red line) and in non‐dialysis patients on the left (blue line) with different scales of the y axis for the 2 populations. HD indicates hemodialysis; Non HD, non‐hemodialysis.
Dialysis patients were younger (67±13 years versus 71±15 years) and had significantly higher percentages of females (55.6% versus 53.1%, P<0.001), and patients of black and Hispanic races compared with non‐dialysis patients (35.2% versus 14.6%, and 16.7% versus 7.6%, P<0.001 for all). Dialysis patients also had significantly higher prevalence of atherosclerotic risk factors, anemia and heart failure but similar prevalence of atrial fibrillation compared with non‐dialysis patients (Table 1). There was a similar increase in the utilization of thrombolytics in dialysis and non‐dialysis patients between 2003 and 2014 (0.6–5.6%, and 1.1–7.2%, respectively, P trend<0.001 for all) (Figure 3). Mechanical thrombectomy remained uncommonly utilized in both group (0.2% and 0.5% in dialysis and non‐dialysis patients, respectively).
Table 1.
Baseline Characteristics of Acute Ischemic Stroke Patients Stratified by Dialysis Status
| Unmatched Cohorts | Matched Cohorts | |||||
|---|---|---|---|---|---|---|
| Non‐Dialysis (n=916 368) | Dialysis (n=13 642) | P Value | Non‐Dialysis (n=11 118) | Dialysis (n=11 118) | P Value | |
| Age—mean (SD), y | 71 (14) | 67 (13) | <0.001 | 67 (13) | 67 (13) | 0.861 |
| 18 to 44 | 4.7% | 5.7% | <0.001 | 5.1% | 5.3% | 0.301 |
| 45 to 65 | 28.7% | 37.8% | 37.2% | 37.6% | ||
| 66 to 84 | 49.5% | 51% | 52.5% | 51.5% | ||
| >85 | 17.1% | 5.4% | 5.2% | 5.6% | ||
| Female | 53.1% | 55.6% | <0.001 | 54.4% | 54.9% | 0.499 |
| Race | ||||||
| White | 70.2% | 43.4% | 43.9% | 44.1% | ||
| Black | 16.7% | 35.2% | 35.6% | 35.0% | ||
| Hispanic | 7.6% | 14.6% | 13.8% | 14.1% | ||
| Medical comorbidities | ||||||
| Hypertension | 78.2% | 94.7% | <0.001 | 93.9% | 94.4% | 0.118 |
| Diabetes mellitus | 33.6% | 63.5% | <0.001 | 64.6% | 64.6% | 0.988 |
| Atrial fibrillation | 18.2% | 18% | 0.718 | 18.2% | 18.6% | 0.606 |
| Chronic lung disease | 14.6% | 15.3% | 0.014 | 15.9% | 15.6% | 0.99 |
| Anemia | 10.7% | 44.1% | <0.001 | 45.1% | 45.4% | 0.654 |
| Heart failure | 12.7% | 28% | <0.001 | 27.9% | 27.8% | 0.94 |
| Smoking | 15% | 6% | <0.001 | 6.2% | 6.4% | 0.659 |
| Vascular disease | 8.2% | 16.7% | <0.001 | 16.8% | 17.3% | 0.342 |
| Coronary disease | 14.7% | 21.7% | <0.001 | 22.4% | 22.5% | 0.974 |
| Liver disease | 1% | 2.5% | <0.001 | 2.6% | 2.5% | 0.898 |
| Thrombolytic therapy | 3.8% | 2.5% | <0.001 | 2.8% | 2.8% | 0.99 |
| Thrombectomy | 0.5% | 0.2% | <0.001 | 0.3% | 0.2% | 0.583 |
| Patient and hospital demographics | ||||||
| Teaching hospital | 44% | 48.8% | <0.001 | 49.2% | 49.1% | 0.852 |
| Region of the hospital | <0.001 | 0.649 | ||||
| Northeast | 17% | 16.2% | 18.7% | 18.2% | ||
| Midwest | 22.8% | 20.8% | 16.3% | 16.6% | ||
| South | 42.3% | 44% | 45.2% | 45.1% | ||
| Rural location | 14.4% | 7.2% | <0.001 | 6.4% | 6.5% | 0.868 |
| Primary payer | <0.001 | 0.944 | ||||
| Medicare/Medicaid | 73% | 88.2% | 87.4% | 88.4% | ||
| Private including HMO | 19.4% | 10% | 10.8% | 9.9% | ||
| Self‐pay | 4.7% | 0.8% | 0.7% | 0.8% | ||
| Median household income | <0.001 | 0.739 | ||||
| 1. 0 to 25th percentile | 30.7% | 37.5% | 38.3% | 37.8% | ||
| 2. 26 to 50th percentile | 26.9% | 25.6% | 25.4% | 25.1% | ||
| 3. 51 to 75th percentile | 23.2% | 21.3% | 20.1% | 20.9% | ||
| 4. 76 to 100th percentile | 19.2% | 15.6% | 16.2% | 16.3% | ||
Figure 3.
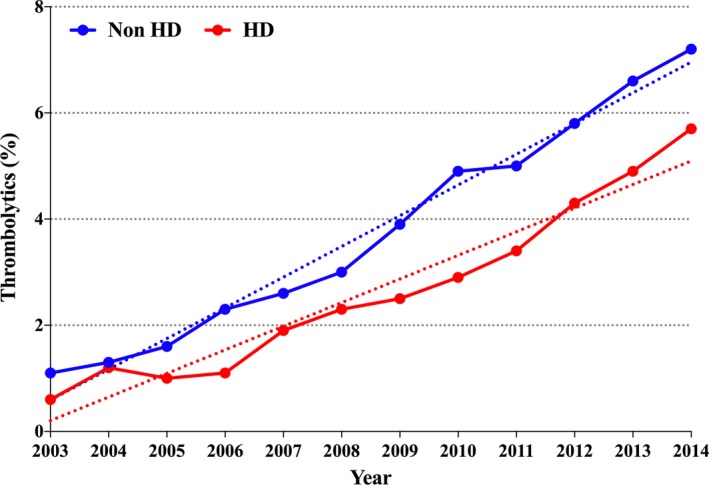
Utilization trend of systemic thrombolysis in dialysis and non‐dialysis patients admitted with acute ischemic stroke. HD indicates hemodialysis; Non HD, non‐hemodialysis.
Dialysis patients were more likely to be treated at teaching and urban hospitals (Table 1). Majority of patients (42.3% versus 44%, P<0.001 in the non‐dialysis versus dialysis group, respectively) resided in southern states. Dialysis patients were more likely to be insured by Medicare (88.2% versus 73%, P<0.001) and to be in the lowest (0–25th percentile) of median household income (37.5% versus 30.7%, P<0.001). After propensity matching for age, sex, comorbidities and hospital characteristic, baseline characteristics became well balanced between the 2 matched groups (Table 1).
Outcomes of the Unmatched Cohorts
In‐hospital mortality was significantly higher in the dialysis group (7.4% versus 5.0%, P<0.001). However, this mortality gap narrowed overtime (Figure 4). Dialysis patients also had higher incidences of gastrointestinal bleeding, blood transfusion and infectious complications with the exception of urinary tract infections, which was higher in the non‐dialysis cohort (Table 2). Hemorrhagic stroke conversion was, however, similar between dialysis and non‐dialysis patients (1.3% versus 1.3%, P=0.83). Surrogates of severe disability (mechanical ventilation, tracheostomy and non‐home discharges) were more frequent in the dialysis group, who also had longer hospitalizations and higher cost of care.
Figure 4.
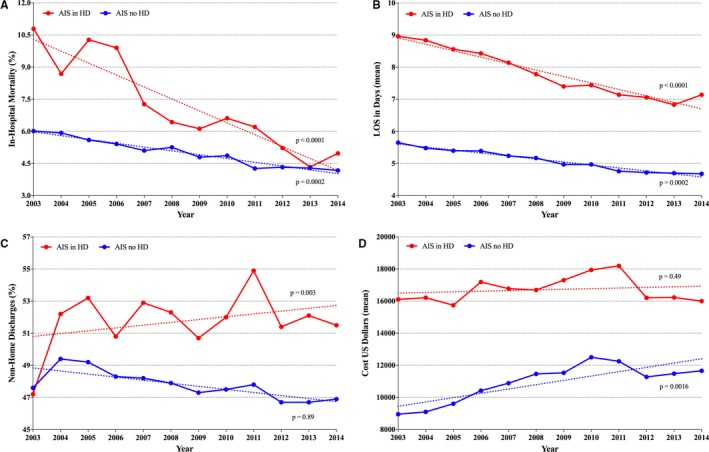
Temporal trends of stroke mortality, resource utilization and cost in dialysis vs non‐dialysis patients between 2003 and 2014. (A) In‐hospital mortality, (B) hospital length of stay, (C) discharge disposition, (D) cost. AIS indicates acute ischemic stroke; HD, hemodialysis.
Table 2.
In‐Hospital Outcomes of Acute Ischemic Stroke Among Dialysis and Non‐Dialysis Patients
| Unmatched Cohorts | Matched Cohorts | |||||
|---|---|---|---|---|---|---|
| Non‐Dialysis (n=916 368) | Dialysis (n=13 642) | P Value | Non‐Dialysis (n=11 118) | Dialysis (n=11 118) | P Value | |
| In‐hospital death | 5% | 7.4% | <0.001 | 5.2% | 7.6% | <0.001 |
| Bleeding complications | ||||||
| Intracranial hemorrhage | 1.3% | 1.3% | 0.826 | 1.4% | 1.3% | 0.727 |
| Craniotomy | 0.2% | 0.1% | <0.001 | 0.1% | 0.1% | 0.23 |
| Blood transfusion | 2.3% | 8.5% | <0.001 | 7.2% | 8.6% | <0.001 |
| Gastrointestinal bleeding | 0.5% | 1.1% | <0.001 | 1% | 1.1% | 0.512 |
| Infectious complications | ||||||
| Acquired pneumonia | 2.9% | 4.5% | <0.001 | 4% | 4.4% | 0.184 |
| Sepsis | 1% | 4% | <0.001 | 2.5% | 4% | <0.001 |
| Urinary tract infection | 12% | 10.1% | <0.001 | 16.9% | 10.1% | <0.001 |
| Severe disability surrogates | ||||||
| Non‐home discharge | 45.4% | 47.9% | <0.001 | 52.5% | 52.6% | 0.677 |
| Gastrostomy | 4.2% | 5.5% | <0.001 | 6% | 5.6% | 0.186 |
| Mechanical ventilation | 1.4% | 2.8% | <0.001 | 2.9% | 2.8% | 0.777 |
| Tracheostomy | 0.1% | 0.1% | 0.997 | 0.1% | 0.1% | 0.152 |
| LOS, median (25th, 75th percentile), day | 4 (2, 6) | 6 (4, 9) | <0.001 | 5 (3, 8) | 6 (4, 9) | <0.001 |
| Cost, median (25th, 75th percentile), $ | 7685 (5178, 12 141) | 11 173 (7180, 18 527) | <0.001 | 10 357 (6552, 15 500) | 11 046 (7584, 17 765) | <0.001 |
$ indicates dollar; LOS, length of stay.
Outcomes of the Matched Cohorts
After vigorous propensity matching adjusting for baseline demographics, clinical co‐morbidities, region, insurance status and hospital characteristics, the 2 groups were well matched (Table 2). In‐hospital mortality remained significantly higher in the dialysis group (7.6% versus 5.2%, P<0.001). However, most complications became non‐significantly different between the 2 groups, with the exception of blood transfusion and sepsis, which were higher in the dialysis cohort and urinary tract infection, which was higher in the non‐dialysis cohort (Table 2). Mean hospital length of stay remained longer (8±9 versus 7±10, P<0.001), and mean hospital cost remained higher ($16 517±$20 255 versus $14 683±$20 597, P<0.001) in the dialysis group. Both sepsis and blood transfusion were significant predictors of in‐hospital mortality in the dialysis cohort (Table 3).
Table 3.
The Contribution of Sepsis and Blood Transfusion to In‐Hospital Mortality in Patients With Ischemic Stroke on Maintenance Dialysis
Discussion
The major findings of this investigation are: (1) Dialysis patients have 8‐fold higher incidence of AIS compared with non‐dialysis patients. However, the incidence of AIS in the dialysis population decreased significantly in the past decade while it remained stable among non‐dialysis patients; (2) Dialysis patients experiencing AIS have distinctive demographics and clinical risk profiles compared with those not on dialysis; (3) In‐hospital mortality following AIS is higher among dialysis than non‐dialysis patients, but this mortality gap narrowed overtime; and (4) AIS in dialysis patients is associated with higher rates of infectious complications and blood transfusion, longer hospitalizations, and higher cost of care.
Patients with end‐stage renal disease on dialysis have higher prevalence of vascular risk factors and cardiovascular events than non‐dialysis population. Their incidence of AIS is therefore anticipated to be higher than the non‐dialysis population. Seliger et al first reported an excess incidence of AIS among the dialysis population in the United States between 1993 and 1998.3 In their study, patients on dialysis had significantly higher odds of experiencing AIS than non‐dialysis patients (odd ratio 6.1, and 10.1 for white males and females and 4.3 and 6.5 for black males and females, P<0.001 for all). Other studies investigating the incidence of AIS in dialysis patients were single center, non‐contemporary or primarily involved non‐US population.2, 8, 16, 17, 18 To our knowledge, this is the largest study examining contemporary rates of AIS in the dialysis and non‐dialysis population. Similar to the findings by Seliger et al, our study shows that the incidence of AIS in dialysis patients is several folds higher than patients who are not on dialysis. Indeed, dialysis patients constituted 1.5% of all patients admitted with AIS in the United States between 2003 and 2014. Nevertheless, the incidence of AIS in dialysis patients decreased by 36% during the study period, while it remained unchanged in non‐dialysis patients. The underlying mechanism of this decrement is unknown, but some potential causes can be speculated. The substantial improvement in dialysis access care, the temporal increase in the number of kidney transplantation, and the widespread use of Erythropoiesis‐Stimulating Agents may have possibly contributed to the decline in AIS rate among dialysis patients in the past decade.19 However, further studies are needed to understand the underlying mechanisms of the excess AIS rate in dialysis patients to identify opportunities for further improvement.
Examination of the differences in the clinical risk profiles and demographics and between dialysis and non‐dialysis patients reveals several intriguing findings: (1) Over 35% of dialysis patients admitted with AIS are of black race. This percentage is higher than the percentage of black individuals in the overall population, it is indeed proportional to their percentage of black patients among dialysis patients in the United States,13 (2) Dialysis patients experiencing AIS are more likely to be cared for at urban, teaching hospital and to be in the lowest quartile of median household income, (3) Although dialysis patients had higher prevalence of hypertension, diabetes mellitus, coronary and vascular diseases, and heart failure, they had lower prevalence of atrial fibrillation, a major risk factor for AIS. This could be related to the lower incidence of atrial fibrillation among black and Hispanic patients, who together constituted 50% of the AIS dialysis population,20 and (4) Despite the higher prevalence of vascular disease and the higher perceived risk of bleeding among dialysis patients, the utilization of thrombolytic increased proportionally in dialysis and non‐dialysis patients. The utilization rates of mechanical thrombectomy were minuscule in both groups, because these data preceded the published trials demonstrating the significant benefit of thrombectomy in selected AIS patients.21
The higher mortality following AIS in dialysis versus non‐dialysis patients has been suggested in prior studies: in a recent analysis from the large Get With The Guidelines database, risk‐adjusted in hospital mortality among dialysis patients admitted with AIS was 56% higher than in patients with normal renal function.5 However, this analysis is limited by only including patients ≥65 years of age and ensured by Medicare, while 45% of dialysis patients admitted with AIS in our study were <65 years of age. In a nationwide cohort of patients admitted with AIS in Taiwan, 30‐day mortality was greater in dialysis patients than in those with normal kidney function (hazard ratio, 2.33; 95% confidence interval=1.80–3.02).22 Our data are in line with the aforementioned studies, demonstrating a 46% increase in risk‐adjusted in‐hospital mortality among dialysis patients admitted with AIS compared with non‐dialysis patients. However, our study shows a persistent decline in post‐AIS morality among dialysis patients in the past decade. Although the granularity of data in this study precludes accurate speculations on the causes of this mortality trend, it is in line with the decreasing mortality rates among dialysis patients overall in the past decade.14
Data on the potential impact of dialysis status on AIS severity and complications are limited. El Husseini et al found that dialysis status was associated with lower odds of home discharge following AIS in patients >65 years of age (odd ratio 0.86; confidence interval 0.79–0.94, P<0.001).5 Non‐homes discharge is an indirect surrogate to severe disability following AIS. In our study, although dialysis patients had higher rates on non‐home discharge overall, no statistically significant differences were seen in discharge status after risk adjustment between dialysis and non‐dialysis patients. Other surrogates of severe disability (gastrostomy, mechanical ventilation, and tracheostomy) were also not statistically significantly different in the propensity matched cohorts. Nevertheless, AIS in dialysis patients was associated with longer hospitalization and higher cost despite risk adjustment. These findings may have important implications on the management of AIS in the dialysis population, in whom certain treatment decisions might be taken or forgone because of fear of bleeding complications or anticipated functional recovery.
Limitations
This study has several limitations. (1) The NIS is an administrative database that gathers data for billing purposes and can be limited by erroneous coding. However, we used ICD‐9‐CM codes for AIS and its complications that have been shown to have high specificity and positive predictive value.23, 24 In addition, the hard, clinical end points used in our analysis (in‐hospital mortality) are difficult to miscode, (2) To ensure accuracy of the diagnosis; we limited the inclusion of patients with AIS to those in whom the primary admission was for AIS. Patients hospitalized for other reasons in whom AIS occurred later in the hospitalization were excluded. Therefore, the true incidence of AIS in both groups is likely higher than what is reported in this study, (3) NIS allows detailed assessment of in‐hospital outcomes. However, baseline laboratory and brain imaging data are not captured. Also, data needed to calculate traditional stroke severity scale numbers are not available in NIS. We used surrogates of stroke severity that have been previously used in administrative databases,25, 26 (4) the potential for unmeasured confounders may bias the outcomes results. However, we believe that our rigorous propensity matching has adequately addressed this selection bias, and (5) lastly, long‐term outcomes beyond hospital discharge are not available in NIS. However, our findings of high but gradually improving rates of inpatient mortality and substantial cost and resource utilization following AIS in hemodialysis patients provide important insight to the clinicians caring for these patients.
Conclusions
Despite the temporal decline, the incidence of ischemic stroke among dialysis patients remains several folds higher than patients not on dialysis. After risk adjustment, rates of in‐hospital mortality, blood transfusion, and sepsis were higher among dialysis patients, who also had longer hospitalizations and accrued a higher cost of care. Further studies are needed to identify appropriate strategies for stroke prevention and optimal management algorithm in dialysis patients.
Disclosures
None.
Supporting information
Table S1. Patient and Hospital Characteristics Included in the Propensity Score Matching
Table S2. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) Codes for Outcomes
Table S3. Calculation of Annual AIS Incidence in the Dialysis and Non‐Dialysis Populations
(J Am Heart Assoc. 2018;7:e008686 DOI: 10.1161/JAHA.118.008686.)
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Power A, Chan K, Singh SK, Taube D, Duncan N. Appraising stroke risk in maintenance hemodialysis patients: a large single‐center cohort study. Am J Kidney Dis. 2012;59:249–257. [DOI] [PubMed] [Google Scholar]
- 3. Seliger SL, Gillen DL, Longstreth WT Jr, Kestenbaum B, Stehman‐Breen CO. Elevated risk of stroke among patients with end‐stage renal disease. Kidney Int. 2003;64:603–609. [DOI] [PubMed] [Google Scholar]
- 4. Wang HH, Hung SY, Sung JM, Hung KY, Wang JD. Risk of stroke in long‐term dialysis patients compared with the general population. Am J Kidney Dis. 2014;63:604–611. [DOI] [PubMed] [Google Scholar]
- 5. El Husseini N, Fonarow GC, Smith EE, Ju C, Schwamm LH, Hernandez AF, Schulte PJ, Xian Y, Goldstein LB. Renal dysfunction is associated with poststroke discharge disposition and in‐hospital mortality: findings from Get With The Guidelines‐Stroke. Stroke. 2017;48:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smeeton NC, Heuschmann PU, Rudd AG, McEvoy AW, Kitchen ND, Sarker SJ, Wolfe CD. Incidence of hemorrhagic stroke in black Caribbean, black African, and white populations: the South London stroke register, 1995–2004. Stroke. 2007;38:3133–3138. [DOI] [PubMed] [Google Scholar]
- 7. Tariq N, Adil MM, Saeed F, Chaudhry SA, Qureshi AI. Outcomes of thrombolytic treatment for acute ischemic stroke in dialysis‐dependent patients in the United States. J Stroke Cerebrovasc Dis. 2013;22:e354–e359. [DOI] [PubMed] [Google Scholar]
- 8. Wetmore JB, Ellerbeck EF, Mahnken JD, Phadnis MA, Rigler SK, Spertus JA, Zhou X, Mukhopadhyay P, Shireman TI. Stroke and the “stroke belt” in dialysis: contribution of patient characteristics to ischemic stroke rate and its geographic variation. J Am Soc Nephrol. 2013;24:2053–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wetmore JB, Phadnis MA, Mahnken JD, Ellerbeck EF, Rigler SK, Zhou X, Shireman TI. Race, ethnicity, and state‐by‐state geographic variation in hemorrhagic stroke in dialysis patients. Clin J Am Soc Nephrol. 2014;9:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ovbiagele B. Chronic kidney disease and risk of death during hospitalization for stroke. J Neurol Sci. 2011;301:46–50. [DOI] [PubMed] [Google Scholar]
- 11. Rowat A, Graham C, Dennis M. Renal dysfunction in stroke patients: a hospital‐based cohort study and systematic review. Int J Stroke. 2014;9:633–639. [DOI] [PubMed] [Google Scholar]
- 12. Synhaeve NE, van Alebeek ME, Arntz RM, Maaijwee NA, Rutten‐Jacobs LC, Schoonderwaldt HC, de Kort PL, van der Vlugt MJ, Van Dijk EJ, Wetzels JF, de Leeuw FE. Kidney dysfunction increases mortality and incident events after young stroke: the FUTURE study. Cerebrovasc Dis. 2016;42:224–231. [DOI] [PubMed] [Google Scholar]
- 13. Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694. [DOI] [PubMed] [Google Scholar]
- 14. End‐stage renal disease in the United States; annual data report. 2017.
- 15. US Census Bureau Data. 2017.
- 16. Sozio SM, Armstrong PA, Coresh J, Jaar BG, Fink NE, Plantinga LC, Powe NR, Parekh RS. Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: the choices for healthy outcomes in caring for ESRD (CHOICE) study. Am J Kidney Dis. 2009;54:468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawamura M, Fijimoto S, Hisanaga S, Yamamoto Y, Eto T. Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis. 1998;31:991–996. [DOI] [PubMed] [Google Scholar]
- 18. Iseki K, Fukiyama K. Predictors of stroke in patients receiving chronic hemodialysis. Kidney Int. 1996;50:1672–1675. [DOI] [PubMed] [Google Scholar]
- 19. Aljohani SAF, Almustapha A, Amin AH, Busu T, Kawsar A, Alkhouli M. Contemporary outcomes of cardioembolic stroke in the United States. Circulation. 2017;136:A19672. [Google Scholar]
- 20. Herrington W, Haynes R, Staplin N, Emberson J, Baigent C, Landray M. Evidence for the prevention and treatment of stroke in dialysis patients. Semin Dial. 2015;28:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sardar P, Chatterjee S, Giri J, Kundu A, Tandar A, Sen P, Nairooz R, Huston J, Ryan JJ, Bashir R, Parikh SA, White CJ, Meyers PM, Mukherjee D, Majersik JJ, Gray WA. Endovascular therapy for acute ischaemic stroke: a systematic review and meta‐analysis of randomized trials. Eur Heart J. 2015;36:2373–2380. [DOI] [PubMed] [Google Scholar]
- 22. Wang IK, Liu CH, Yen TH, Jeng JS, Sung SF, Huang PH, Li JY, Sun Y, Wei CY, Lien LM, Tsai IJ, Sung FC, Hsu CY; Taiwan Stroke Registry Investigators . Renal function is associated with 1‐month and 1‐year mortality in patients with ischemic stroke. Atherosclerosis. 2018;269:288–293. [DOI] [PubMed] [Google Scholar]
- 23. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using International Classification of Diseases, Revisions 9 and 10. Stroke. 2005;36:1776–1781. [DOI] [PubMed] [Google Scholar]
- 24. Ramirez L, Kim‐Tenser MA, Sanossian N, Cen S, Wen G, He S, Mack WJ, Towfighi A. Trends in acute ischemic stroke hospitalizations in the United States. J Am Heart Assoc. 2016;5:e003233 DOI: 10.1161/JAHA.116.003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moradiya Y, Levine SR. Comparison of short‐term outcomes of thrombolysis for in‐hospital stroke and out‐of‐hospital stroke in United States. Stroke. 2013;44:1903–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qureshi AI, Chaudhry SA, Hassan AE, Zacharatos H, Rodriguez GJ, Suri MF, Lakshminarayan K, Ezzeddine MA. Thrombolytic treatment of patients with acute ischemic stroke related to underlying arterial dissection in the United States. Arch Neurol. 2011;68:1536–1542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient and Hospital Characteristics Included in the Propensity Score Matching
Table S2. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) Codes for Outcomes
Table S3. Calculation of Annual AIS Incidence in the Dialysis and Non‐Dialysis Populations


