Abstract
Background
We conducted a systematic review and meta‐analysis of randomized controlled trials (RCTs) comparing patent foramen ovale (PFO) closure, anticoagulation, and antiplatelet therapy to prevent stroke recurrence in patients with PFO‐associated cryptogenic stroke.
Methods and Results
We searched Medline, Cochrane Library, and EMBASE through March 2018. The primary outcome was stroke recurrence. Pooled incidences, hazard ratios, and risk ratios (RRs) were calculated in random‐effects meta‐analyses. PFO closure was associated with a lower risk of recurrent stroke compared with antithrombotic therapy (antiplatelet therapy or anticoagulation: 3560 patients from 6 RCTs; RR=0.36, 95% CI: 0.17–0.79; I2=59%). The effect of PFO closure on stroke recurrence was larger in patients with atrial septal aneurysm or large shunt (RR=0.27, 95% CI, 0.11–0.70; I2=42%) compared with patients without these anatomical features (RR=0.80, 95% CI, 0.43–1.47; I2=12%). Major complications occurred in 2.40% (95% CI, 1.03–4.25; I2=77%) of procedures. New‐onset atrial fibrillation was more frequent in patients randomized to PFO closure versus antithrombotic therapy (RR=4.33, 95% CI, 2.37–7.89; I2=14%). One RCT compared PFO closure versus anticoagulation (353 patients; hazard ratio=0.14, 95% CI, 0.00–1.45) and 2 RCTs compared PFO closure versus antiplatelet therapy (1137 patients; hazard ratio=0.18, 95% CI, 0.05–0.63; I2=12%). Three RCTs compared anticoagulation versus antiplatelet therapy, with none showing a significant difference.
Conclusions
PFO closure is superior to antithrombotic therapy to prevent stroke recurrence after cryptogenic stroke. The annual absolute risk reduction of stroke was low, but it has to be tempered by a substantial time at risk (at least 5 years) in young and middle‐aged patients. PFO closure was associated with an increased risk of atrial fibrillation.
Keywords: anticoagulation, patent foramen ovale, patent foramen ovale closure, stroke
Subject Categories: Anticoagulants, Secondary Prevention, Meta Analysis, Ischemic Stroke
Clinical Perspective
What Is New?
This systematic review and meta‐analysis of randomized controlled trials revealed that in patients 60 years or younger presenting with cryptogenic stroke and a patent foramen ovale (PFO), PFO closure was associated with a lower rate of recurrent stroke compared with medical therapy.
Although a 60% reduction in the risk of recurrent stroke was observed after PFO closure, the annual absolute risk reduction was low (1.0/100 person‐years).
Patients with an associated atrial septal aneurysm or a large shunt seemed to benefit more from PFO closure.
PFO closure was associated with an increased risk of new‐onset atrial fibrillation.
What Are the Clinical Implications?
There is now enough evidence to conclude that PFO closure is superior to antithrombotic therapy to prevent stroke recurrence after confirmed cryptogenic stroke in carefully selected patients aged up to 60 years.
Introduction
Despite extensive pathogenic workup, approximately one third of acute ischemic strokes are classified as cryptogenic.1, 2 Observational studies have demonstrated a strong association between patent foramen ovale (PFO) and cryptogenic stroke,3, 4 suggesting that paradoxical embolism through a PFO may be an important cause of otherwise unexplained ischemic strokes, notably in younger patients.5, 6 Three randomized controlled trials (RCTs) published in 2012 and 2013 (CLOSURE I (STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale),7 PC Trial (Clinical Trial Comparing Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder with Medical Treatment in Patients with Cryptogenic Embolism),8 and RESPECT (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment)9) failed to demonstrate the superiority of PFO closure over antithrombotic therapy (antiplatelet therapy or anticoagulants) to prevent recurrent stroke. Recently, the long‐term analysis of the RESPECT trial10 and 3 other RCTs (Gore REDUCE (Gore Helex septal occluder and antiplatelet medical management for reduction of recurrent stroke or imaging‐confirmed transient ischemic attack in patients with patent foramen ovale),11 CLOSE (Patent Foramen Ovale Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrence),12 and DEFENSE‐PFO (Device Closure Versus Medical Therapy for Cryptogenic Stroke Patients With High Risk Patent Foramen Ovale)13) reported a lower incidence rate of recurrent stroke in patients randomized to PFO closure compared with controls. We performed a systematic review and meta‐analysis of all randomized data allowing the direct comparison of PFO closure, anticoagulation, and antiplatelet therapy to prevent recurrent stroke in patients with cryptogenic stroke and PFO. To this aim, we included published data from all RCTs and unpublished data from the 3‐arm CLOSE trial, allowing head‐to‐head comparison of all the abovementioned therapeutic strategies and calculation of the absolute risk of stroke and corresponding number needed to treat. Because traditional updated meta‐analyses may sometimes lead to false‐positive results due to repeated significance testing,14 we performed trial sequential analyses (TSA), a method similar to interim analyses of a single RCT, in order to determine whether enough evidence has been obtained to reach a conclusion.15, 16
Methods
The present study was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) Statement.17 Upon request, the data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. The analytic plan was prespecified, but the study protocol was not registered. The following comparisons were planned: PFO closure versus antithrombotic therapy (antiplatelet therapy or anticoagulation), PFO closure versus antiplatelet therapy, PFO closure versus anticoagulation, and anticoagulation versus antiplatelet therapy. The end points of interest for the present meta‐analysis were defined before starting the literature search. Our primary end point was fatal or nonfatal recurrent stroke.11 Secondary end points were occurrence of a transient ischemic attack (TIA),18 all‐cause mortality, major bleeding, major procedural complication, and new‐onset atrial fibrillation.
Study Selection, Data Extraction, and Quality Assessment
Studies eligible for the systematic review and meta‐analysis were RCTs published in any language comparing at least 2 of the 3 following therapeutic strategies for preventing recurrent stroke in patients with PFO and cryptogenic stroke or TIA: transcatheter PFO closure, anticoagulation, and antiplatelet therapy. We searched Medline, the Cochrane Library, and EMBASE for articles published between January 1990 and March 2018, using the terms and search strategy detailed in Table S1. Two investigators (G.T., D.C.) independently screened the titles and abstracts of all records, excluded irrelevant articles, and obtained the full texts of the remaining articles to identify those studies meeting the inclusion criteria. Using standardized forms, relevant data for the meta‐analysis were independently extracted by the same investigators, in duplicate (Table and Table S2). Subsequently, the Cochrane Collaboration's tool was used to assess the risk of selection, performance, detection, attrition, and reporting biases among the included RCTs.19 Trials with more than 2 and 4 high‐risk components were considered as having a moderate and high risk of bias, respectively.20
Table 1.
PFO Closure Versus Antithrombotic Therapy: Descriptive Summary of the Design of Randomized Trials Characteristics Included in the Meta‐Analysis
| Closure I (2012)7 | PC Trial (2013)8 | RESPECT (20139; 201710) | Gore REDUCE (2017)11 | CLOSE (2017)12 | DEFENSE‐PFO (2018)13 | |
|---|---|---|---|---|---|---|
| Trial design |
Prospective, randomized, blinded adjudication of outcome events 1:1 |
Prospective, randomized, blinded adjudication of outcome events 1:1 |
Prospective, randomized, blinded adjudication of outcome events 1:1 |
Prospective, randomized, blinded adjudication of outcome events 2:1 |
Prospective, randomized, blinded adjudication of outcome events 1:1:1a |
Prospective, randomized 1:1 |
| Main inclusion criteria |
|
|
|
|
|
|
| Intervention group (PFO Closure) |
Transcatheter PFO closure+ Clopidogrel, 75 mg/d for 6 mo AND aspirin, 81 or 325 mg/d for 2 y |
Transcatheter PFO closure+ Aspirin 100 to 325 mg/d for at least 5 to 6 mo AND ticlopidine 250 to 500 mg/d OR clopidogrel 75 to 150 mg/d for 1 to 6 mo |
Transcatheter PFO closure+ Aspirin 81 to 325 mg/d AND clopidogrel daily for 1 mo, followed by aspirin monotherapy for 5 mo |
Transcatheter PFO closure+ Clopidogrel 75 mg/d for 3 d (with 300‐mg dose if necessary) and then same antiplatelet therapy as in the other study arm (site specific), for the rest of the trial |
Transcatheter PFO closurea+ Aspirin 75 mg/d AND clopidogrel 75 mg/d for 3 mo, followed by single antiplatelet therapy for the rest of the trial |
Transcatheter PFO closure+ Aspirin 100 mg/d AND clopidogrel 75 mg/d for at least 6 mo after the procedure |
| Control group (antithrombotic therapy) | At the investigator's discretion: Warfarin, aspirin, or both | At the investigator's discretion: antiplatelet therapy or anticoagulation | At the investigator's discretion: aspirin or warfarin or clopidogrel or/and aspirin plus dipyridamole |
Antiplatelet therapy. Aspirin OR aspirin plus dipyridamole OR clopidogrel |
Antiplatelet therapy: aspirin OR clopidogrel OR aspirin plus dipyridamole | At the investigator's discretion: single or dual antiplatelet therapy or anticoagulation (warfarin) |
| Primary end point | Composite: stroke or TIA, all‐cause mortality within 30 d, and death from neurologic causes between 31 d and 2 y | Composite: death, nonfatal stroke, TIA, peripheral embolism | Composite: ischemic stroke or early death (closure arm: within 30 d after closure or 45 d after randomization; antithrombotic arm: within 45 d after randomization) | 2 co‐primary end points:
|
Stroke (fatal or nonfatal, ischemic or hemorrhagic) | Composite: stroke, vascular death, major bleeding |
| Hypothesis | Rate of primary end point at 2 y: 6% in the medical arm and 3% in the closure arm | Rate of primary end point: 3%/y in the medical arm and 1%/y in the closure arm | Rate of primary end point at 2 y: 4.3% in the medical arm and 1% in the closure arm | Rate of primary end point at 2 y: 2.9% in the medical arm. 55% lower rate of recurrent stroke in the closure arm |
Rate of primary end point: 3.5%/y in antiplatelet arm 50% lower rate in closure or anticoagulation arm |
Rate of primary end point at 2 y: 15% in the medical arm and 4% in the closure arm |
| Enrollment period | June 2003–Oct 2008 | Feb 2000–Feb 2009 | Aug 2003–Dec 2011 | Dec 2008–Feb 2015 | Dec 2008–Dec 2014 | Sept 2011–Oct 2017 |
| Sites | 87 sites in the United States and Canada | 29 sites in Europe, Canada, Brazil, and Australia | 69 sites in the United States and Canada | 63 sites in Canada, Denmark, Finland, Norway, Sweden, United Kingdom, and United States | 34 sites in France and Germany | 2 sites in South Korea |
| Sample size (PFO closure group; antithrombotic therapy group) | N=909 (447; 462) | N=414 (204; 210) | N=980 (499; 481) | N=664 (441; 223) | N=663a (Groups “1” and “2” in the original publication: 238 vs 235) | N=120 (60; 60) |
| Closure device | Starflex only | Amplatzer only | Amplatzer only | Helex Or Cardioform | At the investigator's discretion: Amplatzer (59%), Intrasept, Premere, Starflex, Figulla, Atriasept, Gore septal occluder | Amplatzer only |
| Sponsor | Industrial (NMT medical) | Industrial (St. Jude Medical) | Industrial (St. Jude Medical) | Industrial (Gore) | Academic (French Ministry of Health) | Academic (Cardiovascular Research Foundation, Seoul, South Korea) |
ASA indicates atrial septal aneurysm; CLOSE, Patent Foramen Ovale Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrence; CLOSURE I, STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale; DEFENSE‐PFO, Device Closure Versus Medical Therapy for Cryptogenic Stroke Patients With High‐Risk Patent Foramen Ovale; Gore REDUCE, Gore Helex septal occluder and antiplatelet medical management for reduction of recurrent stroke or imaging‐confirmed transient ischemic attack in patients with patent foramen ovale; IS, ischemic stroke; N, number; PC trial, Clinical Trial Comparing Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder with Medical Treatment in Patients with Cryptogenic Embolism; PFO, patent foramen ovale; RESPECT, Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment; TEE, transesophageal echocardiography; TIA, transient ischemic attack; TTE, transthoracic echocardiography.
Another intervention group in the 3‐arm CLOSE consisted of oral anticoagulation, the control group being antiplatelet therapy.12 The oral anticoagulation group was not described in the present table focusing on the intervention of PFO closure.
Data Synthesis and Meta‐Analysis
To take into account the time‐to‐event nature of the data, we planned to use the hazard ratio (HR) as principal summary measure. However, whenever no HR was reported, we calculated the risk ratio (RR) based on count data. HRs were obtained from the main articles or supplemental appendixes of published studies. We also performed a post hoc analysis of the CLOSE trial to compare patients randomized to PFO closure versus oral anticoagulation (intention‐to‐treat cohort). For this analysis, survival curves were estimated with the Kaplan–Meier method and HR with 95% confidence interval (CI) was calculated with a Cox model with Firth's penalized likelihood method.
For each end point of the present meta‐analysis, the HRs or RRs in the intention‐to‐treat population of each study and their corresponding 95% CI were pooled. We decided a priori to use a random‐effects model to calculate pooled estimates because we assumed that the true effect size might differ from trial to trial, as by design the following factors differed across studies: (1) devices used in the intervention group; (2) type of antithrombotic medication in the control group; and (3) inclusion criteria regarding the anatomy of PFOs. The DerSimonian and Laird method was used for the main analyses.21 However, because most of the meta‐analyses relied on studies with few events, which may lead to a too‐narrow coverage for the overall CIs, we performed sensitivity analyses based on likelihood approaches (Table S3).22 Heterogeneity across studies was assessed using Cochran's Q (reported as a P value) and the I2 statistics. Heterogeneity was classified as moderate (I2≥30%), substantial (I2≥50%), or considerable (I2≥75%).23 Potential sources of heterogeneity were investigated by (1) stratifying studies according to the type of medical treatment in the control group (antithrombotic therapy or antiplatelet therapy alone); (2) conducting a subgroup analysis based on PFO anatomical features (higher‐risk PFOs, defined as PFO associated with atrial septal aneurysm or large shunt,6, 24 versus lower‐risk PFOs); and (3) by meta‐regression. Potential publication bias was investigated using funnel plots and Egger's test.25
Trial Sequential Analysis
We performed a TSA whenever our meta‐analysis suggested the superiority of one therapeutic strategy over another. Indeed, traditional meta‐analytic methods may sometimes lead to false‐positive results, especially when pooled estimates are updated with the publication of new trials in cumulative meta‐analyses.14 The aim of this approach is to determine whether enough evidence has been obtained to reach a conclusion regarding the superiority of one treatment over another, or if a new RCT addressing the same comparison should be undertaken.15, 16 Briefly, in TSA, a cumulative meta‐analysis of RCTs is similar to several interim analyses of a single RCT, with the construction of specific trial sequential monitoring boundaries by using the Lan–DeMets α spending function approach.15, 16 In the present TSA, the low‐bias heterogeneity‐adjusted information size method was used to determine the required information size.26 A description of the interpretation of the TSA plot to determine whether a given meta‐analysis would correspond to a false‐positive, true‐positive, false‐negative, or true‐positive result is provided in Figure S1.
Pooling Incidence Rates and Calculation of Number Needed to Treat
Incidence rates (absolute risks) of stroke recurrence per 100 person‐years in each treatment arm were obtained from published (CLOSURE I, PC Trial, RESPECT, Gore REDUCE) or unpublished data (CLOSE). For the DEFENSE‐PFO trial, the number of person‐years during the first 2 years of follow‐up was estimated using the published Kaplan–Meier curve with the respective number of patients at risk at each time point. The 95% CI of the incidence was calculated using the Wilson method.27 Estimates of the incidence of recurrent stroke were pooled after Freeman–Tukey double arcsine method and then back transformed onto the original scale.28 The number needed to treat (with its 95% CI) to prevent 1 recurrent stroke during 1 person‐year exposure was calculated based upon the pooled incidence of recurrent stroke in the antithrombotic group and the pooled risk ratio for stroke recurrence (PFO closure versus antithrombotic therapy).29
In patients allocated to PFO closure, the incidence rates of any new‐onset atrial fibrillation and of major procedural complication (as defined in each study's protocol) were calculated for 100 patients treated. Regarding the incidence of new‐onset atrial fibrillation, we performed an exploratory analysis based on the type of PFO closure device by pooling published data from CLOSURE I, PC Trial, RESPECT, Gore REDUCE, and DEFENSE‐PFO with unpublished data from CLOSE, in which the choice of the device was left to the discretion of the investigator. Statistical analysis was performed using STATA 11.0 (Statacorp), SAS 9.4 (Cary, NC), and R 3.4.1 (R Foundation for Statistical Computing).
Results
The PRISMA flowchart of literature search and study selection is presented in Figure S2. We identified 9 articles reporting the results of 8 different RCTs meeting the inclusion criteria.7, 8, 9, 10, 11, 12, 30, 31 Four studies (CLOSURE I,7 PC trial,8 RESPECT,10 and DEFENSE‐PFO13) compared PFO closure with antithrombotic therapy (oral anticoagulation or antiplatelet therapy at the discretion of the investigator). Two studies (Gore REDUCE11 and CLOSE12) compared PFO closure with antiplatelet therapy. The CLOSE study also allowed the post hoc comparison of PFO closure versus anticoagulation. Three studies (PICSS (Patent foramen ovale in cryptogenic stroke study),30 Shariat et al,31 and CLOSE12) compared oral anticoagulation with antiplatelet therapy.
PFO Closure Versus Antithrombotic Therapy
The meta‐analysis of PFO closure versus antithrombotic therapy comprised a total of 3560 patients from 6 studies.7, 8, 10, 11, 12, 13 Key features of the design of included trials are summarized in Table. The upper age limit for enrollment was 60 years in all trials except DEFENSE‐PFO, and median follow‐up duration ranged from 2 to 5.9 years (Table S2). Three trials (PC Trial,8 RESPECT,10 and DEFENSE‐PFO13) used the Amplatzer occluder device, the CLOSURE I study used the Starflex device,7 the Gore REDUCE study used the Cardioform or Helex device,11 and choice of the device was left to the discretion of the investigator in the CLOSE trial.12 For this analysis, the comparator was the one used in the design of each study: antiplatelet therapy or/and anticoagulation at the discretion of the investigator in CLOSURE I, PC Trial, RESPECT, and DEFENSE‐PFO; antiplatelet therapy in CLOSE and Gore REDUCE.
We did not find evidence of publication bias. Overall, none of the included studies was considered to be at high risk of bias (Figure S3). There was no blinding of participants and personnel to the treatment arm, but outcomes were adjudicated in a blinded fashion in all studies except in the DEFENSE‐PFO trial, for which this point was not specified in the original publication.13 The risk of attrition bias was judged sizeable in 3 of 6 studies, because of a relatively high dropout rate (>10%) in comparison to the low incidence of recurrent stroke, and a differential dropout rate between the closure and medical therapy arms.8, 10, 11 A possible selective reporting bias could not be excluded in the PC Trial because the Clinical Events Committee discounted potential primary end point events more often in the antithrombotic therapy group than in the PFO closure group.8, 32
Recurrent stroke
A total of 37 strokes occurred among 1889 patients randomized to PFO closure, compared with 79 strokes among 1671 patients randomized to antithrombotic therapy (pooled RR 0.36, 95% CI, 0.17–0.79, P=0.01; I2=59%; Figure 1A). In the trial sequential analysis (Figure 2), the cumulative Z score curve crossed the monitoring boundary, suggesting a “true‐positive” result regarding the superiority of PFO closure over medical therapy (Figure S1).26 The HR for stroke recurrence was provided in 5 out of 6 RCTs (pooled HR 0.40, 95% CI, 0.20–0.82, P=0.01; I2=54%; Figure 1B).7, 8, 10, 11, 12 The random‐effects pooled incidence of stroke recurrence per 100 person‐years was 0.29 (95% CI, 0.02–0.76; I2=83%) in the PFO closure group (Figure S4) and 1.27 (95% CI, 0.84–1.78; I2=53%) in the antithrombotic therapy group (Figure S5). The number needed to treat to prevent 1 recurrent stroke during 1 person‐year of follow‐up was 131 (95% CI, 101–400).
Figure 1.
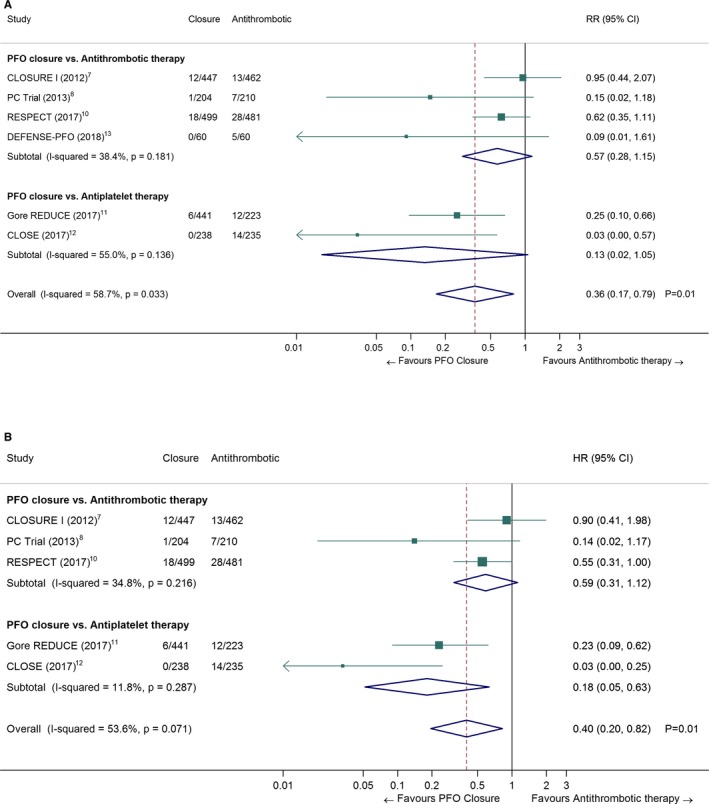
Pooled risk ratio (A) and hazard ratio (B) of recurrent stroke in patients randomized to PFO closure vs antithrombotic therapy (random‐effects meta‐analysis). The closure and antithrombotic columns denote the number of events divided by the total number of patients in each treatment group. The DEFENSE‐PFO trial was not included in (B) because no HR was reported in the original publication. CI indicates confidence interval; CLOSE, Patent Foramen Ovale Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrence; CLOSURE I, STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale; DEFENSE‐PFO, Device Closure Versus Medical Therapy for Cryptogenic Stroke Patients With High‐Risk Patent Foramen Ovale; Gore REDUCE, Gore Helex septal occluder and antiplatelet medical management for reduction of recurrent stroke or imaging‐confirmed transient ischemic attack in patients with patent foramen ovale; HR, hazard ratio; PC trial, Clinical Trial Comparing Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder with Medical Treatment in Patients with Cryptogenic Embolism; PFO, patent foramen ovale; RESPECT, Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment; RR, risk ratio.
Figure 2.
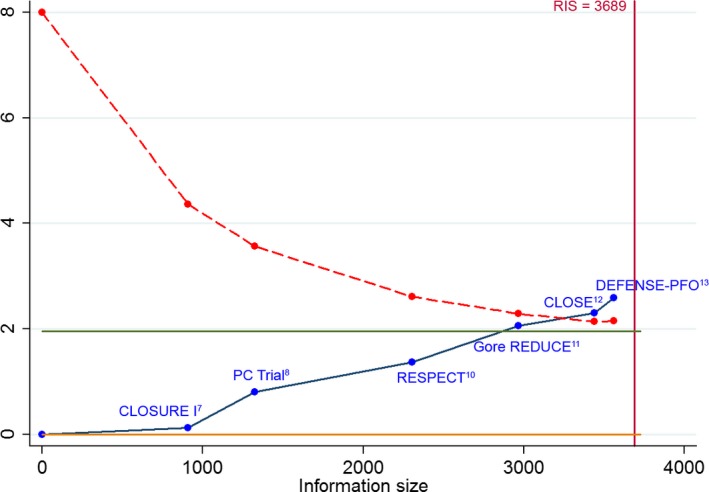
Trial sequential analysis of the risk of recurrent stroke in patients randomized to PFO closure vs antithrombotic therapy. The cumulative Z‐curve crosses the monitoring boundary (red dashed line) before reaching the required information size (RIS, red vertical line), providing evidence for the superiority of PFO closure over antithrombotic therapy to prevent recurrent stroke.26 CLOSE indicates Patent Foramen Ovale Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrence; CLOSURE I, STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale; DEFENSE‐PFO, Device Closure Versus Medical Therapy for Cryptogenic Stroke Patients With High‐Risk Patent Foramen Ovale; Gore REDUCE, Gore Helex septal occluder and antiplatelet medical management for reduction of recurrent stroke or imaging‐confirmed transient ischemic attack in patients with patent foramen ovale; PC trial, Clinical Trial Comparing Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder with Medical Treatment in Patients with Cryptogenic Embolism; PFO, patent foramen ovale; RESPECT, Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment.
There was no significant difference in the effect of PFO closure in studies where only antiplatelet therapy, as opposed to any antithrombotic medication, was allowed in the control group (random‐effects meta‐regression: P=0.16). In a subgroup analysis restricted to studies in which only antiplatelet medication was allowed (Gore REDUCE11 and CLOSE12), PFO closure remained superior to antiplatelet therapy (pooled HR 0.18, 95% CI, 0.05–0.63, P=0.007, I2=12%; Figure 1B). This result was confirmed by the cumulative Z score curve crossing the monitoring boundary in trial sequential analysis (data not shown).
There was a significant difference in the effect of PFO closure to prevent stroke recurrence in patients with higher‐risk anatomical features (atrial septal aneurysm or large shunt, Table S4), as opposed to patients without (Figure 3; random‐effects meta‐regression: P=0.01). In patients with higher‐risk anatomical features, the pooled RR for PFO closure was 0.27 (95% CI, 0.11–0.70, P=0.01; I2=42%), whereas it was 0.80 (95% CI, 0.43–1.47, P=0.41; I2=12%) in patients with lower‐risk anatomical features.
Figure 3.
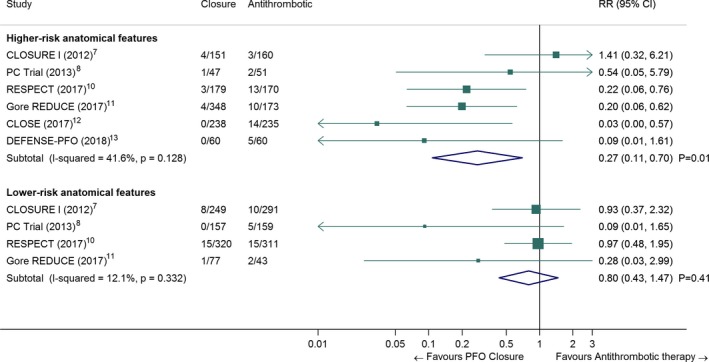
Pooled risk ratio of recurrent stroke in patients randomized to PFO closure vs antithrombotic therapy, according to PFO anatomical features (random‐effects meta‐analysis). For the present meta‐analysis, we defined higher‐risk anatomical features as follows (Table S4): For CLOSURE I, PC trial and RESPECT: presence of an atrial septal aneurysm (ASA), regardless of shunt size, For CLOSE and DEFENSE‐PFO: presence of an ASA and/or a large shunt (i.e., all included patients), For Gore REDUCE: moderate or large shunt (Nota bene: presence or absence of ASA could not be analyzed because it was not recorded in patients randomized to the antiplatelet group). Number of recurrent strokes in each group were extracted from the original publications of the randomized trials or calculated using published data by Kent et al.33 The Closure and Antithrombotic columns denote the number of events divided by the total number of patients in each treatment group. CI indicates confidence interval; CLOSE, Patent Foramen Ovale Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrence; CLOSURE I, STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale; DEFENSE‐PFO, Device Closure Versus Medical Therapy for Cryptogenic Stroke Patients With High‐Risk Patent Foramen Ovale; Gore REDUCE, Gore Helex septal occluder and antiplatelet medical management for reduction of recurrent stroke or imaging‐confirmed transient ischemic attack in patients with patent foramen ovale; PC trial, Clinical Trial Comparing Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder with Medical Treatment in Patients with Cryptogenic Embolism; PFO, patent foramen ovale; RESPECT, Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment; RR, risk ratio.
Transient ischemic attack
A total of 64 TIAs occurred among 1889 patients randomized to PFO closure, compared with 64 TIAs among 1671 patients randomized to medical therapy (pooled RR 0.85, 95% CI, 0.60–1.21, P=0.38; I2=0%; Figure S6).
All‐cause mortality and major bleeding
Vital status was available for 1844 patients randomized to PFO closure and 1667 patients randomized to antithrombotic therapy, of whom 13 and 15 died during follow‐up, respectively (pooled RR 0.79, 95% CI, 0.39–1.60, P=0.51; I2=0%; Figure S7). No death was related to the occurrence of a stroke or a procedural complication. Major bleeding occurred in 34 out of 1820 patients randomized to PFO closure versus 28 out of 1583 patients randomized to antithrombotic therapy (pooled RR 0.97, 95% CI, 0.43–2.20, P=0.94; I2=37%; Figure S8).
Major procedural complications and new‐onset atrial fibrillation
Major procedural complications occurred in 52 out of 1844 patients randomized to PFO closure (random‐effects pooled incidence per 100 patients treated: 2.40, 95% CI, 1.03–4.25; I2=77%, Figure S9). New‐onset atrial fibrillation (irrespective of its duration) was present in 93 out of 1844 patients randomized to PFO closure versus 17 out of 1667 patients randomized to antithrombotic therapy (pooled RR 4.33, 95% CI, 2.37–7.89, P<0.001; I2=14%; Figure S10). An exploratory analysis of the random‐effects pooled incidence of new‐onset atrial fibrillation according to the type of PFO closure device is presented in Figure S11. The pooled incidence of new‐onset atrial fibrillation per 100 patients treated was 4.56 (95% CI, 3.58–5.63; I2=1%). The pooled incidence of new‐onset atrial fibrillation per 100 patients treated with nitinol double disk devices (Amplatzer or Figulla Flex II) was 3.65 (95% CI, 2.48–5.01; I2=0%), compared with 5.61 (95% CI, 4.11–7.29; I2=0%) for patients treated with other devices (P=0.02 for comparison). A descriptive summary of the occurrence of new‐onset atrial fibrillation in the 6 RCTs is presented in Table S5. Altogether, 93 patients randomized to the PFO closure group had new‐onset atrial fibrillation, 5 of whom had a recurrent stroke. Atrial fibrillation was considered to be transient in most instances (Table S5).
PFO Closure Versus Anticoagulation
The 3‐arm CLOSE study was the only one in which patients were randomized to PFO closure or oral anticoagulation, but the head‐to‐head comparison of these 2 groups was not prespecified in the study statistical analysis plan.34 The baseline characteristics of patients randomized to PFO closure (n=173) and anticoagulation (n=180) were similar and have been described in the supplemental appendix of the original publication.12 In the anticoagulation group, 3 patients had a recurrent stroke over a follow‐up of 967 patient‐years, compared with none in the PFO Closure group over a follow‐up of 963 patient‐years (intention‐to‐treat analysis). The Kaplan–Meier cumulative estimates of the probability of stroke‐free survival are presented in Figure 4 (log‐rank test: P=0.08). In Cox regression, the corresponding HR was 0.14 (95% CI, 0.00–1.45; P=0.26). Subgroup analyses based on the type of oral anticoagulant (vitamin K antagonists or direct oral anticoagulants) could not be conducted because of the small number of patients taking direct oral anticoagulants (n=13; 7.2%).
Figure 4.

Kaplan–Meier cumulative estimates of the probability of recurrent stroke in patients randomized to PFO closure vs anticoagulation therapy in the CLOSE trial. This post hoc analysis was performed in the intention‐to‐treat cohort. The inset shows the same data on an enlarged y axis. CI indicates confidence interval; CLOSE, Patent Foramen Ovale Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrence; PFO, patent foramen ovale.
Anticoagulation Versus Antiplatelet Therapy
The PICSS study evaluated transesophageal echocardiographic findings in patients randomly assigned to warfarin or aspirin in the Warfarin‐Aspirin Recurrent Stroke Study.30 Of the 630 patients with stroke enrolled, 98 patients had a cryptogenic stroke associated with a PFO. Four of the 42 patients randomized to warfarin met the primary end point of recurrent stroke or death at 2 years, versus 10 out of the 56 patients randomized to aspirin (HR 0.52, 95% CI, 0.16–1.67, P=0.28). No separate information on recurrent stroke was provided, and this study was therefore not included in a meta‐analysis. In a single‐center randomized controlled trial, Shariat et al compared warfarin and aspirin in patients with PFO and cryptogenic stroke or TIA.31 Five of 21 patients randomized to warfarin had a recurrent stroke or TIA versus 2 of 23 patients randomized to aspirin (HR 0.33, 95% CI, 0.06–1.7). No separate information on recurrent stroke was provided, and this study was therefore also not included in a meta‐analysis. In the CLOSE trial, the comparison of anticoagulation versus antiplatelet therapy was prespecified. As previously reported, in the intention‐to‐treat analysis, 3 out of 187 patients (1011 patient‐years) in the anticoagulation group had a recurrent stroke, versus 7 out 174 patients (926 patient‐years) in the antiplatelet therapy group (HR 0.44, 95% CI, 0.11–1.48).12
Discussion
In our meta‐analysis that included 3560 patients enrolled in 6 RCTs, patients <60 years of age with a cryptogenic ischemic stroke and a PFO had a 64% lower risk of stroke recurrence when assigned to the PFO closure group than to the antithrombotic group. Our trial sequential analysis provides further support to the argument that this conclusion is not a false‐positive result. Indeed, concluding that a cumulative meta‐analysis is significant at the usual nominal 0.05 threshold may correspond to a false‐positive result when the required information size has not been reached, as in the present study.16 By contrast, the TSA provides an adjusted, more stringent level of statistical significance, in order to limit the risk of type I error.15 The absolute risk of stroke recurrence was low in both groups: 0.29 and 1.27 per 100 person‐years in the closure group and the antithrombotic group, respectively. This absolute difference translates into a number needed to treat to prevent 1 recurrent stroke at 1 year of 131. This may seem like a sizeable number but it has to be tempered by a substantial time at risk in those young and middle‐aged patients. Indeed, although there are currently no data available regarding the very long‐term risk (>10 years) of stroke recurrence in patients with PFO‐associated “cryptogenic” stroke, a follow‐up of 5 years or more was achieved in more than 50% of patients enrolled in RESPECT and CLOSE. In those studies, the Kaplan–Meier curve of the antithrombotic therapy group did not suggest a decline in the rate of recurrent stroke over time.
In line with subgroup analyses from the RESPECT trial, our meta‐analysis suggests that patients with atrial septal aneurysm or large shunt may benefit more from PFO closure than patients without those anatomical features. This finding has pathophysiological plausibility, because it has been suggested that increased septal mobility may enhance the probability of paradoxical embolism by mechanically directing blood flow from the inferior vena cava into the PFO.35 It has also been shown that patients with both PFO and atrial septal aneurysm constitute a subgroup at substantial risk for recurrent stroke, compared with patients with PFO alone.6 Considering the low incidence rate of recurrent stroke in patients with cryptogenic stroke and PFO, closure confined to selected patients with anatomical features associated with a higher risk of stroke recurrence could be a more appropriate approach to enhance the benefits of PFO closure. In the CLOSE and DEFENSE‐PFO trials, where only patients with higher‐risk anatomical features could be enrolled, no recurrent stroke was observed after PFO closure. This may suggest that PFO harboring these anatomical features would be more likely causally related to the index stroke and therefore better candidates for PFO closure.
We observed in our meta‐analysis a relatively large heterogeneity between trials that can be partly explained by the choice of antithrombotic treatment in control groups. Indeed, in the antithrombotic groups, antiplatelet drugs or oral anticoagulants were used according to physician preference in all but 2 trials.11, 12 This could have confounded trial results if oral anticoagulants and antiplatelet drugs have different impacts on the risk of stroke recurrence. However, we did not observe a higher risk of stroke recurrence in studies in which only antiplatelets were allowed (1.33% versus 1.30%).
It remains unproven whether oral anticoagulation in some cases could be an alternative to PFO closure to prevent recurrent stroke. CLOSE was the only study allowing a randomized comparison of these 2 strategies. Even though the point estimate seemed to favor PFO closure (HR=0.14), that association was not significant and the wide CI provided very little indication of the underlying effect size, which could range between an unlimitedly large decrease in hazard to a clinically meaningful 45% increased hazard of recurrent stroke. Of note, the effectiveness of direct oral anticoagulants to prevent stroke recurrence in patients with PFO‐associated stroke has not been adequately studied until the current time, because only 13 patients were treated with a direct oral anticoagulant in a randomized trial. However, even if a future randomized controlled trial shows the noninferiority of oral anticoagulation over PFO closure to prevent recurrent stroke, 1 remaining question would be whether long‐term or even lifetime anticoagulation should be maintained in these young and middle‐aged patients.
Our meta‐analysis did not provide evidence for a difference in the risk of recurrent TIA between groups (RR 0.85, 95% CI, 0.60–1.21, P=0.38; I2=0%) in contrast to the protective effect with regard to the risk of stroke. One reason could be that the diagnosis of TIA is difficult because symptoms are often nonspecific and, by definition, are not associated with objective neuroimaging findings.18 TIA remains subject to diagnostic inaccuracy that could lead to background noise responsible for a potential watering‐down effect of PFO closure.36 Another reason could be that the optimal duration of dual antiplatelet therapy after device implantation is unknown. It can be hypothesized that a too‐short dual antiplatelet therapy may expose patients to higher risk of small local thrombus formation, ultimately leading to a TIA. Against this hypothesis, no heterogeneity was found between trials with regard to the TIA end point, in spite of different durations of dual antiplatelet therapy by study design.
Contrasting with a recent analysis of administrative data suggesting a 7% rate of serious periprocedural complications and death after PFO closure,37 we observed a low rate of major procedural complications (2.4%; 95% CI, 1.03–4.25), none of which led to death. Although this result might be explained by a lower prevalence of medical comorbidities and an upper age limit of 60 years in most trials, its interpretation should be subject to caution because a considerable heterogeneity was observed across studies (I2=77%). This heterogeneity may reflect the variations in the definition of major procedural complications across trials, especially regarding the inclusion of atrial fibrillation. New‐onset atrial fibrillation was 4.3 times more frequent in patients assigned to the closure group than in those assigned to the antithrombotic group. Pathophysiology of new‐onset atrial fibrillation is not unique. It has been hypothesized that early events of atrial fibrillation after PFO closure are likely to be triggered by local inflammation.38 Interestingly, our analysis suggests that absolute risks of new‐onset atrial fibrillation could differ according to type of devices used in trials. The Amplatzer septal occluder device might have a better safety profile with regard to the risk of AF compared with other devices. However, comparisons should be interpreted with caution because patients were not randomized according to types of device. Atrial fibrillation related to closure was transient in 2 out of 3 patients, and its clinical relevance, determinants, and overall risk of stroke require further investigation.
Our study has several potential limitations. First, the rates of patients lost to follow‐up and crossover in the included studies were relatively high taking into account the low number of events, which may have led to an attrition bias.32 Second, there were was a slight imbalance in referral for end point adjudication in the PC trial, which might correspond to a reporting bias.8, 32, 33 However, no evidence of selective reporting was reported in the other RCTs included in this meta‐analysis.33 Third, our study has the limitations of aggregate data meta‐analyses. In particular, the definitions of some end points, such as major procedural complications, varied markedly across studies. However, the definition of our primary end point was virtually identical in all included studies.
In conclusion, there is now enough evidence to reasonably conclude that PFO closure is superior to antithrombotic therapy with regard to the risk of stroke recurrence in patients with cryptogenic stroke. Patients with an associated atrial septal aneurysm or a large shunt may benefit more from PFO closure. This is a step forward that will benefit many patients. Further individual participant data meta‐analysis may answer the question of the role of potential effect modifiers. Future studies will also have to address the question of PFO closure in patients not included in trials, such as those over 60 years of age or those with a competitive cause of ischemic stroke, but also the clinical relevance of atrial fibrillation induced by PFO closure.
Sources of Funding
The CLOSE trial was funded by the French Ministry of Health (ClinicalTrials.gov number NCT00562289).
Disclosures
Guérin received Grant/Research Support from Abbott Vascular, Boston scientific and Biotronic, and Consulting Fees/Honoraria from Abbott Vascular, AstraZeneca, Lilly, Actelion, and General Electric. Mas participated in advisory boards and received lectures fees from Bayer, Boerhinger‐Ingelheim, Bristol Myers Squibb, and Daiichi‐Sankyo. The remaining authors have no disclosures to report.
Supporting information
Appendix S1. Patent Foramen Ovale Closure or Anticoagulants Versus Antiplatelet Therapy to Prevent Stroke Recurrence (CLOSE) investigators.
Table S1. Literature Search Strategy
Table S2. PFO Closure Versus Antithrombotic Therapy: Descriptive Summary of Included Patients and Data Extracted for Each End Point of the Present Meta‐Analysis
Table S3. Sensitivity Analyses: Results Obtained With the DerSimonian & Laird Method and With Likelihood Approaches to Meta‐Analysis
Table S4. PFO Anatomical Features in Randomized Controlled Trials
Table S5. Description of the Events of New‐Onset Atrial Fibrillation and Subsequent Risk of Stroke
Figure S1. Guide to the interpretation of a trial sequential analysis (TSA) plot.
Figure S2. PRISMA flow chart of literature search and study selection.
Figure S3. Assessment of the risk of bias among included RCTs, using the Cochrane Collaboration's tool.
Figure S4. Random‐effects pooled incidence of recurrent stroke per 100 person‐years in the PFO closure group.
Figure S5. Random‐effects pooled incidence of recurrent stroke per 100 person‐years in the antithrombotic therapy group.
Figure S6. Pooled RR for TIA in patients randomized to PFO closure vs antithrombotic therapy (random‐effects meta‐analysis).
Figure S7. Pooled RR of all‐cause mortality in patients randomized to PFO closure vs antithrombotic therapy (random‐effects meta‐analysis).
Figure S8. Pooled RR of major bleeding in patients randomized to PFO closure vs antithrombotic therapy (random‐effects meta‐analysis).
Figure S9. Random‐effects pooled incidence of major procedural complication per 100 patients treated in the PFO closure group.
Figure S10. Pooled RR of new‐onset atrial fibrillation in patients randomized to PFO closure vs antithrombotic therapy (random‐effects meta‐analysis).
Figure S11. Random‐effects pooled incidence of new‐onset atrial fibrillation per 100 patients treated in the PFO closure group, according to the closure device (exploratory analysis).
(J Am Heart Assoc. 2018;7:e008356 DOI: 10.1161/JAHA.117.008356.)
References
- 1. Sacco RL, Ellenberg JH, Mohr JP, Tatemichi TK, Hier DB, Price TR, Wolf PA. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989;25:382–390. [DOI] [PubMed] [Google Scholar]
- 2. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, Sacco RL, Connolly SJ; Cryptogenic Stroke EIWG . Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438. [DOI] [PubMed] [Google Scholar]
- 3. Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, Drobinski G, Thomas D, Grosgogeat Y. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–1152. [DOI] [PubMed] [Google Scholar]
- 4. Alsheikh‐Ali AA, Thaler DE, Kent DM. Patent Foramen Ovale in Cryptogenic Stroke. Incidental or Pathogenic? Stroke. 2009;40:2349–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kent DM, Ruthazer R, Weimar C, Mas JL, Serena J, Homma S, Di Angelantonio E, Di Tullio MR, Lutz JS, Elkind MS, Griffith J, Jaigobin C, Mattle HP, Michel P, Mono ML, Nedeltchev K, Papetti F, Thaler DE. An index to identify stroke‐related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J; Patent Foramen O, Atrial Septal Aneurysm Study G . Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740–1746. [DOI] [PubMed] [Google Scholar]
- 7. Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L; for the Closure I Investigators . Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999. [DOI] [PubMed] [Google Scholar]
- 8. Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick‐Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, Wahl A, Windecker S, Juni P; for the PC Trial Investigators . Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083–1091. [DOI] [PubMed] [Google Scholar]
- 9. Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL; for the Respect Investigators . Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100. [DOI] [PubMed] [Google Scholar]
- 10. Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL; for the RESPECT Investigators . Long‐term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377:1022–1032. [DOI] [PubMed] [Google Scholar]
- 11. Sondergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen‐Kudsk JE, Settergren M, Sjostrand C, Roine RO, Hildick‐Smith D, Spence JD, Thomassen L; for the Gore REDUCE Investigators . Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377:1033–1042. [DOI] [PubMed] [Google Scholar]
- 12. Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, Bejot Y, Vuillier F, Detante O, Guidoux C, Canaple S, Vaduva C, Dequatre‐Ponchelle N, Sibon I, Garnier P, Ferrier A, Timsit S, Robinet‐Borgomano E, Sablot D, Lacour JC, Zuber M, Favrole P, Pinel JF, Apoil M, Reiner P, Lefebvre C, Guerin P, Piot C, Rossi R, Dubois‐Rande JL, Eicher JC, Meneveau N, Lusson JR, Bertrand B, Schleich JM, Godart F, Thambo JB, Leborgne L, Michel P, Pierard L, Turc G, Barthelet M, Charles‐Nelson A, Weimar C, Moulin T, Juliard JM, Chatellier G; for the CLOSE Investigators . Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377:1011–1021. [DOI] [PubMed] [Google Scholar]
- 13. Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH, Song JM, Kang DH, Kwon SU, Kang DW, Lee D, Kwon HS, Yun SC, Sun BJ, Park JH, Lee JH, Jeong HS, Song HJ, Kim J, Park SJ. Cryptogenic stroke and high‐risk patent foramen ovale: the DEFENSE‐PFO trial. J Am Coll Cardiol. 2018. Available at: http://www.onlinejacc.org/content/early/2018/02/28/j.jacc.2018.02.046.full. Accessed May 9, 2018. [DOI] [PubMed] [Google Scholar]
- 14. Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive—trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. Int J Epidemiol. 2009;38:287–298. [DOI] [PubMed] [Google Scholar]
- 15. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. J Clin Epidemiol. 2008;61:64–75. [DOI] [PubMed] [Google Scholar]
- 16. Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta‐analysis. BMC Med Res Methodol. 2017;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL; American Heart Association, American Stroke Association Stroke Council, Council on Cardiovascular Surgery Anesthesia, Council on Cardiovascular Radiology Intervention, Council on Cardiovascular Nursing, Interdisciplinary Council on Peripheral Vascular Disease . Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke. 2009;40:2276–2293. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods G, Cochrane Statistical Methods G . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Badhiwala JH, Nassiri F, Alhazzani W, Selim MH, Farrokhyar F, Spears J, Kulkarni AV, Singh S, Alqahtani A, Rochwerg B, Alshahrani M, Murty NK, Alhazzani A, Yarascavitch B, Reddy K, Zaidat OO, Almenawer SA. Endovascular thrombectomy for acute ischemic stroke: a meta‐analysis. JAMA. 2015;314:1832–1843. [DOI] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 22. Jackson D, Bowden J, Baker R. How does the DerSimonian and Laird procedure for random effects meta‐analysis compare with its more efficient but harder to compute counterparts? J Stat Plan Inference. 2010;140:961–970. [Google Scholar]
- 23. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration; 2011. Available at: http://handbook.Cochrane.Org. Accessed May 6, 2018. [Google Scholar]
- 24. Goel SS, Tuzcu EM, Shishehbor MH, de Oliveira EI, Borek PP, Krasuski RA, Rodriguez LL, Kapadia SR. Morphology of the patent foramen ovale in asymptomatic versus symptomatic (stroke or transient ischemic attack) patients. Am J Cardiol. 2009;103:124–129. [DOI] [PubMed] [Google Scholar]
- 25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. J Clin Epidemiol. 2008;61:763–769. [DOI] [PubMed] [Google Scholar]
- 27. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. [Google Scholar]
- 28. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta‐analysis of binomial data. Arch Public Health. 2014;72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mendes D, Alves C, Batel‐Marques F. Number needed to treat (NNT) in clinical literature: an appraisal. BMC Med. 2017;15:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002;105:2625–2631. [DOI] [PubMed] [Google Scholar]
- 31. Shariat A, Yaghoubi E, Farazdaghi M, Aghasadeghi K, Borhani Haghighi A. Comparison of medical treatments in cryptogenic stroke patients with patent foramen ovale: a randomized clinical trial. J Res Med Sci. 2013;18:94–98. [PMC free article] [PubMed] [Google Scholar]
- 32. Li J, Liu J, Liu M, Zhang S, Hao Z, Zhang J, Zhang C. Closure versus medical therapy for preventing recurrent stroke in patients with patent foramen ovale and a history of cryptogenic stroke or transient ischemic attack. Cochrane Database Syst Rev. 2015:CD009938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kent DM, Dahabreh IJ, Ruthazer R, Furlan AJ, Reisman M, Carroll JD, Saver JL, Smalling RW, Juni P, Mattle HP, Meier B, Thaler DE. Device closure of patent foramen ovale after stroke: pooled analysis of completed randomized trials. J Am Coll Cardiol. 2016;67:907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mas JL, Derumeaux G, Amarenco P, Arquizan C, Aubry P, Barthelet M, Bertrand B, Brochet E, Cabanes L, Donal E, Dubois‐Rande JL, Durand‐Zaleski I, Ernande L, Finet G, Fraisse A, Giroud M, Guerin P, Habib G, Juliard JM, Leys D, Lievre M, Lusson JR, Marcon F, Michel P, Moulin T, Mounier‐Vehier F, Pierard L, Piot C, Rey C, Rodier G, Roudaut R, Schleich JM, Teiger E, Turc G, Vuillier F, Weimar C, Woimant F, Chatellier G; CLOSE investigators . Close: closure of patent foramen ovale, oral anticoagulants or antiplatelet therapy to prevent stroke recurrence: study design. Int J Stroke. 2016;11:724–732. [DOI] [PubMed] [Google Scholar]
- 35. De Castro S, Cartoni D, Fiorelli M, Rasura M, Anzini A, Zanette EM, Beccia M, Colonnese C, Fedele F, Fieschi C, Pandian NG. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke. 2000;31:2407–2413. [DOI] [PubMed] [Google Scholar]
- 36. Kitsios GD, Thaler DE, Kent DM. Potentially large yet uncertain benefits: a meta‐analysis of patent foramen ovale closure trials. Stroke. 2013;44:2640–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Merkler AE, Gialdini G, Yaghi S, Okin PM, Iadecola C, Navi BB, Kamel H. Safety outcomes after percutaneous transcatheter closure of patent foramen ovale. Stroke. 2017;48:3073–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chubb H, Whitaker J, Williams SE, Head CE, Chung NA, Wright MJ, O'Neill M. Pathophysiology and management of arrhythmias associated with atrial septal defect and patent foramen ovale. Arrhythm Electrophysiol Rev. 2014;3:168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Patent Foramen Ovale Closure or Anticoagulants Versus Antiplatelet Therapy to Prevent Stroke Recurrence (CLOSE) investigators.
Table S1. Literature Search Strategy
Table S2. PFO Closure Versus Antithrombotic Therapy: Descriptive Summary of Included Patients and Data Extracted for Each End Point of the Present Meta‐Analysis
Table S3. Sensitivity Analyses: Results Obtained With the DerSimonian & Laird Method and With Likelihood Approaches to Meta‐Analysis
Table S4. PFO Anatomical Features in Randomized Controlled Trials
Table S5. Description of the Events of New‐Onset Atrial Fibrillation and Subsequent Risk of Stroke
Figure S1. Guide to the interpretation of a trial sequential analysis (TSA) plot.
Figure S2. PRISMA flow chart of literature search and study selection.
Figure S3. Assessment of the risk of bias among included RCTs, using the Cochrane Collaboration's tool.
Figure S4. Random‐effects pooled incidence of recurrent stroke per 100 person‐years in the PFO closure group.
Figure S5. Random‐effects pooled incidence of recurrent stroke per 100 person‐years in the antithrombotic therapy group.
Figure S6. Pooled RR for TIA in patients randomized to PFO closure vs antithrombotic therapy (random‐effects meta‐analysis).
Figure S7. Pooled RR of all‐cause mortality in patients randomized to PFO closure vs antithrombotic therapy (random‐effects meta‐analysis).
Figure S8. Pooled RR of major bleeding in patients randomized to PFO closure vs antithrombotic therapy (random‐effects meta‐analysis).
Figure S9. Random‐effects pooled incidence of major procedural complication per 100 patients treated in the PFO closure group.
Figure S10. Pooled RR of new‐onset atrial fibrillation in patients randomized to PFO closure vs antithrombotic therapy (random‐effects meta‐analysis).
Figure S11. Random‐effects pooled incidence of new‐onset atrial fibrillation per 100 patients treated in the PFO closure group, according to the closure device (exploratory analysis).


