Abstract
Background
Regular physical activity reduces the risk of cardiovascular events, but most ischemic heart disease (IHD) patients do not obtain enough.
Methods and Results
ACTIVE REWARD (A Clinical Trial Investigating Effects of a Randomized Evaluation of Wearable Activity Trackers with Financial Rewards) was a 24‐week home‐based, remotely monitored, randomized trial with a 16‐week intervention (8‐week ramp‐up incentive phase and 8‐week maintenance incentive phase) and an 8‐week follow‐up. Patients used wearable devices to track step counts and establish a baseline. Patients in control received no other interventions. Patients in the incentive arm received personalized step goals and daily feedback for all 24 weeks. In the ramp‐up incentive phase, daily step goals increased weekly by 15% from baseline with a maximum of 10 000 steps and then remained fixed. Each week, $14 was allocated to a virtual account; $2 could be lost per day for not achieving step goals. The primary outcome was change in mean daily steps from baseline to the maintenance incentive phase. Ischemic heart disease patients had a mean (SD) age of 60 (11) years and 70% were male. Compared with control, patients in the incentive arm had a significantly greater increase in mean daily steps from baseline during ramp‐up (1388 versus 385; adjusted difference, 1061 [95% confidence interval, 386–1736]; P<0.01), maintenance (1501 versus 264; adjusted difference, 1368 [95% confidence interval, 571–2164]; P<0.001), and follow‐up (1066 versus 92; adjusted difference, 1154 [95% confidence interval, 282–2027]; P<0.01).
Conclusions
Loss‐framed financial incentives with personalized goal setting significantly increased physical activity among ischemic heart disease patients using wearable devices during the 16‐week intervention, and effects were sustained during the 8‐week follow‐up.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT02531022.
Keywords: behavioral economics, financial incentives, goal‐setting, ischemic heart disease, physical activity, wearable devices
Subject Categories: Cardiovascular Disease, Exercise, Secondary Prevention
Clinical Perspective
What Is New?
Despite the many benefits of regular physical activity, most ischemic heart disease patients do not participate in exercise‐based cardiac rehabilitation or obtain enough physical activity on their own.
We evaluated a scalable approach to increase physical activity among ischemic heart disease patients by using insights from behavioral economics to design financial incentives and goal setting to address predictable barriers to behavior change and by using wearable devices to remotely monitor behaviors.
The combination of loss‐framed financial incentives and personalized goal setting significantly increased physical activity levels during the 24‐week trial.
What Are the Clinical Implications?
Providing only wearable devices to ischemic heart disease patients is unlikely to lead to significant changes in physical activity.
Combining approaches based on behavioral economic insights with wearable devices can be used to deploy home‐based interventions that significantly change behavior among ischemic heart disease patients.
Ischemic heart disease (IHD) is the leading cause of morbidity and mortality in the United States.1, 2 Regular physical activity reduces the risk of cardiovascular events among patients with IHD.1, 3 For example, participation in exercise‐based cardiac rehabilitation has been demonstrated to reduce mortality by up to 30%.4, 5, 6, 7, 8, 9 However, the majority of eligible patients do not participate in a cardiac rehabilitation program.8, 10, 11 Recent evidence also suggests that IHD patients do not often achieve physical activity goals on their own.12
Wearable devices have received significant attention for their ability to remotely monitor health behaviors such as physical activity.13, 14 However, thus far there is limited evidence of interventions that use these devices to effectively sustain behavior change among high‐risk patients.13, 15, 16 Our previous work found that financial incentive‐based approaches that use mobile technologies can be effective in increasing physical activity,17, 18, 19 but only if they are designed to appropriately leverage insights from behavioral economics—a field that incorporates insights from psychology to design interventions that address predictable barriers to behavior change.20, 21 For example, we found that the framing of financial incentives significantly impacted their effectiveness.18 A “gain‐framed” incentive that used the standard economic approach of rewarding individuals only after physical activity goals were achieved was not effective. However, a “loss‐framed” financial incentive that allocated money upfront to a virtual account, which could be lost if goals were not achieved, led to a 50% relative increase in physical activity.
In this study, our objective was to use a randomized controlled trial to test the effectiveness of loss‐framed financial incentives with personalized goal setting to increase physical activity among IHD patients. We tested a potentially scalable design that recruited patients from 4 hospitals and delivered home‐based interventions remotely by using wearable devices and an automated technology platform.22
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design
ACTIVE REWARD (A Clinical Trial Investigating Effects of a Randomized Evaluation of Wearable Activity trackers with Financial Rewards) was a randomized controlled trial conducted between March 7, 2016 and April 3, 2017 consisting of a 16‐week intervention period (composed of an 8‐week ramp‐up phase followed by an 8‐week maintenance phase) and an 8‐week follow‐up period. The study protocol (Data S1) was approved by the University of Pennsylvania (Philadelphia, PA) Institutional Review Board, and participants provided informed consent.
The study was conducted using Way to Health, a research technology platform at the University of Pennsylvania used previously for physical activity interventions.17, 18, 19, 22 Patients used the study website to create an account, provide informed consent online, and completed baseline eligibility surveys and the MacNew heart disease quality of life questionnaire.23, 24 Patients selected whether to receive study communications by text message, e‐mail, interactive voice recording, or a combination of these. Eligible patients were mailed a wrist‐worn wearable device (Misfit Shine) and authorized access for the study to capture step data. Our previous work demonstrated that these types of devices accurately track step counts.14
All patients received $20 for completing enrollment, $30 for completing the 24‐week trial, and were allowed to keep the wearable device after the trial concluded. Patients were mailed a bank check at the end of each month with accumulated earnings.
Study Sample
Recruitment occurred from February 2, 2016 to September 21, 2016 at the following 4 hospitals in southeastern Pennsylvania: Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, and Lancaster General Hospital. Outreach targeted patients eligible for, but not yet enrolled in, a cardiac rehabilitation program and focused on patients with a recent cardiac catheterization for evaluation of coronary artery disease. Patients were contacted by telephone or during cardiology outpatient visits. Patients were eligible to participate if they were aged ≥18 years, had a history of acute coronary syndrome (unstable angina, non–ST‐segment–elevation myocardial infarction, or ST‐segment–elevation myocardial infarction), or had coronary catheterization for suspected ischemic heart disease that resulted in a definitive diagnosis. After enrollment, we used data from the electronic health record to check for the presence of IHD. Patients were excluded if they were already enrolled in a formal cardiac rehabilitation program within the past 1 year, did not have access to a smartphone or tablet compatible with the wearable device, were admitted to the hospital and were not being discharged to home, or had any other reason that participation was unsafe (eg, hemodynamic instability or New York Heart Association Class III–IV heart failure) or infeasible (eg, inability to provide informed consent).
Baseline Step Count
Before randomization, patients were told to spend 2 weeks getting accustomed to their device. During this run‐in period, we estimated a baseline step count using the second week of data—a method used in previous work.25 The first week of data was ignored to diminish the potential upward bias of the estimate from higher activity during initial device use. To prevent potential mismeasurement, we ignored any daily values less than 1000 steps because evidence indicates that these values are unlikely to represent capture of actual activity.26, 27 If less than 4 days of data were available during the second week (n=5), the patient was contacted to inquire about any device issues and the run‐in period was expanded until at least 4 days of data were captured.
Randomization
Patients with a baseline step count were then electronically randomized to a study arm using block sizes of 4, stratified by age (<65 years versus ≥65 years). All investigators, statisticians, and data analysts were blinded to arm assignments until the study and analysis were completed.
Interventions
Patients in the control arm had their step counts passively monitored by the wearable device, but were not informed of their baseline step count. The wearable device was preset with the goal of 10 000 steps per day and could be adjusted by the patient. The wearable device displayed progress toward that goal using a circular dial, and actual step counts were available within the smartphone application. Patients in this arm received no other interventions.
Patients in the incentive arm received daily feedback on their performance for all 24 weeks. In the ramp‐up incentive phase (weeks 1–8), daily step goals increased gradually from baseline by 15% each week with a maximum goal of 10 000 steps. After 8 weeks, step goals then remained fixed during the maintenance incentive phase (weeks 9–16) and the follow‐up phase without incentives (weeks 17–24). During the 16‐week intervention, patients were offered a loss‐framed financial incentive. Each week, patients were informed upfront that $14 was allocated to a virtual account. Each day the patient achieved his or her step goal, the balance remained unchanged, but each day the step goal was not achieved, the patient was informed that $2 had been deducted. The balance was refreshed with $14 every week on Monday. This design leveraged 4 important psychological principles: Individuals tend to be more motivated by losses than gains,18, 28 favor immediate over delayed gratification,29 try to avoid the feeling of regret,30, 31, 32, 33 and tend to be more driven for aspirational behavior around temporal landmarks such as the beginning of the week (the fresh start effect).34
After the 16‐week intervention period and 24‐week trial, patients in both arms were asked to complete self‐reported surveys on healthcare utilization (participation in cardiac rehabilitation, cardiac catherization, cardiology clinic visit, emergency room visit, and hospital admission) and perceptions of the overall study and wearable device.
Outcome Measures
The primary outcome was change in mean daily steps from baseline to the maintenance incentive phase. Secondary outcomes were change in mean daily steps from baseline to the ramp‐up incentive phase and follow‐up phase.
Statistical Analysis
Power calculations (a priori) were based on previous work17, 18, 19 and assumed a baseline mean step count of 6000 steps in the control group with an SD of 2000 steps, a 15% dropout rate, and a 2‐sided α of 0.05. It was estimated that a sample of 148 patients (74 per arm) would ensure at least 80% power to detect a 1000‐step difference between the incentive and control arms in the change in mean daily steps from baseline to the maintenance incentive phase. However, enrollment was closed with 105 patients because of funding constraints on the timeline. Based on these same assumptions, we had at least 80% power to detect a 1200‐step difference.
All randomly assigned patients were included in the intention‐to‐treat analysis. For each patient on each day of the study (patient‐day level), the number of steps achieved was obtained as a continuous variable. Data could be missing for any day if a patient did not use the activity tracking device or did not upload data. One patient had very high step counts compared to others in the study. After investigation, we learned that the patient frequently played the drums and there have been reports of inaccurate step tracking from this activity.35, 36 Therefore, all of this patient's data were deemed invalid and classified as missing. For the prespecified main analysis, we used multiple imputation for data that were missing and step values less than 1000. We have used this method in previous work17, 18, 19, 25 and in this study because evidence indicates that step values less than 1000 may not represent accurate data capture.26, 27 Five imputations were conducted using the mice package in R (R Foundation for Statistical Computing, Vienna, Austria), which allows for patient random effects with this data structure.37 The following predictors of missing data were included: study arm, week of study, calendar month, baseline step count, age, sex, race/ethnicity, education, marital status, household income, body mass index, days since last cardiac catheterization, most recent ejection fraction, and history of diabetes mellitus, hypertension, hyperlipidemia, smoking, and valvular heart disease. Results were combined using Rubin's standard rules.38 Secondary analyses were conducted using collected data without multiple imputation, both with and without step values less than 1000.
Unadjusted analyses estimated the change in mean daily steps from baseline to each week and each phase (ramp‐up, maintenance, and follow‐up) of the study. In adjusted analyses, we used PROC GLIMMIX in SAS (SAS Institute Inc, Cary, NC) to fit linear mixed‐effects models with a random intercept, patient random effects, and to account for the repeated measures of daily step counts. In the main model, we included baseline step count and fixed effects for calendar month and study arm. To test the robustness of our findings, we also fit a fully adjusted model that included age, sex, race/ethnicity, education, marital status, household income, body mass index, days since last cardiac catheterization, most recent ejection fraction, and history of diabetes mellitus, hypertension, hyperlipidemia, smoking, and valvular heart disease. We assumed a normal distribution and obtained difference in steps between arms for each phase (ramp‐up, maintenance, and follow‐up) using the least squares means (LSMEANS) command. In a post‐hoc exploratory subgroup analysis, we evaluated effects in patients with recent care for IHD by fitting the same models for only patients who had a cardiac catheterization within the 90 days preceding enrolling in the study.
Hypothesis tests were 2‐sided using a significance level of 0.05. Analyses were conducted in SAS (version 9.4; SAS Institute Inc) and R software (version 3.4.0; R Foundation for Statistical Computing).
Results
In this trial, 105 patients with IHD were randomized (Figure 1). Patients had a mean (SD) age of 60 (11) years, 70% were male, and 74% enrolled in the trial within 90 days after a cardiac catheterization. Baseline patient characteristics were well balanced across the study arms (Table 1). Baseline mean daily steps were 6577 (SD, 3084) in control and 7205 (SD, 3246) in the incentive arm, which was not significantly different (P=0.32).
Figure 1.
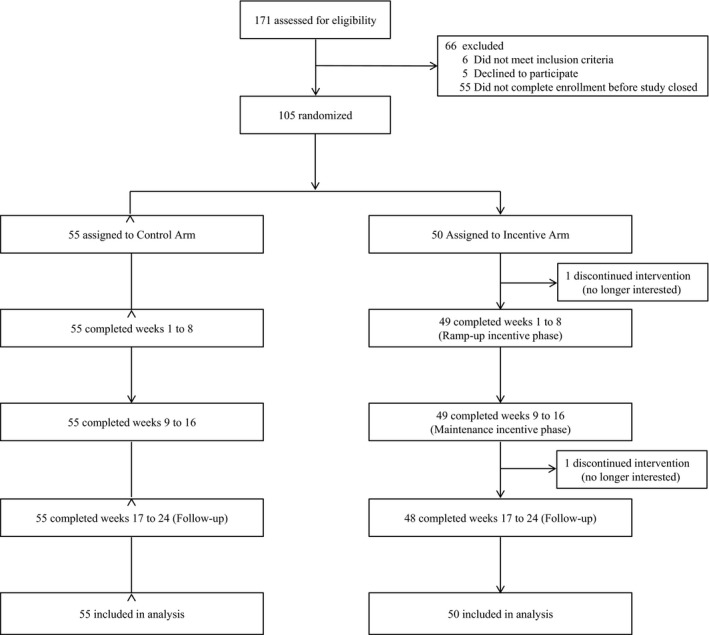
CONSORT diagram. Both arms used a wearable device to track daily steps. Patients in control received no other interventions. Patients in the incentive arm received a personalized step goal and daily feedback for 24 weeks. During the first 16 weeks, patients in the incentive arm also received a $2 per day loss‐framed financial incentive.
Table 1.
Characteristics of the Study Sample
| Characteristic | Control Arm (n=55) | Incentive Arm (n=50) | P Value |
|---|---|---|---|
| Sociodemographics | |||
| Age, y, mean (SD) | 59.1 (11.5) | 60.0 (9.5) | 0.65 |
| Male sex, no. (%) | 37 (67.3) | 36 (72.0) | 0.60 |
| Race/ethnicity, no. (%) | 0.46 | ||
| White non‐Hispanic | 38 (69.1) | 38 (76.0) | |
| Black non‐Hispanic | 14 (23.6) | 8 (16.0) | |
| Other | 3 (5.5) | 4 (8.0) | |
| Education, no. (%) | 0.29 | ||
| Some high school | 3 (5.5) | 3 (6.0) | |
| High school graduate | 10 (18.2) | 12 (24.0) | |
| Some college or specialized training | 18 (32.7) | 8 (7.7) | |
| College graduate | 24 (43.6) | 26 (52.0) | |
| Missing | 0 (0.0) | 1 (2.0) | |
| Marital status, no. (%) | 0.83 | ||
| Single | 11 (20.0) | 12 (24.0) | |
| Married | 35 (63.6) | 29 (58.0) | |
| Other | 9 (16.4) | 9 (18.0) | |
| Insurance, no. (%) | 0.82 | ||
| Private | 28 (50.9) | 24 (48.0) | |
| Medicare | 24 (43.6) | 21 (42.0) | |
| Medicaid | 2 (3.6) | 4 (8.0) | |
| Military | 1 (1.8) | 1 (2.0) | |
| Annual household income, no. (%) | 0.10 | ||
| Less than $50 000 | 21 (38.2) | 18 (36.0) | |
| $50 000 to $100 000 | 17 (30.9) | 7 (14.0) | |
| Greater than $100 000 | 12 (21.8) | 17 (34.0) | |
| Missing | 5 (9.1) | 8 (16.0) | |
| Baseline measures | |||
| Baseline step count, mean (SD) | 6577 (3084) | 7205 (3246) | 0.32 |
| Body mass index, mean (SD) | 30.1 (5.6) | 31.0 (6.8) | 0.48 |
| Diabetes mellitus, no. (%) | 18 (32.7) | 15 (30.0) | 0.76 |
| Ejection fraction, mean % (SD) | 57.8 (9.2) | 58.2 (9.6) | 0.85 |
| Health‐related quality of life score, mean (SD) | 2.7 (1.0) | 2.5 (0.8) | 0.14 |
| Hypertension, no. (%) | 44 (80.0) | 43 (86.0) | 0.42 |
| Hyperlipidemia, no. (%) | 45 (81.8) | 40 (80.0) | 0.81 |
| Previous cardiac catheterization, median days (IQR) | 37 (16.232) | 35 (23.73) | 0.49 |
| Previous cardiac catheterization within 90 days preceding enrollment, no. (%) | 40 (72.7) | 38 (76.0) | 0.78 |
| Smoking, no. (%) | 0.17 | ||
| Past smoker | 28 (50.9) | 19 (38.0) | |
| Active smoker | 6 (10.9) | 3 (6.0) | |
| Valvular heart disease, No. (%) | 4 (7.3) | 8 (16.0) | 0.16 |
Health‐related quality of life score obtained from the MacNew Survey. IQR indicates interquartile range.
One hundred three patients (98%) completed the entire 24‐week study. During the maintenance period, 22.0% of observations were missing and 1.9% of step counts were less than 1000. In the control arm, these rates were higher with 38.6% observations missing and 1.4% of step counts less than 1000 (Table S1).
In the control arm, the unadjusted change in mean daily steps from baseline began near 500 during the first 6 weeks, but then slowly declined throughout the rest of the study (Figure 2). In the incentive arm, the unadjusted change in mean daily steps from baseline began above 1000 in week 1 and increased during the ramp‐up incentive phase to nearly 2000. Mean change in daily steps from baseline remained above 1250 for the maintenance period and then slightly declined in the follow‐up period ranging from 850 to 1250 (Figure 2).
Figure 2.
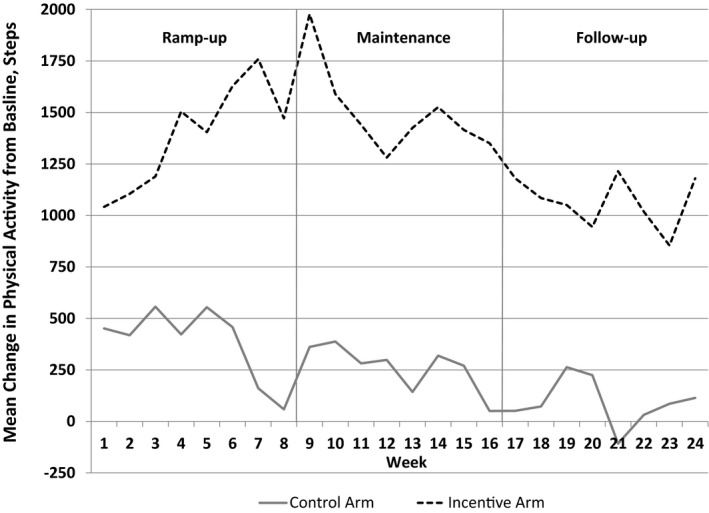
Central illustration, Unadjusted mean change in daily steps from baseline by study arm and week. Data presented are the difference between mean daily steps and mean baseline steps by week for each arm. Gray solid line represents patients in the control arm. Black dashed line represents patients in the financial incentive arm. Solid vertical gray lines represent the end of each study phase.
In the main adjusted model compared with control, patients in the incentive arm had a significantly greater increase in mean daily steps from baseline during ramp‐up (1388 versus 385; adjusted difference, 1061 [95% confidence interval [CI], 386–1736]; P<0.01), maintenance (1501 versus 264; adjusted difference, 1368 [95% CI, 571–2164]; P<0.001), and follow‐up (1066 versus 92; adjusted difference, 1154 [95% CI, 282–2027]; P<0.01). Results were qualitatively similar in the fully adjusted model (Table 2) and in secondary analyses that used collected data (Tables S2 and S3).
Table 2.
Physical Activity Outcomes
| Baseline | Ramp‐up (Weeks 1–8) | Maintenance (Weeks 9–16) | Follow‐up (Weeks 17–24) | |
|---|---|---|---|---|
| Steps per day, Mean (SD) | ||||
| Control arm | 6577 (3084) | 6962 (3364) | 6841 (3254) | 6669 (3091) |
| Incentive arm | 7205 (3246) | 8593 (3204) | 8705 (3107) | 8271 (3003) |
| Main model | ||||
| Difference adjusted for baseline (95% CI) | ··· | 1061 (386, 1736) | 1368 (571, 2164) | 1154 (282, 2027) |
| P value | ··· | <0.01 | <0.001 | <0.01 |
| Fully adjusted model | ||||
| Difference adjusted for baseline (95% CI) | ··· | 742 (−27, 1510) | 1216 (350, 2082) | 1063 (128, 1997) |
| P value | ··· | 0.06 | <0.01 | 0.03 |
Models compare the incentive arm with the control arm using imputed data and the least squares means command. Main model adjusts for baseline step count and fixed effects for calendar month and study arm. Fully adjusted model also added the following covariates: age, sex, race/ethnicity, education, marital status, household income, body mass index, days since last cardiac catheterization, most recent ejection fraction, and history of diabetes mellitus, hypertension, hyperlipidemia, smoking, and valvular heart disease. CI indicates confidence interval.
Seventy‐eight patients (74.3%) had recent care for IHD as indicated by a cardiac catheterization within the 90 days preceding enrolling in the study. Subset analyses among this group found that compared with control, patients in the incentive arm had a significantly greater increase in mean daily steps from baseline during ramp‐up (1331 versus 276; adjusted difference, 1131 [95% CI, 466–1797]; P<0.001), maintenance (1445 versus 142; adjusted difference, 1509 [95% CI, 720–2297]; P<0.001), and follow‐up (1225 versus 4; adjusted difference, 1455 [95% CI, 745–2376]; P<0.001; Table S4).
No adverse events were reported during the entire trial. Total cost of loss‐framed incentives was $5194, which averaged $103.88 per patient. Self‐reported healthcare utilization was similar between arms (Tables S5 and S6). By the end of the intervention, only 8 patients (3 in control and 5 in the incentive arm) reported joining a formal cardiac rehabilitation program. Most patients reported positive perceptions of their experience in the study, but more patients in the incentive arm agreed that they would continue to use the wearable device after the study completed (83.8% versus 5.8%; Table S7).
Discussion
In this trial, we found that loss‐framed financial incentives with personalized goal setting and wearable devices significantly increased physical activity among IHD patients over a 6‐month period including 8 weeks of follow‐up without incentives. To our knowledge, this is 1 of the first studies to demonstrate the successful use of financial incentives and wearable devices to increase physical activity among this high‐risk population. Subset analyses found similar results among the 74% of patients who had recent care for IHD as indicated by a cardiac catheterization within the 90 days preceding enrolling in the study. This intervention remotely monitored patients using an automated technology platform and wearable devices and therefore has the potential to be scaled more broadly.
Our findings reveal several important implications for future intervention design. First, a key element of our study design was the use of loss aversion, a principle from behavioral economics.13, 17, 18, 19, 28, 29, 30, 31, 32, 33, 34 Most previous financial incentive‐based physical activity interventions have used gain‐framed incentives16, 39, 40, 41—individuals earn a reward after the behavior is achieved. However, our previous work among overweight and obese individuals found that loss‐framed incentives were more effective than gain‐framed incentives.18 Results from this trial confirm that loss‐framed incentives can significantly increase physical activity among high‐risk cardiovascular disease patients. We also found that loss‐framed incentives led to sustained effects during the 8‐week follow‐up without incentives (1154 more steps). Whereas physical activity in the incentive arm declined slightly from maintenance to follow‐up, activity during both periods was higher than during the ramp‐up phase (1061 more steps). It is also important to note that physical activity levels in the control arm declined over time, most rapidly during the follow‐up phase. Nonetheless, future studies should evaluate the sustainability of incentive effects over longer‐term periods and could compare incentive designs that vary in magnitude, duration, or frequency. Future studies could also evaluate financial incentives and personalized feedback independently to assess differential effects.
Second, our approach to goal setting was unique from most previous financial incentive‐based intervention studies. Many physical activity interventions that use incentives are designed with static step goals that ask individuals to immediately achieve large step increases.16, 40, 42 In this trial, we used a ramp‐up phase to gradually increase physical activity goals (15% increase per week) and personalized these goals using the patient's baseline step count. This approach mirrors that of cardiac rehabilitation programs.5, 7 Future studies could directly compare immediate versus gradually increasing step goals and evaluate different trajectories for step‐goal increases.
Third, this trial used a home‐based, remotely monitored intervention that could be scaled more broadly by leveraging technology. IHD patients in this study all met eligibility criteria for cardiac rehabilitation, but only 7.6% reported enrolling in a formal program by the end of the intervention. Nationally, cardiac rehabilitation enrollment rates are suboptimal.8, 10, 11 Although our intervention does not substitute for cardiac rehabilitation, it may be an effective method to increase physical activity in patients who are eligible but do not participate in these programs. Future studies could build upon this approach to further test a home‐based, remotely monitored cardiac rehabilitation program.
Our study is subject to several limitations. First, patients were from 4 hospitals in southeastern Pennsylvania and needed access to a smartphone or tablet, which may limit generalizability. Second, we evaluated physical activity using step counts and did not have data on other measures of physical activity, device wear time, or other health outcomes. However, step counts are most commonly displayed by wearable devices and have been successfully used in interventions with small improvements in clinical outcomes across different populations.43 Future studies could evaluate both changes in step counts and other clinical outcomes over longer periods. Third, the control arm had slightly higher missing data rates than the incentive arm. However, our imputation and regression models both used patient random effects to adjust for differential variation across patients and arms. We also found similar results when using imputed and nonimputed data. Fourth, whereas effects were sustained during follow‐up, daily feedback was continued and physical activity did decline slightly. Further evaluations are needed to determine longer‐term sustainability. Fifth, the control arm did not receive daily feedback or personalized goal setting, and so we were unable to isolate the effects of the financial incentive alone. Sixth, in this study, loss‐framed incentives were compared with control and not with gain‐framed incentives. Future studies could compare different ways to frame incentives in this study population to increase physical activity.
Conclusions
In this home‐based, remotely monitored trial of IHD patients using wearable devices, loss‐framed financial incentives with personalized goal setting significantly increased physical activity during the 16‐week intervention, and effects were sustained during the 8‐week follow‐up. Our findings demonstrate that digital health interventions that leverage insights from behavioral economics offer a promising approach to change health behaviors among patients with cardiovascular disease.
Sources of Funding
This study was supported, in part, by grant number UL1TR000003 from the National Center for Advancing Translational Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Science or the National Institutes of Health. The study was also supported, in part, by the Institute for Translational Medicine and Therapeutics (ITMAT) and the University of Pennsylvania Health System through the Penn Medicine Nudge Unit. Misfit Inc donated some of the wearable devices used in this study. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Disclosures
Dr Patel is supported by career development awards from the Department of Veterans Affairs HSR&D and the Doris Duke Charitable Foundation. Dr Patel is founder of Catalyst Health, a technology and behavior change consulting firm. Dr Patel also has received research funding from Deloitte, which is not related to the work described in this article. Ms Ha was supported by a T32 grant from the National Institute on Aging (5‐T32‐AG‐051090‐03). The remaining authors have no disclosures to report.
Supporting information
Data S1. Study Protocol.
Table S1. Missing Data Rates by Arm and Study Period
Table S2. Physical Activity Outcomes Using Only Collected Data Without Multiple Imputation
Table S3. Physical Activity Outcomes Using Only Collected Data Without Multiple Imputation but Excluding Step Values Less Than 1000
Table S4. Physical Activity Outcomes for Patients That Had Their Last Cardiac Catheterization Within the 90 Days Preceding Enrolling in the Study
Table S5. Patients’ Self‐Reported Healthcare Utilization During the 16‐Week Intervention Period
Table S6. Patients’ Self‐Reported Healthcare Utilization During the 8‐Week Follow‐up Period
Table S7. Patients’ Perceptions of the Study and Wearable Device
(J Am Heart Assoc. 2018;7:e009173 DOI: 10.1161/JAHA.118.009173.)
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, Dellavalle R, Danaei G, Ezzati M, Fahimi A, Flaxman D, Foreman, Gabriel S, Gakidou E, Kassebaum N, Khatibzadeh S, Lim S, Lipshultz SE, London S, Lopez, MacIntyre MF, Mokdad AH, Moran A, Moran AE, Mozaffarian D, Murphy T, Naghavi M, Pope C, Roberts T, Salomon J, Schwebel DC, Shahraz S, Sleet DA, Murray, Abraham J, Ali MK, Atkinson C, Bartels DH, Bhalla K, Birbeck G, Burstein R, Chen H, Criqui MH, Dahodwala, Jarlais, Ding EL, Dorsey ER, Ebel BE, Ezzati M, Fahami, Flaxman S, Flaxman AD, Gonzalez‐Medina D, Grant B, Hagan H, Hoffman H, Kassebaum N, Khatibzadeh S, Leasher JL, Lin J, Lipshultz SE, Lozano R, Lu Y, Mallinger L, McDermott MM, Micha R, Miller TR, Mokdad AA, Mokdad AH, Mozaffarian D, Naghavi M, Narayan KM, Omer SB, Pelizzari PM, Phillips D, Ranganathan D, Rivara FP, Roberts T, Sampson U, Sanman E, Sapkota A, Schwebel DC, Sharaz S, Shivakoti R, Singh GM, Singh D, Tavakkoli M, Towbin JA, Wilkinson JD, Zabetian A, Murray, Abraham J, Ali MK, Alvardo M, Atkinson C, Baddour LM, Benjamin EJ, Bhalla K, Birbeck G, Bolliger I, Burstein R, Carnahan E, Chou D, Chugh SS, Cohen A, Colson KE, Cooper LT, Couser W, Criqui MH, Dabhadkar KC, Dellavalle RP, Jarlais, Dicker D, Dorsey ER, Duber H, Ebel BE, Engell RE, Ezzati M, Felson DT, Finucane MM, Flaxman S, Flaxman AD, Fleming T, Foreman, Forouzanfar MH, Freedman G, Freeman MK, Gakidou E, Gillum RF, Gonzalez‐Medina D, Gosselin R, Gutierrez HR, Hagan H, Havmoeller R, Hoffman H, Jacobsen KH, James SL, Jasrasaria R, Jayarman S, Johns N, Kassebaum N, Khatibzadeh S, Lan Q, Leasher JL, Lim S, Lipshultz SE, London S, Lopez, Lozano R, Lu Y, Mallinger L, Meltzer M, Mensah GA, Michaud C, Miller TR, Mock C, Moffitt TE, Mokdad AA, Mokdad AH, Moran A, Naghavi M, Narayan KM, Nelson RG, Olives C, Omer SB, Ortblad K, Ostro B, Pelizzari PM, Phillips D, Raju M, Razavi H, Ritz B, Roberts T, Sacco RL, Salomon J, Sampson U, Schwebel DC, Shahraz S, Shibuya K, Silberberg D, Singh JA, Steenland K, Taylor JA, Thurston GD, Vavilala MS, Vos T, Wagner GR, Weinstock MA, Weisskopf MG, Wulf S, Murray; U.S. Burden of Disease Collaborators . The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation. 2003;107:3109–3116. [DOI] [PubMed] [Google Scholar]
- 4. O'Connor GT, Buring JE, Yusuf S, Goldhabler SZ, Olmstead EM, Paffenbarger RS, Hennekens CH. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80:234–244. [DOI] [PubMed] [Google Scholar]
- 5. Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise‐based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2001;(1):CD001800. [DOI] [PubMed] [Google Scholar]
- 6. Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260:945–950. [PubMed] [Google Scholar]
- 7. Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta‐analysis. J Am Coll Cardiol. 2016;67:1–12. [DOI] [PubMed] [Google Scholar]
- 8. Sandesara PB, Lambert CT, Gordon NF, Fletcher GF, Franklin BA, Wenger NK, Sperling L. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate”. J Am Coll Cardiol. 2015;65:389–395. [DOI] [PubMed] [Google Scholar]
- 9. Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123:2344–2352. [DOI] [PubMed] [Google Scholar]
- 10. Doll JA, Hellkamp A, Ho PM, Kontos MC, Whooley MA, Peterson ED, Wang TY. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med. 2015;175:1700–1702. [DOI] [PubMed] [Google Scholar]
- 11. Parashar S, Spertus JA, Tang F, Bishop KL, Vaccarino V, Jackson CF, Boyden TF, Sperling L. Predictors of early and late enrollment in cardiac rehabilitation, among those referred, after acute myocardial infarction. Circulation. 2012;126:1587–1595. [DOI] [PubMed] [Google Scholar]
- 12. Kronish IM, Diaz KM, Goldsmith J, Moise N, Schwartz JE. Objectively measured adherence to physical activity guidelines after acute coronary syndrome. J Am Coll Cardiol. 2017;69:1205–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel MS, Asch DA, Volpp KG. Wearable devices as facilitators, not drivers, of health behavior change. JAMA. 2015;313:459–460. [DOI] [PubMed] [Google Scholar]
- 14. Case MA, Burwick HA, Volpp KG, Patel MS. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015;313:625–626. [DOI] [PubMed] [Google Scholar]
- 15. Jakicic JM, Davis KK, Rogers RJ, King WC, Marcus MD, Helsel D, Rickman AD, Wahed AS, Belle SH. Effect of wearable technology combined with a lifestyle intervention on long‐term weight loss: the IDEA randomized clinical trial. JAMA. 2016;316:1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finkelstein EA, Haaland BA, Bilger M, Sahasranaman A, Sloan RA, Nang EE, Evenson KR. Effectiveness of activity trackers with and without incentives to increase physical activity (TRIPPA): a randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:983–995. [DOI] [PubMed] [Google Scholar]
- 17. Patel MS, Asch DA, Rosin R, Small DS, Bellamy SL, Eberbach K, Walters KJ, Haff N, Lee SM, Wesby L, Hoffer K, Shuttleworth D, Taylor DH, Hilbert V, Zhu J, Yang L, Wang X, Volpp KG. Individual versus team‐based financial incentives to increase physical activity: a randomized, Controlled Trial. J Gen Intern Med. 2016;31:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel MS, Asch DA, Rosin R, Small DS, Bellamy SL, Heuer J, Sproat S, Hyson C, Haff N, Lee SM, Wesby L, Hoffer K, Shuttleworth D, Taylor DH, Hilbert V, Zhu J, Yang L, Wang X, Volpp KG. Framing financial incentives to increase physical activity among overweight and obese adults: a randomized, controlled trial. Ann Intern Med. 2016;164:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel MS, Volpp KG, Rosin R, Bellamy SL, Small DS, Fletcher MA, Osman‐Koss R, Brady JL, Haff N, Lee SM, Wesby J, Hoffer K, Shuttleworth D, Taylor DH, Hilbert V, Zhu J, Yang L, Wang X, Asch DA. A randomized trial of social comparison feedback and financial incentives to increase physical activity. Am J Health Promot. 2016;30:416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loewenstein G, Asch DA, Volpp KG. Behavioral economics holds potential to deliver better results for patients, insurers, and employers. Health Aff (Millwood). 2013;32:1244–1250. [DOI] [PubMed] [Google Scholar]
- 21. Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA. 2007;298:2415–2417. [DOI] [PubMed] [Google Scholar]
- 22. Asch DA, Volpp KG. On the way to health. LDI Issue Brief. 2012;17:1–4. [PubMed] [Google Scholar]
- 23. Dempster M, Donnelly M, O'Loughlin C. The validity of the MacNew quality of life in heart disease questionnaire. Health Qual Life Outcomes. 2004;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hofer S, Saleem A, Stone J, Thomas R, Tulloch H, Oldridge N. The MacNew heart disease health‐related quality of life questionnaire in patients with angina and patients with ischemic heart failure. Value Health. 2012;15:143–150. [DOI] [PubMed] [Google Scholar]
- 25. Patel MS, Benjamin EJ, Volpp KG, Fox CS, Small DS, Massaro JM, Lee JJ, Hilbert V, Valentino M, Taylor DH, Manders ES, Mutalik K, Zhu J, Wang W , Murabito JM. Effect of a game‐based intervention designed to enhance social incentives to increase physical activity among families: the BE FIT randomized clinical trial. JAMA Intern Med. 2017;177:1586–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bassett DR Jr, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer‐measured physical activity and health behaviors in U.S. adults. Med Sci Sports Exerc. 2010;42:1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kang M, Rowe DA, Barreira TV, Robinson TS, Mahar MT. Individual information‐centered approach for handling physical activity missing data. Res Q Exerc Sport. 2009;80:131–137. [DOI] [PubMed] [Google Scholar]
- 28. Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- 29. O'Donoghue T, Rabin M. Doing it now or later. Am Econ Rev. 1999;89:103–124. [Google Scholar]
- 30. Chapman GB, Coups EJ. Emotions and preventive health behavior: worry, regret, and influenza vaccination. Health Psychol. 2006;25:82–90. [DOI] [PubMed] [Google Scholar]
- 31. Connolly T, Butler D. Regret in economic and psychological theories of choice. J Behav Decis Making. 2006;19:139–154. [Google Scholar]
- 32. Krähmer D, Stone R. Anticipated regret as an explanation of uncertainty aversion. Econ Theory. 2013;52:709–728. [Google Scholar]
- 33. Zeelenberg M, Pieters R. Consequences of regret aversion in real life: the case of the Dutch postcode lottery. Organ Behav Hum Decis Process. 2004;93:155–168. [Google Scholar]
- 34. Dai H, Milkman KL, Riis J. The fresh start effect: temporal landmarks motivate aspirational behavior. Manage Sci. 2014;60:2563–2582. [Google Scholar]
- 35. Chiauzzi E, Rodarte C, DasMahapatra P. Patient‐centered activity monitoring in the self‐management of chronic health conditions. BMC Med. 2015;13:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Motti V, Caine K. Smart Wearables or Dumb Wearables? Understanding how Context Impacts the UX in Wrist Worn Interaction. 34th ACM International Conference on the Design of Communication; 2016. September 23, 2016; Silver Spring, MD: ACM. [Google Scholar]
- 37. Young R, Johnson DR. Handling missing values in longitudinal panel data with multiple imputation. J Marriage Fam. 2015;77:277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 39. Finkelstein EA, Brown DS, Brown DR, Buchner DM. A randomized study of financial incentives to increase physical activity among sedentary older adults. Prev Med. 2008;47:182–187. [DOI] [PubMed] [Google Scholar]
- 40. Harkins KA, Kullgren JT, Bellamy SL, Karlawish J, Glanz K. A trial of financial and social incentives to increase older adults’ walking. Am J Prev Med. 2017;52:e123–e130. [DOI] [PubMed] [Google Scholar]
- 41. Mitchell MS, Goodman JM, Alter DA, John LK, Pakosh MT, Faulkner GE. Financial incentives for exercise adherence in adults: systematic review and meta‐analysis. Am J Prev Med. 2013;45:658–667. [DOI] [PubMed] [Google Scholar]
- 42. Kullgren JT, Harkins KA, Bellamy SL, Gonzales A, Tao Y, Zhu J, Volpp KG, Asch DA, Heisler M, Karlawish J. A mixed‐methods randomized controlled trial of financial incentives and peer networks to promote walking among older adults. Health Educ Behav. 2014;41(1 Suppl):43S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bravata DM, Smith‐Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Sirard JR. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–2304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Study Protocol.
Table S1. Missing Data Rates by Arm and Study Period
Table S2. Physical Activity Outcomes Using Only Collected Data Without Multiple Imputation
Table S3. Physical Activity Outcomes Using Only Collected Data Without Multiple Imputation but Excluding Step Values Less Than 1000
Table S4. Physical Activity Outcomes for Patients That Had Their Last Cardiac Catheterization Within the 90 Days Preceding Enrolling in the Study
Table S5. Patients’ Self‐Reported Healthcare Utilization During the 16‐Week Intervention Period
Table S6. Patients’ Self‐Reported Healthcare Utilization During the 8‐Week Follow‐up Period
Table S7. Patients’ Perceptions of the Study and Wearable Device


