Abstract
Background
Mesenchymal stem cell–derived extracellular vesicles (EVs) are believed to be cardioprotective in myocardial infarct. The objective of this study was to examine the effects of human mesenchymal cell–derived EV injection on cardiac function, myocardial blood flow, and vessel density in the setting of chronic myocardial ischemia.
Methods and Results
Twenty‐three Yorkshire swine underwent placement of an ameroid constrictor on their left circumflex artery. Two weeks later, the animals were split into 2 groups: the control group (CON; n=7) and the EV myocardial injection group (MVM; n=10). The MVM group underwent myocardial injection of 50 μg of EVs in 2 mL 0.9% saline into the ischemic myocardium. Five weeks later, the pigs underwent a harvest procedure, and the left ventricular myocardium was analyzed. Absolute blood flow and the ischemic/nonischemic myocardial perfusion ratio were increased in the ischemic myocardium in the MVM group compared with the CON group. Pigs in the MVM group had increased capillary and arteriolar density in the ischemic myocardial tissue compared with CON pigs. There was an increase in expression of the phospho–mitogen‐activated protein kinase/mitogen‐activated protein kinase ratio, the phospho–endothelial nitric oxide synthase/endothelial nitric oxide synthase ratio, and total protein kinase B in the MVM group compared with CON. There was an increase in cardiac output and stroke volume in the MVM group compared with CON.
Conclusions
In the setting of chronic myocardial ischemia, myocardial injection of human mesenchymal cell–derived EVs increases blood flow to ischemic myocardial tissue by induction of capillary and arteriolar growth via activation of the protein kinase B/endothelial nitric oxide synthase and mitogen‐activated protein kinase signaling pathways resulting in increased cardiac output and stroke volume.
Keywords: angiogenesis, cardiac function, mesenchymal stem cell, microsphere
Subject Categories: Angiogenesis, Animal Models of Human Disease, Stem Cells
Clinical Perspective
What Is New?
This article is the first to demonstrate that in the setting of chronic myocardial ischemia, the myocardial injection of human mesenchymal cell–derived extracellular vesicles increases vessel density and blood flow to ischemic myocardial tissue, resulting in increased cardiac output and stroke volume.
What Are the Clinical Implications?
These data suggest that human mesenchymal cell–derived extracellular vesicles may be used in patients who suffer from chronic myocardial ischemia to increase myocardial blood flow and improve cardiac function.
Introduction
Chronic myocardial ischemia leads to cardiomyocyte apoptosis and necrosis, left ventricular remodeling, heart failure, and reduced ejection fraction.1 Current treatment modalities to increase blood flow to chronically ischemic myocardial tissue include revascularization procedures such as coronary bypass grafting, percutaneous coronary intervention, and transmyocardial revascularization. Patients with chronically ischemic myocardium are often not good candidates for surgical procedures because of advanced comorbidities; furthermore, percutaneous intervention may not be feasible in the setting of small vessels or chronic vessel occlusion. Currently, there are no effective medical therapies to induce angiogenesis as a means to increase blood flow to chronically ischemic myocardium.
Mesenchymal stem cells are multipotent stromal cells that can differentiate into multiple different cell types including endothelial and cardiomyocytes. Phase 1 clinical trials demonstrate that intracoronary infusion of cardiosphere‐derived cells is efficacious in patients with advanced heart failure after myocardial infarction.2, 3 However, larger‐cohort studies have failed to show a lasting clinical benefit.4, 5 Recent evidence suggests that the main benefit of mesenchymal stem cells may be derived from the bioactive molecules or extracellular vesicles they secrete. These extracellular vesicles play a key role in the regulation of physiological processes including tissue repair, immune function, and angiogenesis. Extracellular vesicles are enclosed by a lipid bilayer ranging in size from 30 to 2000 nm in diameter. Microsomes are larger extracellular vesicles ranging in size from 50 to 1000 nm that are generated by budding from the plasma membrane. Exosomes are extracellular vesicles ranging in size from 40 to 120 nm that are derived from the endolysosomal pathways.6 All extracellular vesicles contain numerous small interfering RNA, micro‐RNA, cytokines, lipids, growth factors, and angiogenic factors, rendering them a promising treatment strategy for patients with myocardial ischemia.7 The release of extracellular vesicles from mesenchymal cells is thought to induce angiogenesis, promote proliferation and differentiation of host cells, inhibit apoptosis, modulate immune function, and recruit endogenous stem cells.7 Extracellular vesicles have been shown to be cardioprotective in small and large animal models of acute and chronic myocardial infarct.8, 9, 10, 11 Although acute and chronic myocardial diseases often coincide, myocardial infarct and chronic myocardial ischemia are distinct disease processes.12 Acute myocardial infarction involves ischemic injury and cardiac scar formation if reperfusion is not immediately established. Chronic ischemia is induced if the coronary anatomy is not sufficient to provide adequate perfusion to the myocardium. Both of these processes may lead to chronic cardiac failure. To our knowledge, there are no studies examining the effect of the delivery of extracellular vesicles in a large animal model of chronic myocardial ischemia.
The objective of this study was to examine the effects of the injection of human mesenchymal cell–derived extracellular vesicles on myocardial function, perfusion, and microvascular density in the setting of chronic myocardial ischemia. We hypothesized that the intramyocardial injection of extracellular vesicles would provide a beneficial effect on cardiac function and perfusion by inducing angiogenic and trophic signaling in our porcine model of chronic myocardial ischemia.
Materials and Methods
All animal care and procedures were carried out according to the approved Institutional Animal Care and Use Committee protocol by Rhode Island Hospital (Lifespan). The data, analytic methods, and study materials have been and will continue to be made available to other researchers for purposes of reproducing the results or replicating the procedure.13, 14, 15, 16, 17, 18, 19, 20
Human Mesenchymal Cell–Derived Extracellular Vesicle Isolation
Human mesenchymal cells (Lonza, Allendale, NJ) were cultured according to Lonza recommendations in mesenchymal stem cell growth medium Bulletkit medium (Lonza, Allendale, NJ). At fourth passage, cells were grown until confluence. The mesenchymal stem cell growth medium Bulletkit medium was removed, the cells were washed with sterile PBS, and serum‐free Roswell Park Memorial Institute medium was added for 24 hours before the medium was collected. The extracellular vesicles were then collected from Roswell Park Memorial Institute medium by centrifugation at 100 000g for 30 minutes. The extracellular vesicles were washed with sterile PBS at 100 000g for 30 minutes and then resuspended in 0.5 mL of 10% dimethyl sulfoxide. Samples were stored in a −80° freezer between collection and use. They were then quickly thawed and kept on ice before injection into the pigs. Fifty micrograms of extracellular vesicles was resuspended in 2 mL of sterile normal saline before myocardial injection.
Chronic Myocardial Ischemia Animal Model
Twenty‐three Yorkshire swine (Tufts, Boston, MA) underwent placement of an ameroid constrictor to their left circumflex artery at 11 weeks of age, in a manner similar to that previously reported13, 14, 15 (Figure 1). Pigs received aspirin (10 mg/kg) 1 day before surgery and 5 days postoperatively. Cephalexin (30 mg/kg) was administered 1 day before surgery and 5 days postoperatively. Anesthesia was induced with intramuscular injection of telazol (4.4 mg/kg) and xylazine (2.2 mg/kg). A fentanyl patch was placed before surgery (4 μg/kg). A normal saline intravenous drip was started at a rate of (5 mL/[kg·h]). The pigs were intubated with a cuffed endotracheal tube for mechanical ventilation. Isofluorane (0.75‐3.0 minimum alveolar concentration) was used to maintain anesthesia. The pigs were placed in a supine position. A transverse incision was made along the third rib from the left sternal border extending laterally ≈6 cm. A Kelly clamp was used to enter the parietal pleura. The pericardium was sharply opened, the phrenic nerve was identified and protected, and the lingula of the left lung was retracted from the operative field. A 5.0 prolene suture (Ethicon, Inc, Somerville, NJ) was placed on the edge of the left atrial appendage to expose the atrioventricular groove. A small nick was made in the fat overlying where the left anterior descending (LAD) and left circumflex artery (LCx) bifurcate from the left main coronary artery. Heparin (80 IU/kg) was administered to help prevent thrombin formation. The LCx was dissected free and vessel loops were placed around it. Pressure was applied to the vessel loops to occlude the distal LCx artery for 2 minutes (the electrocardiogram was monitored at this time to evaluate for ST elevations). During occlusion, 5 cc of gold microspheres (Biophysics Assay Laboratory, Worcester, MA) was injected into the left atrial appendage. After 2 minutes, occlusion was discontinued, the ST changes were allowed to return to normal, and an ameroid constrictor (Research Instruments SW, Escondido, CA) was placed on the LCx artery. The ameroid constrictor consisted of a titanium ring with a hydrophilic core; the core slowly closes over 10 to 20 days to induce chronic myocardial ischemia.16 Topical nitroglycerin (1 mL) was applied to prevent spasm of the vessel. The pericardium was closed with silk sutures; the ribs, muscle, and skin were closed in 4 layers with vicryl 1‐0, 2‐0, 3‐0, and 4‐0 sutures, respectively (Ethicon, Inc, Somerville, NJ). Buprenorphine (0.03 mg/kg) was administered intramuscularly 30 minutes before closure. Amiodarone (10 mg/kg) was administered if any arrhythmias developed during the procedure.
Figure 1.
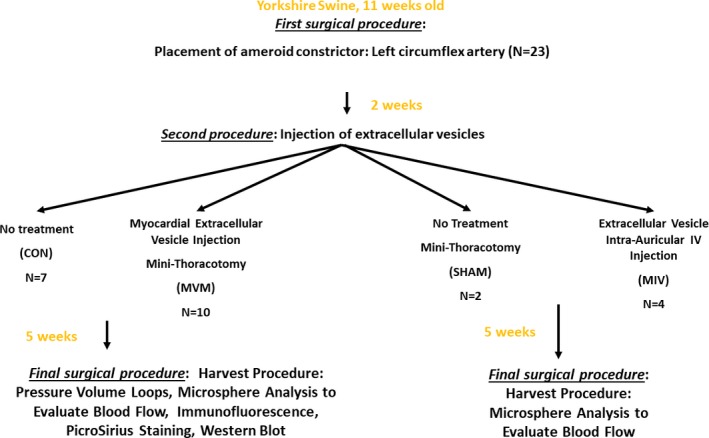
Flow diagram demonstrating the methods.
Injection of Extracellular Vesicles
Two weeks later, the animals were divided into 2 groups: the control group without extracellular vesicle (EV) injection (control group [CON]; n=7) and the extracellular vesicles myocardial injection group (MVM; n=10). The MVM group underwent a minithoracotomy procedure with the incision ≈1 inch below the incision from the ameroid placement procedure. The preoperative and postoperative care, anesthesia, pig positioning, incision opening, and incision closing were performed as described above. Briefly, the pericardium was opened, and the left ventricle and left atrial appendage were identified. The LAD and LCx artery were identified, and the extracellular vesicles (50 μg extracellular vesicles in 2 mL 0.9% saline) were injected into the area of myocardium at risk for ischemia. The incision was then closed.
Saline Injection Group Procedure
In order to determine if the repeat thoracotomy procedure (performed to administer the extracellular vesicles into the myocardium) had an effect on myocardial blood flow, a group of 2 animals underwent an ameroid constrictor placement procedure as described above and, 2 weeks later, underwent another minithoracotomy procedure in which the LAD and LCx artery were identified and 2 mL 0.9% saline was injected into the area of myocardium at risk for ischemia (saline injection; n=2). Five weeks later these pigs underwent a harvest procedure as described below.
Intravenous Injection of Extracellular Vesicles
In order to determine if the intravenous (IV) injection of extracellular vesicles would provide a physiologic effect, in a pilot study 4 animals received an IV injection of extracellular vesicles (MIV; n=4) instead of a thoracotomy. After pigs were sedated, an IV catheter was placed in the auricular vein, and the extracellular vesicles (50 μg extracellular vesicles in 2 mL 0.9% saline) were infused. Sedation was then reversed, and the animals underwent a harvest procedure 5 weeks later as described below.
Harvest Procedure
Five weeks following treatment, all pigs underwent a harvest procedure. The pigs were placed in the supine position. An incision was made in the right inguinal fold. Tissue was separated, and the right femoral artery was identified and dissected free. An 18‐gauge needle was inserted in the right femoral artery, and the Seldinger technique was used to place a 6F catheter sheath. A pressure monitor (6F Metro Top Catheter, AD Instruments, Sydney, Australia) was inserted through the sheath to monitor blood pressure. A median sternotomy was performed. Electrocautery was used to divide the skin to the sternum from the sternal notch to the xiphoid process. Blunt finger dissection was used to release the pericardium from the sternum. A Lebsche sternal chisel was used to open the sternum, and a sternal retractor was placed to separate the rib cage and expose the heart. The pericardium was opened and 3‐0 silk sutures (Ethicon, Inc, Somerville, NJ) were placed in the pericardial tissue to lift the heart. Careful dissection of any adhesions was made so that the left atrium and left ventricle were free. Lutetium microspheres (5 mL) were injected into the left atrial appendage while 10 mL of blood was simultaneously withdrawn from a right femoral artery catheter. With the Seldinger technique, a 6F sheath was placed in the left ventricle, and a pressure‐volume loop (AD Instruments, Sydney, Australia) was introduced to record hemodynamics. At the end of the procedure, the heart was excised from the pig while still beating. The myocardial tissue of the left ventricle was quickly divided into 16 pieces based on location with respect to the LAD and LCx arteries. Pieces from each section were placed in 10% neutral buffered formalin (for paraffin slides used with immunofluorescence and PicroSirius analysis), snap frozen with liquid nitrogen (for Western blot analysis), and air dried (for microsphere analysis).
The Rhode Island Hospital Institutional Animal Care and Use Committee approved and supervised all experiments. The Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals were used to ensure proper care of all animals.17
Myocardial Perfusion
During the first surgical procedure, the LCx artery was isolated and manually occluded for 2 minutes, during which time 5 mL of gold microspheres (Biophysics Assay Laboratory, Worcester, MA) was injected into the left atrial appendage. These microspheres are used to determine the area of the left ventricle that receives blood from the LCx artery. During the harvest procedure, 5 mL of lutetium microspheres was injected into the left atrial appendage while simultaneously 10 mL of blood was withdrawn from a femoral artery catheter. The blood and left ventricular myocardial tissue samples from 10 sections (based on proximity of location to the LAD and LCx arteries) were weighed and dried before being sent to Biophysics Assay Laboratory, Worcester, MA for analysis of blood flow. Blood flow data on the ischemic and nonischemic tissue were determined for each pig. The Mann‐Whitney U test was used to analyze blood flow data, which is reported as CON (median), MVM (median), Mann‐Whitney U, Difference (Actual), (Difference: Hodges‐Lehmann), and P value.
Metabolic Parameters
During each surgical procedure a glucose tolerance test was performed (0.5 g/kg of dextrose 50% was injected, and blood glucose was measured at 30 and 60 minutes), the pigs were measured for length and weight, and blood was taken from the left atrium and sent to the Rhode Island Hospital chemistry laboratory for analysis of cholesterol parameters. Student t test was used to analyze data, which are reported as mean±SEM.
Hemodynamic Measurements
During the harvest procedure, a pressure catheter (AD Instruments, Sydney, Australia) was inserted through a 6F catheter sheath into the left femoral artery for mean arterial pressure analysis. After the sternum was opened, a pressure‐volume catheter (AD Instruments, Sydney, Australia) was inserted through a 6F catheter sheath directly into the left ventricle. Hemodynamic data were recorded and analyzed with Sonosoft Software (Empower Technologies, Inc, Sarasota, FL). Student t test was used to analyze data, which are reported as mean±SEM.
Immunofluorescence
Tissue sections 5 μm thick from tissue that had been paraffin fixed and mounted on slides were used for cluster of differentiation (CD) 31 (Abcam, Cambridge, UK) and α‐smooth muscle actin (α‐SMA) (Abcam, Cambridge, UK) staining. The slides were deparaffinized, baked in a 60°C oven for 1 hour, and rehydrated in water. Heat‐induced antigen retrieval was performed in a rice cooker using antigen‐unmasking solution (Vector H3300; Vector Labs, Burlingame, CA). The slides were then rinsed 3 times in TBS. Immunoblocking was then performed in humidified chambers with Superblock (ThermoFisher, Franklin, MA) at room temperature for 1 hour. Antibody (α‐SMA 1:100) was diluted using TBS‐T with 1% BSA, and 300 μL was applied on each specimen. The slides were coverslipped and placed in the 4°C refrigerator for an overnight incubation. After 24 hours, the coverslips were removed, and slides were rinsed 3 times in TBS with agitation. The secondary antibody (Alexa Fluor 488; Abcam, Cambridge, UK) was applied at room temperature for 1 hour to determine signal. The slides were again rinsed 3 times in TBS, and the second primary (CD31, 1:50) was added to the slides for another overnight incubation. The following day the slides were rinsed 3 times in TBS, and the secondary antibody (Alexa Fluor 594; Abcam, Cambridge, UK) was applied to the slides at room temperature in the humidified chamber for 1 hour to determine signal. The slides were rinsed 3 times in TBS, and then DAPI was applied to each, and each slide was coverslipped. Three red, green, and blue images were acquired per specimen with a Nikon E800 microscope (Nikon Inc, Melville, NY) using a ×20 Plan Apo objective and a Spot RT3 camera (Diagnostic Instruments, Sterling Heights, MI). Image analysis was performed with Image J Software (National Institutes of Health, Bethesda, MD). Arteriolar and capillary densities were determined by defining positive staining via thresholding and measuring vessel counts in each field. Endothelial cells were defined as positive with CD31 staining of 50 to 800 pixels. Arteriolar vessels were defined as positive with α‐SMA staining of 800 or more pixels. Data were analyzed with a Mann‐Whitney U test and are reported as median fold change values compared to control (CON) group: CON (median), MVM (median), Mann‐Whitney U, Difference (Actual), (Difference: Hodges‐Lehmann), and P value.
PicroSirius Red Stain
Tissue sections 5 μm thick from tissue that had been paraffin fixed and mounted on slides were used for PicroSirius red staining (Polysciences, Inc, Warrington, PA). The slides were deparaffinized, baked in a 60°C oven for 1 hour, and rehydrated in water. Nuclei were stained with Weigert hematoxylin and washed with running tap water. PicroSirius stain was applied for 1 hour, and the slides were washed in 2 changes of acidified water. The tissue samples were dehydrated using 3 changes of 100% ethanol. The tissue was then cleared in xylene and mounted in a resinous medium. Three red, green, and blue images were acquired per specimen with a Nikon E800 microscope (Nikon Inc, Melville, NY) using a ×10 Plan Apo objective and a Spot RT3 camera (Diagnostic Instruments, Sterling Heights, MI). Image processing and analysis were performed using iVision color image analysis software (BioVision Technologies, version 4.0.10, Exton, PA). Positive staining was defined by thresholding the red component of the stain, and percentage area was calculated. Data were analyzed with a Mann‐Whitney U test and are reported as median percentage area: CON (median), MVM (median), Mann‐Whitney U, Difference (Actual), Difference (Hodges‐Lehmann), and P value.
Western Blot Analysis
Western blot data were collected per BioRad protocol (Life Science Research, Hercules, CA). Briefly, ischemic and nonischemic left ventricular myocardial tissue lysates were made with radioimmunoprecipitation assay buffer (Boston BioProducts, Ashland, MA). Forty micrograms of protein was fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis using 4% to 15% Tris‐glycine gels (BioRad Life Science Research, Hercules, CA). The fractionated protein was then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). Membranes were blocked for 1 hour with 5% skim milk and incubated overnight at 4°C with primary antibodies in 3% bovine serum assay blocking solution against phosphorylated protein kinase B (AKT) (serine 473), AKT, phosphorylated endothelial nitric oxide synthase (serine 1177) (p‐eNOS), eNOS, phospho‐ p44/42 mitogen‐activated protein kinases (Thr202/Tyr204) (p‐MAPK), p44/42 MAPK, cleaved caspase 3, caspase 3, cleaved caspase 9, caspase 9, phosphorylated BAD (Serine 136), BAD (Cell Signaling, Danvers, MA); 12 to 24 hours later the membranes were incubated with the appropriate horseradish peroxidase–linked secondary antibody for 1 hour at room temperature (Jackson ImmunoResearch, West Grove, PA). Immune complexes were visualized with enhanced chemiluminescence (ThermoFisher, Franklin, MA). Images were captured with a digital camera system (G‐Box, Syngene, Cambridge, UK), and band densitometry was quantified as arbitrary light units using Image J Software (National Institutes of Health, Bethesda, MD). Anti–glyceride‐3‐phosphate dehydrogenase antibody (Cell Signaling) was used on all membranes to correct for loading error. Data were analyzed with Mann‐Whitney U test,and are reported as median fold change values compared to the CON group: CON (median), MVM (median), Mann‐Whitney U, Difference (Actual), Difference (Hodges‐Lehmann), and P value.
Proteomics Analysis
Liquid chromatography–tandem mass spectrometry was performed on a fully automated proteomic technology platform at the Lifespan Core Facility18, 19 that includes an Agilent 1200 Series Quaternary HPLC system (Agilent Technologies, Santa Clara, CA) connected to a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific, Waltham, MA). The liquid chromatography–tandem mass spectrometry setup was used as described previously.20
Data Analysis
By analyzing data from prior protocols, we were able to estimate the smallest sample size needed to obtain statistically significant data. Using a 2‐tailed a‐level of 0.01, a B‐error level of 0.10, and the known standard deviation (0.150 mL/[mg·min]), we obtained a minimum sample size of 7 animals per group. A Mann‐Whitney U test was used to compare the medians among the groups for the metabolic parameters, myocardial blood flow, vessel density, and the Western blot analysis. Data are presented as CON (median), MVM (median), Mann‐Whitney U, Difference (Actual), Difference (Hodges‐Lehmann), and P value. The above data were analyzed using GraphPad Prism 5.0 Software (GraphPad Software Inc, San Diego, CA). Hemodynamic, liver enzymes, glucose, and blood cholesterol data were measured using Student t test and analyzed in Microsoft Excel.
Results
Metabolic Parameters
There was no significant difference in body mass index at the time of the initial ameroid placement procedure (CON 52.8 kg/m2, n=7; MVM 65.0 kg/m2, n=10, 16; 12.11, 17.2, P=0.08) or the harvest procedure between the control and MVM groups (CON 58.5 kg/m2, n=7, MVM 60.1 kg/m2; n=9, 29; 1.6, 1.6, P=0.84) (Figure 2A). There was no difference in percentage change in body mass index between groups (CON 13.2 kg/m2, n=7, MVM −28.0 kg/m2, n=9, 18; −41.2, −32.5, P=0.17). There was no change in glucose tolerance test between groups at the ameroid procedure. However, there was improved glucose tolerance at 60 minutes (CON 202 mg/dL, n=7, MVM 181 mg/dL, n=10, 9.5; −21, −38, P=0.01) in the MVM group compared with the control group 5 weeks later at the harvest procedure (Figure 2B). There was no difference in percentage change between the initial ameroid placement procure and the harvest procedure in aspartate aminotransferase, alkaline phosphate, alanine aminotransferase, total bilirubin, direct bilirubin, total protein, albumin, C reactive protein, insulin, cholesterol, triglycerides, high‐density lipoprotein, low‐density lipoprotein, or fructosamine between the control and MVM group. There was a significant difference in percentage change in alkaline phosphate in the MVM group compared with control (CON 5.72±0.06, n=7; MVM −45.75±0.10, n=10; P<0.01) (Table 1).
Figure 2.
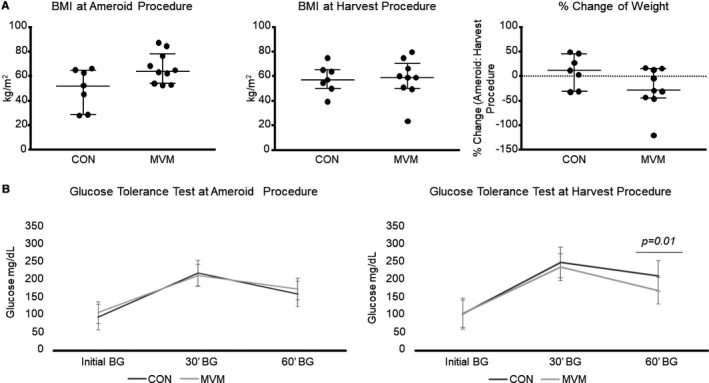
Metabolic parameters of pigs at ameroid and harvest procedures. A, There was no difference in BMI at the initial ameroid placement procedure or the harvest procedure between the control and MVM groups. B, There was no change in glucose tolerance test between groups at the ameroid procedure. There was improved glucose tolerance at 60 minutes in the MVM group compared with the control group 5 weeks later at the harvest procedure. BG indicates blood glucose; BMI, body mass index; CON, control group; MVM, myocardial extracellular vesicle injection group.
Table 1.
Metabolic Parameters
| Metabolic Parameter | CON (% Change) | SEM (±) | MVM (% Change) | SEM (±) | P Value |
|---|---|---|---|---|---|
| Glucose | 3.14 | 0.17 | 2.33 | 0.04 | 0.97 |
| AST | −28.10 | 0.12 | −40.27 | 0.11 | 0.55 |
| Alkaline phosphatase | 5.72 | 0.06 | −45.75 | 0.10 | 0.005a |
| ALT | 21.55 | 0.04 | 6.40 | 0.08 | 0.24 |
| Total bilirubin | 0.00 | 0.00 | −30.00 | 0.10 | 0.08 |
| Direct bilirubin | 0.00 | 0.00 | 0.00 | 0.00 | N/A |
| Total Protein | −0.21 | 0.03 | 4.01 | 0.03 | 0.39 |
| Albumin | −3.80 | 0.05 | −8.81 | 0.04 | 0.51 |
| CRP | −17.14 | 0.17 | 8.44 | 0.07 | 0.23 |
| Insulin | 0 | 0 | 2.12 | 0.03 | 0.67 |
| Cholesterol | −18.14 | 0.06 | 1.09 | 0.08 | 0.17 |
| Triglyceride | −20.01 | 0.27 | −32.16 | 0.33 | 0.82 |
| HDL | −1.22 | 0.05 | 2.06 | 0.09 | 0.81 |
| LDL | −44.44 | 0.11 | −6.94 | 0.11 | 0.08 |
| Fructosamine | 1.39 | 0.0216874 | 3.23 | 0.03 | 0.55 |
There was no difference between the initial ameroid placement and the harvest procedure in AST, ALT, total bilirubin, direct bilirubin, total protein, albumin, CRP, insulin, cholesterol, triglycerides, HDL, LDL or fructosamine between the control (CON) and MVM group. There was a significant decrease in alkaline phosphatase in the MVM group compared with the CON (P=0.005). ALT, alanine aminotransferase; AST, aspartate aminotransferase; CON, control; CRP, C‐reactive protein; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MVM, myocardial extracellular vesicle injection group.
P<0.05.
Hemodynamic Parameters
There were increases in cardiac output (CON 2489±405 mL/min, n=5, MVM 3716±253 mL/min, n=8, P=0.04) and stroke volume (CON 37±5 mL, n=5, MVM 52±3, n=8, P=0.46) and a trend toward increase in stroke work (CON 1492±129 mm Hg×mL, n=5, MVM 2077±251 mm Hg×mL, n=8, P=0.07) in the MVM groups compared with the control, suggesting an improvement in the cardiac function of the MVM group. There was no difference in heart rate or blood pressure between groups (Table 2).
Table 2.
Extracellular Vesicle Injection Improves Cardiac Function
| Hemodynamic Parameter | CON | MVM | P Value |
|---|---|---|---|
| SW, mm Hg×mL | 1493±129 | 2077±251 | 0.07 |
| CO, mL/min | 2498±405 | 3716±253 | 0.04a |
| SV, mL | 37±5 | 52±3 | 0.046a |
| HR, bpm | 67±3 | 71±4 | 0.32 |
| MAP, mm Hg | 55±7 | 58±6 | 0.85 |
There was an increase in cardiac output (P=0.04), and stroke volume (P=0.46), and a trend toward increase in stroke work (P=0.07) in the MVM groups compared with the controls. CO indicates cardiac output; CON, control group; HR, heart rate; MAP, mean arterial pressure; MVM, myocardial extracellular vesicle injection group; SV, stroke volume; SW, stroke work.
P<0.05.
Effect of Extracellular Vesicles on Myocardial Blood Flow
Absolute blood flow (mL/[min·g]) was increased in the chronically ischemic collateral‐dependent myocardium in the MVM group compared with the control group (CON 0.39 mL/[min·g], n=6, MVM 0.62 mL/[min·g], n=9, 3; 0.24, 0.25; P<0.01). There was no difference in absolute blood flow in the normally perfused myocardium (LAD artery territory) between the 2 groups (CON 0.61 mL/[min·g], n=6, MVM 0.59 mL/[min·g], n=10, 30; −0.02, 0.006; P>0.99). There was an increase in myocardial perfusion ratio (LCx artery/LAD artery) in the MVM group compared with the control group (CON 0.62, n=6, MVM 0.94, n=9, 4; 0.32, 0.29; P<0.01). Together, these findings suggest that EV injections improved myocardial perfusion in the ischemic regions of the MVM groups (Figure 3).
Figure 3.
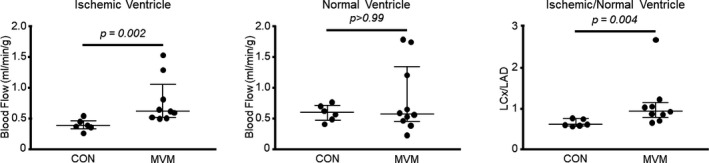
Myocardial extracellular vesicle injection increases blood flow to ischemic myocardial tissue: absolute blood flow (mL/[min·g]) was increased in the ischemic ventricle in the MVM group compared with the control group. There was an increase in myocardial perfusion ratio (left circumflex artery/left anterior descending) in the MVM group compared with the control group. CON indicates control group; LAD, left anterior descending artery; LCx, left circumflex artery; MVM, myocardial extracellular vesicle group.
Effect of Saline Injection on Myocardial Perfusion
In the pigs that underwent placement of an ameroid constrictor followed by injection of saline rather than extracellular vesicles and the pigs that underwent constrictor placement without injection of saline or EV (CON), there was no difference in collateral‐dependent (LCx territory) myocardial blood flow between the CON group and the saline injection group (CON 0.39 mL/[min·g], n=6; saline injection 0.28 mL/[min·g], n=3, 0; −0.15, −0.15; P=0.07). There was no difference in blood flow to the normally perfused myocardium (LAD territory) between the CON and saline injection groups (CON 0.61 mL/[min·g], saline injection 0.31 mL/[min·g], 0, −0.30, −0.30; P=0.07). There was no difference in the myocardial perfusion ratio (LCx artery/LAD artery) between the saline injection group and the CON group (CON 0.62 mL/[min·g], saline injection 0.68 mL/[min·g], 4, 0.06, 0.04; P=0.64). This suggests that the increased collateral‐dependent perfusion observed in the MVM group was due to the injection of extracellular vesicles and not due to the trauma and inflammation resulting from insertions of a needle and injection of fluid.
Intravenous Injection of Extracellular Vesicles
As a pilot study to evaluate the possibility of a less‐invasive means of administration of extracellular vesicles, we evaluated blood flow data in 4 pigs that received IV injections of extracellular vesicles. The absolute blood flow was not significantly increased in the ischemic ventricle of the group that received the IV injections when compared with the control (CON 0.39 mL/[min·g], n=6, IV 0.36 mL/[min·g], n=4, 11; −0.02, −0.04; P=0.91). Although these findings are not conclusive due to small sample size, IV injections of EV appear to have insignificant effects on myocardial perfusion and/or cardiac function.
Myocardial Arteriolar and Capillary Density
Pigs in the MVM group had increased capillary density (as demonstrated by CD31 staining) in the ischemic myocardial tissue compared with the control groups (CON 0.93, n=6, MVM 1.24, n=10, 6; 0.31, 0.32; P<0.01) (1 data point was removed from the CON group because it was more than 2 standard deviations above the mean). There was no change in capillary density between groups in the nonischemic myocardial tissue (CON 0.83, n=7, MVM 0.89, n=10, 34; 0.07, −0.007; P=0.96). There was an increase in arteriolar density (as demonstrated by α‐SMA staining) in the ischemic myocardial tissue between the control and MVM groups (CON 1.07, n=7, MVM 1.37, n=10, 15; 0.30, 0.30; P=0.047), suggesting increased vessel density in EV‐injected ischemic myocardium. There was no change in arteriolar density in the nonischemic myocardial tissue between the control and MVM groups (CON 0.88, n=7, MVM 1.18, n=10, 22.5; 0.29, 0.29; P=0.24) (Figure 4).
Figure 4.
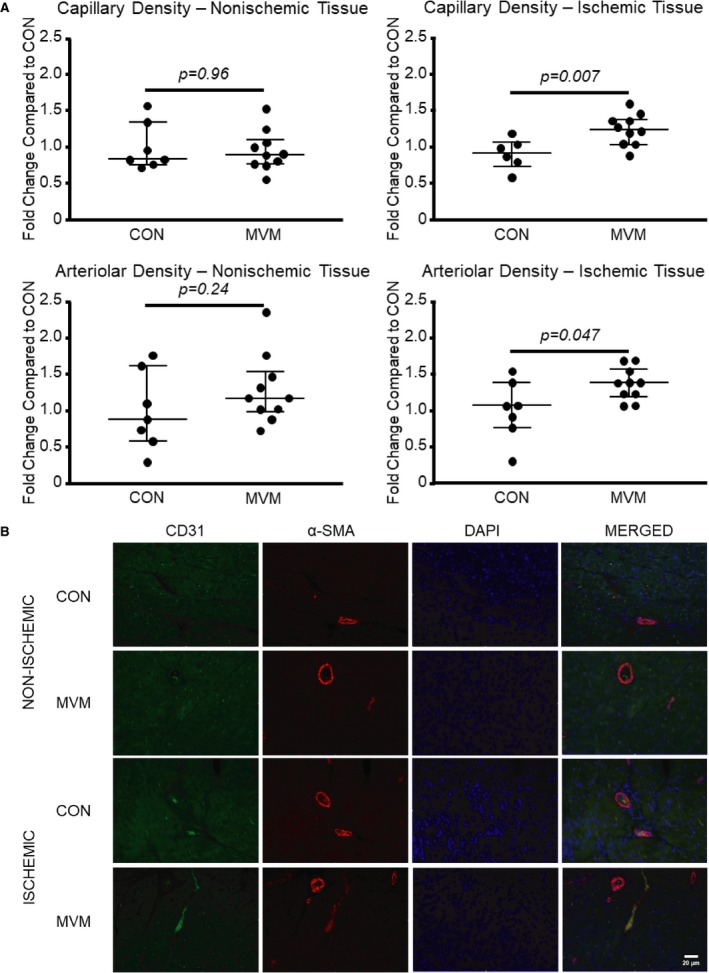
Myocardial extracellular vesicle injection increases arteriolar and capillary density in ischemic myocardial tissue. A, Pigs in the MVM group had increased capillary and arteriolar density in the ischemic myocardial tissue compared with the control groups. B, Representative images of myocardial tissue at ×20 high‐power field. DAPI is blue, CD31 is green, α‐SMA is red. CON indicates control group; MVM, myocardial extracellular vesicle group.
Myocardial Collagen Expression
There was no difference in percentage collagen expression between the MVM and control group in the ischemic tissue (CON 10.46%, n=7, MVM 12.72%, n=10, 27; 2.27, 1.57; P=0.33) or normal ventricle (CON 10.69%, n=7, MVM 11.82% mL/[min·g], n=10, 26; 1.13, 1.11; P=0.42), suggesting no significant changes in the amount of fibrotic tissues. There was no difference in ischemic ventricular to nonischemic ventricular myocardial collagen ratio between groups (CON 1.08, n=7, MVM 1.06, n=10, 38; −0.02, −0.008; P=0.99) (Figure 5).
Figure 5.
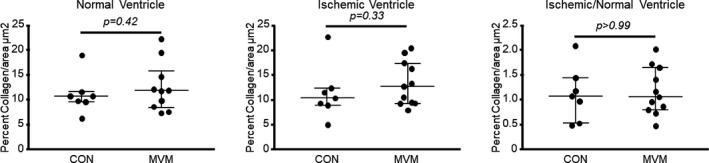
Myocardial extracellular vesicle injection does not affect collagen expression in myocardial tissue. There was no difference in percentage collagen expression between the MVM and CON groups in the ischemic tissue or normal ventricle. There was no difference in ischemic ventricle vs normal ventricle collagen ratio between groups. CON indicates control group; MVM, myocardial extracellular vesicle group.
Proangiogenic Signaling Pathway in Ischemic Myocardial Tissue
There was no significant difference in expression of p‐MAPK (CON 0.87, n=7, MVM 1.39, n=10, 17; 0.51, 0.53; P=0.09) or MAPK (CON 0.93, n=7, MVM 0.95, n=10, 34; 0.02, 0.005; P=0.96) between the MVM and control group. However, there was an increase in expression of the p‐MAPK/MAPK ratio in the MVM group compared with the control group (CON 0.99, n=7, MVM 1.47, n=10, 14, 0.48, 0.61, P=0.04). There was no significant difference in expression of p‐eNOS (CON 1.04, n=7, MVM 1.06, n=10, 27; 0.02, 0.06; P=0.33) or eNOS (1.00, n=7, MVM 0.86, n=10, 26; −0.14, −0.13; P=0.33) between the MVM and control group. There was an increase in expression of the p‐eNOS/eNOS ratio in the MVM group compared with the control group (CON 1.03, n=7, MVM 1.42, n=10, 10; 0.40, 0.46; P=0.01). Together, these results suggest that extracellular vesicles induced activation of eNOS and MAPK signaling pathways in chronically ischemic myocardium. There was an increase in expression of AKT (CON 1.08, n=7, MVM 1.26, n=10, 14; 0.18, 0.22; P=0.04). There was no difference in expression of p‐AKT (CON 0.87, n=7, MVM 0.99, n=10, 32; 0.13, 0.08; P=0.81) or the p‐AKT/AKT ratio (CON 1.00, n=7, MVM 0.98, n=10, 31; −0.02, −0.04; P=0.74). Together, these data suggest an absolute increase in the expression of AKT in the MVM group (Figure 6).
Figure 6.
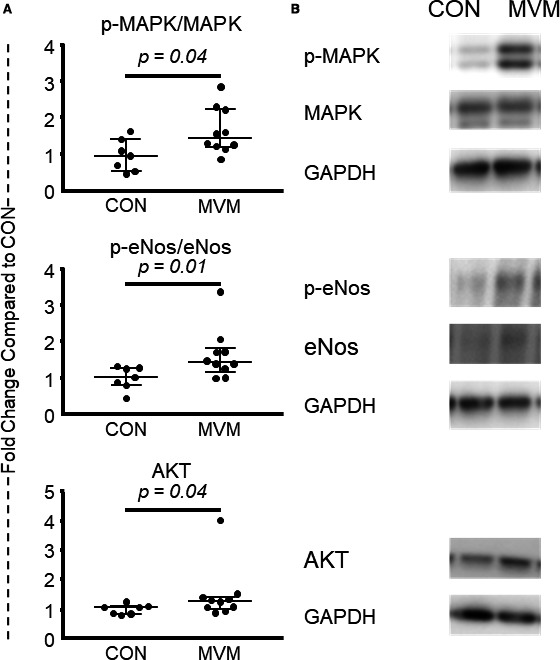
Myocardial extracellular vesicle injection increases proangiogenic protein expression in ischemic myocardial tissue. A, There was no significant difference in expression of p‐MAPK or MAPK between the MVM and control group. There was an increase in expression of the p‐MAPK/MAPK ratio in the MVM group compared with the control group. There was no significant difference in expression of p‐eNOS or eNOS between the MVM and control group. There was an increase in expression of the p‐eNOS/eNOS ratio in the MVM group compared with the control group. There was an increase in expression of AKT. There was no difference inexpression of p‐AKT or the p‐AKT/AKT ratio in the MVM group compared with the control group. B, Representative images from Western blots. CON indicates control group; MVM, myocardial extracellular vesicle group.
Apoptotic Signaling Pathway in Ischemic Tissue
There was no significant difference in expression of p‐BAD (CON 0.98, n=7, MVM 1.21, n=10, 29; 0.23, 0.19; P=0.60), BAD (CON 0.93, n=7, MVM 1.24, n=10, 19; 0.31, 0.30; P=0.13) or the p‐BAD/BAD (CON 1.16, n=7, MVM 0.68, n=10, 24; −0.49, −0.11; P=0.31) ratio between the MVM and control group. There was no significant difference in expression of cleaved caspase 3 (CON 0.90, n=7, MVM 1.20, n=10, 30; 0.30, 0.22; P=0.67), caspase 3 (CON 0.95, n=7, MVM 1.04, n=10, 28; 0.10, 0.10; P=0.54), or the cleaved caspase 3/caspase 3 ratio (CON 0.72, n=7, MVM 0.77, n=10, 32; 0.05, −0.11; P=0.81) between the MVM and control group. There was no significant difference in expression of cleaved caspase 9 (CON 0.97, n=7, MVM 1.01, n=10, 32; 0.04, 0.03; P=0.81), caspase 9 (CON 0.93, n=7, MVM 1.12, n=10, 24; 0.19, 0.18; P=0.31), or the cleaved caspase 9/caspase 9 ratio (CON 0.95, n=7, MVM 0.86, n=10, 33; −0.09, −0.03; P=0.88) between the MVM and control group. These results suggest that EVs do not have significant effects on the signaling pathways that modulate apoptosis.
Angiogenic Signaling Pathways in Nonischemic Tissue
There was no significant difference in expression of p‐MAPK (CON 0.76, n=7, MVM 1.03, n=10, 29; 0.27, 0.11; P=0.60), MAPK (CON 1.05, n=7, MVM 0.98, n=10, 29; −0.08, −0.07; P=0.60), or the p‐MAPK/MAPK (CON 1.12, n=7, MVM 1.28, n=10, 21; 0.15, 0.27; P=0.19) ratio between the MVM and control group. There was no significant difference in expression of p‐eNOS (CON 0.82, n=7, MVM 1.01, n=10, 34; 0.19, 0.02; P=0.96), eNOS (CON 0.97, n=7, MVM 0.96 n=10, 34; −0.02, −0.04; P=0.96), or the p‐eNOS/eNOS (CON 0.98, n=7, MVM 1.20, n=10, 24; 0.21, 0.16; P=0.31) ratio between the MVM and control group. There was no change in expression of p‐AKT (CON 1.02, n=7, MVM 0.75, n=10, 19; −0.27, −0.26; P=0.13), AKT (CON 0.74, n=7, MVM 0.78, n=10, 35; 0.04, 0.002; P>1.0), or the p‐AKT/AKT ratio (CON 0.80, n=7, MVM 0.46, n=10, 21; −0.34, −0.35; P=0.19). Taken together, unlike in ischemic myocardium, EVs do not have positive effects on the signaling pathways in the nonischemic myocardium.
Apoptotic Signaling in the Nonischemic Tissue
There was no significant difference in expression of p‐BAD (CON 0.78, n=7, MVM 0.73, n=10, 25, −0.04, −0.20, P=0.36), BAD (CON 0.68, n=7, MVM 1.27, n=10, 29, 0.59, 0.30, P=0.60) or the p‐BAD/BAD (CON 1.0±0.26, n=7, MVM 1.31±0.64, n=10, P=0.47) ratio between the MVM and control group There was no significant difference in expression of cleaved caspase 3 (CON 0.95, n=7, MVM 1.0, n=10, 31; 0.05, 0.08; P=0.73), caspase 3 (CON 0.82, n=7, MVM 0.91, n=10, 28; 0.09, −0.17; P=0.53), or the cleaved caspase 3/caspase 3 ratio (CON 1.04, n=7, MVM 0.05, n=10, 29; 0.008, 0.11; P=0.60) between the MVM and control group. There was no significant difference in expression of cleaved caspase 9 (CON 0.82, n=7, MVM 0.73, n=10, 28; −0.08, −0.10; P=0.53), caspase 9 (CON 1.03, n=7, MVM 1.04, n=10, 30; 0.01, 0.05; P=0.67), or the cleaved caspase 9/caspase 9 ratio (CON 0.90, n=7, MVM 0.77, 24; −0.13, −0.14; P=0.31) between the MVM and control group. These results suggest that, as in ischemic regions, apoptotic signaling pathways remain unaltered in the nonischemic regions in MVM group of animals.
Proteomic Analysis of Extracellular Vesicles
To evaluate the contents of the extracellular vesicles, a preliminary qualitative proteomic analysis was performed. A total of 967 unique proteins were identified from the extracellular vesicle samples that were used for EV injections. Many known extracellular vesicle biomarker proteins (such as CD44, CD81, CDC42, EGFR, CTNNB1, CAMK2D, CAMK2G, COL1A2, and FLNA)21 were identified. In order to examine whether the levels of proteins present in the EVs modulate in response to the culture conditions, a comparative qualitative proteomic analysis of EV samples isolated from bronchial smooth muscle cells using serum‐starved Roswell Park Memorial Institute medium (S‐NCI) versus rich medium (NS‐NCI) was performed. Venn diagram analysis shows a 52.4% overlap of proteins between the S‐NCI and NS‐NCI EVs, whereas 22% to 25% proteins were nonoverlapping, ie, unique to the samples (Figure S1). Heatmap analysis of serum‐starved (stress) versus nonstarved (nonstress) EVs demonstrated significant changes in the protein levels of growth factors and their receptors (IGFBP5, IGFBP7, PDGFRB, MYDGF, HIC5), redox regulating proteins (catalase, GSTP1, GST01), integrins (ITGB1, ITGB3, ITGB5), integrin‐linked protein, catenin (CTNNA1, CTNNB1), cadherin (CTNND1, CTNNB1), metalloproteinase (CD147), protein translation factors (EEF2, eIF4G3, eIF2S1, eIF3C, eIF4A1, eIF2S3), and cell surface glycoproteins (CDH11, CDH13, CD42, CD81) (Figure S2). Further studies using replicate samples are required for quantitative analysis of EV protein contents and their roles in improving cardiac function in chronically ischemic myocardium.
Discussion
Mesenchymal stem cells are currently under clinical investigation as a treatment to improve myocardial function and perfusion in patients with advanced heart failure after myocardial infarction.2, 3 The main benefit of mesenchymal stem cells may be derived from the bioactive encapsulated molecules they secrete in the form of EVs.7 EVs have been shown to improve blood flow and cardiac function in rodent or porcine models of acute or chronic myocardial infarction.8, 9, 10, 11 We did not observe myocardial infarction in our ameroid model of chronic myocardial ischemia. Chronic myocardial ischemia often affects people for years before they suffer symptoms or have an acute infarction. Therefore, finding a medical therapy to increase blood flow in the setting of chronic ischemia may help to alleviate the cumulative myocardial stress that develops and ultimately to preserve cardiac function.
In this study we examined the effect of myocardial injection of extracellular vesicles on myocardial function, blood flow, and vessel density in the setting of chronic myocardial ischemia. We found that myocardial injection of extracellular vesicles (1) improves cardiac output and stroke volume, (2) increases blood flow to ischemic myocardial tissue, (3) increases both capillary and arteriolar density in the chronically ischemic myocardium, and (4) is associated with increased expression levels of p‐MAPK/MAPK, p‐eNOS/eNOS, and AKT protein expression.
To ensure accurate delivery of the extracellular vesicles to the myocardial tissue, we injected the extracellular vesicles directly into the area of the myocardium at risk for ischemia based on the location of the LAD and LCx arteries. We found that this method effectively increases blood flow to the ischemic territory. This increase in blood flow was associated with increased capillary and arteriolar density in the ischemic myocardium as demonstrated by increased numbers of CD31‐ and α‐SMA–positive cells, respectively. This suggests that the injection of EVs increases myocardial perfusion to ischemic tissue by inducing increased angiogenesis in the chronically ischemic myocardium. Importantly, we found that hearts treated with EV injection demonstrated increased stroke volume and cardiac output compared with the control group, although the procedure had no effect on heart rate or blood pressure (which were within normal limits in both groups). Taken together, these findings suggest that the increase in blood flow to the ischemic tissue results in improved cardiac performance supporting the use of EV injection to alleviate the symptoms in patients with chronic myocardial ischemia (Figure 7).
Figure 7.
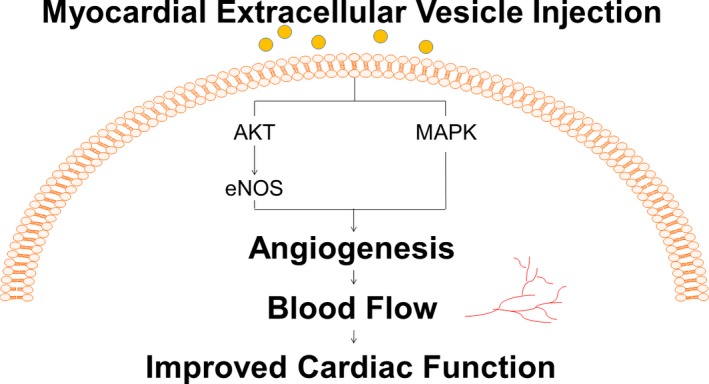
Myocardial extracellular vesicle injection increases myocardial blood flow and cardiac function. In the setting of chronic myocardial ischemia, myocardial injection of human mesenchymal cell–derived extracellular vesicles increases blood flow to the ischemic myocardial tissue by induction of capillary and arteriolar growth via upregulation of the MAPK and AKT/eNOS pathways, resulting in increased cardiac output and stroke volume.
EVs have been shown to contain numerous small interfering RNA, micro‐RNA, cytokines, growth factors, and angiogenic factors.7 Our proteomic data on the extracellular vesicles identified several proteins that are known to be associated with EVs. Our data also demonstrate that EVs contain several proangiogenic growth factors and proteins known to activate signaling pathways. These data also demonstrate that the protein contents of EVs can be modulated depending on the culture conditions/stimuli used for the bone marrow stem cells, suggesting that future studies may help develop effective modalities to treat chronically ischemic myocardium. Using Western blot analysis, we found that there was increased expression of activated, phosphorylated forms of the important proangiogenic proteins p‐MAPK/MAPK and p‐eNOS/eNOS in the MVM pigs compared with the CON. Although we did not see a significant increase in the activated, phosphorylated, form of AKT or the p‐AKT/AKT ratio, we observed an increase in total AKT expression in the ischemic tissue in the MVM group compared with the CON. These findings suggest that protein contents of EVs are plausibly responsible for the improvement in angiogenesis and myocardial blood flow in the ischemic heart. The mechanisms by which EVs improve cardiac function may involve proangiogenic growth factor and proteins including MAPK and AKT/eNOS.
It is interesting to note that we did not see an effect of IV injection of EVs on myocardial blood flow, arteriolar or capillary density, or angiogenesis in ischemic myocardium. This could be explained by the fact that we injected the EVs directly into the area of the chronically ischemic collateral‐dependent myocardium, whereas IV injections were given through auricular veins. EVs are thought to direct themselves to the area of organ injury; however, it is possible that we may need higher dosages of EVs to reach the heart from the auricular vein (as some may be localized to other organs), or that this method of administration is not effective.
We did not see a difference in apoptotic signaling in the ischemic or nonischemic myocardial territory in the MVM group compared to the CON group (as demonstrated by caspase 3, caspase 9, and BAD protein expression). We also found that there was no difference in collagen expression in the ischemic or nonischemic myocardial tissue as demonstrated by PicroSirius staining. This is not surprising given that our animal model of chronic ischemia induces chronic ischemia and not an acute myocardial infarction. Therefore, we would not expect to observe increased apoptosis or fibrosis in the ischemic or nonischemic territory. This may differentiate our large‐animal model from others that use models of acute and chronic myocardial infarction rather than a model of chronic ischemia.11
It is important to note that we injected EVs isolated from human mesenchymal stem cells into nonimmunosuppressed swine and saw no apparent detrimental signs of rejection or toxic effects, which corresponds with literature suggesting that EVs isolated from human mesenchymal stem cells may have immunosuppressive effects.22 Both groups demonstrated normal activity and vital signs. Overall, there were no significant differences in percentage changes of liver function or cholesterol expression between the 2 groups (measured at the initial and harvest surgeries). There was a significant percentage decrease in alkaline phosphatase in the MVM group compared with the control group. However, 1 laboratory value is likely not clinically relevant when all other liver function parameters are normal. There was also no difference in body mass index or percentage change in body mass index (measured at the initial and harvest surgeries). Taken together, this supports the notion that extracellular vesicles do not induce an immune response.
Interestingly, although there was no change in glucose tolerance test results at the ameroid placement procedure (before the pigs were split into 2 groups), we did see an improvement in glucose tolerance at 60 minutes in the MVM group. There is some evidence to suggest that EVs may alleviate obesity, improve glucose tolerance, and can be beneficial in the setting of acute kidney injury.23, 24 However, further studies are required to better understand these effects of EVs.
Limitations
One limitation to our original study is that the CON group underwent ameroid placement but did not undergo a myocardial injection using vehicle (saline). In an effort to address this we performed experiments with a group of pigs who underwent ameroid placement followed by cardiac injections using saline without EVs (n=2). Our results demonstrate that there is no difference in blood flow between the CON and the saline‐injected group, and an increase in blood flow between the MVM versus the CON and saline‐injected groups validated the beneficial effect of EV injection on myocardial blood flow. We initially hypothesized that the IV administration of EVs would have a positive effect. To that end, we first performed a pilot study using 4 animals with IV injection. However, negative outcome of the study prompted us to employ the technique of direct myocardial injection. We observed that the beneficial effect of extracellular injection seen in the MVM group occurred despite the added inflammation and stress caused by the repeat thoracotomy procedure. Future studies will be looking at more translational mechanisms of delivery of EVs such as increased dosages of IV EVs or intramuscular delivery. Once we find the least invasive and most effective mode of delivery of EVs, we will perform appropriate additional control and sham procedures. Another limitation is that a relatively low dose of EVs was delivered to the ischemic myocardium. The large size of the porcine model makes appropriate upscaling of the synthesis of EVs difficult. Future studies will be needed to determine a dose‐response curve once the effective translational mechanism of delivery is determined. It is to be noted that we performed proteomic analysis of EVs using only 2 samples. To determine quantitative analysis, 5 samples in each group should be analyzed. Future studies will be performed to analyze EV proteins with a larger sample size. We used a young, otherwise healthy porcine model of chronic ischemia. Most regenerative therapies are positive in these “normal” models, and nearly all preclinical studies utilizing diseased models such as a high‐fat–fed models are negative, as are nearly all clinical trials.25, 26, 27, 28 Finally, we used male pigs in our current research to decrease variability; in the future we will use female pigs as well. All these issues are currently being addressed in our laboratory.
Conclusion
In the setting of chronic myocardial ischemia, the myocardial injection of human mesenchymal cell–derived EVs increases blood flow to the ischemic myocardial tissue by induction of capillary and arteriolar growth via upregulation of the MAPK and AKT/eNOS signaling pathways, resulting in increased cardiac output and stroke volume.
Sources of Funding
This work was supported in part by the National Heart, Lung, and Blood Institute grant RO1 HL 46716 (Sellke), RO1HL128831‐01A1 (Sellke, Usheva‐Simdjuyska); NIH/NHLBI grant R01HL133624‐01A1 (Abid) and American Heart Association Grant‐in‐Aid 14GRNT20460291 (Abid); and NIH/NIGMS Training Grant 2T32 GM065085‐12 (Potz, Scrimgeour).
Disclosure
None.
Supporting information
Figure S1. Protein expression in bone marrow stem cell (BMSC)‐derived extracellular vesicles. A preliminary proteomic analysis demonstrated 967 proteins in the extracellular vesicle (EV) samples isolated from BSMC using serum‐starved Roswell Park Memorial Institute medium (S‐NCI). We also used EV collected from BSMC using rich medium (nonstarved, termed as NS‐NCI) for proteomic analysis. Venn diagram analysis shows 52.4% overlap of proteins between the S‐NCI and NS‐NCI EVs; 22% to 25% proteins were nonoverlapping between the samples.
Figure S2. A and B, Heatmap analysis of protein levels between serum‐starved extracellular vesicles (EVs) and nonstarved EVs. Significant differences between the expressions levels of PDGFRB, IGFBP5/7, eIF4G3, eIF2S1, eIF3C, eIF4A1, EEF2, eIF2S3, catalase, GSTP1, GSTo1, MYDGF, HIC5, BAG2, MAP4, CD147, CD81, CD9, CD42, ITGA2, ITGA3, ITGA6, ITGA7, ITGA11, ITGB1, ITGB3, ITGB5, ITGAV, integrin‐linked protein, CTNNA1, CTNNB1, CTNND1, CDH11, and CDH13 in nonstarved (nonstress) and starved (stress) EVs were noted in the preliminary study.
Acknowledgments
We would like to thank the laboratory of Peter Quesenberry, Professor of Oncology, Department of Hematology/Oncology at Brown University, Providence, RI, for instrumental help with the EV isolation. We would also like to thank Nagib Ahsan, PhD, Assistant Professor in the Department of Biology and Medicine at Brown University, Providence, RI, for his help with the EV proteomic analysis.
(J Am Heart Assoc. 2018;7:e008344 DOI: 10.1161/JAHA.117.008344.)
This article was the winner of the Vivien Thomas Young Investigator Award at the American Heart Association Scientific Sessions, November 11 to 15, 2017, in Anaheim, CA.
References
- 1. Xin W, Li X, Lu X, Niu K, Cai J. Involvement of endoplasmic reticulum stress‐associated apoptosis in a heart failure model induced by chronic myocardial ischemia. Int J Mol Med. 2011;27:503–509. [DOI] [PubMed] [Google Scholar]
- 2. Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LEJ, Berman D, Czer LSC, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere‐derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marbán L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marbán E. Intracoronary cardiosphere‐derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1‐year results of the CADUCEUS trial. J Am Coll Cardiol. 2014;63:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, Merkely B, Musialek P, Wojakowski W, Andreka P, Horvath IG, Katz A, Dolatabadi D, El Nakadi B, Arandjelovic A, Edes I, Seferovic PM, Obradovic S, Vanderheyden M, Jagic N, Petrov I, Atar S, Halabi M, Gelev VL, Shochat MK, Kasprzak JD, Sanz‐Ruiz R, Heyndrickx GR, Nyolczas N, Legrand V, Guédès A, Heyse A, Moccetti T, Fernandez‐Aviles F, Jimenez‐Quevedo P, Bayes‐Genis A, Hernandez‐Garcia JM, Ribichini F, Gruchala M, Waldman SA, Teerlink JR, Gersh BJ, Povsic TJ, Henry TD, Metra M, Hajjar RJ, Tendera M, Behfar A, Alexandre B, Seron A, Stough WG, Sherman W, Cotter G, Wijns W. Cardiopoietic cell therapy for advanced ischemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham‐controlled CHART‐1 clinical trial. Eur Heart J. 2016;38:648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kasai‐Brunswick TH, Rodrigues A, Andrade R, Barbosa Q, Farjun B, Cristina F, Mesquita P, Silva D, Ramos IP, Suhett G, Brasil GV, Torrentes S, Brito JOR, Passipieri A, Carvalho AB, Carlos A, De Carvalho C. Cardiosphere‐derived cells do not improve cardiac function in rats with cardiac failure. Stem Cell Res. 2017;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev. 2013;12:347–357. [DOI] [PubMed] [Google Scholar]
- 7. Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, Lambrichts I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143:181–196. [DOI] [PubMed] [Google Scholar]
- 8. Liu M, Wang YL, Shang M, Wang Y, Zhang Q, Wang SX, Wei S, Zhang K, Liu C, Wu YN, Liu ML, Song JQ, Liu YX. Flow cytometric analysis of circulating microvesicles derived from myocardial ischemic preconditioning and cardioprotection of ischemia/reperfusion injury in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2015;31:524–531. [PubMed] [Google Scholar]
- 9. Giricz Z, Varga ZV, Baranyai T, Sipos P, Pálóczi K, Kittel Á, Buzás EI, Ferdinandy P. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol. 2014;68:75–78. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, Liu Y, Kim IM, Weintraub NL, Tang Y. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. J Cardiol. 2015;192:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marbán L, Ghaleh B, Marba E. Exosomes secreted by cardiosphere‐derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sagastagoitia J, Sáez Y, Vacas M, Narváez I, de Lafuente J, Molinero E, Escobar A, Lafita M, Iriarte J. Acute versus chronic myocardial ischemia: a differential biological profile study. Pathophysiol Haemost Thromb. 2007;08:91–97. [DOI] [PubMed] [Google Scholar]
- 13. Sabe AA, Potz BA, Elmadhun NY, Liu Y, Feng J, Abid MR, Abbott JD, Senger DR, Sellke FW. Calpain inhibition improves collateral dependent perfusion in a hypercholesterolemic swine model of chronic myocardial ischemia. J Thorac Cardiovasc Surg. 2016;151:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Potz BA, Sabe AA, Elmadhun NY, Feng J, Liu Y, Mitchell H, Quesenberry P, Abid MR, Sellke FW. Calpain inhibition decreases myocardial apoptosis in a swine model of chronic myocardial ischemia. Surgery. 2015;158:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Potz BA, Sabe AA, Elmadhun NY, Feng J, Clements RT, Abid MR, Sellke FW. Glycogen synthase kinase 3β inhibition improves myocardial angiogenesis and collateral‐dependent perfusion in a swine model of metabolic syndrome. J Am Heart Assoc. 2016;5:e003694 DOI: 10.1161/JAHA.116.003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radke PW, Heinl‐Green A, Frass OM, Post MJ, Geddes DM, Alton EW. Evaluation of the porcine ameroid constrictor model of myocardial ischemia for therapeutic angiogenesis studies. Endothelium. 2006;13:25–33. [DOI] [PubMed] [Google Scholar]
- 17. National Research Council . Guide for the Care and Use of Laboratory Animals [Internet]. 8th ed Washington, DC: The National Academies Press; 2011. DOI: 10.17226/12910. [DOI] [Google Scholar]
- 18. Yu K, Salomon AR. HTAPP: high‐throughput autonomous proteomic pipeline. Proteomics. 2011;10:2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu K, Salomon AR. PeptideDepot: flexible relational database for visual analysis of quantitative proteomic data and integration of existing protein information. Proteomics. 2009;9:5350–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahsan N, Belmont J, Chen Z, Clifton JG, Salomon AR. Highly reproducible improved label‐free quantitative analysis of cellular phosphoproteome by optimization of LC‐MS/MS gradient and analytical column construction. J Proteomics. 2017;165:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, Hwang D, Kim KP, Kim DW. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839–849. [DOI] [PubMed] [Google Scholar]
- 22. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell‐derived microvesicles. Stem Cell Rev. 2015;11:150–160. [DOI] [PubMed] [Google Scholar]
- 24. Pan S, Yang X, Jia Y, Li Y, Chen R, Wang M, Cai D, Zhao R. Intravenous injection of microvesicle‐delivery miR‐130b alleviates high‐fat diet‐induced obesity in C57BL/6 mice through translational repression of PPAR‐γ. J Biomed Sci. 2015;22:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suuronen E, Hazra S, Zhang P, Vincent R, Kumarathasan P, Zhang Y, Price J, Chan V, Sellke F, Mesana T, Veinot J, Ruel M. Impairment of human cell‐based vasculogenesis in rats by hypercholesterolemia‐induced endothelial dysfunction and rescue with L‐arginine supplementation. J Thorac Cardiovasc Surg. 2010;139:209–216. [DOI] [PubMed] [Google Scholar]
- 26. Ruel M, Wu GF, Khan TA, Voisine P, Laham RJ, Sellke FW. Inhibition of the cardiac angiogenic response to surgical FGF‐2 therapy in a swine endothelial dysfunction model. Circulation. 2003;108:335–341. [DOI] [PubMed] [Google Scholar]
- 27. Voisine P, Bianchi C, Ruel M, Malik T, Rosinberg A, Feng J, Khan TA, Xu SH, Sandmeyer J, Laham RJ, Sellke FW. Inhibition of the cardiac angiogenic response to exogenous vascular endothelial growth factor. Surgery. 2004;136:407–415. [DOI] [PubMed] [Google Scholar]
- 28. Kuraitis D, Suuronen EJ, Sellke FW, Ruel M. The future of regenerating the myocardium. Curr Opin Cardiol. 2010;25:575–582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Protein expression in bone marrow stem cell (BMSC)‐derived extracellular vesicles. A preliminary proteomic analysis demonstrated 967 proteins in the extracellular vesicle (EV) samples isolated from BSMC using serum‐starved Roswell Park Memorial Institute medium (S‐NCI). We also used EV collected from BSMC using rich medium (nonstarved, termed as NS‐NCI) for proteomic analysis. Venn diagram analysis shows 52.4% overlap of proteins between the S‐NCI and NS‐NCI EVs; 22% to 25% proteins were nonoverlapping between the samples.
Figure S2. A and B, Heatmap analysis of protein levels between serum‐starved extracellular vesicles (EVs) and nonstarved EVs. Significant differences between the expressions levels of PDGFRB, IGFBP5/7, eIF4G3, eIF2S1, eIF3C, eIF4A1, EEF2, eIF2S3, catalase, GSTP1, GSTo1, MYDGF, HIC5, BAG2, MAP4, CD147, CD81, CD9, CD42, ITGA2, ITGA3, ITGA6, ITGA7, ITGA11, ITGB1, ITGB3, ITGB5, ITGAV, integrin‐linked protein, CTNNA1, CTNNB1, CTNND1, CDH11, and CDH13 in nonstarved (nonstress) and starved (stress) EVs were noted in the preliminary study.


