Abstract
Sleep disturbances and disorders have been implicated in cardiovascular morbidity and mortality. Converging evidence suggests that psychosocial factors that confer risk or resilience to cardiovascular disease (CVD) are also related to sleep. Profound differences in sleep among racial/ethnic minorities compared to non-Hispanic whites in the United States suggest that sleep, and its interplay with psychosocial factors, may contribute to observed disparities in CVD and in health and functioning, more broadly. Less understood is the extent to which sleep and psychosocial factors interact to influence the pathophysiology and clinical course of CVD. This paper reviews observational and experimental evidence linking short sleep duration and insomnia, both modifiable sleep disturbances, to CVD, including key physiological mechanisms. Also reviewed is evidence of significant interrelationships among sleep, race/ethnicity, and psychosocial factors known to confer risk or resilience to CVD including depression, psychological stress, and close interpersonal relationships. It is proposed that a transdisciplinary research framework that integrates knowledge, methods, and measures from the fields of psychology and sleep research may be used to catalyze advances in the prevention and treatment of cardiovascular disease. Also discussed are promising new directions, expected challenges, and the importance of training in transdisciplinary science and research approaches.
Keywords: Cardiovascular Disease, Sleep, Psychosocial Factors, Sleep Duration, Insomnia
The fields of psychology and sleep research have evolved largely independent of one another. Many of the conceptual models, measures, research methods, and clinical applications that have traditionally defined psychology focused almost exclusively on psychological, physiological, social, behavioral and environmental factors that occur or are measured during wakefulness. In contrast, sleep research has been primarily driven by a fundamental interest in the functions of sleep. Clinical sleep medicine has, similarly, focused on the etiology and treatment of sleep disorders. In recent years, however, the fields of psychology and sleep have become integrated in substantive ways, as instantiated by the emergence of Behavioral Sleep Medicine as a subspecialty linking health psychology and sleep medicine (Stepanski & Perlis, 2000) and the formal recognition by the American Psychological Association of Sleep Psychology as a specialty in its own right.
The integration of psychology and sleep research is evident in recent advances in our understanding of the interplay between sleep and psychosocial factors such as depression, psychological stress, and close interpersonal relationships and their possible downstream effects on the pathophysiology and clinical course of cardiovascular disease (CVD); the leading cause of death world-wide (Roth et al., 2015). Yet, innovative, high-impact opportunities still exist at the interface of psychology and sleep research. Continued integration of psychological and sleep research approaches holds the potential to greatly improve public health through the identification of unique, multifactorial risk factors and pathways through which our waking and sleeping lives interact to confer resilience or risk to cardiovascular morbidity and mortality. This paper will review evidence linking modifiable sleep disturbances to CVD, including key physiological mechanisms. Evidence of significant interrelationships among sleep and psychosocial factors known to confer risk or resilience to CVD provides additional support for the importance of integrative research. Greater, transdisciplinary, integration of knowledge and methods from the fields of psychology and sleep research will catalyze advances in the prevention and treatment of cardiovascular disease.
Sleep Disturbance as a Risk Factor for Cardiovascular Disease
Sleep is a multidimensional biobehavioral process including components such as sleep duration, continuity, architecture, timing, rhythmicity, regularity, and satisfaction (Buysse, 2014). Individual dimensions of sleep can be measured along a continuum (e.g., hours of sleep), in terms of discrete categories (e.g., short or long sleepers), or described in terms of disorders (e.g., insomnia, sleep apnea). This article will use short sleep duration and insomnia to illustrate opportunities for developing and refining integrative models of the influence of our waking and sleeping lives on the pathophysiology and clinical course of CVD. Short sleep duration and insomnia are both highly prevalent, strongly linked to cardiovascular risk via common physiological mechanisms, and modifiable via nonpharmacological approaches. Not included in this paper is the well-documented impact of sleep apnea on CVD, which has been systematically reviewed elsewhere (e.g., Dong, Zhang, & Qin, 2013). The reader interested in the influence of racial/ethnic disparities on sleep apnea and CVD risk, including the importance of sociocultural context and environment, is referred to recent integrative reviews (Grandner et al., 2016a; Williams et al., 2015).
Sleep Duration
In their 2015 joint consensus statement, the Sleep Research Society (SRS) and American Academy of Sleep Medicine (AASM) recommended that adults regularly obtain 7 hours or more of sleep to promote optimal health and functioning (Watson et al., 2015). Yet, only 65.2% of adult respondents in the 2014 Behavioral Risk Factor Surveillance System survey reported sleeping 7 or more hours per night (Liu et al., 2014). At the other end of the sleep duration spectrum, discrepant evidence linking long sleep duration to adverse health outcomes led the SRS and AASM to state that the health risk of sleeping 9 hours or more was uncertain (Watson et al., 2015).
Sleep duration may be measured by polysomnography (PSG), which is used to quantify the amount of time spent in rapid-eye-movement (REM) and non-REM sleep. While providing a physiological measure of sleep, the cost and burden associated with PSG can be a barrier to its use. Population-based studies typically measure sleep duration by self-report or wrist actigraphy, the latter of which estimates sleep duration based on the pattern and level of accelerometer- assessed activity counts. Behavioral and objective measures of sleep duration, as assessed by wrist actigraphy and PSG, respectively, generally represent the total number of minutes or hours of sleep (typically, nocturnal sleep). Self-reported sleep duration may be measured by retrospective report (e.g., past month) or via daily diaries. While less expensive than PSG and actigraphy, the wording and interpretation of self-report sleep duration questions may differ across studies and participant characteristics may influence responses (Matthews et al., 2018).
The systematic study of sleep and CVD traces its roots back to several reports from the Alameda County Study. Evidence that mortality rates from ischemic heart disease were lowest in men and women who reported habitual sleep durations of 7 to 8 hours inexorably linked sleep duration and cardiovascular health (Wingard, Berkman & Brand, 1982; Wingard & Berkman, 1983). Numerous epidemiological studies have subsequently reported a U-shaped relationship between sleep duration and cardiovascular morbidity and mortality. For example, cross-sectional analysis of self-reported sleep duration and cardiovascular morbidity in 30,000 National Health Interview Survey participants showed increased odds of any CVD in short and long sleepers (Sabanayagam & Shankar, 2010). Multivariate odds ratios of 2.20 and 1.57 were observed in those reporting sleep durations of ≤ 5 or ≥ 9 hours, respectively, after adjusting for known sociodemographic, behavioral, and anthropometric CVD risk factors, as well as CVD and psychiatric history. A 2010 meta-analysis of 15 studies conducted in the United States, Europe, and Asia, with over 400,000 participants and follow-up periods between 6.9 – 25 years, reported significant associations among self-reported habitual sleep duration and clinically confirmed cardiovascular outcomes (Cappuccio, Cooper, D’Elia, Strazzullo, & Miller, 2011). Compared to the reference category of 7 – 8 hours of sleep per night, relative risk for developing or dying from coronary heart disease (CHD) or stroke was between 1.48–1.15 for short sleepers (< 5–6 hours/night) and 1.38–1.65 for long sleepers (>8–9 hours/night).
Reports of increased blood pressure (BP) and arterial stiffness in response to acute sleep deprivation provide experimental support linking short sleep to CVD (Ogawa et al, 2003; Sunbul, Kanar, Durmus, Kivrak, & Sari, 2014). Experimental sleep restriction, which more closely mimics short sleep duration, has been shown to blunt sleep-associated blood pressure dipping and to reduce endothelial-dependent vasodilation in healthy young adults (Sauvet et al., 2015; Yang, Haack, Gautam, Meier-Ewert, & Mullington, 2017). In the only study of its kind to date, Haack and colleagues (2013) evaluated the effects of a six-week sleep extension protocol, compared to a maintenance condition, in a group of 22 adults with prehypertension or hypertension type 1. The sleep extension protocol resulted in significant decreases in beat-to-beat systolic and diastolic blood pressure with no comparable decreases in the maintenance condition.
Insomnia
Insomnia is the most common sleep complaint and disorder, with prevalence estimates of 30% for insomnia symptoms and 5 to 10% for insomnia disorder (see Mai & Buysse, 2008). The American Psychiatric Association definition of insomnia further stipulates that these sleep complaints occur at least 3 nights per week for at least 3 months, despite adequate opportunity for sleep, that they cause significant distress or daytime impairment, and are not attributable to the physiological effects of a drug of abuse or medication (American Psychiatric Association, 2013). Insomnia is diagnosed by clinical interview with a trained clinician and does not require objective findings as measured by PSG. In contrast to insomnia disorder, insomnia symptoms may be assessed by standardized questionnaires such as the Insomnia Severity Index (ISI; Morin, Belleville, Bélanger, & Ivers, 2011), discrete symptoms (e.g., report of difficulty initiating sleep), or actigraphy- or PSG-assessed indices of sleep continuity [e.g., sleep latency (SL), wakefulness after sleep onset (WASO), sleep efficiency (SE; sleep duration/time in bed x 100)].
A 2014 meta-analysis of 17 cohort studies, including 311,260 adult who were free of CVD at baseline, reported significant prospective associations among insomnia (symptoms or disorder) and specific cardiovascular outcomes including myocardial infarction, coronary heart disease, stroke and CVD mortality (Li, Zhang, Hou, & Tang, 2014). Multivariable analyses demonstrated that insomnia was associated with each outcome, with increased relative risk between 28–55% for incident disease and 33% for cardiovascular mortality, compared to individuals without insomnia. Given that CVD develops over years and decades, other studies have focused on links between insomnia and early disease markers. For example, a 2013 meta-analysis of 7 studies, including over 40,000 adults, reported significant associations among symptoms of insomnia and incident hypertension during follow-up periods of one or more years (Meng, Zheng, & Hui, 2013). The relative risks (95% confidence intervals [CI]) for difficulties maintaining sleep and early morning awakenings were 1.20 (1.06–1.36) and 1.14 (1.07–1.20) respectively. Evidence of statistical heterogeneity and publication bias was low in both meta-analyses, supporting the strength of study findings.
A handful of studies have evaluated subclinical indices of CVD in patients with insomnia compared to good sleeper control participants. For example, a laboratory-based study of normotensive adults reported elevated heart rate and blunted day-to-night blood pressure dipping in patients compared to control participants (Lanfranchi et al., 2009). Nakazaki and colleagues (2012) reported that intima-media thickness and carotid plaque scores were significantly elevated in 33 older adults with insomnia disorder compared to 53 good sleeper control participants, after adjusting for other known risk factors. Insomnia symptoms, which are prevalent in the general population, have also been associated with cardiovascular risk factors. A recent randomized clinical trial in 402 adults with comorbid insomnia and hypertension showed significant improvements in blood pressure control in patients treated with Estazolam compared to placebo, with BP compliance rates (<140/90 mmHg) over a 28-day period of 74.5% in the treatment group and 50.5% in the placebo group (Li et al., 2017)
Multidimensional Sleep Disturbances
For the most part, evidence linking sleep to cardiovascular morbidity and mortality is based on individual dimensions of sleep. Yet, individual dimensions of sleep are not experienced in isolation. In one of the most important discoveries in sleep medicine in the past decade, Vgontzas and colleagues identified a subgroup of patients with insomnia at elevated risk for decrements in health and functioning. The defining characteristic of this high-risk phenotype is the combined presentation of chronic insomnia and objectively-assessed short sleep duration (< 6 hours). Their first study reported that adults with the insomnia+objective short sleep phenotype were more likely to present with hypertension, compared to patients with insomnia who slept 6 or more hours in the lab as well as individuals who slept less than 6 hours without insomnia (Vgontzas, Liao, Bixler, Chrousos, & Vela-Bueno 2009a). A prospective study in the same cohort later demonstrated that individuals with the high-risk phenotype had a nearly 4-fold increased odds of developing incident hypertension over a 7.5-year follow-up period (Fernandez-Mendoza et al., 2012). Both cross-sectional and prospective effects were robust to adjustment for standard CVD risk factors as well as objectively-assessed sleep disordered breathing. More recently, Bathgate and colleagues (2016) replicated these findings in a study of 255 adults with insomnia studied at two large university health centers. After adjusting for standard CVD risk factors, the increased risk of reporting hypertension in the insomnia+objective short sleep group was 3.59 (95% CI, 1.58, 8.17), compared to individuals with insomnia who slept 6 or more hours. Extending this work to self-report measures, several large European cohort studies have reported increased risk for registry-based clinical cardiovascular outcomes in individuals characterized by self-reported short sleep duration in combination with subjectively-assessed sleep quality complaints (e.g., Hoevenaar-Blom, Spijkerman, Kromhout, van den Berg, & Verschuren, 2011; Rod et al., 2014).
Physiological Mechanisms Linking Disturbed Sleep to Cardiovascular Disease
Sleep duration and insomnia are known to influence the pathophysiology of CVD via inflammatory, autonomic, and metabolic pathways, among others. These physiological mechanisms are used to illustrate links between sleep and CVD; each may, similarly, inform multidimensional, integrative models of the influence of our waking and sleeping lives on the pathophysiology and clinical course of CVD.
Inflammation
Inflammation plays an important role in the development and progression of CVD (e.g., Willeit et al., 2016). A recent systematic review of 72 studies including more than 50,000 participants concluded that short sleep duration was a significant correlate of increased circulating interleukin-6 (IL-6) levels and long sleep duration was associated with elevated levels of both IL-6 and C-reactive protein (CRP; Irwin, Olmstead, & Carroll, 2016). Elevated CRP levels have also been linked to self-reported and actigraphy-assessed short sleep duration (Grandner et al., 2013; Hall, Lee, & Matthews, 2015a). In the same meta-analysis, laboratory- based sleep deprivation was not associated with increased inflammation, perhaps due to the time course of inflammatory processes. While experimental sleep restriction is associated with acute increases in the activity of upstream pro-inflammatory molecular pathways (e.g., Tumor Necrosis Factor-α (TNFα) messenger RNA and nuclear factor (NF)-κβ activation), downstream effects on circulating levels of IL-6, CRP and TNFα may not be observed during the short follow-up periods employed in experimental sleep restriction/deprivation studies (Irwin et al., 2016). Indeed, a protocol designed to mimic habitual sleep restriction in healthy young adults reported significant increases in circulating proinflammatory cytokines following a week-long restriction from 8 to 6 hours of sleep (Vgontzas et al., 2004).
Inflammation has, similarly, been linked to insomnia disorder. For example, two small studies reported increased nocturnal IL-6 in patients with chronic insomnia relative to good sleeper control participants (Burgos et al., 2006; Vgontzas et al., 2002). Evidence from a randomized controlled trial (n=123) of the comparative efficacy of a multi-component cognitive behavioral therapy for insomnia (CBTI), Tai Chi Chih (TCC), and an attention-control sleep seminar (SS) suggests that insomnia may play a causal role in inflammation. Compared to the SS control condition, CBTI was associated with decreased indices of cellular and systemic inflammation and TCC was associated with decreased cellular inflammation only; however, both active interventions were associated with decreased activity of proinflammatory transcriptional profiles (e.g., reduced NF-κβ activity) (Irwin et al., 2014; Irwin et al., 2015). The multicomponent nature of the CBTI condition, which was modified to include behavioral strategies to enhance mood and daytime activity levels, precludes attributions about the mechanisms linking modified CBTI to inflammation, including differences in inflammation profiles over the follow-up period in both active treatment groups (see Irwin et al., 2014). What we do know is that effective treatment of insomnia was associated with significant decreases in inflammation, irrespective of treatment group allocation. At follow-up, CRP levels were significantly lower in participants whose insomnia remitted, compared to those who still met diagnostic criteria for insomnia (Irwin et al., 2014). In contrast to insomnia disorder, two population-based studies reported that self-reported symptoms of insomnia were not associated with inflammation, measured by CRP, IL-6, or TNF-α, after multivariate adjustment (Laugsand, Vatten, Bjorngaard, Hveem, & Janszky, 2012; Prather, Vogelzangs, & Pennix, 2015).
Autonomic Nervous System Activity
Autonomic nervous system (ANS) activity in the form of decreased parasympathetic activity and increased sympathetic activity is a recognized risk factor for CVD (see Hillebrand et al., 2013). Consistent with the hypothesis that short sleep duration increases CVD risk though autonomic dysfunction, experimental sleep deprivation has been shown to decrease parasympathetic activity and increase sympathetic activity, indexed by high-frequency heart rate variability (HF-HRV) and plasma norepinephrine (NE), respectively (Zhong et al., 2005). Naturalistic observation of on-call physicians, deprived of sleep for 26 hours, revealed diminished HF-HRV and high levels of plasma NE and epinephrine (Tobaldini et al., 2013).
Insomnia has long been described as a disorder characterized by cognitive and somatic hyperarousal (see Bonnet & Arand, 2010). Indeed, early case control studies suggested that parasympathetic activity was significantly lower in patients with insomnia compared to age- and sex-matched participants without insomnia (see Bonnet & Arand, 2010). Yet, as reviewed by Dodds and colleagues (2017), the results of subsequent studies have been highly mixed, perhaps due to the diversity of approaches across studies including differences in diagnostic criteria, sample characteristics, methods and measurement of ANS activity, etc. Converging evidence does suggest ANS dysregulation in patients with the insomnia+objective short sleep phenotype (see Fernandez-Mendoza, 2017).
Metabolic Dysfunction
Chronic metabolic dysfunction in the form of insulin resistance and impaired glucose tolerance is a leading risk factor for CVD morbidity and mortality (e.g., Huang et al., 2014). A seminal study published in 1999 demonstrated that restricting sleep to 4 hours per night over 6 consecutive nights profoundly reduced glucose clearance and insulin response to glucose in a sample of 11 young, lean males to levels usually observed in geriatric patient populations (Spiegel, Leproult, & Van Cauter, 1999). Subsequent experimental and observational studies have continued to replicate and extend these findings, suggesting that sleep curtailment is a critical risk factor for the development of obesity, diabetes, and their downstream effects on cardiovascular health (Van Cauter, Spiegel, Tasali, & Leproult, 2008). Two recent studies in healthy adults with lifestyle-restricted short sleep reported improvements in insulin sensitivity following short-term protocols that extended sleep by approximately one hour per night (Killick et al., 2015; Leproult, Deliens, Gilson, & Peigneux, 2015).
A similar pattern of metabolic dysfunction is seen in insomnia in most, but not all, studies (see Depner, Stothard, & Wright, 2014). Vgontzas and colleagues (2009b) reported that diabetes risk was nearly three times greater in patients with insomnia and laboratory-assessed sleep durations of < 5 hours, compared to adults without insomnia and sleep durations of 6 or more hours of sleep, after adjusting for standard diabetes risk factors as well as symptoms of depression and sleep apnea. A meta-analysis of 6 prospective studies including over 15,000 adults evaluated type 2 diabetes incidence in relation to self-reported symptoms of insomnia (Cappuccio, D’Elia, Strazzullo, & Miller, 2010). Results indicated an increased risk of incident diabetes over a 3- to 32-year follow up in association with symptoms of difficulty initiating [RR = 1.28 (95% CI 1.03–1.60)] and maintaining sleep [RR = 1.48, (95% CI 1.13–1.96)]. The authors further noted that associations among symptoms of insomnia and incident diabetes risk were not attenuated when analyses were restricted to studies with direct clinical assessments (n = 4).
Sleep and Indices of Psychosocial Risk and Resilience
The past 20 years has seen a dramatic increase in the number of studies focused on the psychosocial correlates of sleep. Much of this research has been focused on the impact of psychosocial factors on sleep. As reviewed below, depression, psychological stress, and close interpersonal relationships have each been linked to sleep duration and insomnia. Yet, the observational and/or cross-sectional nature of many of these studies suggests that attributions of causality are premature. Importantly, each of these psychosocial factors influences CVD risk (i.e., depression, psychological stress) or resilience (close interpersonal relationships). Also reviewed is the influence of race/ethnicity on sleep duration and insomnia, including its importance to our understanding of disturbed sleep as a risk factor for cardiovascular morbidity and mortality. A clearer understanding of the extent to which these psychosocial factors influence and are influenced by sleep across racial/ethnic groups is critical to advancing our understanding of the influence of our waking and sleeping lives on the pathophysiology and clinical course of CVD.
Depression
As reflected in the diagnostic criteria for major depressive disorder (MDD), depression and disturbed sleep are highly comorbid (American Psychiatric Association, 2013). Prospective observational and experimental studies suggest that insomnia contributes to the onset and clinical course of MDD. For example, a prospective population-based study of 4,547 Swiss adults found that 17% to 50% of participants with insomnia symptoms lasting 2 weeks or longer subsequently developed incident depression (Buysse et al., 2008). A small RCT that evaluated the impact of antidepressant medication (escitalopram; EsCIT) alone or in combination with CBTI (EsCIT+CBTI) reported higher remission rates of depression and insomnia in the EsCIT+CBTI condition compared to the EsCIT condition (Manber et al., 2008). These data suggest that treatment related remission of insomnia symptoms may have contributed to the remission of depression. With respect to depression and sleep duration, a meta-analysis of 7 prospective studies including 25,272 participants reported that short [RR=131 (95% CI 1.04–1.64)] and long [RR=1.42 (95% CI 1.04–1.92)] sleep duration were both associated with incident depression, despite heterogeneity in the assessment of depression assessment (self-report rating scales or clinical diagnostic interview) and sleep duration categories (referent group was generally between 6–8 hours) (Zhai, Zhang, & Zhang, 2015). Importantly, results were robust to adjustment for major confounding factors including sociodemographics and body mass index.
Psychological Stress
Psychological stress is antithetical to sleep. The stress response involves psychophysiological preparation to fight or flee from perceived threat, whereas mental and physiological quiescence are necessary to the initiation of and maintenance of sleep. A large body of evidence has documented cross-sectional and prospective associations among stress and sleep. For example, disturbed sleep has been associated with numerous indices of psychological stress including academic stress, stressful life events, job and financial strain, lower socioeconomic status, poverty, discrimination, and unfair treatment, among others (e.g., Brindle et al., 2018; Hall et al., 2015b; Patel, Grandner, Xie, Branas, & Gootneratne, 2010). Objectively- assessed difficulty maintaining sleep, as measured by wakefulness after sleep onset, is the dimension of sleep most reliably associated with stress in cross-sectional and prospective studies (Hall, et al., 2015b; Kim & Dimsdale, 2007). Moreover, prospective studies suggest that individual differences in vulnerability to stress-related sleep disturbances precipitate and maintain insomnia (Drake, Pillai, & Roth, 2014; Jarrin, Chen, Ivers, & Morin, 2014). Although stress is not consistently associated with sleep duration in observational studies, experimental sleep deprivation has been shown to modulate physiological (e.g., BP, heart rate, cortisol) responses to acute laboratory stress (Franzen et al., 2011; Minkel et al., 2014) and lower the threshold for reporting events as stressful (Minkel et al., 2012).
Interpersonal Relationships
In their ideal form, interpersonal relationships confer a sense of safety, security, and connection. These feelings, in turn, are important to one’s ability to obtain, fall, and stay asleep which, after all, involves a state of reduced vigilance to one’s surroundings. Numerous studies have now documented significant cross-sectional associations among greater relationship quality, for both married and nonmarried couples, and fewer objectively- and subjectively-assessed symptoms of insomnia (e.g., Robles, Slatcher, Trombello, & McGinn, 2014; Troxel, Buysse, Hall, & Matthews, 2009). The concordance between couples’ sleep and the quality of their interactions appears bidirectionally linked such that greater sleep concordance is associated with more positive partner interactions, including conflict resolution, and vice versa (Gordon & Chen, 2014; Hasler & Troxel, 2010). Parent-child relationships are, similarly, important. A recent study of adolescents reported that close maternal-adolescent relationships buffered the effects of academic stress on self-reported sleep duration and symptoms of insomnia (Van Schalkwijk, Blessinga, & Willemen, Van Der Werf, & Schuengel, 2015). This work, further, suggests that associations between interpersonal relationship quality and sleep may differ for men and women (Hasler & Troxel, 2010; Van Schalkwijk et al., 2015).
Race/Ethnicity
Studies conducted in the United States suggest that sleep differs markedly as a function of self-identified racial/ethnic group. To date, the majority of studies have evaluated sleep in blacks (term refers to individuals who self-identify as African-American or black) compared to whites (term refers to individuals who self-identify non-Hispanic Whites or of European descent), as summarized in a meta-analysis of 14 studies, including 1,010 blacks and 3,156 whites, age 18 or older, without diagnosed or suspected sleep disorders (Ruiter, DeCoster, Jacobs, & Lichstein, 2011). Objectively-assessed sleep duration and sleep efficiency were lower in blacks, with mean effect sizes of −.48 and −.54, respectively. Race/ethnicity differences in subjectively-assessed sleep duration were small (−.23). In another study, genetic ancestry was unrelated to objectively-assessed sleep duration and efficiency (Halder et al., 2017). Decrements in sleep duration and reports of difficulty initiating sleep have also been observed in other racial/ethnic minorities including Latinos, Chinese- and Asian-Americans, and African and Caribbean immigrants (Chen et al., 2015; Egan, Knutson, Pereira, & von Schantz, 2017).
Multiple modifiable factors contribute to observed racial/ethnic differences in sleep duration and insomnia symptoms. As summarized by others, expectations, attitudes, norms, and beliefs about sleep may influence the interpretation of and response to questionnaires or interviews (Adenekan et al., 2013; Williams et al, 2015). Other modifiable sociocultural factors that may contribute to decrements in sleep in racial/ethnic minorities include disparities in socioeconomic status (SES), access to health care, characteristics of the physical environment (e.g., crowding, noise, risk to safety), perceived racism and discrimination (see Grandner, Williams, Knutson, Roberts & Jean-Louis, 2016b). Moderation analyses in the meta-analysis of sleep in blacks and whites by Ruiter and colleagues (2011), similarly, suggest that modifiable risk factors contribute to decrements in sleep duration and, to a lesser extent, sleep efficiency. Setting and employment status had more modest, non-significant, effects on race/ethnicity differences in objectively-assessed sleep efficiency. While not causal, these data suggest that systemic differences that contribute to racial/ethnic disparities in socioeconomic status and health may, similarly, contribute to decrements in sleep in racial/ethnic minorities in the US. That these factors are modifiable suggests that decrements in sleep duration and efficiency in racial/ethnic minorities, including their long-term impact on cardiovascular morbidity and mortality, are amendable to intervention (Egan et al., 2017).
Promising Future Directions and Expected Challenges
Emerging evidence is consistent with the hypothesis that short sleep duration and insomnia, alone or in combination, are associated with subclinical and clinical cardiovascular disease. Plausible physiological mechanisms linking disturbed sleep and CVD risk have been identified including inflammation, ANS dysfunction, and metabolic dysfunction. The mechanisms through which long sleep duration contributes to cardiovascular outcomes are less clear. Unmeasured disease is a plausible explanation, although long sleep duration remained correlated with (all-cause) mortality after adjusting for inflammation in a population-based study of older adults (Hall al., 2015a). Evaluation of the mechanisms linking long sleep to subclinical markers of cardiovascular disease is further complicated by the paucity of “long sleepers” in laboratory sleep studies, which generally have more stringent exclusion criteria compared to population- or community-based sleep studies. Certainly, the preponderance of evidence suggests that disturbed sleep is a plausible risk factor for cardiovascular disease. Systematic research that integrates models, methods, and measures developed and employed by psychologists and sleep researchers has the potential to advance critical knowledge gaps in our basic understanding of how sleep affects cardiovascular disease. This research agenda is, in turn, critical for informing integrative clinical approaches to reduce cardiovascular morbidity and mortality via improved sleep.
The literature linking sleep to cardiovascular disease has several limitations. Most importantly, the majority of studies are based on one measurement time point. Longitudinal assessment of sleep is needed to evaluate the impact of exposure and trajectories, especially given the protracted time course of cardiovascular pathophysiology. For example, while laboratory-based studies that include 3 to 14 days of sleep restriction provide well-controlled opportunities to model the acute effects of insufficient sleep on subclinical disease processes, they cannot model the long-term effects of chronic short sleep duration on subclinical or clinical cardiovascular outcomes. Observational studies have reported that increased exposure to disturbed sleep including chronic insomnia alone or in combination with short sleep duration are associated with clinical disease. Sleep extension paradigms provide the opportunity to study the longer-term effects of exposure to short sleep, although interventions have also been of limited duration (e.g., 2 weeks), with limited physiological assessments.
It remains to be seen whether treatment of insomnia and short sleep duration leads to changes in clinical disease such as observed, for example, in the landmark Lifestyle Heart Trial, which was focused on waking health behaviors (Ornish et al., 1990). Internet-based sleep interventions and the proliferation of wearable technologies represent promising opportunities for person-oriented approaches for optimizing sleep with the goal of improving cardiovascular and overall health. As with other areas of preventive medicine, creative strategies will be needed to enhance long term compliance with sleep-based lifestyle interventions; this challenge may be especially difficult for individuals whose short sleep duration is related to competing demands such as work and family responsibilities or social opportunities promulgated by 24/7 access to electronic media.
Systematic examination of reliable effect modifiers is critical to precision medicine efforts including treatment allocation, development of evidence-based, tailored interventions informed by factors associated with resilience, and early identification of individuals at increased risk for the adverse cardiovascular effects of disturbed sleep. Candidate effect modifiers include age, sex, race/ethnicity, and existing co-morbidities. Yet, systematic evidence is generally lacking with regard to the impact of effect modifiers on physiological pathways linking sleep to cardiovascular disease (e.g., inflammation, ANS and metabolic dysfunction) and sleep intervention effects on subclinical markers of disease. Taking cues from the fields of psychology and sleep research, more recently identified effect modifiers include social isolation and social support (e.g., Cho, Seeman, Kiefe, Lauderdale, & Irwin, 2015) and genetic differences in resilience to sleep loss (Satterfield, Wisor, Field, Schmidt, & Van Dongen, 2015).
For the most part, evidence linking sleep to subclinical markers of CVD and cardiovascular morbidity and mortality is focused on individual dimensions of sleep. Research on cardiovascular risk in participants with the chronic insomnia+objective short sleep phenotype (e.g., Vgontzas, 2009a) is a notable exception to this limitation. However, sleep is a multidimensional biobehavioral process. Conceptual- and unbiased discovery-based approaches may be used to identify multivariate dimensions of sleep associated with cardiovascular morbidity and mortality (e.g, Buysse, 2014; Wallace et al., 2018). This latter approach is now feasible through “big data” approaches to data harmonization, management, analysis, and interpretation.
The past five years have seen the publication of several studies that suggest promise for reducing sleep-related cardiovascular risk. As reviewed above, sleep extension protocols to ameliorate lifestyle-related short sleep duration have been associated with reductions in blood pressure and insulin sensitivity (Haack et al., 2012; Killick et al., 2015; Leproult, et al., 2015). Multi-component CBTI and Tai Chi Chih in older adults with insomnia has been associated with decreases in inflammation and decreased activity of proinflammatory transcriptional profiles (Irwin et al., 2014; Irwin et al., 2015). These data, although extremely preliminary, suggest that behavioral interventions to extend short sleep or to treat insomnia may influence inflammatory, ANS, and metabolic pathways linking disturbed sleep and CVD risk. The extent to which intervention-related changes in physiology benefit cardiovascular risk in the long term remains untested and represents an emerging opportunity for psychology and sleep research/medicine. Building on the work of Irwin and colleagues, the use of randomized clinical trials in short sleepers and patients with insomnia are needed to evaluate the short- and long-term effects of improving sleep on mechanistic pathways and subclinical markers of disease. Moreover, randomized or pragmatic trials to improve sleep in patients with existing disease may be used to improve quality of life and reduce adverse clinical outcomes.
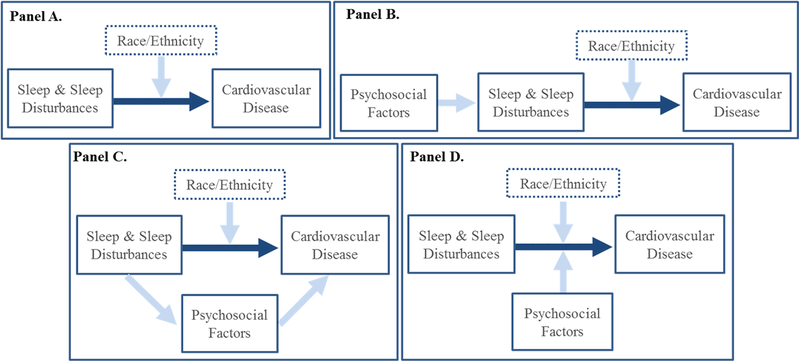
As illustrated in the conceptual model shown in Figure 1, psychosocial factors may serve as mediators or moderators of the sleep-CVD relationship or may impact CVD through its upstream impact on sleep (Figure 1; Panels B-D). To begin disentangling these roles, we offer general directions for future research. First, cross-sectional, observational studies using actigraphy and sleep diaries can be used to establish the extent to which psychosocial factors covary with sleep and CVD. Prospective studies can determine whether changes in these factors covary with changes in sleep and CVD status. Such analyses represent the first step in determining where such factors reside in the conceptual model (Figure 1; Panels B-D). Second, experimental studies can manipulate psychosocial factors and assess changes in sleep, acutely using PSG or over the long-term using actigraphy and diaries. This approach will allow researchers to determine which psychosocial factors, if any, lie upstream of and influence sleep (Figure 1; Panel B). Experimental manipulations of sleep, using PSG or actigraphy, can be used to determine whether psychosocial factors play a mediating role within the causal chain leading from sleep to CVD (Figure 1; Panel C). Finally, examination of synergistic effects among psychosocial factors and disturbed sleep may be used to identify the extent to which psychosocial factors moderate associations among sleep and CVD (Figure 1; Panel D). It should be noted that these general research directions would be increasingly informative if sufficient attention is paid to sample size and statistical power to allow for analyses of the effect of age, sex, and race/ethnicity.
Figure 1.
Conceptual model of the impact of phychosocial factors on the relationship between sleep and sleep disturbances and cardiovascular disease.
Dark blue arrow represents the physiological basis of the relationship between sleep and cardiovascular disease; Panel A represents moderation of the relationship between sleep and cardiovascular disease by race/ethnicity; panel B represents the impact of psychosocial factors on sleep; Panel C represents psychosocial factors as mediators of the relationship between sleep and cardiovascular disease; Panel D represents psychosocial factors as moderators of the relationship between sleep and cardiocvascular disease
As already noted, there are many ways to measure and characterize sleep. No one approach is superior to others, as each has distinct advantages and disadvantages. While PSG provides rich multi-system data, including objective measures of sleep, including duration, continuity, architecture (visual sleep stage scoring and spectral analysis), and other indices of nocturnal physiology (e.g., HRV), its cost and burden to participants generally preclude assessment over more than 3 nights. With the exception of some indices of sleep architecture that are highly stable across nights (Israel et al, 2012), wrist actigraphy and sleep diaries may be better suited to research focused on indices of habitual sleep such as duration and continuity. For the same reasons, actigraphy and sleep diaries are better suited for measures of sleep patterns important to health and functioning such as the regularity and timing of sleep, as well as assessment of multidimensional indices of sleep and sleep health (Buysse, 2014; Taylor et al., 2016). Actigraphy is usually used in conjunction with daily sleep diaries, usually completed before sleep and upon awakening. Sleep diaries can be easily adapted to study objectives and include measures other than sleep (e.g., health behaviors, acute illness, medication use, and mood). As a matter of caution, diary data may be influenced by biases common to self-report assessments.
While dedicated sleep laboratories may be needed for intensive, multi-day observational and experimental protocols that incorporate psychophysiological assessments of sleep and waking behaviors, ambulatory PSG devices may be preferable for research focused on environmental, sociocultural, and ecologically valid indices of relationship functioning on sleep (e.g., Egan et al., 2017; Grandner et al., 2016, Ruiter et al., 2011). Assessment of insomnia does not require objective sleep measures, unless one is focused on multidimensional indices such as the insomnia+objective short sleep phenotype (Vgontzas, et al., 2009a,b). Insomnia disorder may be assessed via clinical interviews pursuant to diagnostic criteria (American Psychiatric Association, 2013) or self-report questionnaires with validated clinical cut-offs such as the Insomnia (Morin et al., 2011). Finally, studies focused on associations among our waking and sleeping lives in relation to CVD (or other mental and physical health outcomes) need to assess sleep disordered breathing. Apnea is elevated in association with numerous psychosocial and sociocultural factors that influence cardiovascular morbidity and mortality (e.g., Grandner et al., 2016a). The STOP-Bang Questionnaire, which uses a combination of self-report, demographic, and anthropometric to quantify apnea risk, may be used to estimate apnea severity in selected populations (Nagappa et al., 2015). Simple, easy to use home testing devices for the measurement of sleep apnea are an extremely cost-effective alternative to laboratory-based assessment of sleep disordered breathing.
Integrative research that advances our understanding of the interrelationships among sleep and psychosocial, environmental, and sociocultural factors and their downstream effects on health and health disparities, including CVD, are currently hampered by the lack of formal training opportunities. Too few graduate programs in psychology provide training in sleep research and/or clinical sleep medicine. Of these, even fewer use a transdisciplinary (or health psychology) approach to provide trainees with the requisite concepts, models and tools for evaluating mechanisms linking sleep and cardiovascular disease (or other medical outcomes). Core graduate level courses in sleep and circadian rhythms biology are essential components of training and may be available in departments of psychology, behavioral health, or neuroscience or in affiliated schools of medicine. Hands-on experience in the conduct and scoring of sleep studies is essential for establish core competencies, whether sleep is measured by behavior (actigraphy) or physiology (PSG). This shortage of opportunities at the graduate level is critical as it represents the first step in the formal training pipeline.
The advent of specialized transdisciplinary postgraduate training programs has begun to seed research on the physiological, psychological, behavioral, social and environmental mechanisms linking sleep to cardiovascular and other diseases (e.g., cancer, diabetes, dementia, Alzheimer’s). However, absent specialized expertise garnered during graduate school, limits to the duration of postdoctoral fellowships often mean that more in-depth training is needed through internal (e.g., institution-based) or external (e.g., National Institutes of Health, National Science Foundation) career development awards. The trend for decreasing numbers of new physician scientists seen in medical research is also reflected in the field of sleep medicine, thereby limiting the bridging of basic and clinical science fostered by medical training. Researchers and clinicians from underrepresented groups, whose understanding of context is critical to the advancement of public health in the United States and abroad, are also sorely needed at the interface of psychology, sleep research, and cardiovascular (medical) science.
Conclusions
This issue of American Psychologist highlights significant advances in our understanding of the influence of psychological, physiological, behavioral, social, and environmental factors on the pathophysiology and clinical course CVD, which remains the leading cause of death worldwide (Roth et al., 2015). This article presents evidence that sleep plays a key role in both the development and progression of CVD. Specifically, short sleep duration and insomnia, alone or in combination, are associated with increased cardiovascular morbidity and mortality, after adjusting for other key risk factors. Inflammation, ANS dysfunction, and metabolic dysfunction represent plausible physiological mechanisms through which disturbed sleep affects CVD. Although the extant literature has primarily focused on disturbed sleep as a risk factor for disease, frameworks that emphasize sleep health (Buysse, 2014) may also be used to emphasize wellness and promote cardiovascular health. Fewer studies have focused on psychosocial factors through which sleep may affect and be affected by CVD. These pathways represent important growth opportunities for psychologists who may have focused their research and/or clinical efforts on risk or resilience factors that, as it happens, occur or have been traditionally measured during wakefulness. We propose that a transdisciplinary research framework that integrates knowledge and methods from the fields of psychology and sleep research will accelerate our understanding of the pathophysiology and clinical course of CVD. In turn, these advances will catalyze advances in the prevention and treatment of CVD.
Acknowledgments
This article was supported by grants R01 HL104607 (MH Hall, PI), R01 AG047139 (MH Hall, DJ Buysse, MPI), and F32 HL137227 & T32 HL082610 (training support to RC Brindle) from the National Institute of Heart, Lung, and Blood Disorders and the National Institute on Aging.
Footnotes
This article is part of a special issue, “Cardiovascular Disease: Psychological, Social, and Behavioral Influences,” published in the Xxxxxx 2018 issue of American Psychologist. Susan M. Czajowski, Catherine M. Stoney, and Peter G. Kaufmann were the scholarly leads. Timothy W. Smith and Anne E. Kazak served as editors of the special issue.
References
- Adenekan B, Pandey A, McKenzie S, Zizi F, Casimir GJ, & Jean-Louis G (2013). Sleep in America: Role of racial/ethnic differences. Sleep Medicine Reviews, 17, 255–262. 10.1016/j.smrv.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Bathgate CJ, Edinger JD, Wyatt JK, & Krystal AD (2016). Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep, 39, 1037–1045. 10.5665/sleep.5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MH, & Arand DL (2010). Hyperarousal and insomnia. Sleep Medicine Reviews, 14, 9–15. 10.1016/j.smrv.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Brindle RC, Cribbet MR, Samuelsson LB, Gao C, Frank E,...Hall MH (2018). The relationship between childhood trauma and poor sleep health in adulthood. Psychosomatic Medicine, 80, 200–207. 10.1097/PSY.0000000000000542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos I, Richter L, Klein T, Fiebich B, Feige B, Lieb K, … Riemann D (2006).Increased nocturnal interleukin-6 excretion in patients with primary insomnia: A pilot study. Brain Behavavior and Immunity, 20, 246–253. 10.1016/j.bbi.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Buysse DJ (2014). Sleep health: Can we define it? Does it matter? Sleep, 37, 9–17. 10.1665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, & Rössler W (2008). Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep, 31, 473–480. 10.1093/sleep/31.4.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, & Miller MA (2010). Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care, 33, 414–420. 10.2337/dc09-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, & Miller MA (2011). Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. European Heart Journal, 32, 1484–1492. 10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcantara C, … Redline S (2015). Racial/ethnic differences in sleep disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep, 38, 877–888. 10.1665/sleep.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Seeman TE, Kiefe CI, Lauderdale DS, & Irwin MR (2015). Sleep disturbance and longitudinal risk of inflammation: Moderating influences of social integration and social isolation in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain Behavior and Immunity, 46, 319–326. 10.1016/j.bbi.2015.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depner CM, Stothard ER, & Wright KP (2014). Metabolic consequences of sleep and circadian disorders. Current Diabetes Reports, 14, 507 10.1007/s11892-014-0507-s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds KL, Miller CB, Kyle SD, Marshall NS, & Gordon CJ (2017). Heart rate variability in insomnia patients: A critical review of the literature. Sleep Medicine Reviews, 33, 88–100. 10.1016/j.smrv.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Dong JY, Zhang YH, & Qin LQ (2013). Obstructive sleep apnea and cardiovascular risk: Meta-analysis of prospective cohort studies. Athersclerosis, 229, 489–495. 10.1016/j.athersclerosis.2013.04.026 [DOI] [PubMed] [Google Scholar]
- Drake CL, Pillai V, & Roth T (2014). Stress and sleep reactivity: A prospective investigation of the stress-diathesis model of insomnia. Sleep, 37, 1295–1304. 10.5665/sleep.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan KJ, Knutson KL, Pereira AC, & von Schantz M (2017). The role of race and ethnicity in sleep, circadian rhythms and cardiovascular health. Sleep Medicine Reviews, 33, 70–78. 10.1016/j.smrv.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, & Bixler EO (2012). Insomnia with objective short sleep duration and incident hypertension: The Penn State Cohort. Hypertension, 60, 929–935. 10.1161/HYPERTENSIONAHA.112.193268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J (2017). The insomnia with short sleep duration phenotype: An update on it’s importance for health and prevention. Current Opinion in Psychiatry, 30, 56–63. 10.1097/yco.0000000000000292 [DOI] [PubMed] [Google Scholar]
- Franzen PL, Gianaros PJ, Marsland AL, Hall MH, Siegle GJ, Dahl RE, & Buysse DJ (2011). Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosomatic Medicine, 73, 679–682. 10.1097/PSY.0b013e31822ff440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Buxton OM, Jackson N, Sands-Lincoln M, Pandey A, & Jean-Louis G (2013). Extreme sleep durations and increased C-reactive protein: Effects of sex and ethnoracial group. Sleep, 36, 769–779e. 10.5665/sleep.2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM & Chen S (2014). The role of sleep in interpersonal conflict: Do sleepless nights mean worse fights? Social Psychological and Personality Science, 5, 168–175. 10.1177/19485550613488952 [DOI] [Google Scholar]
- Grandner MA, Alfonso-Miller P, Fernandez-Mendoza J, Shetty S, Shenoy S, & Combs D (2016a). Sleep: Important considerations for the prevention of cardiovascular disease. Current Opinion in Cardiology, 31, 551–565. 10.1097/hco.0000000000000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Williams NJ, Knutson KL, Roberts D, & Jean-Louis G (2016b). Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Medicine, 18, 7–18. 10.1016/j.sleep.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Serrador J, Cohen D, Simpson N, Meier-Ewert H, & Mullington JM (2013). Increasing sleep duration to lower beat-to-beat blood pressure: A pilot study. Journal of Sleep Research, 22, 295–304. 10.1111/jsr.12011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder I, Matthews KA, Buysse DJ, Strollo PJ, Causer V, Reis SE, & Hall MH (2017). African genetic ancestry is associated with sleep depth in older African Americans. Sleep, 38, 1185–1193. 10.5665/sleep.4888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Casement MD, Troxel WM, Matthews KA, Bromberger JT, … Buysse DJ (2015b). Chronic stress is prospectively associated with sleep in midlife women: The SWAN Sleep Study. Sleep, 38, 1645–1654. 10.5665/sleep.5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Lee L, & Matthews KA (2015a). Sleep duration during the school week is associated with C-reactive protein risk groups in healthy adolescents. Sleep Medicine, 16, 73–78. 10.1016/j.sleep.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP & Troxel WM (2010). Couples’ nighttime sleep efficiency and concordance: Evidence for bidirectional associations with daytime relationship functioning. Psychosomatic Medicine, 72, 794–801. http://dx/doi.org/10.1097.psy.0b013e3181ecd08a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand S, Gast KB, de Mutsert R, Swenne CA, Jukema JW, Middeldorp S, … Dekkers OM (2013). Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: Meta-analysis and dose-response meta-regression. Europace, 15, 742–749. 10.1093/europace/eus341 [DOI] [PubMed] [Google Scholar]
- Hoevenaar-Blom MP, Spijkerman AMW, Kromhout D, van den Berg JF, & Verschuren WMM (2011). Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: The MORGEN Study. Sleep, 34, 1487–92. 10.5665/sleep.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Cai X, Chen P, Mai W, Tang H, Huang Y, & Hu Y (2014). Associations of prediabetes with all-cause and cardiovascular mortality: A meta-analysis. Annals of Medicine, 46, 684–692. 10.3109/07853890.2014.955051 [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, … Nicassio P (2014) Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: A randomized controlled comparative efficacy trial. Sleep, 37, 1543–1552. 10.5665/sleep.4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, … Cole S(2015) Cognitive behavioral therapy and Tai Chi reverse cellular and genomic markers of inflammation in late-life insomnia: A randomized controlled trial. Biological Psychiatry, 78, 721–729. 10.1016/j.biopsych.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, & Carroll JE (2016). Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biological Psychiatry, 80, 40–52. 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel B, Buysse DJ, Krafty RT, Begley A, Miewald J, & Hall MH (2019). Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: For some measures, one night is enough. Sleep, 35, 1285–1291. 10.5665/sleep.2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrin DC, Chen IY, Ivers H, & Morin CM (2014). The role of vulnerability in stress-related insomnia, social support and coping styles on incidence and persistence of insomnia. Journal of Sleep Research, 23, 681–688. 10.1111/jsr.12172 [DOI] [PubMed] [Google Scholar]
- Killick R, Hoyos CM, Melehan KL, Dungan GC 2nd, Poh J, & Liu PY (2015). Metabolic and hormonal effects of ‘catch-up’ sleep in men with chronic, repetitive, lifestyle-driven sleep restriction. Clinical Endocrinology (Oxf), 83, 498–507. 10.1111/cen.12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E-J, & Dimsdale JE (2007). The effect of psychosocial stress on sleep: A review of the polysomnographic literature. Behavioral Sleep Medicine, 5, 256–278. 10.1080/15402000701557383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranchi PA, Pennestri MH, Fradette L, Dumont M, Morin CM, & Montplaisir J (2009). Nighttime blood pressure in normotensive subjects with chronic insomnia: Implications for cardiovascular risk. Sleep, 32, 760–766. 10.1093/sleep/32.6.760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugsand LE, Vatten LJ, Bjorngaard JH, Hveem K, & Janszky I (2012). Insomnia and high-sensitivity C-reactive protein: the HUNT study, Norway. Psychosomatic Medicine, 74, 543–553. 10.1097/PSY.0b013e31825904eb [DOI] [PubMed] [Google Scholar]
- Leproult R, Deliens G, Gilson M, & Peigneux P (2015). Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep, 38, 707–715. 10.5665/sleep.4660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang XW, Hou WS, & Tang ZY (2014). Insomnia and risk of cardiovascular disease: A meta-analysis of cohort studies. International Journal of Cardiology, 176, 1044–1047. 10.1016/j.ijcard.2014.07.284 [DOI] [PubMed] [Google Scholar]
- Li Y, Yang Y, Li Q, Yang X, Wang Y, … Li H (2107). The impact of improvement of insomnia on blood pressure in hypertensive patients. Journal of Sleep Research, 26, 105114 10.1111/jrs.12411 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, & Croft JB (2014) Prevalence of healthy sleep duration among adults - United States. MMWR Morbidity and Mortality Weekly Report, 65, 137–141. http://dx.doi.org/10.15585/mmwr.mm6506a1 [DOI] [PubMed] [Google Scholar]
- Mai E & Buysse DJ (2008). Insomnia: Prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Medicine Clinics, 3, 167–174. 10.1016/j.jsmc.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, & Kalista T (2008). Cognitive behavioral therapy for insomnia enahnces depression outcome in patetients with comorbid major depressive disorder and insomnia. Sleep, 31, 489–495. 10.1093/sleep/31.4.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Patel SR, Pantesco EJ, Buysse DJ, Kamarck TW, Lee L, & Hall MH (2018). Similarities and differences in estimates of sleep duration by polysomnography, actigraphy, diary, and self-reported habitual sleep in a community sample. Sleep Health, 4, 96–103. 10.1016/j.sleh.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Zheng Y, & Hui R (2013). The relationship of sleep duration and insomnia to risk of hypertension incidence: A meta-analysis of prospective cohort studies. Hypertension Research, 36, 985–995. 10.1038/hr.2013.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel JD, Moreta M, Muto J, Htaik O, Jones C, Basner M, & Dinges D (2014). Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Psychology, 33, 1430–1434. 10.1037/a0034219 [DOI] [PubMed] [Google Scholar]
- Minkel JD, Banks S, Htaik O, Moreta MC, Jones CW, McGlinchey EL, … Dinges DF (2012). Sleep deprivation and stressors: Evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion, 12, 1015–1020. 10.1037/a0026781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM ,Belleville G, Bélanger L, Ivers H (2011). The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34, 601–608. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagappa M, Liao P, Wong J, Auckley D, Ramachandran SK, Memtsoudis S,...Chung F (2015). Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: A systematic review and meta-analysis. PLoS One, 14, e0143697 http://dx/doi.org/10.137/journal.pone.0143697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazaki C, Noda A, Koike Y, Yamada S, Murohara T, & Ozaki N (2012). Association of insomnia and short sleep duration with atherosclerosis risk in the elderly. American Journal of Hypertension, 25, 1149–1155. 10.1038/ajh.2012.107 [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Kanbayashi T, Saito Y, Takahashi Y, Kitajima T, … Shimizu T (2003). Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: A study with microneurographic technique. Sleep, 26, 986–9. 10.1093/sleep/26.8.986 [DOI] [PubMed] [Google Scholar]
- Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, … Gould KL (1990). Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet, 336, 129–133. 10.1016/0140-6736(90)91656-U [DOI] [PubMed] [Google Scholar]
- Patel NP, Grandner MA, Xie D, Branas CC, & Gootneratne N (2010). “Sleep Disparity” in the population: Poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health, 10, 475 10.1186/1471-2458-10-475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Vogelzangs N, & Penninx BW (2015). Sleep duration, insomnia, and markers of systemic inflammation: Results from the Netherlands Study of Depression and Anxiety (NESDA). Journal of Psychiatry Research, 60, 95–102. 10.1016/j.jpsychires.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, & McGinn MM (2014). Marital quality and health: A meta-analytic review. Psychological Bulletin, 140, 140–187. 10.1037/a0031859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rod NH, Kumari M, Lange T, Kiviamki M, Shipley M, & Ferrie J (2014). The joint effect of sleep duration and disturbed sleep on cause-specific mortality: Results from the Whitehall II Cohort Study. PLoS ONE, 9, e91965 10.1371/journal.pone.0091965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, … Murray CJL (2015). Global and regional patterns in cardiovascular mortality from 1900 to 2013. Circulation, 132, 1667–78. 10.1161/CirculationAHA.114.008720 [DOI] [PubMed] [Google Scholar]
- Ruiter ME, Decoster J, Jacobs L, & Lichstein KL (2011). Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep Medicine, 12, 209–214. 10.1016/j.sleep.2010.12.010 [DOI] [PubMed] [Google Scholar]
- Sabanayagam C, & Shankar A (2010). Sleep duration and cardiovascular disease: Results from the National Health Interview Survey. Sleep, 33, 1037–1042. 10.1093/sleep/33.8.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterfield BC, Wisor JP, Field SA, Schmidt MA, & Van Dongen HP (2015). TNFalpha G308A polymorphism is associated with resilience to sleep deprivation-induced psychomotor vigilance performance impairment in healthy young adults. Brain Behavior and Immunity, 47, 66–74. 10.1016/j.bbi.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvet F, Drogou C, Bougard C, Arnal PJ, Dispersyn G, … Chennaoui M (2015). Vascular response to 1 week of sleep restriction in healthy subjects: A metabolic response? International Journal of Cardiology, 190, 246–55. http://dx/doi/10.1016/j.ijcard.2015.04.119 [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, & Van Cauter E (1999). Impact of sleep debt on metabolic and endocrine function. Lancet, 354, 1435–1439. 10.1016/S0140-6736(99)01376-8 [DOI] [PubMed] [Google Scholar]
- Stepanski EJ, & Perlis ML (2000). Behavioral sleep medicine: An emerging subspeciality in health psychology and sleep medicine. Journal of Psychosomatic Research, 49, 343–347. 10.1016/S0022-3999(00)00171-9 [DOI] [PubMed] [Google Scholar]
- Sunbul M, Kanar BG, Durmus E, Kivrak T, & Sari I (2104). Acute sleep deprivation is associated with increased arterial stiffness in healthy young adults. Sleep and Breathing, 18, 215–20. 10.1007/s11325-013-0873-9 [DOI] [PubMed] [Google Scholar]
- Taylor BJ, Matthews KA, Hasler BP, Roecklein KA, Kline CE, Buysse DJ, … Hall MH (2016). Bedtime variability and metabolic health in midlife women: The SWAN Sleep Study. Sleep, 39, 457–465. 10.5665/sleep.5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobaldini E, Cogliati C, Fiorelli EM, Nunziata V, Wu MA, Prado M, … Montano N (2013). One night on-call: Sleep deprivation affects cardiac autonomic control and inflammation in physicians. European Journal of Internal Medicine 24, 664–670. 10.1016/j.ejim.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Troxel WM, Buysse DJ, Hall MH, & Matthews KA (2009). Marital happiness and sleep disturbances in a multi-ethnic sample of middle-aged women. Behavioral Sleep Medicine, 7, 2–19. 10.1080/154020000802577736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Spiegel K, Tasali E, & Leproult R (2008). Metabolic consequences of sleep and sleep loss. Sleep Medicine, 9, S23–S28. 10.1016/S1389-9457(08)70013-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schalkwijk FJ, Blessinga AN, Willemen AM, Van Der Werf YD, & Schuengel C (2015). Social support moderates the effects of stress on sleep in adolescents. Journal of Sleep Research, 24, 407–413. 10.1111/jsr.12298 [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Papanicolaou DA, Prolo P, Vela-Bueno A, Kales A, & Chrousos GP (2002). Chronic insomnia is associated with a shift in interlukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism: Clinical and Experimental, 51, 887–892. 10.1053/meta.2002.33357 [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler HM, Lin H, Follet H, & Chrousos GP (2004). Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. The Journal of Clinical Endocrinology & Metabolism, 89, 2119–26. 10.1210/jc.2003.031562 [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Bixler EO, Chrousos GP, & Vela-Bueno A (2009a). Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep, 32, 491–497. 10.5665/sleep/32.4.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, & Bixler EO (2009b). Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care, 32, 1980–1985. 10.2337/dc09-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace ML, Stone K, Smagula SF, Hall MH, Simsek B, … Buysse DJ (2018). Which sleep health characteristics predict all-cause morality in older men? An application of flexible multivariable approaches. Sleep, 41, zsx189 10.1093/sleep/zsx189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, … Tasali E (2015).Recommended amount of sleep for a healthy adult: A joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep, 38, 843–44. 10.5665/sleep.4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit P, Thompson SG, Agewall S, Bergstrom G, Bickel H, Catapano AL, … Sander D (2016). Inflammatory markers and extent and progression of early atherosclerosis: Meta-analysis of individual-participant-data from 20 prospective studies of the PROG-IMT collaboration. European Journal of Preventive Cardiology, 23, 194–205. 10.1177/2047487314560664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NJ, Grandner MA, Snipes A, Rogers A, Williams O, Airhihenbuwa A, Jean-Louis G (2015). Racial/ethnic disparities in sleep health and health care: Importance of the sociocultural context. Sleep Health, 1, 28–35. 10.1016/s.sleh.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard DL, & Berkman LF, & Brand RJ (1982). A multivariate analysis of health-related practices: A nine-year mortality follow-up of the Alameda Country Study. American Journal of Epidemiology, 116, 765–775. 10.1093/oxfordjournals.aje.a113466 [DOI] [PubMed] [Google Scholar]
- Wingard DL & Berkman LF (1983). Mortality risk associated sleeping patterns among adults. Sleep, 6, 102–107. 10.1093/sleep/6.2.102 [DOI] [PubMed] [Google Scholar]
- Yang H, Haack M, Gautam SL, Meier-Ewert HK, & Mullington JM (2017). Repetitive exposure to shortened sleep leads to blunted sleep-associated blood pressure dipping. Journal of Hypertension, 35, 1167–94. 10.1097/HJH.0000000000001284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L, Zhang H, Zhang D (2015). Sleep duration and depression among adults: A metaanalysis of prospective studies. Depression and Anxiety, 32, 664–670. 10.1002/da.22386 [DOI] [PubMed] [Google Scholar]
- Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MN, … Basner RC (2005). Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. Journal of Applied Physiology, 98, 20242032 10.1152/japplphysiol.00620.2004 [DOI] [PubMed] [Google Scholar]