Abstract
Psychological stress still attracts scientific, clinical, and public interest because of its suspected connection to health, particularly cardiovascular health. Psychological stress is thought to arise from appraisal processes that imbue events and contexts with personal significance and threat-related meaning. These appraisal processes are also thought to be instantiated in brain systems that generate and control peripheral physiological stress reactions through visceral motor (brain-to-body) and visceral sensory (body-to-brain) mechanisms. In the short-term, physiological stress reactions may enable coping and adaptive action. Among some individuals, however, the patterning of these reactions may predict or contribute to pathology in multiple organ systems, including the cardiovascular system. At present, however, we lack a precise understanding of the brain systems and visceral control processes that link psychological appraisals to patterns of stress physiology and physical health. This understanding is important: a mechanistic account of how the brain connects stressful experiences to bodily changes and health could help refine biomarkers of risk and targets for cardiovascular disease prevention and intervention. We review research contributing to this understanding, focusing on the neurobiology of cardiovascular stress reactivity and cardiovascular health. We suggest that a dysregulation of visceral motor and visceral sensory processes during stressful experiences may confer risk for poor cardiovascular health among vulnerable individuals. We further describe a need for new interpretive frameworks and markers of this brain-body dysregulation in cardiovascular behavioral medicine.
Keywords: cardiovascular disease, cardiovascular reactivity, psychological stress, visceral control, visceral prediction errors
It sometimes happens that a man falls dead in a fit of violent rage, and it is said, perhaps, that he had a weak heart, which could not stand the strain imposed by his mental state. Nobody seems to think that this is but the culmination of a long series of such fits of madness, which have themselves caused the weakness in question
(Manning, 1895, p. 325).
As reflected in this historical quote, psychological stress and negative emotions have long been thought to increase vulnerability to physical disease, particularly atherosclerotic cardiovascular disease (CVD) and its clinical sequelae (Dimsdale, 2008; Steptoe & Kivimaki, 2012). Psychological stress and aspects of negative emotionality may in fact confer a level of risk for CVD that is on par with the risk conferred by smoking, dietary imprudence, and physical inactivity (Rozanski, 2014). Yet, open questions remain about how psychological stress confers CVD risk. Addressing these questions is important: CVD remains a leading public health burden and the chief cause of premature death in postindustrial nations (Benjamin et al., 2017).
At issue here is the specific question of how the brain constructs psychologically stressful experiences and links such experiences to bodily reactions that could plausibly relate to CVD vulnerability. To this end, we first describe psychological appraisal processes and their roles in constructing stressful experiences and negative emotional states. We then describe how appraisal processes may lead to health-relevant physiological reactions in the body. To retain focus, we selectively emphasize cardiovascular (e.g., blood pressure, heart rate) stress reactions that are mediated by the autonomic nervous system. This focus is based on cumulative evidence that individual differences in cardiovascular stress reactions relate to future CVD risk, clinical events, and mortality (Carroll et al., 2012; Chida & Steptoe, 2010; Steptoe & Kivimaki, 2012). Next, we highlight recent neuroscience studies that aim to characterize the brain systems that are involved in mediating psychological stress appraisals and simultaneously controlling cardiovascular physiology via visceral motor (brain-to-body) and visceral sensory (body-to-brain) autonomic nervous system pathways. We end with a perspective on trait-like (phenotypic) individual differences in cardiovascular stress reactivity. This perspective views cardiovascular stress reactivity in two ways. The first is that cardiovascular stress reactivity results from brain-based and predictive visceral motor commands. These visceral-motor commands feed forward from the brain to calibrate peripheral physiology with anticipated metabolic and behavioral needs of the body that are appropriate to a given context. The second is that cardiovascular stress reactivity provides visceral sensory information, which feeds back to the brain from the body to influence stress appraisals and future visceral motor commands—thus defining a brain-body loop. By this perspective, we postulate that stressful experiences may lead to brain-based visceral prediction errors. Such errors can consist of (i) feed-forward (visceral motor) commands for metabolic support that is contextually inappropriate and (ii) feedback (visceral sensory) information that does not minimize future visceral prediction errors. By these postulates, visceral prediction errors manifest as individual differences in cardiovascular stress reactivity. We end by considering the utility of this perspective for understanding and altering the brain-body mechanisms by which psychological stress impacts cardiovascular physiology and vulnerability to CVD.
Construction of Stressful Experiences by Psychological Appraisals
Perspectives on psychological stress and physical health have evolved over a vast period of time, with continuing debates about how to conceptualize, define, and measure psychological stress and its mechanisms of action (Cohen, Gianaros, & Manuck, 2016). A core assumption of early and recent perspectives is that experiences of psychological stress result from iterative interactions between events, personal evaluations of events, and coping reactions to alter events. These iterative interactions are specifically thought to arise in part from evaluative processes that evoke coordinated changes in behavior, emotional states, and physiology in ways that may predict, precipitate, or protect against disease. For example, a premise of early and still influential perspectives is that individual-by-event interactions are capable of evoking distinct patterns of behavioral, emotional, and physiological stress reactions that might lead to risk for distinct kinds of physical disease across individuals (e.g., cardiovascular disease, asthma, etc.) (e.g., Alexander, 1939; MacLean, 1949; Mason, 1971; Weiner, 1992).
Another common assumption of many perspectives on psychological stress and health derives from Lazarus' (1966) conceptual framework on appraisal processes. In this framework, psychological stress unfolds after an external event (e.g., an insult from a friend) or an internal event (e.g., anticipating or recalling a traumatic experience) is first registered as a ‘demand.’ Such demands may signal preparative needs for coping and adaptation to manage predicted harm. After such demands are registered, they are iteratively appraised (evaluated) with respect to an individual's perceived resources for coping. Mechanistically, appraisal processes are of two types in this framework: (i) Primary appraisals denote the extent to which demands are evaluated for their personal meaning, relevance, and significance (e.g., “How much does this matter?”); and (ii) secondary appraisals denote the extent to which coping resources against such demands are evaluated for their availability (e.g., “What can be done?”). Understood in this way, psychological stress is thus experienced when internal or external demands are appraised as threats—events and contexts that are unpredictable, uncontrollable, and overwhelming with respect to the construed coping resources of the individual.
Importantly, iterative primary and secondary appraisals are specific to the individual— continuously shaped and updated by contextual and idiosyncratic factors. The latter factors can include personal life histories, learning and memory, schemas, intentions, and future expectations. Accordingly, appraisals can be viewed as directly relevant to understanding individual differences in disease vulnerability, insofar as appraisals can link myriad sources of external and internal information (demands) appraised by the individual as threatening to varied behavioral, emotional, and physiological stress response patterns that can plausibly undermine physical health (Cohen et al., 2016).
For the reasons above, appraisals are still widely viewed as central—if not fully understood—mechanisms that construct stressful experiences and stress response patterns that can shape one's health. Next, we describe the possible interplay between appraisals and predictive physiological control mechanisms of the brain. These mechanisms encompass visceral motor and sensory processes that may be adaptive in the short-term, but may also be maladaptive for health over the long-term—particularly for cardiovascular health.
Visceral Sense and Sensibility: Psychological Stress and Predictive Physiological Control
In addition to appraisal processes, perspectives on psychological stress and physical health have long incorporated the concept of physiological homeostasis and its disturbance, which originated with Walter Cannon (1932). Cannon had extended earlier ideas of the physiologist, Claude Bernard, to argue that parameters of physiology that are essential for life (e.g., blood pressure) are monitored and maintained around equilibrium points, referred to as set points. Cannon posited that a set point for a given physiological parameter is determined by the body's ‘knowledge’ of that parameter's expected and near steady-state value. This presumed ‘knowledge’ implied the existence of internal models or expected patterns against which actual parameters of physiology are compared. The process of comparing and keeping physiological parameters close to their steady-state set points—termed homeostasis—was proposed to operate via visceral sensory and visceral motor mechanisms.
From a canonical homeostatic perspective, visceral sensory and motor mechanisms respectively detect and then correct departures from set points by reflexive negative-feedback mechanisms. From this perspective, a psychological stressor can thus be defined as a demand that is first appraised as a threat and then initiates departures in peripheral physiological parameters from their putative homeostatic set points. These departures were conceived of as physiological stress reactions that are necessary to support evolutionarily-conserved behavioral actions meant to cope and maintain life (e.g., ‘fight-or-flight’ and ‘emergency’ reactions). However, a long-debated problem with classical homeostatic perspectives is that they emphasize negative feedback mechanisms and reflexive processes that are inefficient for regulating peripheral physiology and for coping and adapting to varied threats that are appraised by the brain (Sterling, 2012). In this regard, classical homeostatic perspectives seem incompatible with current views of health-relevant physiological stress reactions as proactive—not reflexive— adjustments that are based on predictive brain processes (Sterling, 2012). Finally, such classical perspectives have long been enmeshed with historical and hierarchical perspectives on the autonomic nervous system and its involvement in stress physiology (Lovallo, 2016). To a large extent, the latter perspectives have evolved substantially in recent years.
Evolving views of autonomic control and stress physiology
In brief, the autonomic nervous system can be thought of as an information transfer system, as it bi-directionally traffics messages between brain and body (Lane et al., 2009). Other physiological systems engaged by psychological stressors (e.g., the HPA-axis) may operate over different time scales, but still share similar information transfer characteristics (Lane et al., 2009). With respect to the autonomic nervous system and stress physiology, early views envisioned its sympathetic and parasympathetic arms as operating mostly independent of the brain (e.g., Sheehan, 1936). During stressful experiences, the parasympathetic arm was seen as subordinated and suppressed by the sympathetic arm. The sympathetic arm was seen as dominant and diffusely active—liberating energy for immediate action to preserve life (Jänig, 2006). By such views, the brain may detect threats and trigger peripheral physiological reactions, but it would otherwise leave the two autonomic arms to their own and isolated reflexive devices. Along these lines, the functional organization of autonomic and other physiological reactions to stressors was construed as hierarchical—placing particular brain systems at the ‘top rungs’ of the hierarchy and ascribing little or no importance, integration, or feedback control to peripheral and visceral sensory information ascending from the ‘bottom rungs’ to the ‘top rungs’ (e.g., from the autonomic arms) (Berntson, Gianaros, & Tsakiris, in press).
Our understanding of how the brain regulates autonomic function during stressful experiences has evolved markedly from early interpretive views. This evolution has implications for how we think about the complex and two-way relationships between psychological stress and physical health. Indeed, recent views on autonomic control emphasize granular (vs. diffuse) and integrative (vs. isolated) mechanisms for stress physiology that need not operate ‘hierarchically’ (Berntson & Cacioppo, 2008; Jänig, 2006; Malpas, 2010; Saper, 2002). Advances in anatomy, for example, show that the mechanisms for autonomic control over the viscera (internal organs) are highly differentiated within the brain (Barrett & Simmons, 2015; Craig, 2002; Critchley & Harrison, 2013; Dum, Levinthal, & Strick, 2016). Moreover, recent advances have led to knowledge that autonomic nerve fibers transmit highly specific visceral sensory (body-to-brain) feedback information from the internal organs, such as the heart and blood vessels, to areas of the cerebral cortex. As discussed below, the latter cortical areas that receive such visceral sensory information may be equally important for psychological stress appraisals. Hence, autonomic changes evoked by psychological stressors can be flexible and fine-tuned to control and represent discrete organ functions in a given context by feed-forward (visceral motor) and feedback (visceral sensory) pathways. This view of stressor-evoked autonomic changes contrasts with early views of strictly hierarchical and isolated autonomic control—especially over cardiovascular physiology (Berntson & Cacioppo, 2008; Dampney, 2016). In extension, the brain-based control over cardiovascular physiology via autonomic mechanisms also appears to be more accurately conceptualized as predictive and not strictly reflexive.
To elaborate, visceral sensory (feedback) information conveyed from the internal organs of the body via ascending autonomic pathways can serve as a determinant of future visceral motor outflow of the sympathetic and parasympathetic arms. For example, while visceral sensory information can act as negative feedback input to enable a corrective return to homeostatic set points or ‘targets’, this same sensory information may be bypassed (ignored) by feed-forward (predictive) physiological adjustments away from such targets. This bypassing can serve to update or change homeostatic targets to new and contextually-determined targets. These updated targets may in turn enable metabolic support for anticipated action and coping (Dampney, 2016; Sterling, 2012). Such anticipatory or predictive dynamics that bypass negative-feedback input are made possible by several redundant physiological control mechanisms. These mechanisms are instantiated across autonomic nerve clusters (ganglia) in the periphery, pre-autonomic cell groups in the spinal cord, as well as distributed networks of the brain (Berntson & Cacioppo, 2008). As a result of this organization, during proactive (anticipatory) states, local organ-level homeostatic control mechanisms can be bypassed or suspended. Thus, the autonomic nervous system, like the voluntary (somatic) motor system, can be as proactive as it is reactive or reflexive in the regulation of peripheral stress physiology. Consequently, visceral motor commands from the brain that are relayed by the autonomic nervous system to the internal organs (e.g., heart and vasculature) can be calibrated to meet the predicted metabolic needs of a context and even to predicted visceral sensory input (Barrett & Simmons, 2015; Shivkumar et al., 2016; Taggart, Critchley, van Duijvendoden, & Lambiase, 2016).
To illustrate the above concepts, it is well established that heart rate can increase to support behavior as a result of visceral motor commands. The autonomic arms relay these commands to provide metabolic support for muscle contraction and energy expenditure. However, the degree to which heart rate increases to support behavior can be determined by the degree of anticipated action and anticipated kinesthetic and visceral sensory information, not as a reflexive (homeostatic) response following muscle activation (Jennings, van der Molen, Brock, & Somsen, 1993). Exercise physiology studies show further that cardiovascular changes (e.g., heart rate increases) anticipatory to effortful behaviors are proportionate to expected behavioral exertion (Fisher, Young, & Fadel, 2015). Finally, visceral motor commands from the brain that anticipate behavior appear to alter physiology in these ways by modifying the operating characteristics of homeostatic control mechanisms via predictive neural processes (Dampney, 2016). As we postulate next, the predictive modification of homeostatic control over peripheral physiology during psychological stress may plausibly signal or shape disease vulnerability.
Hierarchies vs. heterarchies in autonomic control
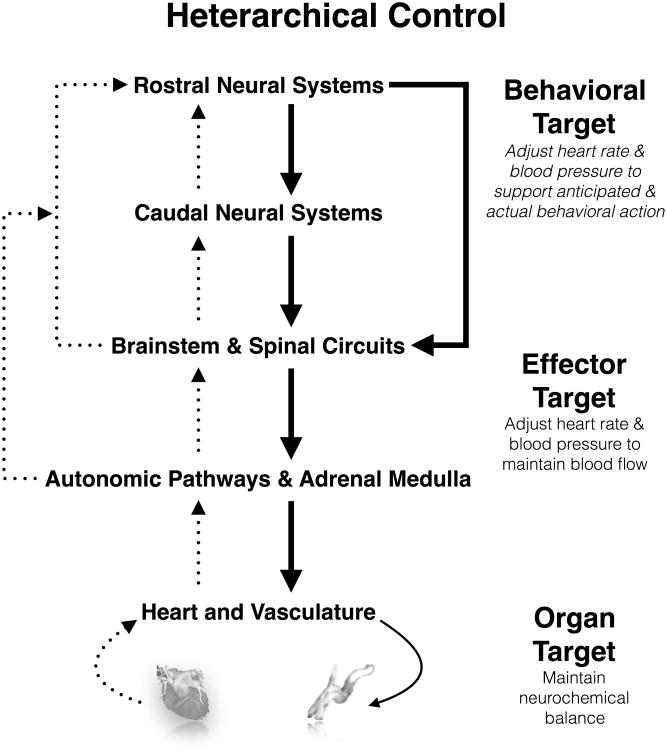
The processes described above concerning the predictive autonomic regulation over physiology can be understood within the framework of heterarchical organization, which differs from the framework of hierarchical organization (Berntson & Cacioppo, 2008; Kleckner & Quigley, 2015). In a heterarchical organizational framework, there are ‘nodes’ of autonomic control that are distributed across peripheral autonomic ganglia, spinal cord neural networks, brainstem processing sites, and more rostral brain regions (e.g., in the cerebral cortex). Specific autonomic control over peripheral physiology can be exerted at multiple nodes within such a regulatory web, and visceral sensory information (afferent physiological feedback) can be used by any other node to implement control. Here, each node may be biased to be responsive to certain forms of actual or expected visceral sensory information, and each node may be capable of initiating certain visceral motor adjustments. Yet, each node's ‘local’ regulatory functions are subject to modulation or ‘bypassing’ by other nodes in the heterarchy. In this fashion, parallel and redundant pathways for autonomic control over parameters of peripheral physiology are able to fine-tune fast, flexible, anticipatory, and context-dependent changes in end organs (e.g., heart and blood vessels). This heterarchical or web-like organization also permits the modification or suspension of relatively automatic and possibly health-maintaining homeostatic (negative-feedback) functions by predictive (anticipatory) neural processes of the brain (Figure 1). From this standpoint, predictive neural processes could shape health and disease vulnerability by chronically biasing visceral motor and sensory homeostatic control loops that operate within a heterarchy. It is noteworthy that the latter postulate aligns with the concepts of allostasis and allostatic load (McEwen, 1998; Sterling & Eyer, 1988).
Figure 1.
Simplified schematic of heterarchical organization of neural influences on the cardiovascular system and targets for visceral homeostatic control. Shown are interacting behavioral, effector, and organ targets of visceral homeostatic control. Behavioral targets correspond to brain-based predictive metabolic support commands for mental and overt behavioral action. These behavioral targets can be conditioned by appraisal processes, as mediated by interactions between rostral and caudal neural systems, including cortical, limbic, midbrain, and brainstem regions. Effector targets correspond to levels of heart rate and blood pressure necessary to sustain metabolic support of tissues. Organ targets correspond to local maintenance of ion and fluid balance in the heart and vasculature. In this heterarchical organization, systems can interact directly and indirectly. As a consequence of this organization, autonomic outflow that is locally organized to maintain homeostasis can be interrupted, bypassed, or influenced by caudal and rostral neural systems to reset or produce ‘non-homeostatic’ target organ state changes (e.g., in the heart and vasculature).
In brief, allostasis refers to the activation of multiple parameters of physiology (e.g., autonomic, neuroendocrine, metabolic and immune) that are thought to enable coping and adaptation to changing environmental demands (Sterling & Eyer, 1988). Importantly, the multiple parameters of physiology that are activated by such demands are thought to influence one another non-linearly in the service of maintaining the long-term stability of end organs (McEwen & Gianaros, 2011). In this way, the construct of allostasis emphasizes the importance of inter-related variability in parameters of physiology to achieve homeostasis over the long-term. Moreover, the construct of allostasis was originally proposed to describe a mechanism for how the brain implements predictive or anticipatory physiological control, thus contrasting with the notions of strictly reflexive and arguably inefficient negative-feedback mechanisms to achieve homeostasis (Sterling, 2012). The derivative construct of allostatic load refers to the purported cumulative burden of repeatedly instantiating allostasis, a load that may manifest in pathology and disease vulnerability (McEwen & Gianaros, 2011).
Allostasis and allostatic load build on earlier conceptions of homeostasis and its chronic disturbance for health, as well as the earlier construct of heterostasis (Selye, 1973). Heterostasis was proposed by Selye to describe evoked and often excessive (e.g., large magnitude) changes in multiple parameters of physiology that can reset or result in entirely new targets of equilibrium (set points) of these parameters. Such changes were viewed to enable coping with threats, consistent with the notion of allostasis. But, such changes were also thought to be pathological over time and under certain conditions, consistent with the notion of allostatic load. As an example, Selye wrote that among “…predisposed individuals, excessive neuroendocrine “emergency” reactions may precipitate a cardiovascular accident” (1973, p.443). On these grounds, an key aspect of the idea of heterostasis, which prefigured allostasis and allostatic load, is that excessive, repeated, or otherwise dysregulated anticipatory or predictive physiological adjustments may confer disease vulnerability (McEwen & Gianaros, 2011). We next expand on the latter proposal from a heterarchical control perspective, emphasizing the importance of brain-based visceral motor commands and visceral sensory information in physiological reactivity to stress and CVD risk.
Cardiovascular Reactivity: A Manifestation of Visceral Prediction Errors and a Candidate Brain-Body Pathway Linking Stress and CVD
Insofar as there are brain systems for appraising threats and orchestrating physiological stress reactions, how is it that these brain systems might also relate to risk for diseases, such as CVD? As reflected in the introductory quote and the ideas of heterostasis, allostasis, and allostatic load, a long-suspected answer is that brain-based physiological reactions to psychological stressors may confer disease risk over time by inducing cumulative damage to organs in the body, including the heart and vasculature (Charvat, Dell, & Folkow, 1964).
This notion is captured by the cardiovascular reactivity hypothesis, according to which patterns of stressor-evoked cardiovascular reactivity may confer CVD risk by contributing to adverse changes in the heart and vasculature that promote hypertension, atherosclerosis, and forms of pathology that presage clinical events (e.g., myocardial infarction, ischemia, stroke) (Krantz & Manuck, 1984; Steptoe & Kivimaki, 2012). The cardiovascular reactivity hypothesis has also been used to characterize stable (trait-like) individual differences in stressor-evoked cardiovascular response patterning. This trait-like patterning (i.e., reactivity) may refer not only to the magnitude (relative size) of evoked changes in cardiovascular physiology (e.g., how much heart rate and blood pressure rise), but also to the duration and temporal profiles of such evoked changes. Finally, this patterning may similarly refer to the underlying determinants of observed changes in cardiovascular physiology (e.g., specific cardiac and vascular determinants of rises in blood pressure for a given individual) (Kasprowicz, Manuck, Malkoff, & Krantz, 1990).
Certain typologies of response patterning that have attracted the most attention include large-magnitude (e.g., exaggerated), small-magnitude (e.g., blunted), non-habituating, and prolonged (e.g., non-recovering) forms of stressor-evoked cardiovascular reactivity. Individual differences in these reactivity patterns attract attention because of epidemiological evidence that individuals who have a tendency (phenotype) to exhibit exaggerated (e.g., large-magnitude, prolonged) stressor-evoked cardiovascular reactivity are at risk for an accelerated progression of atherosclerosis, hypertension, ischemic stroke, and early death (Carroll et al., 2012; Chida & Steptoe, 2010). More recent evidence suggests that blunted cardiovascular reactivity may likewise signal risk for outcomes related to CVD (Ginty, Kraynak, Fisher, & Gianaros, 2017). In addition to other factors, brain-based appraisal processes may partly explain individual differences in stressor-evoked cardiovascular reactivity and their links to CVD (Lovallo & Gerin, 2003). This speculation has motivated human neuroscience studies to define the particular brain systems that are simultaneously involved in appraisal and cardiovascular (and other physiological) reactivity patterns linked to CVD. Below, we expand on a neurobiological perspective informed by these studies. According to this perspective, stressor-evoked cardiovascular reactivity patterns are posited to be manifestations of predictive neural processes that are instantiated in brain systems for appraisal and visceral control. In addition, this perspective incorporates the notion that stressor-evoked cardiovascular reactivity patterns may influence appraisals and predictive neural processes via visceral sensory pathways.
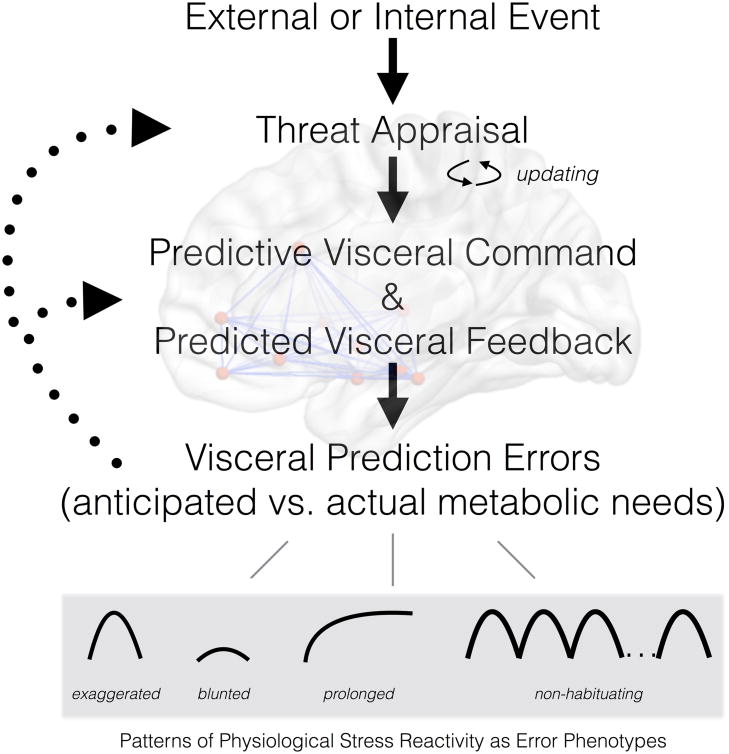
More precisely, we propose that exaggerated cardiovascular stress reactivity is the end product of a visceral prediction error. This ‘error’ is in the direction of a brain-based and visceral motor command to provide metabolic support that is disproportionately greater than the actual metabolic demands and behavioral effort engendered by an appraised stressor or threat. This proposal derives from the notion of cardiac-somatic uncoupling, wherein metabolic and behavioral needs are misaligned with cardiovascular physiology during stressful experiences (Obrist, 1981). Concretely, a surge in systolic blood pressure of 40mmHg prior to delivering a public speech could be viewed as metabolically disproportionate, exaggerated, and inappropriate for the context. In this instance, the brain's predictive error manifests as a cardiovascular reaction that outstrips actual metabolic needs (i.e., metabolic overshoot; Figure 2). These ‘visceral prediction errors’ can be quantified by integrating lab-based stress reactivity protocols with methods of exercise physiology, where the size of a cardiovascular reaction to a psychological stressor is compared with what would be projected based on actual metabolic and energy needs (Ginty et al., 2017).
Figure 2.
A heuristic schematic of the pathways that influence stressor-evoked physiological (e.g., cardiovascular) reactivity. Specific types of reactivity linked to disease risk are conceptualized as outcomes of ‘visceral prediction errors,’ wherein there is a mismatch between anticipated and actual metabolic needs of a context or demand appraised as threatening. Appraisals that generate threat-related meaning are updated according feedback provided by the outcomes of predictive processes.
We just noted a primary visceral prediction error, exaggerated metabolic response, but these prediction errors may take different forms. For example, they may manifest as patterns of blunted cardiovascular reactivity (metabolic undershoot), which also relates to poor physical health outcomes and may reflect chronic or recurrent experiences of psychosocial stress (Chida & Hamer, 2008). Conceptually, blunted reactivity may derive from underestimates of future metabolic needs and perhaps a prediction of excessive corrective visceral sensory feedback. As another example, failure for a given physiological (e.g., cardiovascular) stress reaction to recover or habituate over time (a prolonged or unremitting reaction) might arise from multiple predictive visceral motor commands being issued in parallel, ‘superimposed’ onto one another, or summed over time to sustain the expression of ‘errors’ in peripheral physiology. Finally, prolonged or non-habituating patterns of reactivity may be viewed as a form of impaired visceral prediction error correction or learning. Here, afferent (visceral sensory) feedback provided to the brain about actual or contextual metabolic needs may not serve to minimize ongoing or future visceral prediction errors. Put differently, a prolonged pattern of reactivity that is inappropriate to a context does not result in an updated or otherwise calibrated pattern of stress reactivity that is matched to actual metabolic needs (Kelsey, Soderlund, & Arthur, 2004). This failure of updating or calibration may in part be due to insensitivity to visceral sensory feedback, as well as perseverative processes that maintain threat appraisals over time (Ottaviani et al., 2016).
According to our perspective, stable or phenotypic patterns of cardiovascular reactivity may be thus conceptualized as neurobiological dimensions of individual difference involving the manifestation of so-called visceral prediction errors. These errors are based on visceral motor and sensory mechanisms that may be conditioned and shaped for the individual over time by repeated experiences of psychological stress. We next highlight studies on brain systems that may mediate stress appraisals and cardiovascular reactivity via these mechanisms.
Brain Substrates for Stressor-Evoked Cardiovascular Reactivity
Decades of nonhuman animal studies have detailed the brain systems that control cardiovascular physiology by bidirectional (visceral motor and sensory) autonomic and neuroendocrine mechanisms. Early nonhuman animal research focused specifically on brain systems that mediate cardiovascular changes that accompany what Hess and Brugger (1943) referred to as the affektiven abwehrreaction (defense reaction). These cardiovascular changes thus co-occur with overtly defensive (e.g., fight-or-flight) behaviors, and they are still widely thought to provide metabolic support for defensive behaviors by adjusting heart rate, blood pressure, cardiac contractility, and regional blood flow and volume. From nonhuman animal work, a core subcortical brain circuitry for cardiovascular stress reactivity has been proposed to include the hypothalamus, bed nucleus of the stria terminalis, midbrain periaqueductal gray (PAG), as well as cell groups in the brainstem (pons, medulla). Collectively, these subcortical systems (corresponding to caudal and brainstem circuits in Figure 1) provide for rapid control over autonomic and neuroendocrine outflow to the heart and blood vessels.
More recently, human neuroimaging studies have begun characterizing the forebrain and cortical systems that may be jointly involved in appraising stressors and regulating acute stressor-evoked cardiovascular reactions (corresponding to rostral neural systems in Figure 1). Studies that focus on characterizing these brain systems have involved administering tasks that are appraised as stressful and concurrently evoke changes in cardiovascular physiology. These include tasks that entail preparing speeches under conditions of negative social evaluation (Eisenbarth, Chang, & Wager, 2016); engaging in time-pressured and effortful cognitive performance tasks under conditions of conflict, negative feedback, low behavioral control, and unpredictability (e.g., Akdeniz et al., 2014; Gianaros et al., 2017); as well as tasks that entail processing affective and threatening stimuli (e.g., Dalton, Kalin, Grist, & Davidson, 2005). Importantly, such tasks are comparable to laboratory-based stressors used in epidemiological studies, permitting some comparison and integration across fields. In the latter regard, there is evidence that (i) stressor-evoked cardiovascular reactions evoked in neuroimaging testing environments relate to laboratory-assessed reactions (Gianaros, Jennings, Sheu, Derbyshire, & Matthews, 2007) and (ii) stressor-evoked neural activity is reliable over time (Sheu, Jennings, & Gianaros, 2012). These lines of evidence suggest that individual differences in cardiovascular and neural reactivity to stress may comprise stable and potentially ‘trait-like’ brain-body phenotypes for stress sensitivity and reactivity.
The purported functions of brain systems associated with stressor-evoked cardiovascular reactivity have been reviewed extensively (e.g., Gianaros & Wager, 2015; Ginty et al., 2017; Muscatell & Eisenberger, 2012; Myers, 2016). Here, we briefly describe some functions ascribed to a subset of these systems; namely, the ACC, insula, and amygdala. We focus on these systems for illustrative purposes only and in view of their presumptive functions pertinent to psychological stress appraisals, cardiovascular reactivity, and CVD risk. We emphasize that there is unlikely to be any selective ‘stress circuit’ or ‘stress reactivity network’ of the brain. Rather, it is more likely that context-dependent, distributed, and interactive patterns of activity across these and other brain systems that serve multiple functions are the most plausible determinants of acute physiological, namely cardiovascular, stress reactions (Eisenbarth et al., 2016; Gianaros et al., 2017). Indeed, complex interactions across the ACC, insula, amygdala, and other forebrain areas may be fundamental for coupling appraisal and visceral prediction processes across a range of behavioral states to control peripheral physiology as appropriate to context (Barrett & Simmons, 2015). Here, it is thought that such areas may generate internal predictive models (simulations) of future outcomes and patterns of behavior and physiology that are influenced by context, prior experience, and other life history factors (Kleckner et al., 2017).
Anterior cingulate cortex (ACC)
The ACC is a heterogeneous brain region that has been reliably linked to acute physiological stress reactions and individual differences in cardiovascular reactivity. Broadly stated, sub-regions within the ACC and adjacent vmPFC are thought to coordinate cognitive, affective, and visceral control processes with goal directed behavior (Critchley, 2005). As part of a broader vmPFC network, sub-regions within the ACC appear to ascribe personal meaning to events and contexts, enabling individuals to represent and experience core affective states during stressful experiences (Lindquist, Wager, Kober, Bliss-Moreau, & Barrett, 2012; Roy, Shohamy, & Wager, 2012). Consistent with the perspective offered here, we have proposed that sub-regions within the ACC calibrate and regulate the magnitude and duration of cardiovascular reactions to psychological stressors to support contextually-adaptive behavioral coping processes, and that mis-calibrations (visceral prediction errors) are the substrates for metabolically inappropriate (e.g., blunted, exaggerated, prolonged) cardiovascular reactions (Ginty et al., 2017).
Insula
In addition to the ACC, the insula has long been known to influence cardiovascular function via autonomic pathways, particularly during psychologically stressful and emotional experiences (Oppenheimer & Cechetto, 2016). Afferent (visceral sensory) relays from peripheral organs project to the insula along its caudal-to-rostral (back-to-front) direction to form a ‘viscerotopic’ map (Craig, 2002). This map is thought to support the integration of visceral sensory information with appraisal processes and the concurrent regulation of behavior and physiological activity (Critchley, 2005). Moreover, insular dysfunction appears capable of directly contributing to cardiac pathology under emotional or otherwise stressful experiences. Such pathology includes arrhythmic changes, Takotsubo (broken-heart) syndrome, and direct damage to muscle cells of the heart (Oppenheimer & Cechetto, 2016).
Amygdala
The amygdala is a cell complex that is engaged by stressful and emotional (e.g., threatening) events, and its regulation over cardiovascular physiology has been related to its roles in integrating, storing, and updating sensory and expectation-related (prediction) signals from other brain areas (Gianaros & Wager, 2015). The amygdala is densely networked with ACC, insular, and other areas for visceral control (Dampney, 2016; Öngür & Price, 2000). Animal models show that amygdala lesions block stressor-evoked cardiovascular reactions (Sanders, Wirtz-Nole, DeFord, & Erling, 1994) and these lesions prevent hypertension induced by chronic stress (Fukumori, Nishigori, Goshima, & Kubo, 2004). Although human findings relating amygdala activity to stressor-evoked cardiovascular reactivity vary across studies (Gianaros & Wager, 2015), animal findings seem to complement recent human neuroimaging work showing that stress-related amygdala activity predicts clinical CVD events more strongly than conventional risk factors (Tawakol et al., 2017).
Collectively, converging lines of evidence suggest that psychological stressors engage the amygdala and networked forebrain areas, including the ACC and insula. Importantly, these brain systems appear to link appraisals of these stressors to visceral control mechanisms for cardiovascular stress reactivity, and possibly to individual differences in reactivity.
A Neurobiological Perspective on Cardiovascular Reactivity and CVD Vulnerability
If appraisal and visceral control processes enable adaptive action in the short-term by coordinating physiology with behavior, then how could such processes predict or contribute to CVD risk among vulnerable individuals? The answer may partly lie in the manner by which heterarchical mechanisms for physiological control result in a failure to minimize visceral prediction errors (Figure 2), which then manifest as different patterns of physiological stress reactivity. As noted earlier, these heterarchical mechanisms may include the bypassing or modification of how visceral control loops maintain homeostasis.
A prominent example of such bypassing is the manner in which appraisals of threat appear to modify a major cardiovascular reflex for short-term blood pressure homeostasis: the baroreflex. The baroreflex is a specific homeostatic visceral control loop that constrains heartbeat-to-heartbeat variations in blood pressure around a regulatory set-point. It maintains this constraint via a visceral sensory limb that detects changes in blood pressure and a visceral motor limb that adjusts autonomic outflow to the heart and blood vessels to control heart rate, the force with which the heart beats, and the caliber (degree of constriction) of blood vessels (Gianaros & Wager, 2015). Under homeostatic conditions, blood pressure and heart rate are reciprocally related to each other to support metabolic demands. For example, when blood pressure decreases (as it does when we stand up), heart rate increases as a counter measure to increase cardiac output and thus increase blood pressure (preventing fainting). Contrary to what is observed during homeostatic conditions, however, the reciprocal homeostatic relationship between heart rate and blood pressure can be modified (e.g., suppressed) during psychological stress, and particular set point for blood pressure may be reset to a different level according to the context. In this way, a stress appraisal may lead to joint increases in heart rate and blood pressure, as well as insensitivity to corrective visceral sensory feedback for homeostasis. But how might appraisal-based modifications of visceral control loops, such as the baroreflex, relate to disease risk? And, what are the mechanisms for these modifications?
Answers to these questions were first suggested by animal work showing that forebrain regions that presumably support appraisal processes (rostral neural systems in Figure 1) have direct anatomical projections to cell groups in the midbrain and brainstem (caudal neural systems and brainstem circuits in Figure 1). These projections provide a substrate to rapidly change the control dynamics of the baroreflex to alter the target organ physiology of the heart and vasculature via autonomic pathways, as illustrated in Figure 1 (Berntson, Sarter, & Cacioppo, 1998; Dampney, 2015). This animal work has been extended in human brain imaging studies of baroreflex control (Shoemaker & Goswami, 2015), particularly in work showing that baroreflex suppression by psychological stress relates to stressor-evoked activity in rostral cortical and subcortical brain systems implicated in appraisal: the ACC, insula, amygdala, and other anatomically networked areas (Gianaros, Onyewuenyi, Sheu, Christie, & Critchley, 2012). Such findings agree with the possibility (based on a heterarchical perspective) that rostral forebrain systems for appraisal could partly mediate ensuing physiological reactions by suspending, suppressing, bypassing, or modifying the homeostatic activity of visceral control loops.
A specific possibility, for example, is that disease vulnerability may increase when visceral control mechanisms for heart rate and blood pressure, such as the baroreflex, are chronically or repeatedly suspended by predictions errors that are incommensurate with metabolic need. And, such reactions arising from the predictive suspension of visceral control loops may affect circulatory functions that precipitate acute episodes of ischemia and other clinical phenomena during psychological stress (Dimsdale, 2008; Steptoe & Kivimaki, 2012). Moreover, metabolically inappropriate physiology may result from insensitivity to visceral feedback that would otherwise minimize visceral prediction errors. An etiological role in CVD may thus be suspected when baroreflex control is repeatedly or chronically modified by stressful experiences.
In the latter regard, it is important to underscore the influence of ‘bottom-up’ visceral sensory information provided by the baroreflex and other visceral control loops on appraisal systems of the brain. Hence, it is well established that sensory (afferent) baroreflex activity feeds back to the brain from the heart and blood vessels. Sensitivity to this feedback influences not only blood pressure homeostasis, but also a range of cognitive, emotional, and behavioral processes that are relevant to the appraisal of external and internal events, especially those encoded as threats (Berntson, Sarter, & Cacioppo, 2003; Garfinkel & Critchley, 2016). As depicted in Figure 1, visceral sensory feedback specifically reaches brainstem circuits and caudal neural systems of the midbrain, and it is known to be further processed by rostral neural systems of the forebrain and cerebral cortex implicated in appraisal processes—particularly the amygdala, insula, and areas of the cingulate and prefrontal cortices (Critchley & Harrison, 2013). Based on this body-to-brain feedback, the magnitude of a cardiovascular (e.g., blood pressure) reaction to a stressor can be represented and monitored by distributed neural systems that issue predictive visceral motor commands. In this way, visceral sensory information provided by cardiovascular stress reactions and relayed as part of autonomic-baroreflex pathways may be capable of updating appraisals and minimizing future visceral prediction errors to influence future manifestations of physiological stress reactivity. Finally, such visceral sensory information itself may be predicted or anticipated by brain systems supporting appraisal processes, a phenomenon referred to as interoceptive prediction (Barrett & Simmons, 2015). For instance, discrepancies between predicted and actual interoceptive (visceral sensory) information may account for clinical outcomes relevant to CVD (e.g., physical symptom misinterpretation, anxiety) (Shivkumar et al., 2016). As ‘bottom-up’ afferent signals, it is possible that such discrepancies may become paired via associative processes with appraisals and stressor coping behaviors in a manner that perpetuates or sustains the expression of visceral prediction errors.
From our perspective, brain systems mediating stress appraisal and reactivity processes may thus be viewed as situated within a heterarchical web of predictive feed-forward and feedback physiological control mechanisms. This perspective, however, raises questions about how to disrupt the interplay between appraisals and visceral control processes to reduce CVD risk.
Breaking the Links between Psychological Stress and Cardiovascular Disease
According to our perspective, optimal approaches to reduce CVD risk may necessitate a combination of strategies that alter stress appraisals and optimize visceral feedback to minimize visceral prediction errors and calibrate physiology with contextual metabolic needs.
With respect to the former strategies, interventions to promote cognitive reappraisal may have particularly beneficial effects on cardiovascular outcomes and stress reactivity processes (DeSteno, Gross, & Kubzansky, 2013). Cognitive reappraisal involves changing the meaning of an event in a manner that changes that person's response to that event (Gross & Thompson, 2007). Reappraisal parallels constructs of antecedent and problem-focused coping strategies embodied within stress appraisal theory (Lazarus & Folkman, 1984). Moreover, cognitive reappraisal is a core component of adjunctive and secondary CVD risk reduction programs (e.g., Gulliksson et al., 2011). With respect to our perspective, cognitive reappraisal reliably engages brain systems involved in regulating peripheral physiology, particularly aspects of cardiovascular and immune function. These include the ACC, insula, and amygdala (Buhle et al., 2014). As a result, reappraisal could minimize or prevent appraisal-based visceral prediction errors – adaptively calibrating anticipatory physiological and behavioral reactions to potential threats. This speculation agrees with evidence that individuals who report using reappraisal often exhibit lower C-reactive protein, a marker of systemic inflammation and parameter of CVD risk that is influenced by autonomic mechanisms (Appleton, Stephen L. Buka, Loucks, Gilman, & Kubzansky, 2013). Cognitive reappraisal is also suggested to positively alter physiological stress reactivity and recovery (Jamieson, Berry Mendes, & Knock, 2013). In support of a neurobiological path linking reappraisal to peripheral physiology and CVD risk, neural activity in the ACC observed during reappraisal was recently related to a marker of atherosclerosis, and this relationship was mediated by an inflammatory cytokine under autonomic control: interleukin-6 (Gianaros et al., 2014). Strategies to alter stress appraisals may further extend to other interventions, such as mindfulness and the affirmation of one's values, which favorably change stress physiology and markers of CVD risk (Creswell et al., 2016; Spicer et al., 2016). We suggest that a shared benefit of such ‘top-down’ interventions may be to minimize visceral prediction errors.
In addition to appraisal-based or ‘top-down’ mechanisms, other behavioral tactics may influence stress reactivity and CVD risk via ‘bottom-up’ pathways. An example is physical activity, which exerts cardio-protective effects by complex biological and behavioral processes. The latter encompass stress and emotion-related processes relevant to CVD risk. Physical activity may specifically relate to aspects of stress physiology, including cardiovascular reactivity. Here, meta-analyses provide moderate, but not uniform, evidence that greater engagement in physical activity relates to reductions in stressor-evoked reactivity (Forcier et al., 2006; Jackson & Dishman, 2006). This reduction is especially apparent after a bout of exercise (Hamer, 2012). Interestingly, intervention evidence shows that physical activity increases the feedback sensitivity and effectiveness of the baroreflex (Mameletzi, Kouidi, Koutlianos, & Deligiannis, 2011), which may improve clinical outcomes (La Rovere, Bersano, Gnemmi, Specchia, & Schwartz, 2002). Finally, physical activity robustly affects brain plasticity, particularly in areas involved in visceral motor and sensory control (Bar et al., 2016). Physical activity also favorably affects rostral neural systems of the forebrain that support cognitive functions that underpin successful reappraisal (McEwen & Gianaros, 2011).
Based on existing evidence and the perspective offered here, it may be that ‘bottom-up’ strategies, such as physical activity, ‘prime’ brain systems in ways that make reappraisal-based or other cognitively oriented (‘top-down’) behavioral interventions for CVD more effective. If so, then interventions to reduce psychological stress and its influence on CVD risk could thus be most effective when they (i) integrate psychological and behavioral tactics and (ii) target brain systems that jointly appraise threats and control peripheral physiology (Rozanski, 2014).
Conclusion
In its epidemiological sense, a ‘host’ is what is vulnerable to the influence of a pathogenic agent. Host factors, in turn, shape how the host becomes exposed, susceptible, and responsive to pathogenic agents. Insofar as psychological stress is pathogenic, we broadly suggest that the host and host factors ultimately comprise the machinery of the brain and the mechanisms by which the brain couples stress appraisals with coordinated changes in behavior and peripheral physiology.
In this review, we highlighted evidence for specific brain processes that appear to play simultaneous roles in constructing stressful experiences via appraisals and controlling peripheral physiology via predictive visceral motor and sensory processes. We emphasized that such processes are unlikely to be localized to any particular area or circuit of the brain. We also described a perspective on a particular kind of physiological stress reactivity that relates to CVD risk; namely, stressor-evoked cardiovascular reactivity. By this perspective, we postulated that threat appraisals are essentially ‘prediction ensembles’ that entrain physiology and behavior in accordance with anticipated metabolic needs engendered by a context or demand. Such predictive processes may derive from internal models or prospective simulations of future outcomes anticipated by a given context or demand. And, particular types of stressor-evoked cardiovascular reactivity that are generated by these processes may be conceptualized as manifestations of visceral prediction errors, wherein physiology is not calibrated to match the metabolic or behavioral needs of a given context or demand. As widely hypothesized by others, such physiological-somatic uncoupling may contribute to disease vulnerability and clinical events, or it may serve as a proxy of other phenomena that do so. This perspective is also compatible with others on psychological stress and physical health, particularly those postulating that physiological stress reactivity does not unfold in a vacuum. Instead, it represents a source of information that feeds back to the brain to alter appraisals, information processing, and future patterns of reactivity that can shape health and disease vulnerability over the lifespan (cf., Del Giudice, Ellis, & Shirtcliff, 2011).
Moving forward, there is a need to address questions raised by the perspective presented here. What developmental, genetic, and environmental factors shape individual differences in appraisals, visceral predictions, and feedback processes? From a translational perspective, to what extent are these processes malleable or causally involved in pathology? Does the perspective offered here apply to other forms of physiological reactivity beyond cardiovascular reactivity that are relevant to physical health and that may exhibit diverse forms of ‘error’ over different time scales (e.g., neuroendocrine, immune, etc.)? Notably, too, the term ‘error’ was used here in a relative sense. We appreciate that ‘errors’ as we have described them are not inherently or uniformly maladaptive or inappropriate. Indeed, visceral prediction errors are likely adaptive under some contexts (e.g., where there is a high degree of contextual ambiguity or uncertainty about the future). However, it is possible that they relate to pathology and disease risk when forms of such ‘errors’ are chronically expressed as stable phenotypes. From an evolutionary perspective, for example, predictive visceral control processes for stress reactivity may have enabled coping with threats that necessitated metabolic energy expenditures calibrated to extreme behavioral actions that served to maintain life. An open question is whether ‘modern stressors’ or those that are chronic (e.g., relationship conflict, job strain) that do not necessitate such actions make the expression of mis-calibrations (visceral prediction errors) more common and thus cumulatively pathogenic among some individuals.
It also remains open as to whether and how stressors of modern life might affect sensitivity to visceral sensory information that serves to minimize visceral prediction errors and alter psychological appraisals in the ways that were outlined above. In this regard, future work stimulated by the perspective offered here would be to better understand how predictive visceral motor and sensory processes for stress physiology relate to the leading psychosocial risk factors for CVD. These include psychosocial factors that are associated with prolonged anticipatory states, long-term alterations in psychological appraisals, and exposures to demands that unfold over extended periods of time without needs for excessive metabolic support, including work-related demands, close relationship difficulties, financial problems, caregiving burdens, insufficient social support, and social disconnection (Steptoe & Kivimaki, 2012).
Finally, it remains to be determined whether the neurobiological perspective presented here is relevant to understanding the comorbidity between CVD and stress-related psychiatric disorders (e.g., major depressive disorder, post-traumatic stress disorder) that involve a dysfunction of brain systems for stress appraisals and visceral control. Accordingly, addressing these possibilities and open questions should help to deepen our understanding of the brain-body mechanisms by which psychological stress may influence CVD risk, as well as inform brain-based strategies to predict and possibly reduce the burden of CVD.
Acknowledgments
This work was supported by National Institutes of Health R01 grants HL089850 to Peter Gianaros and HL101959 to J. Richard Jennings. We thank Gary Berntson and Thomas Kraynak for their comments on earlier versions of this manuscript.
Footnotes
The authors declare no conflicts of interest with respect to their authorship or the publication of this article.
Contributor Information
Peter J. Gianaros, Departments of Psychology and Psychiatry and Center for the Neural Basis of Cognition, University of Pittsburgh
J. Richard Jennings, Departments of Psychiatry and Psychology, University of Pittsburgh.
References
- Akdeniz C, Tost H, Streit F, Haddad L, Wust S, Schafer A, et al. Meyer-Lindenberg A. Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA Psychiatry. 2014;71:672–680. doi: 10.1001/jamapsychiatry.2014.35. [DOI] [PubMed] [Google Scholar]
- Alexander F. Factors in essential hypertension: Presentation of a tentative theory. Psychosomatic Medicine. 1939;1:173–179. [Google Scholar]
- Appleton AA, Stephen L, Buka SL, Loucks EB, Gilman SE, Kubzansky LD. Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation. Health Psychology. 2013;32:748–756. doi: 10.1037/a0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar KJ, Herbsleb M, Schumann A, de la Cruz F, Gabriel HW, Wagner G. Hippocampal-brainstem connectivity associated with vagal modulation after an intense exercise intervention in healthy men. Front Neurosci. 2016;10:145. doi: 10.3389/fnins.2016.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nature Reviews Neuroscience. 2015;16:419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Stroke Statistics, S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT. The neuroevolution of motivation. In: Shah JY, Gardner WL, editors. Handbook of Motivation. New York: Guilford Press; 2008. [Google Scholar]
- Berntson GG, Gianaros PJ, Tsakiris M. Interoception and the autonomic nervous system: Bottom-up meets top-down. In: Tsakiris M, De Preester H, editors. The Interoceptive Basis of the Mind. New York, NY: Oxford University Press; in press. [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: The basal forebrain cholinergic link. Behavioral Brain Research. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. European Journal of Neuroscience. 2003;18:2103–2109. doi: 10.1046/j.1460-9568.2003.02967.x. doi:2967[pii] [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Ochsner KN. Cognitive reappraisal of emotion: A Meta-analysis of human neuroimaging studies. Cerebral Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. The wisdom of the body. New York: WW Norton & Company; 1932. [Google Scholar]
- Carroll D, Ginty AT, Der G, Hunt K, Benzeval M, Phillips AC. Increased blood pressure reactions to acute mental stress are associated with 16-year cardiovascular disease mortality. Psychophysiology. 2012;49:1444–1448. doi: 10.1111/j.1469-8986.2012.01463.x. [DOI] [PubMed] [Google Scholar]
- Charvat J, Dell P, Folkow B. Mental Factors and Cardiovascular Diseases. Cardiologia. 1964;44:124–141. doi: 10.1159/000167892. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychological Bulletin. 2008;134:829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cohen S, Gianaros PJ, Manuck SB. A stage model of stress and disease. Perspect Psychol Sci. 2016;11:456–463. doi: 10.1177/1745691616646305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Taren AA, Lindsay EK, Greco CM, Gianaros PJ, Fairgrieve A, et al. Ferris JL. Alterations in resting-state functional connectivity link mindfulness meditation with reduced interleukin-6: A randomized controlled trial. Biological Psychiatry. 2016;80:53–61. doi: 10.1016/j.biopsych.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77:624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Kalin NH, Grist TM, Davidson RJ. Neural-cardiac coupling in threat-evoked anxiety. Journal of Cognitive Neuroscience. 2005;17:969–980. doi: 10.1162/0898929054021094. [DOI] [PubMed] [Google Scholar]
- Dampney RA. Central mechanisms regulating coordinated cardiovascular and respiratory function during stress and arousal. American Journal of Physiology. 2015;309:R429–443. doi: 10.1152/ajpregu.00051.2015. [DOI] [PubMed] [Google Scholar]
- Dampney RA. Central neural control of the cardiovascular system: current perspectives. Advances in Physiology Education. 2016;40:283–296. doi: 10.1152/advan.00027.2016. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neuroscience and Biobehavioal Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSteno D, Gross JJ, Kubzansky L. Affective science and health: The importance of emotion and emotion regulation. Health Psychology. 2013;32:474–486. doi: 10.1037/a0030259. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE. Psychological stress and cardiovascular disease. Journal of the American College of Cardiology. 2008;51:1237–1246. doi: 10.1016/j.jacc.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL. Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proceedings of the National Academy of Sciences, USA. 2016;113:9922–9927. doi: 10.1073/pnas.1605044113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth H, Chang LJ, Wager TD. Multivariate brain prediction of heart rate and skin conductance responses to social threat. J Neurosci. 2016;36(47):11987–11998. doi: 10.1523/JNEUROSCI.3672-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Comprehensive Physiology. 2015;5:475–512. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- Forcier K, Stroud LR, Papandonatos GD, Hitsman B, Reiches M, Krishnamoorthy J, Niaura R. Links between physical fitness and cardiovascular reactivity and recovery to psychological stressors: A meta-analysis. Health Psychology. 2006;25:723–739. doi: 10.1037/0278-6133.25.6.723. [DOI] [PubMed] [Google Scholar]
- Fukumori R, Nishigori Y, Goshima Y, Kubo T. Contribution of the medial amygdaloid nucleus to the development of hypertension in spontaneously hypertensive rats. Neuroscience Letters. 2004;365:128–131. doi: 10.1016/j.neulet.2004.04.066. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Critchley HD. Threat and the body: How the heart supports fear processing. Trends in Cognitive Science. 2016;20:34–46. doi: 10.1016/j.tics.2015.10.005.. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SW, Matthews KA. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension. 2007;49:134–140. doi: 10.1161/01.HYP.0000250984.14992.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Kuan DC, Schirda BL, Jennings JR, Sheu LK, et al. Manuck SB. An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biological Psychiatry. 2014;75:738–745. doi: 10.1016/j.biopsych.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD. Brain systems for baroreflex suppression during stress in humans. Human Brain Mapping. 2012;33:1700–1716. doi: 10.1002/hbm.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Uyar F, Koushik J, Jennings JR, Wager TD, et al. Verstynen T. A brain phenotype for stressor-evoked cardiovascular reactivity. Journal of the American Heart Association. 2017;6:e006053. doi: 10.1161/JAHA.117.006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Wager TD. Brain-body pathways linking psychological stress and physical health. Current Directions in Psychological Science. 2015;24:313–321. doi: 10.1177/0963721415581476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty AT, Kraynak TE, Fisher JP, Gianaros PG. Cardiovascular and autonomic reactivity to psychological stress: Neurophysiological substrates and links to cardiovascular disease. Autonomic Neuroscience. 2017 doi: 10.1016/j.autneu.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford Press; 2007. [Google Scholar]
- Gulliksson M, Burell G, Vessby B, Lundin L, Toss H, Svardsudd K. Randomized controlled trial of cognitive behavioral therapy vs standard treatment to prevent recurrent cardiovascular events in patients with coronary heart disease: Secondary prevention in Uppsala Primary Health Care Project. Archives of Internal Medicine. 2011;171:134–140. doi: 10.1001/archinternmed.2010.510. [DOI] [PubMed] [Google Scholar]
- Hamer M. Psychosocial stress and cardiovascular disease risk: the role of physical activity. Psychosom Med. 2012;74(9):896–903. doi: 10.1097/PSY.0b013e31827457f4. [DOI] [PubMed] [Google Scholar]
- Hess WR, Brugger M. Das subkorticale Zentrum der affektiven Abwehrreaktion. Helv Physiol Acta. 1943;1:33–52. [Google Scholar]
- Jackson EM, Dishman RK. Cardiorespiratory fitness and laboratory stress: a meta-regression analysis. Psychophysiology. 2006;43(1):57–72. doi: 10.1111/j.1469-8986.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- Jamieson JP, Berry Mendes W, Knock MK. Improving acute stress responses: The power of reappraisal. Current Directions in Psychological Science. 2013;22:51–56. [Google Scholar]
- Jänig W. The integrative action of the autonomic nervous system: neurobiology of homeostasis. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- Jennings JR, van der Molen MW, Brock K, Somsen RJ. How are tonic and phasic cardiovascular changes related to central motor command? Biological Psychology. 1993;35(3):237–254. doi: 10.1016/0301-0511(93)90004-r. [DOI] [PubMed] [Google Scholar]
- Kasprowicz A, Manuck S, Malkoff S, Krantz D. Individual differences in behaviorally evoked cardiovascular response: temporal stability and hemodynamic patterning. Psychophysiology. 1990;27:605–619. doi: 10.1111/j.1469-8986.1990.tb03181.x. [DOI] [PubMed] [Google Scholar]
- Kelsey RM, Soderlund K, Arthur CM. Cardiovascular reactivity and adaptation to recurrent psychological stress: replication and extension. Psychophysiology. 2004;41:924–934. doi: 10.1111/j.1469-8986.2004.00245.x. [DOI] [PubMed] [Google Scholar]
- Kleckner IR, Quigley KS. An approach to mapping the neurophysiological state of the body to affective experience. In: Feldman-Barrett L, Russell JA, editors. The psychological construction of emotion. New York: Guilford Press; 2015. [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, et al. Feldman Barrett L. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nature Human Behaviour. 2017;1:0069. doi: 10.1038/s41562-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: a review and methodologic critique. Psychological Bulletin. 1984;96:435–464. [PubMed] [Google Scholar]
- La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106:945–949. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]
- Lane RD, Waldstein SR, Chesney MA, Jennings JR, Lovallo WR, Kozel PJ, et al. Cameron OG. The rebirth of neuroscience in psychosomatic medicine, part I: historical context, methods and relevant basic science. Psychosomatic Medicine. 2009;71:117–134. doi: 10.1097/PSY.0b013e31819783be. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Psychological stress and the coping process. New York: McGraw-Hill; 1966. [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal and coping. New York: Guilford; 1984. [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behavioral and brain sciences. 2012;35(3):121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR. Stress and health: Biological and psycholocial interactions. 3rd. Thousand Oaks, CA: Sage; 2016. [Google Scholar]
- Lovallo WR, Gerin W. Psychophysiological reactivity: mechanisms and pathways to cardiovascular disease. Psychosomatic Medicine. 2003;65:36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- MacLean PD. Psychosomatic disease and the visceral brain: Recent developments bearing on the Papez theory of emotion. Psychosomatic Medicine. 1949;11:338–351. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiological Reviews. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- Mameletzi D, Kouidi E, Koutlianos N, Deligiannis A. Effects of long-term exercise training on cardiac baroreflex sensitivity in patients with coronary artery disease: a randomized controlled trial. Clin Rehabil. 2011;25(3):217–227. doi: 10.1177/0269215510380825. [DOI] [PubMed] [Google Scholar]
- Manning HL. Physiological Effects of Anger. The Journal of Hygiene and Herald of Health. 1895;45:324–326. [Google Scholar]
- Mason JW. A re-evaluation of the concept of nonspecificity in stress theory. Journal of Psychiatric Research. 1971;8:323–333. doi: 10.1016/0022-3956(71)90028-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annual Review of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Eisenberger NI. A social neuroscience perspective on stress and health. Soc Personal Psychol Compass. 2012;6:890–904. doi: 10.1111/j.1751-9004.2012.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B. Corticolimbic regulation of cardiovascular responses to stress. Physiology and Behavior. 2016;172:49–59. doi: 10.1016/j.physbeh.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrist PA. Cardiovascular Psychophysiology: A Perspective. New York, NY: Plenum Press; 1981. [Google Scholar]
- Öngür D, Price J. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Cechetto DF. Insular cortex and the regulation of cardiac function. Comphrehensive Physiology. 2016;6:1081–1133. doi: 10.1002/cphy.c140076. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, Brosschot JF. Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychological Bulletin. 2016;142:231–259. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Science. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanski A. Behavioral cardiology: current advances and future directions. Journal of the American College of Cardiology. 2014;64:100–110. doi: 10.1016/j.jacc.2014.03.047. [DOI] [PubMed] [Google Scholar]
- Sanders BJ, Wirtz-Nole C, DeFord SM, Erling BF. Central amygdaloid lesions attenuate cardiovascular responses to acute stress in rats with borderline hypertension. Physiology and Behavior. 1994;56:709–713. doi: 10.1016/0031-9384(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annual Review of Neuroscience. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Selye H. Homeostasis and heterostasis. Perspectives in Biology and Medicine. 1973;16:441–415. doi: 10.1353/pbm.1973.0056. [DOI] [PubMed] [Google Scholar]
- Sheehan D( Discovery of the autonomic nervous system. Archives of Neurology and Psychiatry. 1936;35:1081–1115. [Google Scholar]
- Sheu LK, Jennings JR, Gianaros PJ. Test-retest reliability of an fMRI paradigm for studies of cardiovascular reactivity. Psychophysiology. 2012;49(7):873–884. doi: 10.1111/j.1469-8986.2012.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen PS, Esler M, et al. Zipes DP. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. Journal of Physiology. 2016;594:3911–3954. doi: 10.1113/JP271870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Goswami R. Forebrain neurocircuitry associated with human reflex cardiovascular control. Frontiers in Physiology. 2015;6:240. doi: 10.3389/fphys.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer J, Shimbo D, Johnston N, Harlapur M, Purdie-Vaughns V, Cook J, et al. Wager TD. Prevention of stress-provoked endothelial injury by values affirmation: a proof of principle study. Annals of Behavioral Medicine. 2016;50:471–479. doi: 10.1007/s12160-015-9756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9(6):360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- Sterling P. Allostasis: a model of predictive regulation. Physiology and Behavior. 2012;106:5–15. doi: 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. New York: John Wiley & Sons; 1988. pp. 629–649. Reprinted from: In File. [Google Scholar]
- Taggart P, Critchley H, van Duijvendoden S, Lambiase PD. Significance of neuro-cardiac control mechanisms governed by higher regions of the brain. Auton Neurosci. 2016;199:54–65. doi: 10.1016/j.autneu.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, et al. Pitman RK. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389:834–845. doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. Perturbing the organism: The biology of stressful experience. Chicago, IL: University Of Chicago Press; 1992. [Google Scholar]