Abstract
Heightened anticipation and fear of movement-related pain has been linked to detrimental fear-avoidance behavior in chronic Low-Back Pain (cLBP). Spinal Manipulative Therapy (SMT) has been proposed to work partly by exposing patients to non-harmful but forceful mobilization of the painful joint, thereby disrupting the relationship between pain anticipation, fear, and movement. Here, we investigated the brain processes underpinning pain anticipation and fear of movement in cLBP, and their modulation by SMT, using functional Magnetic Resonance Imaging. Fifteen cLBP patients and 16 healthy controls (HC) were scanned while observing and rating video clips depicting back-straining or neutral physical exercises, which they knew they would have to perform at the end of the visit. This task was repeated after a single session of spinal manipulation (SMTmanip; cLBP and HC) or mobilization (SMTmobil; cLBP only), in separate visits.
Compared to HC, cLBP patients reported higher expected pain and fear of performing the observed exercises. These ratings, along with clinical pain, were reduced by SMT. Moreover, cLBP, relative to HC, demonstrated higher BOLD signal in brain circuitry that has previously been implicated in salience, social cognition and mentalizing, while observing backstraining, compared to neutral exercises. The engagement of this circuitry was reduced after SMT, and especially SMTmanip, proportionally to the magnitude of SMT-induced reduction in anticipated pain and fear. This study sheds light on the brain processing of anticipated pain and fear of back-straining movement in cLBP, and suggests that SMT may reduce cognitive and affective-motivational aspects of fear-avoidance behavior, along with corresponding brain processes.
Keywords: Pain anticipation, Expectation, Fear of movement, Fear-avoidance, Physical exercise, chronic Low Back Pain, Spinal Manipulative Therapy, Manual Therapy, functional Magnetic Resonance Imaging, fMRI
Heightened anticipation and fear of movement-related pain has been linked to detrimental fear-avoidance behavior in chronic Low-Back Pain (cLBP). Spinal Manipulative Therapy (SMT) has been proposed to work partly by exposing patients to non-harmful but forceful mobilization of the painful joint, thereby disrupting the relationship between pain anticipation, fear, and movement. Here, we investigated the brain processes underpinning pain anticipation and fear of movement in cLBP, and their modulation by SMT, using functional Magnetic Resonance Imaging. Fifteen cLBP patients and 16 healthy controls (HC) were scanned while observing and rating video clips depicting back-straining or neutral physical exercises, which they knew they would have to perform at the end of the visit. This task was repeated after a single session of spinal manipulation (SMTmanip; cLBP and HC) or mobilization (SMTmobil; cLBP only), in separate visits.
Compared to HC, cLBP patients reported higher expected pain and fear of performing the observed exercises. These ratings, along with clinical pain, were reduced by SMT. Moreover, cLBP, relative to HC, demonstrated higher BOLD signal in brain circuitry that has previously been implicated in salience, pain anticipation, social cognition and mentalizing, while observing back-straining, compared to neutral exercises. The engagement of this circuitry was reduced after SMT, and especially SMTmanip, proportionally to the magnitude of SMT-induced reduction in anticipated pain and fear. This study sheds light on the brain processing of anticipated pain and fear of back-straining movement in cLBP, and suggests that SMT may reduce cognitive and affective-motivational aspects of fear-avoidance behavior, along with corresponding brain processes.
Perspective:
This study of chronic Low Back Pain (cLBP) patients investigated how Spinal Manipulative Therapy (SMT) affects clinical pain, expected pain, and fear of physical exercises. The results indicate that one of the mechanisms of SMT may be to reduce pain expectancy, fear of movement, and associated brain responses.
Introduction
Elevated anticipation of motion-related pain is common in chronic Low Back Pain (cLBP)69, and is associated with fear of movement and excessive avoidance behavior, which can be detrimental to health and quality of life, and prevent recovery50. Higher fear-avoidant behavior is associated with higher disability12, 29, 68, 69, 71, and reduction in fear/anxiety can predict successful therapy70. The Fear-Avoidance model of chronic pain posits that chronification of pain is often characterized by a vicious cycle whereby catastrophizing about pain leads to fear of movement and hypervigilance, which in turn can incite hypersensitization and exacerbation of pain, leading to yet more avoidance40. This cycle has been linked to Pavlovian and operant conditioning21, 67, in which pain initially represents an unconditioned response (UR) to a nociceptive unconditioned stimulus, (US), but may be elicited by a progressively wider range of non-harmful movements (CS)27, 46. While a large literature implicates psychological mechanisms of fear-avoidance behavior in pain, less is known about the brain mechanisms involved in the anticipation and fear of movement-evoked pain. Understanding these mechanisms is critically important, since they influence behavioral decisions to approach or avoid situations where a perceived harmful physical movement may occur.
Initial neuroimaging studies of cLBP patients observing back-straining maneuvers have found increased sympathetic responses25 and altered brain processing in circuitry consistent with social cognition, salience and mentalizing, such as ventrolateral (vlPFC) and dorsomedial (dmPFC) prefrontal cortex, mid-anterior insula (m/aINS), middle temporal gyrus (MTG), superior temporal sulcus (STS), and amygdala. Notably, in these studies, participants passively viewed static pictures4, 5, 61 or videos43, 44 depicting people in back-straining positions, without any actual prospect of executing physical activity by the participants themselves. Thus, the behavioral relevance of the context – and in turn fear – may have been limited.
Conditioned responses – such as fear of potentially harmful maneuvers – can be “unlearned” when CS or CR consistently occurs without leading to UR – e.g. a perceived harmful motion is not followed by pain or harm. There is evidence that exposing patients to (feared) non-harmful physical activity, in order to extinguish fear responses, can reduce avoidance behavior in chronic musculoskeletal pain20, 67, 70. Interestingly, one proposed effect of spinal manipulative therapy (SMT), which involves salient sensory and proprioceptive feedback through passive mobilization of spine joints, is that it might help disrupt the association between fear, back-motion, and pain9, 74 However, it has not yet been investigated whether SMT affects motivational aspects relevant to avoidance – such as anticipated pain and fear of movement. Moreover, very little is known about how SMT affects brain processing22.
Here, we investigated the brain-based underpinnings anticipated pain and fear of physical exercises, and the effect of two SMT techniques (grade 3 mobilization and grade 5 manipulation) on these outcomes. We hypothesized that, in cLBP, observation of back-straining, relative to neutral, exercises would elicit brain responses in circuitry involved in social cognition, fear, salience, and pain processing (e.g. the anterior cingulate cortex, insulae, and amygdalae), in addition to visual and fronto-parietal attention regions. Further, we hypothesized that SMT would reduce clinical pain, as well as fear, expected pain of back-straining exercises, and corresponding brain responses to observation of such exercises. Finally, we hypothesized that these effects would be stronger for SMT manipulation relative to mobilization, reflecting a dose-response.
Methods
Subjects
Fifteen patients with chronic Low Back Pain (cLBP, 8 female, mean age ± SD = 37.7±9.7) and 16 individually age- and sex-matched healthy controls (HC, 8 female, age 38.2±10.4) were enrolled. One HC was excluded from final paired analyses as we were not able to recruit an age- and sex-matched cLBP for this individual. All subjects provided written informed consent prior to participation. The study was approved by the Human Research Committee of Massachusetts General Hospital and was conducted in accordance with the Declaration of Helsinki.
Inclusion criteria for cLBP patients included age ≥ 21 and ≤ 65, nonspecific low back pain, diagnosed >6 months prior to enrollment, and ongoing pain that averaged at least 4 on a 0-10 scale of pain during the week prior to enrollment. Exclusion criteria included radicular pain (i.e., pain radiating down below the knee), neural deficit in the lower extremity, positive dural tension signs, surgery within the past year related to back pain, pain management procedures during the study period, contraindications to functional Magnetic Resonance Imaging (fMRI), current or past history of major medical, neurological, or psychiatric illness other than chronic pain, peripheral nerve injury, diabetes, pregnancy, breastfeeding, less than 6 months post-partum, history of head trauma, high blood pressure, use of opioid medications, use of recreational drugs, history of substance abuse, and back pain due to cancer, fracture, or infection. Exclusion criteria for healthy controls were, in addition to those for cLBP, chronic or acute low back pain.
Experimental protocol
The study involved 3 study visits for cLBP patients – an initial behavioral visit, an MRI visit with SMT mobilization (i.e., grade III of the Maitland Joint Mobilization Grading Scale28, and an MRI visit with SMT manipulation (i.e., grade V of the Maitland Joint Mobilization Grading Scale). The order of the MRI visits was counterbalanced across subjects (interval between MRI visits was 24.7±22.9, mean±SD). The HC group completed 2 study visits - an initial behavioral visit and an MRI visit with grade V SMT. Since we did not anticipate SMT-induced changes in clinical outcomes for HC, we did not include an SMT mobilization visit for this group, as the comparison between SMT manipulation and SMT mobilization was aimed at investigating differences in clinical outcomes and associated brain responses. Therefore, our study had a mixed design, with both a within-subject (grade III vs grade V in cLBP patients) as well as a between-subject (Grade V in cLBP vs healthy participants) component. All visits took place at the MRI facilities at Martinos Center for Biomedical Imaging, Massachusetts General Hospital, between September 2014 and November 2016.
Behavioral visit:
Following informed consent, a licensed chiropractor performed a clinical evaluation (including history and physical examination), in order to further characterize the nature of the pain symptoms (or, in the case of healthy volunteers, to exclude the presence of back pain) and to determine the suitability and safety of manual therapy. All participants were also asked to perform a series of back-straining (e.g., sit-ups, thrusts, leg lifts and pelvic tilts) and non-back-straining (e.g., arm-lifts, flexion-extension of the arms) physical exercises repeated 3 times each, rating the intensity (0 = ‘no pain’, 100 = ‘the most intense pain tolerable’) and unpleasantness (0 = ‘neutral’, 100 = ‘extremely unpleasant’) of their back pain after each repetition. The patients’ responses to these exercises were used to guide the selection of subject-specific videos that most reliably exacerbated the patients’ pain for use in the subsequent MRI visits (see below).
MRI visits:
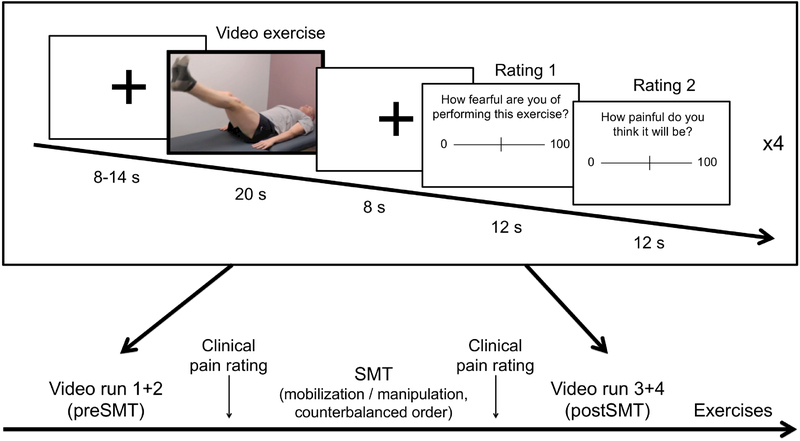
Participants were placed in a supine position in a 3T Siemens Skyra whole-body MRI scanner (Siemens Medical Systems, Erlangen, Germany). In each of 4 separate fMRI runs (~4.5 min each, 2 before and 2 after SMT), participants were shown 4 videos (20 s each) – 2 depicting ‘high back-straining exercises’ (BSE) and 2 depicting ‘low back-straining exercises’ (Neutral) – in a pseudorandomized order (Figure 1, for illustrations of all the video material, see Supplemental Digital Content 1). For each cLBP patient, as well as his/her age- and sex-matched control, the BSE videos depicted an actor (sex-matched to the participant) performing the two back-straining exercises which most reliably elicited pain in the cLBP patient during the behavioral visit; in the control videos, the same actor performed non-back-straining exercises (identical in all participants). We decided to use videos rather than still images, as they are able to show the full dynamic of physical exercises, which likely increases their salience. In order to maximize the emotional impact of observing back-straining exercises, at the beginning of the imaging visit the participants were informed that at the end of the imaging visit they would be asked to perform the exercises they observed in the videos. Eight seconds after each video, the subjects were prompted to rate how much pain they expected from performing the exercise they saw on the previous video clip and how fearful they were of performing that exercise. After the first 2 fMRI runs, participants rated their clinical pain and were temporarily removed from the MRI bore, received SMT (either manipulation or mobilization) while on the scanner bed, rated their clinical pain again, and then were placed back into the MRI bore and completed the 2 final fMRI runs. At the end of the first visit, participants performed 5 repetitions of each of the back-straining and neutral exercises corresponding to the videos they had seen, in keeping with the instructions. The main purpose of this procedure was to ensure that the experimental induction of anticipation of clinical pain exacerbation would be still credible during the subsequent visit.
Figure 1: Experimental design.
Participants viewed 20 s videos showing back-straining or neutral (non-back-straining) exercises in a pseudorandomized order. After each video, they rated fearfulness and expected pain from performing the exercise on Visual Analogue Scales. There were 8 videos (spread over 2 scan runs) before, and 8 videos after SMT. At the end of each scan session, the participants were asked to perform the observed back-straining exercises, which had been established to be painful at the initial training visit for all chronic Low-Back Pain patients.
Psychophysical measures
During the scanning procedures subjects used a button box for ratings, using scales displayed with E-Prime software (v. 1.1, Psychology Software Tools, Sharpsburg, PA). Numerical rating scales (0-100) were used for low-back pain (0 = ‘no pain’, 100 = ‘the most intense pain tolerable’) before and after the preSMT fMRI and the postSMT fMRI runs. The same button box was used for ratings of expected pain from exercise (‘How painful do you think it will be?’ 0 = ‘no pain’, 100 = ‘the most intense pain tolerable’) and fearfulness (‘How fearful are you of performing this exercise?’ 0 = ‘Not at all fearful’, 100 = ‘Extremely fearful’).
At the training visit, participants filled out the following validated questionnaires for facilitating clinical characterization of the sample groups: Tampa scale of Kinesiophobia45 (TSK), Pain Catastrophizing Scale59 (PCS), Beck’s Depression Inventory6 (BDI), Brief Pain Inventory60 (BPI), and treatment credibility (1 = ‘Definitely’, 5 = ‘Definitely not’, Likert scale, modified from Sherman et al.57). Additionally, they rated bothersomeness of low-back pain (0 = ‘Not at all bothersome’, 100 = ‘Extremely bothersome’, VAS), expected relief from SMT (0 = ‘Does not work at all’, 10 = ‘Complete relief, VAS), and desire for relief (0 = ‘No desire for pain relief, 10 = ‘The most intense desire for relief imaginable’).
Psychophysics data analysis
To compare cLBP and HC groups on basic demographic, trait, and clinical characteristics, we performed paired t-tests of age, TSK, PCS, BDI and BPI scores, credibility of SMT, clinical pain at baseline, anxiety at baseline, Low Back Bothersomeness, expected relief from SMT, and desire for relief.
To confirm that cLBP expected more pain from, and were more fearful of, performing back-straining relative to neutral exercises, we performed a mixed-design AN OVA with the factors GROUP (cLBP, HC) and VIDEO EXERCISE (BSE, Neutral), using baseline (preSMT) ratings from the first visit.
To investigate whether SMT affected cLBP patients’ clinical pain, we performed an ANOVA with the factors SMT TECHNIQUE (manipulation, mobilization), and TIME (preSMT, postSMT). SCAN ORDER (manipulation first, mobilization first) was included as a categorical covariate of no interest.
To investigate whether SMT affected cLBP patients’ expected pain and fear of performing exercises, we used two separate repeated measures ANOVAs using each patient’s mean expected pain and fearfulness (averaged across BSE and Neutral videos, separately), with the factors SMT TECHNIQUE (manipulation, mobilization), VIDEO EXERCISE (BSE, Neutral), and TIME (preSMT, postSMT). SCAN ORDER (manipulation first, mobilization first) was included as a categorical covariate of no interest.
We calculated Pearson correlation coefficients to investigate the relationship between SMT-induced change in clinical pain (postSMT - preSMT) vs. change in expected pain and change in fearfulness. To investigate the impact of positive expectation, we calculated Pearson correlation coefficients between expected relief from SMT vs. SMT-induced changes in clinical pain, expected pain, and fearfulness. Behavioral data were analyzed using JASP (Version 0.8.1.5).
MRI data acquisition and preprocessing
BOLD fMRI data were collected using a whole brain, simultaneous multislice, T2*-weighted gradient echo BOLD EPI pulse sequence (TR = 1250 ms, TE = 33ms, flip angle = 65°, voxel size = 2mm isotropic, number of slices = 75, SMS factor = 5). A high resolution structural volume (multi-echo MPRAGE) was collected for the purposes of anatomical localization and spatial normalization (TR = 2530 ms, TE = 1.69 ms, flip angle = 7°, voxel size = 1mm isotropic).
FMRI data processing and analysis was carried out using FEAT (FMRI Expert Analysis Tool) Version 6.0, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). The following preprocessing was applied: slice-timing correction, motion correction using MCFLIRT32, fieldmap-based EPI unwarping using PRELUDE and FUGUE30, 31, non-brain removal using BET58; spatial smoothing (FWHM = 4mm), temporal high-pass filter (0.011 hz as computed by FSL’s cutoffcaic), grand-mean intensity normalization by a single multiplicative factor. All 4 runs were realigned (6 dof) to a common space (the seventh volume of the first fMRI run) before first-level GLM analyses. The transformation matrix for the registration from functional to the high resolution anatomical image was computed using Boundary Based Registration (FreeSurfer’s bbregister tool26). For structural-to-standard space registration, we used the FSL’s Linear registration tool (FLIRT, 12 DOF)32, 33 followed by FSL’s Non-linear registration tool (FNIRT)1. All single-subject analyses were performed in functional space, and registration to standard space (MNI152) was applied before group analyses.
fMRI data analysis
Single-subject GLM analyses were carried out using FILM with local autocorrelation correction. For each run (two preSMT, and two postSMT), we modeled the epochs corresponding to the video presentation (BSE, Neutral) as regressors in a General Lineal Model. In the same design matrix we also modeled rating periods, the first temporal derivative of each time course, and the 6 motion parameters as regressors of no interest. From this first-level analyses we computed a total of 6 contrasts: BSE – rest, Neutral – rest, BSE – Neutral, and their opposites. In a second-level fixed-effect analysis, we averaged the contrast maps across both preSMT runs and across both postSMT runs separately, resulting in two sets of contrasts of parameter estimates for each contrast (preSMT and postSMT).
To investigate brain responses to observing back-straining and neutral exercises, we carried out whole-brain voxelwise group GLMs separately for cLBP and HC from the baseline (preSMT) scans at the first visit, for each of the 6 contrasts. Because the patient and control groups were recruited in a matching-pairs design (with each patient matched for age and sex to a control subject), the group contrasts were evaluated using paired t-tests comparing age / sex matched cLBP and HC groups. Group inference was performed using FLAME (FMRIB’s Local Analysis of Mixed Effects) 1+2, and the resulting statistical maps were cluster corrected for multiple comparisons using a cluster-forming voxel-wise threshold of Z>2.3, and a (corrected) cluster significance threshold of P<0.05.
We then conducted whole-brain voxel-wise GLMs to investigate SMT-induced change in BOLD responses (postSMTBSE-Neutral – preSMTBSE-Neutral) in cLBPmanip relative to cLBPmobil, and cLBPmanip relative to HC.
To investigate whether SMT-induced changes in expected pain and fear of movement were associated with changes in brain responses to these videos, we carried out whole-brain voxelwise regression analyses (postSMTBSE-Neutral – preSMTBSE-Neutral) with SMT-induced changes in expected pain and fearfulness ratings (postSMTBSE-Neutral – preSMTBSE-Neutral) as regressors of interest. These regression analyses were performed separately for SMTmanip and SMTmobil, and for cLBP participants only since the HC group, as anticipated, did not show enough dynamic range in ratings of expected pain (12 out of 15 rated expected pain as 0 both before and after SMT).
Visualization of brain imaging data was produced using FSL’s FSLView for data displayed on volumes (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FslView), Freesurfer’s Freeview for brain surfaces (https://surfer.nmr.mgh.harvard.edu), and Caret for cerebellar surfaces10, 65 (http://www.nitrc.org/projects/caret/).
Results
Demographic, trait, and clinical characteristics
CLBP and HC groups did not significantly differ in age, TSK or BDI scores, perceived credibility of SMT, and expected relief from SMT (Table 1). We found significantly higher PCS scores, clinical pain (baseline VAS, BPI, and Low Back Bothersomeness), baseline anxiety, and desire for relief for cLBP compared to HC. For one LBP patient, we did not obtain BDI, Credibility, clinical pain, and anxiety. However, all participants were retained for data collection of all other data for both imaging visits.
Table 1:
Subject characteristics
| 95% Confidence Interval | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mean±SD (cLBP) | Mean±SD (HC) | t | df | p | Cohen’s d | Lower | Upper | |
| Age | 37.7±9.7 | 38.5±10.1 | 0.580 | 14 | 0.571 | 0.150 | −1.258 | 2.191 |
| Tampa Scale of Kinesiophobia | 33.3±5.7 | 33.1±7.5 | 0.854 | 14 | 0.407 | 0.221 | −2.569 | 5.969 |
| Pain Catastrophizing Scale | 13.6±10.2 | 6.2±6.8 | 2.492 | 14 | 0.026 | 0.644 | 0.972 | 12.962 |
| Beck’s Depression Inventory | 1.9±2.7 | 4.1±4.2 | 1.427 | 13 | 0.177 | 0.382 | −1.082 | 5.296 |
| Perceived credibility of SMT | 1.8±0.6 | 2.1±0.8 | −0.942 | 13 | 0.363 | −0.252 | −0.753 | 0.295 |
| Clinical pain (Baseline, 0-100) | 44.2±18.6 | 0.3±1.2 | 8.484 | 13 | <0.001 | 2.268 | 32.889 | 55.361 |
| Anxiety (Baseline, 0-100) | 12.2±12.3 | 0.8±1.4 | 3.606 | 13 | 0.003 | 0.964 | 4.896 | 19.532 |
| Brief Pain Inventory (Severity, 0-10) | 4.5±1.4 | 0.3±0.4 | 10.768 | 14 | <0.001 | 2.780 | 3.358 | 5.029 |
| Brief Pain Inventory (Interference, 0-10) | 3.2±2.1 | 0.1 ±0.2 | 5.757 | 14 | <0.001 | 1.486 | 1.948 | 4.261 |
| Low back bothersomeness (0-10) | 5.0±1.7 | 0.5±1.3 | 9.049 | 14 | <0.001 | 2.336 | 3.403 | 5.517 |
| Expected relief (0-10) | 5.3±2.8 | 3.6±3.3 | 1.363 | 14 | 0.194 | 0.352 | −0.962 | 4.315 |
| Desire for relief (0-10) | 9.0±1.0 | 1.8±3.4 | 7.150 | 14 | <0.001 | 1.846 | 5.101 | 9.472 |
Abbreviations: cLBP = chronic Low Back Pain; HC = Healthy Control; SMT = Spinal Manipulative Therapy
cLBP patients, relative to HC, expected more pain from, and were more fearful of, performing back-straining exercises
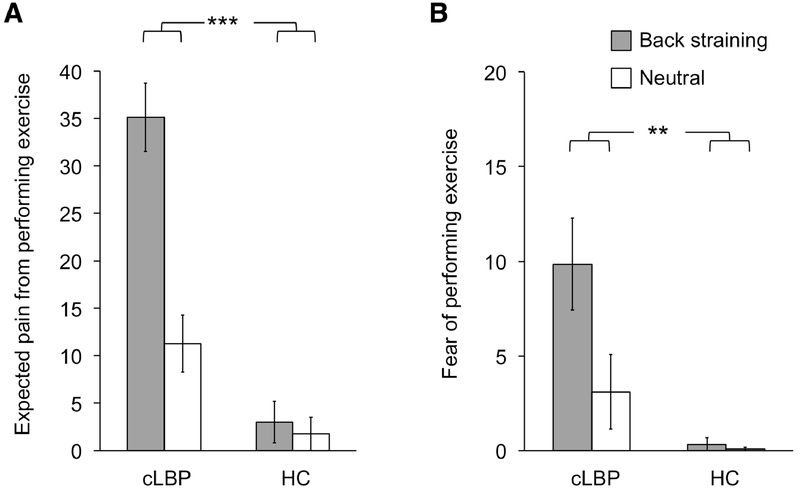
A mixed-design ANOVA confirmed that cLBP, relative to HC, anticipated the exercises depicted in the videos, when performed in first-person at the end of the visit, to be more painful (main effect of GROUP: F(1, 13)=31.54, P<0.001, partial eta squared: η2p=0.71). Furthermore, a main effect of VIDEO EXERCISE confirmed higher expected pain from BSE relative to Neutral (F(1, 13)=35.87, P<0.001, η2p=0.73). Most importantly, there was a GROUP*VIDEO EXERCISE interaction (F(1, 13)=32.38, P<0.001, η2p=0.71). A paired t-test between cLBP and HC revealed that the difference in expected pain between BSE and Neutral was significantly greater (t(14)=5.82, P<0.001, d=1.50) for cLBP (23.84±15.32) compared to HC (1.25±2.60). The mean and SD of the video ratings are presented in Figure 2.
Figure 2: Ratings of videos showing back straining and neutral exercises.
Patients with chronic Low Back Pain (cLBP) expected back straining exercises to be more painful than neutral exercises (A), and were more fearful of performing these exercises (B). There was a significant interaction, confirming that the difference in ratings between videos of back straining and neutral exercises was significantly larger for cLBP compared to Healthy Controls. **p<0.01; ***p<0.005.
A repeated measures ANOVA confirmed higher fear of performing the exercises depicted in the videos for cLBP relative to HC (main effect of GROUP: F(1, 13)=6.88, P=0.021, η2p=0.35). A main effect of VIDEO EXERCISE confirmed higher fearfulness of BSE relative to Neutral exercises (F(1, 13)=20.79, P<0.001, η2p=0.62). Importantly there was a GROUP*VIDEO EXERCISE interaction (F(1, 13)=12.35, P=0.004, η2p=0.49). This difference was significantly greater (t(14)=3.77, P=0.002, d=0.97) for cLBP (6.75±6.01) compared to HC (0.25±1.36).
Brain responses to the observation of back-straining relative to neutral physical exercises
Baseline group brain responses to BSE and Neutral videos, compared to rest, are shown in Suppl. Figure 1. When observing BSE, compared to Neutral exercises, cLBP patients showed higher BOLD signal in multiple regions including Visual areas 1, 4, and 5, Supramarginal Gyrus (SMG), Angular Gyrus (AngGJ, Temporoparietal Junction (TPJJ, a cluster in the Superior Parietal Sulcus (STS) / Middle Temporal Gyrus (MTG) compatible with the extrastriate body area, anterior Insula (aINS), Posterior Cingulate Cortex (PCC), anterior Mid-Cingulate Cortex (aMCC), ventrolateral (vlPFC), dorsomedial (dmPFC), and dorsolateral (dlPFC) Prefrontal Cortices, thalamus, caudate, putamen, cerebellum (Figure 3A).
Figure 3: BOLD responses to observing back straining relative to neutral exercises.
Activation maps show cluster-corrected BOLD responses (voxel-based Z-scores, prior to Spinal Manipulative Therapy) to viewing videos of back straining (BSE) relative to neutral exercises in chronic Low-Back Pain patients (cLBP, A) and age / sex matched healthy controls (HC, B), and in cLBP relative to HC (C). There were no significant voxels in the opposite contrast (HC – cLBP). Bar plots show mean Z-scores within Regions of Interest (ROIs) drawn from the [cLBPBSE-Neutral – HCBSE-Neutral] contrast, indicating the interaction was driven by increased BOLD responses to BSE videos for cLBP. Error bars represent Standard Error of the Mean. aINS = anterior Insula; vlPFC =ventrolateral Prefrontal Cortex; dlPFC = dorsolateral Prefrontal Cortex; STS = Superior Frontal Sulcus; MTG = Middle Temporal Gyrus; TPJ = Temporoparietal Junction.
The HC group also demonstrated higher BOLD signal during BSE relative to Neutral videos, however in a smaller number of regions (V5, SMG, TPJ, lateral PFC, aMCC, thalamus, putamen, and caudate; Figure 3B).
A direct group comparison (cLBP vs HC) of the BSE – Neutral contrast revealed statistically significant clusters in bilateral dlPFC, left vlPFC / alNS, left STS / MTG, left TPJ and dorsomedial PFC, (Figure 3C, top). As illustrated by extracted mean Z-scores in these regions (Figure 3C, bottom), cLBP demonstrated significant activations during observation of back-straining exercises, compared to no activation (or, in some cases, deactivation) when observing the neutral exercises. The controls, on the other hand, showed no significant activation in response to either of the videos, and little or no difference across video type. A binarized mask created from regions significant in the cLBPBSE-Neutral-HCBSE-Neutral contrast was used for the subsequent ROI-based investigation of the effects of SMT on the brain responses to observing exercises.
SMT reduced clinical pain, and expected pain and fear of performing physical exercises
Clinical pain.
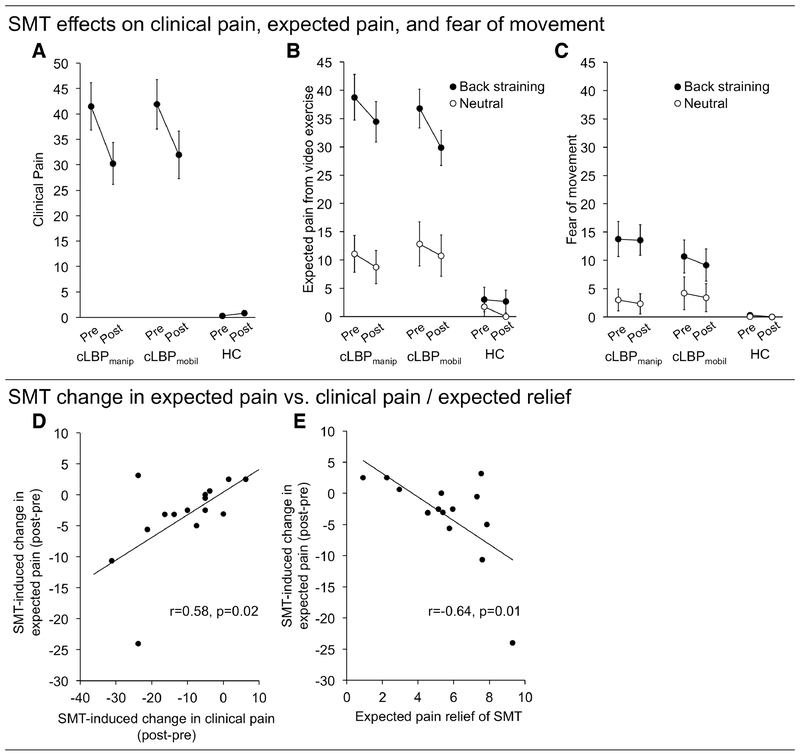
A significant ANOVA main effect of TIME (F(1, 13)=13.34, P=0.003, η2p=0.51) indicated that clinical pain was reduced after SMT (31.12±16.83) relative to the preSMT baseline (41.69±18.11). There was no main effect of SMT TECHNIQUE (F(1,13)=0.25, P=0.63, η2p=0.02) and no TIME*SMT TECHNIQUE interaction (F(1,13)=0.22, P=0.65, η2p=0.02). Thus, the data did not support the hypothesis that the effect of SMT on clinical pain is different between manipulation and mobilization (Figure 4A).
Figure 4: SMT-induced change in clinical pain and expected pain from, and fear of, performing physical exercises.
A) SMT significantly reduced clinical pain (p=0.003), but this pain change did not differ between SMTmanip and SMTmobil (p=0.65). B) SMT reduced expected pain from performing exercises in cLBP (p=0.01). This reduction was significantly stronger for back straining relative to neutral exercises (p=0.03). C) SMT reduced overall fear of performing exercises in cLBP (p=0.046). D) SMT-induced change in clinical pain (postSMT – preSMT) correlated with SMT-induced change in expected pain, such that those with the strongest pain reduction also had the strongest reduction in expected pain of performing back stressing exercises. (E) In cLBP patients, expected pain relief of SMT correlated significantly with SMT-induced change in expected pain from performing exercise (Back straining-Neutral). cLBP = chronic Low-Back Pain; HC = Healthy Control; SMT = Spinal Manipulative Therapy. *p<0.05
Expected pain.
A significant ANOVA main effect of TIME (F(1, 13)=8.99, P=0.01, η2p=0.41) indicated that expected pain from the exercises was reduced after SMT (20.94±16.97) relative to before (24.87±19.08). As hypothesized, there was a significant main effect of VIDEO EXERCISE (F(1,13)=29.85, P<0.001, η2p=0.70), with BSE (34.95±13.86) rated higher than Neutral (10.86±13.15). There was a significant TIME*VIDEO EXERCISE interaction (F(1,13)=6.28, P=0.026, η2p=0.33). In line with our hypothesis, a direct t-test indicated that the SMT-induced reduction was stronger for BSE (Δ[postSMT-preSMT]: −5.66±7.6) than for Neutral (Δ[postSMT-preSMT]: −2.21±2.27) videos (t(14)=1.99, p=0.034, Figure 4B). There was a significant SMT TECHNIQUE*SCAN ORDER interaction (F(1,13)=4.92, p=0.045, η2p=0.27). There were no significant interactions between TIME*SMT TECHNIQUE (F(1,13)=0.25, p=0.62, η2p=0.02) or between TIME*VIDEO EXERCISE*SMT TECHNIQUE (F(1,13)=1.35, p=0.27, η2p=0.09). Thus, the data did not suggest that that the effect of SMT on expected pain was different depending on the SMT technique used.
Fear of movement.
A significant main effect of TIME (F(1,13)=4.89, p=0.046, η2p=0.27) indicated fearfulness was reduced after SMT (7.13±10.47) relative to before (7.91±11.35, Figure 4C). As expected, there was also a main effect of VIDEO EXERCISE (F(1,13)=25.37, p>0.001, η2p=0.66), showing higher fearfulness ratings of BSE (11.80±11.13) relative to Neutral videos (3.24±8.82). There were no other significant main effects or interactions (all Ps>0.06). Thus, the data did not suggest that an SMT-induced reduction in tearfulness was different across different video types, or SMT techniques.
Relationship between clinical pain vs. expected pain and fearfulness.
cLBP patients’ change in clinical pain (postSMT – preSMT) correlated significantly with change in expected pain (postSMTBSE-Neutral – preSMTBSE-Neutral, r=0.58, P=0.02), indicating that patients with the greatest reduction in clinical pain following SMT also had the greatest reduction in expected pain from performing the observed back-straining exercises (Figure 4D). We did not find a statistically significant correlation between change in fearfulness and change in clinical pain (r=0.17, P=0.55).
Relationship between expected relief of SMT vs. SMT outcomes.
cLBP patients’ expected relief of SMT correlated significantly with change in clinical pain (r=0.67, P=0.006) and expected pain from performing exercise (postSMTBSE-Neutral – preSMTBSE-Neutral, r=−0.64, P=0.01). There was no significant correlation between expected relief from SMT and change in fearfulness (r=−0.22, P=0.44).
SMT reduced brain responses in circuitry involved in processing observed back-straining exercises
A whole-brain voxel-wise within-group interaction in the cLBP group (cLBPmanip[postSMT BSE-Neutral – preSMTBSE-Neutral] – cLBPmobil [postSMTBSE-Neutral – preSMTBSE-Neutral]) showed a statistically significant difference in the effect of technique (manipulation vs mobilization) in left STS, right aINS, right SI, right Superior Temporal Gyrus (STG), bilateral dlPFC, vlPFC, vmPFC, pINS, paracingulate, medial occipital cortex, and cerebellum (Figure 5A). Furthermore, a between-group interaction (cLBPmanip[postSMTBSE-Neutral – preSMTBSE-Neutral] – HC [postSMTBSE-Neutral – preSMTBSE-Neutral]) indicated the presence of a statistically significant difference in the effect of manipulation across groups (cLBP vs HC; Figure 5A) in left TPJ and bilateral aINS, vlPFC, dlPFC, STS / MTG, medial occipital cortex, and right cerebellum (Figure 5B). The examination of Z-stat values from these regions suggested that the interaction was driven by a BOLD contrast reduction following SMTmanip, when observing back-straining exercises (BSE-Neutral) for cLBP, compared to an increase for HC (Figure 5C). Exploratory contrasts showing the contrast [postsMTBSE-Neutral – preSMTBSE-Neutral] separately for cLBPmanip and cLBPmobil are shown in Suppl. Figure 2.
Figure 5: Manual therapy reduced BOLD responses to videos showing back straining relative to neutral exercises in cLBP patients.
To investigate SMT effects on BOLD responses to observing back straining exercises (BSE), we compared whole-brain contrast parameters [postSMTBSE-Neutral – preSMTBSE-Neutral] for cLBPmanip, relative to cLBPmobil and HC. cLBPmanip showed a widespread reduction of BOLD contrast relative to cLBPmobil (A) and HC (B). C) To illustrate directionality, mean Z values were extracted from ROIs where the cLBP-HC contrast overlapped with voxels that showed a stronger BOLD signal in response to BSE for cLBP relative to HC at baseline (the [cLBPBSE-Neutral– HCBSE-Neutral] contrast; see Figure 3C). Error bars represent Standard error of the mean. aINS = anterior Insula; vlPFC = ventrolateral Prefrontal Cortex; dlPFC = dorsolateral Prefrontal Cortex; STS = Superior Frontal Sulcus; MTG = Middle Temporal Gyrus; TPJ = Temporoparietal Junction; cLBP = Low Back Pain; HC=Healthy Control; Manip = Grade 5 manipulation; Mobil = Grade 3 mobilization.
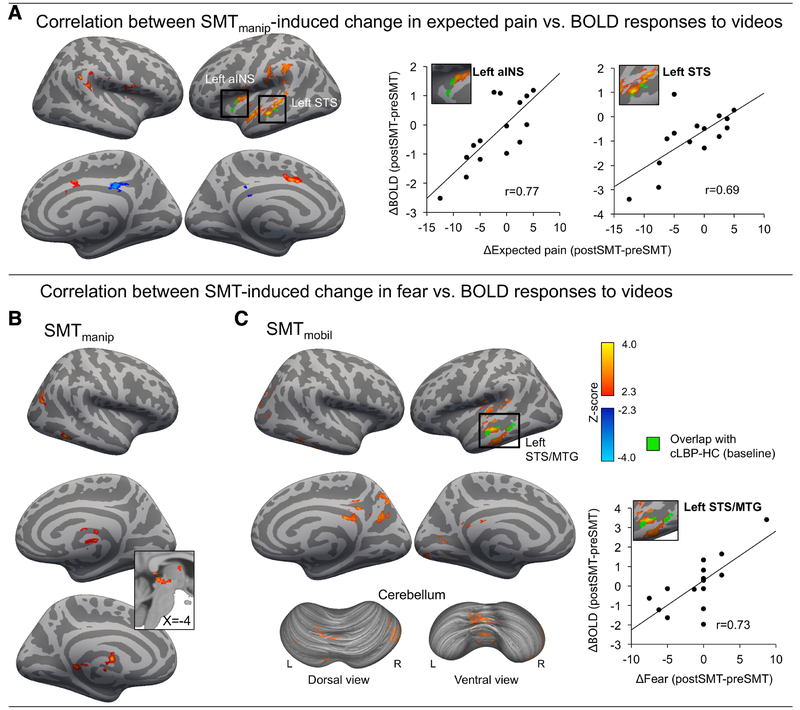
SMT-induced change in expected pain correlated with SMT-induced change in brain responses to observation of back-straining exercises
A whole-brain voxel-wise regression analysis indicated that, for cLBP manip, those patients with the largest SMT-induced reduction in expected pain from performing back-straining exercises also had the largest SMT-induced reduction in BOLD fMRI responses to videos in right TPJ, left STS / STG, left m/aINS, aMCC, and SMA (Figure 6A, left). To illustrate this relationship, we extracted mean Z-scores from two ROIs (aINS and STS) identified by the intersection of the correlation map and with the activation map from the baseline [cLBP BSE-Neutral -HCBSE-Neutral] contrast (Figure 3C), and plotted these values against SMT-induced Δexpected pain (BSE-Neutral) (Figure 6A, right). There was no significant correlation between SMT-induced change in expected pain and BOLD responses to videos for cLBPmobil. For cLBPmanip, there was a significant correlation between SMT-induced change in fear of movement and BOLD responses to videos (BSE-Neutral) in right MTG, right lateral occipital cortex, medial thalamus, and the Periaqueductal Grey matter (Figure 6B). For cLBPmobil, there was a significant correlation between SMT-induced change in fear of movement and BOLD responses to videos (BSE-Neutral) in left STS / MTG, left mid-posterior insula, posterior cingulate cortex, precuneus, medial occipital cortex, and cerebellum (Figure 6C).
Fig. 6: Correlation between SMT-induced change in BOLD responses to videos and change in expected pain from, and fear of, performing back straining exercises.
A) Activation maps (Whole-brain cluster-corrected) show brain regions where SMT-induced ΔBOLD responses (postSMT – preSMT) correlated with Δexpected pain (postSMT – preSMT). To illustrate directionality, scatterplots show ROI-extracted mean Z-scores from two regions (left aINS and left STS, marked green) identified by the intersection of the correlation map and the [cLBPBSE-Neutral–HCBSE-Neutral] activation map at baseline (both cluster-corrected significance-thresholded). There were no significant correlations with change in expected pain for SMTmobil. Furthermore, SMT-induced change in fear of movement correlated with BOLD changes elicited by SMTmanip (B) and SMTmobil (C). To illustrate directionality, ROI-extracted mean Z-scores are shown from Left STS, a region that showed an overlap with the baseline [cLBPBSE-Neutral – HCBSE-Neutral] activation map. aINS = anterior Insula; STS = Superior Temporal Sulcus, MTG = Middle Temporal Gyrus; SMT = Spinal Manipulative Therapy, BSE = Back straining Exercise videos.
Discussion
We used fMRI to investigate brain processes supporting fear of movement and anticipated pain of back-straining exercises in cLBP patients, and modulation of this brain circuitry by spinal manipulative therapy (SMT). cLBP, relative to age- and sex-matched healthy controls (HC), reported higher fear, and anticipated pain, of performing back-straining exercises depicted by observed videos, which was accompanied by increased BOLD-fMRI responses in brain circuitry involved in social processing, emotion regulation, and salience. SMT reduced clinical pain, fear of movement, and expected pain from back-straining exercises. Furthermore, reductions in fear and expected pain correlated with reductions in BOLD-responses to observing back-straining exercises. While there were no differences between SMT techniques on self-report assessment, SMTmanip was associated with stronger reduction in BOLD-responses, relative to SMTmobil and HC. These results shed light on 1) the brain processing underpinning aversive anticipation of back-straining movements in cLBP, and 2) how SMT might affect these motivational processes.
We found that cLBP patients, relative to HC, showed greater BOLD responses in vlPFC, aINS, dmPFC, dlPFC, TPJ, and STS/MTG, when observing videos of individuals performing back-straining relative to neutral exercises. Several of these regions dmPFC, aINS, and dlPFC are known nodes of the salience network7, and have been implicated in pain anticipation23, 48. These regions have also been implicated in goal formation, prediction error processing and top-down modulation of pain37, 47, 52, 72. Furthermore, vlPFC, TPJ and STS have been consistently implicated in mentalizing, theory-of-mind, and social cognition more generally18, 55, 56, 66. Finally, the STS/MTG cluster is consistent with the extrastriate Body Area, which is implicated in processing observed bodies and body parts14, 36.
Previous studies investigating fear of movement in cLBP have produced mixed results. Consistent with our findings, one recent study found that cLBP relative to HC showed increased activation of vlPFC, aINS, STG/STS, and dmPFC/ACC in response to observing images of back-straining relative to neutral activities61. Another study also found increased vlPFC and amygdala activation for videos showing back-straining versus back-neutral activities, albeit no BOLD contrast difference between cLBP and HC44. Another study found increased hippocampal activation for high-fear cLBP patients compared to HC5, while an earlier study found no patient versus HC contrast in fMRI response to still images of back-straining maneuvers4. These discrepancies may be due to protocol differences. Our study differs from earlier reports in two important ways. First, while most previous studies (except ref.61) used a pre-selected pool of aversive stimuli, we used individually tailored back-straining videos for each patient based on which exercises were most painful, maximizing the contrast between back-straining and neutral stimuli. Second, our stimuli were likely more behaviorally relevant than in previous studies, as the participants knew they would be asked to perform the same exercises depicted in the videos. This may have improved our sensitivity in recording relevant brain processes.
We did not find increased BOLD responses in circuitry typically associated with fear, such as amygdala, aMCC, and sgACC. One reason may be that, despite significantly higher cLBP fear ratings for back-straining compared to neutral videos, and compared to HC, the task did not appear to induce intense kinesiophobia, with relatively small magnitude of fear overall, and with several patients reporting no fear at all. Thus, it is still possible that amygdala/sgACC play important roles for chronic pain-related kinesiophobia in real-life situations involving stronger fear54. Moreover, previous studies have also reported limited fear and perceived aversiveness of specifically-designed “fear-evoking” visual stimuli4, 5, 44, some of which nevertheless reported increased BOLD responses in the amygdala42, 44. Future studies may use brainstem-optimized acquisition to investigate the involvement of other key structures important for fear conditioning, such as the posterior and medial hypothalamic nuclei16, 19, 64, 73, dorsal PAG62, 64 and the superior colliculus41.
Notably, we observed higher ratings of expected pain compared to fear, suggesting patients still found the observed exercises aversive, but perhaps on a more cognitive level, with limited affect. Expectations play a crucial role in pain. Expectations about whether pain will improve or worsen following an intervention can lead to hypo- or hyperalgesia, respectively2, 17, 34, 51 Importantly, expectations and beliefs about the outcome of certain actions guide behavioral decisions on whether to approach or avoid72. Interestingly, we found that initial expectations of treatment relief correlated with reduction in expected pain from back-straining exercises (Figure 3E). Fear responses likely play a crucial role in the acquisition of avoidance behavior, and are elicited in situations where such feared movements are likely to occur. Nevertheless, explicit fear may potentially be limited during abstract cognitive evaluation of the outcomes of movement – such as in the context of our study – during which avoidance decisions are often made35.
We found that SMT reduced not only patients’ clinical back pain, but also the aversiveness of the observed back-straining exercises, i.e., both fear and expected pain. Moreover, this correlated with SMT-induced reduction in BOLD responses. Specifically, patients with stronger reduction in expected pain also had stronger reduction in BOLD responses in aINS, STS/STG, aMCC, and SII (SMTmanip) and for reduction in fear and BOLD responses in STS/MTG, pINS, PCC, and cerebellum (SMTmobil). One possibility is that SMT may disrupt the association between low-back movement, fear, and pain9, 74. Within the fear-avoidance framework, SMT elicits salient sensory and proprioceptive input from the painful region (low-back), presumably followed by not only an absence of a US/UR (nociception/pain), but also a reduction of pain. This might help disrupt the association between low-back sensations and fear responses – somewhat reminiscent of exposure therapy – which may in turn reduce the aversiveness of back-straining exercises67. Alternatively, the reduction in fear and expected pain could be a direct consequence of reduced clinical pain, as people are more aversive to movements when in more pain15, 63. If so, we would expect a similar reduction regardless of the location or mode of analgesia13. Future studies should systematically compare these motivational aspects during pain relief from treatments involving exteroceptive/proprioceptive stimuli of the painful limb (such as SMT for cLBP) versus treatment that does not (e.g. pharmacotherapy). According to the fear-avoidance model, fear learning is a key component of chronification of pain (and emerging avoidance behavior), and ‘unlearning’ – e.g. through exposure to motion – is suggested as a key mechanism to reduce avoidance behavior and disability20, 67. Pharmacological treatment alone has limited prolonged efficacy for chronic pain3, potentially due to an inability to target the deeply engrained association between pain anticipation, fear, physical maneuvers, and pain21, 24. SMT, either as monotherapy or potentially in combination with psychological therapy such as cognitive behavioral therapy (CBT), may have the advantage of targeting this learning aspect of chronic pain, along with other putative mechanisms such as counter-irritation and improved local circulation due to reduced muscle tension8, 38.
We did not find evidence that different techniques of SMT had a differential effect on clinical pain, fear of movement, or expected pain. Previous studies have similarly not found notable differences in efficacy between techniques11, 22, 39, 49, 53. However, we did find differences between techniques in brain responses to the observations of back-straining exercises. cLBP relative to HC showed widespread reductions in BOLD responses to back-straining videos after SMTmanip, notably in circuitry identified in the baseline contrast, such as dlPFC, aINS, vlPFC, TPJ, and STS/MTG. Direct comparison indicated that, for cLBP, SMTmanip induced stronger BOLD contrast reductions in dlPFC, vlPFC, aINS, posterior STS/STG, and dmPFC, relative to SMTmobil. Taken together, these results suggest that in cLBP patients, SMTmanip induced stronger reduction of BOLD responses in circuitry involved in processing observed back-straining relative to neutral exercises, compared to SMTmobil. This is in line with our hypothesis that SMTmanip, which involves greater amplitude of manipulation directed to the spine joints, would elicit stronger effects on clinical outcomes. However, the lack of a difference in subjective reports warrants a more cautious interpretation.
There are several limitations in our study. First, we assessed outcomes from only a single session of SMTmanip and SMTmobil. Longitudinal studies involving multiple sessions may better parse SMT-induced changes in brain responses with clinically relevant outcomes. Second, we did not observe group differences (cLBP/HC) in trait kinesiophobia45. Importantly however, cLBP showed significantly higher fear and expected pain from observed back-straining exercises compared to neutral, which is more central for testing our hypotheses. Furthermore, we observed similar trait kinesiophobia scores as previous studies4, 5, 43, 44. Notably, previous studies have also shown no differences in trait kinesiophobia between chronic pain samples and HC44, highlighting the possibility that generalized trait kinesiophobia might not be as relevant for chronic pain as situational kinesiophobia that is being acquired through individualized history of movement, fear, and pain. Another limitation is that the sample size, while similar to those of many other fMRI studies, was relatively limited and may be susceptible to type II errors. As such, this study should be followed up by replication in larger samples. This would also allow more advanced (e.g., mediation and moderation) analyses aimed at more directly evaluating the possible causal relationship between brain and behavioral changes induced by SMT.
In conclusion, we found that observation of back-straining exercises was associated with increased fear and expected pain of performance in cLBP compared to HC, and elicited increased BOLD responses in vlPFC, aINS, dlPFC, dmPFC, TPJ, and STS/MTG. In cLBP patients, both SMTmanip and SMTmobil reduced both clinical pain and aversiveness (fear and expected pain) of observed back-straining exercises. Although the two techniques did not differentially affect these clinical outcomes, SMTmanip relative to SMTmobil elicited stronger overall reduction of brain circuitry involved in appraisal of observed back-straining exercises. Importantly, stronger reduction in averseness (fear and expected pain) was associated with stronger reduction in BOLD responses in this circuitry to observing back-straining exercises. Potentially, SMT might modulate the aversiveness of performing back-straining maneuvers through disruption of the association between these (exteroceptive and proprioceptive) sensations and fear/pain. Future studies should address the effect of therapies such as SMT on motivational aspects and avoidance behavior more directly, and include multiple sessions of therapy.
Supplementary Material
Highlights.
Patients with cLBP show higher BOLD signal when observing physical exercises
SMT reduces clinical pain, expected pain, and fear of movement in cLBP
SMT manipulation and mobilization equally improves clinical outcomes
SMT reduces brain responses to observing back-straining exercises in cLBP
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: This work was supported by the NCMIC foundation (M.L.L.), Norwegian Research Council /Marie Sklodowska-Curie Actions (FRICON/COFUND-240553/F20 to D.M.E.), the National Center for Research Resources (P41RR14075; CRC 1 UL1 RR025758, Harvard Clinical and Translational Science Center); Martinos Computing facilities; NIH S10RR023401; S10RR019307; S10RR019254; S10RR023043. None of the authors have any conflicts of interest.
References
- 1.Andersson J, Jenkinson M, Smith S: Non-linear registration, aka spatial normalisation, FMRIB technical report TR07JA2, 2010. [Google Scholar]
- 2.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. JNeurosci. 30:12964–12977, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballantyne JC, Mao J. Opioid therapy for chronic pain. NEnglJMed. 349:1943–1953, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Barke A, Baudewig J, Schmidt-Samoa C, Dechent P, Kroner-Herwig B. Neural correlates of fear of movement in high and low fear-avoidant chronic low back pain patients: an event-related fMRI study. Pain. 153:540–552, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Barke A, Preis MA, Schmidt-Samoa C, Baudewig J, Kroner-Herwig B, Dechent P. Neural Correlates Differ in High and Low Fear-Avoidant Chronic Low Back Pain Patients When Imagining Back-Straining Movements. J Pain. 17:930–943, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Beck AT, Steer RA, Brown GK: Beck depression inventory-II.(Corporation P, Ed.), San Antonio, TX, 1996. [Google Scholar]
- 7.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc LondBBiolSci. 360:1001–1013, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 14:531–538, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop MD, Torres-Cueco R, Gay CW, Lluch-Girbes E, Beneciuk JM, Bialosky JE. What effect can manual therapy have on a patient’s pain experience? Pain Manag. 5:455–464, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou R, Shekelle P. Will This Patient Develop Persistent Disabling Low Back Pain? JAMA. 303:1295–1302, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Cleland JA, Glynn P, Whitman JM, Eberhart SL, MacDonald C, Childs JD. Short-term effects of thrust versus nonthrust mobilization/manipulation directed at the thoracic spine in patients with neck pain: a randomized clinical trial. Phys Ther. 87:431–440, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Cook AJ, Brawer PA, Vowles KE. The fear-avoidance model of chronic pain: validation and age analysis using structural equation modeling. Pain. 121:195–206, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Coronado RA, Gay CW, Bialosky JE, Carnaby GD, Bishop MD, George SZ. Changes in pain sensitivity following spinal manipulation: a systematic review and meta-analysis. J Electromyogr Kinesiol. 22:752–767, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costantini M, Urgesi C, Galati G, Romani GL, Aglioti SM. Haptic perception and body representation in lateral and medial occipito-temporal cortices. Neuropsychologia. 49:821–829, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Crombez G, Eccleston C, Van Damme S, Vlaeyen JW, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain. 28:475–483, 2012 [DOI] [PubMed] [Google Scholar]
- 16.dos Anjos-Garcia T, Ullah F, Falconi-Sobrinho LL, Coimbra NC. CB1 cannabinoid receptor-mediated anandamide signalling reduces the defensive behaviour evoked through GABA(A) receptor blockade in the dorsomedial division of the ventromedial hypothalamus. Neuropharmacology. 113:156–166, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Ellingsen DM, Wessberg J, Eikemo M, Liljencrantz J, Endestad T, Olausson H, Leknes S. Placebo improves pleasure and pain through opposite modulation of sensory processing. Proc Natl Acad Sci U S A. 110:17993–17998, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 16:693–700, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Falconi-Sobrinho LL, dos Anjos-Garcia T, Elias DH, Coimbra NC. Unravelling cortico-hypothalamic pathways regulating unconditioned fear-induced antinociception and defensive behaviours. Neuropharmacology. 113:367–385, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Fordyce WE, Shelton JL, Dundore DE. The modification of avoidance learning pain behaviors. JBehavMed. 5:405–414, 1982 [DOI] [PubMed] [Google Scholar]
- 21.Gatzounis R, Schrooten MG, Crombez G, Vlaeyen JW. Operant learning theory in pain and chronic pain rehabilitation. Curr Pain Headache Rep. 16:117–126, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Gay CW, Robinson ME, George SZ, Perlstein WM, Bishop MD. Immediate changes after manual therapy in resting-state functional connectivity as measured by functional magnetic resonance imaging in participants with induced low back pain. J Manipulative Physiol Ther. 37:614–627, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geuter S, Koban L, Wager TD. The Cognitive Neuroscience of Placebo Effects: Concepts, Predictions, and Physiology. Annu Rev Neurosci. 40:167–188, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Giles LG, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine. 28:1490–1502; discussion 1502-1493, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Glombiewski JA, Riecke J, Holzapfel S, Rief W, Konig S, Lachnit H, Seifart U. Do patients with chronic pain show autonomic arousal when confronted with feared movements? An experimental investigation of the fear-avoidance model. Pain. 156:547–554, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 48:63–72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvie DS, Moseley GL, Hillier SL, Meulders A. Classical Conditioning Differences Associated With Chronic Pain: A Systematic Review. J Pain. 18:889–898, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Hengeveld E, Banks K, (Eds.): Maitland’s Vertebral Manipulation: Management of Neuromusculoskeletal Disorders, Churchill-Livingstone-Elsevier, 2014. [Google Scholar]
- 29.Houben RM, Leeuw M, Vlaeyen JW, Goubert L, Picavet HS. Fear of movement/injury in the general population: factor structure and psychometric properties of an adapted version of the Tampa Scale for Kinesiophobia. JBehavMed. 28:415–424, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Fast Jenkinson M., automated, N-dimensional phase-unwrapping algorithm. Magnetic Resonance in Medicine. 49:193–197, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson M: Improving the registration of B0-distorted EPI images using calculated cost function weights. In: Int. Conf. on Human Brain Mapping (HBM), Budapest, Hungary, 2004. [Google Scholar]
- 32.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17:825–841, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 5:143–156, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci U S A. 109:15959–15964, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirsch I, Lynn SJ, Vigorito M, Miller RR. The role of cognition in classical and operant conditioning. J Clin Psychol. 60:369–392, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Kitada R, Yoshihara K, Sasaki AT, Hashiguchi M, Kochiyama T, Sadato N. The Brain Network Underlying the Recognition of Hand Gestures in the Blind: The Supramodal Role of the Extrastriate Body Area. JNeurosci. 34:10096–10108, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci. 38:86–95, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Lascurain-Aguirrebena I, Newham D, Critchley DJ. Mechanism of Action of Spinal Mobilizations: A Systematic Review. Spine. 41:159–172, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Leaver AM, Maher CG, Herbert RD, Latimer J, McAuley JH, Jull G, Refshauge KM. A randomized controlled trial comparing manipulation with mobilization for recent onset neck pain. Arch Phys Med Rehabil. 91:1313–1318, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Lethem J, Slade PD, Troup JD, Bentley G. Outline of a Fear-Avoidance Model of exaggerated pain perception--I. Behav Res Ther. 21:401–408, 1983 [DOI] [PubMed] [Google Scholar]
- 41.Li L, Feng XL, Zhou Z, Zhang HQ, Shi QQ, Lei ZG, Shen PL, Yang QN, Zhao BH, Chen SR, Li L, Zhang YL, Wen PJ, Lu ZH, Li X, Xu FQ, Wang LP. Stress Accelerates Defensive Responses to Looming in Mice and Involves a Locus Coeruleus-Superior Colliculus Projection. Current Biology. 28:859-+, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Meier ML, Stämpfli P, Humphreys BK, Vrana A, Seifritz E, Schweinhardt P. The impact of pain-related fear on neural pathways of pain modulation in chronic low back pain. PAIN Reports. 2:e601, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier ML, Stampfli P, Vrana A, Humphreys BK, Seifritz E, Hotz-Boendermaker S. Fear avoidance beliefs in back pain-free subjects are reflected by amygdala-cingulate responses. Front Hum Neurosci. 9:424, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meier ML, Stampfli P, Vrana A, Humphreys BK, Seifritz E, Hotz-Boendermaker S. Neural Correlates of Fear of Movement in Patients with Chronic Low Back Pain vs. Pain-Free Individuals. Front Hum Neurosci. 10:386, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller RP, Kori SH, Todd DD. The Tampa Scale: A measure of kinisophobia. Clin J Pain. 7:51, 1991 [Google Scholar]
- 46.Moseley GL, Vlaeyen JW. Beyond nociception: the imprecision hypothesis of chronic pain. Pain. 156:35–38, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Onat S, Buchel C. The neuronal basis of fear generalization in humans. Nat Neurosci. 18:1811–1818, 2015 [DOI] [PubMed] [Google Scholar]
- 48.Palermo S, Benedetti F, Costa T, Amanzio M. Pain anticipation: An activation likelihood estimation meta-analysis of brain imaging studies. Hum Brain Mapp. 36:1648–1661, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penza CW, Horn ME, George SZ, Bishop MD. Comparison of 2 Lumbar Manual Therapies on Temporal Summation of Pain in Healthy Volunteers. J Pain. 18:1397–1408, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rainville J, Smeets RJ, Bendix T, Tveito TH, Poiraudeau S, Indahl AJ. Fear-avoidance beliefs and pain avoidance in low back pain--translating research into clinical practice. Spine J. 11:895–903, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Reicherts P, Gerdes AB, Pauli P, Wieser MJ. Psychological Placebo and Nocebo Effects on Pain Rely on Expectation and Previous Experience. J Pain. 17:203–214, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Roy M, Shohamy D, Daw N, Jepma M, Wimmer GE, Wager TD. Representation of aversive prediction errors in the human periaqueductal gray. Nat Neurosci. 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salom-Moreno J, Ortega-Santiago R, Cleland JA, Palacios-Cena M, Truyols-Dominguez S, Fernandez-de-las-Penas C. Immediate changes in neck pain intensity and widespread pressure pain sensitivity in patients with bilateral chronic mechanical neck pain: a randomized controlled trial of thoracic thrust manipulation vs non-thrust mobilization. J Manipulative Physiol Ther. 37:312–319, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Salomons TV, Davis KD. Fear avoidance and neuroimaging: falsification or just failure to confirm? Pain. 153:511–512, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev. 42:9–34, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Schurz M, Tholen MG, Perner J, Mars RB, Sallet J. Specifying the brain anatomy underlying temporo-parietal junction activations for theory of mind: A review using probabilistic atlases from different imaging modalities. Hum Brain Mapp. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherman KJ, Hogeboom CJ, Cherkin DC, Deyo RA. Description and validation of a noninvasive placebo acupuncture procedure. J Altern Complement Med. 8:11–19, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 17:143–155, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment. 7:524–532, 1995 [Google Scholar]
- 60.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 5:133–137, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Taylor AM, Harris AD, Varnava A, Phillips R, Taylor JO, Hughes O, Wilkes AR, Hall JE, Wise RG. A Functional Magnetic Resonance Imaging Study to Investigate the Utility of a Picture Imagination Task in Investigating Neural Responses in Patients with Chronic Musculoskeletal Pain to Daily Physical Activity Photographs. PLoS One. 10:e0141133, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. JNeurosci. 22:2748–2752, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trost Z, France CR, Thomas JS. Exposure to movement in chronic back pain: evidence of successful generalization across a reaching task. Pain. 137:26–33, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Ullah F, dos Anjos-Garcia T, dos Santos IR, Biagioni AF, Coimbra NC. Relevance of dorsomedial hypothalamus, dorsomedial division of the ventromedial hypothalamus and the dorsal periaqueductal gray matter in the organization of freezing or oriented and non-oriented escape emotional behaviors. BehavBrain Res. 293:143–152, 2015 [DOI] [PubMed] [Google Scholar]
- 65.Van Essen DC. Windows on the brain: the emerging role of atlases and databases in neuroscience. Curr Opin Neurobiol. 12:574–579, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Van Overwalle F A dissociation between social mentalizing and general reasoning. Neuroimage. 54:1589–1599, 2011 [DOI] [PubMed] [Google Scholar]
- 67.Vlaeyen JW. Learning to predict and control harmful events: chronic pain and conditioning. Pain. 156 Suppl 1:S86–93, 2015 [DOI] [PubMed] [Google Scholar]
- 68.Vlaeyen JW, Kole-Snijders AM, Boeren RG, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 62:363–372, 1995 [DOI] [PubMed] [Google Scholar]
- 69.Vlaeyen JW, Kole-Snijders AM, Rotteveel AM, Ruesink R, Heuts PH. The role of fear of movement/(re)injury in pain disability. J Occup Rehabil. 5:235–252, 1995 [DOI] [PubMed] [Google Scholar]
- 70.Vlaeyen JW, Morley S, Linton SJ, Boersma K, de Jong J: Pain-related fear: exposure based treatment for chronic pain, IASP Press, Seattle, 2012. [Google Scholar]
- 71.Wertli MM, Rasmussen-Barr E, Weiser S, Bachmann LM, Brunner F. The role of fear avoidance beliefs as a prognostic factor for outcome in patients with nonspecific low back pain: a systematic review. Spine J. 14:816–836 e814, 2014 [DOI] [PubMed] [Google Scholar]
- 72.Wiech K, Tracey I. Pain, decisions, and actions: a motivational perspective. FrontNeurosci. 7:46, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilent WB, Oh MY, Buetefisch CM, Bailes JE, Cantella D, Angle C, Whiting DM. Induction of panic attack by stimulation of the ventromedial hypothalamus. J Neurosurg. 112:1295–1298, 2010 [DOI] [PubMed] [Google Scholar]
- 74.Zusman M Mechanisms of Musculoskeletal Physiotherapy. Physical Therapy Reviews. 9:39–49, 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.