Aflatoxin B1 (AFB1), one of most potent and common mycotoxins in human food and animal feed, has hepatotoxic and carcinogenic effects on humans and poultry.
Aflatoxin B1 (AFB1), one of most potent and common mycotoxins in human food and animal feed, has hepatotoxic and carcinogenic effects on humans and poultry.
Abstract
Aflatoxin B1 (AFB1), one of most potent and common mycotoxins in human food and animal feed, has hepatotoxic and carcinogenic effects on humans and poultry. Recent studies indicated that selenium (Se) has a protective effect on apoptosis induced by toxin poisoning. The present study was designed to reveal the ameliorative effects of selenium on the expression of apoptosis related molecules in the jejunum of broilers exposed to an AFB1 diet for 3 weeks. A total of 216 one-day-old healthy Cobb broilers were randomly divided into the control group (0 mg kg–1 AFB1), AFB1 group (0.6 mg kg–1 AFB1), AFB1 + Se group (0.6 mg kg–1 AFB1 + 0.4 mg kg–1 supplement Se) and Se group (0.4 mg kg–1 supplement Se), respectively. TUNEL and flow cytometry assays both indicated that 0.4 mg kg–1 selenium could ameliorate excess apoptosis caused by AFB1 in jejunal cells. Moreover, the expressions of FAS, FASL, TNF-α, TNF-R1, CASPASE-3, CASPASE-8, CASPASE-10, GRP78 and GRP94 analyzed by qRT-PCR demonstrated that 0.4 mg kg–1 selenium restored these parameters to be close to those in the control group. In summary, supplementation of selenium at a concentration of 0.4 mg kg–1 selenium could protect the chicken's jejunum from excess apoptosis caused by 0.6 mg kg–1 AFB1 through down-regulating the expression of death receptor pathway and endoplasmic reticulum pathway related molecules. According to this conclusion, this study may contribute to a better understanding of selenium's protective role against AFB1 poisoning.
Introduction
Aflatoxin B1 (AFB1), synthesized by the toxigenic fungi Aspergillus flavus and Aspergillus parasiticus, has strong hepatotoxic and carcinogenic effects on humans and poultry.1,2 This toxin is a highly unsaturated molecule which can be metabolized by cytochrome P-450 enzymes to reactive exo-8,9-epoxides3,4 that can induce the formation of DNA adducts and subsequently hepatocellular carcinoma in humans.5,6 Due to this, AFB1 was classified by IARC as a carcinogenic substance.7
Apoptosis is one of the several types of programmed cell death and is characterized by a series of morphological changes, including nuclear condensation and fragmentation, as well as plasma membrane blebbing, which lead to the formation of apoptotic bodies.8 AFB1 can activate ROS generation, and subsequently lead to the impairment of mitochondrial functions, resulting in apoptosis.9 Early research has shown that AFB1 caused apoptosis of hepatocytes, thymocytes, jejunal mucosal cells and the bursa of Fabricius cells.10–13
Selenium is an important micronutrient for humans and animals, and selenium deficiency is associated with several disease conditions.14 In humans and animals, cell proliferation and death must be regulated to maintain tissue homeostasis.15 With the biological functions of selenium, including anticarcinogenic activity, protection against oxidant damage or aging, and even a role in reproduction and detoxicity, selenium promotes cell cycle progression and prevents cell death at nutritional doses.16 According to some early reports, sodium selenite exhibited protective effects on AFB1-induced splenic toxicity,17 lesions of the thymus and jejunum,11,12 as well as reduction of the hepatic dysfunction and apoptosis caused by AFB1.18
The jejunum, with a strong ability for digestion and absorption, accounts for a large part of the small intestine. Since the integrity of the jejunum structure is closely associated with its function, any pathological damage of the jejunum will cause dysfunction and affect the ability of digestion and absorption.19 Our previous study revealed that AFB1 can cause pathological changes of the jejunum and may regulate apoptosis through the death receptor and endoplasmic reticulum pathways,20 while Peng X et al. suggested that supplementation of dietary sodium selenite at a concentration of 0.4 mg kg–1 Se may ameliorate AFB1-induced apoptosis through the mitochondrial pathway.12 However, it is still necessary to elucidate whether selenium has protective effects on the jejunum of AFB1-intoxicated broilers by the death receptor and endoplasmic reticulum pathways.
Therefore, this study aimed at exploring the ameliorative effects of selenium on the jejunum of AFB1-intoxicated broilers by detection of the expression of apoptotic molecules related to the death receptor and endoplasmic reticulum pathways, based on the TUNEL assay, flow cytometry and quantitative real-time PCR tests. The results from the present study would provide a reference for the protective effects of selenium against AFB1-caused apoptosis on jejunual cells and may contribute to the detoxification of feed contaminated by AFB1.
Materials and methods
Experimental diet
A total of 216 one-day-old healthy Cobb broilers were purchased from Chia Tai Group (Wenjiang, Sichuan, China), and randomly divided into the control group (0 mg kg–1 AFB1), AFB1 group (0.6 mg kg–1 AFB1), AFB1 + Se group (0.6 mg kg–1 AFB1 + 0.4 mg kg–1 supplement Se) and Se group (0.4 mg kg–1 supplement Se), respectively. 1% feed-grade sodium selenite was mixed into the control diet to formulate Se and AFB1 + Se diets containing 0.4 mg kg–1 Se supplement by a stepwise dilution method. AFB1 was purchased from Sigma-Aldrich (USA, A6636). The basal diet, namely the control diet, was formulated according to the National Research Council (NRC, 1994)21 and Chinese Feeding Standard of Chicken (NY/T33-2004) recommendations (Table 1). The AFB1-contaminated diet was made according to the method described by Kaoud.22 Briefly, 27 mg AFB1 was dissolved into 30 ml methanol, and then a 30 ml mixture was added into 45 kg corn–soybean basal diet to formulate the AFB1 and AFB1 + Se diets, respectively. The equivalent methanol was added into the corn–soybean basal diet to formulate the control diet. Then the methanol in the diets was evaporated at 98 °F (37 °C). Our previous studies demonstrated that 0.6 mg kg–1 AFB1 in diet had obvious adverse effects on broilers, and an appropriate level of Se supplied in the diet (0.4 mg kg–1) could provide optimal protective effects against AFB1-induced toxicity in broilers.12,23 Based on this information, toxin concentrations (0.6 mg kg–1 AFB1) and dietary Se level (0.4 mg kg–1) were chosen. Chickens were fed in cages with electrically heated units and provided with water as well as the aforementioned diet ad libitum for 21 days. The animal protocols used in this work and all procedures of the experiment were performed based on the laws and guidelines of Sichuan Agricultural University Animal Care and Use Committee (Approval No: 2012-024).
Table 1. The composition of the basal diet.
| Composition | Content (%) | Nutrient | Content (%) |
| Corn | 51.95 | Crude protein (CP) | 21.5 |
| Soybean | 39.5 | Methionine (Met) | 0.5 |
| Rapeseed oil | 4.1 | Calcium (Ca) | 1 |
| dl-Methionine | 0.2 | All phosphorus (P) | 0.7 |
| Calcium hydrogen phosphate | 1.85 | Methionine + Cystine (Met + Cys) | 0.84 |
| Calcium carbonate | 1.3 | Lysine (Lys) | 1.15 |
| Sodium chloride | 0.4 | Threonine (Thr) | 0.83 |
| Trace element premix a | 0.5 | Metabolizable energy (ME) (MJ kg–1) | 12.52 |
| Choline | 0.17 | ||
| Multivitamins b | 0.03 | ||
| Total | 100 |
aTrace element premix (mg kg–1): FeSO4·7H2O, 530; CuSO4·5H2O, 30; MnSO4·H2O, 400; ZnSO4·7H2O, 470; KI, 18; NaSeO3, 0.3.
bMultivitamins: Vitamin A, 13 500 IU kg–1; Vitamin D, 3000 IU kg–1; Vitamin E, 24 IU kg–1; Vitamin K3, 3 mg kg–1; pantothenic acid, 15 mg kg–1; folic acid, 1.05 mg kg–1; nicotinamide, 30 mg kg–1; biotin, 0.14 mg kg–1.
Body weight and microscopic quantitative analysis
During the period of experiment, six broilers in each group were randomly chosen and euthanized at 7, 14 and 21 days of age. The body of broilers was weighed and recorded. Then the jejunum (the midpoint between the bile duct entry and Meckel's diverticulum) was immediately fixed in 4% paraformaldehyde. After 24 h for fixation, tissues were dehydrated in alcohol, embedded with paraffin, sectioned at 5 μm, and stained with haematoxylin and eosin (HE) for histological observation. The histological structures of the tissues were observed and photographed with a digital camera (Nikon, DS-Ril, Japan). Microscopic quantitative analysis was carried out as follows: altogether ten measurements were taken per broiler for each parameter in the jejunum stained with HE using Image-Pro Plus 5.1 (USA) image analysis software. The following parameters were determined: the number of absorptive cells (the absorptive cell number per 100 μm along the middle axis of the villus), villus height (the length from the top of the villus to the crypt mouth), villus area {the villus height multiplying villus width (the width of the middle of the villus)}, crypt depth (the length from the crypt mouth to the crypt base following the crypt lumen) and the ratio of the villus height and crypt depth (villus/crypt).
TUNEL assay
TUNEL assay was carried out according to the manufacturer's instruction of the Apoptosis Detection Kit (Boster Corporation, China, MK1020). Briefly, the jejunal paraffin sections were dewaxed with 100% xylene, and rehydrated in successive changes of 100%, 95%, 85% and 75% ethanol. After endogenous peroxidase activity was quenched for 10 min in 3% H2O2 with distilled water at 37 °C, the sections were incubated with proteinase K diluted 1 : 200 in TBS at 37 °C for 5–10 min in a humid chamber. A labeling mixture containing digoxin-dUTP in Terminaldeoxynucleotidyl Transferase (TdT) enzyme buffer was added to the sections and incubated at 37 °C for 2 h. After three successive washings with TBS for 2 min, sections were covered with the anti-digoxin–biotin conjugate diluted 1 : 100 in blocking regent and incubated for 30 min at 37 °C. The tissues were then incubated for 1 h at 37 °C with streptavidin biotin complex (SABC, Boster Corporation, China) diluted 1 : 100 in TBS. Labeling was visualized with 3′3′-diaminobenzidene. The sections were then counterstained with haematoxylin. For the negative control, representative sections were processed in the same way, while incubation with TdT enzyme buffer was omitted. The number of TUNEL-positive cells was evaluated according to the following method. Briefly, photographs of TUNEL staining were taken with a digital microscope camera system (Nikon DS-Ri1, Japan). For each section, five fields of 0.064 mm2 from each area of the image (corresponding to five fields at 400× magnification) were analyzed using computer-assisted image-Pro Plus 5.1 (USA) image analysis software. By selecting the ‘colour-chosen target’ in the option bar of the morphologic analysis system, all TUNEL-positive cells in the field were marked in colour. Then, ‘calculating’ in the option bar was selected to automatically calculate the number.
Cell apoptosis analysis by flow cytometry
At 7, 14, and 21 days of the experiment, six broilers in each group were euthanized, and jejuna were sampled from each chicken to determine the percentage of apoptotic cells with a flow cytometer, similar to the method reported by Chen.24 Briefly, the dissected jejuna were thereupon homogenized to form a cell suspension and filtered, then the cells were washed and resuspended in phosphate buffer at a concentration of 1 × 106 cells per mL. 5 μL Annexin V-fluorescein isothiocyanate (V-FITC) and 5 μL propidium iodide (PI) were added into 100 μL cell suspension, and incubated at 25 °C for 15 min in the dark. 400 μL 1× Annexin binding buffer was added to the mixture, and then the apoptotic cells were assayed with a flow cytometer (BD FACSCalibur) within 1 h. The annexin V-FITC kit was obtained from BD Pharmingen (USA, 556547).
Quantitative real-time PCR (qRT-PCR) analysis
The jejunal mucosae from six broilers in each treatment at 7, 14, and 21 days of the experiment were stored in liquid nitrogen, respectively. Adding liquid nitrogen, the jejunal mucosae were crushed with a pestle to homogenize until powdery, respectively. Total RNA was extracted from the powder of jejunal mucosae using RNAiso Plus (9108/9109, Takara, Otsu, Japan). The mRNA was then reversely transcribed into cDNA using the Prim Script™ RT reagent kit with gDNA Eraser (RR047A, Takara, Otsu, Japan). The cDNA was used as a template for quantitative real-time PCR analysis. For qRT-PCR reactions, 25 μL mixtures were prepared by using SYBR® Premix Ex Taq™ II (DRR820A, Takara, Otsu, Japan), containing 12.5 μL Tli RNaseH Plus, 1.0 μL of forward and 1.0 μL of reverse primer, 8.5 μL RNAasefree water and 2 μL cDNA. Reaction conditions were set to 3 min at 95 °C (first segment, one cycle), 10 s at 95 °C and 30 s at Tm of a specific primer pair (second segment, 44 cycles) followed by 10 s at 95 °C, and 72 °C for 10 s (dissociation curve segment) using a Thermal Cycler (C1000, BIO RAD, CA, USA). The expression of FAS, FASL, TNF-α, TNF-R1, GRP78, GRP94, CASPASE-3, CASPASE-8 and CASPASE-10 mRNAs was analyzed, and β-actin was used as an internal control gene. A sequence of primers was obtained from GenBank of NCBI. Primers were designed with Primer 5, and synthesized by Sangon Biotech (Shanghai, China) (Table 2). The control broiler responses (mRNA amount) were used as reference values for between treatments comparisons within the same control day in each week, respectively. The results were analyzed with the 2–ΔΔCt calculation method.25
Table 2. Primer sequences, corresponding accession numbers and sizes of the amplification products.
| Gene | Primer | Sequences (5′–3′) | Product size (bp) | Accession number |
| CASPASE-3 | F | TGGCCCTCTTGAACTGAAAG | 139 | NM_204725 |
| R | TCCACTGTCTGCTTCAATACC | |||
| CASPASE-8 | F | GTCTCCGTTCAGGTATCTGCT | 143 | NM_204592 |
| R | TCTCAATGAAAACGTCCGGC | |||
| CASPASE-10 | F | CTGGGGGCTCCAAAAGTCC | 204 | XM_421936 |
| R | AAAGGGGGACAAAGCCAACA | |||
| FAS | F | TCCACCTGCTCCTCGTCATT | 78 | NM_001199487 |
| R | GTGCAGTGTGTGTGGGAACT | |||
| FASL | F | GGCATTCAGTACCGTGACCA | 78 | NM_001031559 |
| R | CCGGAAGAGCACATTGGAGT | |||
| GRP78 | F | GGTGTTGCTTGATGTGTGTCC | 134 | NM_205491 |
| R | GCTGATTGTCAGAAGCTGTGG | |||
| GRP94 | F | TGACCTGGATGCAAAGGTGGA | 250 | NM_204289 |
| R | TTAAACCCCACACCATCCCTCAAC | |||
| TNF-α | F | TCAGACCAGATGGGAAGGGA | 127 | AY765397 |
| R | ACTGGGCGGTCATAGAACAG | |||
| TNF-R1 | F | CCTGTCTGTCTTCCCTGTCC | 120 | NM_001030779 |
| R | GGTGCATGGGGTCTTTTCTA | |||
| β-Actin | F | TGCTGTGTTCCCATCTATCG | 178 | L08165 |
| R | TTGGTGACAATACCGTGTTCA |
Statistical analysis
The results were expressed as mean ± standard deviation (X ± SD). Through SPSS 20.0 software (IBM Corp, Armonk, NY, USA) for windows, statistical analyses were performed using one-way analysis of variance or t test, and Dunnett T3 was employed for multiple comparisons. Statistically significant differences were considered at p < 0.05 and marked significance was considered at p < 0.01.
Results
Body weight and microscopic quantitative analysis
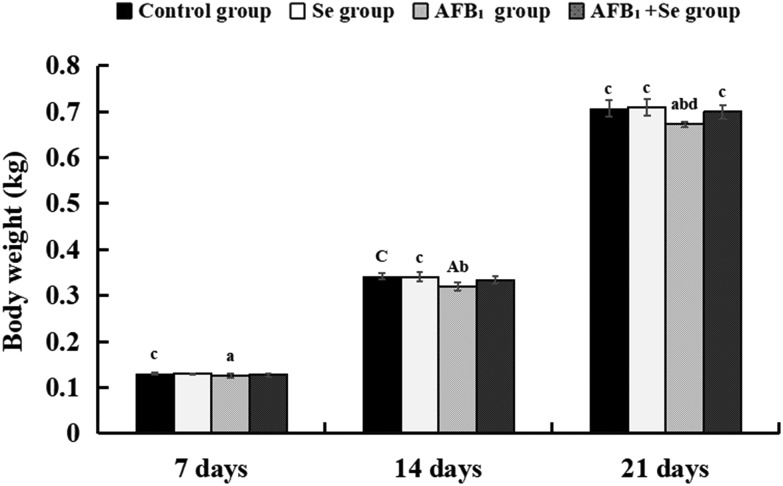
The body weight of broilers in the AFB1 group was significantly decreased at 7, 14 and 21 days of age (p < 0.05 or p < 0.01) when compared with the control group. Compared with the AFB1 group, only at 21 day of age, the body weight of broilers in the AFB1 + Se group was significantly decreased (p < 0.05). Besides, there were no significant differences in these values between the Se group and the control group (p > 0.05) (Fig. 1).
Fig. 1. The body weight of broilers at 7, 14 and 21 days of age. Note: Data are presented with the means ± standard deviation (n = 6). Letters A, B, C and D represent the significant difference (p < 0.01) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively. Letters a, b, c, and d represent the difference (p < 0.05) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively.
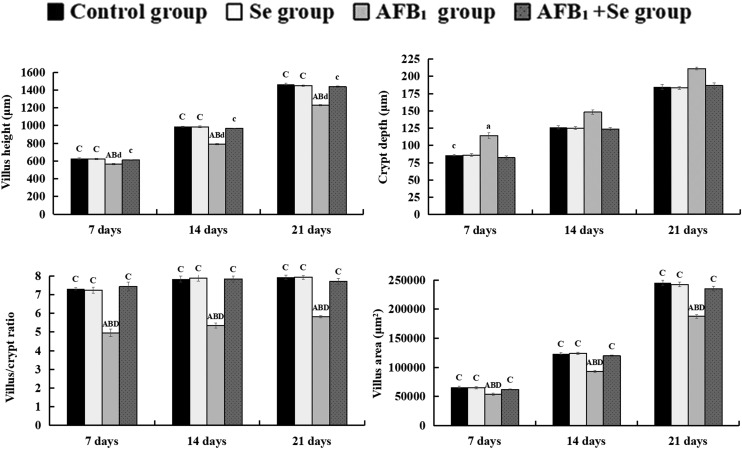
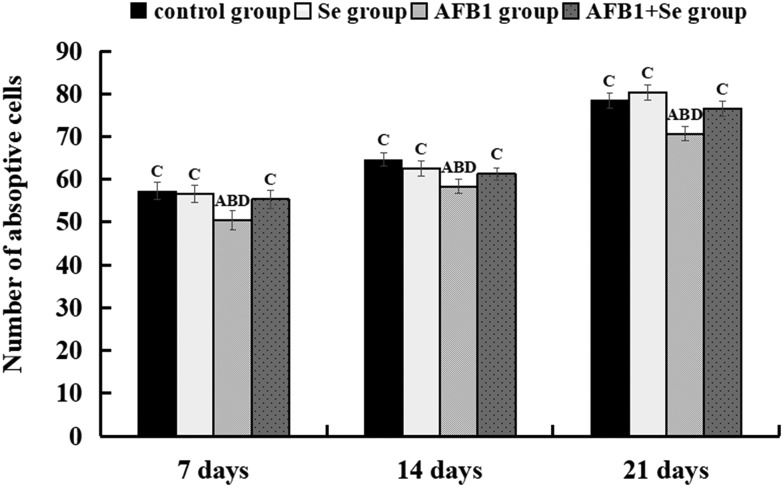
Microscopic quantitative analysis revealed that the villus heights, villus areas and villus/crypt ratios of the AFB1 group were significantly decreased at 7, 14 and 21 days of age (p < 0.05 or p < 0.01), whereas the crypt depths of the AFB1 group were significantly increased when compared with those of the control group, Se group and AFB1 + Se group during the experiment (p < 0.01). Besides, there were no significant differences in these values between the Se group and the control group (p > 0.05) (Fig. 2). Microscopic quantitative analysis also showed that the number of absorptive cells in the AFB1 group was significantly decreased at 7, 14 and 21 days of age (p < 0.01), when compared with the control group. Compared with the AFB1 group, the number of absorptive cells in the AFB1 + Se group was significantly increased at 7, 14 and 21 days of age (p < 0.01). In addition, compared with the control group, there were no significant differences in the Se group (p > 0.05) (Fig. 3).
Fig. 2. The values of microscopic quantitative analysis. (a–d) The values of microscopic quantitative analysis, a–d: the values of villus height, crypt depth, villus area and villus/crypt ratio, respectively. Note: Data are presented with the means ± standard deviation (n = 6). Letters A, B, C and D represent the significant difference (p < 0.01) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively. Letters a, b, c, and d represent the difference (p < 0.05) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively.
Fig. 3. The number of absorptive cells (per 100 μm along the middle axis of the villus) in the jejunum at 7, 14 and 21 days of age. Note: Data are presented with the means ± standard deviation (n = 6). Letters A, B, C and D represent the significant difference (p < 0.01) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively.
TUNEL assay
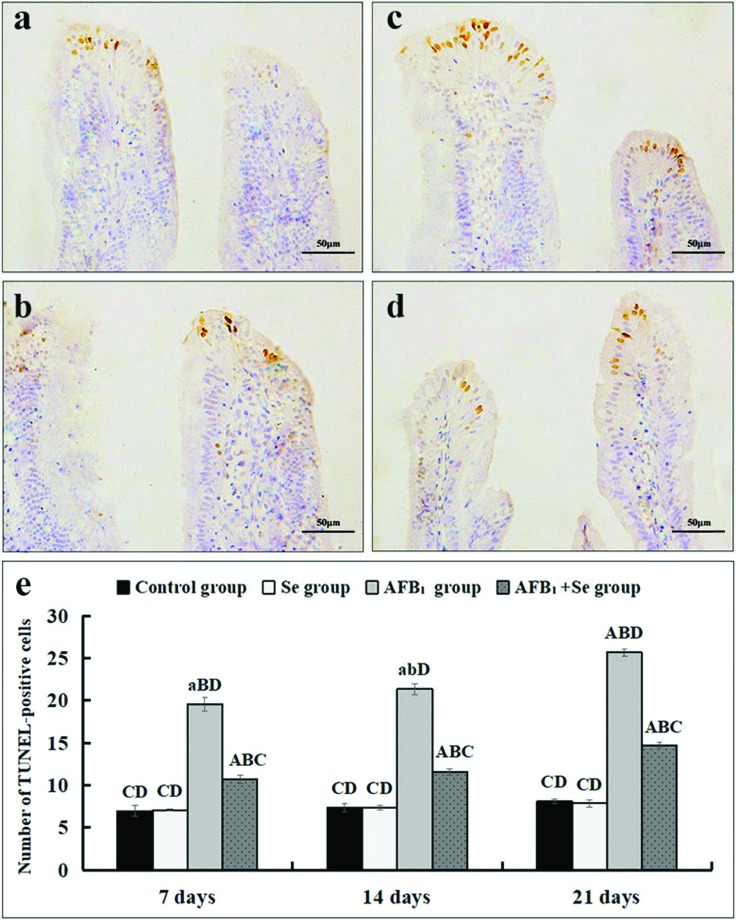
According to Fig. 4, the nuclei of TUNEL-positive cells, mainly distributed in the apical region of the villus, were stained brown by TUNEL assay in four groups. In comparison with the control group and the Se group (Fig. 4a and b), more TUNEL-positive cells were observed in the AFB1 group and the AFB1 + Se group (Fig. 4c and d). When compared with the control group, microscopic quantitative analysis revealed that the number of TUNEL-positive cells in the AFB1 group was significantly increased at 7, 14 and 21 days of age (p < 0.01). Compared with the AFB1 group, the number of TUNEL-positive cells in the AFB1 + Se group was significantly decreased at 14 and 21 days (p < 0.05). In addition, compared with the control group, there were no significant differences in the Se group (p > 0.05) (Fig. 4e).
Fig. 4. The jejunal cell apoptosis by TUNEL assay. (a–d) The representative images of TUNEL-positive cells in the apical regions of jejunal villi in the control group (a), Se group (b), AFB1 group (c) and AFB1 + Se group (d) at 21 days of age (scale bar: 50 μm); (e) the number of TUNEL-positive cells. Note: Data are presented with the means ± standard deviation (n = 6). Letters A, B, C and D represent the significant difference (p < 0.01) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively. Letters a, b, c, and d represent the difference (p < 0.05) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively.
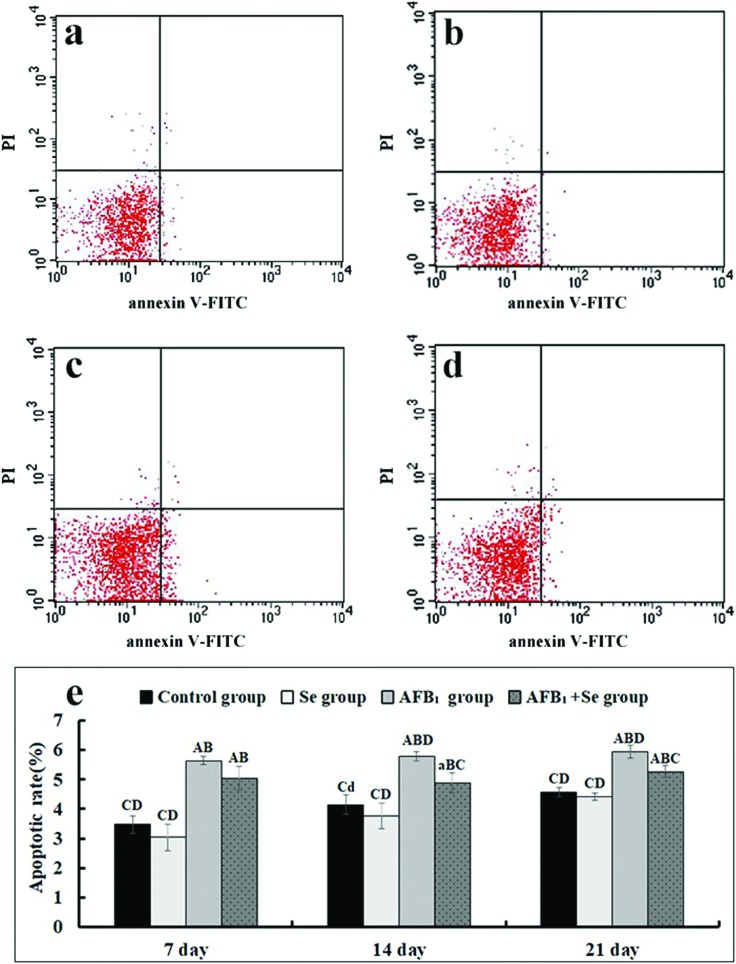
The jejunal cell apoptosis by flow cytometry assay
The percentage of apoptotic cells was quantitatively detected by flow cytometry. Through detection of the total percentage of early (Annexin-V positive and PI negative) and late (both Annexin-V and PI positive) apoptotic cells, apoptotic cell counts were measured. When compared with the control group, the percentages of apoptotic cells of the jejunal cells in the AFB1 group were significantly increased at 7, 14 and 21 days of age (p < 0.01). In comparison with the AFB1 group, the values in the AFB1 + Se group were significantly decreased at 14 and 21 days (p < 0.01). Besides, there were no significant differences in these values between the Se group and the control group (p > 0.05) (Fig. 5e).
Fig. 5. The jejunal cell apoptosis by flow cytometry assay. (a–e) The representative scattergram of apoptotic jejunal cells obtained by flow cytometry assay in the control group (a), Se group (b), AFB1 group (c) and AFB1 + Se group (d) at 21 days of age; (e) apoptotic rates by flow cytometry assay. Note: Data are presented with the means ± standard deviation (n = 6). Letters A, B, C and D represent the significant difference (p < 0.01) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively. Letters a, b, c, and d represent the difference (p < 0.05) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively.
The mRNA expression of cell apoptotic regulatory molecules by qRT-PCR
The mRNA expressions of death receptor pathway related molecules including FAS, FASL, TNF-α, TNF-R1, CASPASE-3, CASPASE-8 and CASPASE-10 in the jejunal mucosa are shown in Fig. 6. When compared with the control group, the mRNA expressions of FAS, TNF-α, TNF-R1, CASPASE-3 and CASPASE-8 in the AFB1 group were significantly increased at 7, 14 and 21 days of age (p < 0.05 or p < 0.01) while those of FASL and CASPASE-10 were only significantly increased at 14 and 21 days of age (p < 0.05 or p < 0.01). In comparison with the AFB1 group, the values in the AFB1 + Se group were significantly decreased at 7, 14 and 21 days of age (p < 0.05 or p < 0.01), except for those of FAS, FASL, TNF-α, CASPASE-3 and CASPASE-10 at 7 days (p > 0.05). Besides, there were no significant differences in these values between the Se group and the control group (p > 0.05).
Fig. 6. The mRNA expressions of FAS, FASL, TNF-α, TNF-R1, CASPASE-3, CASPASE-8 and CASPASE-10 in the jejunal mucosa and expressed as fold change relative to the control group. Note: Data are presented with the means ± standard deviation (n = 6). Letters A, B, C and D represent the significant difference (p < 0.01) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively. Letters a, b, c, and d represent the difference (p < 0.05) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively.
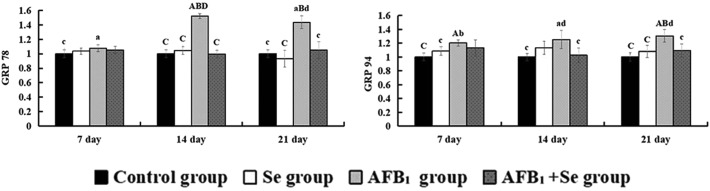
The mRNA expression of GRP78 and GRP94 involved in the endoplasmic reticulum pathway is shown in Fig. 7. When compared with the control group, the mRNA expressions of GRP78 and GRP94 in the AFB1 group were significantly increased at 7, 14 and 21 days of age (p < 0.05 or p < 0.01). In comparison with the AFB1 group, the values in the AFB1 + Se group were significantly decreased at 14 and 21 days of age (p < 0.05 or p < 0.01). In addition, no significant differences in these values were noted between the Se group and the control group (p > 0.05).
Fig. 7. The mRNA expressions of GRP78 and GRP94 in the jejunal mucosa and expressed as fold change relative to the control group. Note: Data are presented with the means ± standard deviation (n = 6). Letters A, B, C and D represent the significant difference (p < 0.01) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively. Letters a, b, c, and d represent the difference (p < 0.05) between the group and control group, Se group, AFB1 group, and AFB1 + Se group, respectively.
Discussion
Selenium (Se), an essential trace element, has kinds of functions such as antioxidant and cancer-preventive effects in both human beings and domestic animals.26 Se mainly incorporates into a selenoprotein that possesses several types of biological functions ranging from antioxidants or oxidoreductases, transport and delivery of selenium to peripheral tissues, protein folding, and endoplasmic reticulum (ER) stress.27–29 Since selenium can antagonize some toxicity caused by oxidative stress, it may have an protective effect on humans or animals which suffer from toxicity of oxidative damage induced by AFB1.18 There have been more and more research studies demonstrating that selenium has acted as an essential element in the dietary prevention of AFB1-casued toxicity in the liver, spleen, bursa of Fabricius and thymus.11,17,30,31 Our early study has revealed that the lesion in the jejunum caused by AFB1 was significantly alleviated with the dietary supplementation of selenium.32 Also, the decreased body weight induced by AFB1 was increased in three +Se groups, consistent with Chen's report.11 The epithelial tissue of the small intestine has a high metabolic rate. The intestinal villi epithelium continues to fall off and is rapidly replaced by the proliferation and migration of the cells of the crypt to the villi to form a new epithelial cell. The abscission of the epithelial tissue and the proliferation of the bottom cells keep a relatively dynamic equilibrium, thus maintaining a normal structure of the intestine. The absorptive cells can promote the effect of nutrient absorption. Intestinal villi can increase the area of absorption and promote digestion and absorption. The villi height determines the number of absorptive cells on the surface of the intestine. The crypt depth directly affects the distance from the cell to the intestinal villi, which affects the rate of metabolism of the intestinal villi. Thus, the assessment of the villi height, crypt depth and the ratio of villi height to crypt depth is a comprehensive indicator of the development of the small intestine. In addition, in order to avoid the disturbance caused by the variation of the intestinal villi diameter, the area of intestinal villi was also measured in this experiment. The size of the intestinal villi area also affects the digestion and absorption function of the small intestine. The jejunum is the longest part of the small intestine, which plays a major role in digestion and absorption. In our research, AFB1 could cause the decrease of the number of absorptive cells, villus height, villus area and the ratio of villus height to crypt depth. However, with the supplementation of selenium, these indexes returned to normal values.
Moreover, excess apoptosis induced by AFB1 was restrained by this element in the jejunum of broilers.12 Due to the role in the detection of DNA fragmentation and distribution of apoptotic cells, TUNEL assay was conducted to identify the excess apoptosis.33 Under a microscope, measurement of the number of TUNEL-positive cells is the microscopic quantitative analysis to evaluate the apoptotic level. In the present study, the number of TUNEL-positive cells in the AFB1 group was significantly increased compared with the control group, while the value of the AFB1 + Se group was lower than that of the AFB1 group. However, no significant differences of the value were observed between the +Se group and the control group. It may result from the antioxidant effects of Se, which is known to compose the active center of GSH-Px, an antioxidant enzyme that protects membrane lipids and macromolecules from oxidative damage produced by peroxides.34 Se supplementation in the diet improves the GSH-Px activity, which enhances the mechanisms of selenium-dependent and selenium-independent ROS scavenging and restores their antioxidative capacity.35 The reduction of reactive oxygen metabolites by GSH-Px helps to maintain the membrane integrity36 and repress ROS-mediate apoptosis37,38 induced by AFB1. These results indicated that 0.4 mg kg–1 Se supplement in the diet could effectively alleviate excess apoptosis caused by 0.6 mg kg–1 AFB1, in line with our early report.12 Our early report indicated that excess apoptosis induced by 0.3 mg kg–1 AFB1 in jejunal cells was only significant at 7 and 14 days of age,12 however, in the present study, this change was observed from 7 to 21 days of age at 0.6 mg kg–1 AFB1 concentration. The different concentration of AFB1 may contribute to this discrepancy. Furthermore, flow cytometry assay, an effective way to detect early and late stages of apoptotic cells,39 was also used in the present research. The trend of the apoptotic level in four groups from 7 to 21 days of age, tested by flow cytometry assay, was similar to that of the results of TUNEL assay. However, the exact mechanism of the protective role of selenium in excess apoptosis in the jejunum caused by AFB1 needs to be elucidated by further research.
AFB1-caused apoptosis can be initiated by the extrinsic and the intrinsic pathways.40 The intrinsic pathway mainly refers to apoptosis taking place via the mitochondria pathway, which was explored in our early research.12 As for the extrinsic pathway, the expression of apoptotic molecules which related to the death receptor and endoplasmic reticulum in the jejunum was also detected in our early study.20 With the up-regulated CASPASES including CASPASE-8, CASPASE-10 and CASPASE-3,41–43 the death receptor pathway can be initiated by the activation of death receptors such as FAS/FASL and TNF-α/TNF-R1.44,45 FAS, a member of the TNFR/nerve growth factor receptor family, can transduce signals during apoptosis.46,47 When bound to the cognate ligand (FASL), FAS/FASL could transmit an apoptotic signal within the cells, then activate the caspase cascade including CASPASE-8/10 and CASPASE-3, and finally lead to apoptosis.48,49 TNF-α exerts its proapoptotic biological activity by binding to type 1 and type 2 receptors (TNF-R1 and TNF-R2).50 After its trimerization, TNF-α/TNF-R1 recruits to the two adapter proteins TNF-R-associated death domain (TRADD) and Fas-associated death domain (FADD), subsequently a death inducing signal complex (DISC) is formed.50–52 Through these two kinds of death receptors, the death receptor pathway is initiated to induce excessive apoptosis. Early documents have shown that selenium could have a protective effect on the decreased expression of death receptor pathway related molecules.53,54 In the present study, our research revealed that the expression levels of FAS, FASL, TNF-α, TNF-R1, CASPASE-3, CASPASE-8 and CASPASE-10 were significantly decreased in the AFB1 + Se group when compared with the AFB1 group. However, no significant differences of these values were observed between the +Se group and the control group. Therefore, it is tempting to speculate that selenium could ameliorate AFB1-induced excess apoptosis through the death receptor pathway in the jejunum of broilers.
ER is an eukaryotic organelle where the synthesis, folding and transportation of proteins take place.55 When the protein folding requirements surpass the processing capacity of the ER, accumulated unfolded proteins will induce ER stress56–58 and trigger the unfolded protein response (UPR), which is a conserved cytoprotective signaling pathway.59,60 Thus, the UPR can enhance the capabilities of the ER to process paraproteins and up-regulate the expression of ER chaperones GRP78 and GRP94, which support the folding of new proteins.61,62 GRP78 and GRP94 are involved in polypeptide translocation across the ER membrane and act as apoptotic regulators by protecting the host cell against ERS-induced cell death.63 Besides, they are responsible for protein folding and assembly, targeting misfolded proteins for degradation, ER Ca2+ binding and controlling the initiation of ER stress sensors.64 If the ER stress persists for a long term, the UPR could lead to apoptosis.65,66 Various cell toxins can stimulate the cell to trigger ER stress and consequently the UPR. According to our early research, AFB1 could cause ER stress and up-regulate the expression of GRP78 and GRP94.20 Some researchers reported that selenium could ameliorate Pb and Cd poisoning by decreasing GRP78 and GRP94.55,67,68 Our results indicated that selenium could alleviate the increased mRNA expressions of GRP78 and GRP94 caused by AFB1, and no significant differences of these values were observed between the +Se group and the control group. This study showed that selenium may have an ameliorative effect on AFB1-indueced ER stress and protect the jejunum from excess apoptosis.
Conclusions
In summary, supplementation of selenium at a concentration of 0.4 mg kg–1 selenium could effectively alleviate the AFB1 poisoning condition, and protect the chicken's jejunum from excess apoptosis caused by 0.6 mg kg–1 AFB1 through down-regulating the expression of death receptor pathway and ER pathway related molecules. According to this conclusion, this study may contribute to a better understanding of selenium's protective role against toxin poisoning.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the program for Changjiang scholars, the University Innovative Research Team (IRT 0848), the Education Department of Sichuan Province (2012FZ0066 and 2013FZ0072) and Huimin project of Chengdu science and technology (2016-HM01-00337-SF).
References
- Abbas H. K., Wilkinson J. R., Zablotowicz R. M. Toxin Rev. 2009;28(2–3):142–153. [Google Scholar]
- Egmond H. P. V., Jonker M. A. Toxin Rev. 2004;23(2–3):273–293. [Google Scholar]
- Bedard L. L., Massey T. E. Cancer Lett. 2006;241(2):174–183. doi: 10.1016/j.canlet.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Johnson W. W., Shimada T. Mutat. Res., Fundam. Mol. Mech. Mutagen. 1998;402(1):121–128. doi: 10.1016/s0027-5107(97)00289-3. [DOI] [PubMed] [Google Scholar]
- Wild C. P., Turner P. C. Mutagenesis. 2002;17(6):471–481. doi: 10.1093/mutage/17.6.471. [DOI] [PubMed] [Google Scholar]
- Richard J. L. Int. J. Food Microbiol. 2007;119(1–2):3–10. doi: 10.1016/j.ijfoodmicro.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Ostry V., Malir F., Toman J., Grosse Y. Mycotoxin Res. 2017;33(1):65–73. doi: 10.1007/s12550-016-0265-7. [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Aaronson S. A., Abrams J. Cell Death Differ. 2009;16(8):1093–1107. doi: 10.1038/cdd.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang W. Anim. Sci. J. 2016;87(12):1490–1500. doi: 10.1111/asj.12550. [DOI] [PubMed] [Google Scholar]
- Ribeiro D. H., Ferreira F. L., Silva V. N. D. Int. J. Mol. Sci. 2010;11(4):1944–1955. doi: 10.3390/ijms11041944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Shu G., Peng X. Food Chem. Toxicol. 2013;59(3):446–454. doi: 10.1016/j.fct.2013.06.032. [DOI] [PubMed] [Google Scholar]
- Peng X., Zhang S., Fang J. Int. J. Environ. Res. Public Health. 2014;11(12):13130–13143. doi: 10.3390/ijerph111213130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S., Wu B., Yu Z. Oncotarget. 2014;7(40):65295–65306. doi: 10.18632/oncotarget.11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D. L., Selenium: its molecular biology and role in human health, Springer Science & Business Media, 2011. [Google Scholar]
- Pucci B., Kasten M., Giordano A. Neoplasia. 2000;2(4):291–299. doi: 10.1038/sj.neo.7900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H. Molecules. 2009;14(3):1263–1278. doi: 10.3390/molecules14031263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Shu G., Peng X. Int. J. Environ. Res. Public Health. 2013;10(7):2834–2844. doi: 10.3390/ijerph10072834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S., Shi D., Clemons-Chevis C. L. Biol. Trace Elem. Res. 2014;162(1–3):296–301. doi: 10.1007/s12011-014-0131-4. [DOI] [PubMed] [Google Scholar]
- Yunus A. W., Razzazi-Fazeli E., Bohm J. Toxins. 2011;3(6):566–590. doi: 10.3390/toxins3060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Zuo Z., Zhu P. Oncotarget. 2017;8(52):89655–89664. doi: 10.18632/oncotarget.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N. J. Appl. Poult. Res. 1994;3:101. [Google Scholar]
- Kaoud H. A. Sci. J. Appl. Res. 2012;1(1):16–21. [Google Scholar]
- Peng X., Yu Z., Liang N. Oncotarget. 2016;7(11):12222–12234. doi: 10.18632/oncotarget.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Cui H., Cui Y. Hum. Exp. Toxicol. 2011;30(7):685–692. doi: 10.1177/0960327110379022. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Papp L. V., Holmgren A., Khanna K. K. Antioxid. Redox Signaling. 2010;12(7):793–795. doi: 10.1089/ars.2009.2973. [DOI] [PubMed] [Google Scholar]
- Maraldi T., Riccio M., Zambonin L. Neurotoxicology. 2011;32(2):180–187. doi: 10.1016/j.neuro.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Saad M. B., Gertner L. R., Bona T. D. Recent Pat. Food, Nutr. Agric. 2009;1(3):243–247. doi: 10.2174/2212798410901030243. [DOI] [PubMed] [Google Scholar]
- Papp L. V., Lu J., Holmgren A. Antioxid. Redox Signaling. 2007;9(7):775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- Shi D., Guo S., Liao S. Biol. Trace Elem. Res. 2012;145(3):325–329. doi: 10.1007/s12011-011-9201-z. [DOI] [PubMed] [Google Scholar]
- Chen K., Jing F., Xi P. Food Chem. Toxicol. 2014;74(74):91–97. doi: 10.1016/j.fct.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Zhang S., Peng X., Fang J. Biol. Trace Elem. Res. 2014;160(1):32–40. doi: 10.1007/s12011-014-0009-5. [DOI] [PubMed] [Google Scholar]
- Ribeiro S., Sharma R., Gupta S. Andrology. 2017;5(3):477–485. doi: 10.1111/andr.12334. [DOI] [PubMed] [Google Scholar]
- Rotruck J. T., Pope A. L., Ganther H. E., Swanson A. B., Hafeman D. G., Hoekstra W. G. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Eberta R., Ulmera M., Zecka S., Meissner-Weigla J., Schneidera D., Stopperb H., Schuppb N., Kassemc M., Jakoba F. Stem Cells. 2006;24:1226–1235. doi: 10.1634/stemcells.2005-0117. [DOI] [PubMed] [Google Scholar]
- Brenneisen P., Steinbrenner H., Sies H. Mol. Aspects Med. 2005;26:256–267. doi: 10.1016/j.mam.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Yeo J. E., Kang S. K. Biochim. Biophys. Acta, Mol. Basis Dis. 2007;1772:1199–1210. doi: 10.1016/j.bbadis.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Zhou Y. J., Zhang S. P., Liu C. W., Cai Y. Q. Toxicol. In Vitro. 2009;23:288–294. doi: 10.1016/j.tiv.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Henry C. M., Hollville E., Martin S. J. Methods. 2013;61(2):90–97. doi: 10.1016/j.ymeth.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Mariño G., NisoSantano M., Baehrecke E. H. Nat. Rev. Mol. Cell Biol. 2014;15(2):81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. H., Kluger M. S., Madge L. A. Am. J. Pathol. 2002;161(4):1485–1495. doi: 10.1016/s0002-9440(10)64424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chun H. J., Wong W. Proc. Natl. Acad. Sci. U. S. A. 2001;98(24):13884–13888. doi: 10.1073/pnas.241358198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. Life Sci. 2001;69(25–26):2957–2964. doi: 10.1016/s0024-3205(01)01404-7. [DOI] [PubMed] [Google Scholar]
- Waring P., Müllbacher A. Immunol. Cell Biol. 1999;77(4):312–317. doi: 10.1046/j.1440-1711.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- Wallach D. Cytokine Growth Factor Rev. 1996;7(3):211–221. doi: 10.1016/s1359-6101(96)00032-9. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Imai Y., Matsumoto T. Cell. 2007;130(5):811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Saradha B., Vaithinathan S., Mathur P. P. Toxicology. 2009;255(3):131–139. doi: 10.1016/j.tox.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Lavrik I. N., Krammer P. H. Cell Death Differ. 2012;19(1):36–41. doi: 10.1038/cdd.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott F. L., Stec B., Pop C. Nature. 2009;457(7232):1019–1022. doi: 10.1038/nature07606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H., Shu H. B., Pan M. G. Cell. 1996;84(2):299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Aggarwal S., Gollapudi S., Gupta S. J. Immunol. 1999;162(4):2154–2161. [PubMed] [Google Scholar]
- Yuan J. Curr. Opin. Cell Biol. 1977;9(2):247–251. doi: 10.1016/s0955-0674(97)80069-5. [DOI] [PubMed] [Google Scholar]
- Miao K., Zhang L., Yang S. Environ. Toxicol. Pharmacol. 2013;36(3):913–920. doi: 10.1016/j.etap.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Valadbeygi A., Naji T., Pirnia A. Cryobiology. 2016;73(2):135–139. doi: 10.1016/j.cryobiol.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Liu L., Yang B., Cheng Y. Biol. Trace Elem. Res. 2015;167(2):308–319. doi: 10.1007/s12011-015-0314-7. [DOI] [PubMed] [Google Scholar]
- Doyle K. M., Kennedy D., Gorman A. M. J. Cell. Mol. Med. 2011;15(10):2025–2039. doi: 10.1111/j.1582-4934.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki H., Nishitoh H., Ichijo H. J. Chem. Neuroanat. 2004;28(1–2):93–100. doi: 10.1016/j.jchemneu.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G. S. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R. K., Li C., Chaudhary S. C. Toxicol. Appl. Pharmacol. 2013;272(3):879–887. doi: 10.1016/j.taap.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ron D. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Lee A. S. Methods. 2005;35(4):373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Lee A. S. Trends Biochem. Sci. 2001;26(8):504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- Zhu G., Lee A. S. J. Cell. Physiol. 2015;230(7):1413–1420. doi: 10.1002/jcp.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadi M., Oulidi A., Gackière F. FASEB J. 2013;27(4):1600–1609. doi: 10.1096/fj.12-218875. [DOI] [PubMed] [Google Scholar]
- Ron D., Walter P. Nat. Rev. Mol. Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Tabas I., Ron D. Nat. Cell Biol. 2011;13(3):184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Pan T., Wan N. J. Inorg. Biochem. 2017;170:169–177. doi: 10.1016/j.jinorgbio.2017.02.022. [DOI] [PubMed] [Google Scholar]
- Wang X., An Y., Jiao W. Biol. Trace Elem. Res. 2017;2017(4):1–10. [Google Scholar]