 The hypothalamic GABAA receptor may be involved in the reproductive toxicity in male offspring due to paternal electromagnetic pulse exposure.
The hypothalamic GABAA receptor may be involved in the reproductive toxicity in male offspring due to paternal electromagnetic pulse exposure.
Abstract
Many studies indicate that parental exposure to an electromagnetic field (EMF) can cause long-term toxicity to the health of the offspring. While concerns have been focused on maternal influence, much less is known regarding the effects of paternal factors. Electromagnetic pulse (EMP) is a special and widely used type of EMF. The present study was designed to investigate the effects of paternal EMP exposure on the reproductive endocrine function of the male rat offspring. Male Sprague Dawley rats were randomly exposed to EMP at 200 kV m–1 for 0, 100 or 400 pulses before mating. The adult male offspring were sacrificed and the structural changes of testes, levels of serum steroid hormones, sperm characteristics, reproductive behaviors, content of the reproductive endocrine-related neurotransmitter GABA and expression of the GABAA receptor were analyzed. The results showed that paternal exposure induced a decrease of testosterone (T), sperm quantity and acrosin activity in the male offspring (p < 0.05). It did not show significant changes in the structure of testes, sperm deformity frequency and reproductive behaviors compared with the sham-exposed group. The content of GABA and the protein and mRNA expression of the hypothalamic GABAA receptor protein increased in the EMP exposure group (p < 0.05). In conclusion, our study shows that under these experimental conditions EMP had a certain degree of influence on the reproductive endocrine function of the male rat offspring, and the hypothalamic GABAA receptor may be involved in the reproductive toxicity of the male offspring.
Introduction
Due to modern technology, humans are inevitably exposed to various types of electromagnetic fields (EMF) with increased intensities, and it has become a major public health issue.1,2 Faced with the increasing exposure to electromagnetic radiation, more and more attention has been drawn to its effects on human health. The male reproductive system is considered to be one of the most sensitive targets of EMF.3,4 The association between EMF exposure and male reproductive dysfunction has been widely studied. Evidence from studies supports a growing claim that electromagnetic radiation may have a detrimental effect on testicular functions and sperm parameters leading to decreased male fertility.5–7 However, data regarding the possible long-term reproductive toxicity in the male offspring due to parental EMF exposure have not been addressed adequately.
Limited data suggested that maternal exposure to EMF may affect the reproductive function of the male offspring. Researchers have reported that exposure to EMF during a critical prenatal period could significantly demasculinize male scent marking behavior or cause less mating frequency in the male offspring.8,9 Prenatal exposure to EMF may adversely affect the testicular development in the offspring and alter sperm quality and biochemical characteristics in sixty-day-old offspring.10,11 However, some experiments showed that exposure to extremely low frequency or radiofrequency EMF during the gestation and lactation periods did not cause abnormal alterations of the reproductive ability in the male offspring.12,13 These conflicting findings could be attributed to the different types and parameters of EMF exposure.
Moreover, the reproductive function changes in the male offspring following paternal EMF exposure are scarce. Electromagnetic pulse (EMP) is a high-energy pulse with an extremely fast rising time and a broad bandwidth.14 Due to its distinguishing properties, it is widely used in medicine, security screening, and military applications.15 The increasing opportunity of EMP exposure calls for worldwide concerns on the potential health influence of humans, especially for some military personnel, workers and researchers who work with or can be exposed to EMP under their occupational conditions. Previously published scientific articles indicated that exposure to EMP could induce potential effects on the male reproductive system and fertility including: dysfunction of the blood–testicle barrier, damage of testicular structures, apoptosis of spermatogenic cells, etc.16–19 Our research team recently revealed that paternal exposure to EMP with a 35 kV m–1 field strength may affect the offspring sex ratio.20 In our present study, male rats were exposed to EMP before mating to explore whether paternal EMP exposure could affect the reproductive endocrine function of the adult male offspring.
The hypothalamic–pituitary–gonadal (HPG) axis is the control core of an animal reproductive system's development and function.21 The HPG axis is regulated by GABAergic signaling at the level of gonadotropin-releasing hormone (GnRH) neurons, which play a pivotal role in the reproductive function. The GABAergic modulation of GnRH neurons can influence the reproductive function in several ways, including the onset of puberty and the regulation of the estrous cycle. The inhibitory neurotransmitter GABA is one of the most important regulators, primarily through the hypothalamic GABAA receptor, regulating GnRH secretion, promoting the secretion of pituitary hormones, and ultimately achieving the regulation of the animal reproductive endocrine function.22,23 Therefore, the hypothalamic GABAA receptor expression plays an important role in the regulation of the reproductive endocrine function. On the basis of these, the aim of the present study was to investigate the effects of paternal EMP exposure on the reproductive endocrine function of the male rat offspring and to explore whether it was associated with GABA and its receptor.
Experimental
Animals
Nine male (weighing 370 to 390 g) and eighteen female (weighing 240 to 260 g) Sprague Dawley rats were purchased from the Experimental Animal Center of the Fourth Military Medical University, China. Rats were housed in cages under laboratory conditions (12 h day/12 h night cycle, temperature: 21 ± 1 °C, relative humidity: 60 ± 7%). All animal procedures were performed in accordance with the PR China Animal Management Rule (Documentation Number 55, 2001, Ministry of Health of PR China) and were approved by the Animal Care Committee of Fourth Military Medical University.
EMP exposure
The EMP was generated using a spark gap pulse generator and transmitted into a gigahertz transverse electromagnetic (GTEM) cell. The EMP generator and the GTEM cell were both devised by the Department of Mechanical Engineering, Southeast University (Nanjing City, Jiangsu Province, China). The apparatus was previously described by Zeng et al.24 Male rats were divided randomly into three groups: sham-exposed group, 100 and 400 pulse exposure group, respectively, receiving 0, 100, and 400 pulses of 200 kV m–1 daily for 7 days. The rats in the sham-exposed group were placed under identical conditions as the EMP exposure group, but without EMP exposure. During exposure, the male rats can move freely in the pure plastic chamber. After 7 days, the exposure was terminated.
Experimental design
After termination of exposure all males were caged together with naive females for a week at a ratio of 2 : 1. The pregnant females were placed inside a separate cage under normal laboratory conditions. After each birth, the offspring were weaned from their mother on P21 (birth day = P0) and were housed at four animals per cage per sex. In our present study only the male offspring were used as research subjects. Female offspring were used for other studies reported elsewhere. For each analysis, 1 or 2 male offspring per litter was used for a total of 8 per group.
Sexual behavior
Between P80 and 88, adult male offspring were evaluated for sexual behavior during the dark phase of their cycle under red-light illumination. For the test, each male was placed into a cage and an estrous female was introduced 5 min later. For each 30 min test, the following behaviors were videotaped and scored: latency (time) to first mount, number of mounts, latency (time) to first ejaculation and number of ejaculations.25
Histopathological evaluation of the testes
Five days after sexual behavioral testing, the male offspring were intraperitoneally injected with 40 mg kg–1 i.p. of sodium pentobarbital for anaesthesia. Then perfusion was carried out through the left ventricle with 0.9% saline followed by 4% formaldehyde. After this, testes were collected and fixed in the same solution for 24 h. Then 3 μm sections were made from testes and were stained with hematoxylin and eosin (H&E) for histopathological analyses after paraffin embedding.
Hormonal analyses
In order to investigate the effects of paternal EMP exposure on sex steroid hormone levels in the male rat offspring, blood was collected after sexual behavior testing. Serum was collected after centrifugation and stored at –20 °C until analysis. Radioimmunoassay (RIA) for serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone (T) and gonadotropin-releasing hormone (GnRH) was carried out using radioimmunoassay kits (Beijing Sino-uk Institute of Biology and Technology) according to the manufacturer's protocol. Hormone levels were analyzed using a γ-counter (GC-911).
Epididymal sperm characteristics
After anesthetization the caudal epididymides of the offspring were quickly isolated from adherent fat and connective tissues, minced with scissors in 1 ml 0.9% NaCl and incubated at 37 °C for 20 minutes enabling the migration of sperm from the epididymides to NaCl. After incubation, the supernatant containing all epididymal sperm was obtained by filtering with a strainer. The number of sperm was counted using a haemocytometer under a light microscope after dilution (1 : 20). The results were presented as the total number of sperm per ml per cauda epididymis (×106).26
For morphological analysis of spermatozoa, the supernatant containing sperm was used to prepare slides. After staining with Eosine Y, the slides were viewed under a light microscope at 400× total magnification. Five hundred spermatozoa per slide were examined and the total abnormality rates were expressed as a percentage.27
Acrosin is an important proteolytic enzyme in the acrosome which plays a vital role in the process of fertilization. The epididymal sperm acrosin activity was evaluated by the gelatinolysis test as described by Henkel et al., which is based on the ability of acrosomal enzymes to hydrolyze a high molecular weight gelatin.28 For evaluation of acrosin activity, 20 μl supernatant was diluted 1 : 10 in PBS. The samples were smeared on precoated gelatin slides and incubated in a moist chamber at 37 °C for 2 hours. The percentage of sperm showing halo formation was calculated per slide.
Hypothalamic content of GABA
The male offspring were anaesthetized and the hypothalamus was immediately removed and stored at –80 °C until high performance liquid chromatography (HPLC) measurement. The tissue was ultrasonically homogenized in 250 μl of 0.4 mol L–1 perchloric acid for 100 mg tissue. The samples were kept at 0 °C for an hour and then centrifuged at 15 000g at 4 °C for 20 min.
The supernatant was filtered and then injected and analysed using an HPLC system (Model 5600A CoulArray, EAS).
Expression of GABAA receptor
Western blot
The hypothalamus was isolated from a rat after narcotism and then homogenized in lysis buffer containing a protease inhibitor for 30 min on ice with gentle rocking. Then the sample was centrifuged at 15 000g for 5 min at 4 °C, and the supernatant was stored at –80 °C. Protein concentrations were determined by a bicinchoninic acid (BCA) protein assay. After this, the protein was boiled for 5 min in 5× loading buffer to run SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
Real-time fluorescence quantitative RT-PCR
The total RNA was extracted using a TRIzol reagent (Invitrogen) according to instructions. The RT reaction was performed using the PrimeScript RT Master Mix (Takara) in 10 μl volume consisting of 0.5 μg total RNA, 2 μl 5× PrimerScript buffer, 0.5 μl oligodT primer, 0.5 μl random 6 mers, 0.5 μl PrimerScript RT Enzyme Mix I and RNase-free water. The RT-PCR was then executed using SYBR Green Realtime PCR Master Mix (Takara) on an ABI 7500 FAST Real-time PCR system. The primers were as follows:
GABA A Rα1 (NM_183326.2) forward primer: 5′-TTTGGAGTGACGACCGTTCTG-3′ and reverse primer: 5′-GCAATAAACCAGTCCATGGCC-3′; β-actin (internal control, NM_031144.2) forward primer: 5′-TCTGTGTGGATTGGTGGCTCT-3′ and reverse primer: 5′-AGAAGCATTTGCGGTGCAC-3′.
The PCR reaction was performed in a 20 μl final mixture containing 10 μM of each primer, a diluted cDNA solution and 10 μl SYBR Green Realtime PCR Master Mix. The thermal cycling conditions were as follows: 95 °C for 5 s, 40 cycles at 95 °C for 5 s and then 62 °C for 34 s. Relative GABAA receptor mRNA expression was calculated using the 2–ΔΔCT method as described previously.29
Statistical analysis
The statistical software SPSS 13.0 was used for statistical analysis. The continuous variables were presented as the means ± SD and were analyzed by ANOVA followed by Dunnett's t-test. For all tests, p < 0.05 was considered to be statistically significant.
Results
Sexual behavior
The evaluation of sexual behaviors including latency (time) to first mount, number of mounts, latency (time) to first ejaculation and number of ejaculations are shown in Table 1. No significant differences in the above sexual behaviors were observed in both groups exposed to EMP compared to the sham-exposed group.
Table 1. Effects of EMP on the reproductive behaviors (n = 8, mean ± SEM).
| Group | Latency to first mount (s) | Number of mounts | Latency to first ejaculation (s) | Number of ejaculations |
| Sham | 31.77 ± 8.28 | 35 ± 6.06 | 449.88 ± 55.27 | 9.75 ± 2.99 |
| 100 pulses | 37.27 ± 10.05 | 33.5 ± 6.24 | 518.44 ± 100.07 | 9.00 ± 1.83 |
| 400 pulses | 33.41 ± 13.26 | 33.25 ± 6.70 | 533.93 ± 72.16 | 8.25 ± 3.77 |
Histopathological evaluation of the testes
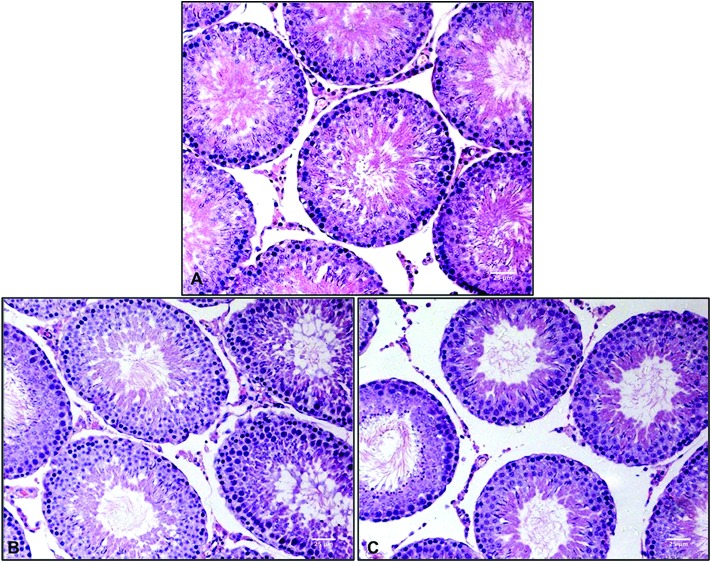
Histopathological evaluation of testis sections was performed using light microscopy. Normal architectures of the seminiferous tubules and interstitial tissue were observed in the sham-exposed group. No obvious histopathological alterations were found in sections from the exposure groups (Fig. 1).
Fig. 1. Histopathological analyses of testis sections stained with H&E (×400). (A) sham-exposed group. (B) 100 pulse group. (C) 400 pulse group.
Steroid hormone assays
Table 2 shows the levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone (T) and gonadotropin-releasing hormone (GnRH). Compared with the sham-exposed group, serum T levels were significantly lower in the male offspring of the two exposure groups. There was no significant alteration in the levels of FSH, LH and GnRH (Table 2).
Table 2. Effects of electromagnetic pulse on the levels of hormones (n = 8, mean ± SEM).
| Group | FSH (mIU ml–1) | LH (mIU ml–1) | T (ng ml–1) | GnRH (pg ml–1) |
| Sham | 14.50 ± 1.70 | 14.80 ± 1.83 | 0.89 ± 0.22 | 48.76 ± 8.84 |
| 100 pulses | 14.20 ± 1.73 | 14.01 ± 1.20 | 0.37 ± 0.29* | 51.59 ± 12.09 |
| 400 pulses | 14.42 ± 1.66 | 13.71 ± 1.53 | 0.60 ± 0.25* | 52.14 ± 13.21 |
Epididymal sperm characteristics
The effects of EMP exposure on sperm parameters are presented in Table 3. The sperm concentration and the percentage of sperm with halo formation were significantly lower in the EMP exposed offspring than in sham-exposed rats (p < 0.05). There was no statistically significant difference in the sperm deformity rate among the groups.
Table 3. Effects of electromagnetic pulse on the epididymal sperm characteristics (n = 8, mean ± SEM).
| Group | Sperm concentration (× 106 ml–1) | Deformity rate (%) | Halo formation rate (%) |
| Sham | 39.5 ± 10.75 | 1.8 ± 0.87 | 35.83 ± 5.35 |
| 100 pulses | 21.5 ± 9.18* | 2.87 ± 0.81 | 15.33 ± 2.02* |
| 400 pulses | 16.33 ± 3.08* | 2.6 ± 0.53 | 19.5 ± 2.55* |
Hypothalamic content of GABA
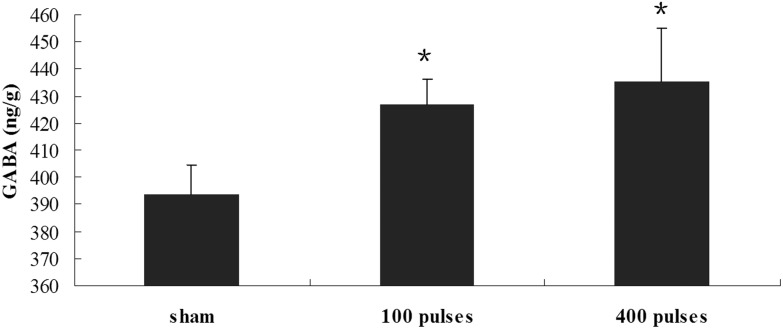
To further explore the effects of paternal EMP exposure on the reproductive endocrine function, the levels of the neurotransmitter GABA were evaluated in the hypothalamus. As shown in Fig. 2, GABA levels were higher in the EMP exposure group than in the sham-exposed group (Fig. 2, p < 0.05).
Fig. 2. Level of hypothalamic GABA in EMP exposure groups and sham-exposed group. *p < 0.05 versus the sham group.
Expression of the GABAA receptor
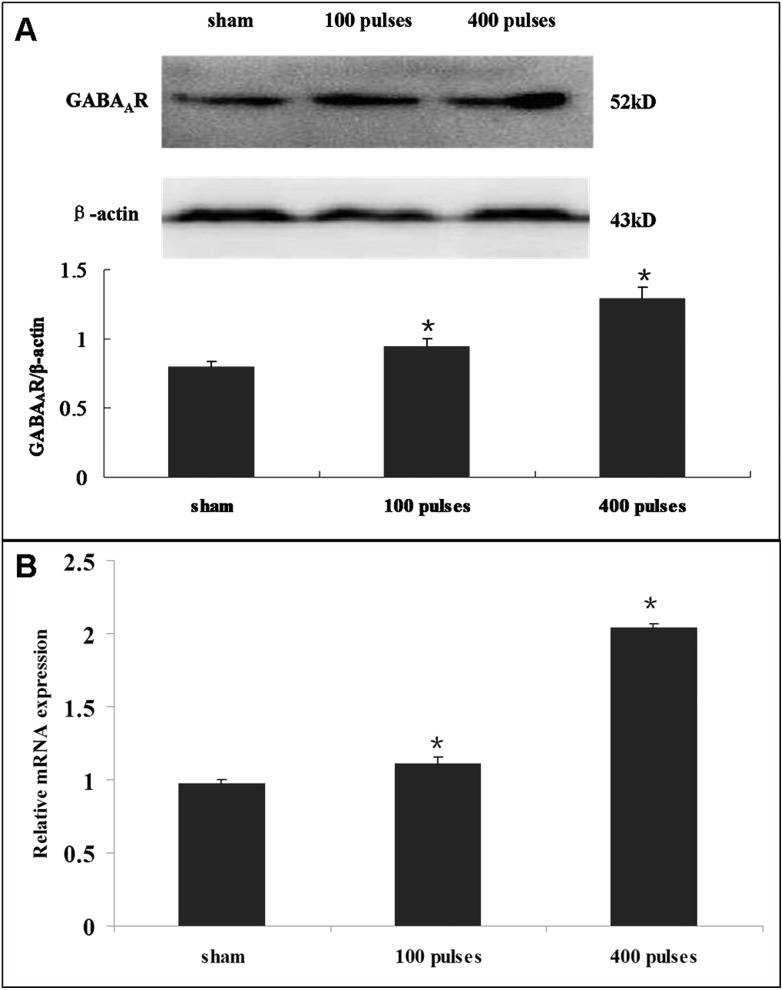
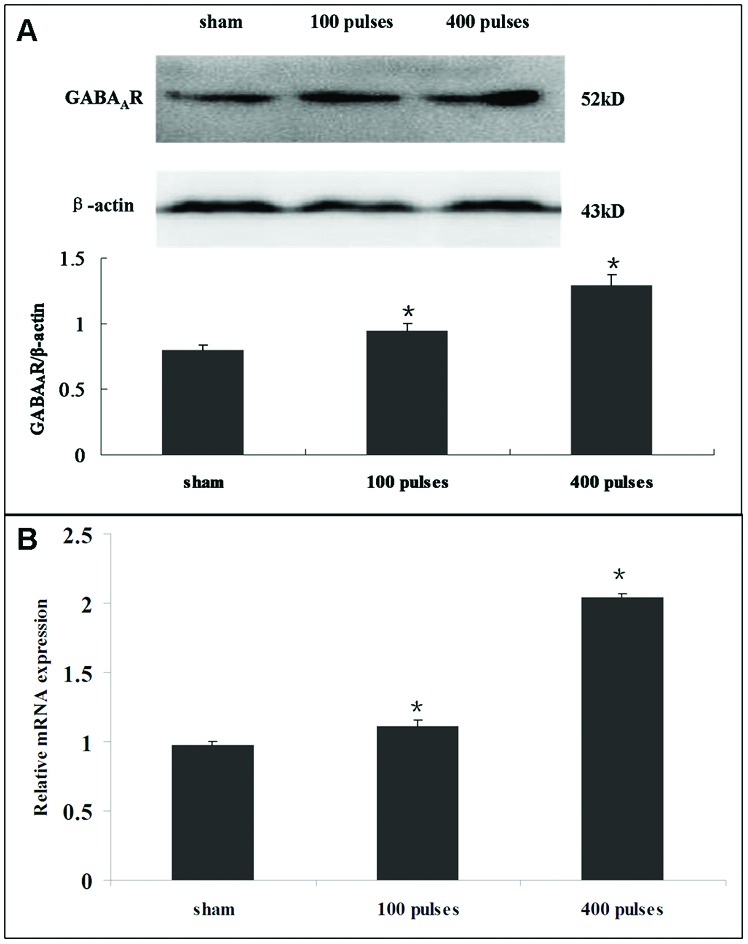
Compared with the sham-exposed group, the western blot results showed that the protein expression of the GABAA receptor was significantly higher in the EMP exposure group (Fig. 3A, p < 0.05). Similarly, the mRNA level of the GABAA receptor increased significantly in the EMP exposure group (Fig. 3B, p < 0.05).
Fig. 3. (A) The effect of EMP on the protein expression level of the GABAA receptor in hypothalamus. The histogram panel shows the relative density compared with GAPDH. (B) The effect of EMP on the mRNA expression level of the GABAA receptor in hypothalamus. Error bars represent the standard deviation of three replicates. *p < 0.05 versus the sham group.
Discussion
The worldwide increasing exposure to EMF draws considerable attention due to its potential impact on human health. The association between parental exposure and the development of the offspring is among the chief concerns. Previous studies presented ambivalent results for maternal exposure effects of EMF on adverse pregnancy outcomes including miscarriage, perinatal death, altered gender ratio, congenital anomalies, etc.30–33 Some studies provided contradictory results that low frequency EMF can have a positive effect on sperm quality, motility, and fertility. These contradictory results were highly dependent on the type and exposure parameters of EMF.34 The findings of studies on offspring development effects associated with paternal exposure are also conflicting. Previous epidemiological and biological studies mainly focussed on the issue that paternal occupational exposure to EMF could be associated with cancer, birth defects and so on in children.35–38 However, no such association was found in some reports.39–41 As a particular EMF, EMP is extensively used in many fields such as navigation, targeting and communication. However, research on the effects of EMP on the long-term reproductive endocrine function after parental exposure is limited.
In our present study, we used rats as research subjects to evaluate the potential long-term adverse effects of paternal exposure to EMP on the reproductive endocrine function of the male offspring. In our study, no obvious differences in the structure of testes and reproductive behaviors in the male offspring were observed between the EMP exposure group and the sham-exposed group. However, paternal exposure to EMP affected the sperm characteristics in the male offspring. Although the sperm deformity rate was not affected, the number of sperm and the halo formation rate were decreased significantly in the EMP exposure group. The number, morphology and function of sperm have been related to male infertility. A threshold concentration of normal sperm is required to achieve conception. The sperm acrosome reaction is a key step of fertilization and defects in the acrosome reaction may result in infertility.42 These results indicated that paternal exposure to different pulses of EMP induced the poor sperm quality in the male offspring, suggesting a reduction in fertilization potential.
We also examined the serum hormone concentrations in the male offspring. As one of the most important hormones, testosterone is crucial to spermatogenesis.43 The synthesis and release of testosterone depend on LH binding to the Leydig cells. LH, along with FSH, is important for the production of spermatozoa. In addition, they can exert negative feedback effects on gonadotropins at the hypothalamic and pituitary levels.44 Previous research studies showed variable results on the effects of EMP on the levels of reproductive hormones. Zeng et al. found that serum levels of LH and T were elevated in irradiated rats (3 × 105 or 4 × 105 pulses, 100 kV m–1).24 However, Li et al. reported that two-week EMP exposure (10 000 pulses, 35 kV m–1) led to the increase of the T level, despite the decrease in the LH level.20 Our present data showed that paternal exposure to EMP led to a significant decrease of T levels in the male offspring, whereas there were no noticeable changes in serum levels of FSH, LH and GnRH. Besides, the T levels were much lower in the 100 pulse exposure group compared to the 400 pulse exposure group. The decrease of T levels may have no direct relationship with the intensity of paternal EMP exposure. Given that LH levels were not decreased accompanied by the decrease of T levels, we speculated that this fluctuation in T levels cannot be simply interpreted from the damage on Leydig cells in the testes. The production of T was regulated by a complex system involving the hypothalamus, anterior pituitary and testes.45 As multiple signaling pathways are involved in the secretion and regulation of hormones, the specific mechanism needs to be further explored.
It is known that the reproductive cycle is regulated by intricate interactions among the hypothalamic–pituitary–gonadal (HPG) axis, which encompasses a small portion of hypothalamic neurons that express GnRH, the gonadotropes of the anterior pituitary gland, and the gonads.46 The activities of GnRH neurons are regulated by steroid hormones, growth factors and neurotransmitters. Among these neurotransmitters, γ-amino butyric acid (GABA) has been recognized as one of the major players. It acts through GABAA receptors to control the GnRH release and gonadotropin secretion.47–49 In the present study, we observed whether paternal EMP exposure could induce the alteration of GABA as a possible mechanism for its adverse effects on the male rat offspring. We assessed the content of GABA and the protein and mRNA levels of the GABAA receptor in the hypothalamus. The results showed that the GABA levels were higher in the EMP exposure group than in the sham-exposed group. In addition, the protein and mRNA levels of the GABAA receptor were significantly higher in the EMP exposure group. This suggested that the alteration of the GABA level and GABAA receptor expression may be part of the mechanism via which paternal EMP exposure results in detrimental effects on the reproductive endocrine function. However, our data showed that the levels of GnRH did not change in the rat offspring after paternal EMP exposure, and the increase of GABA failed to exert feedback regulation. In terms of the complexity of the HPG axis, many other factors should also be considered, and the underlying mechanism should be investigated specifically and comprehensively through more investigations.
Conclusion
In summary, our studies show that paternal EMP exposure had a certain degree of influence on the reproductive endocrine function of the male offspring and the mechanism may be associated with the increasing hypothalamic levels of GABA and GABAA receptor. Although the type of EMP is unique and the intensity is much higher than occupational exposure, more attention should be paid to the long-term effects of the toxicity due to paternal EMP exposure on the health of the offspring.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
This work was supported by the National Basic Research Program of China (No. 2011CB503704), National Natural Science Foundation of China (No. 60971055 and 60871068) and International Science and Technology Cooperation Program of China (No. 2010DFA31900).
References
- Hardell L., Sage C. Biomed. Pharmacother. 2008;62:104–109. doi: 10.1016/j.biopha.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Jalilian H., Teshnizi S. H., Röösli M., Neghab M. Neurotoxicology. 2017:pii: S0161-813X(17)30239-5. doi: 10.1016/j.neuro.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Desai N. R., Kesari K. K., Agarwal A. Reprod. Biol. Endocrinol. 2009;22:114–118. doi: 10.1186/1477-7827-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makker K., Varghese A., Desai N. R. Reprod. Biomed. Online. 2009;18:148–157. doi: 10.1016/s1472-6483(10)60437-3. [DOI] [PubMed] [Google Scholar]
- Li D. K., Yan B., Li Z. Reprod. Toxicol. 2010;29:86–92. doi: 10.1016/j.reprotox.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Deepinder F., Sharma R. K. Fertil. Steril. 2008;89:124–128. doi: 10.1016/j.fertnstert.2007.01.166. [DOI] [PubMed] [Google Scholar]
- Falzone N., Huyser C., Becker P. Int. J. Androl. 2011;34:20–26. doi: 10.1111/j.1365-2605.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- McGivern R. F., Sokol R. Z., Adey W. R. Teratology. 1990;41:1–8. doi: 10.1002/tera.1420410102. [DOI] [PubMed] [Google Scholar]
- Cobb B. L., Jauchem J. R., Mason P. A. Bioelectromagnetics. 2000;21:524–537. doi: 10.1002/1521-186x(200010)21:7<524::aid-bem6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Hancı H., Odacı E., Kaya H., Aliyazıcıoğlu Y., Turan İ., Demir S., Çolakoğlu S. Reprod. Toxicol. 2013;42:203–209. doi: 10.1016/j.reprotox.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Odacı E., Hancı H., Yuluğ E., Türedi S., Aliyazıcıoğlu Y., Kaya H., Çolakoğlu S. Biotech. Histochem. 2016;91(1):9–19. doi: 10.3109/10520295.2015.1060356. [DOI] [PubMed] [Google Scholar]
- Chung M. K., Lee S. J., Kim Y. B., Park S. C., Shin D. H., Kim S. H., Kim J. C. Asian J. Androl. 2005;7(2):189–194. doi: 10.1111/j.1745-7262.2005.00007.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Imai N., Nabae K., Wake K., Kawai H., Wang J., Watanabe S., Kawabe M., Fujiwara O., Ogawa K., Tamano S., Shirai T. Radiat. Res. 2010;173(3):362–372. doi: 10.1667/RR1615.1. [DOI] [PubMed] [Google Scholar]
- Ding G. R., Qiu L. B., Wang X. W. Toxicol. Lett. 2010;196:154–160. doi: 10.1016/j.toxlet.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Li K., Ma S., Ren D., Li Y., Ding G., Liu J., Guo Y., Guo G. Biol. Trace Elem. Res. 2014;158(1):81–86. doi: 10.1007/s12011-014-9903-0. [DOI] [PubMed] [Google Scholar]
- Wang X. W., Ding G. R., Shi C. H., Zhao T., Zhang J., Zeng L. H., Guo G. Z. Biomed. Environ. Sci. 2008;21(3):218–221. doi: 10.1016/S0895-3988(08)60032-X. [DOI] [PubMed] [Google Scholar]
- Wang X. W., Ding G. R., Shi C. H., Zeng L. H., Liu J. Y., Li J., Zhao T., Chen Y. B., Guo G. Z. Toxicology. 2010;276(1):58–63. doi: 10.1016/j.tox.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Qiu L., Chen C., Ding G., Zhou Y., Zhang M. Biomed. Environ. Sci. 2011;24(4):438–444. doi: 10.3967/0895-3988.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Miao X., Wang Y., Lang H., Lin Y., Guo Q., Yang M., Guo J., Zhang Y., Zhang J., Liu J., Liu Y., Zeng L., Guo G. OMICS. 2017;21(2):81–89. doi: 10.1089/omi.2016.0151. [DOI] [PubMed] [Google Scholar]
- Li J. H., Jiang D. P., Wang Y. F., Yan J. J., Guo Q. Y., Miao X., Lang H. Y., Xu S. L., Liu J. Y., Guo G. Z. Environ. Toxicol. Pharmacol. 2017;54:155–161. doi: 10.1016/j.etap.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Dixon J. R., Ahmed S. F. Paediatrics and Child Health. 2007;17:343–348. [Google Scholar]
- Falk S., Wolfgang T., Carsten G. Theriogenology. 2006;66:691–709. [Google Scholar]
- Camille Melón L., Maguire J. J. Steroid Biochem. Mol. Biol. 2016;160:196–203. doi: 10.1016/j.jsbmb.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Ji X., Zhang Y. Electromagn. Biol. Med. 2011;30:205–218. doi: 10.3109/15368378.2011.587929. [DOI] [PubMed] [Google Scholar]
- Chahoud I., Faqi A. S. Reprod. Toxicol. 1998;12:667–671. doi: 10.1016/s0890-6238(98)00051-3. [DOI] [PubMed] [Google Scholar]
- Ribeiro E. P., Rhoden E. L., Horn M. M. J. Urol. 2007;177:395–399. doi: 10.1016/j.juro.2006.08.083. [DOI] [PubMed] [Google Scholar]
- Fernandez C. D., Porto E. M., Arena A. C. Int. J. Androl. 2008;31:427–437. doi: 10.1111/j.1365-2605.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- Henkel R., Müller C., Miska W. J. Androl. 1995;16:272–277. [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Li D. K., Odouli R., Wi S. Epidemiology. 2002;13:9–20. doi: 10.1097/00001648-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Yang M. J., Liu J. Y., Wang Y. F. Theriogenology. 2013;80:18–23. doi: 10.1016/j.theriogenology.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Jauchem J. R. Int. J. Hyg. Environ. Health. 2008;211:1–29. doi: 10.1016/j.ijheh.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Shah S. G., Farrow A. J. Occup. Health. 2014;56(5):323–331. doi: 10.1539/joh.13-0196-ra. [DOI] [PubMed] [Google Scholar]
- Darbandi M., Darbandi S., Agarwal A., Henkle R., Sadeghi M. R. Altern. Ther. Health Med. 2017:pii:pii: AT5423AT5423. [PubMed] [Google Scholar]
- Pearce M. S., Hammal D. M., Dorak M. T. Pediatr. Blood Cancer. 2007;49:280–286. doi: 10.1002/pbc.21021. [DOI] [PubMed] [Google Scholar]
- Blaasaas K. G., Tynes T., Irgens A. Occup. Environ. Med. 2002;59:92–97. doi: 10.1136/oem.59.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mageroy N., Mollerlokken O. J., Riise T. Occup. Environ. Med. 2006;63:92–97. doi: 10.1136/oem.2005.021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baste V., Riise T., Moen B. E. Eur. J. Epidemiol. 2008;23:369–377. doi: 10.1007/s10654-008-9236-4. [DOI] [PubMed] [Google Scholar]
- Ohnishi Y., Mizuno F., Sato T. J. Toxicol. Sci. 2002;27:131–138. doi: 10.2131/jts.27.131. [DOI] [PubMed] [Google Scholar]
- Hug K., Grize L., Seidler A. Am. J. Epidemiol. 2010;171:27–35. doi: 10.1093/aje/kwp339. [DOI] [PubMed] [Google Scholar]
- Mjøen G., Saetre D. O., Lie R. T. Eur. J. Epidemiol. 2006;21:529–535. doi: 10.1007/s10654-006-9030-0. [DOI] [PubMed] [Google Scholar]
- Sigman M., Baazeem A., Zini A. Semin. Reprod. Med. 2009;27(2):115–123. doi: 10.1055/s-0029-1202300. [DOI] [PubMed] [Google Scholar]
- Yu K., Deng S. L., Sun T. C., Li Y. Y., Liu Y. X. Molecules. 2018;23(2):pii: E447. doi: 10.3390/molecules23020447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozlem Nisbet H., Nisbet C., Akar A. Res. Vet. Sci. 2012;93:1001–1005. doi: 10.1016/j.rvsc.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Hackney A. C., Moore A. W., Brownlee K. K. Acta Physiol. Hung. 2005;92(2):121–137. doi: 10.1556/APhysiol.92.2005.2.3. [DOI] [PubMed] [Google Scholar]
- Bliss S. P., Navratil A. M., Xie J. Front. Neuroendocrinol. 2010;31:322–340. doi: 10.1016/j.yfrne.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J., Herbison A. E. Mol. Cell. Endocrinol. 2006;254–255:32–38. doi: 10.1016/j.mce.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Sakuma Y., Kato M. Biol. Reprod. 2009;81:327–332. doi: 10.1095/biolreprod.108.074583. [DOI] [PubMed] [Google Scholar]
- Herbison A. E., Moenter S. M. J. Neuroendocrinol. 2011;23:557–569. doi: 10.1111/j.1365-2826.2011.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]