 Objective: The complex components of PM2.5 including metal elements transported through the blood brain barrier could induce nervous system damage.
Objective: The complex components of PM2.5 including metal elements transported through the blood brain barrier could induce nervous system damage.
Abstract
Objective: The complex components of PM2.5 including metal elements transported through the blood brain barrier could induce nervous system damage. This study discusses the relationship between rats’ learning and memory and changes in the hippocampal neuron histomorphology and neurotransmitter levels induced by PM2.5 exposure. Methods: Male rats were treated with different concentrations of PM2.5 by tracheal perfusion once per week for up to 12 weeks. After the rats were sacrificed, the main metal element contents (Al, Pb, Cu, Mn, As, Cr, Cd, and Ni) of the blood and whole hippocampus, levels of neurotransmitters released in the whole hippocampus and relative receptors, and changes in the hippocampal structure were detected. Results: The results showed that PM2.5 significantly reduced the cognitive learning abilities of rats. PM2.5 exposure increased the contents of hippocampal lead, manganese, and aluminum. The level of glutamic acid was increased in the hippocampal tissues of the 20 mg kg–1 group, in combination with the decreased N-methyl-d-aspartate glutamate receptor (NMDAR) and increased metabotropic glutamate receptor type1 (mGluR1) expression. Increased clearance, a mild disorder of arrangement, and mild edema could be observed in the rat hippocampal neurons treated with PM2.5. Conclusion: PM2.5-induced defects in learning and memory may be related to the morphological abnormalities of the hippocampus and the abnormal expression of neurotransmitters and their receptors.
Introduction
Atmospheric particulate matter (PM) is the primary pollutant most affecting urban air quality in both industrially developed countries and developing countries. The health effects of PM pollution have become the focus of public and national governments.1,2 Fine particulate matter (PM2.5) with an aerodynamic diameter of ≤2.5 μm poses potential hazards due to its large surface area, strong adsorption force, and ability to adsorb heavy metals and various toxic substances, such as polycyclic aromatic hydrocarbons and polychlorinated biphenyls.3,4 PM2.5 can remain suspended in the atmosphere for a long time; not only can it invade the lower respiratory tract, causing inflammation and damage to the respiratory system, but it can also enter through the alveoli into the blood circulation, consequently inducing extensive damage to the body.3,5 PM2.5-related premature deaths throughout the world have increased from 800 000 in 2004 to 3.2 million in 2010.6 Therefore, the health effects of PM2.5 particles have attracted attention and been given emphasis as a top research priority in the world.
In recent decades, studies in the field of international environmental epidemiology have confirmed that exposure to atmospheric particulate matter (inhalable particulate matter, PM10), particularly PM2.5, can lead to an increase in heart and lung system diseases and an increase in total morbidity and mortality. The evidence is consistent with the adverse health effects of short-term exposure to PM2.5 across a range of important health outcomes and diseases. A 10 μg m–3 increment in PM2.5 was associated with a 1.04% increase in the risk of death. Worldwide, there is substantial regional variation from 0.25% to 2.08%. Respiratory causes of death were higher than cardiovascular causes (1.51% vs. 0.84%). Positive associations with mortality were seen for most other causes of death and for cardiovascular and respiratory hospital admissions.7 One European cohort study, which created a total study population of 367 251 participants, observed a significantly increased hazard ratio (HR) for a PM2.5 of 1.07 (95% CI: 1.02–1.13) per 5 μg m–3 by using cohort-specific statistical analyses. Long-term exposure to fine particulate air pollution was associated with natural-cause mortality.8
PM2.5, by its chemical composition, including metal elements, can have an effect on the respiratory, cardiovascular, and neurological systems, induces reproductive toxicity, and is involved in carcinogenesis.9,10 The toxic mechanism of heart and lung damage induced by PM2.5 is caused by oxidative stress effects, local and systemic inflammation, changes in autonomic nervous function, blood circulation state changes, the physical condition of blood vessels, and the direct toxic effect.11 After infiltrating the lung tissue, PM2.5 can cause local oxidative stress and inflammation, damage the lipids, proteins and DNA of biological membranes, and combine with inflammatory factors to cause damage to the respiratory tract, causing reduced lung function and increased respiratory system disease. Particles stimulate the release of lung inflammatory cytokines, which can change the oxidative stress state and the level of inflammation of the circulatory system, prompting the expression of inflammatory cytokines, chemokines, and adhesion molecules, and cause a systemic inflammatory response, which may cause adverse effects on the multifarious tissues and organs, and exert direct toxic effects on multiple systems, including the cardiovascular and nervous systems.11,12 Long-term exposure to particulate air pollution levels typical of exposure in major cities around the globe can alter affective responses and impair cognition.13
This study aimed to determine whether PM2.5 can cause neurotoxicity and the resultant behavioral and neurotransmitter changes. After exposure to PM2.5, the learning and memory abilities of rats and changes in the accumulation of metal elements, neurotransmitters, and related proteins in the hippocampus of rats were detected to clarify the potential neurotoxicity induced by PM2.5 and the primary mechanism.
Materials and methods
Main reagents
Normal saline (NS) was obtained from Shandong Kangning Pharmaceutical Co., Ltd (Shandong, China); glutamate (Glu), serine, glycine, and γ-aminobutyric acid standard substances from Sigma Chemical Co. (USA); absolute ethyl alcohol from Samtec Tianjin Chemical Reagent Co., Ltd (Tianjin, China); methyl alcohol and nitrate (with an excellent level of purity) from Beijing Chemical Works (Beijing, China); and perchloric acid from Tianjin Zhengcheng Chemical Products Co., Ltd (Tianjin, China).
Preparation of a mixed PM2.5 suspension
The atmospheric PM2.5 sample was provided by the environmental monitoring center of Tangshan city and the sampling location was on the roof of that center. The sample was collected from December 2014 to February 2015 during the winter season of the city. An approximately 100 m3 air sample was collected over 24 h on each sampling occasion. The membrane filter carrying PM2.5 was placed into ultra-pure water and the particles were eluted by ultrasonic oscillation. After 30 min of oscillation, the supernatant fluid was filtered through five layers of sterile gauze. The liquids were dried to obtain PM2.5 particles. A control membrane filter was procedurally treated by ultrasonic oscillation in NS as mentioned above and the liquid was utilized in the control animals. The gained PM2.5 particles were observed by electron microscopy and confirmed that their diameters were less than 2.0 μm. And then the PM2.5 particles were weighed and dissolved in NS to make a 4 mg ml–1 stock solution and the liquid was preserved at 4 °C. Before use, the suspensions were subjected to 30 min ultrasonic oscillation to scatter the particles and then sterilized by autoclaving. The procedure was in accordance with our previous study.3
Animal treatment with PM2.5
32 adult specific-pathogen-free (SPF) Sprague-Dawley male rats weighing 200–220 g were purchased from the Institute of Hygiene and Environmental Medicine, Academy of Military Medicine (the license number was SCXK-(Army) 2009-003 and the certificate of conformity number was 0001596). The rats were randomly divided into four groups, namely the control group and three treatment groups. They were allowed food and water ad libitum for one week. After anesthetizing the rats with ether, each rat in the three exposed groups was administered PM2.5 working solution (10 ml per kg body weight) by tracheal perfusion. The dosages of exposure used in this study for the three groups were 5 mg kg–1, 10 mg kg–1, and 20 mg kg–1. Each working solution was freshly prepared by diluting the stock solution with NS. For the control group, each rat was treated by the same method with NS (10 ml per kg body weight), which was processed by oscillation of the control membrane filter. These experimental rats were treated once per week for up to 12 treatments. After the last administration, all rats were euthanized 5 days later by ether anesthesia. The whole blood volumes were gathered and the lungs, liver, kidney, and cerebral cortex were removed. All biological samples were immediately stored at –20 °C. Specimens (0.1 g) were placed into a small beaker and then digested with 4 ml of mixed concentrated acid (perchloric acid: nitric acid at 1: 4) for 12 h. After that, the beakers were placed on one electric hot plate until white crystals appeared on the bottom of the containers. The capacity was filled to 5 ml by adding dilute nitric acid (1%) after cooling. Eventually, the contents of heavy metal elements in these samples were determined using an ICP-MS instrument. The treatment was conducted in accordance with our previous study.3
The detection of heavy metal elements
Agilent 7500a inductively coupled plasma-mass spectrometry (ICP-MS) (Agilent, Santa Clara, USA) was employed to measure the contents of eight kinds of heavy metal elements in these samples. The working conditions and the instrument parameters are listed in Table 1.3 Agilent Calibration Verification Standard solutions were diluted with 1% HNO3 to obtain the standard liquids (STD1). For each heavy metal element, STD1 was diluted to six different concentrations by multiple dilution. For STD1, the minimum concentration was 0 μg L–1 for all of the heavy metal elements and the maximum concentrations were 200 μg L–1 for aluminum (Al), lead (Pb), copper (Cu), manganese (Mn), arsenic (As), and chromium (Cr), and 20 μg L–1 for cadmium (Cd) and nickel (Ni). An internal standard element solution (ISTD, 1 μg ml–1) was prepared by dilution of 10 μg ml–1 Li6, Sc, Ge, Y, In, Tb, and Bi and 1% HNO3 was used as the blank (STD0). The ICP-MS was equipped with an autosampler and an Integrated Sample Introduction System with a Discrete Sampler (ISIS-DS). A Micromist glass concentric nebulizer (Glass Expansion, MA, USA), a quartz torch with a 2.5 mm diameter injector and Shield Torch Technology (Agilent Technologies, CA, USA) were used in the detection. For each rat from the four groups, the hippocampus and whole blood (0.1 g) were detected with the ICP-MS instrument. The method was according to our previous study.3
Table 1. Operating parameters for the 7500a ICP-MS instrument.
| Parameters | Setting |
| Flow rate of carrier gas (L min–1) | 1.14 |
| Sampling depth (mm) | 5.2 |
| Radio-frequency power (W) | 1480 |
| Spray chamber temp (°C) | 2 |
| Sample cone | Nickel skimmer |
| Sampling pattern | Quantitative |
| Scanning mode | Jump peak |
| Times of repetition | 3 |
Neuroethology experiments
Open field test (OFT)
The OFT was performed using the same protocol as previously described14 with minor modifications. The experimental device consisted of an open field reaction tank and an automatic data acquisition and processing system (purchased from Shanghai Xin-ruan Information Technology Co.). The tank size was 100 × 100 × 40 cm2, and the bottom of the box was divided into 25 equilateral squares, sized 4 × 4 cm2. A digital camera covering the entire field was placed above the box. The box was cleaned thoroughly before each animal was tested. The animal was replaced in its home cage immediately after the test. The rats were placed in the open field reaction tank for 3 min, and their movement was recorded using a digital camera. The number of squares crossing by spontaneous moves (SM), the percentage of duration of time spent in the center square (duration = time spent in the center square (s)/total time (s) × 100%), and the number of hind legs standing were analyzed automatically using sports track in XR-Xmaze supermaze software (Shanghai Xin-ruan Information Technology Co.).
Y-type electric maze experiment
After exposure for up to 12 weeks, in a dark room with a quiet environment, the learning and memory ability of rats was also evaluated using the Y-type electric maze test.15,16 The Y-maze (purchased from Zhangjiagang Bio-Medical Instrument Factory) consisted of three arms (regions I–III, 30 × 5 × 20 cm) that converged at an equilateral triangular central area. At the distal end, each arm had a lamp. A safe region was associated with illumination, while other regions featured electrical foot stimulation with an electrical current (50 V, 0.6 mA). The animals were monitored and the rate of learning to escape to the safe arm of the maze was determined for each animal. Upon electrical stimulation, direct escape from the primary region to the safe region was considered to be the correct reaction. The test was then started, and they were placed beside the Y-type electric maze and allowed to adapt to the environment for 5 min, and then testing began randomly, starting from one direction. An electrical current was used for shock administration. The time needed to escape electrical stimulation to the safe region was utilized in order to quantitate learning and memory abilities in the rats. Between every two tests, there was a 30 s rest. The animals were considered to have learned the maze once they made 9/10 correct choices during one continuous test. A smaller value was assumed to reflect improved learning. Rats that did not correctly choose escape during testing with electrical stimulation shock were assigned a lower score.
The determination of neurotransmitter contents in the hippocampus
Neurotransmitter contents, including those of glutamic acid, serine, glycine, and γ-aminobutyric acid in the hippocampus, were detected using high-performance liquid chromatography (HPLC). O-Phthalic aldehyde (OPA), 25 mg, was added to 0.5 ml of methanol solution, and then 40 μl β-mercaptoethanol and 5 ml boric acid-sodium hydroxide buffer (0.4 mol L–1, pH 9.6) were added to form the OPA derivative reagent. After blending, the mixture was stored at 4 °C, avoiding light. Samples (20 μl) and standards of glutamic acid, serine, glycine, and γ-aminobutyric acid were added into 20 μl OPA derivative reagent. The mixture was blended for 30 s and allowed to stand for 80 s, and then 20 μl of samples were loaded onto the HPLC instrument within 2 min. Methanol/NaAc buffer solution was used as the two mobile phases. The velocity was 0.9 ml and the column temperature was set at 29 ± 1 °C. The fluorescence detector was set at an excitation wavelength of 340 nm and an emission wavelength of 450 nm. It was analyzed by Prostar software for peak area, and presented a standard curve for peak area with the standard amino acid concentrations. The concentration of the sample acid was calculated via the external standard method.
The detection of glutamate receptors metabotropic glutamate receptor type1 (mGluR1) and N-methyl-d-aspartate glutamate receptor (NMDAR)
After the animals were sacrificed, the hippocampus was taken out from each rat and the samples were mixed separately and centrifuged at 3000 rpm for 10 min immediately. And then sediment cells were resuspended in 0.5 mL PBS and treated with Tris-buffered ammonium chloride solution to lyse red blood cells. The supernatant of the hippocampus was stored at –80 °C. The glutamate receptors mGluR1 and NMDAR in the hippocampus were detected using the Rat ELISA Kit (Beijing Dong Song Bo Industry Biotechnology Co., Ltd) according to the manufacturer's instructions.
Assessment of hippocampal histopathology
The hippocampal tissue was removed from 4% formaldehyde, followed by regular dehydration, transparent, paraffin embedding, sectioning, and dewaxing. After hematoxylin–eosin (HE) staining, again with dehydration, transparent, and sealing, hippocampal histopathology changes were observed under a light microscope (Nikon Eclipse 80i, Tokyo, Japan) and photos were taken.
Ultrastructural analysis of hippocampal neurons
After complete exposure, a total of three rats randomly selected from each treatment group were analyzed for the hippocampal ultrastructure by transmission electron microscopy (TEM) examination. The hippocampal slices were selected and ultra-thin sections (at a thickness of 400–600 μm) were made as previously described.17,18 These ultrathin sections were stained with lead citrate and uranyl acetate and observed under an FEI Tecnai 12 and Tecnai G2 type electron microscope (Holland). Then, photos of the hippocampus were taken at 10 000–20 000× magnification.
Statistical analysis
All data were analyzed using two-way univariate analysis of variance (ANOVA) followed by Tukey's (equal variances assumed or homogeneity of variance after the variable transformation) or Dunnett's T3 (equal variances not assumed after the variable transformation justification) post hoc test between groups using Statistical Package for Social Sciences software (SPSS version 17.0, Chicago, IL, USA). The results are represented as mean ± SD. All tests were two sided; P < 0.05 was considered statistically significant.
Results
The content of heavy metal elements in PM2.5 fine particles
After collection, the membrane filters carrying PM2.5 particles were processed and their heavy metal elements were detected by ICP-MS. The results are shown in Table 2, which were similar to the results already published in part in our previous study.3 The contents of eight kinds of heavy metal elements in the atmospheric PM2.5 samples ranked from the highest to the lowest level are Al, Pb, Cu, Mn, As, Cr, Cd, and Ni. Component analysis showed the airborne concentrations calculated according to the results of the contents of these heavy metal elements in the membrane filters and they are also shown in Table 2.
Table 2. The contents of eight main heavy metal elements in PM2.5 fine particles.
| Metal elements | Contents in membrane filters (μg g–1) (%) | Airborne concentrations (μg m–3) (%) |
| Aluminum (Al) | 1163 (79.5%) | 2.14 (60.5%) |
| Lead (Pb) | 169 (11.5%) | 0.66 (26%) |
| Copper (Cu) | 113 (7.7%) | 0.41 (17.1%) |
| Manganese (Mn) | 31 (2.1%) | 0.23 (11.8%) |
| Arsenic (As) | 6 (0.4%) | 0.056 (2.2%) |
| Chromium (Cr) | 5 (0.3%) | 0.0283 (1.1%) |
| Cadmium (Cd) | 2 (0.14%) | 0.0065 (0.26%) |
| Nickel (Ni) | 0.5 (0.03%) | 0.0029 (0.11%) |
The effects on the neural behavior of rats induced by PM2.5 particles
The Y-type electric maze and OFT were used to detect the neurobehavioral effect on rats treated with PM2.5. The experimental results from the Y-type electric maze showed that, compared with the control group, error reaction times increased in the 20 mg kg–1 group of rats and the difference was statistically significant (P < 0.05) (Table 3).
Table 3. The Y type electric maze experimental results of rats (mean ± SD, n = 10).
| Groups (mg L–1) | Error reaction times | Latent period (s) |
| Control | 3.80 ± 1.52 | 5.36 ± 1.39 |
| 5 | 5.61 ± 1.79 | 6.24 ± 2.59 |
| 10 | 5.87 ± 2.26 | 6.87 ± 2.66 |
| 20 | 6.27 ± 1.98* | 6.91 ± 2.67 |
| F | 20.75 | 121.17 |
| P | 0.038 | 0.34 |
The OFT results showed that the number of squares crossed was smaller and the residence time in the middle squares was increased in the 20 mg kg–1 group of rats, relative to the control group. These differences were statistically significant (P < 0.05) (Table 4).
Table 4. The open field test results of rats (mean ± SD, n = 10).
| Groups (mg L–1) | Squares crossing times | Upright times | Modification times | Middle squares times (s) |
| Control | 58.72 ± 9.63 | 12.73 ± 4.18 | 3.56 ± 2.46 | 8.57 ± 2.17 |
| 5 | 54.67 ± 8.51 | 11.07 ± 3.72 | 3.75 ± 2.48 | 9.29 ± 2.61 |
| 10 | 53.58 ± 6.34 | 9.57 ± 4.27 | 3.67 ± 1.94 | 10.56 ± 3.83 |
| 20 | 49.80 ± 7.84* | 10.13 ± 4.20 | 3.81 ± 2.15 | 13.69 ± 3.02* |
| F | 112.71 | 1.88 | 1.21 | 23.08 |
| P | 0.021 | 0.17 | 0.31 | 0.045 |
The effect on body weight and hippocampal organ coefficient
PM2.5 particle-treated rats were weighed both at the beginning of exposure and at the end of the treatment. The results showed that no significant difference was detected between the control group and each experimental groups (P = 0.74, F = 0.36; P = 0.07, F = 2.85). After the treatment was completed, the adult rats were killed and the hippocampi were separated and weighed. However, there were no differences detected in the hippocampal organ coefficient (hippocampus weight coefficient (%) = hippocampus weight/body weight × 100%) between the groups (P = 0.198, F = 1.37).
The contents of mainly heavy metal elements in the rat hippocampus
The results of the contents of mainly heavy metal elements in the rat hippocampus are shown in Table 5. Compared with the control group, the lead contents in the rat hippocampi of the three treated groups were significantly increased (P < 0.05). For manganese, the hippocampal tissue contents in the10 mg kg–1 and 20 mg kg–1 groups were much higher than those of the control group (P < 0.05). Furthermore, the content of aluminum in the 20 mg kg–1 group was increased relative to the control group (P < 0.05). However, among the five top-ranked heavy metal elements in the PM2.5-treated samples, there were no difference between the control and treated groups for copper and arsenic element contents (P > 0.05).
Table 5. Heavy metal content in rat hippocampus (mean ± SD, n = 8).
| Groups (mg L–1) | Al (μg g–1) | Pb (μg g–1) | Cu (μg g–1) | Mn (μg g–1) | As (μg g–1) |
| Control | 2.67 ± 0.54 | 2.80 ± 0.67 | 1.80 ± 0.36 | 0.95 ± 0.17 | 0.05 ± 0.014 |
| 5 | 2.93 ± 0.48 | 6.91 ± 0.86* | 1.72 ± 0.35 | 1.06 ± 0.13 | 0.07 ± 0.02 |
| 10 | 3.03 ± 0.32 | 9.48 ± 1.20* | 1.83 ± 0.32 | 1.33 ± 0.20* | 0.072 ± 0.031 |
| 20 | 4.78 ± 0.60* | 18.61 ± 1.93* | 2.08 ± 1.78 | 1.48 ± 0.23* | 0.047 ± 0.01 |
| F | 3.616 | 5.10 | 2.255 | 7.30 | 1.683 |
| P | 0.04 | 0.011 | 0.124 | 0.003 | 0.216 |
Changes in neurotransmitter content in the rat hippocampus
The detection results for the contents of glutamate, serine, glycine, and γ-aminobutyric acid in the rat hippocampus are shown in Table 6. The level of glutamate was significantly increased in the 20 mg kg–1 group compared with the control group, and the differences had statistical significance (P < 0.05).
Table 6. The contents of neurotransmitters in rat hippocampus (mean ± SD, n = 8).
| Groups (mg L–1) | Glutamate (μmol g–1) | Serine (μmol g–1) | Glycine (μmol g–1) | γ-Aminobutyric acid (μmol g–1) |
| Control | 4.96 ± 0.63 | 3172.77 ± 667 | 1807.62 ± 256 | 3163.46 ± 864 |
| 5 | 5.46 ± 0.48 | 3314.69 ± 876 | 1793.93 ± 335 | 3251.58 ± 798 |
| 10 | 5.50 ± 0.39 | 3539.64 ± 864 | 2050.45 ± 345 | 3451.85 ± 962 |
| 20 | 6.00 ± 0.45* | 2866.59 ± 478 | 1832.1 ± 238 | 2980.51 ± 763 |
| F | 5.884 | 2.281 | 1.090 | 0.424 |
| P | 0.003 | 0.058 | 0.182 | 0.737 |
The glutamate receptor (NMDAR and mGluR1) levels in the rat hippocampus
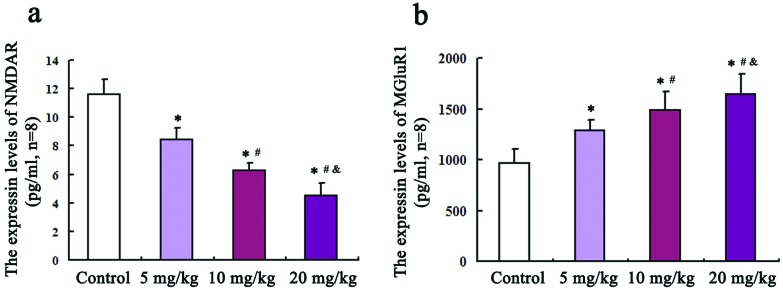
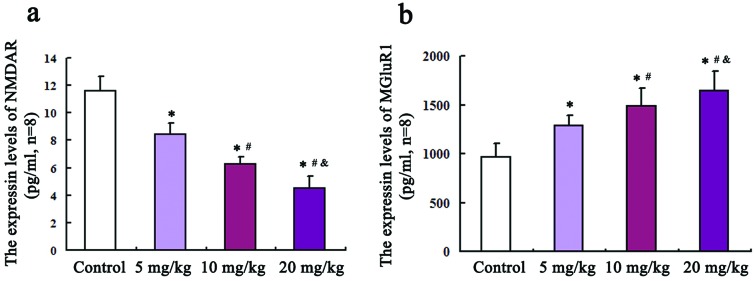
An ELISA method was employed to detect the levels of glutamate receptors NMDAR and mGluR1 in the hippocampus. As shown in Fig. 1(a and b), compared with the control group, the protein levels of NMDAR in 5, 10, and 20 mg kg–1 groups were all remarkably decreased (P = 0.02, F = 3.27; P = 0.013, F = 3.76; P = 0.001, F = 4.58). Inversely, the mGluR1 protein levels in three treated groups are all increased relative to those of the control group and the differences were statistically significant (P = 0.03, F = 462; P = 0.024, F = 589; P = 0.017, F = 741).
Fig. 1. The protein expression levels of NMDAR and mGluR1 in the rat hippocampus. An ELISA method was employed to detect the levels of glutamate receptors NMDAR and mGluR1 in the hippocampus, which are shown in (a) and (b), respectively. Compared with the control group, * P < 0.05; compared with the 5 mg kg–1 group, #P < 0.05; compared with the 10 mg kg–1 group, &P < 0.05.
The pathological changes of hippocampal neurons
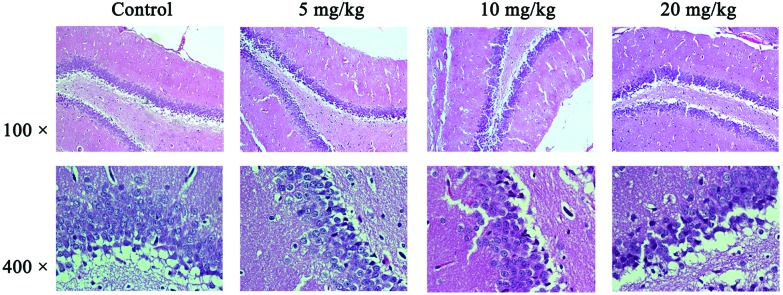
The pathological results were detected by HE staining and observed under an optical microscope (Fig. 2, with 100× for the hippocampus and 400× for the CAI area of the hippocampus). In the hippocampal CAI area of the control group, the normal morphology of neurons, which were orderly arranged, was seen with an unambiguous cell outline, hyperchromatic nuclei, and a rich and uniform cytoplasm. There were no significant differences between the 5 mg kg–1 group and the control group in the cell structure and morphology. For the 10 mg kg–1 group, individual neurons presented pyknosis (the mean number of pyknotic neurons was 9 in the 10 mg kg–1 group vs. 3 in the control group per high power field) and the boundary of the nucleus and cytoplasm was blurred for partial nucleus neurons in the hippocampal CAI area. For the 20 mg kg–1 group of the hippocampal CAI area, the neuron clearance was increased, cells were arranged in slight disorder, individual neurons with nucleus condensation were present, and the hippocampus had slight edema.
Fig. 2. Pathological results in the hippocampal tissues. The results were detected by HE staining and observed under an optical microscope. The changes in tissue histopathology were observed under a light microscope (Nikon Eclipse 80i, Tokyo, Japan) and photos were taken (×100 and ×400, respectively, for each group). Shown are the representative images for the control group (a), 5 mg kg–1 group (b), 10 mg kg–1 group (c), and 20 mg kg–1 group (d), respectively. Neurons presented pyknosis and the blurred boundary between the nucleus and cytoplasm for partial nucleus neurons in the hippocampal CAI area can be seen in the 10 mg kg–1 group. Neurons with nuclear condensation and slight edema in the hippocampus can be seen in the 20 mg kg–1 group.
Ultrastructural changes in hippocampal neurons
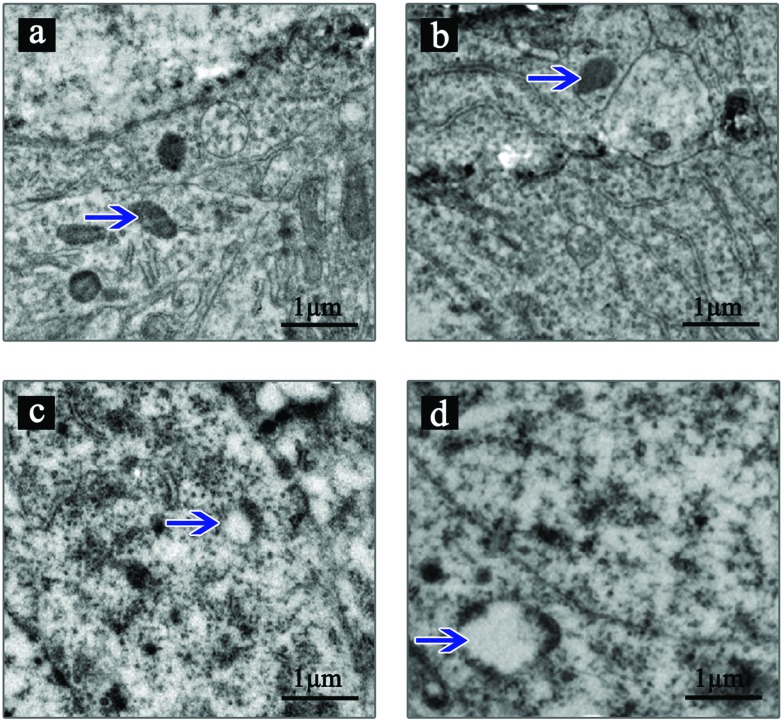
The ultrastructure of hippocampal neurons was observed by TEM (Fig. 3). The results showed that the control group and 5 mg kg–1 group rats with a normal ultrastructure of hippocampal neurons presented an integrated cell and organellar structure. In the 10 mg kg–1 group, mitochondrial swelling can be found in some hippocampal neurons. In the 20 mg kg–1 exposure group, mitochondrial swelling was more obvious and existed with a small number of cavitation mitochondria.
Fig. 3. TEM results of the ultrastructural changes in hippocampal neurons. The ultrastructure of hippocampal neurons was observed by TEM and the photos were taken. Shown are the representative images. The control group (a) and 5 mg kg–1 group (b) have a normal ultrastructure of hippocampal neurons including mitochondria (pointed by blue arrows). Mitochondrial swelling can be found in hippocampal neurons in the 10 mg kg–1 group (c) (pointed by blue arrow). In the 20 mg kg–1 group (d), mitochondrial swelling was more obvious and existed with a small amount of cavitation mitochondria (indicated by blue arrow).
Discussion
Long-term exposure to PM2.5 was related to lower scores in the tests of intelligence.19–21 The previous studies confirmed that Alzheimer's disease and Parkinson's disease are associated with PM2.5 exposure showing that the fine particles are a risk factor for diseases of the central nervous system.22,23
For the present study, a PM2.5 sample was selected from the Tangshan city by an environmental monitoring station and the sampling site was the living quarters. The chronic poisoning rat model was established through PM2.5 exposure by respiratory tract perfusion. To explore the potential danger to the nervous system of rats, PM2.5 particle components, the change in the metal element content in the hippocampus, and the change in neurotransmitters in the hippocampus of rats were detected and analyzed.
Because their diameter is minute, PM2.5 particles can enter through the respiratory tract directly into the bronchi and alveoli and through the pulmonary capillaries into the systemic blood circulation system, with the blood flow to the body causing damage to other systems.24,25 However, the blood brain barrier (BBB) can prevent certain substances in the blood from migrating to the brain tissue.26,27 Larger particles and the macromolecular substances carried by the surface of PM2.5 are mostly blocked by the BBB. Some nanoscale particles and metal elements of PM2.5 can get through the BBB into the brain tissue and cause damage to the brain.28 Additionally, PM2.5 may be linked to cerebrospinal fluid (CSF) perfusion in the brain, and CSF may be another pathway to allow PM2.5 traveling to the hippocampal region.29 In the present study, the detection results of metal elements in the hippocampus showed that Pb, Al, and Mn were the top three metal elements.
Learning and memory are the advanced functions of the brain, and neural behavior experiments are the test for these capabilities in the animal. The Y-type electric maze and the OFT are two commonly used detection methods.30 The present study showed that the experimental rats exposed to PM2.5 displayed significantly decreased space cognitive and exploratory behavior abilities in the new environment. PM2.5 exposure may lead to a decline in the function of learning and memory in rats.
Neurotransmitters are specific chemicals acting as “messengers” in the synaptic transmission between neurons. Glutamic acid (Glu) is one kind of excitatory neurotransmitter with the highest levels and extensive roles in the brain. About half of Glu is involved in regulating synaptic transmission in the central nervous system and regulates almost all brain functions, including learning, memory, cognitive ability, development, and emotion. Glu is widely distributed in the tissues of the central nervous system, such as the cerebral cortex, hippocampus, striatum, and amygdaloid body, which are the areas of vulnerable brain tissue.31 Primary mechanical forces of the initial injury can compromise the BBB and cellular membranes, allowing increased glutamate release into the extracellular space.32 This study showed that Glu increased in the rat hippocampus in the 20 mg kg–1 group. When extracellular accumulation of Glu occurs at a high concentration, it can activate the ionic or metabotropic Glu receptors, causing excitatory neurotoxicity, neuron swelling and necrosis.33,34
After finding that PM2.5 exposure significantly increased the level of Glu in the rat hippocampus, we further tested the levels of neurotransmitter receptors of Glu. NMDA is a type of important ionic excitatory amino acid (excitatory aminoacid) receptor in the central nervous system.35 During the development of the nervous system, NMDAR can not only affect neuronal migration and synaptic plasticity to regulate the development of the central nervous system and play an important physiological role by changing its structure and function, but it also plays a key role in the formation of neuronal circuits. Studies confirmed that NMDAR is a vital receptor over the course of learning and memory.36 In this study, the results showed that the exposure of PM2.5 has an inhibitory effect on NMDAR in the rat hippocampus which may affect the abilities of learning and memory.37
mGluR is a metabolic Glu receptor, which belongs to the family of G protein coupled receptors (GPCRs).38 A related study showed that chronic inhalation of nanoscale (<200 nm) PM could induce neurotoxicity by changing the level of neuronal glutamate receptor subunits (GluA1) in mice hippocampus.39 mGluR1 is a receptor for the excitability amino acid neurotransmitter related to synaptic plasticity in the nervous centralis. Activation of mGluR1 typically occurs in response to strong activity and triggers long-term potentiation (LTP) of synaptic transmission in many brain regions including the hippocampus.40 This study showed that the exposure to PM2.5 could increase mGluR1 expression, causing strong nerve excitability toxicity.
In order to observe the histomorphological changes in the rat hippocampal CAI region induced by PM2.5 exposure, pathological and ultrastructural changes were detected by HE staining and TEM observation. The HE staining results showed that PM2.5 exposure can lead to individual neuron pyknosis with part of the neuron having a blurred border in the neuronal nucleus and cytoplasm. A 20 mg kg–1 dose of PM2.5 exposure even leads to increased clearance between the neurons, disordered arrangement, increased glial cells, cell nucleus condensation and edema of some single neurons in the hippocampal CAI region. Ultrastructural changes in hippocampal neurons exposed to PM2.5 revealed that in some cells, mitochondria swelled and even formed the cavitation of mitochondria, showing mitochondrial damage in hippocampal neurons.
Collectively, metallic elements including Pb, Al, and Mn, which are at high levels in PM2.5 particles, can enter the blood circulation following lung deposition and reach different organs, such as the brain. These metallic elements can cross the BBB to brain tissue and accumulate in the hippocampus, and may induce the abnormal expression of excitatory neurotransmitters and damage to the hippocampus, which affects the learning and memory function of rats.
Author contribution statements
C.Y.J., Z.L.Q., Q.Z.L., X.Q.L, J.L.Z and S.X conceived, designed and carried out the experiments, analyzed the experimental data and wrote the paper; C.Y.J., Q.Z.L, J.S.Z and Y.H.C guided the experiments and prepared all figures and tables; C.Y.J., Z.L.Q., X.Q.L, and Q.Z.L supervised and directed the project. All authors discussed the results and commented on the manuscript and reviewed the manuscript.
Conflicts of interest
There are no conflicts of interest to declare.
Data accessibility
Data are available upon request.
Ethical statement
All of the experimental animal protocols were approved by the animal ethics committee of North China University of Science and Technology, Tangshan, Hebei province, China. The animal methods were conducted in accordance with the approved guidelines (SYXK(JI) 2010-0038).
Funding
This work was supported by a grant from the Tianjin Municipal Health Bureau of Science and Technology Project Foundation (No. 16KG153).
References
- Hu X., Zhang Y., Luo J. Environ. Pollut. 2011;159:1215–1221. doi: 10.1016/j.envpol.2011.01.037. [DOI] [PubMed] [Google Scholar]
- Brown D. M., Petersen M., Costello S. Epidemiology. 2015;26:806–814. doi: 10.1097/EDE.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Liu H., Alattar M. Sci. Rep. 2015;5:16936. doi: 10.1038/srep16936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergham M., Lepers C., Verdin A. Chem. Res. Toxicol. 2012;25:904–919. doi: 10.1021/tx200529v. [DOI] [PubMed] [Google Scholar]
- Terzano C., Di Stefano F., Conti V. Eur. Rev. Med. Pharmacol. Sci. 2010;14:809–821. [PubMed] [Google Scholar]
- Fang D., Wang Q. G., Li H. Sci. Total Environ. 2016;1:1545–1552. doi: 10.1016/j.scitotenv.2016.06.248. [DOI] [PubMed] [Google Scholar]
- Atkinson R. W., Kang S., Anderson H. R. Thorax. 2014;69:660–665. doi: 10.1136/thoraxjnl-2013-204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelen R., Raaschou-Nielsen O., Stafoggia M. Lancet. 2014;383:785–795. doi: 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- Brook R. D., Franklin B., Cascio W. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Peters A., Veronesi B., Calderón-Garcidueñas L. Part. Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A., Holme J. A., Rosas I. BioMed Res. Int. 2013;2013:279371. doi: 10.1155/2013/279371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Deng F., Wei H. Environ. Sci. Technol. 2014;48:3438–3448. doi: 10.1021/es404778w. [DOI] [PubMed] [Google Scholar]
- Fonken L. K., Xu X., Weil Z. M. Mol. Psychiatry. 2011;16:987–995. doi: 10.1038/mp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu L., Jin X., Zhang Y. Environ. Health Perspect. 2015;11:39. [Google Scholar]
- Zhou J., Zhou L., Hou D. Brain Res. 2011;1388:141–147. doi: 10.1016/j.brainres.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Lu S. M., Yu C. J., Liu Y. H. Brain, Behav., Immun. 2015;44:221–234. doi: 10.1016/j.bbi.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang A., He W. Toxicology. 2007;236:208–216. doi: 10.1016/j.tox.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Jiang C., Zhang S., Liu H. NeuroMol. Med. 2014;16:94–105. doi: 10.1007/s12017-013-8260-z. [DOI] [PubMed] [Google Scholar]
- Ying Z., Xu X., Bai Y. Environ. Health Perspect. 2014;122:79–86. doi: 10.1289/ehp.1307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L., Vojdani A., Blaurock-Busch E. J. Alzheimers Dis. 2015a;43:1039–1058. doi: 10.3233/JAD-141365. [DOI] [PubMed] [Google Scholar]
- Schwela D. Rev. Environ. Health. 2000;15:13–42. doi: 10.1515/reveh.2000.15.1-2.13. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L., Franco-Lira M., D'Angiulli A. Environ. Res. 2015b;140:579–592. doi: 10.1016/j.envres.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Palacios N., Fitzgerald K. C., Hart J. E. Environ. Health. 2014;13:80. doi: 10.1186/1476-069X-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Tu Y., Yu Z. Int. J. Environ. Res. Public Health. 2015;12:8187–8197. doi: 10.3390/ijerph120708187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A., Dominici F., Wang Y. Environ. Health. 2014;13:38. doi: 10.1186/1476-069X-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu L. Adv. Drug Delivery Rev. 2012;64:640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Liu C., Fonken L. K., Wang A. Part. Fibre Toxicol. 2014;11:53. doi: 10.1186/s12989-014-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L., Calderon-Garciduenas A., Torres-Jardon R. Prim. Health Care Res. Dev. 2015c;16:329–345. doi: 10.1017/S146342361400036X. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Vojdani A., Blaurock-Busch E. J. Alzheimers Dis. 2015;43(3):1039–1058. doi: 10.3233/JAD-141365. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D., Philipp S., Feldon J. Cogn. Affect. Behav. Neurosci. 2015;15:878–888. doi: 10.3758/s13415-015-0356-5. [DOI] [PubMed] [Google Scholar]
- Luo J., Min S., Wei K. J. Anesth. 2011;25:657–665. doi: 10.1007/s00540-011-1199-z. [DOI] [PubMed] [Google Scholar]
- Hinzman J. M., Thomas T. C., Burmeister J. J. J Neurotrauma. 2010;27:889–899. doi: 10.1089/neu.2009.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo T. G., Li W. K., Zhang Y. H. Mol. Neurobiol. 2015;51:980–994. doi: 10.1007/s12035-014-8753-2. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Yu M. S., Yik S. Y. Cell. Mol. Neurobiol. 2009;29:1233–1244. doi: 10.1007/s10571-009-9419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz J. T., Javitt D. C. Brain Res. Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. H., Qiu Z. Q., Shu W. Q. Toxicol. Lett. 2009;184:121–125. doi: 10.1016/j.toxlet.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Luscher C., Malenka R. C. Cold Spring Harbor Perspect. Biol. 2012;4:997–1001. doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M. R., Hopkins C. R., Brogan J. T. Biochemistry. 2011;50:2403–2410. doi: 10.1021/bi200129s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan T. E., Davis D. A., Iwata N. Environ. Health Perspect. 2011;119:1003–1009. doi: 10.1289/ehp.1002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C., Huber K. M. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.