The aim of this study was to assess the reproductive toxic effects of arsenic on adult Wistar rats exposed to lead during the perinatal period.
The aim of this study was to assess the reproductive toxic effects of arsenic on adult Wistar rats exposed to lead during the perinatal period.
Abstract
The aim of this study was to assess the reproductive toxic effects of arsenic on adult Wistar rats exposed to lead during the perinatal period. The pregnant rats were allowed ad libitum access to tap water containing 819 mg of lead (Pb) per L or without Pb from conception until weaning. Litter size, survival rate and developmental milestones of the pups delivered by Pb exposed dams were comparable to those of the control rats. Conversely, the pups exposed to Pb during the perinatal period exhibited significant delay in cliff avoidance, negative geotaxis, surface righting reflex, ascending wire mesh and testis descent. The control and perinatal Pb-exposed male rats were maintained on tap water containing 2.3 mg of arsenite (As) per L or without arsenite from the pubertal period (post-natal day 55) to adulthood (post-natal day 115) and assessed for reproductive end points. The results revealed that the (1) relative weights of the testis, epididymis, seminiferous tubules and ventral prostate; (2) daily sperm production; (3) epididymal sperm density and (4) numbers of motile, viable, and HOS tail swelled sperm declined significantly in the rats exposed to either Pb or As. The activity levels of testicular 3β- and 17β-hydroxysteroid dehydrogenases were also significantly decreased in the experimental rats. Significant elevation in the levels of reactive oxygen species and lipid peroxidation in association with reduced activities of antioxidant enzymes in the testis and different epididymal regions was recorded in the experimental rats. In the fertility study, although each male in the control and experimental groups produced a copulatory plug and impregnated a female, the mean conception time significantly increased in the experimental groups. The mean number of implantations decreased significantly in the females mated with the experimental males. Moreover, the results of the present study also indicate that reproductive alterations were more deteriorated in the Pb-exposed rats treated with arsenic when compared to individual exposures. In conclusion, the data clearly suggest that reproductive toxicity in male rats exposed to Pb during the perinatal period is exacerbated by As treatment during the pubertal period.
Introduction
Humans and wildlife are inevitably exposed to an array of environmental and dietary chemicals throughout their life. Among the toxic chemicals, metals and/or metalloids are pervasive elements that are ubiquitously present in the environment. Humans are not always exposed to a single element in the environment. Heavy metals such as lead (Pb) and arsenic (As) have attracted the attention of both scientific and public community owing to their environmental, public health and toxicity issues.1–3 Since antiquity, Pb is a well-known non-essential environmental toxic heavy metal that alters a broad range of physiological, biochemical and behavioural functions. The sources of Pb contamination are Pb-based batteries, paints, varnishes, metal products, and industrial and anthropogenic activities.4 The developing nervous system has long been recognized as a primary target site for Pb-induced toxicity.5 The levels of Pb were detected in the placental tissue, umbilical cord, maternal blood and milk samples from women indicating its ability to cross the placental–foetal barrier and cause irreversible damage to the development of the F1 generation.6,7 Previously, it has been reported that gestational and lactational exposure to Pb resulted in developmental and behavioural alterations in rats.8,9 Furthermore, Pb is able to cross the blood–testis barrier and can accumulate in the testis and thereby alter its function.10 Pb poisoning can affect reproductive functions, and though several researchers including us have focussed on spermatogenesis and steroidogenesis,11,12 studies related to the effect of perinatal exposure to Pb on reproduction have received little attention.
Arsenic, a metalloid present abundantly in nature, is well known for its carcinogenic nature in humans.13 Arsenic and its compounds are released into the environment due to human activities and their robust use in industry, agriculture, and medicine and feed additives.14,15 Arsenic intoxication is associated with acute problems like encephalopathy, neuropathy, nausea, vomiting, abdominal pain and severe diarrhea, while chronic exposure to As causes several types of cancers and metabolic syndromes like diabetes and gastrointestinal tract, cardiac and neuronal problems.15 Previously, we have reported that mice exposed to As showed reduction in the weights of reproductive organs, steroidogenesis and spermatogenesis with elevated levels of As in the testis,16 indicating reproductive toxicity. Sarkar et al.17 also reported that acute exposure to arsenic causes rapid and extensive disruption of spermatogenesis.
Many environmental contaminants including metals have been reported to disturb the pro- and anti-oxidant balance of cells, thereby inducing oxidative stress.3,18 Reactive oxygen metabolites such as superoxide anion, singlet oxygen, hydroxyl and peroxyl radicals and hydrogen peroxide are generally considered cytotoxic agents because of their ability to induce oxidative stress. Free radicals or reactive oxygen species (ROS) generated in the tissues are effectively scavenged by the antioxidant defence system that consists of antioxidant enzymes, such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx) and glutathione reductase (GR). ROS are essential for the regulation of several physiological functions, including sperm maturation, and at higher levels, ROS cause damage to sperm and other cytoplasmic organelle membrane structures through peroxidation of phospholipids, proteins, and nucleotides, thereby altering sperm motility and fertilization capacity.19–21
Both Pb and As have been listed in the top ten binary elemental pollutants in soil and water with the majority of metal smelters exposed to this combination of chemicals.22–25 Considering the fact that Pb can cross the placental–foetal barrier and it is present in milk, this study was designed to determine the effect of perinatal exposure to Pb on the reproductive parameters at adulthood in Wistar rats. Furthermore, the study broadened its scope by exposing F1 male progeny to arsenic during their spermatogenic period and examining the reproductive potential at adulthood.
Materials and methods
Procurement and maintenance of animals
Timed-pregnant Wistar rats were purchased from an authorized vendor (M/S Raghavendra Enterprises, Bengaluru, India) and housed individually. The animals were maintained under controlled conditions at 25 ± 2 °C ambient temperature, 55%–60% relative humidity, and 12 h light and 12 h dark cycle and were supplied with a standard pellet diet (purchased from Sai Durga Agencies, Bengaluru, India). All the experiments were conducted in accordance with the guidelines of the Committee for the Purpose of Control and Supervision on Experiments on Animals, Government of India.26 All the experimental protocols were approved by the Institutional Animal Ethical Committee (Regd. No. 438/01/a/CPCSEA/dt.17.07.2001), S.V. University, Tirupati with resolution No: 57/2012/(i)/a/CPCSEA/IAEC/SVU/PSR-KPR dt.08-07-2012. The study was planned and organized as completely double blind.
Chemicals
Sodium arsenite (≥90% purity), androstenedione (≥98% purity), dihydroepiandrosterone (98% purity), NADPH (≥97% purity), NAD (≥98% purity), iodonitrotetrazolium chloride (95% purity), horseradish peroxidase (>90% purity), reduced glutathione (≥98% purity), glutathione reductase (≥90% purity), and oxidized glutathione (≥98% purity) were purchased from Sigma Chemical Company (St Louis, Missouri, USA). Lead acetate (≥99.5% purity), malondialdehyde (purity ≥95%) (MDA), thiobarbituric acid (purity ≥99%) and pyrogallol (purity ≥99%) were purchased from Merck, Darmstadt, Germany.
Experimental design
The pregnant dams were equally randomized into two groups (control and Pb-treated groups) with 10 rats in each group. The rats in the Pb-treated group were allowed ad libitum access to tap water containing 819 mg L–1 lead (0.15% lead acetate) throughout the gestation period. The Pb dose used in the present study is based on our earlier reports.11,18 The rats in the control group were allowed ad libitum access to tap water. All pregnant rats were allowed to deliver pups. The dams in the Pb-treated group were continued on Pb treatment until weaning.
At birth, all litters were culled to eight pups. Whenever possible, only male pups were kept within the litter and females were kept just to maintain equal litter sizes. On postnatal day (PND) 21, all male rats from both control and Pb exposed groups were separated and divided into two equal groups. The first group served as the control and was maintained on tap water and the rats in the second group were maintained on tap water containing 2.3 mg As per L (4 ppm sodium arsenite) from the pubertal period (PND 55) to adulthood (PND 115). The rationale for choosing a 60-day experimental period was to cover one complete spermatogenic cycle which is 60 days in Wistar rats.27
Observations
During the experimental period, the rats were observed daily for overall appearance and signs of toxicity such as postural abnormalities, unusual respiration, vocalization, salivation, head flicking, abnormal appearance of fur, compulsive biting, urination, circling and walking backward.
Developmental milestones and behavioural tasks
Litter size, birth weight and survival rate of the pups were determined. The pups were individually identified by paw tattoo and observed daily for clinical signs of toxicity until the completion of weaning. Incidences of pinna (ear flap) unfolding, lower and upper incisor eruption, fur development and eye opening were determined by an investigator that was blind folded to the treatment. The occurrence of testes descent (by scrotum palpation) was evaluated in all males every morning (8.00–10.00 AM) from PND 25 onwards and the day of testicular descent was recorded.
Two male pups from each mother rat (20 pups from each group) were selected randomly and subjected to the following behavioral tests: cliff avoidance within 30 s (on PND 5), surface righting reflex (on PND 6), negative geotaxis (on PND 7) and ascending wire mesh (on PND 16). All behavioral tests were performed between 08:00 A.M. and 12.00 noon according to the procedures described elsewhere.28
Fertility studies
Twelve rats each from the control and experimental groups were transferred to a mating cage and cohabited with untreated 95 ± 5 day old normal cyclic female rats in a 1 : 1 ratio for a maximum period of 5 days. During cohabitation, the rats were checked for the presence of a vaginal plug and vaginal washings were evaluated for the presence of sperm every morning at 5.30 am. The females with positive plug and/or sperm were separated from the males and assigned day zero of gestation. All the pregnant animals were evaluated for mortality, morbidity and general clinical signs, such as behavioural changes (e.g. agitation, lethargy, hyperactivity and cannibalism), neurological changes (e.g. convulsions, tremors, muscle rigidity and hyper-reflexia) and autonomic signs (e.g. lacrimation, piloerection, pupil size and unusual respiratory pattern).
The pregnant rats from each group were sacrificed on the 18th day of pregnancy. Both horns of the uteri were removed and examined for the number of implantations and the number of live and dead embryos. In addition, both ovaries were removed and the number of corpora lutea was counted. The data were analysed to determine the mating index (number of sperm positive females/number of pairings × 100), fertility index (number of pregnant females/number of sperm positive females × 100), pre-implantation loss (difference between the number of corpora lutea and the number of implantation sites expressed as per number of corpora lutea), and post-implantation loss (difference between the number of implantations and the number of live fetuses expressed as per number of implantations). In addition, the conception time (the interval between the first day of cohabitation and the day of vaginal plug and/or sperm in the vaginal smear) was recorded for each female.
Isolation of tissues and determination of the tissue somatic index and testis volume
The animals were fasted overnight and euthanized by using anaesthetic ether and cervical dislocation. After collecting the blood, other organs such as the liver, brain, kidney, spleen, testes, different parts of epididymis (caput, corpus and cauda), seminal vesicles, and prostate gland were dissected out and immediately weighed to the nearest milligram using a Shimadzu electric balance (Model No: BL-220H; Kyoto, Japan). The volume of the testes was measured by using a water displacement method based on the protocol provided by Saalu et al.29 Tissue somatic index (TSI) was calculated using the following formula:TSI = [weight of the tissue in grams/ weight of the animal in grams] × 100
The cauda epididymis was used for sperm analysis and the testes were used for daily sperm production. The testes were also used for histological studies. The testes and epididymis were also used for biochemical analysis.
Spermatology
Testicular daily sperm production (DSP)
DSP was determined by the method described previously by Blazak et al.30 In brief, testicular parenchyma was homogenized in 50 mL of ice-cold 0.9% NaCl solution containing 0.01% Triton X-100 using a sterilized mortar and pestle. After thorough mixing, the sample was filtered through a metal sieve and the filtrate was used to quantitate the homogenization-resistant spermatids using a Neubauer haemocytometer. The number of sperm produced per gram tissue of testis per day was determined.27
Epididymal sperm analysis
The right caudal part of the epididymis was dissected out from each rat and the spermatozoa were squeezed into 2.0 ml of M199 containing 0.5% bovine serum albumin. The spermatozoa were counted using an improved Neubauer haemocytometer, as described previously by Belsey et al.31 and expressed as millions per mL. Progressive sperm motility was determined within 5 minutes following their isolation from the cauda epididymis by following the method described by Belsey et al.31 and expressed as percent motility of the total sperm quantified. The viability assay was based on the evaluation of the ratio of live to dead spermatozoa using 1% trypan blue solution.32 Furthermore, the membrane integrity of the sperm was determined by exposing the sperm to a hypoosmotic medium (1.351 g fructose and 30.735 g sodium citrate in 100 ml of distilled water) and observed for swelled tails [hypoosmotic swelling (HOS)] under a phase contrast microscope.33 The data were expressed as the percent of the tail swelled sperm counted. The integrity of sperm DNA was determined by an alkaline comet assay.34 The images of the nucleoids from a fluorescence microscope (Zeiss Company, Germany) were captured with a Cool SNAP® Pro colour digital camera. One hundred nucleoids were scored from two separate slides prepared of same sample. The nucleoid length (μm) was determined by using the Image Pro® plus software.
Tissue homogenate preparation
The homogenates were prepared using 0.2 g of testis or epididymis tissue in 2.0 mL of cold (4 °C) Tris-HCl buffer (20 mM, pH 6.8). The samples were processed using a glass–Teflon homogenizer (POLYTRON, PT 1600 E) by passing 5 pulses at 4 °C. They were centrifuged at 800g for 20 min at 4 °C. An aliquot of the supernatant was used for the determination of superoxide anion, hydrogen peroxide and lipid peroxidation levels, while the rest was further centrifuged at 11 000g for 15 min at 4 °C to obtain the supernatant fraction for enzyme assays.
Assay of testicular steroidogenic marker enzymes
The activities of 3β hydroxysteroid dehydrogenase (3β-HSD) (EC 1.1.1.51) and 17β hydroxysteroid dehydrogenase (17β-HSD) (EC 1.1.1.64) were determined in the testicular homogenates by the method of Bergmeyer.35 The enzyme assays were conducted under the conditions following zero order kinetics after preliminary standardization regarding linearity with respect to time of incubation and enzyme concentration. The enzyme activities were expressed in nmol of NAD converted to NADH per mg protein per min (3β-HSD) or nmol of NADPH converted to NADP per mg protein per min (17β-HSD).
Determination of serum testosterone levels
Serum testosterone levels were determined (samples in duplicate) by a chemiluminescent immunoassay (CLIA) using a commercially available autobiotesto kit (catalog number is CL1104-2) purchased from Autobio Diagnostic Co. Ltd, China. The sensitivity of the assay was calculated as 0.002 ng and intra-assay and inter-assay variations were found to be 6.75% and 4.2% respectively.
Determination of superoxide anion levels
The levels of the superoxide anion were measured according the procedure of Prodczasy and Wei36 which is based on the reduction of iodonitrotetrazolium. The reaction mixture was obtained by adding 4.9 mM iodonitrotetrazolium violet, 0.3 mM EDTA and 0.92 mM sodium carbonate (pH 10.2) to 0.2 mL homogenate. The tubes were then incubated for 15 min at room temperature and then kept in boiling water for 1 min. The tubes were cooled to room temperature and then the absorbance was read at 505 nm. The results were expressed as nmoles per gram tissue.
Determination of hydrogen peroxide levels
The level of hydrogen peroxide was determined as described by Pick and Keisari.37 The assay is based on the horseradish peroxidase-mediated oxidation of phenol red by H2O2 which results in the formation of a compound demonstrating increased absorbance at 610 nm. The reaction mixture contained 0.28 mmol phenol red, 8.5 units of horseradish peroxidase and 0.2 mL homogenate. After 5 min of incubation, 1 mL of 0.1 N NaOH was added, and the tube was read at 610 nm. The results were expressed in nmoles of H2O2 per gram of tissue.
Determination of lipid peroxidation (LPO) levels
The level of LPO in the tissues was determined by the method of Ohkawa et al.38 For this, 3.3 ml TBA reagent (0.2 ml of 8.0% SDS, 1.5 ml of 20% acetic acid, 1.5 ml of 0.8% aqueous thiobarbituric acid and 0.1% butylated hydroxyl toluene) was mixed with 0.2 ml tissue supernatant and the mixture was boiled at 95 °C in a water bath for 60 min. The solution was cooled and centrifuged at 2000 rpm for 10 min. The supernatant was then used for recording the absorbance against a blank (distilled water) at 532 nm. The results were expressed as μmoles of malondialdehyde per gram tissue.
Assay of superoxide dismutase
Superoxide dismutase (EC 1.15.1.1) was assayed by the method of Marklund and Marklund.39 Briefly, the assay mixture contained 2.4 ml of tris-hydrochloric acid buffer (50 mM) containing 1 mM EDTA (pH 7.6), 300 μl of pyrogallol (0.2 mM) and 100 μl enzyme source. The increase in absorbance was measured immediately at 420 nm against a reagent blank at 10 s intervals for 3 min on a UV-Vis spectrophotometer (Hitachi U-2001). The activity of the enzyme was expressed as units per mg protein per min at 37 °C.
Assay of catalase
Catalase (EC 1.11.1.6) was assayed by the method of Claiborne.40 Briefly, the assay mixture contained 2.4 ml of phosphate buffer (50 mM, pH 7.0), 10 μl of hydrogen peroxide (19 mM) and 50 μl enzyme source. The decrease in absorbance was measured immediately at 240 nm against a reagent blank at 10 s intervals for 3 min on a UV-Vis spectrophotometer (Hitachi U-2001). The activity of the enzyme was expressed as μmoles of hydrogen peroxide metabolized per mg protein per min at 37 °C.
Assay of glutathione peroxidase
Glutathione peroxidase (GPx) (EC 1.11.1.9) activity was measured by a modification of the method of Paglia and Valentine.41 Briefly, the assay mixture consisted of 50 mM Tris HCl, 0.1 mM ethylenediaminetetraacetic acid, pH 7.6 containing 2 mM GSH, 0.14 mM NADPH and 0.7 U mL–1 GSH reductase. The enzyme source was pre-incubated for 2 minutes at 37 °C in the assay mixture, and the reaction was initiated by the addition of 0.2 mM cumene hydroperoxide. GPx catalyzes the oxidation of glutathione by cumene peroxide. In the presence of glutathione reductase and NADPH the oxidized glutathione (GSSG) is immediately converted into the reduced form with a concomitant oxidation of NADPH to NADP. The decrease in absorbance at 340 nm was measured using a UV-Vis spectrophotometer (Hitachi U-2001) at 37 °C. The activity of the enzyme was expressed as nmoles of NADPH oxidized per mg protein per min.
Assay of glutathione reductase
Glutathione reductase (GR) (EC 1.6.4.2) activity was determined spectrophotometrically with a Hitachi spectrophotometer (U-2001) according to the method of Carlberg and Mannervik.42 The assay system contained 435 mM phosphate buffer, pH 7.3, including 1 mM EDTA, 1 mM GSSG and 0.1 mM NADPH. The activity of the enzyme was expressed as nmoles of NADPH oxidized per mg protein per min.
Determination of the protein content in the enzyme source
The protein content in the enzyme source was estimated by the method of Lowry et al.43 using bovine serum albumin as the standard.
Histological evaluation of testes
The right testis was obtained from the control and experimental rats immediately after their euthanization and fixed individually in Bouin's fluid for 24 h after clearing off the adhering tissues. The fixed specimens were dehydrated by exposing to an ascending series of alcohol and embedded in paraffin blocks after clearing in xylol. Sections of 5 μm thickness were cut and then stained with hematoxylin–eosin44 and examined with an Olympus phase contrast microscope (Model no: BX41TF). Twenty cross sections of stage VII–VIII seminiferous tubules from each animal were analyzed blindly for tubular diameter (basal lamina to basal lamina) and height of the germinal epithelium (basal lamina to neck of elongated spermatozoa), area of seminiferous tubules and inter-tubular space using a computer assisted image analysis system.
Statistical analysis
The data were expressed as mean ± standard deviation and p < 0.05 was considered significant. For the analysis of the reproductive end points in the control and experimental rats, we used one-way analysis of variance (ANOVA) following Tukey's multiple comparison post hoc test. Statistical analysis was performed using the SPSS software for Windows, version 16.0 (SPSS, Chicago, Illinois, USA).
Results
Maternal observations
The pregnant rats in either the control or Pb-exposed group were apparently normal and no visible toxic symptoms were observed in any of the animals. The length of the gestation and lactation period was similar in both the control and Pb-exposed dams (data not shown). The treatment protocol did not cause any abortions in the pregnant animals. No unusual maternal behavior (viz. head flicking, redness around the eyes, biting, licking, self-mutilation and aggressiveness) was observed during experimentation and none of the animals were excluded from the study. Pregnancy length (data not shown) and litter size (number of pups delivered) in the control and Pb-exposed groups were comparable (Table 1).
Table 1. Effect of perinatal exposure to lead on the fertility output of dams and birth weight and survival rate of pups.
| Parameters | Control | Pb exposed |
| Pups per rat# | 12.05 ± 0.82 | 11.68 ± 1.03 (–3.07) |
| No. of live pups per rat# on PND 21 | 11.75 ± 0.50 | 11.17 ± 1.14 (–4.94) |
| Survival rate (%) of pups$ on PND 21 | 97.51 | 95.63 |
| Birth weight of pups$ (g) | 5.92 ± 0.49 | 5.71 ± 0.56 (–3.55) |
Developmental milestones of pups
Survival rate and birth weight of pups in the control and Pb-exposed groups were comparable (Table 1). No general toxicity was observed in any of the pups delivered by either the control or lead-treated dams during weaning. Pinna detachment, eruption of incisors and eye opening of the Pb exposed pups were comparable to those of the control (Table 2).
Table 2. Effect of perinatal exposure to lead on developmental landmarks in male pups.
| Parameter | Control | Pb exposed |
| Anogenital distance a | 0.35 ± 0.05 | 0.33 ± 0.06 (–5.71) |
| Crown–rump length a | 5.71 ± 0.68 | 5.27 ± 0.22 (–7.71) |
| Age at pinna unfolding b | 3.7 ± 0.63 | 3.84 ± 0.39 (3.78) |
| Age at eye slit formation b | 3.6 ± 0.55 | 3.5 ± 0.54 (–2.78) |
| Age at lower incisor eruption b | 3.81 ± 0.39 | 3.95 ± 0.24 (3.67) |
| Age at fur development b | 7.71 ± 0.75 | 7.89 ± 0.34 (2.33) |
| Age at upper incisor eruption b | 10.83 ± 0.73 | 10.23 ± 0.83 (–5.54) |
| Age at eye opening b | 13.33 ± 0.82 | 13.56 ± 0.52 (1.73) |
| Age at testes descent b | 28.67 ± 0.82 | 36.95# ± 0.89 (28.88) |
aExpressed in centimetres.
bExpressed in postnatal days.
Behavioural parameters
The effect of perinatal exposure to Pb on the selected behavioural responses of the pups was explored at various stages of life. The cliff avoidance test for pups was performed on PND 5. Significant delay in cliff avoidance was observed in the perinatal Pb exposed pups compared to the control pups. The pups were tested for surface righting reflex activity on PND 6. The Pb exposed pups were found to be less active than the control pups. The negative geotaxis movement of the pups was tested on PND 7. The Pb exposed pups took more time to turn to 180° than the control pups. The pups were examined for ascending wire mesh activity on PND 16. The Pb exposed pups took more time to reach the edge of the wire mesh than the control group pups (Table 3).
Table 3. Effect of perinatal exposure to lead on the selected behavioural responses of male pups during weaning.
| Parameter | Control | Pb exposed |
| Cliff avoidance | 7.05 ± 0.80 | 23.18* ± 1.89 (228.79) |
| Negative geotaxis | 3.55 ± 0.82 | 23.39* ± 2.88 (558.87) |
| Surface righting | 3.76 ± 0.82 | 11.06* ± 1.71 (194.15) |
| Ascending wire mesh | 29.80 ± 2.35 | 46.20* ± 2.93 (55.04) |
Organ weight profile and volume of testis
No significant change was observed in the body weights, and food and water intake of the experimental rats when compared to the rats in the control group (data not shown). The relative weights of the liver, brain and kidney were comparable (p > 0.05) in both the control and experimental groups (Table 4). Conversely, a significant reduction was observed in the relative weights of the testes (F = 59.006; df = 3, 32; p < 0.0001), caput epididymis (F = 53.432; df = 3, 32; p < 0.0001), corpus epididymis (F = 50.329; df = 3, 32; p < 0.0001), cauda epididymis (F = 195.77; df = 3, 32; p < 0.0001), seminal vesicles (F = 41.534; df = 3, 32; p < 0.0001), and prostate (F = 80.250; df = 3, 32; p < 0.0001) and the volume of the testis (F = 248.51; df = 3, 32; p < 0.0001) in the rats exposed to Pb during the perinatal period, As during the pubertal period and Pb and As sequentially when compared to the control rats (Table 4). Further decline in the relative weights of the testes, caput epididymis, corpus epididymis, cauda epididymis, seminal vesicles, and prostate gland, and the testis volume was observed in the rats sequentially exposed to both Pb during the perinatal period and As at adulthood when compared to individual exposures.
Table 4. Effect of perinatal exposure to lead (Pb) and/or pubertal exposure to arsenic (As) on tissue somatic indices (g %) and testis volume (mL) in adult male rats.
| Tissue | Control | Pb | As | Pb + As |
| Liver | 2.95a ± 0.31 | 2.91a ± 0.21 (–1.36) | 2.56a ± 0.41 (–13.22) | 2.92a ± 0.25 (–1.02) |
| Kidney | 0.69a ± 0.08 | 0.66a ± 0.04 (–4.35) | 0.67a ± 0.02 (–2.90) | 0.63a ± 0.03 (–8.70) |
| Brain | 0.59a ± 0.04 | 0.61a ± 0.10 (3.39) | 0.53a ± 0.06 (–10.17) | 0.52a ± 0.06 (–11.86) |
| Testis | 1.20a ± 0.04 | 1.01b ± 0.10 (–15.83) | 0.92b ± 0.04 (–23.33) | 0.79c ± 0.07 (–34.17) |
| Caput | 1.08a ± 0.11 | 0.94b ± 0.02 (–12.96) | 0.81c ± 0.04 (–25.00) | 0.69d ± 0.07 (–36.11) |
| Corpus | 0.08a ± 0.01 | 0.05b ± 0.01 (–37.50) | 0.05b ± 0.01 (–37.50) | 0.03c ± 0.002 (–62.50) |
| Cauda | 0.20a ± 0.01 | 0.13b ± 0.01 (–35.00) | 0.15c ± 0.01 (–25.00) | 0.09d ± 0.01 (–55.00) |
| Seminal vesicles | 0.42a ± 0.06 | 0.31b ± 0.02 (–26.19) | 0.32b ± 0.03 (–23.81) | 0.22c ± 0.03 (–47.62) |
| Prostate | 0.17a ± 0.01 | 0.12b ± 0.01 (–29.42) | 0.14c ± 0.01 (–17.65) | 0.10d ± 0.01 (–41.18) |
| Volume of testis | 2.06a ± 0.09 | 1.42b ± 0.09 (–31.07) | 1.40b ± 0.05 (–32.04) | 1.22c ± 0.03 (–40.78) |
Spermatology
The testicular DSP (F = 151.53; df = 3, 32; p < 0.0001), cauda epididymal sperm density (F = 443.90; df = 3, 32; p < 0.0001), and motile (F = 301.64; df = 3, 32; p < 0.0001), viable (F = 144.93; df = 3, 32; p < 0.0001) and HOS-tail swelled (F = 319.85; df = 3, 32; p < 0.0001) sperm numbers were significantly decreased in the rats exposed to Pb during their perinatal period or As at their adulthood when compared to the control rats (Fig. 1). Further decrease was observed in the DSP, sperm reserves, and concentration of motile sperm, viable sperm, and HOS-tail swelled sperm of the rats exposed to both Pb and As when compared to the rats exposed to either Pb or As alone (Fig. 1). The sperm nucleoid length showed a significant (F = 203.45; df = 3, 32; p < 0.001) increase in the rats exposed to Pb during their perinatal period or As at their adulthood when compared to the control rats (Fig. 2). Further deterioration of the sperm nucleus was observed in the rats exposed to both Pb and As when compared to the rats exposed to Pb or As alone.
Fig. 1. Effect of perinatal exposure to lead (Pb) and/or pubertal exposure to arsenic (As) on testicular daily sperm production (A), epididymal sperm density (B), motile sperm (C) viable sperm (D), HOS tail swelled sperm (E) and sperm nucleoid length (F) in adult rats. Bars are mean ± S.D. of 9 individuals. Bars with different letters differ significantly from each other at p < 0.05.
Fig. 2. Fluorescence microscopy-derived pictures of the sperm nucleoid of the control rats (a) and the rats exposed to Pb during the perinatal period (b), exposed to As during the pubertal period (c), and exposed to both Pb + As (d) obtained by an alkaline comet assay. For each sample, 100 sperm in two separate slides were scored. A marked increase in tail length was observed in all experimental groups.
Activity levels of steroidogenic marker enzymes and serum testosterone levels
A significant decrease in the activity levels of 3β- (F = 160.14; df = 3, 32; p < 0.0001) and 17β- (F = 108.29; df = 3, 32; p < 0.0001) HSDs was observed in the testis of the rats exposed to Pb during their perinatal period or As at their adulthood when compared to the control rats (Fig. 3). Perinatal exposure to lead along with pubertal exposure to As resulted in an additional decline in the 3β-HSD and 17β-HSD activity levels when compared to individual exposures to either Pb or As at their respective time points (Table 5). Serum testosterone levels were also decreased significantly (F = 221.46; df = 3, 32; p < 0.0001) in all the experimental rats (Table 5).
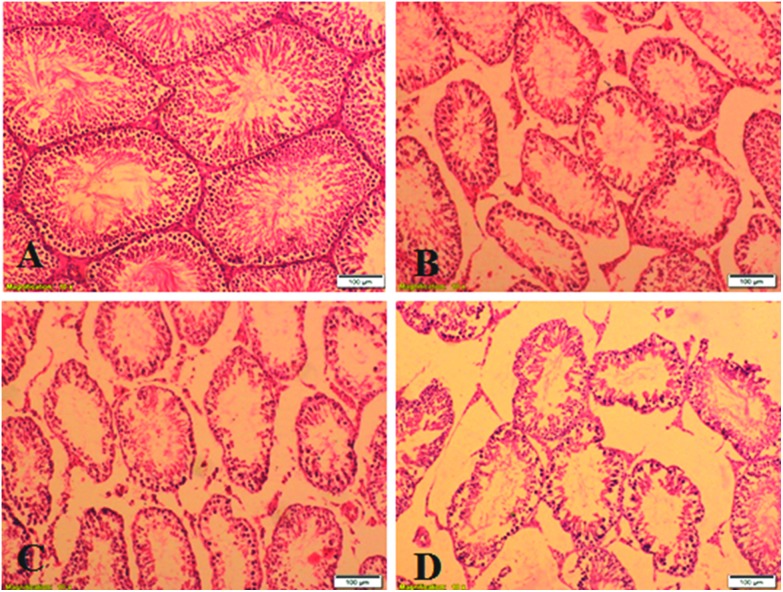
Fig. 3. Photomicrographs of H&E stained sections of the testes from the control rats (A) and the rats exposed to Pb during the perinatal period (B), exposed to As during the pubertal period (C), and exposed to both Pb + As (D).
Table 5. Effect of perinatal exposure to lead (Pb) and/or pubertal exposure to arsenic (As) on the 3β- and 17β-hydroxysteroid dehydrogenase (HSD) activity levels in the testis and serum testosterone levels in rats.
| Parameters | Control | Pb | As | Pb + As |
| 3β-HSD (nmoles of NAD converted into NADH per mg protein per min) | 23.99a ± 1.53 | 12.71b ± 1.58 (–47.02) | 13.72b ± 2.38 (–42.81) | 9.54c ± 1.59 (–60.24) |
| 17β-HSD (nmoles of NADPH converted into NADP per mg protein per min) | 17.37a ± 1.54 | 9.24b ± 1.24 (–46.81) | 11.73c ± 1.01 (–32.47) | 4.82d ± 1.11 (–72.26) |
| Testosterone (ng mL–1) | 4.85a ± 0.58 | 3.11a ± 0.42 (–35.88) | 2.98a ± 0.41 (–38.56) | 1.92a ± 0.21 (–60.41) |
Pro- and anti-oxidant status in the testis and different epididymal regions
The changes in the pro- and anti-oxidant parameters in the testis and different epididymal regions are presented in Tables 6–9. The levels of superoxide anion (F = 54.785 in the testis, F = 122.40 in the caput epididymis, F = 57.024 in the corpus epididymis, and F = 49.153 in the cauda epididymis; df = 3, 32; p < 0.0001), hydrogen peroxide (F = 67.962 in the testis, F = 134.39 in the caput epididymis, F = 54.409 in the corpus epididymis, and F = 134.35 in the cauda epididymis; df = 3, 32; p < 0.0001), and lipid peroxidation (F = 89.370 in the testis, F = 48.437 in the caput epididymis, F = 38.142 in the corpus epididymis, and F = 47.615 in the cauda epididymis; df = 3, 32; p < 0.0001) were significantly elevated in the testis and the caput, corpus, and cauda epididymal parts of the rats exposed to Pb during their perinatal period or As at their adulthood compared to that of the control rats. Further increase was observed in the superoxide anion, hydrogen peroxide and lipid peroxidation levels in the testis, caput epididymis, corpus epididymis and cauda epididymis of the rats exposed to Pb during the perinatal period and treated with As during their pubertal period when compared to the rats treated with either Pb or As alone. Conversely, the activities of antioxidant enzymes such as superoxide dismutase (F = 27.448 in the testis, F = 23.514 in the caput epididymis, F = 11.574 in the corpus epididymis, and F = 19.845 in the cauda epididymis; df = 3, 32; p < 0.0001), catalase (F = 38.966 in the testis, F = 33.432 in the caput epididymis, F = 21.432 in the corpus epididymis, and F = 37.413 in the cauda epididymis; df = 3, 32; p < 0.0001), glutathione peroxidase (F = 119.61 in the testis, F = 104.34 in the caput epididymis, F = 21.432 in the corpus epididymis, and F = 37.413 in the cauda epididymis; df = 3, 32; p < 0.0001), glutathione reductase (F = 49.573 in the testis, F = 49.573 in the caput epididymis, F = 90.704 in the corpus epididymis, and F = 50.006 in the cauda epididymis; df = 3, 32; p < 0.0001) were significantly reduced in the testis and the caput, corpus, and cauda epididymis of the rats exposed to Pb during their perinatal period or As during their pubertal period. Furthermore, the Pb-treated rats exposed to As during their pubertal period showed an additional decrease in the activities of superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase when compared to the rats exposed to Pb during their perinatal period or As during their pubertal period.
Table 6. Effect of perinatal exposure to lead (Pb) and/or pubertal exposure to arsenic (As) on the pro- and anti-oxidant system in the testis of adult male rats.
| Parameter | Control | Pb | As | Pb + As |
| Superoxide anion levels (nmoles per g tissue) | 6.64a ± 1.00 | 10.48b ± 2.31 (57.83) | 11.61b ± 1.33 (74.85) | 16.70c ± 1.79 (151.51) |
| Hydrogen peroxide generation (nmoles per g tissue) | 21.12a ± 2.30 | 32.54b ± 3.48 (54.07) | 33.95b ± 4.27 (60.75) | 44.39c ± 3.52 (110.04) |
| Lipid peroxidation (μmoles malondialdehyde per g tissue) | 13.85a ± 1.355 | 17.01b ± 1.36 (22.82) | 18.37b ± 1.8 (32.64) | 24.53c± 1.07 (77.11) |
| Superoxide dismutase (nmoles pyrogallol oxidized per mg protein per min) | 7.38a ± 0.04 | 6.11b ± 0.04 (–17.21) | 5.21c ± 0.04 (–29.40) | 2.19d ± 0.04 (–70.33) |
| Catalase (nmoles H2O2 metabolized per mg protein min–1) | 7.47a ± 1.09 | 5.17b ± 1.24 (–30.79) | 4.17b ± 1.43 (–44.18) | 1.75c ± 0.65 (–76.57) |
| Glutathione peroxidase (nmoles NADPH oxidized per mg protein per min) | 50.16a ± 2.46 | 36.52b ± 4.39 (–27.19) | 32.24b ± 4.46 (–35.73) | 15.04c ± 4.23 (–70.02) |
| Glutathione reductase (nmoles NADPH oxidized per mg protein per min) | 35.58a ± 4.86 | 27.55b ± 1.89 (–22.57) | 23.25b ± 3.34 (–34.67) | 13.83c ± 3.49 (–61.13) |
Table 7. Effect of perinatal exposure to lead (Pb) and/or pubertal exposure to arsenic (As) on the pro- and anti-oxidant system in the caput of adult male rats.
| Parameter | Control | Pb | As | Pb + As |
| Superoxide anion levels (nmoles per g tissue) | 4.40a ± 0.36 | 6.04b ± 0.54 (37.27) | 7.63c ± 0.85 (73.41) | 10.91d ± 1.06 (147.95) |
| Hydrogen peroxide generation (nmoles per g tissue) | 16.11a ± 3.33 | 25.50b ± 3.33 (58.29) | 33.12c ± 2.59 (105.59) | 42.48d ± 2.18 (163.69) |
| Lipid peroxidation (μmoles malondialdehyde per g tissue) | 10.66a ± 1.89 | 14.21b ± 0.93 (33.30) | 16.85b ± 2.60 (58.07) | 20.37c ± 1.16 (91.09) |
| Superoxide dismutase (nmoles pyrogallol oxidized per mg protein per min) | 6.43a ± 0.04 | 5.23b ± 0.05 (–18.66) | 3.97c ± 0.05 (–38.26) | 1.04d ± 0.04 (–83.83) |
| Catalase (nmoles H2O2 metabolized per mg protein per min) | 5.06a ± 0.8 | 3.21b ± 0.54 (–36.56) | 2.96b ± 1.39 (–41.50) | 0.93c ± 0.46 (–81.62) |
| Glutathione peroxidase (nmoles NADPH oxidized per mg protein per min) | 31.69a ± 2.76 | 22.46b ± 1.84 (–29.13) | 23.14b ± 1.53 (–26.98) | 13.41c ± 2.43 (–57.68) |
| Glutathione reductase (nmoles NADPH oxidized per mg protein per min) | 25.90a ± 1.82 | 18.58b ± 2.42 (–28.26) | 19.51b ± 2.38 (–24.67) | 10.33c ± 3.85 (–60.12) |
Table 8. Effect of perinatal exposure to lead (Pb) and/or pubertal exposure to arsenic (As) on the pro- and anti-oxidant system in the corpus of adult male rats.
| Parameter | Control | Pb | As | Pb + As |
| Superoxide anion levels (nmoles per g tissue) | 5.21a ± 0.91 | 7.11b ± 0.80 (36.47) | 7.87b ± 1.00 (51.06) | 12.05c ± 1.67 (131.29) |
| Hydrogen peroxide generation (nmoles per g tissue) | 12.07a ± 2.48 | 20.26b± 2.69 (67.85) | 21.25b ± 2.06 (76.06) | 29.36c ± 3.93 (143.25) |
| Lipid peroxidation (μmoles malondialdehyde per g tissue) | 8.89a ± 1.35 | 12.16b ± 0.84 (36.78) | 13.65b ± 1.44 (53.54) | 19.06c ± 2.71 (114.40) |
| Superoxide dismutase (nmoles pyrogallol oxidized per mg protein per min) | 4.44a ± 0.07 | 2.97b ± 0.05 (–33.11) | 2.86c ± 0.023 (–35.59) | 0.58d ± 0.01 (–86.94) |
| Catalase (nmoles H2O2 metabolized per mg protein per min) | 4.80a ± 1.00 | 2.95b ± 1.40 (–38.54) | 2.96b ± 1.41 (–38.33) | 0.61c ± 0.42 (–87.29) |
| Glutathione peroxidase (nmoles NADPH oxidized per mg protein per min) | 26.26a ± 3.61 | 17.95b ± 2.62 (–31.65) | 18.02b ± 0.94 (–31.38) | 8.38c ± 1.63 (–68.09) |
| Glutathione reductase (nanomoles NADPH oxidized per mg protein min–1) | 24.82a ± 3.54 | 16.54b ± 1.50 (–33.36) | 15.23b ± 2.45 (–38.64) | 6.00c ± 1.67 (–75.83) |
Table 9. Effect of perinatal exposure to lead (Pb) and/or pubertal exposure to arsenic (As) on the pro- and anti-oxidant system in the cauda of adult male rats.
| Parameter | Control | Pb | As | Pb + As |
| Superoxide anion levels (nmoles per g tissue) | 6.23a ± 0.55 | 9.79b ± 2.05 (57.14) | 9.31b ± 2.01 (49.44) | 15.86c ± 1.83 (154.57) |
| Hydrogen peroxide generation (nmoles per g tissue) | 18.42a ± 2.70 | 26.44b ± 2.89 (43.54) | 28.97b ± 3.43 (57.27) | 45.27c ± 2.57 (145.77) |
| Lipid peroxidation (μmoles malondialdehyde per g tissue) | 10.91a ± 2.09 | 17.25b ± 3.22 (58.11) | 18.49b ± 1.98 (69.48) | 25.74c ± 3.05 (135.93) |
| Superoxide dismutase (nmoles pyrogallol oxidized per mg protein per min) | 7.20a ± 0.08 | 5.88b ± 0.03 (–18.33) | 5.12c ± 0.03 (–28.89) | 1.93d ± 0.03 (–73.19) |
| Catalase (nmoles H2O2 metabolized per mg protein per min) | 6.45a ± 1.92 | 3.92b ± 0.28 (–39.22) | 3.38b ± 0.43 (–47.60) | 1.24c ± 0.68 (–80.78) |
| Glutathione peroxidase (nmoles NADPH oxidized per mg protein per min) | 41.59a ± 4.46 | 21.40b ± 1.84 (–48.55) | 19.19b ± 2.24 (–53.86) | 12.67c ± 1.81 (–69.54) |
| Glutathione reductase (nmoles NADPH oxidized per mg protein per min) | 31.86a ± 4.02 | 21.03b ± 2.64 (–33.99) | 21.77b ± 2.75 (–31.67) | 13.54c ± 3.17 (–57.50) |
Testicular architecture
The photomicrographs of the testis of the control and experimental rats are shown in Fig. 3. The testicular architecture in the control rats showed compactly arranged seminiferous tubules and well-developed germinal epithelium. Each tubule is presented with intact germinal epithelium followed by different stages of germ cells and the lumen is full of sperm (Fig. 3A). Severely atrophied and irregularly placed small sized tubules with large interstitial space were observed in the Pb exposed rats (Fig. 3B). The testicular architecture of the rats exposed to As during their pubertal period showed deranged and small sized seminiferous tubules with depleted germ cells (Fig. 3C). Severely atrophied, irregularly placed and damaged tubules showing large interstitial space with ruptured epithelial membrane, loss of maturation and lumen filled with cell debris and disintegrated sperm were observed in the testis of the rats exposed to both Pb during the perinatal period and As during their pubertal period (Fig. 3D).
Fertility studies
Even though all the females cohabited with the control and experimental males had copulatory plugs (mating index = 100%), the conception time was significantly increased (F = 39.642; df = 3, 28; p < 0.0001) in the females mated with the experimental males (Table 10). The mean number of corpora lutea was comparable in all groups. The mean number of implantations (F = 15.121; df = 3, 28; p < 0.0001) and the mean number of live foetuses (F = 49.563; df = 3, 28; p < 0.0001) per rat decreased significantly in the females mated with the experimental males (Fig. 4). Conversely, pre- and post-implantation losses increased in the females mated with the experimental males.
Table 10. Effect of perinatal exposure to lead (Pb) and/or pubertal exposure to arsenic (As) on reproductive performance in male rats.
| Parameter | Control | Pb | As | Pb + As |
| Conception time (days) | 1.13a ± 0.65 | 2.88b ± 0.60 (154.88) | 3.00b ± 0.71 (165.49) | 4.88c ± 0.78 (331.86) |
| Mating index (%) | 100 (12/12) | 100 (12/12) | 100 (12/12) | 100 (12/12) |
| Fertility index (%) | 100 (12/12) | 87.5 (11/12) | 87.50 (11/12) | 75.0 (9/12) |
| No. of corpora lutea per rat# | 15.04a ± 0.71 | 14.87a ± 0.59 (–1.13) | 15.00a ± 0.82 (–0.26) | 15.33a ± 0.59 (1.93) |
| No. of implantations per rat# | 15.00a ± 0.82 | 11.00b ± 1.74 (–26.67) | 11.5b ± 1.30 (–23.33) | 9.33c ± 0.58 (–37.80) |
| Pre-implantation loss (%) | 6.25 | 25.02 | 23.33 | 39.13 |
| No. of resorptions per rat# | 0 | 0 | 0 | 1 |
| No. of live fetuses per rat# | 14.5a ± 0.5 | 10.33b ± 0.58 (–28.76) | 10.5b ± 1.00 (–27.59) | 5.00c ± 1.73 (–65.52) |
| Post-implantation loss (%) | 3.33 | 6.09 | 8.70 | 48.29 |
Fig. 4. Uterus of females mated with the control males (A), males exposed to Pb during the perinatal period (B), As during the pubertal period (C) or both Pb and As (D) showing foetuses on the 18th day of pregnancy.
Discussion
The developing organism is exquisitely sensitive to stress, and any mammalian assay must include exposure to the test compound in utero and during lactation to fully evaluate its effects on subsequent growth and development. In the present study, maternal toxicity was not observed as a consequence of exposure to Pb at a concentration of 819 mg of Pb per L through drinking water. The fertility output of the dams exposed to Pb was also comparable with that of the controls. Similarly, there were no Pb-associated behavioral findings and no effects of treatment on pregnancy or parturition. These results indicate that this dose of Pb did not cause maternal toxicity. Moreover, the birth weight of the pups and age at pinna unfolding, lower incisor eruption, upper incisor eruption, fur development and eye opening showed no differences between the Pb-exposed groups and the control; conversely, significant delay in the latency time of cliff avoidance, negative geotaxis, surface righting reflex, ascending wire mesh and age at testes descent were observed in the Pb-exposed pups.
In the present study, the following reproductive end points: (1) weights of reproductive organs, (2) numbers of sperm in the testis and epididymis, (3) motility and viability and membrane and chromatin integrity of sperm collected from the tail of the epididymis, (4) steroidogenic enzyme activity levels in the testis, (5) histology of the testis, and (6) fertility were determined in the rats exposed to Pb during the perinatal period. The results revealed that exposure to Pb during the perinatal period caused significant reduction of the relative weights of the testes, cauda epididymis, and caput epididymis on PND 115. Conversely, the relative weight of the liver, kidney and brain did not show any significant changes in any experimental rats indicating that the general metabolic condition of the animals was within the normal range. These results are in agreement with our earlier findings.11,18 The observed reduction in the weights of the testes may be due to the decreased number of germ cells and elongated spermatids, as the weight of the testes is largely dependent on the mass of differentiated spermatogenic cells.45,46 Similar to testis weight, daily sperm production per gram testis was also significantly decreased in rats exposed to Pb during the perinatal period. Moreover, our results also showed a reduced number of epididymal spermatozoa and motile, viable and HOS tail swelled sperm numbers which may be due to reduced androgen production or increased lipid peroxidation in reproductive tissues at their adulthood. Corpas et al.47 and Dorostghoal et al.48 also found that prenatal exposure to Pb perturbs the mitosis of spermatogenic cells and causes alterations in the proliferation of Sertoli cells, thereby decreasing the testicular sperm production and epididymal sperm count of the offspring. It is well established that prenatal and/or neonatal exposure to heavy metals results in smaller testes and reduced testosterone levels in rodents.49 The reduced testicular steroidogenic enzyme activities observed in the rats exposed to Pb during the perinatal period indicate compromised testosterone production which might affect spermatogenesis.18 The results of the present study are also in agreement with earlier reports.50,51
In view of the reported involvement of oxidative stress in reproductive toxicity, in the present study enzymatic and non-enzymatic antioxidant parameters were determined in the testis and different regions of the epididymis of the rats exposed to Pb during the perinatal period. The rats exposed to Pb showed increased levels of superoxide anion, hydrogen peroxide and lipid peroxidation products in association with a significant decrease in the activities of SOD, catalase, GPx and GR in the testes and different regions of the epididymis.
The reduction in the activity levels of SOD, catalase, GPx and GR may reflect the inability of the testis and epididymis to eliminate radicals generated due to stress or due to enzyme inactivation caused by excess ROS production in the testis and epididymis. One of the important aspects of antioxidant enzymes is their nature of synergistic functioning: the antioxidant enzymes catalase and GPx protect SOD against inactivation by hydrogen peroxide. Reciprocally, the SOD protects catalase and GPx against inactivation by superoxide anion. The balance of this enzyme system may be essential to eliminate superoxide anions and peroxides generated in the testis and epididymis. The reduction in the activities of antioxidant enzymes and the increase in ROS could reflect an imbalance in the antioxidant system in the testis and epididymis of the rats exposed to Pb during the perinatal period. It has been reported that oxidative stress is one of the possible mechanisms of action of Pb-induced toxicity and it affects the testis and epididymis, thereby impairing sperm morphology and maturation.52–54 Previous findings in our laboratory also revealed that prenatal exposure to Pb causes disturbances in the testicular pro- and anti-oxidant metabolism of rats as evidenced by an elevation in the lipid peroxidation products and decrease in the activities of antioxidant enzymes.18
Increased lipid peroxidation may indicate an increased generation of ROS, which can cause damage to sperm and other cytoplasmic organelle membrane structures through peroxidation of lipids, proteins and nucleotides, thereby altering sperm motility.55 The mechanism of ROS induced altered sperm motility is still unclear. However, it is hypothesized that H2O2, one of the lipid peroxidation products, might diffuse across the membrane and affect the vital enzymes in the sperm, thereby resulting in decreased sperm motility.56 It can be concluded from the present results that exposure to Pb during the perinatal period generates ROS by decreasing the activities of antioxidant enzymes and increasing lipid peroxidation, thereby causing oxidative stress in the testis and epididymis of adult rats. This may lead to disruption in the functional integrity of cell organelles.
Histopathologically, this study demonstrated that exposure to Pb led to disorganization in the germinal epithelium and the basal membrane of the seminiferous tubule and degeneration and loss of germ cells. It also causes a decrease in the seminiferous tubule diameter and enlarges inter-tubular spaces. In the present study, the reduction in the testicular weights could be due to the germinal cell loss. This histopathological damage may have caused disruption of spermatogenesis.
The important finding of the present study was reduction in fertility in male rats exposed to Pb during the perinatal period. In general, prenatal stress has been suggested to induce inappropriate levels of testosterone and aromatase activity in male rat foetuses during the critical stages of sexual differentiation, from 18–19 days of gestation, thus resulting in abnormal copulatory behaviour and high rates of female lordotic response in male offspring at adulthood.57,58 However, in the present study, though there was a delay in conception time, all the females cohabited with the Pb exposed males had copulatory plugs, indicating that the sexual behaviour is not affected in the Pb exposed male rats. The observed decrease in the numbers of implantations and live foetuses in the female mated rats exposed to Pb during the perinatal period has been attributed to a decrease in testosterone levels in circulation resulting in a decrease in sperm density and deterioration of sperm quality. The impaired sperm motility and deteriorated sperm membrane integrity may result in infertility due to the failure of sperm to reach the site of fertilization as well as their ability to penetrate the zona pellucida.59 The increase in pre-implantation loss observed in the present study may be at least in part due to failure of fertilization, and due to poor sperm quality and lowered sperm density. Thus, the results of the present study indicate that perinatal exposure to Pb alone will affect the reproductive potential at adulthood in rats.
The decrease in the relative weights of reproductive tissues, quality and quantity of sperm and activity levels of steroidogenic marker enzymes in the rats exposed to arsenic (during the pubertal period) might be responsible for the decrease in male fertility. The decreased testicular steroidogenic enzyme activity levels may lead to decreased steroidogenesis in the As exposed rats, which in turn may suppress the reproductive activities in the male rats. Severe deterioration of the testicular architecture was also observed in the rats exposed to As. The levels of lipid peroxidation, H2O2 generation and superoxide anions were also pronounced with a significant decrease in the activity levels of antioxidant enzymes in the testis and epididymis of the As exposed rats indicating As-induced oxidative stress. The reduction in steroidogenesis and spermatogenesis and elevated oxidative stress in the reproductive tissues of the As exposed rats might be responsible for the suppressed fertility. Similar to our findings, exposure to arsenic caused significant reduction in spermatogenesis60,61 and steroidogenesis,62,63 and deterioration of the testicular architecture16,64 and antioxidant defense system in the testes of rats.65,66
Finally, the reproductive perturbations observed in the current study were more protuberant in the Pb-exposed rats treated with As when compared to individual exposures. Only the combinations of sequential exposure to two metals were considered herein. While serving our purpose of providing reference end points, the stresses selected in the present study clearly do not represent all environmental stresses experienced by humans and wildlife. It may happen that several metals are present in high concentrations in nature. The nature of interactions between such stresses may be more complex. However, the present findings reveal the possible role of arsenic in exacerbating reproductive toxicity in rats when combined with lead treatment during the perinatal period.
Guidelines for ethical approval
The authors declare that the experiments were consistent with the guidelines and principles of the Committee for the Purpose of Control and Supervision on Experiments on Animals, Government of India and approved by the Institutional Animal Ethical Committee at S.V. University, Tirupati, India (vide no. IAEC/No-438/01/a/CPCSEA) with resolution No: 57/2012/(i)/a/CPCSEA/IAEC/SVU/PSR-KPR dt.08-07-2012.
Conflicts of interest
The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgments
The authors are grateful to Prof. K. V. S. Sarma, Department of Statistics, Sri Venkateswara University, Tirupati for his help in statistical analysis. The excellent technical assistance of Mr S. Umasankar is also gratefully appreciated. The authors declare that the experiments conducted during these studies comply with the current laws of their country. ChS is grateful to the University Grants Commission for awarding the UGC-PDFSS. KPR acknowledges the University Grants Commission (BSRRFSMS) for awarding a senior research fellowship. PSR is grateful to the UGC, New Delhi for awarding the BSR Fellowship.
References
- Fairbrother A., Wenstel R., Sappington K., Wood W. Ecotoxicol. Environ. Saf. 2007;68:145–227. doi: 10.1016/j.ecoenv.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Dhatrak S. V., Nandi S. S. Indian J. Occup. Environ. Med. 2009;13:60–64. doi: 10.4103/0019-5278.55121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapanti S., Pratap Reddy K., Sreenivasula Reddy P. Environ. Sci. Pollut. Res. 2018;25:13173–13185. doi: 10.1007/s11356-018-1500-x. [DOI] [PubMed] [Google Scholar]
- Fisher I. J., Pain D. J., Thomas V. G. Biol. Conserv. 2006;131:421–432. [Google Scholar]
- Wilson M. A., Johnston M. V., Goldstein G. W., Blue M. E. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5540–5545. doi: 10.1073/pnas.97.10.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer R. A. Environ. Health Perspect. 1990;89:101–105. doi: 10.1289/ehp.9089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saleh I., Shinwari N., Mashhour A., Mohamed G. E. D., Rabah A. Int. J. Hyg. Environ. Health. 2011;214:79–101. doi: 10.1016/j.ijheh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Basha D. C., Rani M. U., Devi C. B., Kumar M. R., Reddy G. R. Int. J. Dev. Neurosci. 2012;30:343–350. doi: 10.1016/j.ijdevneu.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Betharia S., Maher T. J. NeuroToxicology. 2012;33:1117–1127. doi: 10.1016/j.neuro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Apostoli P., Porru S., Bisanti L. Scand. J. Work, Environ. Health. 1999;25:40–43. [PubMed] [Google Scholar]
- Reshma M. A., Sainath S. B., Suneetha Y., Reddy P. S. Ecotoxicol. Environ. Saf. 2011;74:793–799. doi: 10.1016/j.ecoenv.2010.10.044. [DOI] [PubMed] [Google Scholar]
- Shen W., Chen J., Yin J., Wang S. L. Eur. Rev. Med. Pharmacol. Sci. 2016;20:773–780. [PubMed] [Google Scholar]
- Sun H. J., Rathinasabapathi B., Wu B., Luo J., Pu L. P., Ma L. Q. Environ. Int. 2014;69:148–158. doi: 10.1016/j.envint.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Hughes M. F. Toxicol. Lett. 2002;133:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- Ratnaike R. N. Postgrad. Med. J. 2003;79:391–396. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P. S., Rani G. P., Sainath S. B., Meena R., Supriya C. H. J. Trace Elem. Med. Biol. 2011;25:247–253. doi: 10.1016/j.jtemb.2011.08.145. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Hazra J., Upadhyay S. N., Sing R. K., Roy Chowdhury A. Indian J. Physiol. Pharmacol. 2008;52:84–90. [PubMed] [Google Scholar]
- Reshma M. A., Reddy P. S. Int. J. Pharma Bio Sci. 2013;4:893–898. [Google Scholar]
- Schieber M., Chandel N. S. Curr. Biol. 2014;24:453–462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C., Milne S., Leeson H. Reprod. Biomed. Online. 2014;28:684–703. doi: 10.1016/j.rbmo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Ko E. Y., Sabanegh E. S., Agarwal A. Fertil. Steril. 2014;102:1518–1527. doi: 10.1016/j.fertnstert.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Fay R. M., Mumtaz M. M. Food Chem. Toxicol. 1996;34:1163–1165. doi: 10.1016/s0278-6915(97)00090-2. [DOI] [PubMed] [Google Scholar]
- Mejia J. J., Diaz-Barriga F., Calderon J., Rios C., Jiménez-Capdeville M. E. Neurotoxicol. Teratol. 1997;19:489–497. doi: 10.1016/s0892-0362(97)00066-4. [DOI] [PubMed] [Google Scholar]
- Binks K., Doll R., Gillies M., Holroyd C., Jones S. R., McGeoghegan D., Scott L., Wakeford R., Walker P. Occup. Med. 2005;5:215–226. doi: 10.1093/occmed/kqi026. [DOI] [PubMed] [Google Scholar]
- Tchounwou P. B., Clement G. Y., Anita K. P., Dwayne J. S. HHS Public access. 2012;101:133–164. [Google Scholar]
- CPCSEA Indian J. Pharmacol. 2003;35:257–274. [Google Scholar]
- Robb G. W., Amann R. P., Killian G. J. J. Reprod. Fertil. 1978;54:103–107. doi: 10.1530/jrf.0.0540103. [DOI] [PubMed] [Google Scholar]
- Supriya Ch., Reddy P. S. Sci. Nat. 2015;102:26. doi: 10.1007/s00114-015-1274-7. [DOI] [PubMed] [Google Scholar]
- Saalu L. E., Udeh R., Oluyemi K. A., Jewo P. I., Fadeyibi L. O. Int. J. Morphol. 2008;26:1059–1064. [Google Scholar]
- Blazak W. F., Treinen K. A. and Juniewicz P. E., Application of testicular sperm head counts in the assessment of male reproductive toxicity, in Methods in Toxicology, ed. R. E. Chapin and J. J. Heindel, Academic press, San Diego, 1993, 3, pp. 86–94. [Google Scholar]
- Belsey M. A., Moghissi K. S., Eliasson R., Paulsen C. A., Gallegos A. J. and Prasad M. R., Laboratory manual for the examination of human semen and semen cervical mucus interaction, Press Concern, Singapore, 1980. [Google Scholar]
- Talbot P., Chacon R. S. J. Exp. Zool. 1981;215:201–208. doi: 10.1002/jez.1402150210. [DOI] [PubMed] [Google Scholar]
- Jeyendran R. S., Van-Der-Ven H. H., Zaneveld L. J. D. Arch. Androl. 1992;29:105–116. doi: 10.3109/01485019208987714. [DOI] [PubMed] [Google Scholar]
- Varshini J., Srinag B. S., Kalthur G., Krishnamurthy H., Kumar P., Rao S. B. S., Adiga S. K. Andrologia. 2012;44:642–649. doi: 10.1111/j.1439-0272.2011.01243.x. [DOI] [PubMed] [Google Scholar]
- Bergmeyer H. U., Methods of enzymatic analysis, ed. H. U. Bergmeyer, Academic Press, New York, 1974, pp. 447–489. [Google Scholar]
- Prodczasy J. J., Wei R. Biochem. Biophys. Res. Commun. 1988;150:1294–1298. doi: 10.1016/0006-291x(88)90770-x. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. Cell. Immunol. 1981;59:301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Claiborne A., Handbook of methods for oxygen radical research, ed. R. Greenwald, CRC Press, Florida, 1985, pp. 283–284. [Google Scholar]
- Paglia D. E., Valentine W. N. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. J. Biol. Chem. 1975;250:5474–5480. [PubMed] [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Bancraft J. D. and Stevens A., in Theory and practice of histological techniques, ed. J. D. Brancraft and A. Stevens, Churchill Livingstone, New York, 2nd edn, 1982. [Google Scholar]
- Johnson L., Chaturvedi P. K., Williams J. D. Biol. Reprod. 1992;47:1091–1098. doi: 10.1095/biolreprod47.6.1091. [DOI] [PubMed] [Google Scholar]
- Anway M. D., Memon M. A., Uzumcu M., Skinner M. K. J. Androl. 2006;27:868–879. doi: 10.2164/jandrol.106.000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas I., Gaspar I., Martinez S., Codesal J., Candelas S. Reprod. Toxicol. 1995;9:307–313. doi: 10.1016/0890-6238(95)97355-g. [DOI] [PubMed] [Google Scholar]
- Dorostghoal M., Dezfoolian A., Sorooshnia F. Iran. J. Basic Med. Sci. 2011;14:122–131. [Google Scholar]
- Queiroz E. K., Waissmann W. Cad. Saúde Pública. 2006;22:485–493. doi: 10.1590/s0102-311x2006000300003. [DOI] [PubMed] [Google Scholar]
- Reshma Anjum M., Reddy P. S. Int. J. Pharma Biosci. 2013;4:893–898. [Google Scholar]
- Pandya C., Pillai P., Nampoothiri L. P., Bhatt N., Gupta S. Andrologia. 2012;44:813–822. doi: 10.1111/j.1439-0272.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- Patra R. C., Swarup D., Dwivedi S. K. Toxicology. 2001;162:81–88. doi: 10.1016/s0300-483x(01)00345-6. [DOI] [PubMed] [Google Scholar]
- Marchlewicz M., Michalska T., Wiszniewska B. Chemosphere. 2004;57:1553–1562. doi: 10.1016/j.chemosphere.2004.08.102. [DOI] [PubMed] [Google Scholar]
- Bolin C. M., Basha R., Cox D. FASEB J. 2006;20:788–790. doi: 10.1096/fj.05-5091fje. [DOI] [PubMed] [Google Scholar]
- Aitken R. J., Clarkson J. S., Fishel S. Biol. Reprod. 1989;41:183–197. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- Makker K., Agarwal A., Sharma S. Indian J. Med. Res. 2009;129:357–367. [PubMed] [Google Scholar]
- Ward I. L. Science. 1972;175:82–84. doi: 10.1126/science.175.4017.82. [DOI] [PubMed] [Google Scholar]
- Osadchuk L. V., Braastad B. O., Huhtaniemi I., Bakken M. Reprod., Fertil. Dev. 2000;12:119–126. doi: 10.1071/rd99082. [DOI] [PubMed] [Google Scholar]
- De Kretser D. M., O'Bryan M. K., Cram D., McLanchlan R. I. Int. J. Androl. 2000;23:30–33. doi: 10.1046/j.1365-2605.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- Waalkes M. P., Ward J. M., Liu J., Diwan B. A. Toxicol. Appl. Pharmacol. 2003;186:7–17. doi: 10.1016/s0041-008x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Hazra J., Upadhyay S. N., Sing R. K., Roy Chowdhury A. Indian J. Physiol. Pharmacol. 2008;52:84–90. [PubMed] [Google Scholar]
- Meeker J. D., Rossano M. G., Protas B., Padmanahban V., Diamond M. P., Puscheck E., Daly D., Paneth N., Wirth J. J. Fertil. Steril. 2010;93:130–140. doi: 10.1016/j.fertnstert.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Telangb A. G., Malik J. K. Wudpecker J. Pharm. Pharmacol. 2013;2:33–48. [Google Scholar]
- Manna P., Sinha M., Sil P. C. Redox Rep. 2008;13:67–77. doi: 10.1179/135100008X259169. [DOI] [PubMed] [Google Scholar]
- Souza A. C. F., Marchesi S. C., de Almeida Lima G. D., Ferraz R. P., Santos F. C., da Matta S. L., Machado-Neves M. Biol. Trace Elem. Res. 2016;171:354–362. doi: 10.1007/s12011-015-0523-0. [DOI] [PubMed] [Google Scholar]
- Kucukkurt I., Ince S., Demirel H. H., Turkmen R., Akbel E., Celik Y. J. Biochem. Mol. Toxicol. 2015;29:564–571. doi: 10.1002/jbt.21729. [DOI] [PubMed] [Google Scholar]