 The prevalent application of nanoparticles (NPs) has drawn intense concerns about their impact on the environment and human health.
The prevalent application of nanoparticles (NPs) has drawn intense concerns about their impact on the environment and human health.
Abstract
The prevalent application of nanoparticles (NPs) has drawn intense concerns about their impact on the environment and human health. Inhalation of NPs is the major route of NP exposure and has led to adverse effects on the lung. It is of great concern to evaluate the potential hazards of nanoparticles for human health during pulmonary exposure. Here, we proposed a novel 3D human lung-on-a-chip model to recreate the organ-level structure and functions of the human lung that allow to us evaluate the pulmonary toxicity of nanoparticles. The lung-on-a-chip consists of three parallel channels for the co-culture of human vascular endothelial cells and human alveolar epithelial cells sandwiching a layer of Matrigel membrane, which recapitulate the key features of the alveolar capillary barrier in the human lung. Cell–cell interaction, cell–matrix interaction and vascular mechanical cues work synergistically to promote the barrier function of the lung-on-a-chip model. TiO2 nanoparticles and ZnO nanoparticles were applied on the lung-on-a-chip to assay their nanotoxicity on both epithelial cells and endothelial cells. Junction protein expression, increased permeability to macromolecules, dose dependent cytotoxicity, ROS production and apoptosis were assayed and compared on the chip. This lung-on-a-chip model indicated its versatile application in human pulmonary health and safety assessment for nanoparticles, environment, food and drugs.
Introduction
The prevalent application of manufactured nanoparticles (NPs) has drawn increasing concerns about the potential environmental health and safety effects from NP exposure.1–4 Inhalation is one of the primary routes by which human bodies are exposed to NPs.4 Nanoparticle inhaled exposures are associated with pulmonary disorders, such as asthma, pulmonary edema, emphysema, chronic obstructive pulmonary disease, and even lung cancer.5–7 The toxicity of nanoparticles is determined by their physical and chemical properties.8 The small size of the inhaled nanoparticles allows them to deposit and accumulate deep in the alveoli and be taken up by the lung epithelial cells.4,6,9 The cellular mechanism of nanoparticle induced toxicity on pulmonary epithelial and endothelial cells includes oxidative stress, mitochondrial injury, DNA damage, and inflammatory responses.10,11 Furthermore, a small proportion of the deposited nanoparticles could cross the alveolar-capillary barrier and translocate to other organs.6 The toxicities of nanoparticles are dependent on their physiochemical properties. For example, TiO2-NPs are normally recognized as a safe nanomaterial and have been widely applied in the food industry, medicine and architecture. In vivo and in vitro studies have demonstrated that TiO2-NPs have limited adverse effects.12,13 However, ZnO-NPs, another widely-used nanoparticle in medical care and the dietary industry, are toxic to mammalian cells, which has been evidenced by in vitro studies. Exposure to ZnO-NPs could lead to the death of alveolar epithelial cells, and disruption of tight junctions and plasma membranes.14–16 Therefore, identifying the pulmonary toxicity of different nanoparticles has become an emerging issue.
Currently, most pulmonary toxicity testing approaches to NPs are highly dependent on in vivo animal models and in vitro models. Animal models integrate the dynamic components of the respiratory system, blood circulation, immune system and other factors that can influence the toxicity screening.17,18 However, these models are costly, time-consuming and inconvenient to perform quantitative studies in vivo. Besides, animal models are poor representatives of certain human physiological/pathological features, which limits the extrapolation of evidence from animal models.19,20 In contrast, in vitro models for nanotoxicity tests utilize cultured human primary pulmonary cells or cell lines to perform quantitative, high-throughput analysis, providing cell-type specific mechanistic information.21–27 The results from in vitro assays do not correspond well to in vivo comparative results,28–30 since these simplified Petri-dish based cell culture models lack complex cellular architect (e.g. alveolar-capillary barrier) and the microenvironment (cell–cell interaction, cell–matrix interaction, mechanical cues, etc.), of the living lung, which are in close correlation with specific gene expression and organ level functionality.31–33 Therefore, it is urgent to develop organomimetic models for nanotoxicity screening.
Advances in microengineering and cell culture have made it possible to create organ-on-a-chip microdevices to mimic the microarchitectures and functionalities of specific tissues and organs.34 Multiple factors, including cell–cell cross-talk, cell–matrix communications and biophysical cues, have been integrated to recapitulate the physiological features of whole organs. Lung-on-a-chip has been developed to study complex human lung physiology and pathology in an organ-specific context.35–37 The lung-on-a-chip is generally achieved by fabricating a sandwich chip containing two apposed channels, separated by a thin porous polymer membrane, which are lined with human pulmonary epithelial cells and human vascular endothelial cells on opposite sides. However, these lung-on-a-chip models using polymer membranes for the cell attachment were unable to recreate the cell–extra cellular matrix (ECM) interaction in vivo.
In this study, we developed a biomimetic 3D lung-on-a-chip to provide pulmonary toxicity assays of nanoparticles. The lung-on-a-chip consists of two flanking parallel channels lined with human pulmonary alveolar epithelial cells and human umbilicus endothelial cells, respectively, and a middle channel filled with 3D Matrigel. The endothelial cells experience fluid flow to mimic the blood circulation in vivo. By co-culturing epithelial cells and endothelial cell on opposite sides of the 3D ECM membrane, we resembled the key structural features and functions of the alveolar-capillary barrier in vivo. In our study, ZnO and TiO2 nanoparticles were delivered to the epithelial cells, mimicking acute pulmonary nanoparticle exposure. We examined the alteration of the alveolar barrier in barrier integrity, barrier permeability and junction protein expression in the presence of ZnO and TiO2 nanoparticles with different concentrations. We assessed the generation of reactive oxygen stress and apoptosis of epithelial and endothelial cells with different concentrations ZnO and TiO2 nanoparticles. We demonstrate that this system provides a powerful platform for the study of lung physiology and nanotoxicity tests.
Results
Design and fabrication of 3D lung-on-a-chip
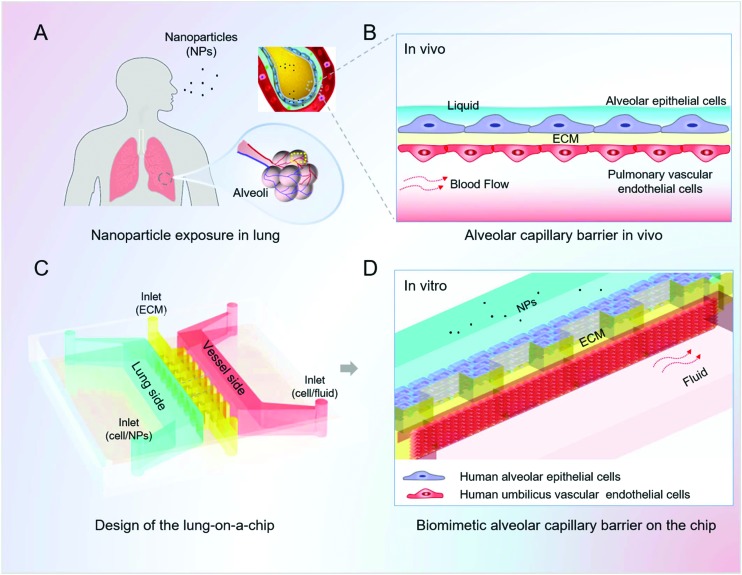
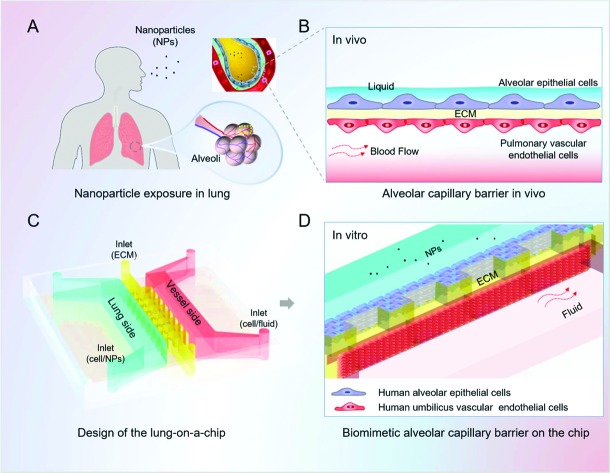
We designed and fabricated a microfluidic device that consists of three parallel channels for the infusion of lung alveolar epithelial cells, human vascular endothelial cells and the extra cellular matrix (Fig. 1). The alveolus channel was lined with human pulmonary alveolar epithelial cells (HPAEpiCs), representing the alveolar side of the alveolar capillary barrier. The vessel channel was lined with HUVECs and experienced fluid flow, representing the capillaries of the lung in vivo. Matrigel was located in the microchannel between the flanking channels to support cell adhesion as well as provide an alveolar microenvironment for the function of the alveolar capillary barrier.
Fig. 1. Design and assembly of the lung-on-a-chip model for nanotoxicity testing mimicking the human alveolar-capillary barrier in vitro. A. Diagram of the nanoparticle exposure to human lung. The small size of the inhaled nanoparticles allows them to deposit and accumulate deep in the alveoli; B. schematic illustration of the alveolar-capillary barrier in vivo. The barrier is composed of the alveolar epithelial cells, pulmonary vascular endothelial cells and extracellular matrix (ECM), which acts as the primary defence preventing the invasion of nanoparticles; C. the design and structure of the lung-on-a-chip; D. zoom-in figure shows the artificial alveolar-capillary barrier on the chip. The flanking channels are lined with human alveolar epithelial cells and vascular endothelial cells, respectively, representing the lung side and blood vessel side of the alveolar capillary barrier in vivo. Matrigel is located between the epithelial and endothelial cells, forming a layered structure of the barrier. The endothelial cells in the vessel channel experience fluidic flow to mimic the dynamic blood flow in the living lung.
Characterization of the lung-on-a-chip
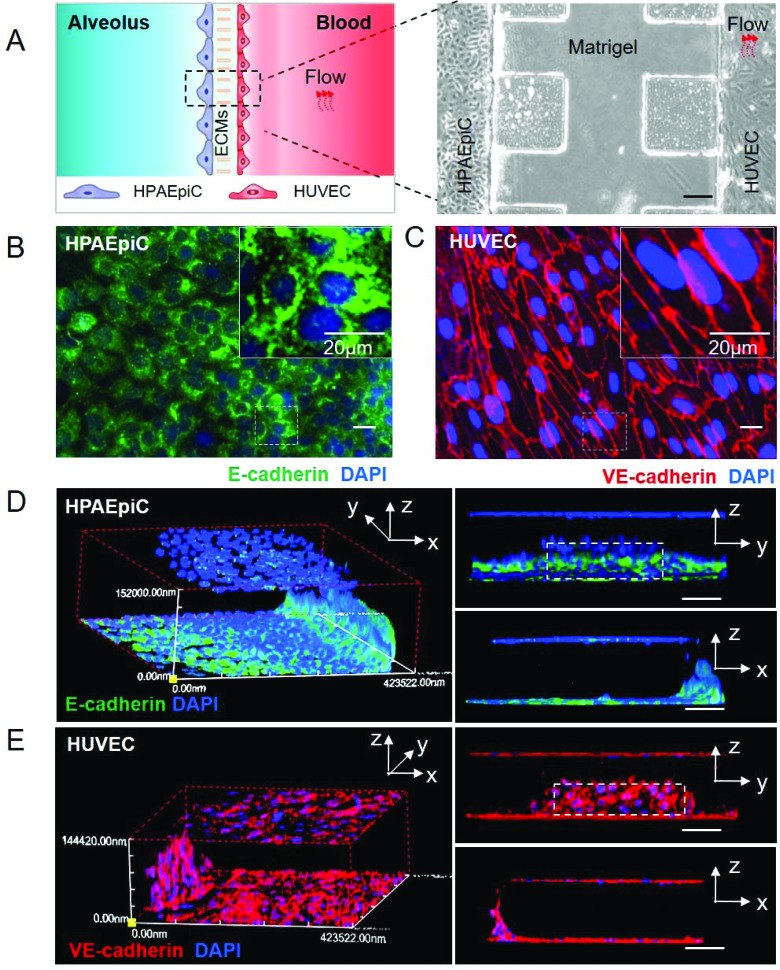
After 3 days’ co-culture and perfusion, the alveolar channel and vessel channel were lined with confluent HPAEpiCs and HUVECs respectively. To illustrate the structure of the alveolar-capillary barrier on the chip, HPAEpiCs and HUVECs were stained with E-cadherin and VE-cadherin for confocal imaging. E-Cadherin is an important adherens junction protein in epithelial cells and crucial for the epithelial barrier,38 and VE-cadherin participates in the formation of adherens junctions between the human lung endothelium and accounts for the maintenance of the endothelial barrier function.39,40 As demonstrated in Fig. 2B and C, the monolayer of HPAEpiCs was positive for E-cadherin expression and the monolayer of HUVECs was positive for VE-cadherin expression. Both junction proteins were localized in peripheral regions of the cells at cell-to-cell contact. The 3D reconstruction of fluorescent confocal images (Fig. 2D and E) validated the intact structure of the alveolar-capillary barrier on the chip which recapitulates the barrier structure in vivo.
Fig. 2. Alveolar-capillary barrier on a chip. After 3 days’ co-culture, HPAEpiCs and HUVECs were fixed and immunostained with E-cadherin antibody and VE-cadherin antibody, respectively. A. Bright-field image of alveolar capillary barrier on the chip, scale bar: 200 μm; B and C. the expression of junction proteins (E-cadherin and VE-cadherin) under continuous flow, scale bar: 20 μm; D and E. three-dimensional reconstructions of alveolar-capillary barrier from fluorescence images; scale bar: 100 μm.
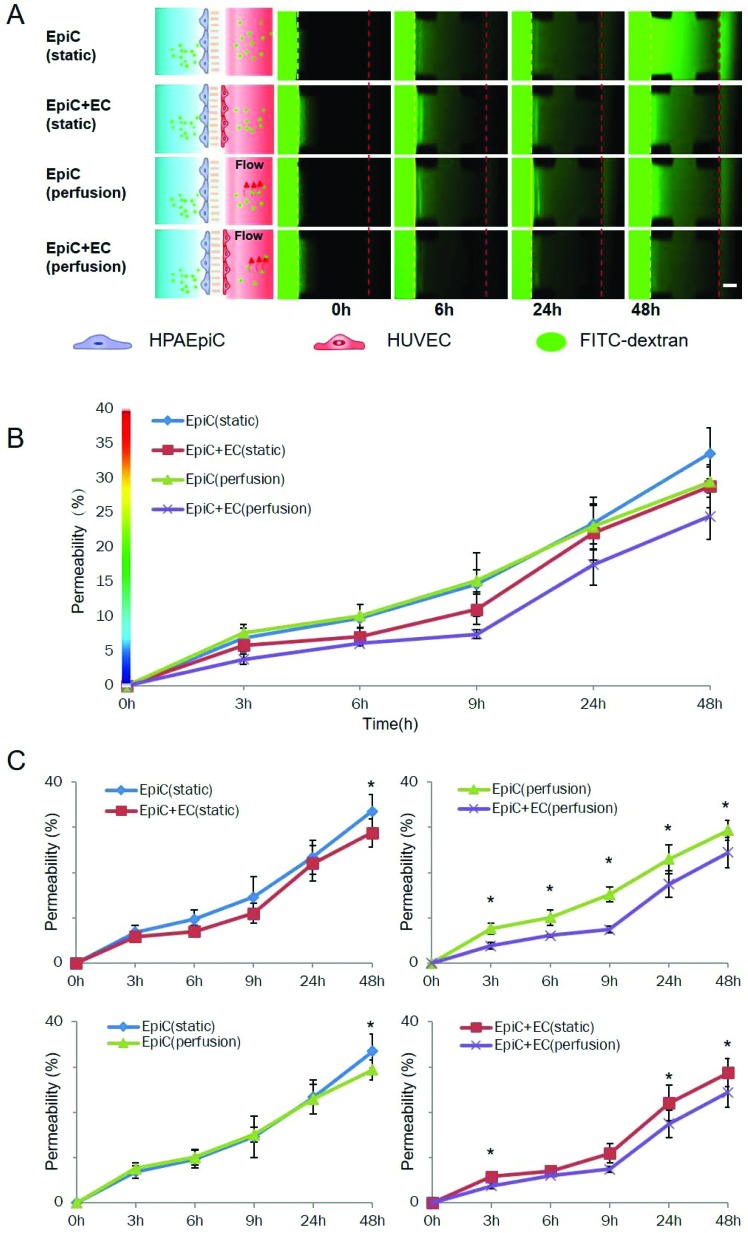
The living lung in vivo is also characterized by the barrier function restricting the passage of foreign materials. By using a fluorescently labelled dextran (10 kDa), we examined the permeability of our lung-on-a-chip model to macro molecules. Fig. 3A shows the time lapse images of fluorescent dextran diffusion through the alveolar-capillary barrier on the chip under static and dynamic cultures with a monoculture of epithelial cells or coculture of epithelial and endothelial cells. As demonstrated in Fig. 3B and C, the diffusion of FITC-dextran is significantly diminished by the co-culture of epithelial cells and endothelial cells under dynamic conditions. Under static conditions, co-culture of the epithelial cells and endothelial cells did not diminish the diffusion significantly in 48 h compared with the monoculture of epithelial cells. In contrast, when a fluidic flow was introduced to the endothelial cells, the co-culture model was able to diminish the diffusion as early as 3 h after the addition of FITC-dextran. The perfusion was able to diminish the diffusion in the presence of endothelial cells. However, without the endothelial cells, the perfusion did not affect the permeability in the monoculture model.
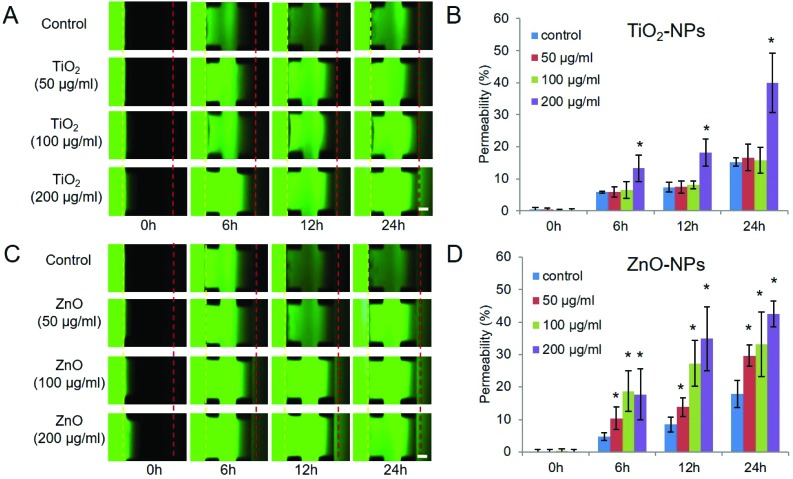
Fig. 3. Evaluation of the barrier function of the 3D lung-on-a-chip under different culture conditions. 3 days after cell loading, a culture medium with 10 μM FITC-dextran (10 kDa) was added on the alveolar channel, and the distribution of FITC-dextran on the chip was recorded by fluorescent microscopy at various time points (0 h, 3 h, 6 h, 9 h, 24 h and 48 h) to determine the permeability of the alveolar capillary barrier on the chip under various conditions. A. Time-lapse images of FITC-dextran across the alveolar-capillary interface into the vessel channel under various conditions. EpiC: HPAEpiC; EC: HUVEC; scale bar: 200 μm; B–C: quantitative analysis of the permeability of alveolar capillary barriers under different conditions. * indicates p < 0.05; error bars: S.D., n = 3.
Modelling nanoparticle exposure on the chip
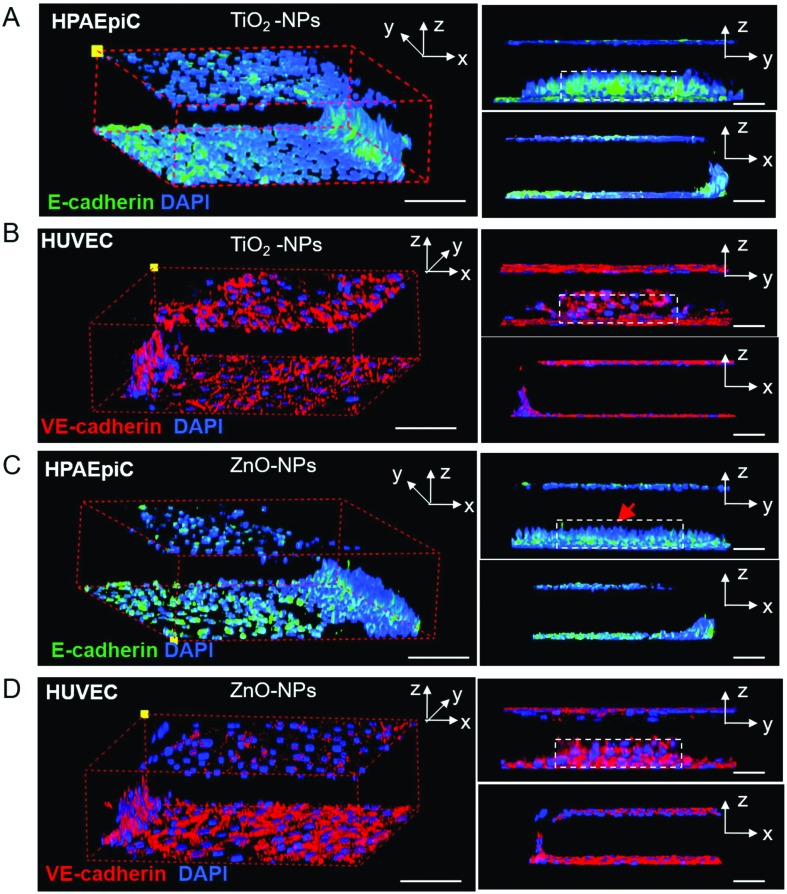
When inhaled nanoparticle aerosols are delivered to the alveoli of the lung, they are deposited in the fluidic layer on the surface of the epithelial cells and subsequently interact with the alveolar-capillary barrier. To mimic the invasion of inhaled nanoparticles in the lung on our lung-on-a-chip model, we dispersed the nanoparticles in the culture medium and injected nanoparticle solutions into the alveolar channel. The epithelial cells were exposed to TiO2-NPs (200 μg ml–1) and ZnO NPs (200 μg ml–1) for 24 h, respectively. After that, the epithelial cells were stained with the E-cadherin antibody and the endothelial cell were stained with the VE-cadherin antibody. The integrity changes of the alveolar-capillary barrier were demonstrated by 3D confocal reconstruction fluorescent images (Fig. 4). The alveolar-capillary barrier on the chip remains intact after exposure to TiO2-NPs (200 μg ml–1, Fig. 4A and B). TiO2-NPs exhibited limited effects on the expression of the junction protein E-cadherin between the epithelial cells (Fig. S1†). On the vascular side, the integrity of the endothelial cell layer was sustained. In contrast, under the treatment of ZnO-NPs (200 μg ml–1) for 24 h, disruption of the barrier was observed (Fig. 4C and D). The expression of E-cadherin decreased and discontinued intercellular junctions were observed between epithelial cells (Fig. S1†). It was noticeable that the ZnO-NPs showed limited effects on the integrity of the endothelial barrier on the lung-on-a-chip model.
Fig. 4. Morphology and integrity of the alveolar-capillary barrier on the chip 24 hours after nanoparticle exposure (200 μg ml–1). A. TiO2-NPs’ effect on the expression of E-cadherin of HPAEpiCs; B. TiO2-NPs's effect on the expression of VE-cadherin of HUVECs; C. ZnO-NPs’ effect on the expression of E-cadherin of HPAEpiCs, red arrow indicates the disrupt on the epithelial barrier; D. ZnO-NPs’ effect on the expression of VE-cadherin of HUVECs. Dashed boxes indicate the epithelial or endothelial barrier structure on the chip. Scale bar: 100 μm.
The toxicity of nanoparticles is associated with their physiochemical properties. Though several in vitro studies have implicated that TiO2-NPs might induce cell death of the pulmonary epithelium, the results vary by what cell lines and types of TiO2-NPs (size, anatase or rutile) were used.41 In our experiment, the TiO2-NPs were about 25 nm in diameter and rutile, which is less toxic to cells. Exposure to the TiO2-NPs exhibited limited disruption of the barrier on the chip, which was in accordance with previous reports. On the other hand, acute exposure to ZnO NPs caused the disruption of the alveolar-capillary barrier. ZnO-NPs induced disruption of the epithelial cell layer, but not the vascular cell layer.
Nanoparticle exposure increased the permeability of the lung-on-a-chip
We next examined the effect of TiO2-NPs and ZnO-NPs on the permeability of the lung-on-a-chip model. The permeability of the lung-on-a-chip model was measured in a temporal manner after the TiO2-NPs and ZnO-NPs were delivered to the epithelial cells at different conditions for 24 hours (Fig. 5). The medium that contained nanoparticles was carefully removed and the alveolar channels were rinsed with fresh medium, followed by the addition of FITC-dextran. Fig. 5A shows time-lapse images of the diffusion of FITC-dextran through the alveolar-capillary barrier on the chip with the addition of TiO2-NPs at different concentrations. As demonstrated in Fig. 5B, a high concentration (200, 100 μg ml–1) of TiO2-NPs increased the permeability of the barrier to FITC-dextran significantly. But at lower concentrations (0, 50 μg ml–1, 100 μg ml–1), the diffusion of FITC-dextran was not changed. Compared with the result in Fig. 4, 200 μg ml–1 TiO2-NP exposure increased the permeability of the barrier while the intercellular junction remained intact. The intercellular junctions between epithelial cells are closely related to the barrier function of the alveolar-capillary barrier, and the assembly involves multiple proteins, such as E-cadherin, claudin family proteins, etc. We assumed that the TiO2 NP exposure might not affect the E-cadherin expression but other junction proteins or channel proteins, resulting in the increased permeability of the alveolar capillary barrier on the chip.
Fig. 5. NPs’ effect on the permeability of the alveolar capillary barrier on the chip. After 24 hours’ exposure to TiO2-NPs/ZnO-NPs, HPAEpiCs and HUVECs were washed with RPMI1640 medium and the FITC-dextran was added in the alveolar channel. The distribution of FITC-dextran on the chip was recorded by fluorescent microscopy at various time points (0 h, 6 h, 12 h, 24 h) to determine the permeability of the alveolar capillary barrier on the chip. A. Time-lapse images of FITC-dextran across the alveolar capillary barrier into the vessel channel with TiO2-NP treatment of different concentrations; B. statistical analysis of the permeability of the alveolar capillary barrier with TiO2-NP treatments; C. time-lapse images of FITC-dextran across the alveolar-capillary interface into the vessel channel with ZnO-NP treatment of different concentrations. Scale bar: 200 μm; D. statistical analysis of the permeability of the alveolar capillary barrier with ZnO NP treatments. * indicates p < 0.05; error bar: SD, n = 3.
Fig. 5C and D show the effect of ZnO-NPs on the permeability of the alveolar-capillary barrier at various concentrations. An increase of the permeability was detected at any concentration of Zn-NPs (50 μg ml–1, 100 μg ml–1, 200 μg ml–1). In addition, ZnO-NPs increased the permeability of the barrier on the chip in a dose-dependent manner. The higher the dose of Zn-NPs, the faster the dextran diffused.
Increased ROS generation with nanoparticles exposure
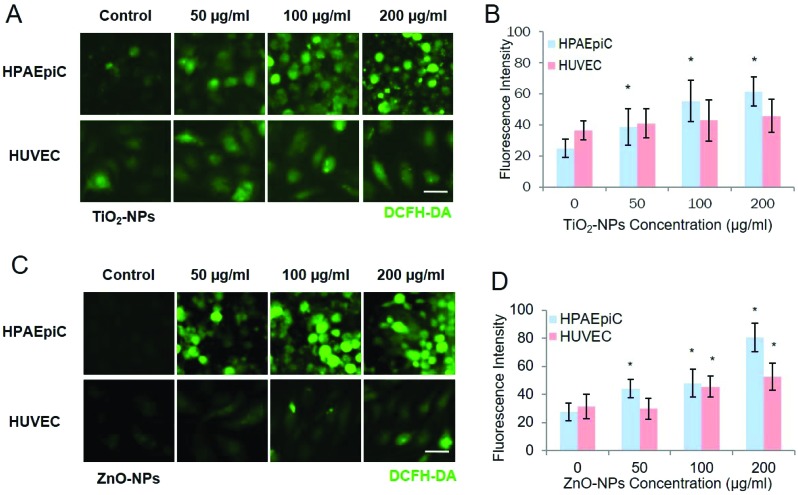
Fig. 6 shows the ROS generation of epithelial cells and endothelial cells induced by TiO2-NPs and ZnO-NPs respectively. After exposure to TiO2-NPs, the ROS generated by epithelial cells increased in a dose-dependent way, which agreed with previous in vitro and in vivo results.41 However, the ROS generated by endothelial cells were not significantly changed with the TiO2-NP exposure. In contrast, with ZnO-NP exposure, both the epithelial cells and endothelial cells on the chip exhibited dose-dependent increase of ROS.
Fig. 6. ROS generation in HPAEpiCs and HUVCEs after nanoparticle exposure at different concentrations on the chip. After 24 hours’ exposure to TiO2-NPs and ZnO-NPs, the ROS generation in HPAEpiCs and HUVECs was analysed via DCFH-DA staining. A. Fluorescent images of TiO2-NP-induced ROS responses of HPAEpiCs and HUVECs; particle diameter: 25 nm; B. statistical analysis of TiO2 NP-induced ROS production by HPAEpiCs and HUVECs. * indicates p < 0.05; C. fluorescent images of ZnO-NP-induced ROS responses of HPAEpiCs and HUVECs on the chip; particle diameter: 40 nm; D. statistical analysis of ZnO-NP-induced ROS generation by HPAEpiCs and HUVECs. Scale bar: 50 μm; * indicates p < 0.05; error bar: S.D. n = 3.
These observations were in accordance with the permeability changes under the treatment of nanoparticles with different concentrations. The direct interaction between epithelial cells and nanoparticles leads to the uptake of nanoparticles. The intrinsic features of the nanoparticles, e.g. small size and high surface area per unit mass, bring a higher reactivity and possibility for the formation of free radicals, which could cause oxidative stress on cells.5,11,42 Compared with TiO2-NPs, ZnO-NPs are considered highly toxic, because of the Zn2+ release after Zn exposure. The Zn2+ could penetrate the epithelial barrier and the Matrigel membrane, interacting with the endothelial cell layers and consequently increasing the ROS in endothelial cells.
Increased apoptosis of HPAEpiCs with nanoparticle exposure
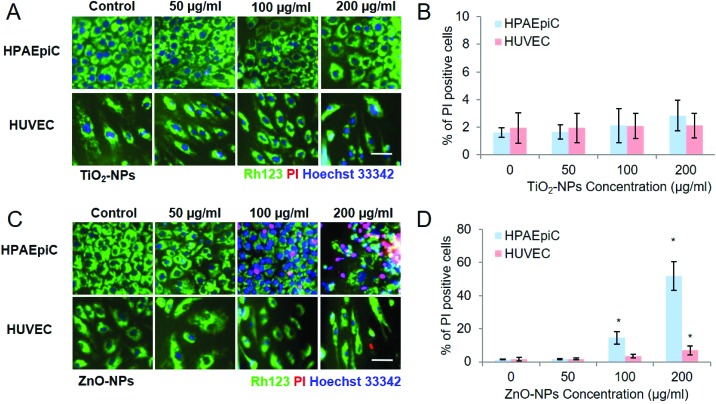
Fig. 7 shows that apoptosis of HPAEpiCs and HUVECs was detected after exposure to TiO2-NPs and ZnO-NPs, respectively. For TiO2-NPs, few apoptotic cells were detected on either HPAEpiCs or HUVECs. While with Zn-NP exposure, increased apoptotic HPAEpiCs were detected in a dose-dependent manner at concentration of 100 μg ml–1 and 200 μg ml–1 for HUVECs. Apoptosis of HUVECs was detected with 200 μg ml–1 ZnO-NP exposure. The cell death rate on the alveolar side was higher than that on the vessel side, which corresponded with the ROS assay.
Fig. 7. Nanoparticle-induced apoptosis of HPAEpiCs and HUVECs on the chip. After exposure to NPs for 24 hours, both HPAEpiCs and HUVCEs were washed with serum free RPMI1640 medium twice and incubated with RH123 and Hoechst 33342 for 20 min. Then the cells were washed with PBS three times and incubated with PI for 15 min before imaging. A. Fluorescent images of TiO2-NP-induced apoptosis of HPAEpiCs and HUVECs on the chip; particle diameter: 25 nm; B. statistical analysis of TiO2 NP-induced apoptosis of HPAEpiCs and HUVECs. * indicates p < 0.05; C. fluorescent images of Zn-NP-induced apoptosis of HPAEpiCs and HUVECs on the chip; particle diameter: 40 nm; D. statistical analysis of ZnO-NP-induced apoptosis of HPAEpiCs and HUVECs. Scale bar: 50 μm; * indicates p < 0.05; error bar: S.D. n = 3.
Given that few apoptotic cells were detected, the treatment with 200 μg ml–1 TiO2-NPs caused an increase of alveolar-capillary permeability and ROS generation on epithelial cells, and we reasoned that the oxidative stress on the HPAEpiCs may be responsible for the disruption of the barrier function on the chip with TiO2-NP exposure. As for the ZnO-NPs, the cell death of epithelial cells and endothelial cells disputed the structural integrity of the barrier, which inevitably compromised the barrier function on the chip. When HPAEpiCs were mono-cultured on the chip (Fig. S2†), ZnO NPs led to obvious dose-dependent apoptosis. However, when co-cultured with HUVECs on the vascular channel, the apoptotic HPAEpiCs were significantly decreased. Further analysis, including tracking NP diffusion and uptake by HUVEC, and paracrine factor identification, could help to elucidate the protective mechanism.
Discussion
The purpose of our study was to establish a physiological relevant model of the human lung to examine acute lung injury after nanoparticle exposure. We have developed a dynamic 3D lung on a chip model allowing coculture of human alveolar epithelial cells and human vascular endothelial cells in compartmentalized channels. This lung-on-a-chip model mimics the structural and functional features of the alveolar-capillary barrier by recapitulating the cell–ECM interaction, cell–cell communication, and fluidic stimuli in native alveoli that influence the function of barrier.
Our lung-on-a-chip resembled the essential cell types in the formation of the alveolar-capillary barrier and the disruption after nanoparticle-induced injury. The barrier in vivo is mainly composed of pneumocytes (AT I and AT II), endothelial cells and the basement membrane (Fig. 1A and B). In alveolar epithelium, AT II cells are responsible for the pulmonary functions and pathological reactions induced by toxicants. The pulmonary vascular endothelium plays an important role in gas exchange and fluid balance between the blood and the interstitial tissues in the lung.40,43 Our model cocultured immortalized HPAEpiCs derived from human primary AT II cells and HUVECs to resemble both essential cell types that participate in the formation of the alveolar-capillary barrier. Stable and confluent cell layers were achieved to replicate the layered epithelium and endothelium barrier structure in vivo. The barrier function of the lung-on-a-chip model was also significantly improved by the co-culture of epithelial cells and endothelial cells (Fig. 3).
To better mimic the organotypic features of the human lung, we also recreated the ECM niche and fluidic cues in the alveoli in vivo. A trans-well based co-culture model employed a synthetic porous polymer membrane to provide a compartmentalized niche for cell proliferation and differentiation and resembled the basement membrane.35,44,45 However, these polymers provided a limited capacity to recreate the cell–ECM communication, which plays crucial roles in cell differentiation, morphogenesis, regeneration and function.31,40 To replicate the cellular interactions with native extracellular matrices in alveoli, we introduced a layer of Matrigel between the epithelial cell layer and endothelial cell layer to resemble the basement membrane. Both epithelial cells and endothelial cells formed intact cell layers (Fig. 2) with a positive expression of specific junction proteins. In our model, the endothelial cells experienced flow perfusion to mimic the circulating blood. The perfusion provided nutrients and oxygen to the lung-on-a-chip and removed metabolic waste more sufficiently. In addition, the perfusion introduced flow shear stress on the endothelial cells and benefitted the formation and function of the endothelial barrier.46–48
Taken together, ECM, co-culture and perfusion work synergistically to promote the barrier function of the lung-on-a-chip model, which was evidenced by the selective permeabilities to macromolecules under variable combinations of these factors. Our model could be extended to the study of complex cell–cell, cell–matrix, and cell–signalling interactions, as well as constructions of other tissue–tissue interfaces.
Beyond recapitulating the layered structure and organotypic function of the alveolar-capillary barrier, our model showed the potential value in nanotoxicity tests. During pulmonary exposure to NPs, the small size of the NPs allows them to penetrate into the deep sites of the alveolar cavity and consequently translocate to the other parts of the human body. The intrinsic features of nanoparticles, e.g. small size and high surface area per unit mass, bring a higher reactivity and possibility for the formation of free radicals which could cause oxidative stress on the cells.5,11,42 The accumulation of oxidative stress would consequently lead to the apoptosis of cells and the dysfunction of barriers. Both ZnO-NPs and TiO2-NPs exhibited dose-dependent toxicity on the epithelial cells and endothelial cells, including ROS generation and apoptosis. The results indicated that the ZnO-NPs are more toxic than TiO2-NPs, which is in accordance with previous studies10,26,28 The influence of NPs on the barrier function was also assayed in a visualized manner. In accordance with the cytotoxicity assay, the permeability of the alveolar barrier on the chip elevated as the concentration of NPs increased, indicating the dysfunction of the barrier. Compared with TiO2-NPs, ZnO-NPs triggered the increase of the permeability at a lower concentration. As demonstrated above, our lung-on-a-chip model provided a reliable platform for assessing the NP-induced pulmonary injury.
In our model, the NP-induced injuries on the epithelial cells and endothelial cells were evaluated simultaneously within the context of the layered structure. Since the NPs were initially in direct contact with the epithelial cells, the epithelial cells experienced more severe damage than the endothelial cells did. The results of the ROS generation and apoptosis assays address the differences caused by spatial differences. In addition, in our lung-on-a-chip model, co-cultured HPAEpiCs with HUVECs showed fewer apoptotic cells than monocultured HPAEpiCs with a treatment of 200 μg ml–1 ZnO-NPs (Fig. S2†). This finding implied that the cell–cell interaction (Fig. S2†) could influence the outcome of toxicity tests, which could help to improve the predictivity and reliability of the in vitro model for toxicity screening.
Conclusions
In summary, we proposed a 3D human lung-on-a-chip model that allows for the evaluation of nanotoxicity. We recreate the biomimetic 3D alveolar-capillary barrier on the chip by co-culturing human lung epithelial cells and human vascular endothelial cells on a 3D Matrigel membrane with flow cues, recapitulating the structural and functional features of native alveoli. The combination of multiple factors provides a valid biomimetic in vitro platform for modelling the NP exposure of the human lung and investigating the nanotoxicity in a physiological relevant manner. Under nanoparticle exposure (TiO2-NPs and ZnO-NPs), cellular morphology, junction protein expression, ROS generation and apoptosis of epithelial and endothelial cells were analysed simultaneously.
This work provides a dynamic in vivo-like human lung model not only for the accurate prediction of the potential toxicological impacts of nanoparticles, but also for novel and discrete nanotechnology applications and environmental safety assessments. Future developments of the in vitro human lung model for nanotoxicity investigations could focus on more complex and realistic models to better mimic human responses, and the establishment of multi-organs-on-a-chip to enable a systematic analysis of organ–organ interactions.
Materials and methods
The fabrication of lung-on-a-chip
A schematic of the fabrication is shown in Fig. 1 and the design of the microfluidic chip is shown in Fig. S3.† Firstly, an ∼80 μm thick layer of SU8-3500 photoresist was spin coated onto a silicon wafer and heated at 95 °C for 30 min. The wafer was cooled in air and covered with a designed mask followed by exposure to 15 mJ cm–2 of UV light for 20 s. The wafer was then post-heated at 95 °C for 10 min. After the wafer cooled down, a second layer of SU8 was spin coated (∼220 μm) and heated at 95 °C for 30 min. The cool wafer was covered by another designed mask with careful alignment and exposed to UV for 40 s. After post-heating at 95 °C for 10 min and a slow cooling, the mask was rinsed with ethyl lactate and blow dried with clean air. Before use, the wafer was heated at 180 °C for two hours and slowly cooled. To fabricate the PDMS chip, uncured polydimethylsiloxane (PDMS, Sylgard 184) was mixed with a curing reagent at a ratio of 1 : 10 w/w and poured onto the silicon wafers (Lijing Opto-electronic Ltd). After curing at 80 °C for 30 min, the PDMS layer was peeled from the wafers and wrapped with plastic film for use.
Chip preparation
The chip was cleaned with plasma for 20 s and irreversibly bonded to cover glass. The chips were sterilized by UV for 30 minutes and ready for use. Before the gel injection, chips were cooled on ice. Matrigel was thawed at 4 °C, and carefully injected into the gel channel. The gel was incubated at room temperature for 5 min. The channels were modified with 2% chitosan (w/v, PBS) for 30 minutes and washed by PBS three times. Subsequently, the channels were modified with Collagen I (10 μg ml–1) at 37 °C for 2 hours and washed with PBS three times.
Cell culture
Immortalized human alveolar epithelial cells (HPAEpiCs) were generated from Type II pneumocytes of human lung tissue (purchased form Sciencell Shanghai Corporation) and cultured with RPMI 1640 Medium supplemented with 10% FBS. Human umbilical vein endothelial cells were isolated from the human umbilical cord and cultured with Endothelial Cell Medium (ECM, ScienCell).
Establishment of alveolar epithelial barrier
HPAEpiCs were disassociated with 0.25% trypsin for 3 min and collected after centrifuging at 800g for 3 min. The cells were re-suspended with RPMI1640 medium supplemented with 10% FBS at a density of 1 × 107 cells per ml. 1 × 105 cells were loaded into the alveolus channel and adhered to the gel by side-standing the chip at 37 °C for 1 hour. After that, 1 × 105 HUVECs were injected into the vessel channel following the same procedure as described above. 24 hours after the cell loading, perfusion systems were connected with chips to introduce the ECM through the vessel channel. The flow rate was set to 10 μl h–1 (0.0035 dye per cm2) to mimic the fluid flow in vivo.
Characterization of nanoparticles
TiO2-NPs and ZnO-NPs were dispersed in RPMI 1640 medium supplemented with 10% FBS at a final concentration of 100 μg ml–1. The size distribution and zeta potential of the nanoparticles in culture medium were determined by a Malvern Zetasizer Nano. The shape of nanoparticles was profiled by transmission electron microscopy (TEM, FEI TECHAI G2 F20).
Nanoparticle exposure on the lung-on-a-chip
The nano-sized ZnO particles (1.7 g ml–1, water) were purchased from Sigma and diluted with ultra-pure water at a final concentration of 200 mg ml–1. The nano-sized rutile TiO2 powders were suspended in ultra-pure water (200 mg ml–1). Both solutions were sonicated (50 W, 30 min) to promote the dispersion prior to being diluted in culture medium. The NPs were introduced into chips at the desired concentrations at 37 °C for 24 hours after 3 days of dynamic culture.
Permeability assay
To assess the permeability of the alveolar-capillary barrier on the chip against macromolecules, the fluxes of fluorescent molecules were measured by detecting the fluorescent intensity of FITC-dextran (10 kDa, Sigma) diffused across the alveolar-capillary barrier. After the formation of the barrier, medium that contained 10 μM FITC-dextran was delivered into the alveolus channel. Time-course fluorescent images of the FITC-dextran on the alveolus channel and vessel channel were collected simultaneously. The relative intensities of FITC-dextran penetrating the barrier were measured to profile the permeability of the barrier on the vessel channel at each time point (i.e. the absolute fluorescent intensity in the vessel channel; the absolute fluorescent intensity in the alveolus channel). The fluorescent intensity of FITC-dextran is determined by choosing three random areas on each side using Image-Pro Plus software (Media Cybernetics, USA).
Immunofluorescence analysis
Cells were washed with phosphate-buffered saline (PBS) three times to remove the media residue and fixed with 4% paraformaldehyde at room temperature for 15 min. Thereto, cells were washed with PBS three times and permeabilized with 0.1% (v/v) TritonX-100 (Sigma) in PBS for 15 min. Samples were washed with PBS three times followed by incubation with goat serum (Beyotime Company, China) at room temperature for 1 hour to block non-specific binding of antibodies. After removing the blocking serum, samples were incubated with primary antibodies for E-cadherin (1 : 100 diluted, CST) and VE-cadherin (1 : 200 diluted, CST), respectively, at 4 °C overnight. Subsequently, cells were washed with PBS three times and incubated with Alexa Flour 488/594-conjugated secondary antibodies (1 : 100 diluted, Beyotime Company, China) for 1 hour at room temperature. Prior to fluorescent imaging, samples were washed with PBS three times (5 min for each) and stained with 4′-6-diamidino-2-phenylindole (DAPI, Sigma) for 15 min at room temperature followed by PBS washing. Fluorescent microscopy images were acquired using an inverted fluorescence microscope (IX71, Olympus) and confocal microscope (FV-1000, Olympus).
ROS analysis
The measurements of ROS production on the chip were performed with a Reactive Oxygen Species Assay Kit (Solarbio, Beijing). Briefly, DCFH-DA was diluted in a serum-free RPMI1640 medium at a final concentration of 10 μM in the dark. The cells were washed with PBS twice and incubated with 10 μM DCFH-DA at 37 °C in the dark for 30 min. After incubation, the cells were rinsed with serum-free RPMI 1640 medium twice and incubated with RPMI1640 medium supplemented with 10% FBS. The fluorescence was measured using an inverted fluorescent microscope (IX71, Olympus) at 488 nm excitation and 525 nm emission.
Apoptosis analysis
After exposure to NPs for 24 h, cells were washed with serum free 1640 medium twice and incubated with Hoechst 33342 (4 μg ml–1) and Rh-123 (2 μg ml–1) at 37 °C for 20 min. Then the cells were washed with PBS three times and incubated with PI (2 μg ml–1) for 15 min followed by PBS washing. Cells were imaged with an inverted fluorescence microscope. Images were analysed with ImageJ v1.50 to calculate the PI positive cells and Hoechst positive cells. For each experimental set, >80 cells were analysed.
Statistical analysis
Statistical significance was assessed by two-tailed paired student's t-tests, one-way ANOVA (alpha = 0.05). Comparisons with p < 0.05 were determined to be significant.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDPB0305, XDA16020900), Key Program of the Chinese Academy of Sciences (KFZD-SW-213), National Key R&D Program of China (No. 2017YFB0405400), International Science & Technology Cooperation Program of China (No. 2015DFA00740), National Nature Science Foundation of China (No. 91543121, 81573394, 31671038), and Innovation Program of Science and Research from the DICP, CAS (DICP TMSR201601).
Footnotes
†Electronic supplementary information (ESI) available: ESI figures. See DOI: 10.1039/c8tx00156a
References
- Gwinn M. R., Vallyathan V. Environ. Health Perspect. 2006;114:1818–1825. doi: 10.1289/ehp.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T., Li N. and Nel A. E., in Annual Review of Public Health, 2009, vol. 30, pp. 137–150. [DOI] [PubMed] [Google Scholar]
- Xia T., Li N., Nel A. E. Annu. Rev. Public Health. 2009;30:137–150. doi: 10.1146/annurev.publhealth.031308.100155. [DOI] [PubMed] [Google Scholar]
- Yang W., Peters J. I., Williams 3rd R. O. Int. J. Pharm. 2008;356:239–247. doi: 10.1016/j.ijpharm.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Madl A. K., Plummer L. E., Carosino C., Pinkerton K. E. Annu. Rev. Physiol. 2014;76:447–465. doi: 10.1146/annurev-physiol-030212-183735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Muralikrishnan S., Ng C. T., Yung L. Y., Bay B. H. Exp. Biol. Med. 2010;235:1025–1033. doi: 10.1258/ebm.2010.010021. [DOI] [PubMed] [Google Scholar]
- Lu X., Zhu T., Chen C., Liu Y. Int. J. Mol. Sci. 2014;15:17577–17600. doi: 10.3390/ijms151017577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava V., Gusain D., Sharma Y. C. Ind. Eng. Chem. Res. 2015;54:6209–6233. [Google Scholar]
- Card J. W., Zeldin D. C., Bonner J. C., Nestmann E. R. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2008;295:L400–L411. doi: 10.1152/ajplung.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Lee Y. K., Jung M., Kim K. H., Chung N., Ahn E. K., Lim Y., Lee K. H. Inhalation Toxicol. 2007;19(Suppl 1):59–65. doi: 10.1080/08958370701493282. [DOI] [PubMed] [Google Scholar]
- Manke A., Wang L., Rojanasakul Y. BioMed Res. Int. 2013;2013:942916. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrez A., Horvath L., Smajda R., Salicio V., Pasquier N., Forro L., Schwaller B. ACS Nano. 2009;3:2274–2280. doi: 10.1021/nn9002067. [DOI] [PubMed] [Google Scholar]
- Liu R., Liu H. H., Ji Z., Chang C. H., Xia T., Nel A. E., Cohen Y. ACS Nano. 2015;9:9303–9313. doi: 10.1021/acsnano.5b04420. [DOI] [PubMed] [Google Scholar]
- Cho W. S., Duffin R., Poland C. A., Howie S. E., MacNee W., Bradley M., Megson I. L., Donaldson K. Environ. Health Perspect. 2010;118:1699–1706. doi: 10.1289/ehp.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Qin X., Wang B., Xu G., Qin Z., Wang J., Wu L., Ju X., Bose D. D., Qiu F., Zhou H., Zou Z. Cell Death Dis. 2017;8:e2954. doi: 10.1038/cddis.2017.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W. S., Duffin R., Howie S. E., Scotton C. J., Wallace W. A., Macnee W., Bradley M., Megson I. L., Donaldson K. Part. Fibre Toxicol. 2011;8:27. doi: 10.1186/1743-8977-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop L., Cena L., Orandle M., Yanamala N., Dahm M. M., Birch M. E., Evans D. E., Kodali V. K., Eye T., Battelli L., Zeidler-Erdely P. C., Casuccio G., Bunker K., Lupoi J. S., Lersch T. L., Stefaniak A. B., Sager T., Afshari A., Schwegler-Berry D., Friend S., Kang J., Siegrist K. J., Mitchell C. A., Lowry D. T., Kashon M. L., Mercer R. R., Geraci C. L., Schubauer-Berigan M. K., Sargent L. M., Erdely A. ACS Nano. 2017;11:8849–8863. doi: 10.1021/acsnano.7b03038. [DOI] [PubMed] [Google Scholar]
- Warheit D. B., Laurence B. R., Reed K. L., Roach D. H., Reynolds G. A., Webb T. R. Toxicol. Sci. 2004;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- Warheit D. B., Sayes C. M., Reed K. L., Swain K. A. Pharmacol. Ther. 2008;120:35–42. doi: 10.1016/j.pharmthera.2008.07.001. [DOI] [PubMed] [Google Scholar]
- de Oliveira M. V. J. Biomed. Sci. 2016;5:1. [Google Scholar]
- AshaRani P. V., Low Kah Mun G., Hande M. P., Valiyaveettil S. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- Limbach L. K., Li Y., Grass R. N., Brunner T. J., Hintermann M. A., Muller M., Gunther D., Stark W. J. Environ. Sci. Technol. 2005;39:9370–9376. doi: 10.1021/es051043o. [DOI] [PubMed] [Google Scholar]
- Sayes C. M., Wahi R., Kurian P. A., Liu Y., West J. L., Ausman K. D., Warheit D. B., Colvin V. L. Toxicol. Sci. 2006;92:174–185. doi: 10.1093/toxsci/kfj197. [DOI] [PubMed] [Google Scholar]
- Limbach L. K., Wick P., Manser P., Grass R. N., Bruinink A., Stark W. J. Environ. Sci. Technol. 2007;41:4158–4163. doi: 10.1021/es062629t. [DOI] [PubMed] [Google Scholar]
- Bhattacharya K., Davoren M., Boertz J., Schins R. P., Hoffmann E., Dopp E. Part. Fibre Toxicol. 2009;6:17. doi: 10.1186/1743-8977-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E. J., Yi J., Chung K. H., Ryu D. Y., Choi J., Park K. Toxicol. Lett. 2008;180:222–229. doi: 10.1016/j.toxlet.2008.06.869. [DOI] [PubMed] [Google Scholar]
- Choi S. J., Oh J. M., Choy J. H. J. Inorg. Biochem. 2009;103:463–471. doi: 10.1016/j.jinorgbio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Sayes C. M., Reed K. L., Warheit D. B. Toxicol. Sci. 2007;97:163–180. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- Pampaloni F., Reynaud E. G., Stelzer E. H. Nat. Rev. Mol. Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- Seagrave J., McDonald J. D., Mauderly J. L. Exp. Toxicol. Pathol. 2005;57(Suppl 1):233–238. doi: 10.1016/j.etp.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Koval M., Ward C., Findley M. K., Roser-Page S., Helms M. N., Roman J. Am. J. Respir. Cell Mol. Biol. 2010;42:172–180. doi: 10.1165/rcmb.2008-0270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J. R., Hogan B. L. Annu. Rev. Cell Dev. Biol. 2011;27:20. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- Koval M. Annu. Rev. Physiol. 2013;75:17. doi: 10.1146/annurev-physiol-030212-183809. [DOI] [PubMed] [Google Scholar]
- Bhatia S. N., Ingber D. E. Nat. Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- Huh D., Matthews B. D., Mammoto A., Montoya-Zavala M., Hsin H. Y., Ingber D. E. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D., Leslie D. C., Matthews B. D., Fraser J. P., Jurek S., Hamilton G. A., Thorneloe K. S., McAlexander M. A., Ingber D. E. Sci. Transl. Med. 2012;4:159ra147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Li K., Zhang X., Liu C., Guo B., Wen W., Gao X. Lab Chip. 2018;18:486–495. doi: 10.1039/c7lc01224a. [DOI] [PubMed] [Google Scholar]
- van Roy F., Berx G. Cell. Mol. Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotta M., Trani M., Dejana E. Dev. Cell. 2013;26:441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J., Matthay M. A. Annu. Rev. Physiol. 2013;75:23. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- Shi H., Magaye R., Castranova V., Zhao J. Part. Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrand A. M., Rahman M. F., Hussain S. M., Schlager J. J., Smith D. A., Syed A. F. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2010;2:544–568. doi: 10.1002/wnan.103. [DOI] [PubMed] [Google Scholar]
- West J. B., Mathieu-Costello O. Annu. Rev. Physiol. 1999;61:543–572. doi: 10.1146/annurev.physiol.61.1.543. [DOI] [PubMed] [Google Scholar]
- Nesmith A. P., Agarwal A., McCain M. L., Parker K. K. Lab Chip. 2014;14:3925–3936. doi: 10.1039/c4lc00688g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benam K. H., Villenave R., Lucchesi C., Varone A., Hubeau C., Lee H. H., Alves S. E., Salmon M., Ferrante T. C., Weaver J. C., Bahinski A., Hamilton G. A., Ingber D. E. Nat. Methods. 2016;13:151–157. doi: 10.1038/nmeth.3697. [DOI] [PubMed] [Google Scholar]
- Tarbell J. M. Cardiovasc. Res. 2010;87:320–330. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredberg J. J., Kamm R. D. Annu. Rev. Physiol. 2006;68:507–541. doi: 10.1146/annurev.physiol.68.072304.114110. [DOI] [PubMed] [Google Scholar]
- Seebach J., Dieterich P., Luo F., Schillers H., Vestweber D., Oberleithner H., Galla H.-J., Schnittler H.-J. Lab. Invest. 2000;80:1819. doi: 10.1038/labinvest.3780193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.