Abstract
Mutations in the gene BEST1 usually cause bestrophinopathies, such as the rare progressive diseases Best vitelliform macular dystrophy (BVMD) and autosomal recessive bestrophinopathy (ARB). This study aimed to investigate the clinical characteristics of patients with BVMD or ARB carrying BEST1 mutations. A total of 12 probands including 9 patients with a clinical diagnosis of BVMD and 3 patients with a clinical diagnosis of ARB were recruited for genetics analysis. All patients underwent detailed ophthalmic examination. All coding exons of the BEST1 gene were screened by PCR-based DNA sequencing. Programs of PolyPhen-2, SIFT, and MutationTaster were used to analyze the potential pathogenicity of the mutations in BEST1. In the 9 unrelated patients with BVMD, one heterozygous BEST1 mutation was revealed in 8 patients and two compound heterozygous mutations in 1 patient. In the 3 unrelated patients with ARB, two compound heterozygous mutations were revealed in 2 patients and three compound heterozygous mutations in 1 patient. Molecular analyses identified a total of 15 mutations, including 3 novel mutations (c.424A>G p.S142G, c.436G>A p.A146T, and c.155T>C p.L52P). Antivascular endothelial growth factor (VEGF) drugs were given to two affected eyes, especially those also exhibiting choroidal neovascularization (CNV), and no serious adverse events occurred. Our study indicates that there is wide genotypic and phenotypic variability in patients with BVMD or ARB in China. The screening of BEST1 gene is significant for the precise diagnosis of BVMD and ARB.
1. Introduction
Best vitelliform macular dystrophy (BVMD; OMIM153700) is a maculopathy characterized by the deposition of yellowish, lipofuscin-like or vitelliform lesions that often show considerable morphologic variability in different stages of the disease [1]. The lesion usually evolves through previtelliform, vitelliform, pseudohypopyon, vitelliruptive, and atrophic/cicatricial stages with time [2]. Electrooculography (EOG) with an Arden ratio of light-peak to dark-trough less than 1.5 is usually a specific clinical diagnostic indicator for BVMD [3]. BVMD is inherited in an autosomal dominant fashion but with variable expressivity [4]. The incidence was noted to be 1.5-20/100000 [5, 6]. The disease was first described by the physician Friedrich Best in 1905 [7].
As for autosomal recessive bestrophinopathy (ARB; OMIM611809), Schatz described a patient with yellowish vitelliform lesion identified with biallelic variants of the BEST1 gene in 2006 [8]. Burgess et al. first reported ARB as a distinct retinal disease associated with the BEST1 gene in 2008 [9]. As opposed to multifocal Best disease and classic Best disease which have autosomal dominant inheritance, the parents in many ARB cases do not have fundus findings and their EOG is normal [10]. OCT imaging highlights both the serous detachment and the hyperreflectivity of the vitelliform lesions. Some patients also present with a cystoid macular edema and, like the vitelliform lesions, the central scar is hyperreflective [1, 4]. It was reported that some cases were hyperopic or had shallow anterior chamber angles that predisposed them to angle-closure glaucoma [11]. EOG shows an absent or markedly reduced light-peak. Reduced amplitudes of electroretinograms (ERGs) are also associated with ARB [12]. However, ERG may remain relatively normal for a long time [11].
The BEST1 gene (OMIM 607854, formerly named VMD2: OMIM 153700), which contains 11 exons and is located on chromosome 11q12-13, has been identified as the disease-causing gene for a variety of diseases called bestrophinopathies, such as BVMD, ARB, retinitis pigmentosa, and autosomal dominant vitreoretinochoroidopathy (ADVIRC) [13, 14]. It is expressed predominantly in retinal pigment epithelium (RPE), and the mRNA encodes the 585-amino acid protein bestrophin-1 [15]. Bestrophin-1 is presumed to function as a Cl- channel activated by Ca2+, an inhibitor of the intracellular voltage-dependent Ca2+ channel, and a channel that also transports HCO3- [16, 17]. More than 300 mutations have been identified to be associated with bestrophinopathies thus far.
Currently, there are no concrete treatments that halt the progressive maculopathy in bestrophinopathy [10]. Appropriate therapies like drug treatment and gene therapy to achieve a better prognosis for bestrophinopathy have been explored for decades [10]. In this study, we investigated the genotypes, clinical characteristics, and therapeutic options of patients with bestrophinopathy in our department to both better understand the disease and improve clinical management.
2. Methods
2.1. Patients and Clinical Data
The research received institutional approval by the Ethical Committee and Institutional Review Board of Peking University People's Hospital. The study conformed to the Declaration of Helsinki and was carried out in accordance with institutional guidelines. Informed consent was obtained from all participants enrolled in the study. In total, 12 unrelated Chinese patients were recruited in this study from 2012 to 2017. In this study, 4 sporadic patients and 8 patients with family members were analyzed. Detailed ophthalmic examination, including measurement of best corrected visual acuity (BCVA), slit-lamp biomicroscopy, dilated fundus examination, fundus photography, fundus autofluorescence (FAF) imaging, fluorescein angiography (FA), indocyanine green angiography (ICGA), and optical coherence tomography (OCT, Heidelberg Engineering, Heidelberg, Germany), was conducted. Ten patients underwent EOG examinations and 6 patients underwent full-field ERG. Clinical treatments were recorded during the follow-up visit.
The diagnoses of BVMD and ARB were based on a combination of genetic tests of BEST1 and ophthalmic examination. BVMD was diagnosed as follows: juvenile-to-adult onset metamorphopsia or vision loss, macular lesion showing vitelliform, vitelliruptive, pseudohypopyon, atrophic or cicatricial changes, and an abnormal EOG Arden ratio below 1.5. ARB was diagnosed based on yellow deep retinal/retinal pigment epithelial deposits present and reduced light-rise on EOG, associated with reduced amplitudes of ERG and an autosomal recessive inheritance pattern.
2.2. Molecular Methods
Peripheral blood samples were collected from all probands and some of their family members for genetic analysis. Genomic DNA was extracted from the peripheral blood samples using an Agilent SureSelect Target Enrichment System Kit (Agilent, USA). Polymerase chain reaction (PCR) was performed using Goldstar Taq MasterMix (Cwbio, PRC) to amplify the exons of the BEST1 gene (NM_004183.3). Samples were sequenced directly by loading the sequencing reaction product into NEXTSEQ500 (Illumina, USA).
The potential pathogenicity of novel missense mutations was investigated using the programs PolyPhen-2 (Polymorphism Phenotype; http://genetics.bwh.harvard.edu/pph/), SIFT (Sort Intolerant from Tolerant, http://sift.jcvi.org/), and MutationTaster (http://www.mutationtaster.org) as the standards and guidelines for the interpretation of sequence variants suggested by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology in 2015. Finally, we verified the novel mutations using 100 heathy controls without any eye disease.
2.3. Statistical Methods
Results were expressed as frequencies and percentages for categorical variables and as mean ± SD for continuous variables.
3. Results
3.1. Mutation Analysis of the BEST1 Gene
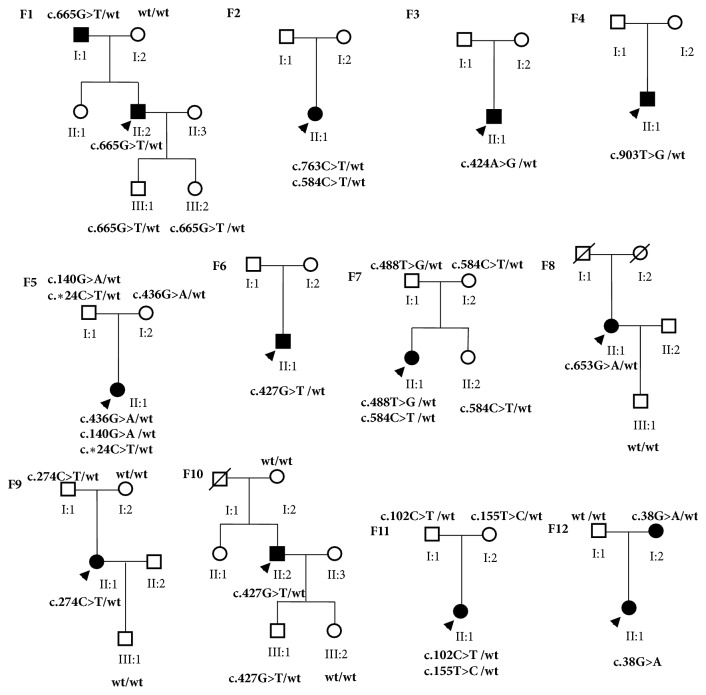
Direct sequencing analysis revealed a total of 15 BEST1 mutations, including 13 (86.7%) missense mutations, 1 (6.7%) splicing mutation c.∗24C>T, and 1 (6.7%) synonymous mutation c.102C>T/p.G34G (Table 1). Of these mutations, 8 different mutations were solely detected in patients with BVMD, 6 mutations were solely identified in patients with ARB, and one missense mutation (c.584 C>T p. A195V) was found in both patients with BVMD and those with ARB. In the 9 unrelated patients with BVMD, one heterozygous BEST1 mutation was revealed in 8 patients and two compound heterozygous mutations in 1 patient. In the 3 unrelated patients with ARB, two compound heterozygous mutations were revealed in 2 patients and three compound heterozygous mutations in 1 patient. Pedigrees of families with BEST1 mutations in this study were shown in Figure 1. Three missense mutations (c.424A>G p.S142G, c.436G>A p.A146T, and c.155T>C p.L52P) had not been previously reported in the literature or registered in the Ensembl database (http://www.ensembl.org/index.html) or the Human Gene Mutation Database (HGMD, http://www.hgmd.cf.ac.uk/ac/index.php). The missense mutation c.424A>G (p.S142G) was identified in BVMD patient without other mutation, and c.436G>A (p.A146T) and c.155T>C (p.L52P) were found in ARB patients combined with other BEST1 mutations. None of the mutations were found in our 100 controls. The 3 novel mutations were predicted to be damaging by PolyPhen-2 (scores of 0.999, 1.000, and 0.794) and predicted to affect protein function by SIFT (scores of 0.00, 0.00, and 0.01). Program MutationTaster predicted that both the amino acid sequence and the splice site would be changed by the 3 novel mutations. And besides, protein features might be changed. Based on the ACMG standards and guidelines for the interpretation of sequence variants in 2015, the three novel missense mutations c.424A>G (p.S142G), c.436G>A (p.A146T), and c.155T>C (p.L52P) were all considered pathogenic.
Table 1.
Mutations of BEST1 genetic testing results1.
| Patient Number | Position | Nucleotide Change | Amino Acid Change | Mutation Type | Novel | Phenotype | Disease Reported | Population Frequency |
|---|---|---|---|---|---|---|---|---|
| 1 | Exon 6 | c.665G>T | G222V | Missense | No | BVMD | LCA | absent |
|
| ||||||||
| 2 | Exon 7 | c.763C>T | R255W | Missense | No | BVMD | BVMD | 0.0000505 |
| Exon 5 | c.584C>T | A195V | Missense | No | BVMD ARB |
0.0002381 | ||
|
| ||||||||
| 3 | Exon 4 | c.424A>G | S142G | Missense | Yes | BVMD | - | absent |
|
| ||||||||
| 4 | Exon 8 | c.903T>G | D301E | Missense | No | BVMD | BVMD | absent |
|
| ||||||||
| 5 | Exon 4 | c.436G>A | A146T | Missense | Yes | ARB | - | absent |
| Exon 2 | c.140G>A | R47H | Missense | No | AVMD | 0.00001626 | ||
| 3 prime UTR | c.∗24C>T | - | Splicing | No | Not provided | 0.0002208 | ||
|
| ||||||||
| 6 | Exon 4 | c.427G>T | V143F | Missense | No | BVMD | AVMD | absent |
|
| ||||||||
| 7 | Exon 5 | c.488T>G | M163R | Missense | No | ARB | ARB | absent |
| Exon 5 | c.584C>T | A195V | Missense | No | BVMD | 0.0002381 | ||
|
| ||||||||
| 8 | Exon 6 | c.653G>A | R218H | Missense | No | BVMD | BVMD | 0.00001218 |
|
| ||||||||
| 9 | Exon 4 | c.274C>T | R92C | Missense | No | BVMD | BVMD | absent |
|
| ||||||||
| 10 | Exon 4 | c.427G>T | V143F | Missense | No | BVMD | AVMD | absent |
|
| ||||||||
| 11 | Exon 2 | c.102C>T | G34G | Synonymous | No | ARB | ARB | 0.00001627 |
| Exon 3 | c.155T>C | L52P | Missense | Yes | - | absent | ||
|
| ||||||||
| 12 | Exon 2 | c.38G>A | R13H | Missense | No | BVMD | BVMD | 0.00001445 |
1NM_004183.3.UTR: untranslated region. Blank section means no data available. LCA: leber congenital amaurosis. BVMD: best vitelliform macular dystrophy. AVMD: adult vitelliform macular dystrophy. ARB: autosomal recessive bestrophinopathy.Population frequency was acquired in gnomAD database.
Figure 1.
Pedigrees of families with BEST1 mutations in this study. Blackened symbols: affected individuals; arrow below the symbol: the proband.
3.2. Clinical Features of the Patients Carrying Different BEST1 Mutations
The demographic and clinical information is listed in Tables 2 and 3. Data were collected from 2012 to 2017. Twelve probands from 12 unrelated Chinese families were recruited, and their clinical phenotypic images were shown in Figure 2. Nine patients, including 6 males and 3 females, were diagnosed with BVMD, and 3 female patients were diagnosed with ARB. The median age at onset of BVMD was 30 years (range, 4-49 years) and the median age at onset of ARB was 10 years (range, 5-11 years). The mean visual acuity (VA) was 0.48±0.35 in patients with BVMD (patients 2 and 4 were excluded, as their VA data were lost) and 0.35±0.21 in patients with ARB.
Table 2.
Clinical data of patients with BVMD.
| Patient Number | Gender | Age (years) | Phenotype | Stages | Therapies | VA (first visit) | VA (after therapies) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| OD | OS | OD | OS | OD | OS | |||||
| 1 | Male | 30 | BVMD | vitelliform | vitelliform | - | 0.8 | 0.12 | - | - |
|
| ||||||||||
| 2 | Female | 34 | BVMD | vitelliform | vitelliruptive | - | defect | defect | - | - |
|
| ||||||||||
| 3 | Male | 8 | BVMD with OS CNV | vitelliruptive | atrophic/cicatricial | OS IVB∗1 | 0.6 | 0.07 | 0.6 | 0.1 |
|
| ||||||||||
| 4 | Male | 20 | BVMD | vitelliruptive | pseudohypopyon | - | defect | defect | - | - |
|
| ||||||||||
| 6 | Male | 25 | BVMD with OD CNV | atrophic/cicatricial | vitelliruptive | OD IVB∗3/IVC ∗1/IVR∗1 OS IVC∗1 |
0.25 | 0.8 | 1.0 | 1.0 |
|
| ||||||||||
| 8 | Female | 49 | BVMD | vitelliruptive | vitelliruptive | OU PDT∗1 | 0.5 | 0.5 | 0.4 | 0.4 |
|
| ||||||||||
| 9 | Female | 30 | BVMD | vitelliruptive | vitelliruptive | - | 1.0 | 1.2 | - | - |
|
| ||||||||||
| 10 | Male | 38 | BVMD | pseudohypopyon | vitelliruptive | - | 0.25 | 0.25 | - | - |
|
| ||||||||||
| 12 | Male | 4 | BVMD with OD fundus hemorrhage | atrophic/ cicatricial |
vitelliform | - | 0.075 | 0.4 | - | - |
BVMD: best vitelliform macular dystrophy. VA: visual acuity. OD: oculus dexter, right eye. OS: oculus sinister, left eye. OU: oculus uterque, binoculus. CNV: choroidal neovascularization. IVB: intravitreal injection of bevacizumab. IVC: intravitreal injection of conbercept. IVR: intravitreal injection of ranibizumab. PDT: photodynamic therapy. Asterisks mean times of therapy. Blank sections mean no data available.
Table 3.
Clinical data of patients with ARB.
| Patient Number | Gender | Age (years) | Phenotype | Therapies | VA (first visit) | VA (after therapies) | ||
|---|---|---|---|---|---|---|---|---|
| OD | OS | OD | OS | |||||
| 5 | Female | 10 | ARB with OU CNV | OD IVB∗2 OS IVB∗1 |
0.05 | 0.6 | 0.2 | 0.63 |
| 7 | Female | 11 | ARB with OU retinoschisis | OU IVR∗1 | 0.5 | 0.5 | 0.5 | 0.63 |
| 11 | Female | 5 | ARB with OD CNV | - | 0.2 | 0.25 | - | - |
ARB: autosomal recessive bestrophinopathy. VA: visual acuity. OD: oculus dexter, right eye. OS: oculus sinister, left eye. OU: oculus uterque, binoculus. CNV: choroidal neovascularization. IVB: intravitreal injection of bevacizumab. IVR: intravitreal injection of ranibizumab. Asterisks mean times of therapy. Blank sections mean no data available.
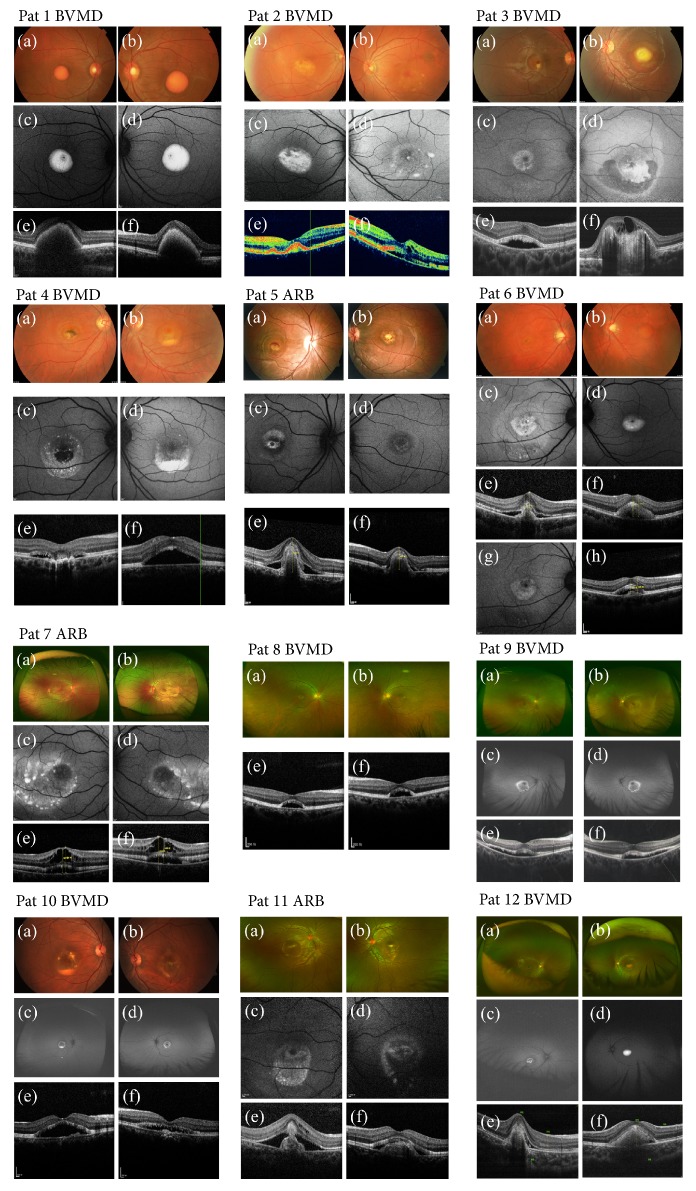
Figure 2.
Clinical phenotypic images of the probands in this study. (a) OD fundus appearance. (b) OS fundus appearance. (c) OD FAF images. (d) OS FAF images. (e) OD macular OCT images. (f) OS macular OCT images. (g) OD FAF image of patient 6 after therapy of intravitreal injection of anti-VEGF. (h) OD OCT image of patient 6 after therapy of intravitreal injection of anti-VEGF.
EOG was performed in 14 eyes of 7 patients with BVMD and 6 eyes of 3 patients with ARB (Table 4). The average EOG Arden ratio of light-peak to dark-trough was 1.130±0.247 (median 1.112, range 1.154-1.885) in BVMD eyes and 1.002±0.095 (median 0.981, range 0.911-1.112) in ARB eyes. The Arden radio was reduced (<1.5) in 13 BVMD eyes (13/14, 92.9%) and 6 ARB eyes (6/6, 100%). Six eyes of 3 BVMD patients (6/6, 100%) showed that absence of reduced amplitudes in ERG including the proband 1 identified the mutation c.665G>T (p.G222V) previously reported in leber congenital amaurosis. Three eyes of 3 ARB patients (3/6, 50%) showed reduced amplitudes of ERG.
Table 4.
The data of the EOG Arden ratio of BVMD or ARB patients.
| Patient Number | Phenotype | EOG (Arden ratio) | |
|---|---|---|---|
| OD | OS | ||
| 1 | BVMD | 1.154 | 1.204 |
|
| |||
| 2 | BVMD | 1.100 | 1.037 |
|
| |||
| 3 | BVMD | 1.885 | 0.892 |
|
| |||
| 5 | ARB | 0.922 | 1.038 |
|
| |||
| 6 | BVMD | 1.127 | 1.153 |
|
| |||
| 7 | ARB | 0.924 | 0.911 |
|
| |||
| 8 | BVMD | 1.105 | 1.119 |
|
| |||
| 9 | BVMD | 0.961 | 0.902 |
|
| |||
| 10 | BVMD | 0.910 | 1.284 |
|
| |||
| 11 | ARB | 1.107 | 1.112 |
EOG: electrooculogram.
Three eyes of 3 BVMD patients (3/18, 16.7%), whose ages were 8, 25, and 4, exhibited choroidal neovascularization (CNV) or fundus hemorrhage. Three eyes of 2 ARB patients (3/6, 50%) also exhibited CNV and 2 eyes of one patient (2/6, 33.3%) exhibited retinoschisis. Antivascular endothelial growth factor (VEGF) drugs, including bevacizumab, conbercept, and ranibizumab, were given to 3 eyes of 2 BVMD patients and 4 eyes of 2 ARB patients, especially those that also exhibited CNV or retinoschisis. Visual acuity was improved in 3 BVMD eyes (3/3) and 3 ARB eyes (3/4) after anti-VEGF therapy and no serious adverse events occurred. Two BVMD eyes accepted photodynamic therapy (PDT) with no adverse events, while the visual acuity declined from 0.5 to 0.4 after therapy.
3.3. Family Members
In total, 23 family members including 13 carriers underwent examinations, among which 2 family members with identified BEST1 mutation were affected. For families 2, 3, 4, and 6, genetic data were available from one proband of each family. In family 1 (BVMD), a heterozygous c.665G>T (p.G222V) missense mutation was detected in 4 members. The proband's father (I-1) was found to have vitelliform changes at the macula, whereas his 4-year-old son (III-1) and 6-year-old daughter (III-2) had normal fundus appearances. In family 9 (BVMD), the fundus of the proband's father (I-1) with a heterozygous c.274C>T (p.R92C) mutation was found to be normal. In family 12 (BVMD), the proband's mother (I-2) with a heterozygous c.38G>A (p.R13H) mutation was found to have a reduced Arden ratio, while her fundus examination was normal. In family 10 (BVMD), the fundus of the proband's 8-year-son (III-1) with a heterozygous c.427G>T (p.V143F) mutation was found to be normal. The fundi of the other members without the mutation were all normal. In families 5, 7, and 11 (ARB), the parents with mutations were all found to have normal Arden ratio and normal fundus appearances. The latter three families showed recessive inheritance.
4. Discussion
The study confirms that BEST1 gene mutation is the primary factor in the development of BVMD and ARB. In this study, we identified a novel missense mutation c.424A>G (p.S142G) in association with a BVMD proband and two novel missense mutations, c.436G>A (p.A146T) and c.155T>C (p.L52P), in association with 2 ARB probands. It is reported that more than 300 distinct BEST1 mutations have been found in sporadic patients and families affected by various bestrophinopathies [18, 19]. In previous study in Chinese patients, many novel mutations have also been identified including c.763C>T (R255W) and c.488T>G (M163R) in this study [20, 21]. Here our findings expand the mutational spectrum of BEST1 and suggest that the mutations of BEST1 in Chinese people may be different in comparison to other ethnic groups.
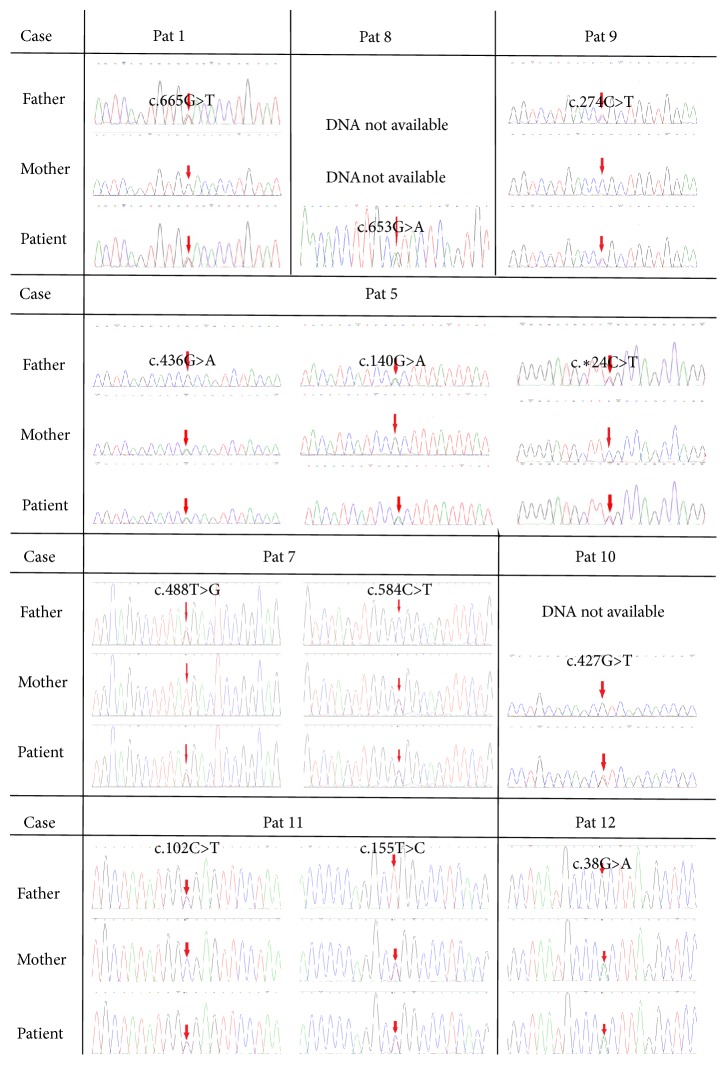
In total, 15 mutations in BEST1 were found in this study. The known mutations reported were associated with leber congenital amaurosis (LCA), BVMD, adult vitelliform macular dystrophy (AVMD), or ARB [15, 22–27]. Sanger confirmation of the identified BEST1 variants in probands with family members is shown in Figure 3. Of the 15 mutations, 8 were associated with BVMD, 6 were associated with ARB, and 1 mutation c.584C>T (p.A195R) was associated with BVMD and ARB. In our study, missense mutations were the leading cause of bestrophinopathy, accounting for 100% of all mutations identified in BVMD and 71.4% in ARB. It is noteworthy that the missense mutation c.424A>G (p.S142G) associated with a BVMD patient and the mutations c.436G>A (p.A146T) and c.155T>C (p.L52P) associated with 2 ARB patients were novel. None of the mutations were present in HGMD, the Ensembl database, and the genic testing of 100 healthy controls. The other mutations identified in the study have been previously reported in different countries such as America and China et al. [28, 29]. In previous reports, cases with compound heterozygous variants were reported, which was rare in BVMD [30, 31]. In our study, we found a patient with two compound heterozygous missense mutations and a typical phenotype for BVMD. The patient with identified BEST1 mutations c.763C>T (p.R255W) and c.584C>T (p.A195V) was a 34-year-old female. It was reported that c.763C>T (p.R255W) was identified in BVMD [20] and c.584C>T (p.A195V) was identified both in BVMD and ARB [23, 25]. There was absence of reduced amplitude of ERG in both eyes. The remaining 3 patients with compound BEST1 mutations expressed the phenotype for ARB. In one of the patients three mutations were identified, among which the splicing mutation c.∗24C>T was considered to be benign. There is wide genetic and phenotypic variability in Chinese patients with BVMD and ARB.
Figure 3.
Sanger confirmation of the identified BEST1 variants. Sequence chromatograms of patients and their parents (where available) are shown.
Bestrophin-1 encoded by the BEST1 gene is an integral membrane protein localized predominantly to the basolateral membrane of RPE [32]. Numerous missense mutations in genes encoding integral membrane proteins are related to defective cellular trafficking [33, 34]. A study investigated whether 13 missense mutations, located in four mutational hot-spots of bestrophin-1, affect plasma membrane targeting in polarized MDCK II cells [29]. All mutants mentioned in that study showed a significant reduction in anion conductance, indicating that disease-associated missense mutations in bestrophin-1 affect cellular trafficking and anion conductance and thus may be a common cause of bestrophinopathy. Similarly, it is reported that splicing mutations may interfere with exon splicing of mRNA, leading to an altered genic product [35]. BEST1 knock-in mice model has been produced and exhibited a phenotype similar to BVMD [36]. Compared to BVMD, ARB has been theorized to be associated with a null phenotype for BEST1. However, the BEST1 knock-out mice model did not exhibit bestrophinopathy [37]. Thus whether ARB is a true “null” phenotype needs to be explored. Anyhow, further studies on the effects of the mutation on cellular function are necessary.
Our study included 9 probands from 9 families diagnosed with BVMD and 3 probands from 9 families diagnosed with ARB. The median age at onset was 30 years (range 4-49) in BVMD and 10 years (5-11) in ARB. The age of BVMD at onset varied widely which was similar to previous report, while ages at onset of our ARB probands were relatively young [11]. The mean VA was 0.48±0.35 in patients with BVMD and 0.35±0.21 in patients with ARB. The average EOG Arden ratio was 1.130±0.247 in BVMD and 1.002±0.095 in ARB, which were obviously reduced compared to normal values. Eyes with reduced Arden radio (<1.5) composed large proportions of 92.9% (13/14) in the BVMD sample and 100% (6/6) in the ARB sample. EOG of the right eye in a BVMD patient was normal, and the patient was an 8-year-old male with an identification of BEST1 mutation c.424A>G (p.S142G). The reason why this mutation did not cause reduced Arden ratio of EOG in one eye needs to be explored. EOG evaluates the function of the outer retina and RPE using the RPE's response to changing illumination [38]. The result infers that the function of RPE cells was impaired in the development of most BVMD and ARB.
There is no concrete treatment for a clinical patient suffering from bestrophinopathy at the moment. Studies in the laboratory suggested that treatment with valproic acid was able to increase the rate of photoreceptor outer segment degradation in an iPSC-RPE model through changing the level of exosome secretion, protein oxidation, and free-ubiquitin [39]. Our goal of therapy was controlling the CNV or retinal hemorrhage secondary to BVMD or ARB. In our study, a patient (patient 6) had CNV secondary to BVMD in the right eye. The VA was 0.25 on initial presentation. After acceptance of intravitreal injection of bevacizumab (IVB) 3 times and conbercept (IVC) and ranibizumab (IVR) once, his BCVA improved to 1.0 while CNV was reduced according to FAF and OCT examinations (Figure 2 (g), (h)). There were no complications after the therapy during the two-year follow-up. In total, anti-VEGF drugs, including bevacizumab, conbercept, and ranibizumab, were given to 3 eyes of 2 BVMD patients and 4 eyes of 2 ARB patients, especially those that also exhibited CNV or retinoschisis. Visual acuity was improved in 3 BVMD eyes (3/3) and 3 ARB eyes (3/4) after anti-VEGF therapy and no serious adverse events occurred. Similarly, previous study also described patient with CNV caused by BVMD who obtained good visual acuity after anti-VEGF treatment [40]. The outcome suggests that anti-VEGF drugs may be an appropriate way to control CNV secondary to bestrophinopathy.
Several gene therapy trials in the retina are currently underway and gene therapy has been shown to be effective for some diseases such as RPE65-Leber congenital amaurosis [41]. Gene therapy in animal models will provide a basis for gene-directed therapy. Our study provided data on genetic mutations and clinical features to assist the exploration of gene therapy in patients with bestrophinopathy. However, there are still many challenges in gene therapy, including an appropriate time of gene intervention and the management of complications and future adverse events during the course of treatment.
In conclusion, we found a novel causative missense mutation, c.424A>G (p.S142G), associated with BVMD and two novel causative missense mutations, c.436G>A (p.A146T) and c.155T>C (p.L52P), associated with ARB. Management of CNV secondary to BVMD or ARB should be taken into consideration to prevent progression and improve visual acuity. Our results expand the data on the mutational findings and clinical characteristics of the disease. We believe that our work will facilitate the diagnosis, clinical therapy, and genetic counseling of BVMD and ARB. Furthermore, we will explore the concrete pathogenesis of the mutations in this investigation that caused BVMD and ARB in cell and animal model in a future study.
Acknowledgments
This work was supported by Beijing Municipal Science and Technology Commission in the form of Science and Technology Program of Beijing (No. Z171100002217081, Z161100000516037). National Natural Science Foundation of China provided financial support in the form of National Natural Science Foundation of China (Grant NO. 81470651, 81770943). This work was also supported by National Key Research and Development Program (2016YFC0904801, 2017YFC0111204).
Contributor Information
Lvzhen Huang, Email: huanglvzhen@126.com.
Mingwei Zhao, Email: zhaomingwei64@163.com.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
The sponsor had no role in the design or conduct of this research. The manuscript was presented as a conference paper in the 10th Chinese Congress of Research in Vision and Ophthalmology.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Boon C. J. F., Theelen T., Hoefsloot E. H., et al. Clinical and molecular genetic analysis of best vitelliform macular dystrophy. Retina. 2009;29(6):835–847. doi: 10.1097/IAE.0b013e31819d4fda. [DOI] [PubMed] [Google Scholar]
- 2.Mohler C. W., Fine S. L. Long-term Evaluation of Patients with Best's Vitelliform Dystrophy. Ophthalmology. 1981;88(7):688–692. doi: 10.1016/S0161-6420(81)34965-3. [DOI] [PubMed] [Google Scholar]
- 3.Tian L., Sun T., Xu K., Zhang X., Peng X., Li Y. Screening of BEST1 gene in a chinese cohort with best vitelliform macular dystrophy or autosomal recessive bestrophinopathy. Investigative Ophthalmology & Visual Science. 2017;58(9):3366–3375. doi: 10.1167/iovs.17-21999. [DOI] [PubMed] [Google Scholar]
- 4.Boon C. J. F., Klevering B. J., Leroy B. P., Hoyng C. B., Keunen J. E. E., den Hollander A. I. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Progress in Retinal and Eye Research. 2009;28(3):187–205. doi: 10.1016/j.preteyeres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Nordstr S., Nordström S. Hereditary macular degenerationea population survey in the country of Vsterbotten. Hereditary macular degenerationea population survey in the country of Vsterbotten. 1974:78–41. doi: 10.1111/j.1601-5223.1974.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 6.Bitner H., Schatz P., Mizrahi-Meissonnier L., Sharon D., Rosenberg T. Frequency, genotype, and clinical spectrum of best vitelliform macular dystrophy: Data from a national center in Denmark. American Journal of Ophthalmology. 2012;154(2):403–e4. doi: 10.1016/j.ajo.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Best F. II. Über eine hereditäre maculaaffektion. Ophthalmologica. 1905;13(3):199–212. doi: 10.1159/000290318. [DOI] [Google Scholar]
- 8.Schatz P., Klar J., Andréasson S., Ponjavic V., Dahl N. Variant phenotype of Best vitelliform macular dystrophy associated with compound heterozygous mutations in VMD2. Ophthalmic Genetics. 2006;27(2):51–56. doi: 10.1080/13816810600677990. [DOI] [PubMed] [Google Scholar]
- 9.Burgess R., Millar I. D., Leroy B. P., et al. Biallelic Mutation of BEST1 Causes a Distinct Retinopathy in Humans. American Journal of Human Genetics. 2008;82(1):19–31. doi: 10.1016/j.ajhg.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson A. A., Guziewicz K. E., Lee C. J., et al. Bestrophin 1 and retinal retinal desease. Progress in Retinal and Eye Research. 2017;58:45–69. doi: 10.1016/j.preteyeres.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boon C. J. F., Van Den Born L. I., Visser L., et al. Autosomal recessive bestrophinopathy: Differential diagnosis and treatment options. Ophthalmology. 2013;120(4):809–820. doi: 10.1016/j.ophtha.2012.09.057. [DOI] [PubMed] [Google Scholar]
- 12.Nakanishi A., Ueno S., Hayashi T., et al. Clinical and genetic findings of autosomal recessive bestrophinopathy in Japanese cohort. American Journal of Ophthalmology. 2016;168:86–94. doi: 10.1016/j.ajo.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Lee C. S., Jun I., Choi S.-I., et al. A novel BEST1 mutation in autosomal recessive bestrophinopathy. Investigative Ophthalmology & Visual Science. 2015;56(13):8141–8150. doi: 10.1167/iovs.15-18168. [DOI] [PubMed] [Google Scholar]
- 14.Glavač D., Jarc-Vidmar M., Vrabec K., Ravnik-Glavač M., Fakin A., Hawlina M. Clinical and genetic heterogeneity in Slovenian patients with BEST disease. Acta Ophthalmologica. 2016;94(8):e786–e794. doi: 10.1111/aos.13202. [DOI] [PubMed] [Google Scholar]
- 15.Caldwell G. M., Kakuk L. E., Griesinger I. B., et al. Bestrophin gene mutations in patients with best vitelliform macular dystrophy. Genomics. 1999;58(1):98–101. doi: 10.1006/geno.1999.5808. [DOI] [PubMed] [Google Scholar]
- 16.Sun H., Tsunenari T., Yau K.-W., Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proceedings of the National Acadamy of Sciences of the United States of America. 2002;99(6):4008–4013. doi: 10.1073/pnas.052692999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal R., Bakall B., Kinnick T., et al. Expression of bestrophin-1, the product of the VMD2 gene, modulates voltage-dependent Ca2+ channels in retinal pigment epithelial cells. The FASEB Journal. 2006;20(1):178–180. doi: 10.1096/fj.05-4495fje. [DOI] [PubMed] [Google Scholar]
- 18.Marchant D., Yu K., Bigot K., et al. New VMD2 gene mutations identified in patients affected by Best vitelliform macular dystrophy. Journal of Medical Genetics. 2007;44(3):p. e70. doi: 10.1136/jmg.2006.044511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sodi A., Passerini I., Simonelli F., Testa F., Menchini U., Torricelli F. A novel mutation in the VMD2 gene in an Italian family with Best maculopathy. Journal Français d'Ophtalmologie. 2007;30(6):616–620. doi: 10.1016/S0181-5512(07)89667-7. [DOI] [PubMed] [Google Scholar]
- 20.Wong R. L. M., Hou P., Choy K.-W., et al. Novel and homozygous best1 mutations in chinese patients with best vitelliform macular dystrophy. Retina. 2010;30(5):820–827. doi: 10.1097/IAE.0b013e3181c700c1. [DOI] [PubMed] [Google Scholar]
- 21.Tian R., Yang G., Wang J., Chen Y. Screening for BEST1 gene mutations in Chinese patients with bestrophinopathy. Molecular Vision. 2014;20:1594–1604. [PMC free article] [PubMed] [Google Scholar]
- 22.Lotery A. J., Namperumalsamy P., Jacobson S. G., et al. Mutation analysis of 3 genes in patients with Leber congenital amaurosis. JAMA Ophtalmology. 2000;118(4):538–543. doi: 10.1001/archopht.118.4.538. [DOI] [PubMed] [Google Scholar]
- 23.Lotery A. J., Munier F. L., Fishman G. A., et al. Allelic variation in the VMD2 gene in Best disease and age-related macular degeneration. Investigative Ophthalmology & Visual Science. 2000;41:1291–1296. [PubMed] [Google Scholar]
- 24.Krämer F., White K., Pauleikhoff D., et al. Mutations in the VMD2 gene are associated with juvenile-onset vitelliform macular dystrophy (Best disease) and adult vitelliform macular dystrophy but not age-related macular degeneration. European Journal of Human Genetics. 2000;8(4):286–292. doi: 10.1038/sj.ejhg.5200447. [DOI] [PubMed] [Google Scholar]
- 25.Kinnick T. R., Mullins R. F., Dev S., et al. Autosomal recessive vitelliform macular dystrophy in a large cohort of vitelliform macular dystrophy patients. Retina. 2011;31(3):581–595. doi: 10.1097/IAE.0b013e318203ee60. [DOI] [PubMed] [Google Scholar]
- 26.Bakall B., Marknell T., Ingvast S., et al. The mutation spectrum of the bestrophin protein - Functional implications. Human Genetics. 1999;104(5):383–389. doi: 10.1007/s004390050972. [DOI] [PubMed] [Google Scholar]
- 27.Davidson A. E., Sergouniotis P. I., Burgess-Mullan R., et al. A synonymous codon variant in two patients with autosomal recessive bestrophinopathy alters in vitro splicing of BEST1. Molecular Vision. 2010;16:2916–2922. [PMC free article] [PubMed] [Google Scholar]
- 28.Kousal B., Chakarova F., Black G. C., et al. Minimal ocular findings in a patient with Best disease caused by the c.653G>A mutation in BEST1. Ceská a Slovenská oftalmologie. 2011;97:170–174. [PubMed] [Google Scholar]
- 29.Milenkovic V. M., Röhrl E., Weber B. H. F., Strauss O. Disease-associated missense mutations in bestrophin-1 affect cellular trafficking and anion conductance. Journal of Cell Science. 2011;124(17):2988–2996. doi: 10.1242/jcs.085878. [DOI] [PubMed] [Google Scholar]
- 30.Katagiri S., Hayashi T., Ohkuma Y., et al. Mutation analysis of BEST1 in Japanese patients with Best's vitelliform macular dystrophy. British Journal of Ophthalmology. 2015;99(11):1577–1582. doi: 10.1136/bjophthalmol-2015-306830. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L., Grob S., Corey R., et al. A novel compound heterozygous mutation in the BEST1 gene causes autosomal recessive Best vitelliform macular dystrophy. Eye (Basingstoke) 2012;26(6):866–871. doi: 10.1038/eye.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marmorstein A. D., Marmorstein L. Y., Rayborn M., Wang X., Hollyfield J. G., Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proceedings of the National Acadamy of Sciences of the United States of America. 2000;97(23):12758–12763. doi: 10.1073/pnas.220402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aridor M., Hannan L. A. Tarffic jam: A compendium of human diseases that affect intracellular transport processes. Traffic. 2000;1(11):836–851. doi: 10.1034/j.1600-0854.2000.011104.x. [DOI] [PubMed] [Google Scholar]
- 34.Jaya Kausalya P., Amasheh S., Günzel D., et al. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. The Journal of Clinical Investigation. 2006;116(4):878–891. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasai H., Aoyama Y., Otsuka H., et al. Single-nucleotide substitution T to A in the polypyrimidine stretch at the splice acceptor site of intron 9 causes exon 10 skipping in the ACAT1 gene. Molecular Genetics and Genomic Medicine. 2017;5(2):177–184. doi: 10.1002/mgg3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Stanton J. B., Wu J., et al. Suppression of Ca2+ signaling in a mouse model of Best disease. Human Molecular Genetics. 2010;19(6):1108–1118. doi: 10.1093/hmg/ddp583.ddp583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marmorstein L. Y., Wu J., McLaughlin P., et al. The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (Best-1) The Journal of General Physiology. 2006;127(5):577–589. doi: 10.1085/jgp.200509473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Constable P. A., Bach M., Frishman L. J., Jeffrey B. G., Robson A. G. ISCEV Standard for clinical electro-oculography (2017 update) Documenta Ophthalmologica. 2017;134(1) doi: 10.1007/s10633-017-9573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh R., Kuai D., Guziewicz K. E., et al. Pharmacological modulation of photoreceptor outer segment degradation in a human iPS cell model of inherited macular degeneration. Molecular Therapy. 2015;23(11):1700–1711. doi: 10.1038/mt.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chhablani J., Jalali S. Intravitreal bevacizumab for choroidal neovascularization secondary to Best vitelliform macular dystrophy in a 6-year-old child. European Journal of Ophthalmology. 2012;22(4):677–679. doi: 10.5301/ejo.5000095. [DOI] [PubMed] [Google Scholar]
- 41.Cideciyan A. V., Aleman T. S., Boye S. L., et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proceedings of the National Acadamy of Sciences of the United States of America. 2008;105(39):15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.