Abstract
Objective
To compare the efficacy and safety of dapagliflozin and dapagliflozin plus saxagliptin vs glimepiride as add‐on to metformin in patients with type 2 diabetes.
Research design and methods
This 52‐week, multicentre, double‐blind, active‐controlled study (NCT02471404) randomized (1:1:1) patients (n = 939; HbA1c 7.5%‐10.5%) on metformin monotherapy (≥1500 mg/day) to add‐on dapagliflozin 10 mg, dapagliflozin 10 mg plus saxagliptin 5 mg, or glimepiride 1 to 6 mg (titrated). The primary efficacy end point was change in HbA1c from baseline to Week 52.
Results
Baseline mean age, diabetes duration and HbA1c were 58.4 years, 7.0 years and 8.3%, respectively. Adjusted mean HbA1c change from baseline was −1.20% with dapagliflozin plus saxagliptin and −0.82% with dapagliflozin, vs −0.99% with glimepiride (mean dose at Week 52, 4.6 mg). Changes in body weight (−3.2 kg and −3.5 kg vs +1.8 kg) and systolic blood pressure (SBP; −6.4 mm Hg and −5.6 mm Hg vs −1.6 mm Hg) were significantly greater with dapagliflozin plus saxagliptin and dapagliflozin than with glimepiride. FPG decreased significantly with dapagliflozin plus saxagliptin compared with glimepiride (−2.1 mmol/L vs −1.5 mmol/L) and was similar with dapagliflozin (−1.6 mmol/L) compared with glimepiride. Confirmed incidence of hypoglycaemia was lower with dapagliflozin regimens than with glimepiride (0 and 1 vs 13 patients) and fewer patients required rescue. Genital infections were more frequent with dapagliflozin; other AE profiles were similar.
Conclusions
Dapagliflozin, saxagliptin and metformin improved glycaemic control compared with glimepiride plus metformin; add‐on of dapagliflozin alone showed efficacy similar to that of glimepiride. Both dapagliflozin regimens decreased body weight and SBP, with a lower incidence of hypoglycaemia compared with glimepiride.
Keywords: dapagliflozin, DPP‐IV inhibitor, phase III study, SGLT2 inhibitor, sulphonylureas, type 2 diabetes
1. INTRODUCTION
Attainment of optimal glycaemic control with a low risk of hypoglycaemia and with body weight reduction is a major goal of type 2 diabetes management.1, 2 Metformin is the first‐line pharmacotherapy recommended for glycaemic control in patients with type 2 diabetes and has proven efficacy in achieving clinically relevant reduction in glycated haemoglobin (HbA1c) levels.1 Most patients eventually require treatment with 2 or more antidiabetes agents to maintain adequate control of blood glucose levels,3 and international treatment guidelines recommend stepwise intensification of therapy through add‐on to metformin.1, 4
The latest generation of oral antidiabetes drugs, including sodium‐glucose cotransporter‐2 (SGLT‐2) inhibitors and dipeptidyl peptidase‐4 (DPP‐4) inhibitors, have demonstrated efficacy and safety in patients with inadequate glycaemic control with metformin monotherapy.5, 6, 7, 8, 9, 10 Moreover, in a 24‐week study, triple therapy with concomitant addition of dapagliflozin, an SGLT‐2 inhibitor, plus the DPP‐4 inhibitor saxagliptin to metformin led to greater improvements in glycaemic control than add‐on of either agent alone.11 These improvements were achieved without increased risk of hypoglycaemia compared with dual therapy, and both dapagliflozin‐containing regimens were associated with the benefit of weight loss.11 Dapagliflozin‐containing treatment regimens, therefore, may be a promising second‐line treatment strategy for patients with type 2 diabetes and inadequate glycaemic control.
Sulphonylureas are widely used as second‐line therapy for type 2 diabetes. Although their efficacy as antidiabetes agents is established, drawbacks include hypoglycaemia, weight gain and limited long‐term efficacy. Furthermore, data from some studies have suggested a possible association between sulphonylurea use and adverse cardiovascular outcomes.12, 13, 14 Use of dapagliflozin‐containing treatment regimens as second‐line therapy may provide an alternative option to sulphonylureas, with a more favourable side‐effect profile and additional beneficial effects on body weight and blood pressure control. In a head‐to‐head study, dapagliflozin and a sulphonylurea (glipizide) demonstrated similar efficacy, with the benefits of sustained reductions in body weight and systolic blood pressure (SBP), greater glycaemic durability and a lower rate of hypoglycaemia with dapagliflozin compared to glipizide after 1 year of treatment.15 These effects continued to be observed in extensions of up to 4 years.15, 16, 17
The primary aim of this study was to test the hypothesis that dapagliflozin, either alone or in combination with saxagliptin, is non‐inferior to titrated glimepiride, a sulphonylurea, in terms of glucose‐lowering effect in patients with type 2 diabetes who have inadequate glycaemic control with a maximum tolerated dose of metformin background therapy. Additional testing was undertaken to assess whether addition of dapagliflozin plus saxagliptin is superior to glimepiride plus metformin, given that triple therapy regimens have previously been shown to be more efficacious than dual therapy. Other indicators of glycaemic control, metabolic parameters, body weight and blood pressure were assessed as secondary end points.
2. RESEARCH DESIGN AND METHODS
2.1. Study design
This was a 52‐week, multicentre, randomized, parallel‐group, double‐blind, double‐dummy, active‐controlled phase 4 study, the DapaZu study (NCT02471404), that was conducted at 194 centres in Germany, the Czech Republic, Hungary, Poland and Slovakia. Local regulatory authorities and the responsible ethics committees/institutional review boards of the participating centres approved the study protocol and all participants provided written informed consent. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and Good Clinical Practice guidelines of the International Conference on Harmonisation.
2.2. Patients
Men and women, aged 18 to <75 years, were eligible for inclusion in the study if they fulfilled the following criteria: diagnosis of type 2 diabetes; stable metformin dose (≥1500 mg/day) for ≥8 weeks prior to enrollment; BMI ≤45 kg/m2; fasting plasma glucose (FPG) ≤ 270 mg/dL (≤15 mmol/L); C‐peptide ≥1.0 ng/mL (≥0.33 nmol/L); HbA1c, 7.5% to 10.5%. Major exclusion criteria included: a cardiovascular event during the 3 months prior to screening; creatinine clearance (CrCl) rate of <60 mL/minute; severe uncontrolled hypertension, defined as SBP ≥180 mm Hg and/or diastolic blood pressure ≥110 mm Hg at any visit up to and including randomization; presence or history of severe congestive heart failure (New York Heart Association Class III and IV), decompensated or acute congestive heart failure, and/or left ventricular ejection fraction ≤40%.
2.3. Treatments
The study design is shown in Figure 1. Patients receiving metformin monotherapy were randomized (1:1:1) to 1 of 3 treatment arms by a computer‐generated random sequence. These comprised dapagliflozin 10 mg; dapagliflozin 10 mg plus saxagliptin 5 mg; and glimepiride 0 to 6 mg (titrated). Matching placebos were used in each group and all study personnell were blinded to the randomization scheme during the study. Dapagliflozin and saxagliptin were taken orally, once daily, at fixed doses throughout the treatment period. Glimepiride treatment was initiated at 1 mg/day and was titrated upwards or downwards in 1 mg increments at subsequent visits, in accordance with prescribing information. The glimepiride/placebo dose was titrated to achieve an individualized FPG target, agreed by the patient and investigator at the start of treatment; a general target of approximately 110 mg/dL (6.1 mmol/L) was proposed in the study protocol. In order to reflect real‐life clinical practice as closely as possible, glimepiride titration was allowed for the duration of the study.
Figure 1.
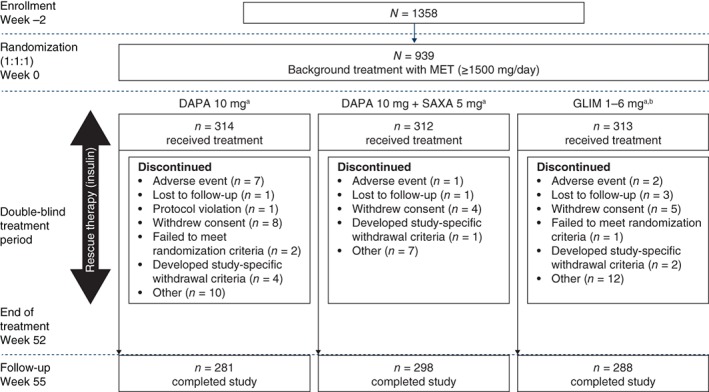
Study design and patient disposition. aDrug regimens were administered once daily and matching placebos were included. bGLIM treatment began at 1 mg/day and was titrated (up or down) in 1 mg increments at subsequent visits, as needed. Abbreviations: DAPA, dapagliflozin; GLIM, glimepiride; MET, metformin; SAXA, saxagliptin
In this study, hypoglycaemic events were categorized according to five definitions: (1) hypoglycaemic event, typical symptoms of hypoglycaemia, accompanied by blood glucose ≤3.9 mmol/L (≤70 mg/dL); (2) major hypoglycaemic episode, symptomatic event with blood glucose ≤3.0 mmol/L (≤54 mg/dL) with a need for external (third party) assistance; (3) confirmed hypoglycaemic event, typical symptoms of hypoglycaemia with a blood glucose reading of ≤2.8 mmol/L (≤50 mg/dL); (4) asymptomatic hypoglycaemic event, measured plasma glucose ≤3.9 mmol/L (≤70 mg/dL) without typical hypoglycaemia symptoms; (5) other hypoglycaemic episode, symptoms of hypoglycaemia without a blood glucose reading or with blood glucose >3.0 mmol/L (>70 mg/dL). Patients were eligible for open‐label rescue with basal insulin for the entire duration of the treatment phase (criteria for initiation of rescue therapy: weeks 0 to 12, FPG > 240 mg/dL [13.3 mmol/L]; weeks 12 to 24, FPG > 200 mg/dL [11.1 mmol/L]; weeks 24 to 52, HbA1c > 8.0%). In patients who had not reached their maximum tolerated dose of glimepiride/placebo, up‐titration of glimepiride/placebo was attempted before rescue with insulin was initiated.
2.4. End points and assessments
The primary efficacy end point was absolute change in HbA1c from baseline to end of the 52‐week treatment period. Key secondary end points included the proportion of patients reporting confirmed hypoglycaemic episodes during the 52‐week treatment period, changes from baseline in total body weight and FPG at week 52, and the time to rescue during the treatment period. Additional secondary end points included the proportion of patients who achieved HbA1c < 7.0% at week 52, the proportion of patients who achieved ≥5% weight reduction, and the changes in waist circumference and in SBP at week 52. Safety end points included the incidence of adverse events (AEs) and hypoglycaemia, as well as findings from physical examinations, electrocardiograms (ECGs) and clinical laboratory evaluations.
2.5. Statistical methods
The study was designed to demonstrate non‐inferiority of dapagliflozin‐containing treatment regimens relative to glimepiride in terms of the primary efficacy variable. Assuming no difference in dapagliflozin and dapagliflozin plus saxagliptin vs glimepiride with regard to HbA1c‐lowering efficacy, as well as a standard deviation (SD) of 1.0, it was estimated that approximately 930 randomized patients would provide ~95% power to demonstrate non‐inferiority.
All efficacy analyses were conducted on the full analysis set (FAS), which comprised randomized patients who received at least 1 dose of study medication and for whom there were a baseline value and at least 1 post‐baseline efficacy value. Analyses were performed using values prior to rescue treatment or discontinuation. The primary efficacy variable was analysed using a mixed‐model repeated measures (MMRM) model with fixed effects for treatment group and covariates. Non‐inferiority of dapagliflozin plus saxagliptin to glimepiride was assessed first, followed by non‐inferiority of dapagliflozin alone to glimepiride, based on a prespecified margin of 0.3%. Superiority of dapagliflozin plus saxagliptin to glimepiride was also assessed. Sensitivity analysis was conducted for the primary end point by repeating the primary efficacy analysis using data from the per protocol set, which comprised patients in the FAS who did not have relevant protocol deviations.
Analyses of secondary efficacy variables (change from baseline in body weight and FPG) were performed using an MMRM model similar to that used for the primary end point. The proportion of patients with confirmed hypoglycaemia was analysed using Fisher's exact test and time to rescue was analysed using a Cox proportional hazards model. Dapagliflozin‐containing treatment groups were tested sequentially vs glimepiride, with dapagliflozin plus saxagliptin compared with glimepiride prior to dapagliflozin alone for all end points with the exception of change in body weight.
Statistical comparisons were performed using a comparison‐wise type 1 error of 5% (two‐sided). To control the overall type 1 error of the study, a hierarchical closed testing procedure was employed: if non‐inferiority of the primary end point was demostrated, testing was performed for key secondary efficacy variables according to the sequential order pre‐specified in the study protocol. Any given key secondary end point was considered for significance only if the key secondary end point before it demonstrated statistical significance. For other secondary variables and exploratory variables, nominal P values were reported without significance testing.
Safety analyses were performed on the safety analysis set, which comprised patients who received at least 1 dose of study medication, regardless of rescue treatment; safety data were summarized using descriptive statistics.
3. RESULTS
3.1. Patient disposition and characteristics
The first patient was enrolled on September 21, 2015 and the last patient completed the study on March 13, 2017. A total of 1358 patients were enrolled and 939 patients were randomized, of whom 867 (92.3%) completed the study (Figure 1). The main reasons for study discontinuation included patient withdrawal (17 patients [1.8%]), adverse events (10 patients [1.1%]) and development of study‐specific withdrawal criteria (7 patients [0.7%]).
Baseline demographics and diabetes characteristics (Table 1) were balanced across treatment groups. Most patients (98.9%) were Caucasian; overall, 63.9% were men, and the mean (SD) patient age and BMI were 58.4 (8.6) years and 32.9 (5.1) kg/m2, respectively. Mean (SD) duration of type 2 diabetes was 7.0 (5.4) years and mean estimated glomerular filtration rate (eGFR) at baseline was similar between groups (86‐88 mL/min/1.73m2). More than half of the patients (56.4%) were taking a metformin dose of 2000 to 2500 mg/day.
Table 1.
Participant demographics and baseline characteristics (randomized analysis set)
| Variable | DAPA + MET (n = 314) |
DAPA + SAXA + MET
(n = 312) |
GLIM + MET
(n = 313) |
Total
(n = 939) |
|---|---|---|---|---|
| Women (%) | 112 (35.7) | 122 (39.1) | 105 (33.5) | 339 (36.1) |
| Age (years) | 57.4 (9.4) | 59.2 (7.9) | 58.6 (8.4) | 58.4 (8.6) |
| Weight (kg) | 97.7 (18.9) | 95.3 (17.4) | 97.3 (17.9) | 96.8 (18.1) |
| BMI (kg/m2) | 33.1 (5.2) | 32.5 (5.1) | 33.0 (5.1) | 32.9 (5.1) |
| SBP (mm Hg) | 138.4 (14.3) | 138.8 (14.1) | 138.8 (13.2) | 138.6 (13.9) |
| Duration of type 2 diabetes (years) | 6.9 (5.2) | 7.3 (5.9) | 6.7 (5.1) | 7.0 (5.4) |
| HbA1c (%) | 8.3 (0.7) | 8.3 (0.7) | 8.3 (0.8) | 8.3 (0.7) |
| FPG (mmol/L) | 10.6 (2.3) | 10.5 (2.0) | 10.4 (2.1) | 10.5 (2.1) |
| eGFR (MDRD) (mL/min/1.73m2) | 87.2 (19.4) | 88.0 (19.6) | 86.0 (17.5) | 87.1 (18.9) |
| C‐peptide (ng/mL) | 0.93 (0.36) | 0.92 (0.37) | 0.93 (0.34) | 0.93 (0.36) |
| History of hypertension (%) | 251 (79.9) | 253 (81.1) | 256 (81.8) | 760 (80.9) |
| Antihypertensive drug usea | ||||
|---|---|---|---|---|
| n = 313 | n = 312 | n = 312 | n = 937 | |
| ACE inhibitors (plain or combinations) | 143 (45.7) | 163 (52.2) | 164 (52.6) | 470 (50.2) |
| Angiotensin II antagonists (plain or combinations) | 96 (30.7) | 70 (22.4) | 74 (23.7) | 240 (25.6) |
| Beta‐blockers (selective) | 114 (36.4) | 110 (35.3) | 104 (33.3) | 328 (35.0) |
| Thiazides (plain) | 39 (12.5) | 41 (13.1) | 48 (15.4) | 128 (13.7) |
Data are presented as mean (SD) or number (%).
Abbreviations: ACE, angiotensin‐converting‐enzyme; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; GLIM, glimepiride; HbA1c, glycated haemoglobin; MDRD, Modification of Diet in Renal Disease; MET, metformin; SAXA, saxagliptin; SBP, systolic blood pressure; SD, standard deviation.
Includes medications initiated after double‐blind treatment initiation or prior to double‐blind treatment initiation, but continued during the double‐blind treatment phase. Data are from the safety analysis set.
3.2. Study drug treatment
The mean (SD) titrated glimepiride dose at Week 52 was 4.6 (1.8) mg; the maximum dose was 6 mg for 164 patients (52.6%) and 7.7% of patients were receiving 1 mg at Week 52. The glimepiride dose was down‐titrated to 0 mg in the case of four patients (1.3%).
3.3. Efficacy
3.3.1. Primary efficacy end point
Adjusted mean change from baseline in HbA1c at 52 weeks was −0.82% for dapagliflozin alone and −1.20% for dapagliflozin plus saxagliptin, compared with −0.99% for glimepiride when added to baseline metformin monotherapy (Table 2 and Figure 2A). Non‐inferiority, based on a prespecified margin of 0.3%, was demonstrated for both dapagliflozin‐containing treatment groups, relative to glimepiride, at Week 52. The change in HbA1c from baseline was statistically significantly greater (P = 0.001) with dapagliflozin plus saxagliptin than with glimepiride, thereby demonstrating superiority of dual add‐on (triple therapy) over glimepiride plus metformin. Figure 2B shows adjusted mean changes from baseline in HbA1c over the 52‐week treatment period. The major benefits of treatment, in terms of HbA1c reduction, were achieved within the first 12 weeks in all treatment arms.
Table 2.
Primary and secondary efficacy end points at 52 weeks prior to rescue (FAS)
| Efficacy end point (week 52) | DAPA + MET (n = 311) | DAPA + SAXA + MET (n = 312) | GLIM + MET (n = 309) |
|---|---|---|---|
| Primary end point | |||
| Change in HbA1c | |||
| Baseline mean, % (SD) |
n = 309 8.3 (0.7) |
n = 311 8.3 (0.7) |
n = 305 8.3 (0.8) |
| Adjusted mean change from baseline, % (SE) | −0.82 (0.05) | −1.20 (0.05) | −0.99 (0.05) |
| Difference from GLIM + MET (95% CI) | 0.16 (0.03, 0.30) |
−0.21 (−0.34, 0.08) |
|
| Superiority P value vs GLIM + MET | N/Aa | 0.001 | |
| Key secondary end points | |||
| Confirmed hypoglycaemia b | |||
| Patients with ≥1 hypoglycaemic event, number (%) |
n = 311 0 (0.0) |
n = 312 1 (0.3) |
n = 309 13 (4.2) |
| Difference from GLIM + MET (95% CI) | −4.2 (−6.5, 2.0) |
−3.9 (−6.2, −1.6) |
|
| P value vs GLIM + METc | < .001 | < .001 | |
| Weight (kg) d | |||
| Baseline Mean (SD) |
n = 311 97.9 (18.9) |
n = 312 95.3 (17.4) |
n = 308 97.5 (17.9) |
| Adjusted mean change from baseline (SE) | −3.5 (0.2) | −3.2 (0.2) | 1.8 (0.2) |
| Difference from GLIM + MET (95% CI) | −5.3 (−5.9, −4.7) |
−4.9 (−5.5, −4.3) |
|
| P value vs GLIM + MET | < .001 | < .001 | |
| FPG (mmol/L) d | |||
| Baseline Mean (SD) |
n = 309 10.6 (2.3) |
n = 311 10.4 (2.0) |
n = 308 10.4 (2.1) |
| Adjusted mean change from baseline (SE) | −1.6 (0.1) | −2.1 (0.1) | −1.5 (0.1) |
| Difference from GLIM + MET (95% CI) | −0.1 (−0.4, 0.2) | −0.6 (−0.9, −0.3) |
|
| P value vs GLIM + MET | .374 | < .001 | |
| Rescue treatment e | |||
| Number of patients rescued (%) | 58 (18.6) | 26 (8.3) | 66 (21.4) |
| Hazard ratio (95% CI) vs GLIM + MET | 0.95 (0.67,1.35) | 0.36 (0.23, 0.57) |
|
| P value vs GLIM + MET | .777 | < .001 | |
| Additional secondary efficacy end points | |||
| Patients achieving HbA1c < 7.0% | |||
| Number of patients (%, adjusted for baseline HbA1c) | 68 (20.3) | 129 (40.3) | 107 (33.9) |
| 95% CI for percentage adjusted | 16.1, 25.2 | 34.8, 46.0 | 28.7, 39.6 |
| Odds ratio (95% CI) vs GLIM + MET | 0.50 (0.34, 0.71) |
1.31 (0.94, 1.84) |
|
| P value vs GLIM + MET | < .001 | .112 | |
| Patients achieving HbA1c < 7.0% without confirmed hypoglycaemia | |||
| Number of patients (%, adjusted for baseline HbA1c) | 68 (20.3) | 128 (40.0) | 99 (31.2) |
| 95% CI for percentage adjusted | 16.2, 25.2 | 34.5, 45.7 | 26.1, 36.8 |
| Odds ratio (95% CI) vs GLIM + MET | 0.56 (0.39, 0.81) | 1.47 (1.05, 2.06) |
|
| P value vs GLIM + MET | .002 | .027 | |
| Patients with a weight reduction ≥ 5% | |||
| Number of patients (%, adjusted for baseline weight) | 70 (22.5) | 82 (25.7) | 11 (3.5) |
| 95% CI for percentage adjusted | 18.1, 27.5 | 21.1, 30.9 | 2.0, 6.2 |
| Odds ratio (95% CI) vs GLIM + MET | 7.96 (4.29, 16.22) | 9.50 (5.15, 19.27) |
|
| P value vs GLIM + MET | < .001 | < .001 | |
| Change in waist circumference (cm) | |||
| Baseline Mean (SD) |
n = 312 111.0 (13.4) |
n = 310 109.3 (12.4) |
n = 310 111.6 (13.1) |
| Adjusted mean change from baseline (SE) | −1.8 (0.3) | −2.5 (0.3) | 1.0 (0.3) |
| Difference from GLIM + MET (95% CI) | −2.8 (−3.7, −1.9) |
−3.4 (−4.3, −2.6) |
|
| P value vs GLIM + MET | < .001 | < .001 | |
| SBP (mm Hg) | |||
| Baseline Mean (SD) |
n = 309 138.4 (14.3) |
n = 312 138.8 (14.1) |
n = 308 138.8 (13.1) |
| Adjusted mean change from baseline (SE) | −5.6 (0.8) | −6.4 (0.7) | −1.6 (0.8) |
| Difference from GLIM + MET (95% CI) | −4.0 (−6.1, −1.9) |
−4.8 (−6.9, −2.8) |
|
| P value vs GLIM + MET | < .001 | < .001 | |
Abbreviations: CI, confidence interval; DAPA, dapagliflozin; FAS, full analysis set; FPG, fasting plasma glucose; GLIM, glimepiride; HbA1c, glycated haemoglobin; MET, metformin; MMRM, mixed‐model repeated measures; SAXA, saxagliptin; SD, standard deviation; SE, standard error.
P values for non‐inferiority were not calculated; non‐inferiority was assessed using the two‐sided 95% CI of the adjusted mean difference between dapagliflozin or dapagliflozin plus saxagliptin and glimepiride, using a prespecified margin of 0.3%.
Confirmed hypoglycaemia: typical symptoms with glucose ≤2.8 mmol/L (≤50 mg/dL).
Fisher's exact test (separate tests for each pair‐wise comparison).
MMRM model with terms for treatment, baseline (weight or FPG), week, treatment‐by‐week interaction and baseline‐by‐week interaction.
Cox proportional hazards model with term for treatment. The Efron method was used in cases where patients had identical rescue times. Time to rescue treatment is censored at the earliest occurrence of the following: discontinuation of study medication, study completion or time of insulin initiation.
Figure 2.
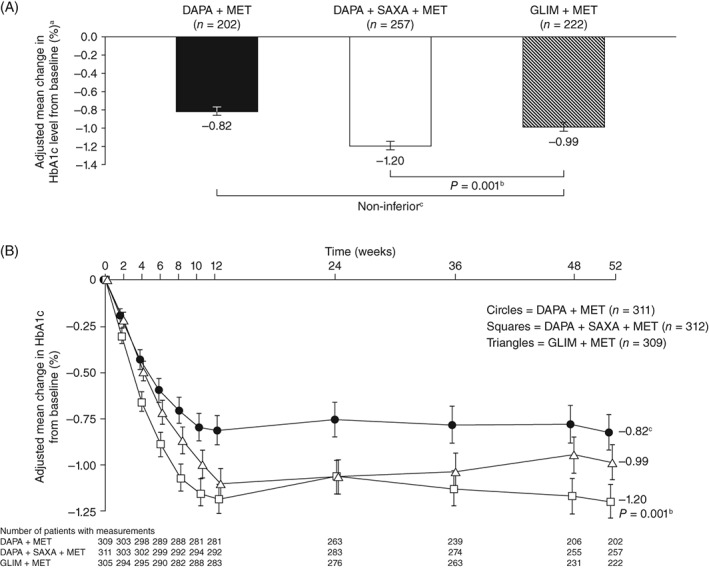
A, HbA1c, adjusted mean change (±SE) from baseline at week 52 and B, adjusted mean change (±SE) from baseline over time (FAS). aAll values are given as least‐squares mean ±standard error. bSuperiority P value vs GLIM + MET (two‐sided). cDAPA + MET was non‐inferior to GLIM + MET, based on a prespecified non‐inferiority margin of 0.3%. Abbreviations: DAPA, dapagliflozin; FAS, full analysis set; GLIM, glimepiride; HbA1c, glycated haemoglobin; MET, metformin; SAXA, saxagliptin; SE, standard error
3.3.2. Secondary end points
The proportion of patients experiencing at least one episode of confirmed hypoglycaemia during the 52‐week treatment period was low across all groups (<5%) and was significantly lower in both dapagliflozin‐containing treatment groups than in the glimepiride group (Table 2). Total body weight decreased from baseline to Week 52 in both dapagliflozin‐containing treatment groups, whereas it increased in the glimepiride group (Table 2). Reductions in FPG from baseline to Week 52 were statistically significantly greater with dapagliflozin plus saxagliptin than with glimepiride as add‐on therapy, and dapagliflozin was non‐inferior to glimepiride as add‐on therapy (Table 2). The time courses to onset of changes in FPG and body weight were similar to those for HbA1c, in that the majority of the treatment effect was achieved by Week 12 with both dapagliflozin‐containing treatment regimens (Figure S1). The number of patients (proportions) who met rescue criteria during the treatment period were 58 (18.6%), 26 (8.3%) and 66 (21.4%) in the dapagliflozin, dapagliflozin plus saxagliptin and glimepiride add‐on to metformin groups, respectively (Table 2).
The proportion of patients who achieved HbA1c < 7.0% at Week 52, regardless of hypoglycaemia, was greater with add‐on dapagliflozin plus saxagliptin than with add‐on glimepiride (Table 2). The proportion of patients with a weight reduction of ≥5% from baseline to Week 52 was higher in the dapagliflozin and dapagliflozin plus saxagliptin groups compared with the glimepiride group (Table 2). Mean waist circumference decreased with dapagliflozin‐containing regimens, whereas it increased with glimepiride (Table 2). Moreover, there were greater reductions in SBP from baseline to Week 52 with dapagliflozin and dapagliflozin plus saxagliptin add‐on therapies than with glimepiride (Table 2 and Figure S2).
3.4. Hypoglycaemia
The overall incidence of hypoglycaemia was substantially lower with dapagliflozin‐containing treatment regimens than with glimepiride add‐on to metformin. In total, there were 10, 19 and 329 hypoglycaemic events with add‐on dapagliflozin, dapagliflozin plus saxagliptin and glimepiride, respectively (Table 3). Notably, no episodes of major hypoglycaemia occurred in any treatment group and there was little difference in the incidence of hypoglycaemia prior to and after rescue. The distribution of hypoglycaemic events over time is shown in Figure S2.
Table 3.
Treatment‐emergent adverse events (safety analysis set; data regardless of rescue therapy)
| Adverse event category | Number (%) of patients | ||
|---|---|---|---|
|
DAPA + MET
(n = 313) |
DAPA + SAXA + MET
(n = 312) |
GLIM + MET
(n = 312) |
|
| At least 1 AE | 188 (60.1) | 158 (50.6) | 170 (54.5) |
| AE leading to discontinuation of study medication | 27 (8.6) | 12 (3.8) | 13 (4.2) |
| Hospitalization for heart failure | 0 | 0 | 1 (0.3) |
| AEs of special interest | |||
| UTIs | 24 (7.7) | 13 (4.2) | 12 (3.8) |
| Genital infections | 25 (8.0) | 15 (4.8) | 2 (0.6) |
| Hypoglycaemia, number of events (proportion of total events in each category, %)a prior to rescue | |||
| Overall events N = 358 |
10 (2.8) | 19 (5.3) | 329 (91.9) |
| Major hypoglycaemiab
N = 0 |
0 | 0 | 0 |
| Episode of hypoglycaemiac
N = 224 |
1 (0.4) | 7 (3.1) | 216 (96.4) |
| Other episode of hypoglycaemiad
N = 65 |
7 (10.8) | 10 (15.4) | 48 (73.8) |
| Confirmed hypoglycaemiae
N = 26 |
0 | 1 (3.8) | 25 (96.2) |
| Asymptomatic hypoglycaemiaf
N = 69 |
2 (2.9) | 2 (2.9) | 65 (94.2) |
Abbreviations: AE, adverse event; DAPA, dapagliflozin; GLIM, glimepiride; MET, metformin; SAE, serious adverse event; SAXA, saxagliptin; UTI, urinary tract infection.
Percentages reflect total number of each type of event across all treatment groups.
Major hypoglycaemic episode: symptomatic episode requiring external assistance with glucose <3.0 mmol/L (<54 mg/dL).
Hypoglycaemia: symptomatic episode with glucose ≤3.9 mmol/L (≤70 mg/dL).
Other episode of hypoglycaemia: symptomatic episode, with or without glucose >3.9 mmol/L (>70 mg/dL).
Confirmed hypoglycaemia: typical symptoms with glucose ≤2.8 mmol/L (≤50 mg/dL).
Asymptomatic hypoglycaemia: event with absence of symptoms but with glucose ≤3.9 mmol/L (≤70 mg/dL).
3.5. Safety and tolerability
Overall, the proportion of patients reporting an AE was similar between treatment groups (range, 50.6%‐60.1%) (Table 3). In the add‐on dapagliflozin, add‐on dapagliflozin plus saxagliptin and add‐on glimepiride groups, UTIs were reported by 24 (7.7%), 13 (4.2%) and 12 (3.8%) patients, respectively. These events were all mild or moderate in severity. Genital infections were reported in 25 (8.0%), 15 (4.8%) and 2 (0.6%) patients in the dapagliflozin, dapagliflozin plus saxagliptin and glimepiride add‐on therapy groups, respectively. In the add‐on dapagliflozin plus saxagliptin group, one patient experienced an event of increased blood ketone body concentration. The event was mild in intensity and pH values during the event were reported to be within the normal range; the patient did not discontinue study medication.
A total of 52 AEs led to discontinuation of study treatment. These events occurred in 27 (8.6%), 12 (3.8%) and 13 (4.2%) patients in the dapagliflozin, dapagliflozin plus saxagliptin and glimepiride groups, respectively (Table 3). Serious AEs (SAEs) were reported in 39 (12.5%), 22 (7.1%) and 35 (11.2%) patients in the dapagliflozin, dapagliflozin plus saxagliptin and glimepiride groups, respectively. One SAE (UTI) in the add‐on dapagliflozin group was considered by the investigator to be related to the study treatment. SAEs leading to study drug discontinuation were dispersed across multiple system organ classes and included renal, urinary and cardiac disorders. These events were reported by 13 (4.2%) and 3 (1.0%) patients in the add‐on dapagliflozin and add‐on glimepiride groups, respectively. No SAEs leading to study drug discontinuation occurred in the dapagliflozin plus saxagliptin group.
Overall, there were no clinically meaningful drug effects on haematological or clinical chemistry parameters in any treatment group. No clinically meaningful changes from baseline were observed for lipid parameters, vital signs or ECG variables in any group during the treatment period. A total of 12 (3.8%), 6 (1.9%) and 7 (2.2%) patients reported AEs of renal impairment/failure in the dapagliflozin, dapagliflozin plus saxagliptin and glimepiride groups, respectively. The preferred term with the highest incidence was decreased CrCl (2.9%, 1.3% and 1.3% with add‐on dapagliflozin, dapagliflozin plus saxagliptin and glimepiride, respectively). One SAE of renal impairment/failure occurred in one patient in the dapagliflozin group on Day 1. This patient had chronic kidney disease and eGFR was 53.8 mL/min/1.73m2 on study day −15 and 17.2 mL/min/1.73m2 on study day 1. Following discontinuation of the study drug on Day 7, eGFR was 42.3 mL/min/1.73m2 when assessed on Day 27. The SAE was assessed as not being causally related to the study medication.
4. DISCUSSION
This study evaluated the efficacy and safety of add‐on therapy with dapagliflozin vs glimepiride in metformin‐treated patients with type 2 diabetes who had inadequate glycaemic control. We considered two regimens: either dapagliflozin alone or in combination with saxagliptin, as a combination of an SGLT‐2 and a DPP‐4 inhibitor has been shown previously to lead to superior improvements in glycaemic control than that achieved with either agent alone.
For the primary end point of change from baseline in HbA1c at Week 52, non‐inferiority was demonstrated for the combination of dapagliflozin and metformin compared with glimepiride plus metformin. Superiority was met for this end point for triple therapy with dapagliflozin, saxagliptin and metformin vs glimepiride plus metformin. Similar trends were observed for key secondary end points; with dapagliflozin‐containing regimens, superiority was met for the proportion of patients reporting confirmed hypoglycaemic episodes during treatment, as well as for the change from baseline in total body weight at Week 52. Both dapagliflozin‐containing regimens decreased FPG from baseline to Week 52 to an equal or greater extent than glimepiride plus metformin. All treatment regimens were well tolerated, and the safety profile of the dapagliflozin‐saxagliptin combination was in line with that of the individual drugs, without additional side effects. Findings from this study are in accordance with those from previous studies of dapagliflozin and saxagliptin when administered as part of either dual or triple therapy with metformin.6, 7, 11, 18 The results were also in line with previous head‐to‐head comparisons of dapagliflozin and other SGLT‐2 inhibitors vs sulphonylureas.15, 16, 17, 19, 20
In this study, dapagliflozin‐containing treatment regimens were associated with the additional benefit of weight loss, compared with glimepiride therapy which was associated with weight gain during treatment. Over 20% of patients in both dapagliflozin treatment groups achieved ≥5% weight loss, compared with only 3.5% of patients in the add‐on glimepiride group. This is of importance, given that body weight reduction is a key treatment goal in patients with type 2 diabetes.1 Dapagliflozin‐containing regimens also reduced SBP, an effect that has been observed previously with SGLT‐2 inhibitor treatment21, 22 and might be attributable to the osmotic, natriuretic and weight‐reducing effects of these agents.23, 24 This is likely to be beneficial to patients with type 2 diabetes, given that achievement of blood pressure targets is associated with reduced risk of microvascular and macrovascular diabetes complications, including cardiovascular disease.25
Dapagliflozin‐containing treatment regimens were well tolerated during the study. Although there were more UTIs and genital infections in the dapagliflozin groups than in the glimepiride group, these were all mild or moderate in intensity and the frequency was consistent with previous reports.11, 26, 27 In line with some previous reports, the frequency of both types of infection was lower in the dapagliflozin plus saxagliptin combination arm compared with the dapagliflozin arm. Several hypotheses for the mechanism underlying this phenomenon have been proposed, the simplest being that addition of a DPP‐4 inhibitor augments overall glucose control and thereby reduces SGLT‐2 inhibitor‐mediated glucosuria.28 However, there are arguments that challenge this hypothesis and dedicated experimental studies are required to provide greater clarity.
Treatment with SGLT‐2 inhibitors has been associated with transient reductions in eGFR. More patients receiving dapagliflozin plus metformin experienced decreased CrCl leading to study discontinuation than did patients receiving glimepiride (2.9% vs 1.3%) However, the study criterion for discontinuation because of decreased CrCl was strict and did not allow CrCl to decrease below 60 mL/min if not reversed within 1 week of detection. Previous studies have shown that the initial effect of SGLT‐2 inhibitors on renal filtration is reversible and is associated with beneficial effects such as reduced albumin excretion and protection of renal function in patients with micro‐ and macroalbuminuria.29, 30, 31, 32
An important adverse effect of sulphonylureas is the invariable induction of hypoglycaemia, which is associated with numerous detrimental patient outcomes.33 In this study, there were substantially fewer hypoglycaemic episodes in patients taking dapagliflozin‐containing regimens than in patients receiving glimepiride plus metformin. This is consistent with findings from a previous study16 and with the mechanism of action of dapagliflozin, which does not depend on insulin secretion or action. A total of 329 hypoglycaemic events occurred in 87 patients in the glimepiride group, indicating that many patients in this treatment group experienced recurrent hypoglycaemia; these events may have prevented up‐titration of glimepiride. The low risk of hypoglycaemic events is an important advantage of add‐on dapagliflozin therapy, given the observed links between occurrence of hypoglycaemia and poor adherence to glucose‐lowering therapy.34 Further, dapagliflozin‐containing regimens are without some of the practical disadvantages associated with sulphonylureas, including the need for careful titration and frequent glucose monitoring.
The enhanced glucose‐lowering efficacy of triple therapy with dapagliflozin plus saxagliptin concomitantly added to metformin is also worthy of mention. There is a growing body of evidence to suggest that combining antidiabetes agents that have complementary mechanisms of action can achieve greater efficacy than add‐on of each agent alone for patients with high HbA1c levels, and dual and triple therapies have been shown to have similar safety profiles.11, 26, 27
A key strength of this study is the up‐titration of glimepiride to reflect real‐world clinical practice. Although titration of sulphonylureas is essential to balance antihyperglycaemic efficacy with the risk of hypoglycaemia, it is not typical for clinical trials to allow continuous titration throughout the study. Potential limitations of the study include the aforementioned inability to monitor long‐term effects of study treatments on renal function, because of the strict criteria for discontinuation.
Overall, triple therapy with dapagliflozin, saxagliptin and metformin was superior to dual therapy with glimepride and metformin in terms of HbA1c reductions and improvements in multiple other metabolic variables. Addition of dapagliflozin alone improved glycaemic control to an extent similar to that of glimepiride, but with the additional benefits of weight loss and improvements in SBP. Dapagliflozin‐ containing regimens were well tolerated and were associated with a substantially lower incidence of hypoglycaemia compared with glimepiride add‐on to metformin therapy, with the added benefit of weight loss and improvements in blood pressure. These findings suggest the utility of dapagliflozin‐containing combination therapy in this patient population as an alternative to sulphonylurea add‐on to metformin.
Conflict of interest
D. M.‐W. is a member of speakers' bureaux and advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, Novo Nordisk and Sanofi. M. K. is a member of speakers' bureaux and advisory boards for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk and Sanofi. K. C. has received honoraria and consulting fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Johnson & Johnson, Novo Nordisk, Medtronic and Roche. D. S. has no conflicts of interest to disclose. K. R. is an employee of AstraZeneca. E. J., R. G.‐S., R. K. and C. D. S. are employees of and stockholders in AstraZeneca. S. J. has received honoraria, research support and consulting fees from Abbott, Amgen, AstraZeneca, Bayer, Berlin Chemie, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo Inc., Janssen, Johnson & Johnson, Lilly, Merck, Merck Sharp & Dohme, Novartis, Novo Nordisk, Orexigen, Pfizer, Roche, Sanofi‐Aventis and Servier. J. S. has served on advisory boards and/or speakers' bureaux for Amgen, AstraZeneca, Bayer, Berlin‐Chemie, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Ipsen, Janssen, LifeScan, Merck, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi‐Aventis and Takeda; and has received research support from Amgen, Eli Lilly, GlaxoSmithKline, Intarcia, Ipsen, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi Aventis, Servier and Takeda. N. Dronamraju is an employee of AstraZeneca. K. Csomós has no conflicts of interest to disclose.
Author contributions
D. M.‐W., K. R., E. J., R. G.‐S., C. D. S., N. D. and K. C. contributed to the study concept, conduct and design. D. M.‐W., M. K., K. C., K. R., E. J., R. K., C. D. S., S. J., J. S. and N. D. contributed to data analysis and interpretation. All authors wrote, reviewed and edited the manuscript. D. M.‐W is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
REFERENCES
- 1. American Diabetes Association . Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1):S1‐S132. [DOI] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429‐442. [DOI] [PubMed] [Google Scholar]
- 3. Fonseca VA, Kulkarni KD. Management of type 2 diabetes: oral agents, insulin, and injectables. J Am Diet Assoc. 2008;108:S29‐S33. [DOI] [PubMed] [Google Scholar]
- 4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm ‐ 2017 executive summary. Endocr Pract. 2017;23:207‐238. [DOI] [PubMed] [Google Scholar]
- 5. DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649‐1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add‐on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double‐blind, placebo‐controlled 102‐week trial. BMC Med. 2013;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2010;375:2223‐2233. [DOI] [PubMed] [Google Scholar]
- 8. Kawalec P, Mikrut A, Lopuch S. The safety of dipeptidyl peptidase‐4 (DPP‐4) inhibitors or sodium‐glucose cotransporter 2 (SGLT‐2) inhibitors added to metformin background therapy in patients with type 2 diabetes mellitus: a systematic review and meta‐analysis. Diabetes Metab Res Rev. 2014;30:269‐283. [DOI] [PubMed] [Google Scholar]
- 9. Taskinen MR, Rosenstock J, Tamminen I, et al. Safety and efficacy of linagliptin as add‐on therapy to metformin in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled study. Diabetes Obes Metab. 2011;13:65‐74. [DOI] [PubMed] [Google Scholar]
- 10. Rosenstock J, Seman LJ, Jelaska A, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add‐on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15:1154‐1160. [DOI] [PubMed] [Google Scholar]
- 11. Rosenstock J, Hansen L, Zee P, et al. Dual add‐on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double‐blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376‐383. [DOI] [PubMed] [Google Scholar]
- 12. Evans JM, Ogston SA, Emslie‐Smith A, Morris AD. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia. 2006;49:930‐936. [DOI] [PubMed] [Google Scholar]
- 13. Selvin E, Bolen S, Yeh HC, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med. 2008;168:2070‐2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta‐analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15:938‐953. [DOI] [PubMed] [Google Scholar]
- 15. Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add‐on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52‐week, double‐blind, active‐controlled noninferiority trial. Diabetes Care. 2011;34:2015‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nauck MA, Del Prato S, Duran‐Garcia S, et al. Durability of glycaemic efficacy over 2 years with dapagliflozin versus glipizide as add‐on therapies in patients whose type 2 diabetes mellitus is inadequately controlled with metformin. Diabetes Obes Metab. 2014;16:1111‐1120. [DOI] [PubMed] [Google Scholar]
- 17. Del Prato S, Nauck M, Duran‐Garcia S, et al. Long‐term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add‐on therapy to metformin in patients with type 2 diabetes: 4‐year data. Diabetes Obes Metab. 2015;17:581‐590. [DOI] [PubMed] [Google Scholar]
- 18. Rosenstock J, Gross JL, Aguilar‐Salinas C, et al. Long‐term 4‐year safety of saxagliptin in drug‐naive and metformin‐treated patients with type 2 diabetes. Diabet Med. 2013;30:1472‐1476. [DOI] [PubMed] [Google Scholar]
- 19. Cefalu WT, Leiter LA, Yoon K‐H, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet. 2013;382:941‐950. [DOI] [PubMed] [Google Scholar]
- 20. Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. Comparison of empagliflozin and glimepiride as add‐on to metformin in patients with type 2 diabetes: a 104‐week randomised, active‐controlled, double‐blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691‐700. [DOI] [PubMed] [Google Scholar]
- 21. Sjostrom CD, Johansson P, Ptaszynska A, List J, Johnsson E. Dapagliflozin lowers blood pressure in hypertensive and non‐hypertensive patients with type 2 diabetes. Diab Vasc Dis Res. 2015;12:352‐358. [DOI] [PubMed] [Google Scholar]
- 22. Yamout H, Perkovic V, Davies M, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol. 2014;40:64‐74. [DOI] [PubMed] [Google Scholar]
- 23. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sjostrom CD, Hashemi M, Sugg J, Ptaszynska A, Johnsson E. Dapagliflozin‐induced weight loss affects 24‐week glycated haemoglobin and blood pressure levels. Diabetes Obes Metab. 2015;17:809‐812. [DOI] [PubMed] [Google Scholar]
- 25. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703‐713. [PMC free article] [PubMed] [Google Scholar]
- 26. Mathieu C, Herrera Marmolejo M, Gonzalez Gonzalez JG, et al. Efficacy and safety of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:1134‐1137. [DOI] [PubMed] [Google Scholar]
- 27. Mathieu C, Ranetti AE, Li D, et al. Randomized, double‐blind, phase 3 trial of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38:2009‐2017. [DOI] [PubMed] [Google Scholar]
- 28. Fadini GP, Bonora BM, Sarangdhar M, Rigato M, Avogaro A. DPP‐4 inhibitors moderate the risk of genitourinary tract infections associated with SGLT2 inhibitors. Diabetes Obes Metab. 2018;20:740‐744. [DOI] [PubMed] [Google Scholar]
- 29. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 30. Heerspink HJ, Johnsson E, Gause‐Nilsson I, Cain VA, Sjostrom CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin‐angiotensin blockers. Diabetes Obes Metab. 2016;18:590‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323‐334. [DOI] [PubMed] [Google Scholar]
- 33. Shafiee G, Mohajeri‐Tehrani M, Pajouhi M, Larijani B. The importance of hypoglycemia in diabetic patients. J Diabetes Metab Disord. 2012;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pollack MF, Purayidathil FW, Bolge SC, Williams SA. Patient‐reported tolerability issues with oral antidiabetic agents: associations with adherence; treatment satisfaction and health‐related quality of life. Diabetes Res Clin Pract. 2010;87:204‐210. [DOI] [PubMed] [Google Scholar]
Supporting information
Figure S1. Adjusted mean changes (± SE) from baseline over time for secondary efficacy variables: FPG (panel A) and body weight (panel B). aDAPA + MET was non‐inferior to GLIM + MET; bSuperiority P value vs GLIM + MET (two‐sided). DAPA, dapagliflozin; FPG, fasting plasma glucose; GLIM, glimepiride; MET, metformin; SAXA, saxagliptin; SE, standard error
Figure S2. Adjusted mean change (± SE) from baseline over time for SBP (FAS) (panel A) and cumulative incidence of hypoglycemic events over timea (panel B). aSafety analysis set, data prior to rescue. Data on the number of patients with available at each time point are not available. DAPA, dapagliflozin; FAS, full analysis set; GLIM, glimepiride; MET, metformin; SAXA, saxagliptin; SBP, systolic blood pressure.
ACKNOWLEDGEMENTS
We are very grateful to all participating patients and study centres. Medical writing support was provided by Lucy Ambrose, DPhil of Oxford PharmaGenesis, Oxford, UK, with funding from AstraZeneca.
Müller‐Wieland D, Kellerer M, Cypryk K, et al. Efficacy and safety of dapagliflozin or dapagliflozin plus saxagliptin versus glimepiride as add‐on to metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:2598–2607. 10.1111/dom.13437
Funding information The study was funded by AstraZeneca.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Adjusted mean changes (± SE) from baseline over time for secondary efficacy variables: FPG (panel A) and body weight (panel B). aDAPA + MET was non‐inferior to GLIM + MET; bSuperiority P value vs GLIM + MET (two‐sided). DAPA, dapagliflozin; FPG, fasting plasma glucose; GLIM, glimepiride; MET, metformin; SAXA, saxagliptin; SE, standard error
Figure S2. Adjusted mean change (± SE) from baseline over time for SBP (FAS) (panel A) and cumulative incidence of hypoglycemic events over timea (panel B). aSafety analysis set, data prior to rescue. Data on the number of patients with available at each time point are not available. DAPA, dapagliflozin; FAS, full analysis set; GLIM, glimepiride; MET, metformin; SAXA, saxagliptin; SBP, systolic blood pressure.


