Abstract
Alpha oscillations are often reported to be amplified during working memory (WM) retention, serving to disengage sensory areas to protect internal representations from external interference. At the same time, contemporary views of WM postulate that sensory areas may often also be recruited for retention. I here review recent evidence that during such ‘perceptual’ WM, alpha oscillations in mnemonically relevant sensory areas are not amplified but attenuated instead. I will argue that such attenuated alpha states serve a mnemonic role and, further, that larger attenuation may support item‐specific attentional prioritisation within perceptual WM. In critically evaluating this role, I also consider (and argue against) four alternatives to a strictly mnemonic account of the available data that may also prove useful to consider in future research. Finally, I highlight key implications of these data for the study of WM and for our understanding of the functional roles of states of attenuated alpha oscillations in cognition.
Keywords: attentional prioritisation, electroencephalography, neuronal oscillations, sensory recruitment, working memory retention
Introduction
Alpha oscillations (8–12 Hz) provide a window into the engagement of the underlying neural circuity within macroscopic magneto‐ and encephalography (M/EEG) measurements in healthy humans (Berger, 1929; Hari & Salmelin, 1997) – with states of attenuated (vs. amplified) alpha oscillations generally being associated with increased (vs. decreased) cortical engagement (Klimesch et al., 2007; Jensen & Mazaheri, 2010; Foxe & Snyder, 2011; Haegens et al., 2011a; Hanslmayr et al., 2012). Because alpha oscillations are particularly well characterised in the brain's visual, somatosensory and auditory processing areas (e.g. Hari & Salmelin, 1997; Haegens et al., 2015), this role has been particularly well documented in relation to the gating of sensory information in service of adaptive perception.
In the realm of working memory (WM), alpha oscillations are typically reported to be amplified during retention (Klimesch et al., 1999; Jensen et al., 2002; Tuladhar et al., 2007; Haegens et al., 2010; Johnson et al., 2011; Bonnefond & Jensen, 2012; Obleser et al., 2012; Spitzer & Blankenburg, 2012), with larger increases in amplitude (e.g. Jensen et al., 2002; Tuladhar et al., 2007) as well as interareal and cross‐frequency phase synchronisation (Palva et al., 2010; Siebenhühner et al., 2016) with higher WM loads. There is growing consensus (Roux & Uhlhaas, 2014; Payne & Sekuler, 2014 for reviews; but see Palva & Palva, 2007) that these modulations too may reflect sensory gating, whereby internal representations are protected from (potential) interfering external sensory input through disengagement of the areas that are receptive to this input. In support of this, the amplification of posterior alpha oscillations during WM retention is more pronounced when prospective visual distractors are expected to cause larger interference (Bonnefond & Jensen, 2012), and similar distractor dependence has recently been documented in the auditory domain (Wöstmann et al., 2017).
In a traditional view of WM, in which representations are retained predominantly outside the sensory areas (as discussed in e.g. Sreenivasan et al., 2014), sensory disengagement appears a sensible and powerful protective instrument. Yet, increasing evidence suggests that WM retention may often also recruit sensory areas (e.g. Awh & Jonides, 2001; Pasternak & Greenlee, 2005; Harrison & Tong, 2009; Kuo et al., 2009; Serences et al., 2009; Sreenivasan et al., 2014; D'Esposito & Postle, 2015), particularly when the fine‐grained sensory properties of the memoranda are retained (Lee et al., 2013; Christophel et al., 2017). To reconcile sensory recruitment during such ‘perceptual’ WM with the postulated alpha‐mediated sensory disengagement, we are invited to revisit the link between alpha oscillations and WM in at least two ways.
First, the utility of alpha amplification for distractor inhibition may be largely restricted to circumstances in which non‐sensory properties (e.g. semantic category labels) are retained, or in which retained memoranda engage a different sensory brain areas than the area from which alpha oscillations are considered. Indeed, amplification of posterior alpha oscillations appears particularly prominent in tasks where the content of WM is verbal, or somatosensory (Jensen et al., 2002; Haegens et al., 2010; Bonnefond & Jensen, 2012; see also Gevins et al., 1997; but see Johnson et al., 2011), but where the sources of this amplification localise to visual brain areas (Tuladhar et al., 2007; Haegens et al., 2010; Bonnefond & Jensen, 2012). Second, it raises the possibility that modulations of alpha oscillations during WM may also serve a genuine mnemonic function – whereby attenuated alpha states (marking sensory engagement, or ‘recruitment’) actively support WM retention.
Building on earlier reviews on the role of alpha oscillations in WM that focused and converged on a protective (disengaging) role for states of amplified alpha oscillations during WM (Payne & Sekuler, 2014; Roux & Uhlhaas, 2014), I here review recent evidence for this complementary mnemonic role for states of attenuated alpha oscillations during perceptual WM. I first highlight recent evidence showing that, when mnemonic information is relevant to a particular sensory area, alpha oscillations measured from this area are not amplified but attenuated instead. In addition, I discuss recent evidence that such attenuated alpha states may not only support WM retention but may also support attentional prioritisation within WM and govern item accessibility. I finally consider (and, where possible, counter) four alternative interpretations of the available data that challenge a strictly mnemonic interpretation and highlight key implications of the reviewed work.
A mnemonic role for attenuated alpha states in perceptual working memory
A number of recent studies have demonstrated that alpha oscillations are not only modulated in sensory modalities that are irrelevant to the WM retention (e.g. in visual areas during verbal retention; as in Jensen et al., 2002; Tuladhar et al., 2007; Bonnefond & Jensen, 2012) but also in modalities that are considered relevant for retention. For example, on the basis of the popular lateralised visual change‐detection task (as in Vogel & Machizawa, 2004), several studies have now demonstrated that posterior (visual) alpha oscillations show a corresponding lateralisation that sustains throughout the retention interval – whereby alpha amplitude is lower contralateral vs. ipsilateral to the retinotopic location of the retained items at encoding (Grimault et al., 2009; Sauseng et al., 2009; Lozano‐Soldevilla et al., 2014). In a similar vein, studies have reported a relative attenuation of alpha in mnemonically relevant vs. mnemonically irrelevant sensory modalities (Spitzer & Blankenburg, 2012; van Ede et al., 2017a), as well as when retaining spatial location as opposed to visual identity (Jokisch & Jensen, 2007), temporal order (Roberts et al., 2013) or relational (Ikkai et al., 2014) information.
Still, as the core results in these studies typically entail a relative amplitude difference between mnemonically relevant vs. irrelevant areas, it often remains difficult to distinguish cortical engagement by attenuated alpha oscillations in relevant areas (supporting mnemonic retention) from cortical disengagement by amplified alpha oscillations in irrelevant areas (supporting distractor inhibition). Complementary evidence suggests that both scenarios may be at play. For example, in the lateralised change‐detection task, alpha lateralisation appears particularly sensitive to the number of irrelevant items (Sauseng et al., 2009) suggesting a role in distractor inhibition. At the same time, in the context of a related lateralised visual WM task, performance appears best predicted by alpha states in visual sites contralateral to the probed item's retained location (with lower amplitude predicting better performance; van Ede et al., 2017b; see also Spitzer & Blankenburg, 2012), thus arguing for a role in mnemonic retention.
To further disentangle these possibilities, my colleagues and I recently investigated alpha modulations in both visual and somatosensory brain areas during retention of either visual or somatosensory sequences. In addition to directly comparing visual with somatosensory retention, we also compared each separately to a control condition with no retention demand (i.e. load 0). This yielded an unambiguous pattern of results: during retention of visual sequences, alpha (as well as beta) oscillations were almost exclusively attenuated in visual brain areas, whereas during retention of somatosensory sequences, this was the case in somatosensory brain areas (van Ede et al., 2017a). In both modalities, these WM related attenuations of alpha and beta oscillations scaled with load (see also Fukuda et al., 2015; Erickson et al., 2017). These results could not simply be accounted for by differences in sensory processing, as sensory stimulation was equated between control and WM trials, between visual and somatosensory WM trials and between trials with different WM loads (van Ede et al., 2017a, for details).
As schematically depicted in Fig. 1, when directly comparing the results of this study to those in the landmark study by Jensen et al. (2002), an important insight emerges. In both cases, alpha amplitude during WM retention shows prominent modulation by WM load. However, the sign of these modulations is opposite. When participants engage in verbal retention (even when information is encoded visually, as in Jensen et al., 2002; Tuladhar et al., 2007), the amplitude of posterior (putatively visual) alpha increases with load, whereas when they engage in visual retention (as argued in van Ede et al., 2017a), alpha amplitude now decreases with load (for more demonstrations of alpha attenuation during WM see also: Medendorp et al., 2007; Fukuda et al., 2015; Erickson et al., 2017). The direction of modulation thus appears to be highly dependent on the nature of the memoranda (as well as the source of the alpha oscillations under consideration; as in e.g. Spitzer & Blankenburg, 2012; van Ede et al., 2017a).
Figure 1.
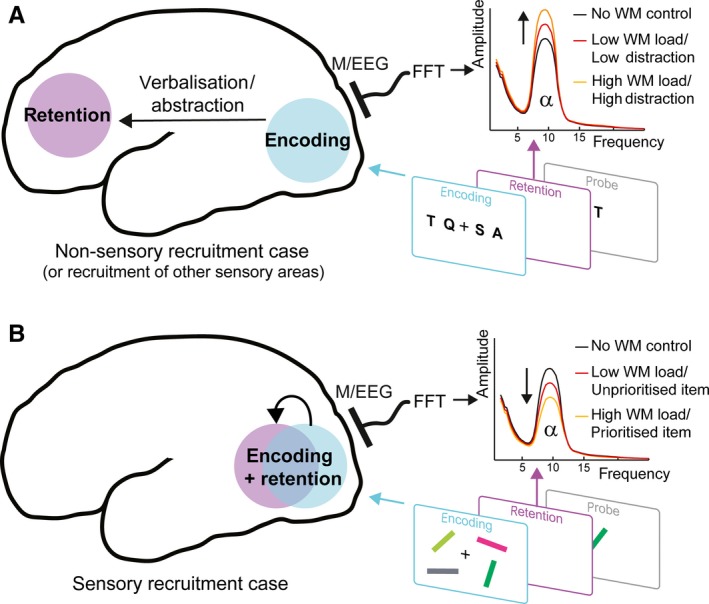
Alpha amplitude during working memory retention as a function of sensory recruitment, external distraction and item‐specific prioritisation. In scenario (A), the memory array contains verbal material that is encoded in visual areas but retained elsewhere (e.g. in prefrontal, or language areas). In M/EEG recordings from the visual areas during the retention interval, alpha power increases with load (as in e.g. Jensen et al., 2002), as well as with the level of (anticipated) external distraction (as in e.g. Bonnefond & Jensen, 2012). In contrast, in scenario (B), the memory array contains visual items that, this time, are not only encoded but also retained in visual areas (because the task requires the retention of the precise visual identity – orientations and colours – of the items). Alpha power in M/EEG recordings from the visual areas now decreases with load (as in e.g. van Ede et al., 2017a) and decreases further when items are placed in a prioritised state based on current attentional demands (as in e.g. Myers et al., 2015). How (expected) distraction in the sensory recruitment case (scenario B) and item‐specific prioritisation in the non‐sensory recruitment case (scenario A) affect alpha remains to be more systematically investigated and is therefore not included in the schematic. M/EEG, Magneto/Electroencephalography; FFT, fast Fourier transform.
Several recent studies have further demonstrated a link between alpha oscillations during retention and the content‐specific identities of WM representations using sophisticated forward encoding modelling of visual stimulus features such as location and orientation (e.g. Foster et al., 2016; Fukuda et al., 2016). This work too argues for a mnemonic role for alpha oscillations during perceptual WM and shows that this role extends beyond the modality and spatial location of the mnemonic items to also include their feature‐specific identity (Fukuda et al., 2016). It remains to be evaluated, however, precisely what aspects of the alpha oscillations (spatial patterns of attenuation, amplification, or both, as well as orthogonal aspects such as changes in peak frequency) contribute to the reconstruction of the memorised stimulus features.
Attenuated alpha states reflect behaviourally relevant prioritisation within perceptual working memory
Over the past decade, it has become clear that attentional selection and prioritisation continue to operate on perceptual representations, even after they have been encoded into WM (Oberauer, 2002; Griffin & Nobre, 2003; Landman et al., 2003; Zokaei et al., 2014; Souza & Oberauer, 2016; Myers et al., 2017). Ample evidence in the perceptual domain suggests that modulations of alpha oscillations may play a key role in such attentional processes (Foxe et al., 1998; Worden et al., 2000; Thut et al., 2006; Jones et al., 2010; van Ede et al., 2011, 2012; Haegens et al., 2011b), with lower amplitude of relevant sensory areas predicting better perceptual performance (e.g. van Dijk et al., 2008; Jones et al., 2010; van Ede et al., 2012). This raises the possibility that, in addition to mnemonic retention, attenuated alpha states may also play a role in mnemonic prioritisation and, accordingly, impact mnemonic performance (at least in cases of perceptual WM).
Support for this hypothesis was recently provided by a number of related studies. The majority of these studies investigated this using retro‐cues (i.e. cues presented during WM retention) that inform which item will (most likely) be probed after an additional delay (see Souza & Oberauer, 2016 for a review of retro‐cueing). As for the lateralised change‐detection tasks described above (where attentional selection occurs during encoding), these studies all report similar lateralisation of alpha oscillations relative to the original location of the retro‐cued item (Spitzer & Blankenburg, 2011; Poch et al., 2014; Myers et al., 2015; Schneider et al., 2015; Wallis et al., 2015; Mok et al., 2016; Wolff et al., 2017). These lateralised alpha modulations do not, however, appear to be exclusive to situations in which mnemonic prioritisation is directed by retro‐cues. They can also be dynamically steered by internally guided temporal expectations, when prioritisation is directed by subjective estimates of elapsed time (van Ede et al., 2017b), or by the expected order in which items will become relevant for visual search (de Vries et al., 2017).
Prioritisation in WM is known to have a profound influence on performance (Souza & Oberauer, 2016 for review). If states of attenuated alpha oscillations indeed support such mnemonic prioritisation (with larger attenuation in populations retaining the prioritised item; as also indicated in Fig. 1), then one would expect such states (at the time of probing) to be predictive of performance on a trial‐by‐trial basis. My colleagues and I recently reported precisely this (van Ede et al., 2017b; see also Poliakov et al., 2014; Backer et al., 2015). In our data, this predictive influence (lower amplitude predicting faster WM access times upon probing) was particularly prominent in sites contralateral to the location of the probed item at encoding (despite the fact that the probe was always presented centrally) and to the interval immediately preceding the probe. Moreover, this influence was particularly prominent on item accessibility, or decision time (as was the influence of our attentional manipulation), and we have now replicated this effect (F. van Ede, S. Chekroud, AC. Nobre, unpublished data). Together, these data suggest that, even when several mnemonic items can all be recalled with high precision, the immediate availability of these items is variable and appears to be tracked by the degree of alpha attenuation in a spatiotopically preserved manner.
Finally, it is noteworthy that the vast majority of studies linking attention to WM (as well as alpha to attention) regard spatial‐based prioritisation mechanisms. It has recently been shown that visual feature dimensions (such as orientation and colour of all items in the array) can also be dynamically up‐ and downregulated during visual WM retention (Ye et al., 2016; Niklaus et al., 2017). Whether alpha modulations also support such feature dimension‐based attentional prioritisation (as reported for the perceptual domain; see Snyder & Foxe, 2010), remains an interesting target for future research.
Alternative accounts to mnemonic retention and prioritisation
While it is tempting to attribute the reviewed work on alpha modulations during WM retention intervals to a mnemonic role, I will argue that such ‘temporal localisation’ to the retention interval is in itself not sufficient to warrant this inference. There are at least four alternative accounts that need to be considered. I go over these below and highlight the available counter‐evidence for each. This serves not only to rule out these alternatives (although there is probably not a single study that can compellingly counter all of them) but also to outline relevant considerations when designing and interpreting future experiments that target mnemonic and attentional roles of neural activity during WM retention.
Mnemonic retention vs. probe anticipation
Ample evidence points to a key role for modulations of alpha oscillations in anticipatory attention of upcoming perceptual events (e.g. Foxe et al., 1998; Worden et al., 2000; Thut et al., 2006; Jones et al., 2010; van Ede et al., 2011, 2012; Haegens et al., 2011b). As WM retention periods are often followed by a perceptual probe stimulus (or a probe display containing multiple items), alpha modulations observed during retention need not reflect retention per se but may reflect probe anticipation instead. This is particularly a concern in tasks in which mnemonic items are probed at their encoded locations (and/or modality), and in which probe displays also scale with load (as is typically the case in the popular visual change‐detection task; as described in Luck & Vogel, 1997; Vogel & Machizawa, 2004). Although probe anticipation may thus account for a fair share of all reports of alpha modulations during WM retention, not all documented alpha modulations during retention can be explained by probe anticipation. For example, alpha modulations scale with load, even when the probe displays are independent of load (as in Jensen et al., 2002; van Ede et al., 2017a) and appear highly specific to the modality of the retained memoranda (e.g. visual vs. somatosensory), even if probe displays are always visual (van Ede et al., 2017a). Also, the lateralisation (relative to the side of the mnemonic items at encoding) of alpha oscillations during WM retention does not appear to rely on lateralised probe anticipation, as this is observed even when items are probed centrally through their colour (van Ede et al., 2017b).
Mnemonic retention vs. lingering of sensory encoding or retro‐cue processing
In the opposite direction of probe anticipation, one must also consider potential lingering of neural responses related to perceptual encoding – as well as to processes of attentional selection during encoding. It is well known that sensory input itself also attenuates alpha oscillations (possibly followed by a rebound) and that such state changes can linger for at least up to a second after this input (e.g. Hari & Salmelin, 1997; Cheyne et al., 2003). Such lingering of encoding responses into the retention interval is particularly problematic when sensory input is not well matched between conditions (such as when different load conditions have different number of items at encoding, or when items are only presented in one sensory modality, but when retention activity is compared between modalities). However, even when sensory input is matched, one is still confronted with potential lingering of the attentional selection (of the mnemonically relevant items) during encoding. There are several arguments against this potential alternative account (in addition to the observation that alpha modulations appear to sustain throughout retention intervals of three or more seconds; e.g. Jensen et al., 2002; van Ede et al., 2017a). Foremost, as reviewed above, alpha modulations also occur following retro‐cues that enable selection of the relevant item(s) only after encoding (but see below). Moreover, it has recently been observed that lateralised modulations of alpha during retention are sensitive to attentional switches between items that occur more than one‐second after encoding and that are guided solely by internal estimates of elapsed time (van Ede et al., 2017b). Finally, if alpha states during retention reflected mere lingering from (attentional selection during) encoding, then, intuitively, mnemonic performance would be best predicted by alpha amplitude during and immediately after encoding. In contrast, this relation appears particularly pronounced immediately prior to the probe (van Ede et al., 2017b; but see Poliakov et al., 2014).
Building on the sensory encoding case, lateralised alpha modulations following retro‐cues may sometimes relate to processing of the retro‐cue itself, as opposed to reflecting shifts in the focus of attention during WM retention. However, the attentional lateralisation of alpha during WM retention appears specific to the modality and location of the retained memoranda, even when the retro‐cue is presented in another modality and does not contain any spatial information (i.e. Spitzer & Blankenburg, 2011) and occurs even when attention is directed via internally guided temporal expectations as opposed to retro‐cues (van Ede et al., 2017b; de Vries et al., 2017).
Mnemonic retention vs. oculomotor behaviour
As was already discovered by Hans Berger, posterior alpha oscillations are profoundly amplified by closing one's eyes (Berger, 1929). This immediately raises the question whether posterior alpha modulations during WM retention may merely reflect modulations in the degree to which the eyes (or pupils) are open. Indeed, closing one's eyes might be another effective (but from a cognitive neuroscience point of view less interesting) way of blocking out potential distracting visual input. If so, these modulations would be expected to be largely confined to posterior alpha oscillations. In contrast, alpha modulations have been described in multiple sensory modalities, where they appear to be selectively modulated depending on the nature of the retained memoranda (e.g. Spitzer & Blankenburg, 2012; van Ede et al., 2017a; Wöstmann et al., 2017). Moreover, potential changes in eye‐opening and pupil size cannot account for lateralised patterns of alpha oscillations relative to the spatiotopic location of the retained and prioritised memoranda (e.g. Sauseng et al., 2009; Spitzer & Blankenburg, 2011; Myers et al., 2015). In the latter case, however, mnemonically driven gaze shifts are a concern instead. Indeed, even when there may be nothing to look at after encoding, participants may still engage in gaze shifts to and between items’ encoded locations (Ferreira et al., 2008; Williams et al., 2013). However, correcting for such gaze shifts appears to preserve alpha lateralisation during WM retention (van Ede et al., 2017b). Moreover, gaze shifts cannot easily account for alpha lateralisation in somatosensory sites during somatosensory WM (Spitzer & Blankenburg, 2011).
Mnemonic retention vs. WM guided interactions with incoming sensations
Although laboratory studies of WM often aim to minimise sensory input during WM retention (such as by having people stare at a blank screen while retaining visual items in WM), it is also evident that it is not possible to deprive participants from sensory input altogether. This raises the possibility that modulations of alpha oscillations during WM retention reflect some interaction between the current content of WM and the (residual) sensory input registered during the retention interval (i.e. WM guided interactions with incoming sensations). In situations in which incoming sensory input can serve WM retention (e.g. the border of a computer monitor may provide a useful spatial reference frame), alpha may attenuate as a result of attentively processing this input. It is debatable, however, whether this constitutes a genuine alternative to a mnemonic interpretation. If we consider that mnemonic retention of perceptual representations may inherently entail a ‘projection’ of these representations onto ongoing perceptual streams (which will be particularly pertinent in the rich sensory environments encountered in everyday situations), then we may wish to attribute the neural signatures of this interaction to WM as well. Still, the degree to which such interactions between WM representations and incoming sensations shape alpha modulations during retention remains a largely unexplored territory (although some first important steps are being made; e.g. de Vries et al., 2017).
Implications and future directions
Alpha oscillations and cognition
On face value, attenuated alpha states in mnemonically relevant sensory areas may appear simply as the flipside of amplified alpha states in mnemonically irrelevant sensory areas. However, whereas the latter may still be attributed to a sensory gating function (albeit in the context of WM), this is not the case for the former. As I have argued, such states of attenuated alpha oscillations in early sensory areas may serve a genuine mnemonic function instead – as has also been argued in the context of long‐term memory retrieval (e.g. Hanslmayr et al., 2012; Staresina et al., 2016; Waldhauser et al., 2016). This bears implications for our understanding of the functional role of attenuated alpha states in cognition. For example, it has recently been argued that such states predominantly reflect an ‘excitability bias’ that merely increases the likelihood of perceiving sensations amidst a noisy background and that may even lead to non‐veridical percepts (Lange et al., 2013; Iemi et al., 2017). In contrast, the reviewed work suggests that such states are also functionally relevant for WM, in cases where the items are clearly registered at encoding. This is the case not only for attenuated alpha states prior to WM encoding (Myers et al., 2014), but, as reviewed throughout, also for such states during the ensuing retention period. Beyond mere excitability biases with a predominant sensory gating function, such states in sensory areas may thus also serve mnemonically relevant computations (I have focused on retention and prioritisation, but this may possibly also extend to computations such as refreshing, updating and manipulating) on previously encoded perceptual representations.
A key open question regards the precise nature of the neural computations associated with (or enabled by) states of attenuated alpha oscillations (as well as their means of carrying over into measurable modulations in extracranial M/EEG). While the answer to this important question remains largely unknown, it is worth pointing out that these neural computations are unlikely to carry only a mnemonic function. Indeed, it is well known that sensory processing also attenuates alpha oscillations and that such attenuation can also be instantiated during the mere anticipation of upcoming processing demands (e.g. Foxe et al., 1998; Worden et al., 2000; Thut et al., 2006; Jones et al., 2010; van Ede et al., 2011, 2012; Haegens et al., 2011b). It is thus likely that, rather than ‘coding’ for the items in WM themselves, such states enable neural computations that facilitate information processing, be it in the context of perception, action, WM, and so on. Influential models posit that alpha oscillations may be the consequence of rhythmic pulses of inhibition in which individual cycles contain only relatively short ‘windows of opportunity’ (when inhibition dies off) for neurons to fire and exchange information (Klimesch et al., 2007; Jensen & Mazaheri, 2010; Jensen et al., 2014; Gips et al., 2016). If so, their attenuation may reflect reduced inhibitory pulsing, creating longer windows of opportunity and enhancing (the capacity for) information processing and transmission. Another, potentially complementary, possibility is that attenuated alpha states are associated with the decorrelation (desynchronisation) of neuronal firing rates (potentially through the segregation into multiple alpha subnetworks at a finer spatial scale), which may increase the coding capacity of the corresponding neuronal population (Zohary et al., 1994; Hanslmayr et al., 2012). Clearly, more work is needed to investigate these possibilities and how they relate to mnemonic as well as other cognitive processes and neural computations.
In future studies, it will also be informative to assess whether states of attenuated (or amplified) alpha oscillations causally contribute to WM retention and prioritisation. This could be achieved by experimentally manipulating these states through, for example, brain stimulation techniques (see Thut & Miniussi, 2009; Thut et al., 2012) and evaluating whether and how this impairs or facilitates memory performance (depending on the location and modality of the mnemonic items as well as the stimulation site, timing and frequency).
Working memory and distractibility
Provided that states of attenuated alpha oscillations have traditionally been associated with increased susceptibility to incoming sensations, the question arises how potentially distracting sensory input is countered in situations in which WM retention involves attenuation of alpha oscillations. This is particularly pertinent for distractors that occupy the same modality and location as the retained memoranda. One implication of the reviewed work may be that the high perceptual resolution gained from retaining items in early sensory areas (putatively through attenuated alpha states) inherently comes at the cost of increased susceptibility to sensory interference from similar material (in line with e.g. Sreenivasan & Jha, 2007; Chumbley et al., 2008; Rademaker et al., 2015). At the same time, the fact that distractors that are perceptually more similar to retained WM representations lead to more interference does not mean that such distraction cannot be overcome at all. Indeed, there is evidence that perceptually similar items may sometimes even receive more inhibition than less similar items (Sreenivasan & Jha, 2007; Kiyonaga & Egner, 2016). Whether and how alpha modulations during retention can support this remains to be investigated and will depend a lot on the degree of spatial and feature specificity with which alpha can be modulated within mnemonically relevant sensory areas.
Another, and perhaps less intuitive, prediction that follows is that even placing an item in the focus of attention (i.e. prioritising an item) may come at the cost of increased susceptibility of this item to sensory interference (for which there is some evidence: Hu et al., 2014; although counter‐evidence is documented too: Makovski et al., 2008; Souza et al., 2016). In future research, it will be interesting to evaluate the extent to which such increased susceptibility to interference is associated with the extent to which alpha oscillations are attenuated (vs. amplified) in the mnemonically relevant sensory areas. It will further be interesting to investigate circumstances under which participants may trade in their high‐fidelity perceptual representations to more abstract representations (putatively gaining distractor resilience at the cost of perceptual resolution; see Lee et al., 2013; Christophel et al., 2017) and to see whether alpha oscillations track such transitions.
Conclusion
I have argued that the association between alpha oscillations and WM is critically shaped by the nature of the memoranda, in conjunction with the source of the alpha oscillations under consideration. When a particular sensory area is not engaged for WM retention, alpha oscillations in this area may be amplified to suppress sensory interference. In contrast, when WM requires the retention of the fine‐grained perceptual representation and engages a particular sensory area, alpha oscillations in this area are attenuated instead. Focusing on the latter case, I have argued that such states of attenuated alpha oscillations serve a genuine and behaviourally relevant mnemonic function. Thus, in addition to a role for such ‘sensory states’ in gating incoming sensations, such states may also support neural computations associated with active retention and dynamic prioritisation in memory. At the same time, I have raised (and where possible countered) four alternatives to a strictly mnemonic account of the available data that, I hope, will also prove useful to consider in designing and interpreting future research.
Conflict of Interest
The author has no conflict of interest.
Author contributions
FvE researched the data and wrote the review.
Supporting information
Acknowledgements
Freek van Ede was supported by a Marie Skłodowska‐Curie Fellowship from the European Commission (code: ACCESS2WM). I also wish to thank Nick Myers, Bernhard Spitzer and Sammi Chekroud for their thoughtful comments and stimulating discussions in the course of preparing this review.
Edited by Ali Mazaheri
Reviewed by Sam Doesburg, Simon Fraser University, Canada; and Kartik Sreenivasan, New York University Abu Dhabi, United Arab Emirates
The associated peer review process communications can be found in the online version of this article.
References
- Awh, E. & Jonides, J. (2001) Overlapping mechanisms of attention and spatial working memory. Trends Cogn. Sci., 5, 119–126. [DOI] [PubMed] [Google Scholar]
- Backer, K.C. , Binns, M.A. & Alain, C. (2015) Neural dynamics underlying attentional orienting to auditory representations in short‐term memory. J. Neurosci., 35, 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, H. (1929) Über das elektroenkephalogramm des menschen. Arch. Psychiat. Nerven., 87, 527–570. [Google Scholar]
- Bonnefond, M. & Jensen, O. (2012) Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr. Biol., 22, 1969–1974. [DOI] [PubMed] [Google Scholar]
- Cheyne, D. , Gaetz, W. , Garnero, L. , Lachaux, J.P. , Ducorps, A. , Schwartz, D. & Varela, F.J. (2003) Neuromagnetic imaging of cortical oscillations accompanying tactile stimulation. Brain Res. Cogn. Brain Res., 17, 599–611. [DOI] [PubMed] [Google Scholar]
- Christophel, T.B. , Klink, P.C. , Spitzer, B. , Roelfsema, P.R. & Haynes, J.D. (2017) The distributed nature of working memory. Trends Cogn. Sci., 21, 111–124. [DOI] [PubMed] [Google Scholar]
- Chumbley, J.R. , Dolan, R.J. & Friston, K.J. (2008) Attractor models of working memory and their modulation by reward. Biol. Cybern., 98, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito, M. & Postle, B.R. (2015) The cognitive neuroscience of working memory. Annu. Rev. Psychol., 66, 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk, H. , Schoffelen, J.M. , Oostenveld, R. & Jensen, O. (2008) Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J. Neurosci., 28, 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede, F. , de Lange, F. , Jensen, O. & Maris, E. (2011) Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha‐ and beta‐band oscillations. J. Neurosci., 31, 2016–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede, F. , Köster, M. & Maris, E. (2012) Beyond establishing involvement: quantifying the contribution of anticipatory α‐ and β‐band suppression to perceptual improvement with attention. J. Neurophysiol., 108, 2352–2362. [DOI] [PubMed] [Google Scholar]
- van Ede, F. , Jensen, O. & Maris, E. (2017a) Supramodal theta, gamma, and sustained fields predict modality‐specific modulations of alpha and beta oscillations during visual and tactile working memory. J. Cognitive Neurosci., 29, 1455–1472. [DOI] [PubMed] [Google Scholar]
- van Ede, F. , Niklaus, M. & Nobre, A.C. (2017b) Temporal expectations guide dynamic prioritization in visual working memory through attenuated alpha oscillations. J. Neurosci., 37, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, M.A. , Albrecht, M.A. , Robinson, B. , Luck, S.J. & Gold, J.M. (2017) Impaired suppression of delay‐period alpha and beta is associated with impaired working memory in schizophrenia. Biol. Psychiat., 2, 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, F. , Apel, J. & Henderson, J.M. (2008) Taking a new look at looking at nothing. Trends Cogn. Sci., 12, 405–410. [DOI] [PubMed] [Google Scholar]
- Foster, J.J. , Sutterer, D.W. , Serences, J.T. , Vogel, E.K. & Awh, E. (2016) The topography of alpha‐band activity tracks the content of spatial working memory. J. Neurophysiol., 115, 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe, J.J. & Snyder, A.C. (2011) The role of alpha‐band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol., 2, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe, J.J. , Simpson, G.V. & Ahlfors, S.P. (1998) Parieto‐occipital approximately 10 Hz activity reflects anticipatory state of visual attention mechanisms. NeuroReport, 9, 3929–3933. [DOI] [PubMed] [Google Scholar]
- Fukuda, K. , Mance, I. & Vogel, E.K. (2015) Alpha power modulation and event‐related slow wave provide dissociable correlates of visual working memory. J. Neurosci., 35, 14009–14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, K. , Kang, M.S. & Woodman, G.F. (2016) Distinct neural mechanisms for spatially lateralized and spatially global visual working memory representations. J. Neurophysiol., 116, 1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins, A. , Smith, M.E. , McEvoy, L. & Yu, D. (1997) High‐resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb. Cortex, 7, 374–385. [DOI] [PubMed] [Google Scholar]
- Gips, B. , van der Eerden, J.P. & Jensen, O. (2016) A biologically plausible mechanism for neuronal coding organized by the phase of alpha oscillations. Eur. J. Neurosci., 44, 2147–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, I.C. & Nobre, A.C. (2003) Orienting attention to locations in internal representations. J. Cognitive Neurosci., 15, 1176–1194. [DOI] [PubMed] [Google Scholar]
- Grimault, S. , Robitaille, N. , Grova, C. , Lina, J.M. , Dubarry, A.S. & Jolicoeur, P. (2009) Oscillatory activity in parietal and dorsolateral prefrontal cortex during retention in visual short‐term memory: additive effects of spatial attention and memory load. Hum. Brain Mapp., 30, 3378–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens, S. , Osipova, D. , Oostenveld, R. & Jensen, O. (2010) Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Hum. Brain Mapp., 31, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens, S. , Nácher, V. , Luna, R. , Romo, R. & Jensen, O. (2011a) α‐Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc. Natl. Acad. Sci. USA, 108, 19377–19382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens, S. , Händel, B.F. & Jensen, O. (2011b) Top‐down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J. Neurosci., 31, 5197–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens, S. , Barczak, A. , Musacchia, G. , Lipton, M.L. , Mehta, A.D. , Lakatos, P. & Schroeder, C.E. (2015) Laminar profile and physiology of the α rhythm in primary visual, auditory, and somatosensory regions of neocortex. J. Neurosci., 35, 14341–14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr, S. , Staudigl, T. & Fellner, M.C. (2012) Oscillatory power decreases and long‐term memory: the information via desynchronization hypothesis. Front. Hum. Neurosci., 6, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari, R. & Salmelin, R. (1997) Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci., 20, 44–49. [DOI] [PubMed] [Google Scholar]
- Harrison, S.A. & Tong, F. (2009) Decoding reveals the contents of visual working memory in early visual areas. Nature, 458, 632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y. , Hitch, G.J. , Baddeley, A.D. , Zhang, M. & Allen, R.J. (2014) Executive and perceptual attention play different roles in visual working memory: evidence from suffix and strategy effects. J. Exp. Psychol. Human, 40, 1665–1678. [DOI] [PubMed] [Google Scholar]
- Iemi, L. , Chaumon, M. , Crouzet, S.M. & Busch, N.A. (2017) Spontaneous neural oscillations bias perception by modulating baseline excitability. J. Neurosci., 37, 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikkai, A. , Blacker, K.J. , Lakshmanan, B.M. , Ewen, J.B. & Courtney, S.M. (2014) Maintenance of relational information in working memory leads to suppression of the sensory cortex. J. Neurophysiol., 112, 1903–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, O. & Mazaheri, A. (2010) Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci., 4, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, O. , Gelfand, J. , Kounios, J. & Lisman, J.E. (2002) Oscillations in the alpha band (9‐12 Hz) increase with memory load during retention in a short‐term memory task. Cereb. Cortex, 12, 877–882. [DOI] [PubMed] [Google Scholar]
- Jensen, O. , Gips, B. , Bergmann, T.O. & Bonnefond, M. (2014) Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends Neurosci., 37, 357–369. [DOI] [PubMed] [Google Scholar]
- Johnson, J.S. , Sutterer, D.W. , Acheson, D.J. , Lewis‐Peacock, J.A. & Postle, B.R. (2011) Increased alpha‐band power during the retention of shapes and shape‐location associations in visual short‐term memory. Front. Psychol., 2, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch, D. & Jensen, O. (2007) Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J. Neurosci., 27, 3244–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S.R. , Kerr, C.E. , Wan, Q. , Pritchett, D.L. , Hämäläinen, M. & Moore, C.I. (2010) Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J. Neurosci., 30, 13760–13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyonaga, A. & Egner, T. (2016) Center‐Surround inhibition in working memory. Curr. Biol., 26, 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch, W. , Doppelmayr, M. , Schwaiger, J. , Auinger, P. & Winkler, T. (1999) ‘Paradoxical’ alpha synchronization in a memory task. Brain Res. Cogn. Brain Res., 7, 493–501. [DOI] [PubMed] [Google Scholar]
- Klimesch, W. , Sauseng, P. & Hanslmayr, S. (2007) EEG alpha oscillations: the inhibition‐timing hypothesis. Brain Res. Rev., 53, 63–88. [DOI] [PubMed] [Google Scholar]
- Kuo, B.C. , Rao, A. , Lepsien, J. & Nobre, A.C. (2009) Searching for targets within the spatial layout of visual short‐term memory. J. Neurosci., 29, 8032–8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman, R. , Spekreijse, H. & Lamme, V.A. (2003) Large capacity storage of integrated objects before change blindness. Vision Res., 43, 149–164. [DOI] [PubMed] [Google Scholar]
- Lange, J. , Oostenveld, R. & Fries, P. (2013) Reduced occipital alpha power indexes enhanced excitability rather than improved visual perception. J. Neurosci., 33, 3212–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.H. , Kravitz, D.J. & Baker, C.I. (2013) Goal‐dependent dissociation of visual and prefrontal cortices during working memory. Nat. Neurosci., 16, 997–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano‐Soldevilla, D. , ter Huurne, N. , Cools, R. & Jensen, O. (2014) GABAergic modulation of visual gamma and alpha oscillations and its consequences for working memory performance. Curr. Biol., 24, 2878–2887. [DOI] [PubMed] [Google Scholar]
- Luck, S.J. & Vogel, E.K. (1997) The capacity of visual working memory for features and conjunctions. Nature, 390, 279–281. [DOI] [PubMed] [Google Scholar]
- Makovski, T. , Sussman, R. & Jiang, Y.V. (2008) Orienting attention in visual working memory reduces interference from memory probes. J. Exp. Psychol. Learn., 34, 369–380. [DOI] [PubMed] [Google Scholar]
- Medendorp, W.P. , Kramer, G.F. , Jensen, O. , Oostenveld, R. , Schoffelen, J.M. & Fries, P. (2007) Oscillatory activity in human parietal and occipital cortex shows hemispheric lateralization and memory effects in a delayed double‐step saccade task. Cereb. Cortex, 17, 2364–2374. [DOI] [PubMed] [Google Scholar]
- Mok, R.M. , Myers, N.E. , Wallis, G. & Nobre, A.C. (2016) Behavioral and neural markers of flexible attention over working memory in aging. Cereb. Cortex, 26, 1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, N.E. , Stokes, M.G. , Walther, L. & Nobre, A.C. (2014) Oscillatory brain state predicts variability in working memory. J. Neurosci., 34, 7735–7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, N.E. , Walther, L. , Wallis, G. , Stokes, M.G. & Nobre, A.C. (2015) Temporal dynamics of attention during encoding versus maintenance of working memory: complementary views from event‐related potentials and alpha‐band oscillations. J. Cognitive Neurosci., 27, 492–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, N.E. , Stokes, M.G. & Nobre, A.C. (2017) Prioritizing information during working memory: beyond sustained internal attention. Trends Cogn. Sci., 21, 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklaus, M. , Nobre, A.C. & van Ede, F. (2017) Feature‐based attentional weighting and spreading in visual working memory. Sci. Rep., 7, 42384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberauer, K. (2002) Access to information in working memory: exploring the focus of attention. J. Exp. Psychol. Learn., 28, 411–421. [PubMed] [Google Scholar]
- Obleser, J. , Wöstmann, M. , Hellbernd, N. , Wilsch, A. & Maess, B. (2012) Adverse listening conditions and memory load drive a common oscillatory network. J. Neurosci., 32, 12376–12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva, S. & Palva, J.M. (2007) New vistas for alpha‐frequency band oscillations. Trends Neurosci., 30, 150–158. [DOI] [PubMed] [Google Scholar]
- Palva, J.M. , Monto, S. , Kulashekhar, S. & Palva, S. (2010) Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc. Natl. Acad. Sci. USA, 107, 7580–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak, T. & Greenlee, M.W. (2005) Working memory in primate sensory systems. Nat. Rev. Neurosci., 6, 97–107. [DOI] [PubMed] [Google Scholar]
- Payne, L. & Sekuler, R. (2014) The importance of ignoring: alpha oscillations protect selectivity. Curr. Dir. Psychol. Sci., 23, 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch, C. , Campo, P. & Barnes, G.R. (2014) Modulation of alpha and gamma oscillations related to retrospectively orienting attention within working memory. Eur. J. Neurosci., 40, 2399–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov, E. , Stokes, M.G. , Woolrich, M.W. , Mantini, D. & Astle, D.E. (2014) Modulation of alpha power at encoding and retrieval tracks the precision of visual short‐term memory. J. Neurophysiol., 112, 2939–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademaker, R.L. , Bloem, I.M. , De Weerd, P. & Sack, A.T. (2015) The impact of interference on short‐term memory for visual orientation. J. Exp. Psychol. Human, 41, 1650–1665. [DOI] [PubMed] [Google Scholar]
- Roberts, B.M. , Hsieh, L.T. & Ranganath, C. (2013) Oscillatory activity during maintenance of spatial and temporal information in working memory. Neuropsychologia, 51, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux, F. & Uhlhaas, P.J. (2014) Working memory and neural oscillations: α‐γ versus θ‐γ codes for distinct WM information? Trends Cogn. Sci., 18, 16–25. [DOI] [PubMed] [Google Scholar]
- Sauseng, P. , Klimesch, W. , Heise, K.F. , Gruber, W.R. , Holz, E. , Karim, A.A. , Glennon, M. , Gerloff, C. et al (2009) Brain oscillatory substrates of visual short‐term memory capacity. Curr. Biol., 19, 1846–1852. [DOI] [PubMed] [Google Scholar]
- Schneider, D. , Mertes, C. & Wascher, E. (2015) On the fate of non‐cued mental representations in visuo‐spatial working memory: Evidence by a retro‐cuing paradigm. Behav. Brain Res., 293, 114–124. [DOI] [PubMed] [Google Scholar]
- Serences, J.T. , Ester, E.F. , Vogel, E.K. & Awh, E. (2009) Stimulus‐specific delay activity in human primary visual cortex. Psychol. Sci., 20, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenhühner, F. , Wang, S.H. , Palva, J.M. & Palva, S. (2016) Cross‐frequency synchronization connects networks of fast and slow oscillations during visual working memory maintenance. Elife, 5, e13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, A.C. & Foxe, J.J. (2010) Anticipatory attentional suppression of visual features indexed by oscillatory alpha‐band power increases: a high‐density electrical mapping study. J. Neurosci., 30, 4024–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza, A.S. & Oberauer, K. (2016) In search of the focus of attention in working memory: 13 years of the retro‐cue effect. Atten. Percept. Psycho., 78, 1839–1860. [DOI] [PubMed] [Google Scholar]
- Souza, A.S. , Rerko, L. & Oberauer, K. (2016) Getting more from visual working memory: retro‐cues enhance retrieval and protect from visual interference. J. Exp. Psychol. Human, 42, 890–910. [DOI] [PubMed] [Google Scholar]
- Spitzer, B. & Blankenburg, F. (2011) Stimulus‐dependent EEG activity reflects internal updating of tactile working memory in humans. Proc. Natl. Acad. Sci. USA, 108, 8444–8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer, B. & Blankenburg, F. (2012) Supramodal parametric working memory processing in humans. J. Neurosci., 32, 3287–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan, K.K. & Jha, A.P. (2007) Selective attention supports working memory maintenance by modulating perceptual processing of distractors. J. Cognitive Neurosci., 19, 32–41. [DOI] [PubMed] [Google Scholar]
- Sreenivasan, K.K. , Curtis, C.E. & D'Esposito, M. (2014) Revisiting the role of persistent neural activity during working memory. Trends Cogn. Sci., 18, 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina, B.P. , Michelmann, S. , Bonnefond, M. , Jensen, O. , Axmacher, N. & Fell, J. (2016) Hippocampal pattern completion is linked to gamma power increases and alpha power decreases during recollection. Elife, 5, e17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut, G. & Miniussi, C. (2009) New insights into rhythmic brain activity from TMS‐EEG studies. Trends Cogn. Sci., 13, 182–189. [DOI] [PubMed] [Google Scholar]
- Thut, G. , Nietzel, A. , Brandt, S.A. & Pascual‐Leone, A. (2006) Alpha‐band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J. Neurosci., 26, 9494–9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut, G. , Miniussi, C. & Gross, J. (2012) The functional importance of rhythmic activity in the brain. Curr. Biol., 22, R658–R663. [DOI] [PubMed] [Google Scholar]
- Tuladhar, A.M. , ter Huurne, N. , Schoffelen, J.M. , Maris, E. , Oostenveld, R. & Jensen, O. (2007) Parieto‐occipital sources account for the increase in alpha activity with working memory load. Hum. Brain Mapp., 28, 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, E.K. & Machizawa, M.G. (2004) Neural activity predicts individual differences in visual working memory capacity. Nature, 428, 748–751. [DOI] [PubMed] [Google Scholar]
- de Vries, I.E.J. , van Driel, J. & Olivers, C.N.L. (2017) Posterior α EEG dynamics dissociate current from future goals in working memory‐guided visual search. J. Neurosci., 37, 1591–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhauser, G.T. , Braun, V. & Hanslmayr, S. (2016) Episodic memory retrieval functionally relies on very rapid reactivation of sensory information. J. Neurosci., 36, 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis, G. , Stokes, M. , Cousijn, H. , Woolrich, M. & Nobre, A.C. (2015) Frontoparietal and cingulo‐opercular networks play dissociable roles in control of working memory. J. Cognitive Neurosci., 27, 2019–2034. [DOI] [PubMed] [Google Scholar]
- Williams, M. , Pouget, P. , Boucher, L. & Woodman, G.F. (2013) Visual‐spatial attention aids the maintenance of object representations in visual working memory. Mem. Cognition, 41, 698–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, M.J. , Jochim, J. , Akyürek, E.G. & Stokes, M.G. (2017) Dynamic hidden states underlying working‐memory‐guided behavior. Nat. Neurosci., 20, 864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden, M.S. , Foxe, J.J. , Wang, N. & Simpson, G.V. (2000) Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha‐band electroencephalography increases overoccipital cortex. J. Neurosci., 20, RC63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöstmann, M. , Lim, S.J. & Obleser, J. (2017) The human neural alpha response to speech is a proxy of attentional control. Cereb. Cortex, 27, 3307–3317. [DOI] [PubMed] [Google Scholar]
- Ye, C. , Hu, Z. , Ristaniemi, T. , Gendron, M. & Liu, Q. (2016) Retro‐dimension‐cue benefit in visual working memory. Sci. Rep., 6, 35573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary, E. , Shadlen, M.N. & Newsome, W.T. (1994) Correlated neuronal discharge rate and its implications for psychophysical performance. Nature, 370, 140–143. [DOI] [PubMed] [Google Scholar]
- Zokaei, N. , Ning, S. , Manohar, S. , Feredoes, E. & Husain, M. (2014) Flexibility of representational states in working memory. Front. Hum. Neurosci., 8, 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


