Abstract
Background
Dietary interventions and cohort studies relating tree nut consumption to blood glucose levels suggest a possible effect of walnuts.
Objective
To examine the associations between walnut consumption and diabetes risk using data from the National Health and Nutrition Examination Survey.
Methods
National Health and Nutrition Examination Survey data on adults conducting 24‐hour dietary recall was pooled across the years 1999 through 2014. Diabetes status or risk was based on self‐report, medication use, fasting plasma glucose levels, and haemoglobin A1c (HbA1c) levels. Individuals were characterized based on reported consumption of walnuts, mixed‐nuts, or no nuts.
Results
After adjustment for covariates, walnut consumers showed lower risk for diabetes compared with non‐nut consumers based on self‐report (odds ratio of 0.47, 95% confidence interval [CI] 0.31‐0.72) as well as fasting blood glucose (relative risk ratio 0.32, CI 0.17‐0.58) and HbA1c (relative risk ratio 0.51, CI 0.27‐0.99). For each standard deviation of increase in walnut intake, prevalence of diabetes dropped 47%. The gender by walnut interaction suggests that the effect may be more potent among women than men (dose response P = .061).
Conclusions
Both among individuals with known diabetes and those diagnosed based on elevated diabetes blood markers, the prevalence of individuals with diabetes was significantly lower among the walnut consumers. A possible gender‐specific effect invites further attention.
Keywords: diabetes mellitus, epidemiology, haemoglobin A1c, NHANES, nut consumption, plasma glucose
Abbreviations
- FPG
fasting plasma glucose
- HbA1c
haemoglobin A1c
- NHANES
National Health and Nutrition Examination Survey
- NN
no nuts
- ON
other nuts
- RRR
relative risk ratio
- WwHC
walnuts with high certainty
- WwON
walnuts with other nuts
1. BACKGROUND
Walnuts have received much attention for their effects on cardiovascular health. Eight clinical trial studies have specifically tested whether the addition of walnuts to the diet under experimental conditions impacts biomarkers of diabetes risk or fasting insulin levels among populations with type 2 diabetes, metabolic syndrome, polycystic ovarian syndrome, or among healthy subjects.1, 2, 3, 4, 5, 6, 7, 8 In these experiments, between 30 and 108 g of walnuts were given per day to a total of 162 females and 132 males for a duration of 4 days to 12 months. Significant decreases were seen in levels of haemoglobin A1c (HbA1c) (P < .0006) and insulin response time (P = .018) among patients with polycystic ovarian syndrome at a given 36 g of walnuts per day.2 Fasting insulin dropped significantly among patients with type 2 diabetes supplemented with 30 g of walnuts per day for 12 months (P = .046).6 This lends support to the hypothesis that the addition of walnuts at a dose of 30 g/day or more may be beneficial in the prevention and control of type 2 diabetes in efficacy trials.2, 6 Further support comes from the use of walnut hydrosol extract to control blood sugar of persons with diabetes mellitus. Physicians found a significant decrease in both average daily blood sugar (ranging from 12 to 31 mg/dL) and the required insulin dose (4‐8 units).9
Recently meta analyses have been published on the overall effects of different food classes on both heart disease and type 2 diabetes10, 11 These studies have found protective effects of nuts/seeds, warranting further research into nuts, and subsequently specific nut studies. Studies of free‐living populations have generally grouped all nuts (including peanuts, a known legume) in their study of associations of nut consumption with diabetes.12, 13, 14, 15 The only epidemiologic study to date that specifically investigated how walnut consumption influenced risk of diabetes found an inverse relationship when controlling for body mass index.14 To further explore the association between walnut consumption and risk of type 2 diabetes, we used data from the National Health and Nutrition Examination Survey (NHANES) (1999‐2014), which represents the US population. We identified diabetic individuals through self report, laboratory glucose, and HbA1c levels commonly used to identify pre‐diabetes or diabetes. These data were used to discern the relationship between walnut consumption as compared with other nuts (ON) and non‐nut consumption and diabetes risk, in the US population.
2. METHODS
2.1. Participants
2.1.1. NHANES sample
The NHANES is a cross‐sectional, probability survey administered to the US civilian population aged from 0 to 85 years. This study used continuous NHANES data collected from 1999 to 2014, covering 8 cycles of data collection (N = 82 091). Participants were excluded from analysis for being under 18 (N = 34 735) and not having data on either day of the 24‐hour food intake (N = 6533). In an attempt to have a control group free of nut consumers, individuals who consumed peanuts, but not ON on the day of recall, were excluded from the analysis (N = 6702). After exclusions, our analytic sample N was 34 121, representing a population size of approximately 176 million individuals. All analyses were weighted using a 16‐year sampling weight created from the 24‐hour dietary recall weights in accordance with the NHANES Analytic and Reporting Guidelines.16
A subset of adults conducted repeat 24‐hour recalls by telephone 3 to 10 days after the baseline dietary assessment. A high level of concordance was noted (~82%) in the walnut consumption classification between both days.
2.2. Measures
2.2.1. Dietary intake interview
Dietary intake interviews were conducted in‐person, in the NHANES mobile examination clinics. The specific types of food as well as the amount of foods consumed were recalled by survey participants. To help in assessing the amount of food consumed, recall cue items such as food charts, measuring cups, and rulers were provided. Survey participants were requested to report all foods and beverages consumed during the past 24 hours from midnight to midnight. National Health and Nutrition Examination Survey dietary data collected from 1999 to 2002 reported individual food intake for 1 day, whereas data collected from 2003 to 2014 reported individual food intake of a subset for 2 days. To maximize our ability to detect walnut consumers, data from both days were used in the 2003 to 2014 data, such that participants reporting walnut consumption in either interview were classified as consumers and gram amounts were averaged between the days. Analyses were conducted using the day 1 dietary interviews weights when a single recall was available.
The NHANES dietary data reports US Department of Agriculture food codes for all foods reported in the individual dietary intake interviews from 1999 to 2014. US Department of Agriculture food codes were used to identify foods that described or included walnuts by searching for the word “walnut” in the food code label descriptors and to identify foods that described or included ON by searching for the words “nut,” “almond,” “macadamia,” “alpine,” “cashew,” “pecan,” “pistachio,” and “granola.”
2.2.2. Walnut consumption groups
The food codes used to identify walnut consumption are listed in Table S1. As in our previous publication on walnut consumption and cognition in NHANES,17 foods consisting entirely or mostly of clearly identifiable walnuts were classified as “walnuts with high certainty” (WwHC) and food codes describing nut mixes and foods that are likely to include walnuts but also ON were classified as “walnuts with other nuts” (WwON) so that 2 levels of confidence were created, the latter being diluted by ON and with estimates of amounts of walnut consumed totalled per individual across all foods consumed in that group. Foods codes describing nuts that are not walnuts (ie cashew nuts and pistachios) or nut‐containing foods that do not commonly contain walnuts (eg granola) were classified as “ON” to allow comparisons of walnut associations with other nut associations. All other foods that did not include walnuts or ON were classified as “no nuts” (NN). Participants were categorized as members of either the WwHC, WwON, ON, or NN groups. Those who consumed at least one WwHC food were categorized to the WwHC group. Participants who did not consume WwHC foods but did consume WwON foods were categorized to the WwON group. Those who did not consume WwHC or WwON foods but did consume other nut foods were included in the ON group. Lastly, participants who did not indicate eating any nut‐containing foods were classified as members of the NN group.
2.2.3. Walnut consumption amounts
To determine the amount of walnuts consumed for each participant of the WwHC and WwON groups, the food item weights (in grams) were totalled. In instances where the food item weight was reflective of more than just walnuts (eg mixed nuts and muffin with nuts), a percentage of the total weight was used. For these items, the percent weight due to walnuts was determined by the investigators based on common recipes.
2.2.4. Diabetes outcomes
Five diabetes definitions were used in this study. Three definitions were based on laboratory results of fasting plasma glucose (FPG) and glycohemoglobin, and 2 definitions were based on questionnaire responses to diabetes questions and medication usage. Multiple definitions were used to capture both subjective and objective diabetes diagnoses and to examine the robustness of our findings.
Self‐reported diabetes
The NHANES questionnaire asked participants if a doctor had ever told them they had diabetes. Responses were dichotomized as diabetic/non‐diabetic, with self‐report “borderline diabetes” collapsed with self‐reported non‐diabetes. Those that reported taking or brought to the interview any of the following: chlorpropamide, diazoxide, glipizide, glyburide, insulin, tolazamide, metformin, acarbose, glimepiride, miglitol, troglitazone, repaglinide, rosiglitazone maleate, pioglitazon, and nateglinide, were classified as a person with diabetes.
Fasting plasma glucose
The NHANES laboratory data from 1999 to 2012 provided FPG values for interviewed participants. In accordance with guidelines by the American Diabetes Association,18 participants with FPG greater than 125 mg/dL were classified as persons with diabetes, participants with FPG greater than 100 mg/dL and less than or equal to 125 mg/dL were classified as borderline diabetes, and participants with FPG less than 100 were classified as persons without diabetes.
Glycohemoglobin
The NHANES laboratory data from 1999 to 2012 also provided data on percentage glycohemoglobin (HbA1c) for interviewed participants. Participants with HbA1c greater than or equal to 6.5% (48 mmol/mol) were classified as persons with diabetes, participants with levels greater than or equal to 5.7% (39 mmol/mol) and less than 6.5% (48 mmol/mol) were classified as borderline diabetes, and participants with levels less than 5.7% (39 mmol/mol) were classified as persons without diabetes.18
Fasting plasma glucose and haemoglobin A1c
A definition combining high levels of both FPG and HbA1c was used to identify a second group of undiagnosed and poorly controlled diabetes. Participants who had diabetes based on either the FPG definition or the A1c definition were classified as having diabetes for the combined definition. Participants were determined to be without diabetes based on both the FPG definition and the A1c definition. Participants who did not classify as having diabetes based on either FPG or A1c level and did not classify as not having diabetes based on both FPG and A1c levels were categorized as borderline diabetes under the combined definition.
2.2.5. Covariates
Covariates used in the adjusted analyses were age, gender, race, years of education, body mass index, alcohol consumption, and levels of physical activity. The upper bound for the age data collected was 85+ years for NHANES years 1999 to 2007 and 80+ years for NHANES years 2008 to 2014. For the purposes of this study, age was winsorized to 80 years to be consistent across all cycles used. To maintain consistency across the NHANES cycles, 4 categories were used for race (non‐Hispanic White, non‐Hispanic Black, Hispanic, and other). Participants categorized as Mexican American and Hispanic were considered Hispanic. Years of education was calculated as a continuous variable ranging from 0 to 16.
In years 1999 to 2006, vigorous physical activity was defined as activity that causes heavy sweating and large increases in breathing or heart rate, while moderate physical activity was defined as light sweating or slight to moderate increase in breathing or heart rate. National Health and Nutrition Examination Survey participants were asked if they did any vigorous or moderate activities for at least 10 minutes over the past 30 days, and were coded based on these answers for the study. In years 2007 to 2012, participants were asked if their work involved vigorous or moderate intensity activity for at least 10 minutes and if they did any vigorous or moderate intensity recreational activities for at least 10 minutes continuously.
Alcohol consumption was analysed as a dichotomous variable to maintain consistency across all NHANES cycles used in this study based on their report of the average number of drinks in the last 12 months.
2.3. Statistical analysis
The associations of walnut consumption with diabetes outcomes were assessed using binary (self‐report diabetes definition) and multinomial (composite self‐report and objective diabetes definitions) logistic regression models. Effects for the walnut consumption groups were based on comparisons to the no nut group. Separate logistic (and multinomial logistic) regression models were built for each of the 5 diabetes outcomes with “non‐diabetes” used as the reference outcome category in each model. Models were additionally adjusted for the aforementioned covariates. Walnut consumption amounts were standardized so that effects are reported per 1 standard deviation of the total walnut grams, which is equivalent to ~1.5 tablespoons of walnuts (a small handful). Walnut consumption amount analyses were conducted among groups reporting any walnut consumption (WwON and WwHC) to assess a dose‐response of walnut consumption. Because the walnut consumption amount analyses represented a smaller subset of the sample, the survey sampling properties (strata and primary sampling units) had insufficient sample size to conduct the variance estimation in 2 of the 118 sampling strata. These 2 strata were excluded from the walnut consumption amount analyses, resulting in a loss of 32 participants. Interactions of walnut consumption groups and amounts with gender and race were additionally explored. All analyses were weighted using the 16‐year NHANES sample day 1 dietary weights and used the NHANES strata and population sampling units to account for the complex survey design. Data management was conducted in SAS version 9.4, The SAS Institute (Cary, NC) and statistical analyses were conducted in Stata version 13.1.19
3. RESULTS
3.1. Description of sample
Characteristics of the 34 121 participants included in the analysis are presented in Table 1. These values reflect application of the appropriate sample weights for the US population over this 15‐year time period. About 51% of the sample was female. About 67% self‐identified as non‐Hispanic White, 12% non‐Hispanic Black, and 14% Hispanic. The majority of participants reported having participated in moderate physical activity within the last 30 days (58%), while a smaller proportion participated in vigorous physical activity within the last 30 days (38%). Diabetes risk among non‐nut consumers differed widely depending upon the measure used: 9.4% based on self‐report, 4.0% based on elevated fasting blood glucose, and 7.3% based on elevated HbA1c levels. These percentages were at least 50% lower among walnut consumers: 4.5%, 1,2%, and 3.2%, respectively (Table 2).
Table 1.
Description of walnut consumptiona and covariates
| Totals, N = 34 121 | Not a Nut Consumerb 77.4% (n = 27 038) | Other Nut Consumerc 10.5% (n = 3 078) | Walnuts With Other Nuts Consumerd 11.9% (n = 3 480) | Walnuts With High Certainty Consumere 2.0% (n = 525) | |
|---|---|---|---|---|---|
|
Grams of walnuts Consumed, Mean ± SD (n) |
4.9 ± 6.9 (4 005) | 3.6 ± 4.0 (3 480) | 12.4 ± 12.4 (525) | ||
| Age, Mean ± SD (n) | 46 ± 17 (34 121) | 45 ± 18 (27 038) | 47 ± 15 (3 078) | 49 ± 16 (3 480) | 54 ± 13 (525) |
| Female, % (n) | 51% (17 115) | 50% (13 141) | 57% (1 773) | 55% (1 866) | 66% (335) |
| White, % (n) | 67% (14 859) | 64% (10 974) | 74% (1 570) | 78% (1 978) | 86% (337) |
| Black, % (n) | 12% (7 468) | 13% (6 318) | 7% (484) | 8% (595) | 5% (71) |
| Hispanic, % (n) | 14% (9 509) | 16% (8 040) | 12% (738) | 8% (658) | 5% (73) |
| Other race, % (n) | 6% (2 285) | 6% (1 706) | 7% (286) | 6% (249) | 5% (44) |
|
Years of education, Mean ± SD (n) |
13 ± 2 (34 121) | 13 ± 2 (27 038) | 14 ± 2 (3 078) | 14 ± 2 (3 480) | 14 ± 2 (525) |
|
Body mass index kg/m2, Mean ± SD (n) |
28 ± 7 (33 537) | 29 ± 7 (26 535) | 28 ± 6 (3 047) | 28 ± 6 (3 438) | 27 ± 5 (517) |
| Ever smoked, % (n) | 24% (7 025) | 27% (6 098) | 16% (422) | 14% (464) | 9% (41) |
| Never consumed alcohol, % (n) | 32% (12 053) | 33% (9 803) | 27% (976) | 29% (1 112) | 27% (162) |
|
Participate in moderate Physical activity, % (n) |
58% (17 851) | 55% (13 396) | 66% (1 895) | 67% (2 191) | 75% (369) |
|
Participate in vigorous Physical activity, % (n) |
38% (11 384) | 36% (8 737) | 43% (1 210) | 40% (1 239) | 41% (198) |
Walnut consumption was determined through self‐report of all food consumed in the past 24 hours.
All individuals whose food codes that did not include walnuts or other nuts were classified as “no nuts” (NN).
Individuals consuming nuts other than walnuts (ie cashew nuts and pistachios) or nut‐containing foods that do not commonly contain walnuts (eg granola) were classified as “other nuts” (ON).
Classified based on food codes describing nut mixes and foods that commonly include walnuts were classified as “walnuts with other nuts” (WwON).
Food codes that clearly indicated walnuts were classified as “walnuts with high certainty” (WwHC).
Table 2.
Description of diabetes outcomes within walnut consumption categories
| No Nuts | Other Nuts | Walnuts with Other Nuts | Walnuts with High Certainty | |
|---|---|---|---|---|
| Self‐report definition a | ||||
| Diabetes, % (n) | 9.4% (3 071) | 8.4% (273) | 9.1% (352) | 4.5% (45) |
| FPG definition b | ||||
| Borderline diabetes, % (n) | 13.7% (4 014) | 12.8% (423) | 14.4% (537) | 14.6% (85) |
| Diabetes, % (n) | 4.0% (1 435) | 2.8% (124) | 3.9% (169) | 1.2% (15) |
| A1C definition c | ||||
| Borderline diabetes, % (n) | 17.6% (5 607) | 19.2% (691) | 18.4% (792) | 21.3% (139) |
| Diabetes, % (n) | 7.3% (2 643) | 4.5% (202) | 6.7% (293) | 3.2% (31) |
| FPG and or A1C definition d | ||||
| Borderline diabetes, % (n) | 25.9% (7 796) | 27.6% (945) | 27.7% (1 100) | 32.3% (188) |
| Diabetes, % (n) | 8.6% (3 080) | 5.3% (238) | 8.5% (358) | 3.8% (38) |
Self‐report classification as diabetic based on being told by a doctor.
Participants with fasting plasma glucose (FPG) > 125 mg/dL classified as diabetic and FPG > 100 mg/dL and ≤125 mg/dL were classified as borderline diabetic.
Glycohemoglobin (haemoglobin A1c, HbA1c) ≥ 6.5% (48 mmol/mol) classified as diabetic and ≥5.7% (39 mmol/mol) and <6.5%(48 mmol/mol) classified as borderline diabetic.
Individuals classified as having high levels on FPG and/or HbA1c.
3.2. Associations of walnut consumption with diabetes outcomes
Table 3 shows the results of the multivariable logistic (and multinomial logistic) regression models of diabetes and walnut consumption. Figure 1 provides a visual comparison of the effect of walnut consumption on diabetes by diabetes definition. For the self‐report diabetes outcome, participants who consumed WwHC foods had half the odds of diabetes (relative risk ratio [RRR] = 0.47 95%, confidence interval = [0.31, 0.72], P < .001) compared with participants who consumed NN, after adjusting for covariates. Additionally, those who consumed ON were 27% less likely to have poorly controlled diabetes relative to participants who consumed NN (RRR = 0.73, P = .006). Among walnut consumers, every standard deviation increase in walnut gram consumption, correlated with a 12% decrease in the odds of self‐reported diabetes, although this was not statistically significant (P = .187). No significant difference in diabetes risk for participants who consumed WwON or ON were noted as compared with participants who consumed NN.
Table 3.
Multivariable associations of walnut consumption with diabetes outcomes
|
Other Nuts Ref = No Nuts |
Walnuts With Other Nuts Ref = No Nuts |
Walnuts With High Certainty Ref = No Nuts |
Grams of Walnutse | |||||
|---|---|---|---|---|---|---|---|---|
| Self‐report definition a | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Ref = Non‐diabetes | ||||||||
| Diabetes | 1.01 (0.82, 1.25) | .930 | 1.01 (0.84, 1.20) | .952 | 0.47 (0.31, 0.72) | <.001 | 0.88 (0.73, 1.06) | .187 |
| FPG definition b | RRR (95% CI) | P | RRR (95% CI) | P | RRR (95% CI) | P | RRR (95% CI) | P |
| Ref = Non‐diabetes | ||||||||
| Borderline diabetes | 0.92 (0.79, 1.07) | .274 | 1.00 (0.88, 1.14) | .949 | 0.98 (0.69, 1.41) | .930 | 0.95 (0.84, 1.07) | .408 |
| Diabetes | 0.78 (0.57, 1.09) | .142 | 1.01 (0.79, 1.30) | .937 | 0.32 (0.17, 0.58) | <.001 | 0.63 (0.47, 0.86) | .003 |
| A1C definition c | RRR (95% CI) | P | RRR (95% CI) | P | RRR (95% CI) | P | RRR (95% CI) | P |
| Ref = Non‐diabetes | ||||||||
| Borderline diabetes | 1.17 (1.01, 1.35) | .033 | 1.00 (0.88, 1.14) | .984 | 1.12 (0.83, 1.51) | .449 | 1.01 (0.89, 1.14) | .863 |
| Diabetes | 0.77 (0.60, 0.98) | .037 | 1.00 (0.81, 1.23) | .981 | 0.51 (0.27, 0.99) | .045 | 0.94 (0.76, 1.16) | .535 |
| FPG and or A1C definition d | RRR (95% CI) | P | RRR (95% CI) | P | RRR (95% CI) | P | RRR (95% CI) | P |
| Ref = Non‐diabetes | ||||||||
| Borderline diabetes | 1.09 (0.96, 1.24) | .179 | 1.05 (0.93, 1.18) | .420 | 1.19 (0.91, 1.57) | .206 | 1.00 (0.89, 1.14) | .938 |
| Diabetes | 0.72 (0.57, 0.92) | .009 | 1.08 (0.89, 1.30) | .433 | 0.50 (0.27, 0.92) | .025 | 0.85 (0.67, 1.08) | .189 |
| RRR, relative risk ratio; OR, odds ratio; CI, confidence interval. | ||||||||
Models adjusted for age, gender, race, body mass index, education, smoking, alcohol use, and exercise.
Self‐report classification as diabetic based on being told by a doctor.
Participants with fasting plasma glucose (FPG) > 125 mg/dL classified as diabetic and FPG > 100 mg/dL and ≤125 mg/dL were classified as borderline diabetic.
Glycohemoglobin (haemoglobin A1c, HbA1c) > 6.5% (48 mmol/mol) classified as diabetic and ≥5.7% (39 mmol/mol) and <6.5%(48 mmol/mol) classified as borderline diabetic.
Individuals classified as having high levels on FPG and/or HbA1c.
Per 1 standard deviation.
Figure 1.
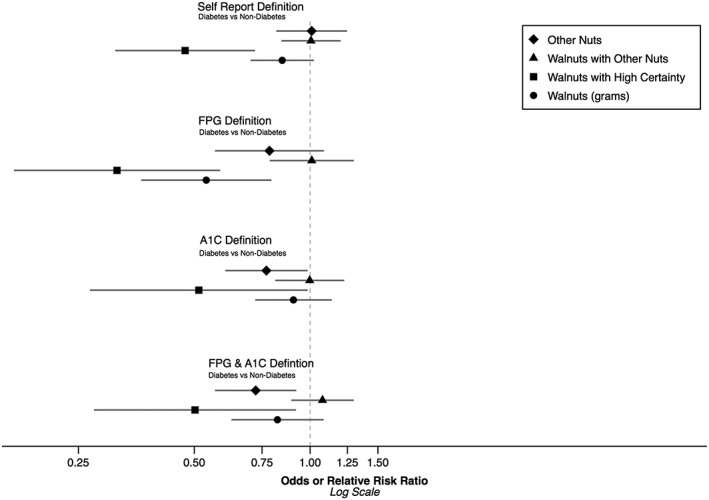
Decreased risk of diabetes among walnut consumers
Using the FPG definition, participants who consumed WwHC showed a 68% lower relative risk of diabetes compared with participants who consumed NN (P < .001). For each standard deviation increase in walnut gram consumption, there was a 37% reduced relative risk of diabetes (P = .003). When assessing diabetes based on the A1c or the combined FPG and A1c definition, participants who consumed WwHC had a 49% and 50% reduced relative risk of diabetes compared with participants who consumed NN respectively (P = .045 and P = .025). However, among the walnut consumers, there was not a significant dose‐response association using the walnut consumption amount for either definition (RRR = 0.94, P = .54 and RRR = 0.85, P = .19).
None of the biochemistry‐based diabetes definitions showed associations between WwON and diabetes prevalence relative to the no nut group. The ON group showed a significant reduction in diabetes prevalence relative to the NN group for both the A1c (RRR = 0.77, P = .037) and combined FPG and A1c definition (RRR = 0.72, P = .009). Associations of walnut or nut consumption with borderline diabetes was not present for any definition except A1c, which showed an increased prevalence of borderline diabetes for other nut consumers relative to the no nut group (RRR = 1.17, P = .033).
Unadjusted analyses, although not presented in Table 3, were conducted and showed results consistent with the magnitude, direction, and statistical significance of our covariate adjusted analyses.
Examination of interactions with gender presented in Table 4 and illustrated in Figure 2 suggests differences between men and women. Among persons consuming WwHC, there is suggestion of a greater effect in women than men. When examined as a dose‐response, the interaction was of borderline significance (P = .061), showing the same trend of females showing lower diabetes prevalence with increasing walnut consumption.
Table 4.
Multivariable interactions with gender
| Walnut Group | Walnut Grams | |
|---|---|---|
| Self‐report definition a | Gender interaction P‐value | Gender interaction P‐value |
| .144 | .061 | |
| FPG definition b | Gender interaction P‐value | Gender interaction P‐value |
| Borderline diabetes | .405 | .706 |
| Diabetes | .633 | .782 |
| A1C definition c | Gender interaction P‐value | Gender interaction P‐value |
| Borderline diabetes | .036 | .747 |
| Diabetes | .692 | .307 |
| FPG and or A1C definition d | Gender interaction P‐value | Gender interaction P‐value |
| Borderline diabetes | .121 | .261 |
| Diabetes | .625 | .368 |
Models adjusted for age, gender, race, body mass index, education, smoking, alcohol use, and exercise.
Self‐report classification as diabetic based on doctor diagnosis.
Participants with fasting plasma glucose (FPG) > 125 mg/dL classified as diabetic and FPG > 100 mg/dL and ≤125 mg/dL were classified as borderline diabetic.
Glycohemoglobin (haemoglobin A1c, HbA1c) ≥ 6.5% (48 mmol/mol) classified as diabetic and ≥5.7% (39 mmol/mol) and <6.5%(48 mmol/mol) classified as borderline diabetic.
Individuals classified as having high levels on FPG and/or HbA1c.
Figure 2.
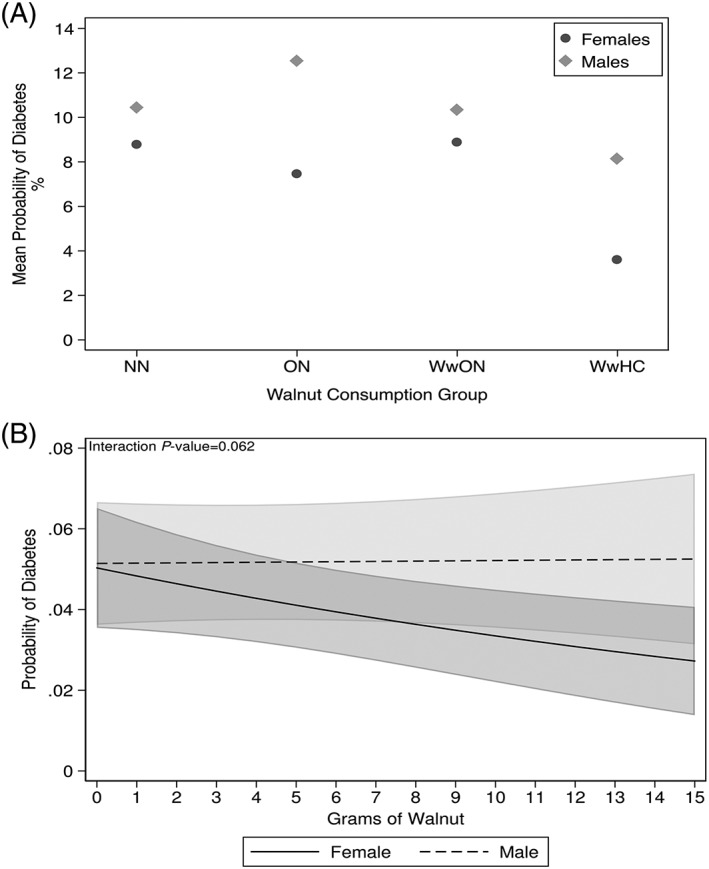
Decreased risk of diabetes in female walnut consumers versus male. Including 95% CIs
4. DISCUSSION
Against a background of meta‐analyses showing heterogeneity in the relationship between nut consumption and diabetes mellitus, we examined this with regard to both nuts in general and walnuts specifically in the US population. Individuals who knew they had diabetes as well as those as yet undiagnosed and those with borderline risk of diabetes were examined.
Previously, the Nurses Health Studies I and II found, after 10 years of follow up, inverse associations between walnut consumption and risk of type 2 diabetes. These were attenuated after adjusting for body mass index.20 Since the participants were all female nurses, external generalizability of the study is limited. In the broader population of NHANES, we also found an inverse association between self‐reported diabetes mellitus and walnut consumption across the entire population. When we stratified by gender, there was indication that the association with walnuts might be greater among women than men.
Additionally, in our NHANES analyses, when FPG levels were used to diagnose diabetes, a strong protective association was noted. This biomarker‐based analysis is less vulnerable to information bias and changes in behaviour related to a known diagnosis as might be the case when individuals change their diet in the hopes of controlling their disease. When elevated A1c was used instead of fasting glucose levels, nut associations were not significant. This is not surprising considering discrepancies between the diagnostic values of A1c levels as compared with plasma glucose levels. An in‐depth assessment of the use of HbA1c for diagnostic purposes found that the standard ADA cutoffs of HbA1c for diabetes and prediabetes exhibited low sensitivity in identifying patients diagnosed using both FPG and 2‐hr glucose.21 Within the NHANES population, the use of HbA1c resulted in large false‐negative rates ranging from 65% to 75%. Misdiagnosis rates increased with age and were disproportionally higher in non‐Hispanic whites and Mexican Americans when based on HbA1c levels as compared with fasting glucose, resulting in potentially biases assessments. The authors concluded that HbA1c values below 6.5% (48 mmol/mol) and 5.7% (39 mmol/mol) do not reliably exclude the presence of diabetes and prediabetes, respectively. This supports the use of fasting blood glucose as the stronger diagnostic tool. As FPG is the marker more closely associated with walnut consumption, we suggest that the association between FPG and walnut consumption is more likely to be of clinical relevance.
Our findings are largely consistent with those of PREDIMED, the randomized trial conducted in Spain, which suggested that a Mediterranean diet supplemented with 30 g/day nuts (50% walnuts, 25% almonds, and 25% hazelnuts) significantly reduced risk the incidence of type 2 diabetes compared with the low‐fat control diet. However, our findings suggest that walnuts might be outperforming ON. The greater the certainty of walnut consumption and the greater the quantity consumed, the stronger the association.
Results from clinical trials, which tested the effect of walnuts on glycaemic control parameters, did not exhibit improvement in diabetic parameters with walnut supplementation.4, 6, 7 In the longest of the 3 studies, lasting 12 months, a significant decrease in plasma insulin was found (P = .046) in the walnut group.6
Two other studies of whether a walnut enriched diet affected individuals with metabolic syndrome found no significant changes in fasting glucose regardless of rather high supplementation of walnut of either 48 or 63 to 108 g/day.1, 5 These studies differed in length (4 days compared with 8 weeks) and study design (crossover versus parallel, respectively). Researchers inferred that weight management is more important than any beneficial effects of walnuts.
Another 8‐week crossover clinical trial, which was performed on healthy Caucasians lasting 8 weeks, did not find any significant differences in fasting glucose, insulin, HbA1c, or HOMA‐IR when a Western diet was enriched with 43 g of walnuts per day.8 A 6‐week‐long dietary intervention among women with polycystic ovarian syndrome did not find differences in fasting glucose, insulin, or HOMA‐IR between the walnut treatment group (receiving 36 g of walnuts per day) and the control group.2 However, the researchers did see a significant (P < .0006) decrease in HbA1c and a faster insulin response time (P = .0182).
One possible mechanism relates to a possbiel walnut‐brain interaction impacting hunger. A recent well‐designed, randomized, placebo‐controlled, double‐blind, cross‐over functional magnetic resonance imaging‐based dietary trial on 10 patients consumed walnuts versus placebo as a smoothie for 5 days. Decreased feelings of hunger and appetite and increased activation of the right insula to highly desirable food cues was found.22 Thus, walnuts may impact appetite and indirectly reduce risk of diabetes. Another theory proposes a role of inflammation and/or oxidative stress in cardiometabolic disorders. A recent cohort study examined the influence of adherence to the Mediterranean diet on oxidative stress and inflammatory biomarkers. In a 10‐year follow‐up, the incidence of diabetes was related to medium and high adherence to a Mediterranean diet, with decrease diabetes risks of 49% and 62% respectively, compared with low adherence. Their results suggest a possible impact of diet on the inflammatory mechanisms causing diabetes.23
The contrasting findings among short‐term clinical trials and effects seen in large free living populations might be explained by either the trial duration, the timing of effect being to late in the course of the disease, or the effect of dietary changes being too subtle in a population that is already heavily treated. Alternatively, in NHANES, walnut consumers may differ in other ways that are not adequately controlled for in the analyses. The largest concern with NHANES is its cross‐sectional nature. Inverse causality might exist due to diagnosed individuals knowing their diagnosis, which may change behaviour. We approached this challenge by using multiple measures of diabetes, some of which were unrelated to knowledge of the condition. Even here, when using fasting glucose, irrespective of self‐report status, we found a significant association. The substantiation in PREDIMED, of lower diabetes incidence under an intervention which included 15 g/day of walnut, adds confidence to a causal assumption.
In the NHANES population, a significant limitation is the intraindividual variation in consumption of specific foods and amounts consumed. The categorization of estimated portion sizes and analysis of nut consumption was based on 24‐hour recall. There is a likelihood of an underestimation of the effect because of the possibility of individuals who normally consume nuts not eating them on the specific report days. As the analysis found a significant relationship even given these limitations, the effect noted is likely diluted by measurement error and may actually be stronger.
Along with the change in beliefs that nuts, being a source of high calories, are undesirable transitioning to belief that nuts are valuable sources of health fats, fibre, nut consumption is changing in the United States. Fortunately, the NHANES data and analyses in this study largely predate this swing in behaviour. The disadvantage, however, is the relatively lower prevalence of walnut consumers in the older rounds of NHANES. Because of this we are unable to examine effects robustly among subgroups. We detect interactions, but our analyses are not powered to adequately explore them.
Another advantage of the older consumption behaviour, which was not likely driven by health awareness, is that consumption is not likely to be biased by disease concerns. This report adds to the growing body of knowledge suggesting positive effects on health of walnut consumption behaviour.
CONFLICT OF INTEREST STATEMENT
The analyses were supported partially by the California Walnut Commission who had, in concurrence with UCLA policy, no role in or influence on the findings, their writing, or publication.
ETHICS STATEMENT
This study involved secondary data analysis of a nationally representative publicly available dataset. The study we conducted was exempt from institutional review for this reason. We have conducted multiple food‐based analyses to determine healthy dietary patterns. This was conducted because of our interest in the value of nuts in the diet in light of their caloric contributions to the diet. We did receive financial support from the California Walnut Commission but in line with UCLA policies they had no involvement in the analyses, interpretation, writing, or editing of the manuscript or publishing of the findings.
Supporting information
Table S1: Walnut Food Codes Used
ACKNOWLEDGEMENTS
The analyses were supported partially by the California Walnut Commission.
Arab L, Dhaliwal SK, Martin CJ, Larios AD, Jackson NJ, Elashoff D. Association between walnut consumption and diabetes risk in NHANES. Diabetes Metab Res Rev. 2018;34:e3031 10.1002/dmrr.3031
REFERENCES
- 1. Brennan AM, Sweeney LL, Liu X, Mantzoros CS. Walnut consumption increases satiation but has no effect on insulin resistance or the metabolic profile over a 4‐day period. Obesity. 18(2009):1176‐1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kalgaonkar S, Almario RU, Gurusinghe D, et al. Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS. Eur J Clin Nutr. 2011;65(3):386‐393. [DOI] [PubMed] [Google Scholar]
- 3. Katz DL, Davidhi A, Ma Y, Kavak Y, Bifulco L, Njike VY. Effects of walnuts on endothelial function in overweight adults with visceral obesity: a randomized, controlled, crossover trial. J Am Coll Nutr. 2012;31(6):415‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma Y, Njike VY, Millet J, et al. Effects of walnut consumption on endothelial function in type 2 diabetes subjects: a randomized controlled crossover trial. Diabetes Care. 2010;33(2):227‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mukuddem‐Peterson J, Stonehouse Oosthuizen W, Jerling JC, Hanekom SM, White Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: a controlled feeding trial. Br J Nutr. 2007;97(6):1144‐1153. [DOI] [PubMed] [Google Scholar]
- 6. Tapsell LC, Batterham MJ, Teuss G, et al. Long‐term effects of increased polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr. 2009;63(8):1008‐1015. [DOI] [PubMed] [Google Scholar]
- 7. Tapsell LC, Gillen LJ, Patch CS, Batterham M. Including walnuts in a low‐fat/modified‐fat diet improves HDL cholesterol‐to‐total cholesterol ratios in patients with type 2 diabetes. Diabetes Care. 2004;27(12):2777‐2783. [DOI] [PubMed] [Google Scholar]
- 8. Wu L, Piotrowski K, Rau T, et al. Walnut‐enriched diet reduces fasting non‐HDL cholesterol and apolipoprotein B in healthy Caucasian subjects: a randomized controlled crossover clinical trial. Metabolism. 2014;63(3):382‐391. [DOI] [PubMed] [Google Scholar]
- 9. Moravej H, Salehi A, Razavi Z, et al. Chemical composition and the effect of walnut hydrosol on glycemic control of patients with type 1 diabetes. Int J Endocrinol Metab. January 2016;14(1):e34726 10.5812/ijem.34726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317(9):912‐924. 10.1001/jama.2017.0947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Micha R, Shulkin ML, Peñalvo JL, et al. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and Meta‐analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS One. 2017;12(4):e0175149 10.1371/journal.pone.%200175149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bullard KMK, Ali MK, Imperatore G, et al. Receipt of glucose testing and performance of two US diabetes screening guidelines, 2007‐2012. PLoS One. 2015;10(4):e0125249 10.1371/journal.pone.%200125249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo K, Zhou Z, Jiang Y, Li W, Li Y. Meta‐analysis of prospective studies on the effects of nut consumption on hypertension and type 2 diabetes mellitus. J Diabetes. 2015;7(2):202‐212. [DOI] [PubMed] [Google Scholar]
- 14. Luo C, Zhang Y, Ding Y, et al. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all‐cause mortality: a systematic review and meta‐analysis. Am J Clin Nutr. 2014;100(1):256‐269. [DOI] [PubMed] [Google Scholar]
- 15. Zhou D, Yu H, He F, et al. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: a systematic review and meta‐analysis of prospective studies. Am J Clin Nutr. 2014;100(1):270‐277. [DOI] [PubMed] [Google Scholar]
- 16. Johnson CL. National Health and Nutrition Examination Survey: analytic guidelines In: Vital and Health Statistics. Data Evaluation and Methods Research; 2013:1‐24. [PubMed] [Google Scholar]
- 17. Arab L, Ang A. A cross sectional study of the association between walnut consumption and cognitive function among adult US populations represented in NHANES. J Nutr Health Aging. March 2015;19(3):284‐290. 10.1007/s12603-014-0569-2 [DOI] [PubMed] [Google Scholar]
- 18. Classification and Diagnosis of Diabetes. Diabetes Care. 2014;38(Supplement 1):S8. [DOI] [PubMed] [Google Scholar]
- 19. StataCorp . College Station, TX: StataCorp LP; 2013. (Stata Statistical Software: Release 13).
- 20. Pan A, Sun Q, Manson JE, Willet WC, Hu FB. Walnut consumption is associated with lower risk of type 2 diabetes in women. J Nutr. 2013;143(4):512‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo F, Moellering DR, Garvey WT. Use of HbA1c for diagnoses of diabetes and prediabetes: comparison with diagnoses based on fasting and 2‐Hr glucose values and effects of gender, race, and age. Metab Syndr Relat Disord. February 10, 2014;12(5):258‐268. 10.1089/met.2013.0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farr OM, Tuccinardi D, Upadhyay J, Oussaada SM, Mantzoros CS. Walnut consumption increases activation of the insula to highly desirable food cues: a randomized, double‐blind, placebo‐controlled, cross‐over fMRI study. Diabetes Obes Metab. 2017;20(1):173‐177. 10.1111/dom.13060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koloverou E, Panagiotakos DB, Pitsavos C, et al. Adherence to Mediterranean diet and 10‐year incidence (2002‐2012) of diabetes: correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metab Res Rev. 2016;32(1):73‐81. 10.1002/dmrr.2672 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Walnut Food Codes Used


