Abstract
Background
Human papillomaviruses (HPVs) are strongly associated with the development of cervical carcinoma, and the distribution of HPV genotypes varies regionally.
Methods
To investigate the distribution characteristics of different genotypes of HPV infection in women in Wuhan, China, a total of 13 775 patients were enrolled over 2 years.
Results
Of these, 2436 patients were infected with HPVs, and the total infection rate was 17.68%. The infection rate of high‐risk HPV (HR‐HPV) was significantly higher (13.96%) than that of single low‐risk HPV (LR‐HPV; 3.72%). Among the HR‐HPV infections, the most common genotype was HPV 52 with an infection rate of 4.23%, followed by HPVs 16, 58, 39, and 51. The most common LR‐HPV genotype was HPV 81, followed by HPVs 6, 11, and 44. Patients under the age of 25 years were found to have the highest HPV infection rate (P < .05). After the age range of 51‐55 years, a downward trend in total HPVs and HR‐HPVs was observed. The HPV infection rate for a single genotype was higher than that for multiple HPVs (P < .01), and the detection rates in summer and winter were significantly higher than those in spring and autumn.
Conclusions
The results demonstrate that the distribution characteristics of various HPV genotype infections are associated with region and age and may be related to season. These data could be the basis for further epidemiological analysis into the control and prevention of HPV infection in this region.
Keywords: genotype distribution, human papillomavirus, infection, membrane‐based flow‐through hybridization technology, surveillance
1. INTRODUCTION
As the second most common malignant tumor in the world,1 cervical cancer is known to be a serious threat to women’s health. In 2012, there were over 500 thousand new cases of cervical cancer diagnosed worldwide and over 26 thousand related deaths.2 Based on DNA sequencing data analysis, more than 200 types of human papillomaviruses (HPVs) have been confirmed, of which 85 genotypes of HPVs are well characterized, and 120 isolates need to be validated as new genotypes.3, 4 According to a report of the International HPV Reference Center, the HPV family comprises 202 different genotypes.5 HPVs preferentially infect the mucosa of the genitals, upper‐respiratory tract, and the skin,6 and they are recognized as important pathogenic factors of cervical cancer and precancerous lesions.6, 7
Based on the association of HPV with cervical cancer or the risk of carcinogenesis, all HPV genotypes can be divided into different subgroups of mucosal HPVs: high‐risk types of HPVs (HR‐HPV), such as HPVs 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82, which are classified as carcinogenic to humans5, 8; low‐risk types of HPVs (LR‐HPV), such as HPVs 6, 11, 28, 32, 40, 42, 43, 44, 54, 55, 57, 61, 62, 71, 72, 74, 81, 83, 84, 86, 87, and 89; probable/possible HR‐HPVs (pHR‐HPV), such as HPVs 68, 26, 53, 66, 82, 70, 67, and 73.5 The HR‐HPVs are the causes of approximately 5% of all cancers in humans and one‐third of all virus‐induced tumors.6 In contrast with HR‐HPV infections, LR‐HPV infections remain unknown, and they are classified as low risk because they have little or no association with cancer.9 HPV infections, especially persistent infections from HR‐HPVs, can lead to cervical intraepithelial neoplasia and cervical cancer10, 11 by remaining in the cervix long enough to cause abnormalities.12, 13, 14
As the primary etiologic agents of cervical cancer are known to be HPVs, this type of cancer and other HPV‐associated malignancies might be prevented or treated by HPV vaccines.15 Previous epidemiological studies have shown that the prevalence of HPV infection and genotype distribution differs among regions,16, 17 and a molecular epidemiological study of HPVs could provide useful data to support reasonable local vaccination strategies.18 There is a great need for more local data on HPV infections and genotype distribution in women of different populations, which can then be used to evaluate the efficacy of the HPV vaccine in different populations19 and contribute to the development of cervical cancer screening.20 China covers a large territory and has many ethnic groups, and the distribution characteristics of HPVs in different regions are not the same. Wuhan is a city in central China with a 2014 population of 10.34 million, and it is one of the largest cities in China. As China’s population is unvaccinated and represents 19% of the total world population,21 a large sample size epidemiological study on HPV infection and subtype distribution in this region should be performed to guide the prevention and treatment of cervical cancer. In this study, a total of 13 775 patients from our outpatient department were identified for a molecular epidemiological survey, which may provide evidence for further etiological diagnosis, treatment and prevention in this region.
2. MATERIALS AND METHODS
2.1. Study population
The participants in this study consisted of 13 775 women who received an annual routine gynecological examination from January 2014 to December 2015 at an outpatient clinic of the Department of Gynecology of Wuhan Medical and Health Center for Women and Children in the city of Wuhan. The patients visited the outpatient clinic for various reasons, including physical examination, infertility, vaginitis, cervicitis, undiagnosed abdominal pain, irregular vaginal bleeding, genital warts, cervical intraepithelial neoplasia and pregnancy. Clinical data were collected from the participants, and a molecular survey of HPVs was conducted. All the women enrolled in this study were required to have a sexual history, and the study excluded those who were pregnant at the time of enrollment, had visited a doctor within the previous 2 months and received cervical physical therapy and hormone treatment, had received oral or vaginal treatment for urogenital infection in the previous 6 months, or had a history of total or partial uterine or cervical resection. Retrospective observation showed that all the participants were between 16‐83 years old, and they were divided into 8 age groups, each covering a 5‐year range.
2.2. Ethical standards
The clinical protocol of this study was approved by the Clinical Research Committee of the Wuhan Medical and Health Center for Women and Children, and the Human Research Advisory Committee of the Tongji Medical College of the Huazhong University of Science and Technology for Medical Sciences. The participants were informed of the protocol objective and procedures, and those interested in participating were asked to sign a written informed consent with the understanding that they could withdraw consent at any time. Prior to enrollment in the study, written informed consent was obtained from all the participants in accordance with the guidelines of the Chinese National Ethics Regulation Committee. The authors assert that all the study procedures were in compliance with the ethical standards of the relevant national and institutional committees on human experimentation and with the World Medical Association’s Declaration of Helsinki of 1975, revised in 2008.
2.3. Specimen collection and management
On the day of sample collection, the participants could not be menstruating, be receiving pelvic radiotherapy, have had vaginal administration or irrigation in the previous 3 days, or have been sexually active within the prior 24 hours. After the cervix uteri were fully exposed with the aid of a vaginal speculum, a specially designed cervical brush (known as a cytobrush) gently scoured the cervix uteri through a series of 5 clockwise rotations to obtain the cervical secretions and cells needed for further testing. The samples were placed in a cell preservation solution and stored at −80°C until HPV DNA extraction and genotyping could be performed. The HPV genotype testing of the samples was completed within a week.
2.4. HPV DNA extraction, PCR amplification and genotyping
The entire detection process included HPV DNA extraction, polymerase chain reaction (PCR) amplification and HPV genotyping. Genomic DNA was first extracted from the collected samples using a DNA extraction kit (Chaozhou Hybribio Biochemical Co., Ltd., Chaozhou, China) according to the manufacturer’s instructions. All the DNA samples were amplified using the Real‐Time PCR system ABI 7500 (Applied Biosystems, USA) using a PCR reagent kit (Chaozhou Hybribio Biochemical Co., Ltd.). HPV genotyping was performed with a HybriMax HPV GenoArray Test Kit (HybriBio Ltd., Chaozhou, China) according to the manufacturer’s instructions.21, 22, 23, 24, 25, 26 Positive and negative controls were run in each PCR analysis process to control for possible contamination and accuracy. This method was used to differentiate 21 genotypes of HPVs, including 14 HR‐HPV types: HPVs 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68; one pHR‐HPV type: HPV 53; 5 LR‐HPV types: HPVs 6, 11, 42, 43, 44; and 81, equivalent to CP8304. In the final step of this process, different genotypes were identified by adding NBT/BCIP solution to display the results, with a clearly visible indigo dot indicating the positive presence of HPV. The position of the HPV genotype probes on the microarray chip determined the result, and multiple dots suggested coinfection or multiple HPV infections.22, 26
2.5. Statistical analysis
All the data were analyzed using SPSS 17.0 software package (GraphPad Software, San Diego, CA, USA). Measurement data such as participant age were presented as the mean ± SD. The chi‐square test was used to compare HPV prevalence across groups. Data were considered to be statistically significant when the P value was <.05.
3. RESULTS
3.1. Overall prevalence of HPV infections
A total of 13 775 samples from consecutively selected study participants who underwent routine molecular biological examination were analyzed by HPV genotyping. The mean age of the study population was 32.98 ± 8.58 years, with an age range from 16 to 83 years. Of the 13 775 women enrolled in the study, 2436 (17.68%) were infected with HPVs, which included 1923 (13.96%) HR‐HPVs (including coinfections) and 513 (3.72%) single infections of LR‐HPVs, with the positive rate of HR‐HPVs being significantly higher than that of LR‐HPVs (P < .05). Of the entire participant population, the numbers of genotype‐positive individuals and their corresponding infection rates, in descending order, are as follows: HR‐HPVs 52, 16, 58, 39, 51, 53, 68, 18, 33, 66, 31, 59, 56, 45, and 35 were 582 (4.23%), 353 (2.56%), 335 (2.37%), 236 (1.71%), 231 (1.68%), 227 (1.65%), 155 (1.13%), 140 (1.02%), 140 (1.02%), 86 (0.55%), 72 (0.52%), 68 (0.48%), 59 (0.43%), and 48 (0.35%), respectively; LR‐HPVs 81, 6, 11, 44, 43, and 42 were 326 (2.43%), 76 (0.62%), 66 (0.49%), 40 (0.29%), 15 (0.06%), and 8 (0.03%), respectively (Figure 1). The results also showed that among all the patients infected with HR‐HPVs, HPV 52 was the most common genotype, followed by HR‐HPVs 16, 58, 81, 39, and 51, while HPV 81 was the most common genotype in LR‐HPVs, followed by HPVs 6, 11, and 44.
Figure 1.
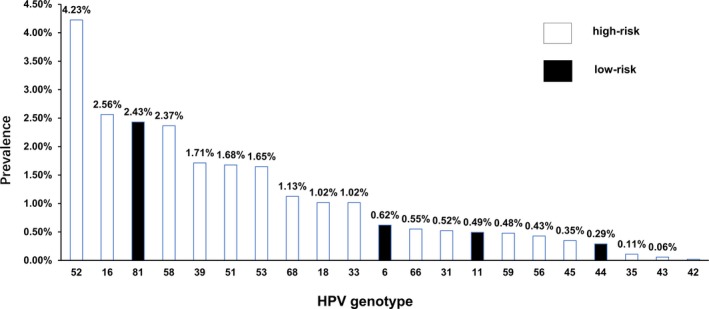
Positive infection rate distributions of different HPV infections
3.2. Distribution characteristics (constituent ratio) of HPV‐positive infections
HPV‐positive individuals had a mix of single and multiple infections from different HPV genotypes, and coinfections included multiple HPV genotypes such as HR‐HPV with HR‐HPV, HR‐HPV with LR‐HPV, and LR‐HPV with LR‐HPV. Among the 2436 patients infected with HPV, constituent ratio analysis showed that the numbers of positive infection with HR‐HPVs 52, 16, 58, 39, 51, 53, 68, 18, 33, 66, 31, 59, 56, 45, and 35 were 582 (21.35%), 353 (12.95%), 326 (11.96%), 236 (8.66%), 231 (8.47%), 227 (8.33%), 155 (5.69%), 140 (5.14%), 140 (5.14%), 76 (2.79%), 72 (2.64%), 66 (2.42%), 59 (2.16%), 48 (1.76%), and 15 (0.55%), respectively (Figure 2A), and LR‐HPVs 81, 6, 11, 44, 43, and 42 were 335 (62.04%), 86 (15.93%), 68 (12.59%), 40 (7.41%), 8 (1.48%), and 3 (0.56%), respectively (Figure 2B).
Figure 2.

Constituent ratio analysis of different genotypes of HR‐HPVs and LR‐HPVs
A review of the constituent ratio of the entire group of HPV‐positive patients showed that HPV 52 was the most common genotype, followed by HR‐HPVs 16, 58, 81, 39, and 51, with HPV 81 ranked first among LR‐HPVs, followed by HPVs 6, 11, and 44 (Figure 2A,B).
3.3. Age‐specific distribution of HPV infection
The HPV‐infected patients, with an average age of 32.98 ± 8.58 years, were divided into the following 8 age groups: ≤25 years, 26‐30 years, 31‐35 years, 36‐40 years, 41‐45 years, 46‐50 years, 51‐55 years, and ≥56 years. The infection rates of these groups were 22.65% (499/2203), 16.27% (725/4456), 15.69% (467/2976), 16.65% (286/1717), 17.61% (199/1130), 20.61% (128/621), 20.05% (71/354), and 19.18% (61/318), respectively (Table 1). Additionally, the rates of positive HPV infection from HR‐HPVs (including single infections of HR‐HPV and multiple infections of HR‐HPV with HPV and HR‐HPV with LR‐HPV) and LR‐HPVs (including single infections of LR‐HPV and multiple infections of LR‐HPV with LR‐HPV) are shown in Table 1 and indicate positive infection rates of HR‐HPVs and LR‐HPVs, ranging from 12.80% (in age group 31‐35 years) to 17.84%, (in age group ≤25 years), and from 2.89% (in age group 31‐35 years) to 5.35% (in age group ≥56 years). Among all 8 age groups, the infection rates of the ≤25 years group were the highest (P < .05). The infection rates of HR‐HPVs were significantly higher than those of LR‐HPVs across the different age groups (in each case, P < .05).
Table 1.
Age‐specific distribution characteristics of HPV infections
| Age group | Average of ages ( ± s) | Total number | Total infection rate | HR‐HPV infection rate | Single LR‐HPV infection rate |
|---|---|---|---|---|---|
| Positive number (positive rate) | Positive number (positive rate) | Positive number (positive rate) | |||
| ≤25 y | 23.37 ± 1.75 | 2203 | 499 (22.65%) | 393 (17.84%) | 106 (4.81%) |
| 26‐30 y | 27.91 ± 1.36 | 4456 | 725 (16.27%) | 572 (12.84%) | 153 (3.43%) |
| 31‐35 y | 32.76 ± 1.40 | 2976 | 467 (15.69%) | 381 (12.80%) | 86 (2.89%) |
| 36‐40 y | 37.76 ± 1.40 | 1717 | 286 (16.65%) | 225 (13.10%) | 61 (3.55%) |
| 41‐45 y | 42.94 ± 1.39 | 1130 | 199 (17.61%) | 152 (13.45%) | 47 (4.16%) |
| 46‐50 y | 47.58 ± 1.43 | 621 | 128 (20.61%) | 98 (15.78%) | 30 (4.83%) |
| 51‐55 y | 52.29 ± 1.30 | 354 | 71 (20.05%) | 58 (16.38%) | 13 (3.67%) |
| ≥56 y | 60.96 ± 5.32 | 318 | 61 (19.18%) | 44 (13.84%) | 17 (5.35%) |
An examination of the distribution trend of HPV infections shows that after one infection peak at the age of ≤25 years, the trend is downward from >25 years to 35 years, followed by an upward trend from age 31 to 50 years (Figure 3). For the period of 46‐56 years, this upward trend in HR‐HPV infections persisted, but the total infection rate and the rate of LR‐HPV infections begin to decline. After the age of 56 years, the infection rate of total HPVs and HR‐HPVs declines, while the rate of LR‐HPVs appears to rise. The rates of HR‐HPV, LR‐HPV, and overall HPV infections in different age groups indicates that once women reach middle age, there is a downward trend in HPV infection, while the infection rates for LR‐HPVs remained fairly stable across the age groups observed (Figure 3). Figure 4 displays the prevalence of the most common 5 genotypes of HR‐HPV infections by age, which indicates that infection rates of HR‐HPVs 16, 39, and 51 increased in the 51‐55 year age group. Notably, of the various genotypes of HR‐HPVs, the infection rate of HPV 16 was found to be the highest (4.70%) in those over 56 years old (P < .05; Figure 4).
Figure 3.
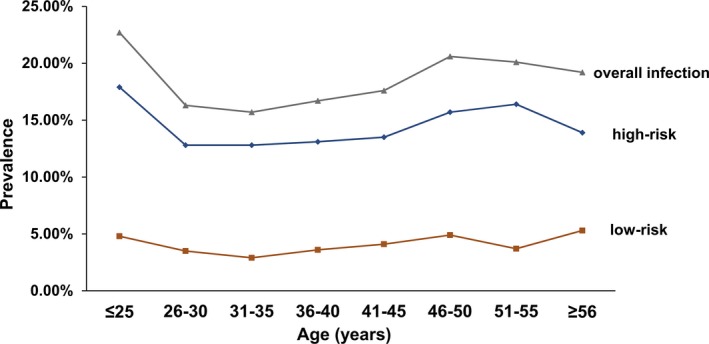
Age‐specific prevalence of HPV infections. Overall, high‐risk and low‐risk infections represent the positive infections of total HPV, HR‐HPV, and LR‐HPVs genotypes, respectively
Figure 4.
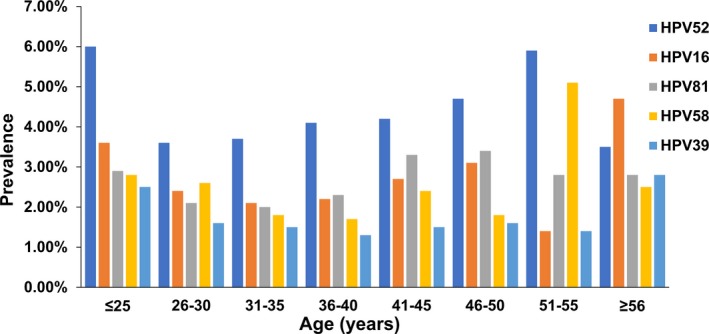
Age‐specific prevalence of the 5 most common HPV genotype infections
3.4. Distribution characteristics of single and multiple infections
The analysis of single and multiple HPV infections across the entire sample of 13 775 subjects (2436 HPV‐positive cases, infection rate of 17.68%) showed that the infection rates of single and multiple HPVs were 13.38% (1843/13 775) and 4.30% (593/13 775), respectively. Among the 2436 HPV‐positive patients, the constituent ratio of single HPV infections (75.66% [1843/2436]) was significantly higher than that of multiple HPV infections (24.34% [593/2436]). In this study, 1‐7 HPV genotypes were detected that involved single, multiple or coinfection, with infection rates of 13.38%, 3.11%, 0.82%, 0.24%, 0.11%, 0.01%, and 0.01%, with corresponding constituent HPV infection ratios of 75.66%, 17.61%, 4.64%, 1.35%, 0.62%, 0.08%, and 0.04%, respectively (Table 2). In actuality, only one case with 7 genotypes of HPV infection was found in this study.
Table 2.
Comparison of the constituent ratios of single and multiple infections of positive HPV infections
| Genotype/genotypes | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Positive number | 1843 | 429 | 113 | 33 | 15 | 2 | 1 |
| Constituent ratio | 75.66% | 17.61% | 4.64% | 1.35% | 0.62% | 0.08% | 0.04% |
Genotype/genotypes represent from 1 to 7 genotypes of HPVs.
Among HR‐HPV infections, the infection rates of single HPVs 52, 16, 39, and 18 were significantly higher than those of multiple genotypes of HR‐HPV infections (in each case, P < .05), and among infection rates of LR‐HPVs, only the multiple infection rate of HPV 11 was significantly higher than its single infection rate (P < .05; Figure 5).
Figure 5.
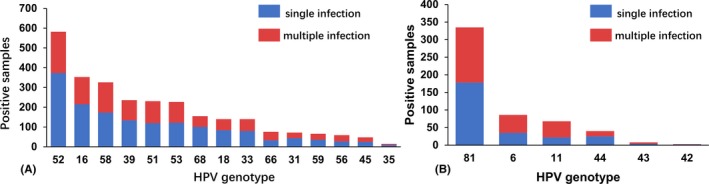
Distribution of single and multiple infections of HR‐HPV/HR‐HPV and LR‐HPV/LR‐HPV genotypes
3.5. Seasonal distribution characteristics
All the participants in this HPV genotyping study were divided by season into spring, summer, autumn, and winter groups of 3292, 3076, 4129, and 3278 women, respectively. The HPV‐positive numbers of the 4 seasonal groups were 507, 559, 740, and 630, with positive detection rates of 15.40%, 18.17%, 17.92%, and 19.22%, respectively (Table 3). Statistical analysis suggests that HPV detection rates in summer and winter are significantly higher than those in spring and autumn (P < .05). Figure 6 shows the ranking of the most common HPV genotypes, including HR‐HPVs and LR‐HPVs, found in women from the different seasonal groups, and these HPV distribution characteristics are clearly similar to those observed year‐round.
Table 3.
Comparison of HPVs infection in different season of 1 year
| Season | Total number | Positive number | Positive rate (%) | HR‐HPV rate (%) | Single LR‐HPV rate (%) |
|---|---|---|---|---|---|
| Spring | 3292 | 507 | 15.40 | 12.19 | 3.21 |
| Summer | 3076 | 559 | 18.17 | 14.21 | 3.96 |
| Autumn | 4129 | 740 | 17.92 | 14.14 | 3.78 |
| Winter | 3278 | 630 | 19.22 | 15.28 | 3.94 |
Figure 6.
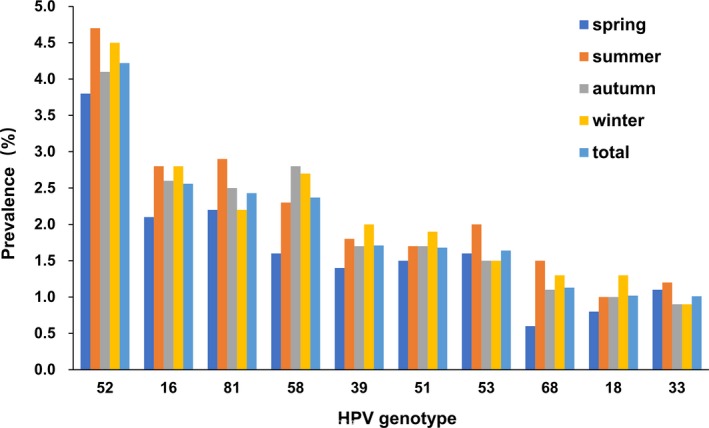
The prevalence of the most common HR‐HVP genotypes in the 4 seasons
4. DISCUSSION
A number of previous studies have confirmed that HPV infection is a causal and necessary factor for cervical cancer,3, 27, 28 and persistent HPV infections in particular are primarily associated with the development of this type of cancer in women.5 A positive HPV DNA test result in women indicates a risk of developing cervical cancer.22 This type of cancer is considered the second most common malignancy in women worldwide and represents 9.8% of all cancer in women.28 To prevent the development of HPV infections in women, HPV vaccines have been made available since 2006 in many countries.29 However, no current vaccine has been developed that considers the distribution and prevalence of the different HPV genotypes, even though these characteristics significantly vary by region.18 Therefore, regional HPV surveillance is needed for the effective vaccination and reasonable prevention of HPV infections, especially HPV‐associated cancer.
With the developments in laboratory medicine and the sensitive detection technology available in clinical practice, multiple infections by different HPV genotypes, including HR‐HPV and LR‐HPV, can be easily and accurately identified, making large public health screening for HPV genotyping in a clinic or health center possible. With its very efficient technique for molecular hybridization, flow‐through hybridization has been recognized as an appropriate tool for HPV genotyping.22, 30, 31 In this study, a newly developed technique that combines flow‐through hybridization and gene chip was used to perform HPV genotyping in women. This method, called HybriMax, can be used to simultaneously identify 21 different HPV genotypes, including 14, 5, and 2 genotypes of HR‐HPVs, LR‐HPVs and unknown‐risk HPVs, respectively. This identification is particularly useful for detecting multiple or coinfections of different HPV genotypes,21, 23, 24, 25 and HybriMax is a reasonable or even ideal technique for performing HPV genotyping in a clinical setting.25
HPV infections, especially persistent infections from HR‐HPVs, are known to cause abnormalities in the cervix12, 13, 14 and lead to cervical cancer.6, 10, 11 Among the multiple HPV genotypes worldwide, the 5 most common were found to be HPVs 16, 18, 31, 58, and 52.28 In this study, the total HPV infection rate was 17.68%, which is consistent with that of Asian states and global statistics, especially the data on developing countries. With regard to HPV genotypes of women of this region, we found that the total infection rate with single HPVs (13.38%) is significantly higher than that with multiple HPVs (4.30%), which indicates that single HPV infection is predominant in the women visiting this metropolitan hospital. However, compared with most common HPV genotypes worldwide, the epidemiological pattern seen in this study is different, with HPVs 52, 16, 58, 81, and 39 ranking as the 5 most common HPVs, which together represent 50% of all HPV infections globally.12 HPV 52 is the most common HR‐HPV genotype, followed by HPVs 16, 58, 39, and 51. These findings are partly consistent with the data on HPV prevalence in Asian regions such as Japan, Taiwan and Shanghai, where the most common HPVs are ranked HPVs 52, 16, 51, 35, and 1811 and HPVs 52, 16, 58, 53, and 33.18 Given that China covers a vast territory, the prevalence patterns differ regionally, even among different regions of mainland China. For example, different prevalence rankings of HPVs are seen in Beijing with HPVs 16, 58, 33, and 3632 and Tianjin with HPVs 16, 58, 18, and 6620 (both are part of Northern China); Shanghai with HPVs 52, 16, 58, 53, and 3316 (Eastern China); Zhejiang Province with HPVs 16, 58, 18, and 5221 (Southern China); Wuhan with HPV 52, followed by HPVs 16, 58, 39, 51, and 53 (Central China), as seen in this study; Yunnan province with geographically and ethnically diverse distribution characteristics33 (Western China). In sum, these regional differences may be due to variation in the ranking of the prevalence estimates of these HPV genotypes by region.
In the present study, the participants ranged in age from 16 to 83 years, with the average age being 32.98 ± 8.58. In addition, women infected with HPV, especially HR‐HPV, were as young as 16 years old, which may indicate that sexual activity in women starts early today. As there is a close relationship between HPV infection and sexual activity,28, 34 and previous analysis has indicated that HPV infection may be acquired relatively quickly after the first sexual intercourse, it is extremely important that females at an early age, even before the start of sexual activity, participate in a school‐based vaccination program.34 In addition, the results showed that HR‐HPV infection can occur in women ranging in age from 16 to 83 years, which indicated that HPV may infect women of all ages.
As this is an epidemiological investigation, composition ratio analysis is necessary, especially for the analysis of the prevalence of positive HPV infections. A total of 2436 cases of HPV infection across 13 775 women were confirmed, among which the infection rates of HR‐HPVs (13.96%) were significantly higher than those of LR‐HPVs (3.72%), indicating that in a large number of individuals, the majority of HPV infections are of HR‐HPVs. Constituent ratio analysis of the different HPV genotypes of the HPV‐positive patients showed that the most common 5 HR‐HPV infections were HR‐HPVs 52, 16, 58, 39, and 51, with infection rates of 21.35%, 12.95%, 11.96%, 8.66%, 8.47%, respectively (Figure 2A). Although LR‐HPVs are suggested to have little or no association with cancers,9 we reported and calculated the data on LR‐HPVs to further investigate the coinfection of HR‐HPV/HPVs with LR‐HPV/HPVs and found that the total infection rates of LR‐HPVs were lower than those of HR‐HPVs, and the infection rate of HPV 81 ranks third of all the HPV genotypes, just after HPVs 52 and 16.
To explore the relationship between HPV infection and women’s ages, previous studies have divided the participants into several groups, usually in 5‐year intervals. These investigations confirmed that the highest HPV prevalence worldwide was in women under 34 years old and that the infection rate decreased in women aged 35‐44 years, followed by an increasing trend for women aged 45‐54 years, except in Asia where infection rates continued to decline.28 Our results show that the prevalence ratio of HPV infection decreased from 20.78% in women aged ≤25 years to the lowest ratio of 14.16% in those aged 31‐35 years and increased to 19.14% in women 46‐50 years old. A so‐called “Asia exception”14 showed a downward trend in infection rates after the age range of 46‐50 years. Some previous studies have suggested that the prevalence of HPV infection is highest in women aged 20‐24 years because of sexual activity,20 which supports our results for women aged ≤25 years. The characteristics of the age‐specific distribution were similar to those seen in the global and Asian HPV infection trends. Compared with the typical upward trend of HPV infection after the age range 46‐50 years globally because of the impaired immune response in women of this age group,28 a downward trend was observed in Asian women, which was named the “Asia exception”. This exception may be due to less frequent sexual activity in Asian women, especially older women.35
Although cervical cancer is a typical aging disease with a starting age from 15 years worldwide, the incidence of this disease was observed to peak in 2 age ranges, 25‐44 years and 75‐79 years, with a 5‐year mortality delay.36, 37 These statistics may explain our results showing that among all the infection rates of the various HR‐HPV genotypes, that of HPV 16 (4.70%) in the age group >56 years was the highest (P < .05; Figure 4) because HPV 16 is one of the most common genotypes associated with cervical cancer,9 and HPVs 16 and 18 are together responsible for 70% of cervical cancers and precancerous cervical lesions.38
With respect to the distribution of HPV infections, both single and multiple HPV infections were detected in this study. As described above, single HPV infections were documented in 76.14% of HPV‐positive women, while multiple infections were observed in 24.86%. The most common HPV genotype associated with multiple or coinfections was HPV 52, followed by HPVs 58 and 16 in HR‐HPVs, and HPV 81 in LR‐HPVs. As the risk of low‐grade squamous intraepithelial lesions (LSIL) was found to increase with the coinfection of HPV 16 and other genotypes,20 the results here may indicate that multiple HPV infections, especially infections involving HR‐HPVs, should receive more clinical attention. The present study also shows that the total, single, and multiple HPV infection rates (15.60%, 7.10%, and 22.65%, respectively) in the ≤25 age group were the highest among all the age groups, which may be because HPV is a sexually transmitted disease, and there is a relatively high frequency of sexual intercourse and a greater number of sexual partners in younger women.18 Notably, while a downward trend in total, single and multiple HPV infection rates was observed in women aged 26‐46 years, those rates in women over 46 years old were maintained at a high level. These 2 infection rate peaks observed in the periods of frequent sexual activity and menopause may be associated with sexual activity, the immune system response and the body’s ability to rid itself of HPVs.28
Although the claim is still controversial that multiple HPV infections are associated with a higher risk of cervical cancer than in women with single HPV infection, a few previous studies have demonstrated that multiple infections significantly increase the severity of neoplasia, particularly for HR–HR‐HPV infections.39 A strong correlation exists between the incidence of multiple HPV infections and precancerous and cervical lesions in women:40 The proportion of multiple infections increased as the severity of the cervical cytological diagnosis increased.41 Furthermore, treatment failure was nearly 5‐fold higher in cases of multiple infections than was that of single infections, and multiple HPV infections correlated more prominently with a lack of treatment than did single type infections.42 Therefore, multiple HPV infections, especially HR‐HR‐HPV infections, demand increased attention as these women are at risk of treatment failure and need reasonable and suitable treatment plans or intervention measures to prevent exacerbation of the infection and even the subsequent carcinogenesis.
Few studies have attempted to epidemiologically investigate this virus‐related disease in association with the impact of different seasons throughout the year. In this study, HR‐HPV infection was the common HPV infection detected across all 4 seasons, and the infection rate of HR‐HPV was significantly higher than that of LR‐HPV in different seasons within the year. Although the distribution of infection rates of HR‐HPVs and LR‐HPVs coincided with that of total HPVs, the detection rates in the summer and winter were greater than those in the spring and autumn. Even we could not provide an appropriate and scientific explanation for the season‐specific prevalence rates, but it may be an interventional factor associated with HPV infection that requires further investigation.
Finally, this study is only a 2‐year analysis of the prevalence of various HPV infection genotypes in women locally. There are a few limitations with respect to the data in this report. We do not have the pathological or cytological analyses of the participants’ cervix. A follow‐up study should be done to track changes in genotype, cervical pathology and cytology as there is a close relationship between cervical carcinoma and long‐term persistent HR‐HPV infections. In addition, we have only conducted statistical analysis of patients admitted to our hospital, and some patients were enrolled based on the reason that they came to the outpatient center, which indicates that these results may not represent the general female population in Wuhan; thus, more detailed data from other hospitals in Wuhan are needed to confirm our findings.
5. CONCLUSIONS
In summary, this study showed a high prevalence of HPV genotypes 52, 16, 58, 39, 51, and 53 in HR‐HPVs, and 81, 6, 11, 44, and 43 in LR‐HPVs in Wuhan, China. There were 2 prevalence peaks of HPV infection among women aged ≤25 and 46‐55 years. Beyond the age range of 51‐55 years, both total and HR‐HPV infection rates decline, while the low infection rate of LR‐HPV remains fairly stable. Rates of single infections were significantly higher than rates of coinfection in almost all single or multiple genotype infections. Over the course of a year, the infection detection rates in the summer and winter were greater than those in the spring and autumn. The data from this study may serve as a baseline for further monitoring and can be used to emphasize the complexity of HPV genotype distribution and the importance of local HPV surveys for vaccination strategies and the national cervical cancer prevention program.
Xiang F, Guan Q, Liu X, et al. Distribution characteristics of different human papillomavirus genotypes in women in Wuhan, China. J Clin Lab Anal. 2018;32:e22581 10.1002/jcla.22581
Funding information
This work was supported by the Open Research Fund Program of the State Key Laboratory of Virology of China (2015KF010), the National Natural Science Foundation of China (81201604) and the Natural Science Foundation of Hubei Province (2012FFB05303, 2012FFB05301).
Feiyan Xiang and Qing Guan equally contributed to this study.
Contributor Information
Chun‐Hui Yuan, Email: Chunhuii.yuen@whu.edu.cn.
Yun Xiang, Email: xiangyun5272008@163.com.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed March 1, 2015. [Google Scholar]
- 3. zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111:581‐587. [DOI] [PubMed] [Google Scholar]
- 4. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boumba LM, Qmichou Z, Mouallif M, et al. Human papillomavirus genotypes distribution by cervical cytologic status among women attending the General Hospital of Loandjili, Pointe‐Noire, Southwest Congo (Brazzaville). J Med Virol. 2015;87:1769‐1776. [DOI] [PubMed] [Google Scholar]
- 6. Ghittoni R, Accardi R, Chiocca S, et al. Role of human papillomaviruses in carcinogenesis. Ecancermedicalscience. 2015;9:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dillner J. Prevention of human papillomavirus‐associated cancers. Semin Oncol. 2015;42:272‐283. [DOI] [PubMed] [Google Scholar]
- 8. Le HH, Bi X, Ishizaki A, et al. Human papillomavirus infection in male patients with STI‐related symptoms in Hanoi, Vietnam. J Med Virol. 2016;88:1059‐1066. [DOI] [PubMed] [Google Scholar]
- 9. Doorbar J. Model systems of human papillomavirus‐associated disease. J Pathol. 2016;238:166‐179. [DOI] [PubMed] [Google Scholar]
- 10. McLaughlin‐Drubin ME, Meyers J, Munger K. Cancer associated human papillomaviruses. Curr Opin Virol. 2012;2:459‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chandra R. Relevance of persistent infection with high‐risk HPV genotypes in cervical cancer progression. MLO Med Lab Obs. 2013;45:40, 42, 44. [PubMed] [Google Scholar]
- 12. Cogliano V, Baan R, Straif K, et al. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. [DOI] [PubMed] [Google Scholar]
- 13. Haverkos HW. Multifactorial etiology of cervical cancer: a hypothesis. MedGenMed. 2005;7:57. [PMC free article] [PubMed] [Google Scholar]
- 14. Castle PE, Rodriguez AC, Burk RD, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;339:b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roden RB, Ling M, Wu TC. Vaccination to prevent and treat cervical cancer. Hum Pathol. 2004;35:971‐982. [DOI] [PubMed] [Google Scholar]
- 16. Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991‐998. [DOI] [PubMed] [Google Scholar]
- 17. Clifford GM, Rana RK, Franceschi S, et al. Human papillomavirus genotype distribution in low‐grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1157‐1164. [DOI] [PubMed] [Google Scholar]
- 18. Gu Y, Yi M, Xu Y, et al. Genotype distribution characteristics of high‐risk human papillomaviruses in women from Shanghai, China. Epidemiol Infect. 2016;144:1482‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stanley M. Prophylactic HPV vaccines: prospects for eliminating ano‐genital cancer. Br J Cancer. 2007;96:1320‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen X, Wallin KL, Duan M, et al. Prevalence and genotype distribution of cervical human papillomavirus (HPV) among women in urban Tianjin, China. J Med Virol. 2015;87:1966‐1972. [DOI] [PubMed] [Google Scholar]
- 21. Xu XX, Zhou JS, Yuan SH, et al. Distribution of HPV genotype in invasive cervical carcinoma and cervical intraepithelial neoplasia in Zhejiang Province, Southeast China: establishing the baseline for surveillance. Int J Environ Res Public Health. 2015;12:10794‐10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tao P, Zheng W, Wang Y, et al. Sensitive HPV genotyping based on the flow‐through hybridization and gene chip. J Biomed Biotechnol. 2012;2012:938780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liao Y, Zhou Y, Guo Q, et al. Simultaneous detection, genotyping, and quantification of human papillomaviruses by multicolor real‐time PCR and melting curve analysis. J Clin Microbiol. 2013;51:429‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teng WQ, Chen XP, Xue XC, et al. Distribution of 37 human papillomavirus types in parotid gland tumor tissues. Oncol Lett. 2014;7:834‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang DY, Yin YP, Feng TJ, et al. HPV infections among MSM in Shenzhen, China. PLoS One. 2014;9:e96364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. An HJ, Cho NH, Lee SY, et al. Correlation of cervical carcinoma and precancerous lesions with human papillomavirus (HPV) genotypes detected with the HPV DNA chip microarray method. Cancer. 2003;97:1672‐1680. [DOI] [PubMed] [Google Scholar]
- 27. Bosch FX, Lorincz A, Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta‐analysis. Lancet Infect Dis. 2007;7:453‐459. [DOI] [PubMed] [Google Scholar]
- 29. Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007‐2013, and postlicensure vaccine safety monitoring, 2006‐2014–United States. MMWR Morb Mortal Wkly Rep. 2014;63:620‐624. [PMC free article] [PubMed] [Google Scholar]
- 30. Pei F, Chen XP, Zhang Y, et al. Human papillomavirus infection in nasal polyps in a Chinese population. J Gen Virol. 2011;92(Pt 8):1795‐1799. [DOI] [PubMed] [Google Scholar]
- 31. Shigehara K, Sasagawa T, Doorbar J, et al. Etiological role of human papillomavirus infection for inverted papilloma of the bladder. J Med Virol. 2011;83:277‐285. [DOI] [PubMed] [Google Scholar]
- 32. Li C, Wu M, Wang J, et al. A population‐based study on the risks of cervical lesion and human papillomavirus infection among women in Beijing, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 2010;19:2655‐2664. [DOI] [PubMed] [Google Scholar]
- 33. Baloch Z, Yuan T, Wang B, et al. Ethnic and geographic variations in HPV prevalence and genotype distribution in north‐western Yunnan, China. J Med Virol. 2016;88:532‐540. [DOI] [PubMed] [Google Scholar]
- 34. Castellsague X, Paavonen J, Jaisamrarn U, et al. Risk of first cervical HPV infection and pre‐cancerous lesions after onset of sexual activity: analysis of women in the control arm of the randomized, controlled PATRICIA trial. BMC Infect Dis. 2014;14:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Addis IB, Van Den Eeden SK, Wassel‐Fyr CL, et al. Sexual activity and function in middle‐aged and older women. Obstet Gynecol. 2006;107:755‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lei T, Mao WM, Lei TH, et al. Incidence and mortality trend of cervical cancer in 11 cancer registries of china. Chin J Cancer Res. 2011;23:10‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hong Y, Zhang C, Li X, et al. HPV and cervical cancer related knowledge, awareness and testing behaviors in a community sample of female sex workers in China. BMC Public Health. 2013;13:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pirek D, Petignat P, Vassilakos P, et al. Human papillomavirus genotype distribution among Cameroonian women with invasive cervical cancer: a retrospective study. Sex Transm Infect. 2015;91:440‐444. [DOI] [PubMed] [Google Scholar]
- 39. Pista A, Oliveira A, Verdasca N, et al. Single and multiple human papillomavirus infections in cervical abnormalities in Portuguese women. Clin Microbiol Infect. 2011;17:941‐946. [DOI] [PubMed] [Google Scholar]
- 40. Sb PAK. Prevalence and distribution of single and multiple HPV infections in cervical cancer and precancerous lesion from Daegu and Gyeongbuk province. Obstet Gynecol Sci. 2008;51:1128‐1136. [Google Scholar]
- 41. Al‐Awadhi R, Chehadeh W, Jaragh M, et al. Distribution of human papillomavirus among women with abnormal cervical cytology in Kuwait. Diagn Cytopathol. 2013;41:107‐114. [DOI] [PubMed] [Google Scholar]
- 42. Munagala R, Dona MG, Rai SN, et al. Significance of multiple HPV infection in cervical cancer patients and its impact on treatment response. Int J Oncol. 2009;34:263‐271. [PubMed] [Google Scholar]


