Abstract
Microdialysis is a method to study the extracellular space in vivo, based on the principle of diffusion. It can be used to measure various small molecules including the neuroregulator adenosine. Baseline levels of the compounds measured with microdialysis vary over studies. We systematically reviewed the literature to investigate the full range of reported baseline concentrations of adenosine and adenosine monophosphate in microdialysates. We performed a meta‐regression analysis to study the influence of flow rate, probe membrane surface area, species, brain area and anaesthesia versus freely behaving, on the adenosine concentration. Baseline adenosine concentrations in microdialysates ranged from 0.8 to 2100 nM. There was limited evidence on baseline adenosine monophosphate concentrations in microdialysates. Across studies, we found effects of flow rate and anaesthesia versus freely behaving on dialysate adenosine concentrations (p ≤ 0.001), but not of probe membrane surface, species, or brain area (p ≥ 0.14). With increasing flow rate, adenosine concentrations decreased. With anaesthesia, adenosine concentrations increased. The effect of other predictor variables on baseline adenosine concentrations, for example, post‐surgical recovery time, could not be analysed because of a lack of reported data. This study shows that meta‐regression can be used as an alternative to new animal experiments to answer research questions in the field of neurochemistry. However, current levels of reporting of primary studies are insufficient to reach the full potential of this approach; 63 out of 133 studies could not be included in the analysis because of insufficient reporting, and several potentially relevant factors had to be excluded from the analyses. The level of reporting of experimental detail needs to improve.
Keywords: adenosine, meta‐analysis, microdialysis, systematic literature review
Abbreviations used
- AMP
adenosine monophosphate
- anova
analysis of variance
- ATP
adenosine triphosphate
- DNA
deoxyribonucleic acid
- EROS
Early Review Organizing Software
- HPLC
high pressure liquid chromatography
- RNA
ribonucleic acid
- SD
standard deviation
- SEM
standard error of the mean
- SR
systematic literature review
Microdialysis is a simple and elegant method to study biological processes in the extracellular space in vivo. It is based on the principle of diffusion; molecules diffuse through a semi‐permeable membrane into a continuously flowing isotonic fluid. Sampling at regular intervals (minutes) allows the investigator to monitor concentrations of the molecule of interest over time (hours–days). Microdialysis can be used to measure various small molecules including neurotransmitters. Besides by biological variation, concentrations of these molecules in the dialysate are affected by, for example, flow rate, membrane type and membrane surface area (Westerink and Cremers 2007). Concentrations therefore can vary substantially between laboratories, but also between individual animals within groups.
Adenosine is a purine nucleoside that is a building block for deoxyribonucleic acid and ribonucleic acid. As a main metabolite of adenosine triphosphate, it is also involved in the cellular energy metabolism. Besides, it is a neuroregulator with specific receptors in the brain, which can be antagonized by caffeine. Many studies have shown that adenosine is involved in the sleep–wake regulation (e.g. Basheer et al. 1999; Blanco‐Centurion et al. 2006; Sharma et al. 2010b; Vazquez‐DeRose et al. 2014). The first report of adenosine being analysed by microdialysis appeared in 1982 (Zetterstrom et al. 1982). So far, an overview of the variation in baseline dialysate concentrations of adenosine is lacking. Such an overview could improve comparisons between laboratories. This could benefit the design of future primary studies.
Systematic literature review (SRs) are common practice in clinical research and are considered to provide the highest level of evidence (Hooijmans et al. 2010a). Most clinical SRs determine the effectiveness of an intervention. In animal research, SRs are still less common, and most animal SRs focus on specific interventions or descriptions of the available animal models. While SRs of research techniques used in animal studies are even less common, an SR is the optimal method to acquire a complete overview of the past use of a technique.
SRs differ from narrative reviews by using a thorough transparent methodology to retrieve, select and analyse all relevant papers for the review (de Vries et al. 2014b). While many interesting narrative reviews on microdialysis are available (refer to the microdialysis handbook by Westerink and Cremers (2007) for an excellent selection), only few SRs of the microdialysis technique have been performed to date. Examples comprise cocaine‐induced dopamine increases in the nucleus accumbens (Frank et al. 2008); ethanol‐induced alterations in glutamate and GABA (Fliegel et al. 2013) and monoamines (Brand et al. 2013); and serotonin neurotransmission after selective serotonin reuptake inhibitors (Fritze et al. 2017).
To provide evidence at an integrated level on the observed range of baseline adenosine levels in microdialysates, we performed an SR. The nucleotide adenosine monophosphate (AMP) can be measured simultaneously with adenosine because of close chemical resemblance (Ballarin et al. 1989). Because we expected many studies to report both compounds, we extended our review to include AMP concentrations.
Microdialysis reflects changes in the extracellular concentrations of the analysed molecules, but does not inform us of their exact concentrations, because the recovery of the compound of interest is < 100% (Westerink and Cremers (2007)). In this SR, we do not aim to estimate the actual in vivo concentrations. We restrict ourselves to providing a complete overview of concentrations in brain dialysates. Besides, to study the influence of potential explanations for differences in baseline concentrations, such as flow rate, probe membrane surface, species, brain area and anaesthesia versus freely behaving, we conducted a meta‐regression analysis.
Materials and methods
On 25 January 2016, we registered the protocol (Van der Mierden et al. 2016) for an SR addressing the research question: ‘What is the reported range of concentrations of adenosine and AMP in intra‐cerebral microdialysates?'. The protocol describes the used methodology for this SR and is compliant with the Transparency and Openness Promotion (TOP) guidelines. It is available on the protocol section of the SYRCLE website (http://www.SYRCLE.nl).
Search and selection
We developed an extensive search strategy for finding all relevant animal studies on adenosine or AMP using microdialysis for three databases: PubMed, EmBase and Web of Science. The search strategies are described in our protocol (Van der Mierden et al. 2016). For PubMed and EmBase, we used the previously published filters for animal studies (Hooijmans et al. 2010b; de Vries et al. 2014a). For Web of Science no animal filter was used, as no validated animal filter is available. Searches were performed on 25 January 2016. Duplicates and triplicates were removed manually. Reference lists of included papers were checked for additional papers.
Screening of titles and abstracts was performed in Early Review Organising Software (Institute of Clinical Effectiveness and Health Policy, Buenos Aires, Argentina) by two independent reviewers (SvdM and CHCL). Screening of full‐text papers was performed by three independent reviewers (SvdM, SAS and CHCL); each paper was checked for eligibility by two of these three reviewers. Discrepancies were resolved by discussion among the reviewers. We excluded studies on other techniques than microdialysis (e.g. biosensors and studies using precursors to microdialysis such as ventricular, cortical cup and push‐pull perfusion), studies measuring other substances than adenosine or AMP (e.g. cyclic‐AMP (Mork and Geisler 1994) and adenosine metabolites (Salem and Hope 1999), extra‐cerebral microdialysis studies, human and in vitro studies and (in the full‐text screening phase only) papers not containing primary study data. We also excluded retro‐dialysis studies that did not report baseline values without adenosine in the perfusion fluid. We included primary studies measuring baseline AMP and/or adenosine by microdialysis in animals other than humans, also when they did not report their baseline data.
Study characteristics and quality assessment
We extracted data on the animal model (e.g. species and sex), the technique (microdialysis; e.g. flow rate and probe length) and two outcome measures: baseline concentration of adenosine and AMP in microdialysate. Study characteristics were tabulated (Appendices S1–S3). The full list of extracted study characteristics is provided in our protocol (Van der Mierden et al. 2016). To obtain an indication of the average reporting quality of the included studies, percentages of reported study characteristics were determined per paper.
Data analysis
In this paper, concentrations are given in nM. We converted concentrations to nM where necessary. When papers reported correction for recovery, we converted the concentrations back to the concentrations in dialysate. If concentration data were only available graphically, Universal Desktop Ruler (AVPSoft, https://avpsoft.com/products/udruler/) was used to estimate the values. Probable typographical errors in the dose of the anaesthetic (Berman et al. 2000) and the flow rate (McKenna et al. 2007) were corrected. Probable typographical errors in Nomoto et al. (1999) and Nomoto et al. (2000) could not be corrected, and these data were excluded from Table 1 and subsequent analyses. Data from (Masana et al. 1989) and (Tominaga et al. 1992) were extracted as far as possible without understanding Japanese. All other included papers were in English. Of the corrected papers, only the one by Berman et al. (2000) was included in the meta‐regression. We did not perform a sensitivity analysis excluding these data.
Table 1.
Baseline adenosine in dialysates from lowest to highest concentration
| Study reference | Adenosine (nM) | SEM (nM) |
|---|---|---|
| Masana et al. (1989) | 0.8 | 0.1 |
| Savelyev et al. (2012) | 1.28 | 0.08 |
| Kalinchuk et al. (2011)A | 2.3 | 0.7 |
| Wigren et al. (2007) | 4.3 | 0.4 |
| Nagel and Hauber (2004) | 4.8 | 0.38 |
| Kalinchuk et al. (2011)B | 6 | 2 |
| Okada et al. (2003) | 6 | 1.7 |
| Carrozzo et al. (2012) | 7 | 7 |
| Lehmann et al. (1987) | 10 | 9 |
| Van Wylen et al. (1986) | 10 | |
| Nagel and Hauber (2002) | 10.51 | 2.61 |
| Melani et al. (2012)A | 11.72 | 0.2 |
| Ballarin et al. (1991)A | 12 | 0.9 |
| Basheer et al. (1999) | 12.1 | 2.3 |
| Melani et al. (1999a) | 13 | 1 |
| Fu et al. (2015) | 13.1 | 1.5 |
| Melani et al. (2012)B | 13.15 | 0.4 |
| Ballarin et al. (1991)B | 14 | 2 |
| Gianfriddo et al. (2003) | 14 | 1 |
| Pazzagli et al. (1995)A | 15.5 | 0.2 |
| Matsumoto et al. (1992)A | 16 | |
| Pazzagli et al. (1995)B | 16.6 | 0.2 |
| Pinna et al. (2002) | 17 | 2 |
| Song et al. (2012)A | 18 | 3 |
| Melani et al. (2003) | 19 | 2 |
| Bennett et al. (2003)A | 20.3 | 3.76 |
| Nelson et al. (2009)A | 20.9 | 2.5 |
| Gianfriddo et al. (2004)A | 23 | 2 |
| Melani et al. (1999b) | 25 | 2 |
| Bennett et al. (2000) | 25–100 | |
| Sharma et al. (2010a) | 26.9 | 9.4 |
| Song et al. (2012)B | 28 | 5 |
| Lutz and Kabler (1997) | 29–73 | 4–9 |
| Lydic et al. (2010) | 29.9 | 4.4 |
| Butcher et al. (1987) | 30 | 10 |
| Porkka‐Heiskanen et al. (1997) | 30 | 9.5 |
| Maysinger et al. (1992)B | 31 | 21 |
| Gianfriddo et al. (2004)B | 32 | 3 |
| Porkka‐Heiskanen et al. (2000) | 32.8 | 3 |
| Matsumoto et al. (1992)B | 33 | |
| Pazzagli et al. (1994) | 34.9 | 3 (?) |
| Bennett et al. (2003)B | 39 | 7 |
| Mijangos‐Moreno et al. (2014)B | 39 | 4 |
| Moss et al. (1995) | 40–190 | |
| Nelson et al. (2009)B | 42 | 6.3 |
| Andine et al. (1990) | 47 | |
| Hagberg et al. (1987) | 49.9 | 9.1 (?) |
| Dux et al. (1990)A | 51 | 6 |
| Scheller et al. (1996) | 52 | 6 |
| Aden et al. (2004) | 53 | 14 |
| Grabb et al. (1998)D | 60 | 10 |
| Herrera‐Marschitz et al. (1994)A | 60 | 18 (?) |
| Koos et al. (1997) | 65 | 12 |
| Miranda et al. (2014) | 67 | 3 |
| Murillo‐Rodriguez et al. (2004)B | 67 | 1 |
| Kjellmer et al. (1989)B | 68 | 7 |
| Perez‐Pinzon et al. (1993) | 68 | 29 |
| Chen et al. (1993) | 70 | |
| Herrera‐Marschitz et al. (1994)B | 70 | 15 (?) |
| Murillo‐Rodriguez et al. (2008) | 70 | 4.22 |
| Berman et al. (2000)B | 69.7 | 9.8 |
| Berman et al. (2000)C | 69.8 | 5.2 |
| Zhu et al. (2001)B | 71.7 | 84.9 |
| Dux et al. (1990)B | 72.8 | 11 |
| Park et al. (1988) | 80 | |
| Berman et al. (2000)D | 81.7 | 14.6 |
| Kaku et al. (2001) | 82.3 | 3 |
| Peigen and Jing (1997)A | 83 | 9 |
| Peigen and Jing (1997)B | 84 | 6.9 |
| Peigen and Jing (1997)C | 87 | 7.3 |
| Deng et al. (2003) | 87–360 | |
| Morimoto et al. (1991) | 90 | 15 |
| Skarphedinsson et al. (1989) | 90 | |
| Chen et al. (1992) | 100 | 10 |
| Hillered et al. (1989) | 100 | 0 |
| Kaku et al. (1994)A | 100 | 20 |
| Kaku et al. (1994)B | 100 | 20 |
| Dobolyi et al. (1997) | 100–300 | |
| Berman et al. (2000)A | 106.1 | 25.9 |
| Kjellmer et al. (1989)A | 107 | 19 |
| Maysinger et al. (1992)A | 107 | 23 |
| Chen and Stone (1991) | 110 | 10 |
| Ruth et al. (1993) | 110 | 5.8 |
| Schulte et al. (2004) | 110 | 45 |
| Murillo‐Rodriguez et al. (2004)A | 112 | 2 |
| Mijangos‐Moreno et al. (2014)A | 120 | 2 |
| Mijangos‐Moreno et al. (2015) | 120 | 3 |
| Watson et al. (1999) | 120 | 17.4 |
| Valtysson et al. (1998) | 130 | |
| Britton et al. (1999)A | 140 | |
| Ballarin et al. (1989) | 144 | 60 |
| Kim et al. (2010) | 165 | 19.7 |
| Carswell et al. (1997) | 184 | 10.5 |
| Britton et al. (1999)B | 190 | |
| Yan et al. (1995) | 190 | 40 |
| Ballarin et al. (1987) | 200 | 30 |
| Gidday et al. (1996) | 200 | 28 |
| Grabb et al. (1998)A | 200 | |
| Headrick et al. (1994) | 200 | |
| Cui et al. (2013) | 204 | 18.5 |
| Blanco‐Centurion et al. (2006) | 216 | 57 |
| Cui et al. (2016) | 230 | 9.5 |
| Pazzagli et al. (1993)A | 240 | 30 (?) |
| Dobolyi et al. (1998) | 285 | 8 |
| Grabb et al. (1998)B | 300 | 100 |
| Li et al. (2011)A | 310 | |
| Li et al. (2011)B | 320 | |
| Pazzagli et al. (1993)B | 330 | 20 (?) |
| Zhang and Niu (1994) | 330 | 50 |
| Zetterstrom et al. (1982) | 340 | |
| Kondoh et al. (1999) | 390 | 90 |
| Richter et al. (1999) | 390 | 14.4 |
| Grabb et al. (1998)C | 400 | |
| Sciotti and Van Wylen (1993a) | 420 | 30 |
| Dohmen et al. (2001)A | 480 | |
| Dohmen et al. (2001)B | 480 | |
| Li et al. (2011)C | 480 | 70 |
| Materi and Semba (2001) | 480 | |
| Sciotti and Van Wylen (1993b)A | 530 | 70 |
| Sciotti and Van Wylen (1993b)B | 570 | 69.3 |
| Sciotti et al. (1992) | 600 | 100 |
| Zhu et al. (2001)A | 619.9 | 212.2 |
| Northington et al. (1992) | 636.5 | 53.6 |
| Dobolyi et al. (1999)A | 650 | 140 |
| Nilsson et al. (1990)A | 730 | |
| Nilsson et al. (1990)B | 730 | |
| Nilsson et al. (1990)C | 730 | |
| Dobolyi et al. (1999)B | 740 | 100 |
| Tominaga et al. (1992) In Japanese) | 800 | 160 (?) |
| Slezia et al. (2004) | 950 | 210 |
| Dobolyi et al. (2000) | 970 | 40 |
| Sciotti et al. (1993)A | 1600 | |
| Sciotti et al. (1993)B | 2100 | 350 |
Capital letters after the year of publication within the literature reference indicate separate subgroups within the same paper. Lower case letters indicate separate papers. Question marks indicate uncertainty on the reported value being an SEM or an SD.
When papers reported several subsequent baseline concentrations with the corresponding standard error of the mean (SEM) or standard deviation (SD), the last baseline measurement (i.e. before starting treatment) with the corresponding SEM was included in Table 1. If only a single mean value or a baseline range was presented, this was included in Table 1. When a range of adenosine concentrations was given, we report the lower and upper boundary of the range. For papers describing several separate experimental groups, the groups were treated as independent experiments (indicated by capital letters in the study‐ID), unless only overall average baseline concentrations were provided.
In microdialysis studies, several brain regions can be measured simultaneously within the same animal. When the same brain region was sampled on both sides of the brain, an average adenosine concentration and the corresponding SEM was calculated; we conservatively assumed a correlation of one between both sides, and calculated the mathematical average. (Borenstein et al. 2009). When different brain regions were measured within the same animal, we treated the brain region as the experimental unit (instead of the animal). That is, if adenosine was measured in multiple brain regions (partially) within the same animals, values are reported for all brain regions separately, and included in the meta‐regression analysis. Six papers reported data from two brain regions that were (partially) measured within the same animals.
To determine if the sample of studies included in our meta‐regression was representative of the full sample of included studies, baseline adenosine levels were compared between included and excluded studies with the non‐parametric independent samples Mann–Whitney U test, using SPSS (IBM Corp. IBM SPSS Statistics for Windows. Armonk, NY, USA) Boxplots were also created in SPSS.
Meta‐regression
While writing the protocol, we did not anticipate sufficient data to be available for a meta‐regression. Therefore, the analysis described below was designed after finishing the data‐extraction, and thus was not pre‐specified. Meta‐regression analysis was performed in R version 3.4.2 (R_Core_Team 2016) with the metafor package (Viechtbauer 2010), using the rma function (knha = TRUE). We used the method suggested by Higgins et al. to transform the reported average adenosine concentrations and corresponding SDs to account for the skew of the data (Higgins et al. 2008).
The following moderator variables were included: probe surface area (estimated by probe length × π × probe diameter), flow rate, species, brain area and anaesthesia versus freely behaving. The analysis of variance function was used to test for overall effects of categorical moderators. Because several brain regions had only been measured in a single study, different brain areas were pooled for the analyses: the basal ganglia were pooled with the basal forebrain; nuclei in the brainstem were pooled as such; thalamic and subthalamic nuclei were pooled as thalamus; and all cortical regions were pooled as cortex. As we could not include post‐surgical recovery period in our analyses, we show the baseline adenosine concentrations by recovery period in a boxplot. Because dialysis experiments mostly start either after 24 h or after 1 week of recovery, we plotted studies with recovery periods ≤ 24 h next to those with periods > 24 h.
A sensitivity analysis was performed in which we replaced SDs with a value below 4 by the value 4 (nM).
Results
Search and selection
Our search in PubMed retrieved 539 references, that in Embase 616 and that in Web of Science 758. After removal of duplicates, 981 references remained for title‐abstract screening. Of these, 196 were screened full‐text, and 128 were included in this review. From the reference lists of the included papers, we identified another four papers on intracerebral microdialysis of adenosine. An overview of the flow of papers is provided in Fig. 1.
Figure 1.
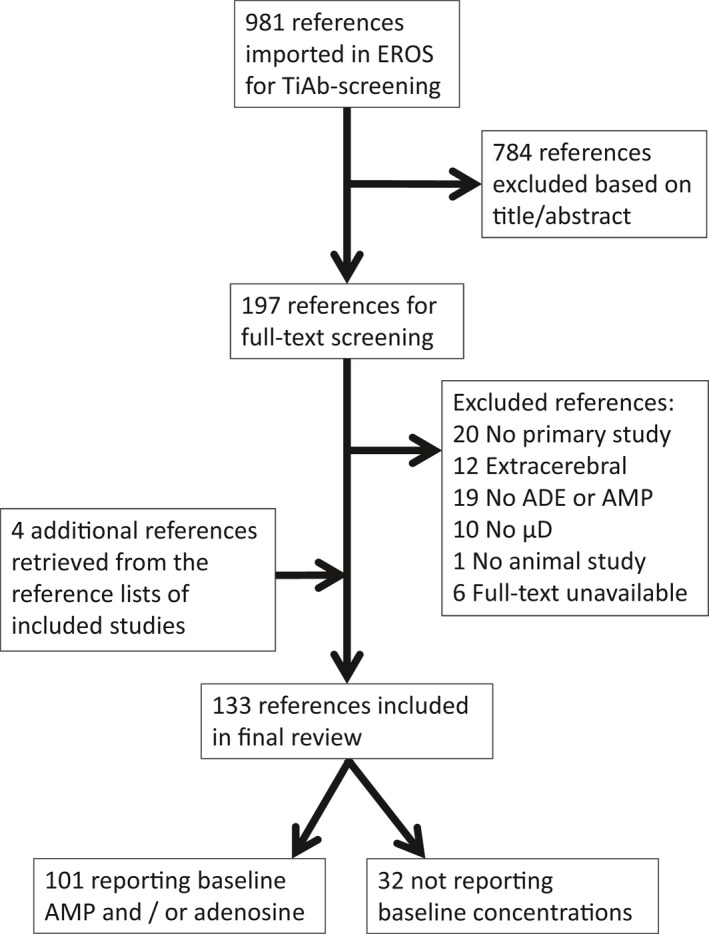
Flow scheme of retrieved and included references. Note that only two papers report baseline adenosine monophosphate concentrations.
Study characteristics and quality assessment
Our search retrieved 132 papers describing intracerebral microdialysis of adenosine or AMP, their characteristics are listed in Appendix S1 (those describing an actual baseline adenosine concentration in dialysate), Appendix S2 (those describing adenosine microdialysis without a baseline concentration) and Appendix S3 (those describing an actual baseline AMP concentration in dialysate). The reporting quality of the included studies is expressed by the percentages of papers reporting specific items.
Of the 132 papers, 110 (83%) reported the number of animals used, which ranged from 3 to 58 per paper. Ninety‐seven papers (73%) were on rats; 43 on Sprague‐Dawleys, 37 on Wistars, 2 on F344s, 1 on Albino Sprague‐Dawleys, 1 on Long‐Evans and 12 left the strain unspecified. Mice were used in 9 papers (7%); C57BL/6 in 5, other strains in 3 and 1 paper left the strain unspecified. The remaining papers were on cats (8), pigs (7), sheep (4), gerbils (3), marmosets (2), rabbits (1) and turtles (1).
The sex of the animals used was reported in 99 papers (75%); 92 were in male animals only, 1 in female animals only and 6 in both sexes. The authors of 55 papers (42%) provided some information on the animal housing, mostly just the temperature and humidity. The light‐dark cycle was reported in 45 papers (34%), but only 25 (19%) provided the actual times. For dialysis experiments in freely behaving animals, post‐surgical recovery ranged from 16 h to > 3 weeks. The post‐surgical recovery period was described by 44 papers (33%); 2 only mentioned ‘later’.
Recovery rate of the probe was described to some extent in 46 papers (35%), and ranged from 2.6% to 47.8%. Sampling bin time was reported by 108 papers (82%), and lasted from 1 to 60 min. Information on the analysis method of adenosine was provided by 124 papers (94%); 120 used some form of high pressure liquid chromatography (HPLC), one used mass spectrometry, one used capillary electrophoresis and one used a radioimmunoassay. Total dialysis experiment duration could be retrieved from 102 papers (77%) and ranged from 30 min to > 54 h.
Adenosine data
Our search retrieved 98 papers reporting actual baseline adenosine concentrations in dialysate for one or more experimental groups. Absolute adenosine concentrations in brain dialysates, ranging from 0.8 to 2100 nM, were reported for 133 experimental animal groups in the 98 papers, and are listed in Table 1.
Of the 98 papers, 63 papers reported the SEM and 16 the SD. There were four papers reporting SEM or SD without indicating which measure was used, and for one paper (in Japanese), we were not sure, all indicated by a question mark in Table 1.
AMP data
Of the papers retrieved by our search, only two presented the absolute AMP concentrations in brain dialysate, for three experimental groups in total. Brain dialysate AMP concentration ranged from 14 to 940 nM. Details on these studies are provided in Appendix S3.
Meta‐regression of the effect of experimental settings on dialysate adenosine concentrations
Included studies
The 74 studies reporting the moderators probe surface area (i.e. probe length and diameter), flow rate, species, brain area and anaesthesia versus freely behaving, as well as the baseline adenosine concentration in dialysate and the corresponding SEM or SD (all listed in Table 1), were included in the meta‐regression. A box‐plot of the adenosine concentrations for included and excluded studies is provided in Fig. 2. The reported adenosine concentrations were not significantly different between included (k = 74) and excluded (k = 59) studies (p = 0.099).
Figure 2.
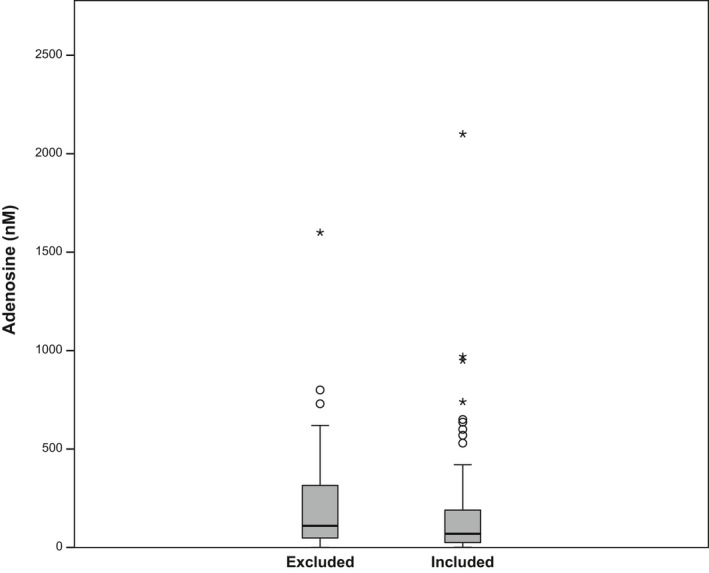
Adenosine concentrations (nM) of studies included and excluded in the meta‐regression. The horizontal bar in the boxplot reflects the median value, the box the 25th and 75th percentile, the whiskers the range excluding outliers, the circles outliers (outside 1.5× the interquartile range) and asterisks extreme outliers (outside 3× the interquartile range). The reported adenosine concentrations were not significantly different between included (k = 74) and excluded (k = 60) experimental animal groups (p = 0.099).
Of the 74 studies included in the meta‐regression, 25 were in freely behaving animals, 49 in anaesthetised animals. For the 25 studies in freely behaving animals, post‐surgical recovery time was reported in 20 (range: 24 h–3 weeks). For the 49 studies in anaesthetized animals, post‐surgical recovery time was only reported in 2 (both 24 h). Post‐surgical recovery time could thus not be analysed in our meta‐regression analysis. To provide an indication of the effect of post‐surgical recovery duration on the baseline adenosine concentrations, we plotted them for the 20 studies in freely behaving animals reporting post‐surgical recovery time in Fig. 3. No statistical analyses were performed because of the low numbers of studies.
Figure 3.
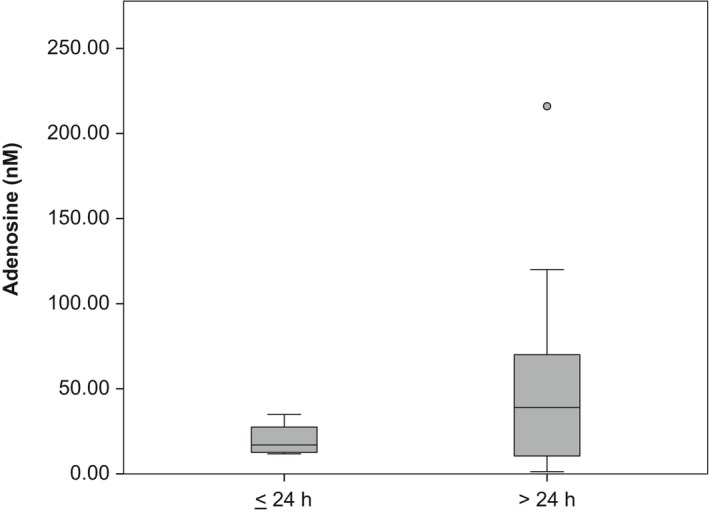
Adenosine concentrations (nM) of studies in freely behaving animals by duration of post‐surgical recovery (≤ 24 h vs. > 24 h). The horizontal bar in the boxplot reflects the median value, the box the 25th and 75th percentile, the whiskers the range excluding outliers and the circle an outlier (outside 1.5× the interquartile range). k = 7 for experimental animal groups with post‐surgical recovery ≤ 24 h and k = 13 for those with recovery > 24 h. No statistical analyses were performed because of the low numbers of studies.
Meta‐regression of adenosine concentrations in dialysate
The meta‐regression included five moderators; two numerical and three categorical. The observed values of these moderators are presented in Table 2. Only anaesthesia and flow rate significantly affected dialysate adenosine concentrations (Table 2). These results were confirmed by the sensitivity analysis. Heterogeneity was high; I 2 = 99.73%.
Table 2.
Moderators in the meta‐regression
| Moderator | Range or values | Estimate from MR (SE) | p‐Value from MR |
|---|---|---|---|
| Flow rate (N) | 0.1–3 μL/min | −0.74 (0.19) | < 0.001 |
| Probe surface area (N) | 0.24–2.84 mm2 | 0.13 (0.32) | 0.681 |
| Anaesthesia (C) | None, injection, inhalation, combined (injection & inhalation) | < 0.001 | |
| Injection versus none | 2.51 (0.44) | < 0.001 | |
| Inhalation versus none | 1.50 (0.43) | 0.008 | |
| Combined versus none | 2.13 (0.78) | 0.008 | |
| Species (C) | Cat, mouse, pig, rat, sheep | 0.672 | |
| Cats versus rats | 1.37 (1.02) | 0.186 | |
| Mice versus rats | 0.35 (0.84) | 0.677 | |
| Pigs versus rats | 0.74 (0.90) | 0.414 | |
| Sheep versus rats | 0.18 (0.91) | 0.840 | |
| Brain region (C) | Basal forebrain, brainstem, cortex, hippocampus, thalamusa | 0.145 | |
| Brainstem versus basal forebraina | −0.98 (0.68) | 0.153 | |
| Cortex versus basal forebraina | −0.26 (0.62) | 0.682 | |
| Hippocampus versus basal forebraina | 0.74 (0.50) | 0.145 | |
| Thalamus versus basal forebraina | 0.76 (0.66) | 0.253 |
N, numerical moderator; C, categorical moderator; MR, meta‐regression.
Refer to the methods section for information on pooling of brain regions.
Figure 4 shows a bubble plot of predicted and actual adenosine concentrations by flow rate, reflecting the overall decrease in concentration with increasing flow rate. Figure 5 shows a plot of the predicted and actual adenosine concentrations by type of anaesthesia, reflecting an increase in adenosine with all types of anaesthesia compared to non‐anaesthetised (i.e. freely behaving).
Figure 4.
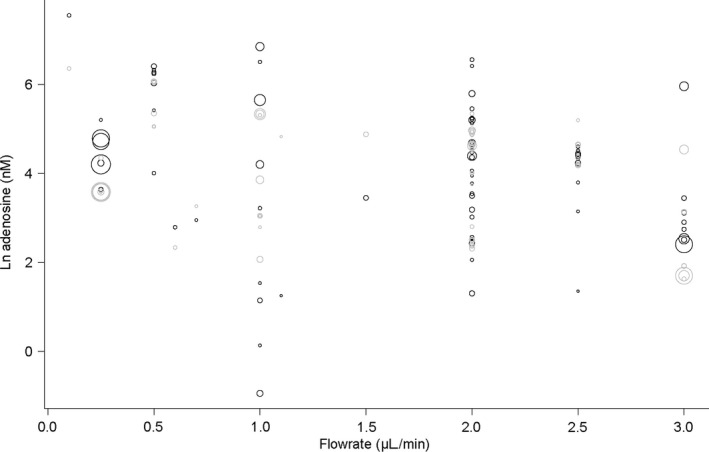
Predicted and actual adenosine concentration by flow rate. Black bubbles reflect actual values, grey bubbles values predicted by the regression model. Bubble size reflects precision (inverse variance) of the studies. Statistical details are provided in the text and Table 2.
Figure 5.
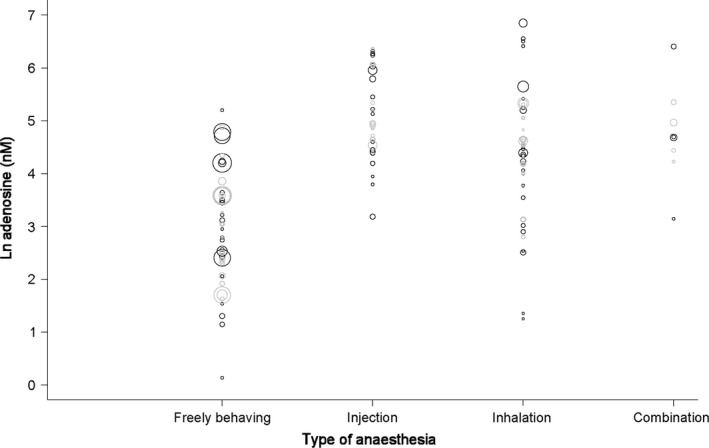
Predicted and actual adenosine concentration by type of anaesthesia. Black bubbles reflect actual values, grey bubbles values predicted by the regression model. Bubble size reflects precision (inverse variance) of the studies. Statistical details are provided in the text and Table 2.
Discussion
This SR shows that the reported baseline concentrations of adenosine in microdialysates from laboratory animals cover a wide range; from 0.8 to 2100 nM. We expected the baseline values to cover a smaller range. To the best of our knowledge, no overview of baseline adenosine concentrations has been published before. The range of concentrations observed in our SR seems to be larger than the range described in, for example, a narrative review of acetylcholine (Pepeu and Giovannini 2007) and a SR of noradrenaline, serotonin and dopamine (Brand et al. 2013), both restricted to a subset of studies with more comparable experimental designs. Most reviews on microdialysis do not describe baseline levels.
Although we did include thesaurus terms for AMP in our searches (MEdical Subject Heading ‘AMP’ in PubMed and ‘adenosine phosphate’ in Embase), besides searching for it in the title, abstract and keywords, we only found two papers reporting absolute AMP concentrations in brain dialysates. We expect that more authors have measured AMP in dialysate, but possibly it was not reported as the main compound of interest. For future SRs on AMP, we recommend extensive a priori scoping searches and adaptation of the search strategy.
While analysing the data for this review, we came across a few older synonyms for the term ‘microdialysis’ that we did not include in our search. In future searches for microdialysis studies, we recommend including the terms ‘chemitrode’, ‘dialytrode’, ‘brain dialysis’, ‘intracerebral dialysis’, ‘intracranial dialysis’, ‘transcranial dialysis’ and ‘implanted perfused hollow fibre’. From the four papers that were retrieved from the reference lists, three (Zetterstrom et al. 1982; Van Wylen et al. 1986; Park et al. 1988) would have been found by searches in PubMed, Embase and Web of Science, had these terms been included. From the same four papers, only one conference abstract (Chen and Stone 1991) is not indexed in any of these databases.
Compared to other substances, microdialysis of adenosine has the added challenge of its source not being purely neurogenic. As a building block for deoxyribonucleic acid and ribonucleic acid and the main metabolite of adenosine triphosphate, the adenosine measured in the dialysates could originate from metabolic processes (Melani et al. 2014) independent of neurotransmission. Our meta‐regression shows that anaesthesia and flow rate affect the baseline dialysate adenosine concentrations. Our finding that adenosine concentrations decrease with increasing flow rate are in line with the basic concept of diffusion (i.e. with a higher flow rate there is less time for analytes to diffuse over the membrane, resulting in lower concentrations) and with experimental findings on other substances (e.g. Rooyackers et al. 2013 and reviewed by Fliegel et al. 2013).
Preceding experimental work shows the effect of anaesthesia on the extracellular levels of various neurotransmitters (reviewed in de Souza Silva et al. 2007). In line with these findings, all types of anaesthesia increase the concentration of adenosine according to our meta‐regression results. While microdialysis studies on adenosine have been performed since 1982, microdialysis of freely behaving animals was uncommon in the early stages. Most of the included studies using anaesthesia were published before the year 2000, most of the studies in freely behaving animals afterwards (refer to Appendix S1). Methodology generally improves over time, which could result in increased recovery; newer studies could find higher baseline concentrations. We thus cannot exclude the effect of anaesthesia on baseline adenosine levels being confounded to some extent by year of publication. The actual effect may thus be larger than the observed effect.
In addition, studies measuring under anaesthesia may well start immediately after surgery, while studies in freely behaving animals will usually implement a recovery period before the onset of measurements. The interval between surgery can affect the adenosine concentrations; adenosine decreases substantially over time since surgery (Pazzagli et al. 1993). This decrease could become even more pronounced with the development of gliosis over the course of several days after surgery. Figure 3 shows, however, that for the included sample of studies, increased lag times result in more variation between the measured adenosine values, but not in clearly decreased concentrations. For our analysis, this limits the confounding by post‐surgical lag time for freely behaving animals. Of note, analyses of the effects of anaesthesia are further complicated by other surgery‐related factors, such as stress, and variables that are inherently associated with anaesthesia, such as the lack of movement, that could potentially explain part of the observed increases in adenosine concentrations.
Based on tissue sections and HPLC with electrochemical detection, baseline levels of purines have been shown to vary significantly between brain regions as well as between different mouse strains (Pani et al. 2014). We tabulated the brain regions as mentioned by the authors, and collated these into the wide categories for the meta‐regression. While this results in the loss of information, the number of studies for most brain regions is still rather low for the analyses. At this stage, the effect of brain region on baseline dialysate adenosine concentration remains unclear.
Different types of chromatography with ultraviolet or fluorescence detection are the most common method for determination of nucleotides and nucleosides, including AMP and adenosine (Khlyntseva et al. 2009). One hundred and twenty‐one of the papers included in this review used some form of HPLC. The advantage of HPLC is that it allows for a rapid determination of several substances simultaneously. The disadvantage of fluorescent detection is the necessity of converting substances into the fluorescent form (by means of derivatisation with chloracetaldehyde), which may lead to hydrolysis of nucleotides and, thus, to loss of substance during sample preparation. While the reporting of the basic type of analysis method in microdialysis papers is relatively high, the description of the analysis methods can be rather minimal. As the analysis methods compare dialysate concentrations to a given standard, we do not expect major effects and did not include them as a moderator in the meta‐regression.
We did not pre‐specify our meta‐regression in our registered protocol, because we did not anticipate sufficient data to be available. Even though the analysis was designed and conducted post hoc, and even though we included only those moderators for which sufficient data were available, we do not expect it to have biased our conclusions.
Microdialysis baseline concentrations depend strongly on, for example, exact probe location and arousal level of the animal. Because of this, many authors only report effects as percentages of baseline, without reporting baseline levels, resulting in exclusion of 33 papers from our meta‐regression. The low levels of reporting of experimental details resulted in another 63 being excluded from the meta‐regression.
The low levels of reporting of experimental details also limited the moderators that could be included in the analyses. Before data‐extraction, we considered including the following additional moderators: membrane type or permeability, sterilisation procedure, sample storage before analysis, washout time, post‐operational recovery time and clock‐time of measurements. These factors are known to affect dialysate concentrations of certain compounds (Westerink and Cremers 2007), by physiological mechanisms, by degradation of adenosine over time or by variations in recovery because of, for example, glial cells around the probe. These factors were, however, reported too infrequently to analyse.
We did not perform a risk of bias assessment for this SR, as we analyse baseline concentrations, completely disregarding any subsequent intervention. Most risk of bias‐tools (e.g. Hooijmans et al. 2014) focus strongly on interventions and presuppose a comparison between an experimental group and control group; these are therefore not relevant to our research question. We did provide an indication of average study quality by calculating the percentages of studies reporting relevant study characteristics.
Publication bias can result from a relative surplus in the literature of studies showing positive effects of an intervention. We did not formally analyse publication bias as it seems hardly relevant when addressing baseline concentrations. For microdialysis studies, baseline concentrations are not always reported; many authors choose to report percentages of baseline only, and this could be more common when finding extremely high or low baseline values. We expect the reporting of extreme baseline concentrations, however, to be equally affected on both sides of the distribution of dialysate adenosine concentrations.
Our meta‐regression is a proof of principle for quantification of the effects of experimental methodology (in microdialysis experiments) on the outcome measure (adenosine concentration in microdialysate) at the meta‐level, with data from literature, without using new animals. This is one of the few SRs and meta‐analyses characterizing microdialysis as an experimental method in laboratory animal studies. To the best of our knowledge, this paper describes the first SR of microdialysis of adenosine.
Other SRs of the microdialysis technique include, for example (Brand et al. 2013), whose fixed‐effect meta‐analysis showed that baseline microdialysate concentrations of noradrenaline, serotonin and dopamine hardly vary by sex, strain, brain region or state of consciousness, but that they increase throughout the brain after ethanol. Besides, a meta‐analysis by (Frank et al. 2008) indicated that cocaine‐induced dopamine overflow depends on dose, brain region and on route of administration, but not on species (rats vs. mice), strain, age, sex, or calcium concentration of the perfusion medium. Furthermore, according to the meta‐analyses by Fliegel et al. (2013), dialysate glutamate and GABA concentrations change with exposure to and withdrawal from ethanol. Moreover, meta‐regression has shown a relationship between serotonin and duration of selective serotonin reuptake inhibitor treatment in the frontal cortex in rats (Fritze et al. 2017). Combined, these results show the power of meta‐analysis as an alternative to performing new animal experiments in answering various research questions in the field of neurochemistry.
We here provide a complete overview of the baseline adenosine concentrations in microdialysis studies, showing a wide range of reported concentrations that would not necessarily be clear in a narrative review. We show that meta‐regression, including experimental methods as moderators, can be used as an alternative to new animal experiments to answer research questions in the field of neurochemistry. To reach the full analytical potential of this and other types of meta‐analyses, the levels of reporting of experimental detail need to improve.
Acknowledgments and conflict of interest disclosure
Funding for this project was provided by ZonMw (40‐42600‐98‐301); R2N, Federal State of Lower Saxony and the DFG (FOR2591, BL 953/11‐1). The authors would like to thank Alice Tillema for help in optimizing the search strategy, Matthijs Feenstra for proofreading an earlier draft of this manuscript, and Judith van Luijk for proofreading the protocol and checking a small sample of concentration calculations for accuracy. The authors have no conflict of interest to declare.
Supporting information
Appendix S1. Study characteristics of papers reporting baseline adenosine concentrations.
Appendix S2. Study characteristics of papers and abstracts not reporting absolute baseline adenosine concentrations or only reporting them in low‐resolution figures.
Appendix S3. Adenosine monophosphate concentrations and corresponding study characteristics.
References
- Aden U., O'Connor W. T. and Berman R. F. (2004) Changes in purine levels and adenosine receptors in kindled seizures in the rat. NeuroReport 15, 1585–1589. [DOI] [PubMed] [Google Scholar]
- Andine P., Rudolphi K. A., Fredholm B. B. and Hagberg H. (1990) Effect of propentofylline (HWA 285) on extracellular purines and excitatory amino acids in CA1 of rat hippocampus during transient ischaemia. Br. J. Pharmacol. 100, 814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarin M., Herrera‐Marschitz M., Casas M. and Ungerstedt U. (1987) Striatal adenosine levels measured ‘in vivo’ by microdialysis in rats with unilateral dopamine denervation. Neurosci. Lett. 83, 338–344. [DOI] [PubMed] [Google Scholar]
- Ballarin M., Reiriz J., Ambrosio S., Camps M., Blesa R. and Mahy N. (1989) Acute effects of 1‐methyl‐1,4‐phenylpyridinium ion (MPP+) on purine metabolism in rat striatum studied in vivo using the microdialysis technique. Brain Res. 483, 184–187. [DOI] [PubMed] [Google Scholar]
- Ballarin M., Fredholm B. B., Ambrosio S. and Mahy N. (1991) Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol. Scand. 142, 97–103. [DOI] [PubMed] [Google Scholar]
- Basheer R., Porkka‐Heiskanen T., Stenberg D. and McCarley R. W. (1999) Adenosine and behavioral state control: adenosine increases c‐Fos protein and AP1 binding in basal forebrain of rats. Mol. Brain Res. 73, 1–10. [DOI] [PubMed] [Google Scholar]
- Bennett H. J., White T. D. and Semba K. (2000) Activation of metabotropic glutamate receptors increases extracellular adenosine in vivo. NeuroReport 11, 3489–3492. [DOI] [PubMed] [Google Scholar]
- Bennett H. J., White T. D. and Semba K. (2003) Activation of cholinergic and adrenergic receptors increases the concentration of extracellular adenosine in the cerebral cortex of unanesthetized rat. Neuroscience 117, 119–127. [DOI] [PubMed] [Google Scholar]
- Berman R. F., Fredholm B. B., Aden U. and O'Connor W. T. (2000) Evidence for increased dorsal hippocampal adenosine release and metabolism during pharmacologically induced seizures in rats. Brain Res. 872, 44–53. [DOI] [PubMed] [Google Scholar]
- Blanco‐Centurion C., Xu M., Murillo‐Rodriguez E., Gerashchenko D., Shiromani A. M., Salin‐Pascual R. J., Hof P. R. and Shiromani P. J. (2006) Adenosine and sleep homeostasis in the basal forebrain. J. Neurosci. 26, 8092–8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M., Hedges L. V., Higgins J. P. T. and Rothstein H. R. (2009) Multiple outcomes or time‐points within a study, in Introduction to Meta‐Analysis (Borenstein M., Hedges L.V., Higgins J.P.T. and Rothstein H.R., eds), pp. 225–238. John Wiley & Sons Ltd., West Sussex, UK. [Google Scholar]
- Brand I., Fliegel S., Spanagel R. and Noori H. R. (2013) Global ethanol‐induced enhancements of monoaminergic neurotransmission: a meta‐analysis study. Alcohol. Clin. Exp. Res. 37, 2048–2057. [DOI] [PubMed] [Google Scholar]
- Britton D. R., Mikusa J., Lee C. H., Jarvis M. F., Williams M. and Kowaluk E. A. (1999) Site and event specific increase of striatal adenosine release by adenosine kinase inhibition in rats. Neurosci. Lett. 266, 93–96. [DOI] [PubMed] [Google Scholar]
- Butcher S. P., Hagberg H., Sandberg M. and Hamberger A. (1987) Extracellular purine catabolite and amino acid levels in the rat striatum during severe hypoglycemia: effects of 2‐amino‐5‐phosphonovalerate. Neurochem. Int. 11, 95–99. [DOI] [PubMed] [Google Scholar]
- Carrozzo M. M., Troisi L., Cannazza G., Cazzato A. S., Braghiroli D., Parenti C., Guiducci S. and Zoli M. (2012) An improved LC‐S/MS method for the quantitation of adenosine concentration in mice brain microdialysates. J. Pharm. Biomed. Anal. 70, 563–566. [DOI] [PubMed] [Google Scholar]
- Carswell H. V., Graham D. I. and Stone T. W. (1997) Kainate‐evoked release of adenosine from the hippocampus of the anaesthetised rat: possible involvement of free radicals. J. Neurochem. 68, 240–247. [DOI] [PubMed] [Google Scholar]
- Chen Y. and Stone T. W. (1991) Release of endogenous adenosine and related purines from the rat hippocampus in vivo. Br. J. Pharmacol. 104, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Graham D. I. and Stone T. W. (1992) Release of endogenous adenosine and its metabolites by the activation of NMDA receptors in the rat hippocampus in vivo. Br. J. Pharmacol. 106, 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. S., Fujitaki J. and Dixon R. (1993) An improved assay for adenosine in rat brain microdialysates using microbore high performance liquid chromatography. J. Liq. Chromatogr. 16, 2791–2796. [Google Scholar]
- Cui M., Bai X., Li T., Chen F., Dong Q., Zhao Y. and Liu X. (2013) Decreased extracellular adenosine levels lead to loss of hypoxia‐induced neuroprotection after repeated episodes of exposure to hypoxia. PLoS ONE 8, e57065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M., Ding H., Chen F., Zhao Y., Yang Q. and Dong Q. (2016) Mdivi‐1 protects against ischemic brain injury via elevating extracellular adenosine in a cAMP/CREB‐CD39‐dependent manner. Mol. Neurobiol. 53, 240–253. [DOI] [PubMed] [Google Scholar]
- Deng Q., Watson C. J. and Kennedy R. T. (2003) Aptamer affinity chromatography for rapid assay of adenosine in microdialysis samples collected in vivo. J. Chromatogr. A 1005, 123–130. [DOI] [PubMed] [Google Scholar]
- Dobolyi A., Reichart A., Szikra T. and Juhasz G. (1997) Purine and pyrimidine nucleoside content of the neuronal extracellular space in rat ‐ an in vivo microdialysis study. Clin. Biochem. 30, 252–253. [DOI] [PubMed] [Google Scholar]
- Dobolyi A., Reichart A., Szikra T., Szilagyi N., Kekesi A. K., Karancsi T., Slegel P., Palkovits M. and Juhasz G. (1998) Analysis of purine and pyrimidine bases, nucleosides and deoxynucleosides in brain microsamples (microdialysates and micropunches) and cerebrospinal fluid. Neurochem. Int. 32, 247–256. [DOI] [PubMed] [Google Scholar]
- Dobolyi A., Szikra T., Kekesi A. K., Kovacs Z. and Juhasz G. (1999) Uridine is released by depolarization and inhibits unit activity in the rat hippocampus. NeuroReport 10, 3049–3053. [DOI] [PubMed] [Google Scholar]
- Dobolyi A., Reichart A., Szikra T., Nyitrai G., Kekesi K. A. and Juhasz G. (2000) Sustained depolarisation induces changes in the extracellular concentrations of purine and pyrimidine nucleosides in the rat thalamus. Neurochem. Int. 37, 71–79. [DOI] [PubMed] [Google Scholar]
- Dohmen C., Kumura E., Rosner G., Heiss W. D. and Graf R. (2001) Adenosine in relation to calcium homeostasis: comparison between gray and white matter ischemia. J. Cereb. Blood Flow Metab. 21, 503–510. [DOI] [PubMed] [Google Scholar]
- Dux E., Fastbom J., Ungerstedt U., Rudolphi K. and Fredholm B. B. (1990) Protective effect of adenosine and a novel xanthine derivative propentofylline on the cell damage after bilateral carotid occlusion in the gerbil hippocampus. Brain Res. 516, 248–256. [DOI] [PubMed] [Google Scholar]
- Fliegel S., Brand I., Spanagel R. and Noori H. R. (2013) Ethanol‐induced alterations of amino acids measured by in vivo microdialysis in rats: a meta‐analysis. In Silico Pharmacol. 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S. T., Krumm B. and Spanagel R. (2008) Cocaine‐induced dopamine overflow within the nucleus accumbens measured by in vivo microdialysis: a meta‐analysis. Synapse 62, 243–252. [DOI] [PubMed] [Google Scholar]
- Fritze S., Spanagel R. and Noori H. R. (2017) Adaptive dynamics of the 5‐HT systems following chronic administration of selective serotonin reuptake inhibitors: a meta‐analysis. J. Neurochem. 142, 747–755. [DOI] [PubMed] [Google Scholar]
- Fu X. Y., Zhang Z. D., Liang K. et al (2015) Subclavian steal syndrome decreases neurogenesis in the cerebellar cortex and affects cognitive function in rabbits. Exp. Ther. Med. 10, 1455–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfriddo M., Corsi C., Melani A., Pezzola A., Reggio R., Popoli P. and Pedata F. (2003) Adenosine A(2A) antagonism increases striatal glutamate outflow in the quinolinic acid rat model of Huntington's disease. Brain Res. 979, 225–229. [DOI] [PubMed] [Google Scholar]
- Gianfriddo M., Melani A., Turchi D., Giovannini M. G. and Pedata F. (2004) Adenosine and glutamate extracellular concentrations and mitogen‐activated protein kinases in the striatum of Huntington transgenic mice. Selective antagonism of adenosine A(2A) receptors reduces transmitter outflow. Neurobiol. Dis. 17, 77–88. [DOI] [PubMed] [Google Scholar]
- Gidday J. M., Kim Y. B., Shah A. R., Gonzales E. R. and Park T. S. (1996) Adenosine transport inhibition ameliorates postischemic hypoperfusion in pigs. Brain Res. 734, 261–268. [PubMed] [Google Scholar]
- Grabb M. C., Sciotti V. M., Gidday J. M., Cohen S. A. and Van Wylen D. G. L. (1998) Neurochemical and morphological responses to acutely and chronically implanted brain microdialysis probes. J. Neurosci. Methods 82, 25–34. [DOI] [PubMed] [Google Scholar]
- Hagberg H., Andersson P. and Lacarewicz J. (1987) Extracellular adenosine, inosine, hypoxanthine, and xanthine in relation to tissue nucleotides and purines in rat striatum during transient ischemia. J. Neurochem. 49, 227–231. [DOI] [PubMed] [Google Scholar]
- Headrick J. P., Bendall M. R., Faden A. I. and Vink R. (1994) Dissociation of adenosine levels from bioenergetic state in experimental brain trauma: potential role in secondary injury. J. Cereb. Blood Flow Metab. 14, 853–861. [DOI] [PubMed] [Google Scholar]
- Herrera‐Marschitz M., Luthman J. and Ferre S. (1994) Unilateral neonatal intracerebroventricular 6‐hydroxydopamine administration in rats: II. Effects on extracellular monoamine, acetylcholine and adenosine levels monitored with in vivo microdialysis. Psychopharmacology 116, 451–456. [DOI] [PubMed] [Google Scholar]
- Higgins J. P., White I. R. and Anzures‐Cabrera J. (2008) Meta‐analysis of skewed data: combining results reported on log‐transformed or raw scales. Stat. Med. 27, 6072–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillered L., Hallstrom A., Segersvard S., Persson L. and Ungerstedt U. (1989) Dynamics of extracellular metabolites in the striatum after middle cerebral artery occlusion in the rat monitored by intracerebral microdialysis. J. Cereb. Blood Flow Metab. 9, 607–616. [DOI] [PubMed] [Google Scholar]
- Hooijmans C. R., Leenaars M. and Ritskes‐Hoitinga M. (2010a) A gold standard publication checklist to improve the quality of animal studies, to fully integrate the Three Rs, and to make systematic reviews more feasible. Altern. Lab. Anim. 38, 167–182. [DOI] [PubMed] [Google Scholar]
- Hooijmans C. R., Tillema A., Leenaars M. and Ritskes‐Hoitinga M. (2010b) Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab. Anim. 44, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmans C. R., Rovers M. M., de Vries R. B., Leenaars M., Ritskes‐Hoitinga M. and Langendam M. W. (2014) SYRCLE's risk of bias tool for animal studies. BMC Med. Res. Methodol. 14, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku T., Hada J. and Hayashi Y. (1994) Endogenous adenosine exerts inhibitory effects upon the development of spreading depression and glutamate release induced by microdialysis with high K+ in rat hippocampus. Brain Res. 658, 39–48. [DOI] [PubMed] [Google Scholar]
- Kaku T., Jiang M. H., Hada J., Morimoto K. and Hayashi Y. (2001) Sodium nitroprusside‐induced seizures and adenosine release in rat hippocampus. Eur. J. Pharmacol. 413, 199–205. [DOI] [PubMed] [Google Scholar]
- Kalinchuk A. V., McCarley R. W., Porkka‐Heiskanen T. and Basheer R. (2011) The time course of adenosine, nitric oxide (NO) and inducible NO synthase changes in the brain with sleep loss and their role in the non‐rapid eye movement sleep homeostatic cascade. J. Neurochem. 116, 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlyntseva S. V., Bazel Y. R., Vishnikin A. B. and Andruch V. (2009) Methods for the determination of adenosine triphosphate and other adenine nucleotides. J. Anal. Chem. 64, 657–673. [Google Scholar]
- Kim T., Vijay R., Kalinchuk A., Messing R. O., Choi D., Dworak M., McCarley R. W. and Basheer R. (2010) Sleep‐wake regulation in type 1 equilibrative nucleoside transporter knockout mice. Sleep 33, A14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellmer I., Andine P., Hagberg H. and Thiringer K. (1989) Extracellular increase of hypoxanthine and xanthine in the cortex and basal ganglia of fetal lambs during hypoxia‐ischemia. Brain Res. 478, 241–247. [DOI] [PubMed] [Google Scholar]
- Kondoh S., Nagasawa S., Kawanishi M., Yamaguchi K., Kajimoto S. and Ohta T. (1999) Effects of ebselen on cerebral ischemia and reperfusion evaluated by microdialysis. Neurol. Res. 21, 682–686. [DOI] [PubMed] [Google Scholar]
- Koos B. J., Kruger L. and Murray T. F. (1997) Source of extracellular brain adenosine during hypoxia in fetal sheep. Brain Res. 778, 439–442. [DOI] [PubMed] [Google Scholar]
- Lehmann A., Hagberg H., Huxtable R. J. and Sandberg M. (1987) Reduction of brain taurine: effects on neurotoxic and metabolic actions of kainate. Neurochem. Int. 10, 265–274. [DOI] [PubMed] [Google Scholar]
- Li H., Yan Z., Zhu J., Yang J. and He J. (2011) Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology 60, 252–258. [DOI] [PubMed] [Google Scholar]
- Lutz P. L. and Kabler S. (1997) Release of adenosine and ATP in the brain of the freshwater turtle (Trachemys scripta) during long‐term anoxia. Brain Res. 769, 281–286. [DOI] [PubMed] [Google Scholar]
- Lydic R., Guzick S., Brummett C. and Baghdoyan H. (2010) Microdialysis delivery of buprenorphineto rat pontine reticular formation does not alter adenosine levels. J. Pain 1, S32. [Google Scholar]
- Masana Y., Morimoto K., Hayakawa T., Shimizu H., Shimada N., Nii Y., Yoshimine T., Mogami H. and Hashimoto T. (1989) Extracellular purine catabolites and tissue nucleotides and purine catabolites during progression and recovery of ischemia. No To Shinkei 41, 687–693. [PubMed] [Google Scholar]
- Materi L. M. and Semba K. (2001) Inhibition of synaptically evoked cortical acetylcholine release by intracortical glutamate: involvement of GABAergic neurons. Eur. J. Neurosci. 14, 38–46. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Graf R., Rosner G., Shimada N. and Heiss W. D. (1992) Flow thresholds for extracellular purine catabolite elevation in cat focal ischemia. Brain Res. 579, 309–314. [DOI] [PubMed] [Google Scholar]
- Maysinger D., Herrera‐Marschitz M., Goiny M., Ungerstedt U. and Cuello A. C. (1992) Effects of nerve growth factor on cortical and striatal acetylcholine and dopamine release in rats with cortical devascularizing lesions. Brain Res. 577, 300–305. [DOI] [PubMed] [Google Scholar]
- McKenna J. T., Tartar J. L., Ward C. P., Thakkar M. M., Cordeira J. W., McCarley R. W. and Strecker R. E. (2007) Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience 146, 1462–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani A., Pantoni L., Corsi C., Bianchi L., Monopoli A., Bertorelli R., Pepeu G. and Pedata F. (1999a) Striatal outflow of adenosine, excitatory amino acids, gamma‐aminobutyric acid, and taurine in awake freely moving rats after middle cerebral artery occlusion: correlations with neurological deficit and histopathological damage. Stroke 30, 2448–2454; discussion 2455. [DOI] [PubMed] [Google Scholar]
- Melani A., Corsi C., Gimenez‐Llort L., Martinez E., Ogren S. O., Pedata F. and Ferre S. (1999b) Effect of N‐methyl‐D‐aspartate on motor activity and in vivo adenosine striatal outflow in the rat. Eur. J. Pharmacol. 385, 15–19. [DOI] [PubMed] [Google Scholar]
- Melani A., Pantoni L., Bordoni F., Gianfriddo M., Bianchi L., Vannucchi M. G., Bertorelli R., Monopoli A. and Pedata F. (2003) The selective A(2A) receptor antagonist SCH 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res. 959, 243–250. [DOI] [PubMed] [Google Scholar]
- Melani A., Corti F., Stephan H., Muller C. E., Donati C., Bruni P., Vannucchi M. G. and Pedata F. (2012) Ecto‐ATPase inhibition: ATP and adenosine release under physiological and ischemic in vivo conditions in the rat striatum. Exp. Neurol. 233, 193–204. [DOI] [PubMed] [Google Scholar]
- Melani A., Pugliese A. M. and Pedata F. (2014) Adenosine receptors in cerebral ischemia. Int. Rev. Neurobiol. 119, 309–348. [DOI] [PubMed] [Google Scholar]
- Mijangos‐Moreno S., Poot‐Ake A., Arankowsky‐Sandoval G. and Murillo‐Rodriguez E. (2014) Intrahypothalamic injection of cannabidiol increases the extracellular levels of adenosine in nucleus accumbens in rats. Neurosci. Res. 84, 60–63. [DOI] [PubMed] [Google Scholar]
- Mijangos‐Moreno S., Poot‐Ake A., Guzman K., Arankowsky‐Sandoval G., Arias‐Carrion O., Zaldivar‐Rae J., Sarro‐Ramirez A. and Murillo‐Rodriguez E. (2015) Sleep and neurochemical modulation by the nuclear peroxisome proliferator‐activated receptor alpha (PPAR‐alpha) in rat. Neurosci. Res. 105, 65–69. [DOI] [PubMed] [Google Scholar]
- Miranda M. F., Hamani C., de Almeida A. C. et al (2014) Role of adenosine in the antiepileptic effects of deep brain stimulation. Front. Cell Neurosci. 8, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto K., Shimizu H., Hayakawa T., Shimada N., Nii Y., Masana Y., Kato A. and Mogami H. (1991) Purine catabolites in cerebral interstitial fluid during progression of and recovery from ischemia. Neurol. Med. Chir. 31, 129–134. [DOI] [PubMed] [Google Scholar]
- Mork A. and Geisler A. (1994) Lithium in situ decreases extracellular levels of cyclic AMP in the dorsal hippocampus of living rats. Pharmacol. Toxicol. 74, 300–302. [DOI] [PubMed] [Google Scholar]
- Moss I. R., Yan S., Laferriere A. and Zhang C. (1995) Microdialyzed enkephalin and adenosine, breathing and hypoxia in developing swine. FASEB J. 9, A666–A666. [Google Scholar]
- Murillo‐Rodriguez E., Blanco‐Centurion C., Gerashchenko D., Salin‐Pascual R. J. and Shiromani P. J. (2004) The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience 123, 361–370. [DOI] [PubMed] [Google Scholar]
- Murillo‐Rodriguez E., Liu M., Blanco‐Centurion C. and Shiromani P. J. (2008) Effects of hypocretin (orexin) neuronal loss on sleep and extracellular adenosine levels in the rat basal forebrain. Eur. J. Neurosci. 28, 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel J. and Hauber W. (2002) Effects of salient environmental stimuli on extracellular adenosine levels in the rat nucleus accumbens measured by in vivo microdialysis. Behav. Brain Res. 134, 485–492. [DOI] [PubMed] [Google Scholar]
- Nagel J. and Hauber W. (2004) Reverse microdialysis of a dopamine D‐2 receptor antagonist alters extracellular adenosine levels in the rat nucleus accumbens. Neurochem. Int. 44, 609–615. [DOI] [PubMed] [Google Scholar]
- Nelson A. M., Battersby A. S., Baghdoyan H. A. and Lydic R. (2009) Opioid‐induced decreases in rat brain adenosine levels are reversed by inhibiting adenosine deaminase. Anesthesiology 111, 1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson P., Hillered L., Ponten U. and Ungerstedt U. (1990) Changes in cortical extracellular levels of energy‐related metabolites and amino acids following concussive brain injury in rats. J. Cereb. Blood Flow Metab. 10, 631–637. [DOI] [PubMed] [Google Scholar]
- Northington F. J., Matherne G. P., Coleman S. D. and Berne R. M. (1992) Sciatic nerve stimulation does not increase endogenous adenosine production in sensory‐motor cortex. J. Cereb. Blood Flow Metab. 12, 835–843. [DOI] [PubMed] [Google Scholar]
- Nomoto M., Shimizu T., Iwata S., Kaseda S. and Fukuda T. (1999) Metabolism of adenosine increase in the striatum in common marmoset parkinsonism induced by MPTP. Adv Neurol. 80, 125–8. [PubMed] [Google Scholar]
- Nomoto M., Kaseda S., Iwata S., Shimizu T., Fukuda T. and Nakagawa S. (2000) The metabolic rate and vulnerability of dopaminergic neurons, and adenosine dynamics in the cerebral cortex, nucleus accumbens, caudate nucleus, and putamen of the common marmoset. J Neurol. Sep; 247 Suppl 5:V16‐22. [DOI] [PubMed] [Google Scholar]
- Okada T., Mochizuki T., Huang Z. L., Eguchi N., Sugita Y., Urade Y. and Hayaishi O. (2003) Dominant localization of adenosine deaminase in leptomeninges and involvement of the enzyme in sleep. Biochem. Biophys. Res. Comm. 312, 29–34. [DOI] [PubMed] [Google Scholar]
- Pani A. K., Jiao Y., Sample K. J. and Smeyne R. J. (2014) Neurochemical measurement of adenosine in discrete brain regions of five strains of inbred mice. PLoS ONE 9, e92422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T. S., Van Wylen D. G., Rubio R. and Berne R. M. (1988) Brain interstitial adenosine and sagittal sinus blood flow during systemic hypotension in piglet. J. Cereb. Blood Flow Metab. 8, 822–828. [DOI] [PubMed] [Google Scholar]
- Pazzagli M., Pedata F. and Pepeu G. (1993) Effect of K+ depolarization, tetrodotoxin, and NMDA receptor inhibition on extracellular adenosine levels in rat striatum. Eur. J. Pharmacol. 234, 61–65. [DOI] [PubMed] [Google Scholar]
- Pazzagli M., Corsi C., Latini S., Pedata F. and Pepeu G. (1994) In vivo regulation of extracellular adenosine levels in the cerebral cortex by NMDA and muscarinic receptors. Eur. J. Pharmacol. 254, 277–282. [DOI] [PubMed] [Google Scholar]
- Pazzagli M., Corsi C., Fratti S., Pedata F. and Pepeu G. (1995) Regulation of extracellular adenosine levels in the striatum of aging rats. Brain Res. 684, 103–106. [DOI] [PubMed] [Google Scholar]
- Peigen K. and Jing S. (1997) The in vivo changes of extracellular fluid adenosine on brain ischemia/reperfusion and the effects of batroxobin on these changes in rats [Chinese]. Chin. J. Neurol. 30, 165–168. [Google Scholar]
- Pepeu G. and Giovannini M. G. (2007) Changes in acetylcholine extracellular levels during cognitive processes, in Handbook of Microdialysis – Methods, Applications and Perspectives. Handbook of Behavioral Neuroscience (Huston J. P., ed.), pp. 377–396. Elsevier – Academic Press, Amsterdam, The Netherlands. [Google Scholar]
- Perez‐Pinzon M. A., Nilsson G. E. and Lutz P. L. (1993) Relationship between ion gradients and neurotransmitter release in the newborn rat striatum during anoxia. Brain Res. 602, 228–233. [DOI] [PubMed] [Google Scholar]
- Pinna A., Corsi C., Carta A. R., Valentini V., Pedata F. and Morelli M. (2002) Modification of adenosine extracellular levels and adenosine A(2A) receptor mRNA by dopamine denervation. Eur. J. Pharmacol. 446, 75–82. [DOI] [PubMed] [Google Scholar]
- Porkka‐Heiskanen T., Strecker R. E., Thakkar M., Bjorkum A. A., Greene R. W. and McCarley R. W. (1997) Adenosine: a mediator of the sleep‐inducing effects of prolonged wakefulness. Science 276, 1265–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka‐Heiskanen T., Strecker R. E. and McCarley R. W. (2000) Brain site‐specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience 99, 507–517. [DOI] [PubMed] [Google Scholar]
- R_Core_Team (2016) R: A Language and environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, or online at https://www.R-project.org/ [accessed on 29 October 2016]. [Google Scholar]
- Richter D. W., Schmidt‐Garcon P., Pierrefiche O., Bischoff A. M. and Lalley P. M. (1999) Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetised cats. J. Physiol. 514, 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooyackers O., Blixt C., Mattsson P. and Wernerman J. (2013) Continuous glucose monitoring by intravenous microdialysis: influence of membrane length and dialysis flow rate. Acta Anaesthesiol. Scand. 57, 214–219. [DOI] [PubMed] [Google Scholar]
- Ruth V. J., Park T. S., Gonzales E. R. and Gidday J. M. (1993) Adenosine and cerebrovascular hyperemia during insulin‐induced hypoglycemia in newborn piglet. Am. J. Physiol. Heart Circ. Physiol. 265, H1762–H1768. [DOI] [PubMed] [Google Scholar]
- Salem A. and Hope W. (1999) Role of endogenous adenosine in the expression of opiate withdrawal in rats. Eur. J. Pharmacol. 369, 39–42. [DOI] [PubMed] [Google Scholar]
- Savelyev S. A., Rantamaki T., Rytkonen K. M., Castren E. and Porkka‐Heiskanen T. (2012) Sleep homeostasis and depression: studies with the rat clomipramine model of depression. Neuroscience 212, 149–158. [DOI] [PubMed] [Google Scholar]
- Scheller D., Kolb J., Peters U. and Tegtmeier F. (1996) The measurement of extracellular inorganic phosphate gives a more reliable indication for severe impairment of cerebral cell function and cell death than the measurement of extracellular lactate. Acta Neurochir. Suppl. 67, 28–30. [DOI] [PubMed] [Google Scholar]
- Schulte G., Sommerschild H., Yang J., Tokuno S., Goiny M., Lovdahl C., Johansson B., Fredholm B. B. and Valen G. (2004) Adenosine A(1) receptors are necessary for protection of the murine heart by remote, delayed adaptation to ischaemia. Acta Physiol. Scand. 182, 133–143. [DOI] [PubMed] [Google Scholar]
- Sciotti V. M. and Van Wylen D. G. (1993a) Attenuation of ischemia‐induced extracellular adenosine accumulation by homocysteine. J. Cereb. Blood Flow Metab. 13, 208–213. [DOI] [PubMed] [Google Scholar]
- Sciotti V. M. and Van Wylen D. G. L. (1993b) Increases in interstitial adenosine and cerebral blood flow with inhibition of adenosine kinase and adenosine deaminase. J. Cereb. Blood Flow Metab. 13, 201–207. [DOI] [PubMed] [Google Scholar]
- Sciotti V. M., Roche F. M., Grabb M. C. and Van Wylen D. G. L. (1992) Adenosine receptor blockade augments interstitial fluid levels of excitatory amino acids during cerebral ischemia. J. Cereb. Blood Flow Metab. 12, 646–655. [DOI] [PubMed] [Google Scholar]
- Sciotti V. M., Park T. S., Berne R. M. and Van Wylen D. G. L. (1993) Changes in extracellular adenosine during chemical or electrical brain stimulation. Brain Res. 613, 16–20. [DOI] [PubMed] [Google Scholar]
- Sharma R., Engemann S. C., Sahota P. and Thakkar M. M. (2010a) Effects of ethanol on extracellular levels of adenosine in the basal forebrain: an in vivo microdialysis study in freely behaving rats. Alcohol. Clin. Exp. Res. 34, 813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Engemann S., Sahota P. and Thakkar M. M. (2010b) Role of adenosine and wake‐promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. J. Neurochem. 115, 782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarphedinsson J. O., Sandberg M., Hagberg H., Carlsson S. and Thoren P. (1989) Relative cerebral ischemia in SHR due to hypotensive hemorrhage: cerebral function, blood flow and extracellular levels of lactate and purine catabolites. J. Cereb. Blood Flow Metab. 9, 364–372. [DOI] [PubMed] [Google Scholar]
- Slezia A., Kekesi A. K., Szikra T., Papp A. M., Nagy K., Szente M., Magloczky Z., Freund T. F. and Juhasz G. (2004) Uridine release during aminopyridine‐induced epilepsy. Neurobiol. Dis. 16, 490–499. [DOI] [PubMed] [Google Scholar]
- Song P., Mabrouk O. S., Hershey N. D. and Kennedy R. T. (2012) In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography‐mass spectrometry. Anal. Chem. 84, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Silva M. A., Muller C. P. and Huston J. P. (2007) Microdialysis in the brain of anesthesized vs. freely moving animals, in Handbook of Microdialysis – Methods, Applications and Perspectives. Handbook of Behavioral Neuroscience (Huston J. P., ed.), pp. 71–91. Elsevier – Academic Press, Amsterdam, The Netherlands. [Google Scholar]
- Tominaga M., Tsujimoto S., Takeshima T., Miyamoto K., Ishida Y., Nishitani M. and Sakaki T. (1992) Purine catabolites and pial arteriolar diameter in transient cerebral ischemia. No To Shinkei 44, 807–812. [PubMed] [Google Scholar]
- Valtysson J., Persson L. and Hillered L. (1998) Extracellular ischaemia markers in repeated global ischaemia and secondary hypoxaemia monitored by microdialysis in rat brain. Acta Neurochir. 140, 387–395. [DOI] [PubMed] [Google Scholar]
- Van der Mierden S., Savelye S. A., De Vrie R. B. and Leenaars C. H. C. (2016) Adenosine Concentrations in Microdialysates, Vol. 2016. SYRCLE, RadboudUMC, Nijmegen, the Netherlands, or online at http://www.SYRCLE.nl [accessed on July 2018]. [Google Scholar]
- Van Wylen D. G., Park T. S., Rubio R. and Berne R. M. (1986) Increases in cerebral interstitial fluid adenosine concentration during hypoxia, local potassium infusion, and ischemia. J. Cereb. Blood Flow Metab. 6, 522–528. [DOI] [PubMed] [Google Scholar]
- Vazquez‐DeRose J., Schwartz M. D., Nguyen A. T., Warrier D. R., Gulati S., Mathew T. K., Neylan T. C. and Kilduff T. S. (2014) Hypocretin/orexin antagonism enhances sleep‐related adenosine and GABA neurotransmission in rat basal forebrain. Brain Struct. Funct. 221, 923–940. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. (2010) Conducting meta‐analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. or online at http://www.jstatsoft.org/v36/i03/ [accessed on 29 October 2016]. [Google Scholar]
- de Vries R. B., Hooijmans C. R., Tillema A., Leenaars M. and Ritskes‐Hoitinga M. (2014a) Updated version of the Embase search filter for animal studies. Lab. Anim. 48, 88. [DOI] [PubMed] [Google Scholar]
- de Vries R. B., Wever K. E., Avey M. T., Stephens M. L., Sena E. S. and Leenaars M. (2014b) The usefulness of systematic reviews of animal experiments for the design of preclinical and clinical studies. ILAR J. 55, 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C. S., White S. E., Homan J. H., Kimura K. A., Brien J. F., Fraher L., Challis J. R. G. and Bocking A. D. (1999) Increased cerebral extracellular adenosine and decreased PGE(2) during ethanol‐induced inhibition of FBM. J. Appl. Physiol. 86, 1410–1420. [DOI] [PubMed] [Google Scholar]
- Westerink B. H. C. and Cremers T. I. F. H. (2007) Handbook of Microdialysis – Methods, Applications and Perspectives, Vol. 16: Handbook of Behavioral Neuroscience. Elsevier – Academic Press, Amsterdam, The Netherlands. [Google Scholar]
- Wigren H. K., Schepens M., Matto V., Stenberg D. and Porkka‐Heiskanen T. (2007) Glutamatergic stimulation of the basal forebrain elevates extracellular adenosine and increases the subsequent sleep. Neuroscience 147, 811–823. [DOI] [PubMed] [Google Scholar]
- Yan S., Laferriere A., Zhang C. and Moss I. R. (1995) Microdialyzed adenosine in nucleus tractus solitarii and ventilatory response to hypoxia in piglets. J. Appl. Physiol. 79, 405–410. [DOI] [PubMed] [Google Scholar]
- Zetterstrom T., Vernet L., Ungerstedt U., Tossman U., Jonzon B. and Fredholm B. B. (1982) Purine levels in the intact rat brain. Studies with an implanted perfused hollow fibre. Neurosci. Lett. 29, 111–115. [DOI] [PubMed] [Google Scholar]
- Zhang J. and Niu X. (1994) Changes of monoamines, purines and amino acids in rat striatum as measured by intercerebral microdialysis during ischemia/reperfusion. Chin. Med. Sci. J. 9, 225–229. [PubMed] [Google Scholar]
- Zhu Y. X., Wong P. S. H., Zhou Q., Sotoyama H. and Kissinger P. T. (2001) Identification and determination of nucleosides in rat brain microdialysates by liquid chromatography/electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 26, 967–973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Study characteristics of papers reporting baseline adenosine concentrations.
Appendix S2. Study characteristics of papers and abstracts not reporting absolute baseline adenosine concentrations or only reporting them in low‐resolution figures.
Appendix S3. Adenosine monophosphate concentrations and corresponding study characteristics.


