Abstract
IL‐5 is an important cytokine for priming and survival of mature eosinophils and for proliferation and maturation of their progenitors. Hence, IL‐5(Rα) targeting will be increasingly used in diseases where eosinophils are the key immune effector cells such as eosinophilic asthma (EA), hypereosinophilic syndrome (HES), eosinophilic esophagitis (EE), and eosinophilic granulomatosis with polyangiitis (EGPA). Therefore, several neutralizing monoclonal antibodies directed against IL‐5 (mepolizumab and reslizumab) and its receptor IL‐5Rα (benralizumab) have found or will find their way to the clinic. While the clinical effect of these drugs has been extensively investigated and reviewed, the understanding of the underlying immunological and hematological mechanisms remains less clear. This review will discuss the translational outcomes of treatment with these monoclonal antibodies in humans to shed light on the mechanisms underlying the main immunological and hematological findings from these clinical trials in humans.
Keywords: anti‐IL‐5, asthma, asthma therapy, eosinophils, IL‐5
1.
Highlights.
IL‐5 targeted therapy (mepolizumab or reslizumab) results in a clear attenuation of eosinophils in blood and a less distinct attenuation of eosinophils in (bronchial) tissue. Basophils are attenuated in blood.
Plasma and sputum IL‐5 levels are increased after treatment. A possible source of elevated levels of IL‐5 might be due to immune complexes between IL‐5 and anti‐IL‐5.
Early eosinophil progenitors (CD34+ and IL‐5Rα+ cells) might increase in blood and decrease in sputum after treatment.
2. INTRODUCTION
Therapies targeted on the IL‐5 pathway are a good extension in the treatment of patients with severe eosinophilic asthma.1 This therapeutical approach has also shown promising results in the treatment of nasal polyps, hypereosinophilic syndrome, eosinophilic granulomatosis with polyangiitis, and other hypereosinophilic disorders.2, 3, 4 For patients with severe eosinophilic asthma treatment with IL‐5‐targeting drugs result in a lower rate of exacerbations and a decrease in use of glucocorticoids. However, the quality of life and prebronchodilator FEV1 hardly improve in a clinically relevant way.1, 5, 6, 7, 8, 9, 10, 11, 12, 13 In addition, only half of the patients with eosinophilic granulomatosis with polyangiitis reached a remission.3 The lack of a complete clinical response in both diseases is difficult to understand as long as some important immunological and hematological issues of the therapy remain to be established. One of the key questions is whether eosinophils that remain in the body during IL‐5‐targeted therapy are an intrinsically different nonresponsive subset or residual “normal” cells, supporting the view that IL‐5 is not critical in human eosinophilopoiesis. A third possibility is that residual eosinophils are found because of under dosing of the monoclonal antibodies such as recently suggested.3, 14, 15 To gather translational data on IL‐5 inhibition: A systemic literature search in PubMed was performed on October 2017 with the following query: (mepolizumab OR nucala OR reslizumab OR benralizumab OR cinqaero OR “anti‐il‐5” OR “anti‐il‐5r” OR “anti il‐5” OR “anti il 5” OR “anti interleukin 5” OR “anti‐interleukin 5” OR “anti‐interleukin‐5”). This query yielded 749 articles that were assessed for relevance and validity on the basis of title and abstract firstly and on full text secondly.
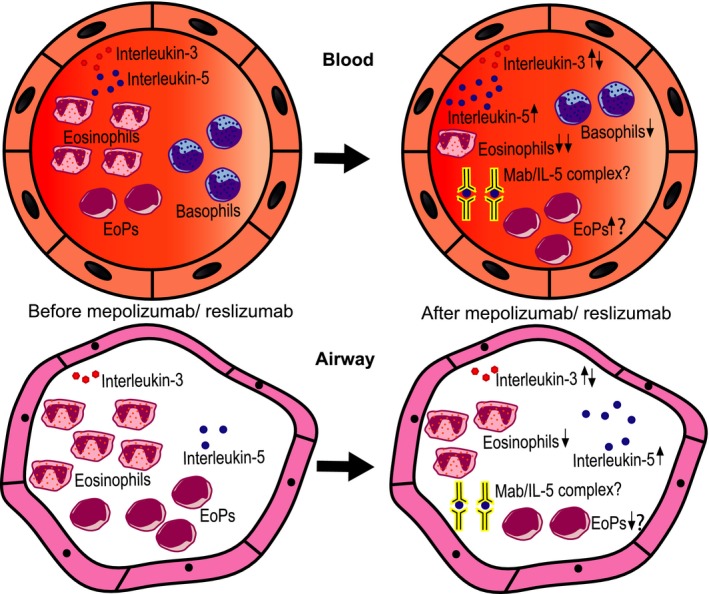
IL‐5 targeted therapy results in an attenuation of eosinophils and basophils in blood and an attenuation of eosinophils and EoPs in the airways. Plasma and sputum levels of IL‐5 increase, possibly due to the formation of immune complexes between IL‐5 and anti‐IL‐5.
3. THE FUNCTION OF INTERLEUKIN‐5 IN HOMEOSTASIS
3.1. Receptor of IL‐5 and its signal transduction
Interleukin‐5 is a cytokine which is produced as a dimer and secreted by multiple cells such as Th2 cells, mast cells, ILC2 cells, and eosinophils.16 It binds to the IL‐5‐specific α subunit—interleukin‐5Rα (CD125)—that is part of a heterodimeric receptor with the common β subunit (CD131). This latter subunit is shared with the heterodimeric IL‐3 receptor (CD123) and GM‐CSF receptor (CD116).17 The common β subunit does not express any ligand binding site but confers high‐affinity ligand binding to intracellular signaling.18 Upon binding of IL‐5 to its receptor, juxtamembranous tyrosine kinases phosphorylate the βc receptor upon which at least 3 major signaling pathways are activated: JAK/STAT, MAPK, and PI‐3K.19 All 3 pathways eventually lead to rapid reprogramming of gene expression and a plethora of cellular responses ranging from proliferation of eosinophil progenitors to priming of cytotoxicity by mature cells.20 Interestingly, IL‐5 with a charge reversal mutation at position 12 (E12K)21 and GM‐CSF with a similar mutation at position 21 (E21R),22 which are important for receptor binding, fail to stimulate tyrosine phosphorylation but can still affect survival.21 This finding reveals the existence of 2 distinct mechanisms of receptor activation, one of which is α‐chain specific.
3.2. Expression of IL‐5 receptors
In humans, the effects of IL‐5 are restricted to basophils and eosinophils.23 The expression of IL‐5Rα on basophils is threefold lower compared to mature eosinophils and their differentiation is not dependent on this cytokine.24 Therefore, the receptor is best characterized in the context of eosinophils. The receptor is both expressed on mature eosinophils and their progenitors including the eosinophil lineage–specific myeloblast (CD34+ and IL‐5Rα+ cells).25 As stimulation of CD34+/IL‐5Rα+ cells only produces eosinophils, it is yet not clear at what stage the IL‐5Rα is upregulated on basophils.25
In tissue, the level of IL‐5Rα on eosinophils is lower compared to blood eosinophils.26, 27 It seems plausible that the receptor is shed after migration to the tissue, because the amount of soluble IL‐5Rα is increased in tissue. This hypothesis is supported by the finding that IL‐5Rα is shed from the surface of healthy control eosinophils in vitro upon interaction with IL‐5. This is probably due to proteolytic cleavage.28 Whether IL‐5Rα expression is also found on type 2 innate lymphoid cells (ILC2) is still a subject of debate, because the results in literature are inconsistent.29, 30
3.3. The role of Il‐5R in differentiation and proliferation
IL‐5 stimulates eosinophil colony formation in bone marrow samples in vitro. IL‐3 and GM‐CSF can also give rise to eosinophil colonies in vitro, but unlike the other cytokines, IL‐5 is the most eosinophil specific.31, 32 The concentrations of IL‐3 and GM‐CSF in vitro need to be 10‐fold higher in order to create eosinophils from marrow mononuclear cells instead of solely neutrophils or monocytes.32 The formation of basophil colonies is also stimulated by IL‐5 in HL‐60 cells albeit to a lesser extent.32, 33
In vivo, the situation is more complex. Murine experiments show a central role of IL‐5 for reactive eosinophilia rather than differentiation per se, as IL‐5 knockout mice do contain mature eosinophils in peripheral blood. They, however, do not exhibit eosinophilia after parasite infection. In culture systems of human CD34+ progenitor cells, IL‐5 only induces transient proliferation and maturation of eosinophil precursors, which also suggests that IL‐5 is a relatively late‐acting factor for eosinophil proliferation rather than a factor for early differentiation.34 All these experiments imply that other factors drive differentiation of eosinophil precursors and IL‐5 is a major growth factor for IL‐5R expressing cells, at least in mice.34
3.4. The IL‐5R and survival
In vitro the presence of IL‐5 and GM‐CSF increases mature eosinophil survival up to 10 days by inhibition of apoptosis.35, 36, 37 The apoptotic eosinophils are normally recognized and phagocytosed by macrophages or to a lesser extent by small‐airway epithelial cells in a process generally referred to as efferocytosis.38, 39 In vivo the average circulatory lifespan of eosinophils was estimated to be between 11 and 63 hours. This large range is probably due to ex vivo manipulation of cells,40 the use of toxic label,40, 41, 42 and the difference in kinetics between homeostasis and pathological conditions.43 The effect of IL‐5 on these kinetics is unknown, but a clear negative correlation between eosinophil apoptosis and sputum IL‐5 levels was found in asthmatic patients, which suggests that IL‐5 is involved in inhibition of apoptosis in eosinophils in vivo.44 Another important receptor involved in eosinophil survival is Siglec‐8. In contrast to IL‐5, GM‐CSF, and IL‐3, activation of Siglec‐8 by Mab or glycan ligands induced cell death.45 Interestingly, this ROS‐dependent regulated cell death only occurred after priming of cells with IL‐5, GM‐CSF, or IL‐33.46 This finding suggests that steady state and resident eosinophils are less susceptible for Siglec‐8‐induced cell death.45
4. IL‐5‐TARGETED THERAPY: HOW AND WHAT ARE THE OPTIONS OR ALTERNATIVES?
Mepolizumab and reslizumab are both humanized monoclonal antibodies (Mab) that bind to and block the function of circulating IL‐5 and consequently prevent binding of IL‐5 to its receptor. Mepolizumab is an IgG1 kappa Mab registered to be administered via a subcutaneous injection with a fixed dose of 100 mg, whereas reslizumab is a IgG4 kappa Mab which is registered to be injected intravenously with a weight‐adjusted concentration of 3 mg/kg.47 Benralizumab is an IgG1 kappa antibody directed against the IL‐5Rα. The published data imply that benralizumab can completely deplete eosinophils and their (late) bone marrow progenitors by induction of antibody‐dependent cell‐mediated cytotoxicity (ADCC) executed by natural killer cells and/or macrophages both in vitro and in nonhuman primates in vivo.24 As phase 3 trials show promising results, this drug will probably be approved soon in the treatment of severe eosinophilic asthma as well.1 Omalizumab is a humanized monoclonal antibody that binds to Cε3 of IgE and blocks this protein from binding to its receptor that is located on mast cells and basophils.48 The required doses are dependent on body weight and serum levels of IgE. As IgE is important in the Th2 allergic immune response, omalizumab is prescribed for patients with severe allergic asthma with high levels of aero‐allergen specific IgE.49 Both anti‐IL‐5 and anti‐IgE target Th2‐mediated inflammation and are therefore eligible for treating patients with eosinophilic and allergic asthma. A systematic review of available literature has shown that mepolizumab and omalizumab have similar therapeutic effects and tolerability in responsive patients with severe asthma.50 As eosinophilic inflammation is not necessarily mediated through the “classical” IgE pathway,51 anti‐IL‐5 seems to be favorable in all patients with signs of eosinophilic inflammation, but a direct clinical comparison has not been performed yet.
5. KEY THERAPEUTIC EFFECTS OF IL‐5 INHIBITION IN EOSINOPHILIC‐DRIVEN DISEASES
All asthma trials have in common that the effect of IL‐5(R)‐targeted therapy primarily leads to a decrease in disease exacerbations and steroid dependence rather than a direct effect on asthma characteristics such as changes in BHR and lung function.1 For chronic rhinosinusitis with polyposis, both reslizumab and mepolizumab led to an improvement in symptoms for most, but not all patients.52, 53 Besides this clinical effect, surgery was no longer required in 30% of patients treated with mepolizumab.54 For eosinophilic esophagitis, anti‐IL‐5 did not lead to improvement of symptoms or histological remission.55 Treating patients with hypereosinophilic syndrome (HES) with mepolizumab resulted in a lower dependence of prednisolone in less than half of the patients.4 Similarly, less than half of the eosinophilic granulomatosis and polyangiitis (EGPA) patients treated with mepolizumab reached complete remission.3 Finally, in atopic dermatitis, mepolizumab did not lead to any clinical significant results, even though eosinophils may play a role in this disease.56
6. INTERLEUKIN‐5‐TARGETED THERAPY: DIFFERENCE BETWEEN MICE AND MEN
There are key differences between murine and human data regarding pathogenesis of asthma and IL‐5‐targeted therapy.23, 57, 58, 59 Therefore, this review focusses on human data rather than murine data.
7. EFFECT OF ANTI‐IL‐5(R) ON EOSINOPHIL AND BASOPHIL NUMBERS IN PERIPHERAL BLOOD AND TISSUES
There has not been a study performed that directly compared mepolizumab and reslizumab in a randomized controlled clinical trial. However, the results in the separate trials were comparable when it comes to reducing the number of circulating eosinophils (Table 1).5, 6, 7, 8, 9, 10, 11 Still, it was speculated that in a certain group of patients with steroid unresponsive asthma, the fixed lower concentration of mepolizumab (100 mg) might be insufficient to reduce the number of eosinophils in the tissues. It was even speculated based on preliminary studies that this low concentration might lead to formation of immune complexes between IL‐5 and mepolizumab potentially activating tissue eosinophils.14, 15 It was indeed found that lower concentrations of antibody were associated with lower reduction of both circulating and tissue eosinophils (Pavord et al 2012 Table 1).6, 60 However, no data to date have directly measured the formation of immune complexes under these conditions and it remains to be established whether immune complexes are a worry in IL‐5‐targeted therapy.
Table 1.
Mean eosinophil numbers in circulation or sputum before and after treatment with IL‐5‐targeted therapy
| Mean blood eosinophil before‐after treatment million/mL (N) | Mean blood eosinophil before‐after placebo million/mL (N) | Duration of treatment (frequency) | Mean % sputum eosinophil before‐after treatment (N) | Mean% sputum eosinophil before‐after placebo (N) | Treatment concentration (road of administration) | |
|---|---|---|---|---|---|---|
| Haldar (2009)5 | 0.32‐0.048 (29) | 0.32‐0.35 (32) | 52 wks (Q4W) | 6.8%‐0.95% (29) | 5.46%‐2.78% (32) | Mepolizumab‐ 750 mg (IV) |
| Pavord (2012)6 | 0.25‐0.03 (156) | 0.28‐ND (155) | 52 wks (Q4W) | 5.8%‐0.48% (21) | 6.8%‐ND (24) | Mepolizumab‐ 750 mg (IV) |
| Pavord (2012)6 | 0.25‐0.06 (153) | 0.28‐ND (155) | 52 wks (Q4W) | 13.9%‐8.9% (18) | 6.8%‐ND (24) | Mepolizumab‐ 75 mg (IV) |
| Bjermer (2016)7 | 0.59‐0.06 (102) | 0.60‐0.57 (103) | 16 wks (Q4W) | ‐ | ‐ | Reslizumab‐ 3.0 mg/kg (IV) |
| Castro (2011)8 | 0.50‐0.10 (52) | 0.50‐0.50 (50) | 12 wks (Q4W) | 10.7%‐0.49% (16) | 8.5%‐5.2% (15) | Reslizumab‐ 3.0 mg/kg (IV) |
| Castroa (2015)9 | 0.62‐0.14 (477) | 0.66‐0.52 (476) | 52 wks (Q4W) | ‐ | ‐ | Reslizumab‐ 3.0 mg/kg (IV) |
| Corren (2016)11 | 0.28‐0.021 (344) | 0.28‐0.28 (83) | 16 wks (Q4W) | ‐ | ‐ | Reslizumab‐ 3.0 mg/kg (IV) |
| Bleeckera (2016)10 | 0.45‐0.00 (202) | 0.46‐0.38 (202) | 48 wks (Q4W) | ‐ | ‐ | Benralizumab‐ 30 mg (SC) |
| Fitzgeralda (2016)12 | 0.47‐0.00 (234) | 0.47‐0.39 (238) | 56 wks (Q4W) | ‐ | ‐ | Benralizumab‐ 30 mg (SC) |
| Naira (2017)13 | 0.46‐0.00 (63) | 0.54‐0.34 (66) | 28 wks (Q4W) | 4.8%‐0.15% (8) | 4.9‐12.15% (4) | Benralizumab‐ 30 mg (SC) |
IV, intravenous; Q4W, every 4 wks; ND, not determined; SC, subcutaneous.
Mean of 2 cohorts.
Median instead of mean.
Benralizumab depletes circulating eosinophils completely, likely through ADCC, although in some patients, small number circulatory cells still remained.10, 12, 13, 61 However, the number of mucosal and/or submucosal airway eosinophils was less affected by the treatment when compared to the effect on the number of circulating cells.13, 62 It is too early to speculate how to interpret this counterintuitive finding, but the concept of the parallel presence of homeostatic and inflammatory eosinophils in the airways of eosinophilic asthmatics might shed some light on the tissue‐dwelling cells in the future.63
Next to altering eosinophil levels, 750 mg of mepolizumab also reduced the number of basophils in the tissue and bone marrow, although this reduction was less pronounced compared to eosinophils.64 It is, therefore, likely that basophils rely on different cytokines such as IL‐3.65, 66
The effect of mepolizumab on mast cells in tissue remains uncertain, because inconsistent results have been published.24, 64, 67 Evidence that mast cells express the IL‐5Rα receptor has only been provided in vitro in very limited number of studies.68
Surprisingly, even though the expression of IL‐5Rα is threefold lower on basophils, still a complete depletion of also basophils in peripheral blood is seen after treatment with benralizumab.24, 62 This suggests that even though basophils rely on IL‐3, a low membrane expression of IL‐5Rα can already induce clearance through ADCC.
8. IL‐5 IS NOT ESSENTIAL FOR EOSINOPHIL AND BASOPHIL DIFFERENTIATION AND PRODUCTION DURING HOMEOSTASIS
It is now tempting to speculate why in response to all above‐mentioned therapies a small amount of eosinophils can still be found in sputum or bronchial tissue in patients with asthma.5, 6, 8, 13, 62 Also treatment of patients with eosinophilic esophagitis with mepolizumab induced a reduction in the number of eosinophils in circulation and in esophageal tissue when compared to placebo but did not lead to complete depletion of the cells. Interestingly, the number of homeostatic eosinophils in the duodenum was not altered by mepolizumab even after treatment with a high concentration of mepolizumab (1500 mg).69 It is possible that IL‐5Rα expression can be redundant by co‐expression of IL‐3 and GM‐CSF receptors and the presence of sufficient amounts of IL‐3 or GM‐CSF. It might even be that certain tissue eosinophils lose their IL‐5Rα such as described for cells found in the BAL after segmental allergen challenge.70 To date, the data in the literature are not yet sufficient to reach such conclusion, but it is relevant to emphasize that tissue eosinophils are found in homeostasis without any indication that IL‐5 is driving this response. The IL‐5Rα expression on these resident eosinophils of the duodenum seems lower, compared to circulating eosinophil.69 This is not likely caused by homologous desensitization as it is completely unclear what the source of IL‐5 would be under these conditions, but ILC2 cells are a putative source.16
9. EFFECT OF ANTI‐IL‐5(RECEPTOR)MAB'S ON EOSINOPHIL PROGENITORS IN AND OUTSIDE THE BONE MARROW
Eosinophilopoiesis takes place in the bone marrow particularly under homeostasis. Under pathological conditions such as found in severe asthma, eosinophil progenitors (CD34+ and IL‐5Rα‐positive progenitors) have also been found in tissue and blood. This supports the hypothesis that extramedullary eosinophilopoiesis can take place.71 In fact, in severe prednisolone‐dependent asthma, the amount of eosinophil lineage–committed progenitor cells in sputum was over 700‐fold higher compared to healthy controls, supporting a role of extramedullary hematopoiesis in eosinophil‐driven disease.72
In the bone marrow, mepolizumab (administered 750 mg intravenously) reduced both the mature and late immature eosinophils (myelocytes and metamyelocytes) significantly compared to placebo while the number of EoPs (early progenitors) was not attenuated in blood or bone marrow.73 It can be hypothesized that IL‐5 mainly influences eosinophil proliferation and maturation of relatively late progenitors rather than the reduction of early progenitors themselves.73 However, the relative amount of late immature and mature eosinophils in the bone marrow was still high after 2 months of therapy (mean of 1.9% and 1.1%, respectively). This lack of complete depletion of relatively late progenitors might be due to a role of other cytokines, such as IL‐3 and GM‐CSF.73 Indeed, treatment with GM‐CSF also leads to eosinophilia.74
Sehmi et al72 showed an increase in blood levels of eosinophil lineage–committed progenitors compared to basal levels in patients who received 6 months of 100 mg mepolizumab by subcutaneous injection and not in patients who received placebo. This finding contradicts the fact that IL‐5 can upregulate its own receptor on CD34+ cells, because a reduction rather than in increase in EoPs would be expected.72, 75 Another finding of the same study was that the number of EoPs and mature eosinophils in sputum was not attenuated in these patients with severe asthma. The authors hypothesized that this might be due to insufficient bioavailability within the tissue of 100 mg mepolizumab opposed to 750 mg. Indeed, in a comparable cohort of patients with severe asthma and prednisolone dependence, the same investigators showed that the number of early progenitors (EoPs) in blood was not attenuated by a low dose of mepolizumab in contrast to a weight‐adjusted relatively high dose of intravenous reslizumab.14 The authors speculated that if also mepolizumab would have been administered high enough (weight‐adjusted), the number of EoPs would in fact be diminished with a consequent attenuation of sputum eosinophils. This hypothesis is, however, contradicted by the finding of an earlier study in which unaltered levels of EoPs in blood and bone marrow were found after treatment with 750 mg mepolizumab.73 In sputum, the amount of CD34+/IL‐5Rα+ cells was also attenuated after treatment with 750 mg mepolizumab, but this was also seen with placebo.73
In contrast to the findings obtained with anti‐IL‐5 antibodies, a complete depletion of eosinophils and their early progenitors in the bone marrow was seen in a very small cohort of 4 patient with asthma that were treated with a single dose of 1 mg/kg benralizumab (anti‐IL‐5Rα) via an intravenous injection.62 It is possible that this difference in the amount of bone marrow eosinophils and late progenitors in comparison with mepolizumab is caused by antibody‐dependent cell‐mediated cytotoxicity.24 Similarly, EoPs are also strongly attenuated in blood and sputum after treatment with benralizumab.61
10. EFFECT OF ANTI‐IL‐5(RECEPTOR) ON CELLULAR PRIMING AND ACTIVATION
IL‐5‐targeted therapy also affects the release of eosinophil basic proteins in these compartments. For instance, it has been described that eosinophil cationic protein (ECP) is found in enhanced levels in serum of asthma patients.76 Also increased concentrations of major basic protein (MBP),77 eosinophil cationic protein (ECP),78 and eosinophilic peroxidase (EPO) were found in tissue in this patient group.70, 76 These cytotoxic proteins can affect extracellular matrix (ECM)proteins in the reticular basement membrane (EMB).79 In addition, they can modulate TGF‐β1 expression by airway eosinophils. The concentrations of these mediators are blunted in BAL‐fluid of patients with atopic asthma after treatment with mepolizumab.79 This suggests that anti‐IL‐5 also reduces the degree of activation of eosinophils in the tissue. This hypothesis is supported by the finding that treatment with anti‐IL‐5 leads to inhibition of the activation of eosinophils by anti‐IL‐5 in a segmental antigen challenge model.80 In this study, the intermediate upregulation of β2‐integrin and the upregulation of P‐selectin glycoprotein ligand‐1 (PSGL‐1) on circulating eosinophils was decreased after mepolizumab.80 Eosinophils of patients treated with mepolizumab have also shown a reduced eotaxin‐induced shape change ex vivo.81
Treatment with anti‐IL‐5Rα antibodies (benralizumab) also caused a decreased concentration of ECP and eosinophil‐derived neurotoxin (EDN) in serum, meaning that despite ADCC, no harmful proteins are released in blood.82
All of the above suggest that anti‐IL‐5(Rα) not only attenuates eosinophil numbers but also reduces priming and/or activation of eosinophils and can therefore diminish inflammation and remodeling as well.
Despite these findings, the situation with priming and activation in vivo is more complex than these in vitro data suggest. This complexity is illustrated by the finding that the expression of activation‐associated markers on eosinophils is not all pointing at complete suppression of eosinophil priming by IL‐5(Rα)‐targeted therapy. This is particularly shown by the expression of β1‐integrin in their active configuration in blood eosinophils, which was not altered by treatment with mepolizumab.80
Also, the amount of major basic protein (MBP) in tissue was not found to drop with reduction in eosinophils.64 A possible explanation could be that the remaining eosinophils are still able to produce sufficient amounts of MBP or there is a different source of MBP‐1.83 Alternative tissue sources of MBP other than placenta are poorly defined although mRNA of the MBP gene (PRG1) has been found in multiple tissues.84 Similarly, unexpected was the finding that the rise of bronchoalveolar eosinophils after an allergen challenge was strongly reduced after administration of 750 mg of mepolizumab intravenously, whereas the remaining eosinophils still showed an IL‐5 signature (enhanced expression of CD23, CD44, and CD69).70
11. EFFECT OF IL‐5(Rα)‐TARGETED THERAPY ON IL‐5 LEVELS IN BLOOD AND TISSUE
Treatment with anti‐IL‐5 (mepolizumab or reslizumab) eventually results in an increase in plasma levels of IL‐5 (bound to Mab?) in patients with asthma, hypereosinophilic syndrome, and eosinophilic gastroenteritis even though there might be a slight decrease just after the start of treatment.81, 85 This increase was also partially found in sputum of patients with asthma treated with mepolizumab but was surprisingly not seen in patients treated with reslizumab.14 The authors of the latter article concluded that this difference was probably due to a low local concentration of mepolizumab (and not of reslizumab) which consequently would have resulted in the formation of immune complexes because the target antigen would still be in excess. These presumably long‐lived immune complexes would then be a stable source of IL‐5. Although this may be a plausible hypothesis, direct proof is lacking. An increase in the plasma level of IL‐5 was also seen with high doses of mepolizumab (750 mg) or an intermediate dose with reslizumab (1 mg/kg) in a small study.81, 85 These data do not rule out the formation of immune complexes, but alternative hypotheses such as the existence of a feedback/feedforward mechanism are equally plausible. Indeed, after treatment with mepolizumab, there was a suggestion that both IL‐5‐producing CD4+ and CD8+ cells increased in peripheral blood.81 This result has not been reproduced in another smaller study with reslizumab.85 It is, therefore, unfortunate that in the study of Mukherjee et al,14 no plasma levels of IL‐5 were presented. An argument against a dominant role of immune complexes in modulating IL‐5 levels in vivo came from data of a study on treating patients with benralizumab. Also in these patients, treatment with this antibody independently of the dose (25‐200 mg) resulted in a clear increase in serum level of IL‐5 while treating a similar cohort of patients with placebo did not.82 The increase in serum/plasma IL‐5 regardless of its cause during treatment with anti‐IL‐5(Rα) might have clinical consequences. For, there is a possibility that after termination of therapy, a rebound eosinophilia may arise. This has been suggested before for patients with hypereosinophilic syndrome, eosinophilic esophagitis, and nasal polyps receiving a single dose of reslizumab (varying dose of 1‐3 mg/kg).53, 85, 86, 87 Remarkably, this rebound eosinophilia was absent in asthma patients treated with a higher single dose of reslizumab: 1 mg/kg instead of 0.3 mg/kg.87 Moreover, in 2 studies in which patients were treated with multiple doses of mepolizumab at a higher dose of 10 mg/kg or 750 mg, a clear rebound phenomenon was also absent.81, 88 Apparently, multiple pathways are in control of eosinophilia. Interesting in this regard is the finding that treatment with GM‐CSF induces eosinophilia but only transiently.89 Nonetheless, these data implicate that discontinuation of the anti‐IL‐5 therapy should always be well monitored, because in some patients, aggravation of symptoms has been described, particularly when steroids were tapered before cessation of the IL‐5(Rα)‐targeted therapy.81, 88
12. EFFECT OF IL‐5(Rα)‐TARGETED THERAPY ON IL‐5Rα AND IL‐3Rα EXPRESSION LEVELS ON EOSINOPHILS
A next level of complexity in IL‐5(Rα)‐targeted therapy is the influence on the expression of cytokine receptors on eosinophils in vivo. Unfortunately, the effect of IL‐5‐targeted therapy on IL‐5Rα expression on eosinophils has not been sufficiently established. The only data available imply that anti‐IL‐5 therapy resulted in a small in the IL‐5Rα on eosinophils, but the results were not convincing.81 This study of Stein et al81 evaluated patients with different forms of eosinophilic inflammation that were treated with a high dose of mepolizumab. They found an 18% in IL‐5Rα‐expression on blood eosinophils together with an increase rather than a decrease in plasma IL‐5. However, in 2 other placebo‐controlled studies performed in different cohorts, this effect of anti‐IL‐5 on the expression of IL‐5R was absent.76, 90 A last relevant study was performed by Kelly et al70 showing the same but not statistically significant trend of an increase in IL‐5Rα on eosinophils in asthma patients that were treated with a single dose of 750 mg of mepolizumab after an antigen challenge. It is not completely clear why IL‐5Rα expression on eosinophils would increase after anti‐IL‐5, but an autoregulatory pathway could be a possible explanation. Another explanation could be that IL‐5 induced receptor shedding is naturally inhibited when less IL‐5 is available, but as there is an increase in IL‐5 after IL‐5 inhibition, this explanation is less likely.
Surprisingly and still not fully understood in the study of Kelly et al70 was the downregulation of IL‐3Rα expression on blood eosinophils in asthma patients after treatment with mepolizumab. The authors speculated that this change might be induced by mepolizumab during eosinophilopoiesis by yet poorly understood mechanism(s). It might also be possible that IL‐5 neutralization could have led to selective homing of IL‐3Rαhigh cells into the tissue.70 Supporting this hypothesis was the finding that IL‐3Rα expression on BAL eosinophils after the antigen challenge was not attenuated. Alternatively, one might argue that the low expression was caused by in situ eosinophilopoiesis from mobilized (early) progenitors under the influence of IL‐3 and/or GM‐CSF instead of IL‐5 leading to cells with specific expression profiles. This hypothesis is supported by a recent study63 potentially showing the existence of 2 distinct eosinophil subsets also in humans. This study of Mesnil et al63 found difference in IL‐3Rα expression between lung parenchymal and peribronchial eosinophils of healthy human subjects and asthmatic patients. It is therefore tempting to speculate that these resident eosinophils are more dependent on IL‐3 instead of IL‐5. This might explain why tissue eosinophilia persists in patients treated with IL‐5(Rα)‐targeted therapy despite low levels of eosinophils in peripheral blood.69
13. CONCLUSION
13.1. How to link the clinical action of IL‐5(Rα)‐targeted therapy with the cell biology of IL‐5?
Mechanisms of anti‐IL‐5 treatment are diverse and not fully understood (see graphical abstract and figure 1).
Figure 1.
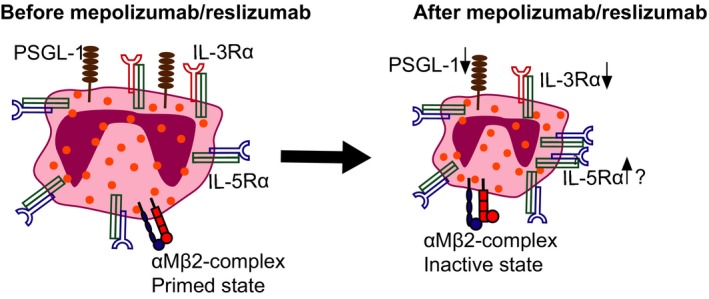
Treatment with anti‐IL‐5 results in a decrease in IL‐3Rα expression on eosinophils in blood, while the expression of IL‐5Rα might increase. Besides eosinophils change from a primed state to a more inactive state, which is characterized by a reduced eotaxin‐induced shape change and smaller size of eosinophils; downregulation of PSGL‐1 and a change of configuration of the (αM)β2‐complex
It is tempting to speculate why IL‐5‐targeted therapy is only beneficial for a part of the disease spectrum in patients with eosinophilic‐driven diseases. Firstly, eosinophils seem, especially in the tissue, only partially dependent on IL‐5. It is still unclear whether these cells are the resident cells63 or that redundancy with IL‐3 and GM‐CSF is present in the system. Several important issues need to be addressed before robust conclusions can be drawn. These include the following: (i) demonstration of the relative importance of local eosinophilopoiesis in affected tissues outside the bone marrow; (ii) direct identification of IL‐5/anti‐IL‐5 immune complexes during anti‐IL‐5 therapy; and (iii) local survival of eosinophils due to other cytokines than IL‐5 such as IL‐3 or GM‐CSF. It is also important to emphasize that inhibiting IL‐5 does not lead to complete disarmament of eosinophils which means that remaining eosinophils are still able to release toxins that can cause tissue damage. Lastly, it is possible that eosinophil‐dominated inflammation in the tissue is not exclusively mediated by eosinophils. This latter observation is illustrated for eosinophilic asthma where patients might still experience symptoms and exacerbations because of neutrophils.91 Also a (near to) complete depletion of eosinophils in patients with eosinophilic asthma receiving benralizumab does not clearly lead to a better improvement in the reduction in asthma exacerbations compared to the treatment with anti‐IL‐5.1 Interestingly, patients with severe “eosinophilic” asthma that are treated with dupilumab (anti‐IL‐4 receptor α‐subunit shared between IL‐4 and IL‐13 receptors) show a comparable or maybe an even better clinical improvement while the number of eosinophils in circulation and in sputum remains unchanged.92 This indicates that even within the spectrum of Th2‐driven diseases, the pathogenesis of inflammation leading to tissue damage is complex. It is, therefore, not particularly surprising that single mediator antagonists only affect part of the disease spectrum in patients with complex immune‐mediated diseases.
CONFLICTS OF INTEREST
Both authors declare that they have no conflict of interests.
Hassani M, Koenderman L. Immunological and hematological effects of IL‐5(Rα)‐targeted therapy: An overview. Allergy. 2018;73:1979–1988. 10.1111/all.13451
REFERENCES
- 1. Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti‐IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rivero A, Liang J. Anti‐IgE and anti‐IL5 biologic therapy in the treatment of nasal polyposis: a systematic review and meta‐analysis. Ann Otol Rhinol Laryngol. 2017;126:739‐747. [DOI] [PubMed] [Google Scholar]
- 3. Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med. 2017;376:1921‐1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215‐1228. [DOI] [PubMed] [Google Scholar]
- 5. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double‐blind, placebo‐controlled trial. Lancet. 2012;380:651‐659. [DOI] [PubMed] [Google Scholar]
- 7. Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789‐798. [DOI] [PubMed] [Google Scholar]
- 8. Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo‐controlled study. Am J Respir Crit Care Med. 2011;184:1125‐1132. [DOI] [PubMed] [Google Scholar]
- 9. Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double‐blind, randomised, placebo‐controlled, phase 3 trials. Lancet Respir Med. 2015;3:355‐366. [DOI] [PubMed] [Google Scholar]
- 10. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high‐dosage inhaled corticosteroids and long‐acting beta2‐agonists (SIROCCO): a randomised, multicentre, placebo‐controlled phase 3 trial. Lancet. 2016;388:2115‐2127. [DOI] [PubMed] [Google Scholar]
- 11. Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 Study of Reslizumab in Patients With Poorly Controlled Asthma: effects Across a Broad Range of Eosinophil Counts. Chest. 2016;150:799‐810. [DOI] [PubMed] [Google Scholar]
- 12. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti‐interleukin‐5 receptor alpha monoclonal antibody, as add‐on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2016;388:2128‐2141. [DOI] [PubMed] [Google Scholar]
- 13. Nair P, Wenzel S, Rabe KF, et al. Oral Glucocorticoid‐Sparing Effect of Benralizumab in Severe Asthma. N Engl J Med. 2017;376:2448‐2458. [DOI] [PubMed] [Google Scholar]
- 14. Mukherjee M, Aleman PF, Kjarsgaard M, et al. Weight‐adjusted intravenous reslizumab in severe asthma with inadequate response to fixed‐dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38‐46. [DOI] [PubMed] [Google Scholar]
- 15. Mukherjee M, Lim HF, Thomas S, et al. Airway autoimmune responses in severe eosinophilic asthma following low‐dose Mepolizumab therapy. Allergy Asthma Clin Immunol. 2017;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nussbaum JC, Van Dyken SJ, von Moltke J, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Broughton SE, Nero TL, Dhagat U, et al. The betac receptor family ‐ Structural insights and their functional implications. Cytokine. 2015;74:247‐258. [DOI] [PubMed] [Google Scholar]
- 18. Adachi T, Alam R. The mechanism of IL‐5 signal transduction. Am J Physiol. 1998;275:C623‐C633. [DOI] [PubMed] [Google Scholar]
- 19. Martinez‐Moczygemba M, Huston DP. Biology of common beta receptor‐signaling cytokines: IL‐3, IL‐5, and GM‐CSF. J Allergy Clin Immunol. 2003;112:653‐665. [DOI] [PubMed] [Google Scholar]
- 20. Coffer PJ, Koenderman L. Granulocyte signal transduction and priming: cause without effect? Immunol Lett. 1997;57:27‐31. [DOI] [PubMed] [Google Scholar]
- 21. McKinnon M, Page K, Uings IJ, et al. An interleukin 5 mutant distinguishes between two functional responses in human eosinophils. J Exp Med. 1997;186:121‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hercus TR, Bagley CJ, Cambareri B, et al. Specific human granulocyte‐macrophage colony‐stimulating factor antagonists. Proc Natl Acad Sci USA. 1994;91:5838‐5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takatsu K. Interleukin‐5 and IL‐5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:463‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kolbeck R, Kozhich A, Koike M, et al. MEDI‐563, a humanized anti‐IL‐5 receptor alpha mAb with enhanced antibody‐dependent cell‐mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344‐1353. [DOI] [PubMed] [Google Scholar]
- 25. Mori Y, Iwasaki H, Kohno K, et al. Identification of the human eosinophil lineage‐committed progenitor: revision of phenotypic definition of the human common myeloid progenitor. J Exp Med. 2009;206:183‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gregory B, Kirchem A, Phipps S, et al. Differential regulation of human eosinophil IL‐3, IL‐5, and GM‐CSF receptor alpha‐chain expression by cytokines: IL‐3, IL‐5, and GM‐CSF down‐regulate IL‐5 receptor alpha expression with loss of IL‐5 responsiveness, but up‐regulate IL‐3 receptor alpha expression. J Immunol. 2003;170:5359‐5366. [DOI] [PubMed] [Google Scholar]
- 27. Liu LY, Sedgwick JB, Bates ME, et al. Decreased expression of membrane IL‐5 receptor alpha on human eosinophils: I. Loss of membrane IL‐5 receptor alpha on airway eosinophils and increased soluble IL‐5 receptor alpha in the airway after allergen challenge. J Immunol. 2002;169:6452‐6458. [DOI] [PubMed] [Google Scholar]
- 28. Liu LY, Sedgwick JB, Bates ME, et al. Decreased expression of membrane IL‐5 receptor alpha on human eosinophils: II. IL‐5 down‐modulates its receptor via a proteinase‐mediated process. J Immunol. 2002;169:6459‐6466. [DOI] [PubMed] [Google Scholar]
- 29. Wright AKA, Weston C, Rana BMJ, Brightling CE, Cousins DJ. Human group 2 innate lymphoid cells do not express the IL‐5 receptor. J Allergy Clin Immunol. 2017;140:1430‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith SG, Chen R, Kjarsgaard M, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75‐86. [DOI] [PubMed] [Google Scholar]
- 31. Sanderson CJ, Warren DJ, Strath M. Identification of a lymphokine that stimulates eosinophil differentiation in vitro. Its relationship to interleukin 3, and functional properties of eosinophils produced in cultures. J Exp Med. 1985;162:60‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin‐5 (IL‐5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL‐1, IL‐3, IL‐6, and GMCSF. Blood. 1989;73:1504‐1512. [PubMed] [Google Scholar]
- 33. Denburg JA, Silver JE, Abrams JS. Interleukin‐5 is a human basophilopoietin: induction of histamine content and basophilic differentiation of HL‐60 cells and of peripheral blood basophil‐eosinophil progenitors. Blood. 1991;77:1462‐1468. [PubMed] [Google Scholar]
- 34. Sanderson CJ. Interleukin‐5, eosinophils, and disease. Blood. 1992;79:3101‐3109. [PubMed] [Google Scholar]
- 35. Yamaguchi Y, Suda T, Ohta S, Tominaga K, Miura Y, Kasahara T. Analysis of the survival of mature human eosinophils: interleukin‐5 prevents apoptosis in mature human eosinophils. Blood. 1991;78:2542‐2547. [PubMed] [Google Scholar]
- 36. Horie S, Okubo Y, Hossain M, et al. Interleukin‐13 but not interleukin‐4 prolongs eosinophil survival and induces eosinophil chemotaxis. Intern Med. 1997;36:179‐185. [DOI] [PubMed] [Google Scholar]
- 37. Owen WF Jr, Rothenberg ME, Silberstein DS, et al. Regulation of human eosinophil viability, density, and function by granulocyte/macrophage colony‐stimulating factor in the presence of 3T3 fibroblasts. J Exp Med. 1987;166:129‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kankaanranta H, Moilanen E, Zhang X. Pharmacological regulation of human eosinophil apoptosis. Curr Drug Targets Inflamm Allergy. 2005;4:433‐445. [DOI] [PubMed] [Google Scholar]
- 39. Duffin R, Leitch AE, Fox S, Haslett C, Rossi AG. Targeting granulocyte apoptosis: mechanisms, models, and therapies. Immunol Rev. 2010;236:28‐40. [DOI] [PubMed] [Google Scholar]
- 40. Farahi N, Singh NR, Heard S, et al. Use of 111‐Indium‐labeled autologous eosinophils to establish the in vivo kinetics of human eosinophils in healthy subjects. Blood. 2012;120:4068‐4071. [DOI] [PubMed] [Google Scholar]
- 41. Steinbach KH, Schick P, Trepel F, et al. Estimation of kinetic parameters of neutrophilic, eosinophilic, and basophilic granulocytes in human blood. Blut. 1979;39:27‐38. [DOI] [PubMed] [Google Scholar]
- 42. Parwaresch MR, Walle AJ, Arndt D. The peripheral kinetics of human radiolabelled eosinophils. Virchows Arch B Cell Pathol. 1976;1:57‐66. [DOI] [PubMed] [Google Scholar]
- 43. Dale DC, Hubert RT, Fauci A. Eosinophil kinetics in the hypereosinophilic syndrome. J Lab Clin Med. 1976;87:487‐495. [PubMed] [Google Scholar]
- 44. Xu J, Jiang F, Nayeri F, Zetterstrom O. Apoptotic eosinophils in sputum from asthmatic patients correlate negatively with levels of IL‐5 and eotaxin. Respir Med. 2007;101:1447‐1454. [DOI] [PubMed] [Google Scholar]
- 45. Kano G, Almanan M, Bochner BS, Zimmermann N. Mechanism of Siglec‐8‐mediated cell death in IL‐5‐activated eosinophils: role for reactive oxygen species‐enhanced MEK/ERK activation. J Allergy Clin Immunol. 2013;132:437‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Na HJ, Hudson SA, Bochner BS. IL‐33 enhances Siglec‐8 mediated apoptosis of human eosinophils. Cytokine. 2012;57:169‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Menzella F, Lusuardi M, Galeone C, Taddei S, Facciolongo N, Zucchi L. Mepolizumab for severe refractory eosinophilic asthma: evidence to date and clinical potential. Ther Adv Chronic Dis. 2016;7:260‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shields RL, Whether WR, Zioncheck K, et al. Inhibition of allergic reactions with antibodies to IgE. Int Arch Allergy Immunol. 1995;107:308‐312. [DOI] [PubMed] [Google Scholar]
- 49. Caruso M, Morjaria J, Emma R, Amaradio MD, Polosa R. Biologic agents for severe asthma patients: clinical perspectives and implications. Intern Emerg Med. 2018;13:155‐176. [DOI] [PubMed] [Google Scholar]
- 50. Cockle SM, Stynes G, Gunsoy NB, et al. Comparative effectiveness of mepolizumab and omalizumab in severe asthma: An indirect treatment comparison. Respir Med. 2017;123:140‐148. [DOI] [PubMed] [Google Scholar]
- 51. Morita H, Moro K, Koyasu S. Innate lymphoid cells in allergic and nonallergic inflammation. J Allergy Clin Immunol. 2016;138:1253‐1264. [DOI] [PubMed] [Google Scholar]
- 52. Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti‐IL‐5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989‐995. [DOI] [PubMed] [Google Scholar]
- 53. Gevaert P, Lang‐Loidolt D, Lackner A, et al. Nasal IL‐5 levels determine the response to anti‐IL‐5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133‐1141. [DOI] [PubMed] [Google Scholar]
- 54. Bachert C, Sousa AR, Lund VJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol. 2017;140:1024‐1031. [DOI] [PubMed] [Google Scholar]
- 55. Sawas T, Dhalla S, Sayyar M, Pasricha PJ, Hernaez R. Systematic review with meta‐analysis: pharmacological interventions for eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;41:797‐806. [DOI] [PubMed] [Google Scholar]
- 56. Oldhoff JM, Darsow U, Werfel T, et al. Anti‐IL‐5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60:693‐696. [DOI] [PubMed] [Google Scholar]
- 57. Kumar RK, Foster PS. Are mouse models of asthma appropriate for investigating the pathogenesis of airway hyper‐responsiveness? Front Physiol. 2012;3:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee JJ, Jacobsen EA, Ochkur SI, et al. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol. 2012;130:572‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kobayashi T, Iijima K, Kita H. Marked airway eosinophilia prevents development of airway hyper‐responsiveness during an allergic response in IL‐5 transgenic mice. J Immunol. 2003;170:5756‐5763. [DOI] [PubMed] [Google Scholar]
- 60. Pouliquen IJ, Kornmann O, Barton SV, Price JA, Ortega HG. Characterization of the relationship between dose and blood eosinophil response following subcutaneous administration of mepolizumab. Int J Clin Pharmacol Ther. 2015;53:1015‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sehmi R, Lim HF, Mukherjee M, et al. Benralizumab attenuates airway eosinophilia in prednisone‐dependent asthma. J Allergy Clin Immunol. 2018;141:1529‐1532. [DOI] [PubMed] [Google Scholar]
- 62. Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132:1086‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mesnil C, Raulier S, Paulissen G, et al. Lung‐resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126:3279‐3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Flood‐Page PT, Menzies‐Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti‐interleukin‐5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199‐204. [DOI] [PubMed] [Google Scholar]
- 65. Kampfer SS, Odermatt A, Dahinden CA, Fux M. Late IL‐3‐induced phenotypic and functional alterations in human basophils require continuous IL‐3 receptor signaling. J Leukoc Biol. 2017;101:227‐238. [DOI] [PubMed] [Google Scholar]
- 66. Yamada T, Sun Q, Zeibecoglou K, et al. IL‐3, IL‐5, granulocyte‐macrophage colony‐stimulating factor receptor alpha‐subunit, and common beta‐subunit expression by peripheral leukocytes and blood dendritic cells. J Allergy Clin Immunol. 1998;101:677‐682. [DOI] [PubMed] [Google Scholar]
- 67. Otani IM, Anilkumar AA, Newbury RO, et al. Anti‐IL‐5 therapy reduces mast cell and IL‐9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2013;131:1576‐1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dahl C, Hoffmann HJ, Saito H, Schiotz PO. Human mast cells express receptors for IL‐3, IL‐5 and GM‐CSF; a partial map of receptors on human mast cells cultured in vitro. Allergy. 2004;59:1087‐1096. [DOI] [PubMed] [Google Scholar]
- 69. Conus S, Straumann A, Bettler E, Simon HU. Mepolizumab does not alter levels of eosinophils, T cells, and mast cells in the duodenal mucosa in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:175‐177. [DOI] [PubMed] [Google Scholar]
- 70. Kelly EA, Esnault S, Liu LY, et al. Mepolizumab attenuates airway eosinophil numbers, but not their functional phenotype in asthma. Am J Respir Crit Care Med. 2017;196:1385‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gauvreau GM, Denburg JA. Hemopoietic progenitors: the role of eosinophil/basophil progenitors in allergic airway inflammation. Expert Rev Clin Immunol. 2005;1:87‐101. [DOI] [PubMed] [Google Scholar]
- 72. Sehmi R, Smith SG, Kjarsgaard M, et al. Role of local eosinophilopoietic processes in the development of airway eosinophilia in prednisone‐dependent severe asthma. Clin Exp Allergy. 2016;46:793‐802. [DOI] [PubMed] [Google Scholar]
- 73. Menzies‐Gow A, Flood‐Page P, Sehmi R, et al. Anti‐IL‐5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003;111:714‐719. [DOI] [PubMed] [Google Scholar]
- 74. Leentjens J, Kox M, Koch RM, et al. Reversal of immunoparalysis in humans in vivo: a double‐blind, placebo‐controlled, randomized pilot study. Am J Respir Crit Care Med. 2012;186:838‐845. [DOI] [PubMed] [Google Scholar]
- 75. Tavernier J, Van der Heyden J, Verhee A, et al. Interleukin 5 regulates the isoform expression of its own receptor alpha‐subunit. Blood. 2000;95:1600‐1607. [PubMed] [Google Scholar]
- 76. Buttner C, Lun A, Splettstoesser T, Kunkel G, Renz H. Monoclonal anti‐interleukin‐5 treatment suppresses eosinophil but not T‐cell functions. Eur Respir J. 2003;21:799‐803. [DOI] [PubMed] [Google Scholar]
- 77. Frigas E, Loegering DA, Solley GO, Farrow GM, Gleich GJ. Elevated levels of the eosinophil granule major basic protein in the sputum of patients with bronchial asthma. Mayo Clin Proc. 1981;56:345‐353. [PubMed] [Google Scholar]
- 78. Virchow JC Jr, Holscher U, Virchow C Sr. Sputum ECP levels correlate with parameters of airflow obstruction. Am Rev Respir Dis. 1992;146:604‐606. [DOI] [PubMed] [Google Scholar]
- 79. Flood‐Page P, Menzies‐Gow A, Phipps S, et al. Anti‐IL‐5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Johansson MW, Gunderson KA, Kelly EA, Denlinger LC, Jarjour NN, Mosher DF. Anti‐IL‐5 attenuates activation and surface density of beta(2) ‐integrins on circulating eosinophils after segmental antigen challenge. Clin Exp Allergy. 2013;43:292‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stein ML, Villanueva JM, Buckmeier BK, et al. Anti‐IL‐5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL‐5 and IL‐5 receptor levels. J Allergy Clin Immunol. 2008;121:1473:1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pham TH, Damera G, Newbold P, Ranade K. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med. 2016;111:21‐29. [DOI] [PubMed] [Google Scholar]
- 83. Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406‐17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. The human protein atlas. In: PRG2 gene; 2018.
- 85. Kim YJ, Prussin C, Martin B, et al. Rebound eosinophilia after treatment of hypereosinophilic syndrome and eosinophilic gastroenteritis with monoclonal anti‐IL‐5 antibody SCH55700. J Allergy Clin Immunol. 2004;114:1449‐1455. [DOI] [PubMed] [Google Scholar]
- 86. Klion AD, Law MA, Noel P, Kim YJ, Haverty TP, Nutman TB. Safety and efficacy of the monoclonal anti‐interleukin‐5 antibody SCH55700 in the treatment of patients with hypereosinophilic syndrome. Blood. 2004;103:2939‐2941. [DOI] [PubMed] [Google Scholar]
- 87. Kips JC, O'Connor BJ, Langley SJ, et al. Effect of SCH55700, a humanized anti‐human interleukin‐5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med. 2003;167:1655‐1659. [DOI] [PubMed] [Google Scholar]
- 88. Haldar P, Brightling CE, Singapuri A, et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12‐month follow‐up analysis. J Allergy Clin Immunol. 2014;133:921‐923. [DOI] [PubMed] [Google Scholar]
- 89. Olver IN, Hercus T, Lopez A, et al. A phase I study of the GM‐CSF antagonist E21R. Cancer Chemother Pharmacol. 2002;50:171‐178. [DOI] [PubMed] [Google Scholar]
- 90. Conus S, Straumann A, Simon HU. Anti‐IL‐5 (mepolizumab) therapy does not alter IL‐5 receptor alpha levels in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2009;123:269‐270. [DOI] [PubMed] [Google Scholar]
- 91. Denlinger LC, Sorkness RL, Lee WM, et al. Lower airway rhinovirus burden and the seasonal risk of asthma exacerbation. Am J Respir Crit Care Med. 2011;184:1007‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium‐to‐high‐dose inhaled corticosteroids plus a long‐acting beta2 agonist: a randomised double‐blind placebo‐controlled pivotal phase 2b dose‐ranging trial. Lancet. 2016;388:31‐44. [DOI] [PubMed] [Google Scholar]


