Summary
Type 1 diabetes (T1D) is an autoimmune disease, which is characterized by the destruction of islet β cells in the pancreas triggered by genetic and environmental factors. In past decades, extensive familial and genome‐wide association studies have revealed more than 50 risk loci in the genome. However, genetic susceptibility cannot explain the increased incidence of T1D worldwide, which is very likely attributed by the growing impact of environmental factors, especially gut microbiome. Recently, the role of gut microbiome in the pathogenesis of T1D has been uncovered by the increasing evidence from both human subjects and animal models, strongly indicating that gut microbiome might be a pivotal hub of T1D‐triggering factors, especially environmental factors. In this review, we summarize the current aetiological and mechanism studies of gut microbiome in T1D. A better understanding of the role of gut microbiome in T1D may provide us with powerful prognostic and therapeutic tools in the near future.
Keywords: environmental factors, genetics, gut microbiome, immune system, type 1 diabetes
1. INTRODUCTION OF TYPE 1 DIABETES
Type 1 diabetes (T1D) is an organ‐specific autoimmune disease due to T lymphocytes and other immune cell infiltrating and attacking pancreatic β cells, resulting in the destruction of β cells and progression to insulin deficiency.1 Type 1 diabetes is consisted of 2 subtypes. The majority of T1D is the autoimmune type (T1A), with a smaller proportion being the non‐autoimmune type, also known as the idiopathic type 1 diabetes (T1B). In clinical practice, islet cell autoantibodies, including anti‐insulin autoantibody (IAA), glutamic acid decarboxylase antibody (GADA), anti‐protein tyrosine phosphatase like protein (IA‐2A) and zinc transporter 8 antibody (ZnT8A) are used to diagnose T1A and identify high‐risk subjects.2, 3, 4 Epidemiologically, incidence of T1D increases at a rate of 3% to 5% per year.5 Moreover, multiple factors influence the susceptibility to T1D, including genetic and environmental factors. Although gender is a very important factor in a variety of autoimmune diseases (women are generally more susceptible than men), there is no gender difference in the prevalence of T1D in humans.6, 7, 8
With regard to genetic factors, more than 50 T1D susceptibility genes have been uncovered by familial linkage analysis and genome‐wide association studies. Notably, not every gene contributes to T1D susceptibility equally. Particularly, the HLA‐DR and ‐DQ genes account for approximately 40% to 50% of the disease risk.9, 10, 11 Other susceptibility genes, including PTPN22, IL2Ra, and CTLA4 are also interrelated closely with T1D.12, 13, 14
Environmental factors are considered to be another essential modulator of T1D.15, 16 In the islets of newly diagnosed diabetic children, an abundance of IFN‐γ and HLA could be found, indicating that viral infection may be involved in T1D. Subsequent studies demonstrated that viruses, especially, enterovirus and Coxsackie B virus, may speed up the progression of T1D, potentially by directly inducing insulitis or activating immune system through molecular mimicry of islet autoantigens.17, 18 Diet is another environmental factor influencing disease susceptibility. In non‐obese diabetic (NOD) mice, gluten‐free diet delayed diabetes, and early dietary exposure to cow's milk proteins accelerated T1D, which is possibly because of the fact that albumin in cow's milk is a molecular mimic of ICA‐1, a surface protein on β cells.19 Vitamin D is thought to be the protective factor against T1D, and the deficiency of vitamin D may increase the risk of T1D.20, 21 In addition, antibiotics have been used to treat infections for more than 50 years. In the last few years, researchers believed that the abuse of antibiotics is correlated with the increased incidence of T1D, but its exact role is still controversial. While some literature suggested antibiotics usage in early life increased T1D susceptibility, another study reported antibiotics protected from T1D in Bio‐Breeding diabetes‐prone (BB‐DP) rat or in NOD mice by modulating gut microbiome.22, 23, 24 Mikkelsen and colleagues showed that antibiotics usage within 1 year had nothing to do with the risk of developing T1D.25
2. GUT MICROBIOME AND TYPE 1 DIABETES
2.1. Introduction of gut microbiome
Gut microbiome contains approximately 500 to 1000 different bacterial species and 100 trillion (1014) bacteria residing in gastrointestinal tract. Because of the symbiotic relationship between gut microbiome and our body, gut microbiome also is called commensal bacteria.26 Microbes in the gut and intestine are generally divided into Gram‐positive (G+) and Gram‐negative (G−) populations.27, 28 Usually, gastrointestinal tracts are dominated by 4 bacterial phyla based on 16S rRNA sequencing, including Firmicutes, Bacteroides, Proteobacteria, and Actinobacteria.29 Firmicutes and Bacteroidetes constitute the most abundant phyla in the adult's gut and intestine, and Actinobacteria is predominant in the gut of breast‐fed infants.30 Bifidobacterium is the most abundant bacteria in Actinobacteria and considered to be probiotic microorganisms. Here we quote the definition of microbiota and microbiome by Samantha and Whiteside.31 Microbiota refers to microorganisms in a particular environment and can be detected by 16S rRNA sequencing. However, microbiome refers to a larger range, including not only 16S rRNA region but also the whole bacterial genome and products.31
2.2. The role of gut microbiome in T1D
Gut microbiome maintains a constantly dynamic and homeostatic condition. However, at the same time, it can be affected by multiple factors. During childbirth, gut microbiome in infants is largely determined by delivery mode. A study showed that gut microbes in vaginally delivered infants were closer to the mother's vaginal microbiome. In contrast, bacteria in the gut of infants who were delivered through C‐section were similar to those on mother's skin surface.32 Additionally, Biasucci and colleagues demonstrated that caesarean delivery affected the early biodiversity of intestinal bacteria of the newborn, indicating that the mode of delivery could influence the composition of intestinal bacteria substantially.33 After childbirth, gut microbiome is largely affected by diet, antibiotics usage, medicine, and even pH value of drinking water. For example, high‐fat and high‐sugar diet affected the incidence of diabetes by causing dysbiosis of gut microbiota, particularly the decreased Bifidobacteria spp.34 In NOD mice, treatment with a broad‐spectrum antibiotic altered the composition of gut microbes and decreased the proportion of regulatory T cells (Tregs) in the intestinal lamina propria, resulting in an increased incidence of T1D.22 On the contrary, Hu and colleagues reported that NOD mice received with antibiotics were protected from T1D development. In particular, when mothers were treated with antibiotics, the offspring could also be protected from T1D with an unknown mechanism.35 Another study showed that although antibiotic treatment in NOD mice changed insulin sensitivity as well as the composition of gut microbes, gut permeability was not altered in the following 8 weeks.36 Therefore, the impact of antibiotic treatment on gut microbiome and T1D is far from conclusive and may depend on the specific antibiotic used and time window of usage. Proton pump inhibitors are mainly used to depress gastric acid production and treat peptic ulcers. Treatment of proton pump inhibitors lowered gut microbial diversity. 37 The pH value of drinking water also influenced the composition and diversity of intestine bacteria.38, 39 In conclusion, multiple factors can affect gut microbiome. The dysregulation of gut microbiome is closely involved in the pathogenesis of T1D.40
Gut microbiome is regarded as another essential modulator of T1D susceptibility in recent years, illustrated by a very quickly growing number of studies reported. In 2008, Wen and colleagues found that specific pathogen free (SPF) condition protected myeloid differentiation primary response gene88 (MyD88)–deficient NOD mice from T1D. However, germ‐free (GF) environment reversed the disease protection in those mice, indicating that gut microbiome might be a critical factor in T1D pathogenesis.41 The following studies showed that Bacillus cereus could delay T1D onset and decrease disease incidence in GF NOD mice and BB‐DP rats.42 Bacterium A. muciniphila protected NOD mice from T1D, especially during infancy, suggesting that early postpartum was a critical time for the microbial protective role to take place.24 However, some peptides secreted by certain bacteria in the gut mimicked pancreatic autoantigen and activated diabetogenic CD8 T cells to accelerate insulitis in NOD mice.43 Similarly, Citrobacter rodentium was shown to promote the development of T1D.29 All these studies demonstrated that gut microbiome may be a pivotal modulator in T1D pathogenesis.
2.3. Taxonomic changes of gut microbiome in T1D pathogenesis
Taxonomic study of gut microflora is usually performed by 16S rRNA sequencing to identify the classification of phylum, family, genus, and species of gut microbes. Taxonomic changes of gut microbiome in T1D have been uncovered by a number of longitudinal as well as cross‐sectional case‐control studies in humans and experiments in animals (Table 1). In 2011, a longitudinal case‐control study was carried out by Knip and colleagues to explore the relationship between gut microbiome and T1D. The 16S pyrosequencing data showed that children with positive islet‐autoantibodies had higher Bacteroidetes/Firmicutes ratio and lower Shannon diversity existed in the gut microbiome.44, 45 Further studies indicated that Bacteroides dorei and vulgatus were significantly accumulated in children with high risk of T1D46, 47 and associated with autoantibody positivity.48, 49 The abundance of short‐chain fatty acids (SCFAs)‐producing bacteria as well as lactate‐producing bacteria was reduced in T1D patients.50 The reduced number of Lactobacillus and Bifidobacterium could also be observed at the onset of T1D.51 Similar results were replicated in animals, illustrated by the fact that more Lactobacillus and Bifidobacterium could be found in bio‐breeding diabetes‐resistant rat but less in T1D‐prone BB‐DP rats.52
Table 1.
The summary of changes in gut microbiome and possible effects in T1D
| Changes in Gut Microbes | Possible Effects or Function | Refs |
|---|---|---|
|
Clostridium perfringens ↑ Dialister invisus ↑ Gemella sanguinis ↑ Bifidobacterium longum ↑ Prevotella ↓ Akkermansia ↓ Bifidobacterium adolescentis ↓ Roseburia faecis ↓ Faecalibacterium prausnitzii ↓ |
Intestinal integrity ↓ Gut permeability ↑ Inflammation ↑ T1D predisposition ↑ |
29, 48
50, 57 |
|
Leptotrichia goodfellowii ↑ Bacillus cereus ↑ Enterobacter mori LMG 25706 ↑ |
Molecular mimicry Activate diabetogenic T cells T1D risk ↑ |
43 |
| Bacteroidetes/Firmicutes ↑ |
Occurs before and at the Diagnosis of T1D |
45 |
|
Bacteroides dorei ↑ Bacteroides vulgatus ↑ Faecalibacterium prausnitzii ↓ |
Involved in multiple autoimmune diseases Disruption of the epithelial layer? Manipulation of immune system? |
46, 47 |
|
Bifidobacteria ↓ Bacteroides genus ↑ |
Butyrate ↓ Bacterial translocation ↑ T1D predisposition ↑ |
48 |
|
Bacteroides adolescentis ↓ Roseburia faecis ↓ |
Regulatory T cells ↓ T1D risk ↑ |
48, 49 |
| Diversity ↓ |
SCFAs ↓ Inflammation ↑ T1D predisposition ↑ |
54, 55 |
Several cross‐sectional case‐control studies have been performed to reveal the differences of gut microbiome between T1D patients and healthy control subjects. When compared with healthy subjects, more phylum Bacteroidetes and lower abundance of 2 dominant Bifidobacterium species were found in T1D children.47 Mejía‐León and Barca compared gut microbiome in patients with newly diagnosed T1D, patients with T1D duration, and healthy controls. The results showed that that newly diagnosed T1D patients had an increased abundance of Bacteroides, whereas healthy control possessed more Prevotella.53
The changes in the composition of gut microflora in T1D result in the altered diversity of gut microbes. Several studies recruited children with islet autoantibody positivity and later progressed to clinical diagnosis of T1D and found the reduced diversity of intestinal bacteria before the disease onset.54 Similarly, reduced diversity of gut microbes was found in diabetic Sprague‐Dawley rats with Streptozotocin injection.55 All these data demonstrated that alteration in gut bacteria is closely related to T1D in both humans and animal models.
2.4. Mechanism of gut microbiome in T1D pathogenesis
Gut microbiome is numerous, diverse, and dynamic. It is critical to determine its role in T1D predisposition and pathogenesis. However, the mechanism is very complicated and remains elusive so far. To our knowledge, gut microbiome may exert function by affecting intestinal permeability, molecular mimicry, and modulating innate and adaptive immune system.
2.4.1. Intestinal permeability
The intestinal barrier maintains mucosa permeability and separates the luminal antigens from the interior of the body. When intestinal barrier is disrupted, intestinal permeability may increase. Studies showed that when intestinal barrier is compromised, pancreatic‐draining lymph node T cells, particularly diabetogenic CD8+ T cells, would be activated and proliferate, promoting insulitis. It has been reported that intestinal permeability in children with T1D was significantly higher than that in controls.56 Maffeis and colleagues chose 10 healthy subjects and 10 children at risk for T1D to participate in a case‐control study to examine the relationship between intestinal permeability and T1D. The results showed that 3 bacteria, including Dialister invisus, Gemella sanguinis, and Bifidobacterium longum, were associated with altered intestinal permeability in T1D.57 In addition, many other studies revealed that the changes of certain microbes, such as Clostridium perfringens, Dialister invisus, Gemella sanguinis, and Bifidobacterium longum, were related to compromised gut integrity and increased T1D risk.29, 48, 50, 57 When the intestinal permeability is increased, intestinal toxins, food antigens, and infection factors may translocate from gastrointestinal lumen to intestinal mucosal components, and finally to the pancreatic lymph nodes to induce or exacerbate T1D.29
2.4.2. Molecular mimicry
Considering the various and numerous bacteria in the gut, it is not easy to estimate the amount of bacterial proteins or metabolites produced. Of note, a number of bacterial proteins have been demonstrated to share the similar molecular structure with self‐antigen in pancreas. An illustration is Mgt protein of Leptotrichia goodfellowii and islet‐specific glucose‐6‐phosphatase‐related protein (IGRP). IGRP protein is a member of G6Pase family and specifically expressed in pancreas. IGRP206‐214 peptide (VYLKTNVFL) is an important epitope that can activate diabetogenic NY8.3 T cells. Leptotrichia goodfellowii is a member of Fusobacteria. Interestingly, although Fusobacteria usually composes a small portion in the gut microbiome (<0.1%), it is closely related to autoimmune disease, inflammatory bowel diseases (IBDs), and skin ulcers.58, 59, 60
A fragment of Mgt protein in L. goodfellowii (267‐275, TYLKTNVFT) shares sequence similarity with IGRP206‐214. Experimentally, Mgt267‐275 activated NY8.3 T cells and accelerated T1D in NOD mice. In addition to L. goodfellowii, Flavobacteriia bacterium, Bacillus cereus, and Enterobacter mori LMG 25706 also possessed homologue peptides of IGRP206‐214, which were functional in vitro and in vivo.43 These findings illustrate that certain diabetogenic microbes exist in the gut and can induce or speed up the occurrence of T1D through molecular mimicry.
Notably, molecular mimicry is an important mechanism of gut microbiome not only for T1D but also for many other autoimmune diseases. Systemic lupus erythematosus is an autoimmune disease characterized by multi‐system damage. Unlike T1D, B cells and autoantibodies play an essential role in disease pathogenesis. Hevia and colleagues recruited 20 remission patients and matched healthy control. They found a lower Firmicutes/Bacteroidetes ratio in the gut microbes from Systemic lupus erythematosus patients, but the exact mechanism was not further studied.61 Following studies demonstrated that B. fragilis, a member of Bacteroides bacteria phylum, can recognize and stimulate VH4‐34‐encoded IgG+ autoreactive B cells.62 Interestingly, molecular mimicry does not always exacerbate autoimmune diseases. Bacteroides express integrase that contains a low‐avidity mimotope for IGRP206‐214. This mimotope helps to recruit diabetogenic CD8+ T cells to gut and further suppress IBD by targeting gut dendritic cells.63 Therefore, molecular mimicry generally is a very important mechanism for gut microbiome to induce autoimmune diseases, but its exact role may depend on different diseases and scenarios.
2.4.3. Innate immune system
Multiple lymph cells infiltrate into the pancreas during early insulitis, including macrophages. Pancreatic macrophages reside in intra‐islet vessels and are in close contact with β cells.64 Pancreatic macrophages capture dense core and present islet granules to islet reactive CD4+ T cells, contributing to the destruction of β cells.65 Ferris and colleagues found that macrophages in NOD islets possessed an activated state, presenting with the elevated sensitivity to stimuli and increased expression of inflammatory transcripts, like Cxcl9, Ccl5, and Cd40. More importantly, injection of lipopolysaccharide (LPS), a layer of lipid polysaccharide in the outermost layer of gram negative bacteria, resulted in rapid inflammation in the pancreas, indicating that islet macrophages can sense and respond to gut microbiome and accelerate T1D.64
Gut microbiome possesses multiple pathogen‐associated molecular patterns, such as LPS, lipoproteins, peptidoglycan, and their nucleic acids. The reorganization of pathogen‐associated molecular patterns depends on pattern‐recognition receptors in the host, particularly toll‐like receptors.66 Gut microbes can trigger different toll‐like receptors to induce both pro‐diabetogenic and anti‐diabetogenic signals. Toll‐like receptor‐induced signals are mediated by signalling adaptors, such as MyD88, to promote cellular responses to LPS.67, 68 Myeloid differentiation primary response gene88–deficient NOD mice in SPF environment were protected from diabetes. Surprisingly, T1D was developed in the GF Myd88‐negative NOD mice, indicating that the T1D‐protective effect required signals from gut microbiome.69
2.4.4. Adaptive immune system
In addition to innate immunity, the interaction between gut microbes and adaptive immunity is essential for the development and pathogenesis of T1D. Some particular gut bacteria have the capacity to regulate T cell subsets and function. Listeria can induce Th1 response, and segmented filamentous bacteria can augment Th17 responses. The altered schaedler flora and consortia of Clostridia have the capability to induce regulatory T cells.70, 71 Furthermore, altered gut microflora can increase the number of type 1 regulatory T (Tr1) cells in the intestine. These Tr1 cells can migrate into the periphery, inhibiting the activation of effector T cells and decreasing diabetes incidence.72
So far the exact mechanism for the bacteria mentioned above to promote the differentiation of naïve T cells into different helper T cells is largely unknown. Short‐chain fatty acids, which are secreted by gut microbes, have been proved to exert an important role. Short‐chain fatty acids, including acetate, propionate, and butyrate, are produced by bacterial fermentation of cellulose that cannot be digested by host enzymes.73 Short‐chain fatty acids may support epithelial cell integrity as well as the function of adaptive immune cells (e.g., promoting peripheral regulatory T‐cell generation).74, 75 Mariño and colleagues reported that mice dieted with acetate and butyrate were protected from T1D. They further demonstrated that acetate markedly decreased the frequency of autoreactive T cells in lymphoid tissues and butyrate increased the number and function of regulatory T cells. Acetate and butyrate also maintained intestinal integrity and decreased the concentration of diabetogenic cytokines in the serum, like IL‐21.76 As mentioned earlier, SPF rather than GF Myd88 negative NOD mice showed delayed onset of T1D. Correspondingly, the concentration of acetate and butyrate in the serum of SPF Myd88 deficient NOD mice was much higher when compared with those in GF transgenic NOD mice. To further explore the potential mechanism, GF Myd88‐deficient NOD mice were fed with acetate or butyrate for 5 weeks. The reduced frequency of autoreactive T cells and the increased number of Treg cells were found in the spleen and colon, suggesting that SCFAs produced by gut microbes are essential in disease protection.76 Bacteroides fragilis could induce Treg cells and enhance its suppressive function, which also required butyrate.77, 78
In addition to T cells, SCFAs are able to influence B cell activity.79 Recently, Kim and colleagues demonstrated that SCFAs regulated the energy metabolism of B cells in the gut, and promoted B cells differentiating into plasma cells and memory B cells through BCR‐activating antigens, resulting in more IgG and IgA production.80, 81 Meanwhile, IgA can coat a substantial amount of gut microbes to protect from exotic pathogens and maintain the homeostasis of the intestine. This is essential for bacteria colonization in the gut of neonates. Therefore, IgA can be used as a marker for colonization of gut microbiome.70
3. GUT MICROBIOME AS A BIOMARKER FOR T1D
We have shown that gut microbiome is closely related to the occurrence and development of T1D. As mentioned earlier, the composition of intestinal microflora is altered before the onset of T1D, including a reduced microbial diversity and increased Bacteriodetes in subjects with pre‐T1D.9 Specifically, the abundance of B.dorei in Bacteriodetes is increased and can be a useful predictor for T1D in Finland. Furthermore, those Bacteroides strains are resistant to common antibiotics and correlated with high‐protein and high‐fat diets.46 However, an important issue is that the composition of gut microbes largely depends on environmental factors (e.g., geographic location, eating habits, and sanitary conditions). Therefore, the results found in Finland may not be able to apply to other geographical locations. In addition, we need to evaluate the advantage of gut microbiome in T1D prediction, when compared with islet autoantibodies that have already been used in the clinical practice to predict T1D. The value of gut microbiome in T1D prediction requires further studies.
4. T1D TREATMENT AND GUT MICROBIOME
Besides disease prediction, the application of gut microbiome to treat T1D seems promising. In animals, the incidence of T1D in disease‐prone mice could be dramatically reduced when cohorting with normal mice or orally gavage with faecal samples from healthy mice.43 Oral probiotic administration could also prevent diabetes development in NOD mice.82 Interestingly, a recent study reported that accurate regulation of gut microbiome by using tungstate, which could specifically inhibit molybdenum‐cofactor‐dependent microbial respiratory pathways, relieved IBD with minimal side effects.83 The transformation or precision editing of gut microbiome may be applied to treat T1D in the future. In humans, several clinical trials using probiotics to treat T1D are undergoing, which are summarized in Table 2.
Table 2.
The summary of gut microbiome in clinical trials of type 1 diabetes
| NCT No. | Trial Name | Interventions | Intervention Model | Outcome Measures | Estimated Enrollment | Sponsors | Status |
|---|---|---|---|---|---|---|---|
| NCT03423589 | Modulation of type 1 diabetes susceptibility through the use of probiotics | VSL#3 | Single group assignment | Transcriptional analysis, gut microbiota | 30 participants | Medical College of Wisconsin | Recruiting |
| NCT02903615 | Optimizing health in type 1 diabetes | Novel diet: Prebiotic fibre focus, lower carbohydrate, Mediterranean‐style. Standard diet | Parallel assignment | Glucose control | 20 participants | Garvan Institute of Medical Research | Recruiting |
| NCT02605148 | TEFA family prevention: Gluten‐free diet to preserve Beta‐cell function (TEFA) | Gluten free diet with probiotics, omega 3 fatty acid, vitamin D supplement | Parallel assignment | β‐cell function, glucose control | 60 participants | Lund University | Recruiting |
| NCT02442544 | Prebiotic fibre supplement in T1DM children |
Prebiotic, Placebo |
Parallel assignment | Glucose control, gut microbiota | 38 participants | University of Calgary | Active, not recruiting |
| NCT03032354 | Probiotics in newly recognized type 1 diabetes | Lactobacillus rhamnosus GG and Bifidobacterium lactis BB12, placebo | Parallel assignment | β‐cell function | 96 participants | Medical University of Warsaw | Active, not recruiting |
The modulation of gut microbiome may be a valuable approach for T1D treatment. Nevertheless, T1D treatment with drugs might affect gut microbiome the other way round. Currently, insulin is commonly used for glycemic control in T1D patients, while certain immunomodulators and traditional Chinese medicine are in the pre‐clinical or clinical trials. Their effects on gut microbiome are discussed below.
4.1. Insulin
The previous literature showed that several drugs commonly used in the treatment of type 2 diabetes, such as metformin, liraglutide, and saxagliptin, had an impact on the composition of gut microbiome.84, 85 Insulin administration is commonly used in patients with T1D, but whether insulin treatment can affect gut microbiome is still unknown. A recent article investigated it by using the NOD mouse model. From 4 to 9 weeks of age, mice were given by gavage porcine insulin twice a week, followed by once a week of insulin for 21 weeks. Faeces were collected and gut microflora composition was determined by 16 s RNA sequencing. As a result, they found that oral insulin administration had no effect on gut microbiota.86 The possible reason could be that most of insulin was degraded when it passed through the intestine, causing its low bioavailability. However, in clinical practice, insulin is commonly subcutaneously injected in T1D patients. So far, whether insulin administration can affect gut microbiome in T1D patients has not been reported yet.
4.2. Immunomodulators
Since T1D is an autoimmune disease, several immunomodulators were accessed for their potential in T1D immunotherapy. Among those, the anti‐CD20 chimeric mAb (rituxizumab that depletes most of B cells), the humanized anti‐CD3 mAbs (teplizumab and otelixizumab), glutamic acid decarboxylase 65 (to induce antigen specific tolerance), DiaPep277 (the peptide corresponding to the human heat shock protein 60437–460), and abatacept (CTLA‐4‐Ig fusion protein that inhibits T cell activation) have been evaluated in clinical trials.87, 88, 89, 90, 91, 92 Although most of immunomodulators showed only modest success in clinical studies, their effects on gut microbiome have not been known yet.
In mouse models, several studies suggest that certain immunomodulators can alter gut microbiome. In allogeneic skin graft mouse, teplizumab administration protected against disease by increasing IL‐10–producing T cells. Interestingly, those T cells were required to migrate into the intestine to exert the protective function. If the migration was blocked, the treatment effects of teplizumab were abolished, indicating that gut microbiome might be involved in teplizumab‐mediated disease protection.93 The following study found that alteration of gut microbiome during anti‐CD3 mAb treatment or cohousing experiments significantly boosted intestinal IL‐10–producing Tr1 cells and decrease T1D incidence.72 Further studies may be required to clarify how gut microbiome is altered after anti‐CD3 mAb treatment.
4.3. Traditional Chinese medicine
Traditional Chinese medicine, also called herbal medicine (HM), has been used in worldwide for a thousand years. Most HMs are taken orally, which may preferentially alter gut microflora. An interplay between HMs and gut microbes might exist. On one hand, gut bacteria play an essential role in HM therapy by biotransforming HM chemicals into effective smaller units that can be better absorbed.46 On the other hand, HM chemicals improve the composition of intestinal flora and pathological condition. So far, most HMs have not been studied in T1D yet, except very few reports. Ganoderma lucidum could drop the increased Firmicutes/Bacteriodetes ratio to normal level.94 Berberine, a plant benzylisoquinoline alkaloid, showed the capability to protect NOD mice from T1D by modulating immune system.53 Artemisinin and Danzhi Jiangtang Capsule could improve the function islet β cells by increasing β‐cell mass and reducing pancreatic β‐cell apoptosis, respectively.95, 96 However, whether and how gut microbiome is altered in HMs‐mediated disease protection is unknown yet.
5. CONCLUSION AND PERSPECTIVE
Currently, the role and mechanism of gut microbiome in T1D attract more and more attention. A rapid growing number of publications have been reported in recent years. Although great progress has been made, many details are still missing and a large number of questions need to be answered.
Genome‐wide association studies have identified more than 50 T1D‐related susceptibility genes. Among them, HLA genes contribute to disease susceptibility substantially in both humans and NOD mice. Replacement of NOD IA‐g7 with C57BL/6 Eα16 prevented T1D in transgenic NOD mice, because of the altered antigen presentation for islet autoantigens. However, a recent article reported that the disease protection in Eα16/NOD mice also depended on gut microbiome, illustrated by the fact that GF or antibiotics‐treated Eα16/NOD lost the disease protection. These data indicate that an interplay between T1D susceptibility genes and gut microbiome may exist and play a crucial role in the pathogenesis of T1D.97
Notably, many susceptibility genes (e.g., IL‐10, IFIH1, TNFAIP3, PTPN2, and FUT2) are shared between T1D and IBD.98, 99 Whether those genes have an impact on gut microflora in T1D is unknown. If the impact exists, how to establish the link between susceptibility genes, altered gut bacteria, and T1D pathology requires further studies.
To sum up, T1D is an autoimmune disease triggered by both genetic and environmental factors. In past decades, a substantial progression has been made to clarify the genetics risk factors by using genome‐wide association studies. But genetics alone is not sufficient to explain why the prevalence of T1D increases at a rate of 3% to 5% per year, when considering genetics in population to be relatively stable. In recent years, the importance of environmental factors in T1D, especially gut microbiome, has been realized. Growing attention has been paid to clarify the taxonomic and functional changes of gut microbiome in the disease pathogenesis, and the interplay between gut microbiome with immune system, which is becoming the hotspot of both research and clinical trials in the field.
Gut microbiome, especially SCFAs, are important to maintain intestinal barrier and immune homeostasis. Evidence so far has demonstrated that dysbiosis of gut microbiome increases T1D predisposition. In humans or animals, the decreased diversity of gut microbiome occurs before disease onset and remains after the diagnosis of T1D. Bacteroidetes and Firmicutes are dominant in the faecal samples. The ratio of Firmicutes to Bacteroidetes is decreased in T1D patients. Dialister invisus, Gemella sanguinis, and Bifidobacterium longum are associated with intestinal permeability. Once the balance of gut microflora is disrupted, the compromised intestinal mucosa permeability may lead to outer or bacterial antigen leakage, boosting excessive immune response or activating autoreactive T cells by molecular mimicry (Figure 1).
Figure 1.
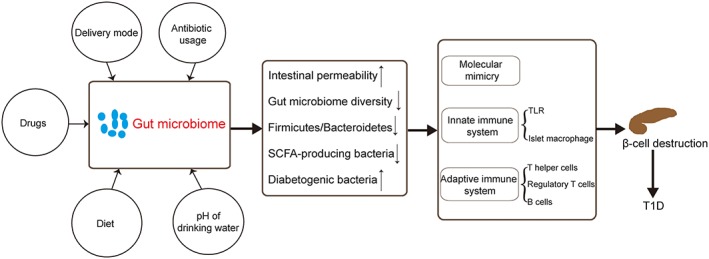
The role of gut microbiome in type 1 diabetes. Multiple factors can influence gut microbiome, resulting in an increase in intestinal permeability, a decrease in gut microbiome diversity, and Firmicutes/Bacteroidetes ratio. Consequently, these changes may destroy islet β cells through molecular mimicry, excessive activation of innate immunity, and adaptive immunity
In light of these results, gut microbiome may be a critical modulator in T1D predisposition and pathogenesis. Continuous studies are required to obtain a better understanding on the roles and mechanisms of gut microbiome in T1D, which may provide a valuable tool to predict and treat T1D in the future.
CONFLICT OF INTEREST
Authors Zheng P, Li Z, and Zhou Z declare that they have no conflict of interest.
ETHICAL APPROVAL
This review article cites the peer‐reviewed articles of our group and other groups. Therefore, the study designs are detailed in the primary articles, and this review does not include a study design with the direct ethical statements of the human study or animal study designs. For our own published studies cited in this article, all animal studies go through the office of human studies or the animal studies offices to confirm the ethical treatment of humans and animals in research.
ACKNOWLEDGEMENTS
We thank Ms Yani Peng for language embellishment of the manuscript. This work is supported by the National Natural Science Foundation of China (grant no. 81500600, 81670716, and 81461168031), the National Science and Technology Infrastructure Program (2015BAI12B13), and the Graduate innovation project of Central South University (grant no. 502211703).
Zheng P, Li Z, Zhou Z. Gut microbiome in type 1 diabetes: A comprehensive review. Diabetes Metab Res Rev. 2018;34:e3043 10.1002/dmrr.3043
REFERENCES
- 1. Boldison J, Wong FS. Immune and pancreatic beta cell interactions in type 1 diabetes. Trends Endocrinol Metab. 2016;27(12):856‐867. [DOI] [PubMed] [Google Scholar]
- 2. Yi B, Huang G, Zhou ZG. Current and future clinical applications of zinc transporter‐8 in type 1 diabetes mellitus. Chin Med J (Engl). 2015;128(17):2387‐2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang L, Luo S, Huang G, et al. The diagnostic value of zinc transporter 8 autoantibody (ZnT8A) for type 1 diabetes in Chinese. Diabetes Metab Res Rev. 2010;26(7):579‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chao C, Huang G, Li X, et al. Change of glutamic acid decarboxylase antibody and protein tyrosine phosphatase antibody in Chinese patients with acute‐onset type 1 diabetes mellitus. Chin Med J (Engl). 2013;126(21):4006‐4012. [PubMed] [Google Scholar]
- 5. Wang Z, Xie Z, Lu Q, et al. Beyond genetics: what causes type 1 diabetes. Clinic Rev Allerg Immunol. 2016;52(2):273‐584. [DOI] [PubMed] [Google Scholar]
- 6. Amur S, Parekh A, Mummaneni P. Sex differences and genomics in autoimmune diseases. J Autoimmun. 2012;38:254‐265. [DOI] [PubMed] [Google Scholar]
- 7. Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994;96(5):457‐462. [DOI] [PubMed] [Google Scholar]
- 8. Yurkovetskiy L, Burrows M, Khan AA, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gülden E, Wong FS, Wen L. The gut microbiota and type 1 diabetes. Clin Immunol. 2015;159(2):143‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pociot F, McDermott MF. Genetics of type 1 diabetes mellitus. Genes Immun. 2002;3(5):235‐249. [DOI] [PubMed] [Google Scholar]
- 11. Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity. 2010;32(4):468‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Belle TL, Coppieters KT, Coppieters MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91(1):79‐118. [DOI] [PubMed] [Google Scholar]
- 13. Zheng P, Kissler S. PTPN22 silencing in the NOD model indicates the type 1 diabetes‐associated allele is not a loss‐of‐function variant. Diabetes. 2013;62(3):896‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xie Z, Chang C, Zhou Z. Molecular mechanisms in autoimmune type 1 diabetes: a critical review. Clin Rev Allergy Immunol. 2014;47(2):174‐192. [DOI] [PubMed] [Google Scholar]
- 15. Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol. 2016;12(3):154‐167. [DOI] [PubMed] [Google Scholar]
- 16. Knip M. Environmental triggers and determinants of beta‐cell autoimmunity and type 1 diabetes. Rev Endocr Metab Disord. 2003;4(3):213‐223. [DOI] [PubMed] [Google Scholar]
- 17. Kaufman DL, Erlander MG, Clare‐Salzler M, Atkinson MA, Maclaren NK, Tobin AJ. Autoimmunity to two forms of glutamate decarboxylase in insulin‐dependent diabetes mellitus. J Clin Invest. 1992;89(1):283‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoon JW, Onodera T, Notkins AL. Virus‐induced diabetes mellitus. XV. Beta cell damage and insulin‐dependent hyperglycemia in mice infected with coxsackie virus B4. J Exp Med. 1978;148(4):1068‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luopajarvi K, Savilahti E, Virtanen SM, et al. Enhanced levels of cow's milk antibodies in infancy in children who develop type 1 diabetes later in childhood. Pediatr Diabetes. 2008;9(5):434‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Du T, Zhou Z, You S, et al. Regulation by 1, 25‐dihydroxy‐vitamin D3 on altered TLRs expression and response to ligands of monocyte from autoimmune diabetes. Clin Chim Acta. 2009;402(1‐2):133‐138. [DOI] [PubMed] [Google Scholar]
- 21. Ghazarian L, Diana J, Simoni Y, Beaudoin L, Lehuen A. Prevention or acceleration of type 1 diabetes by viruses. Cell Mol Life Sci. 2013;70(2):239‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Candon S, Perez‐Arroyo A, Marquet C, et al. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin‐dependent diabetes. PLoS One. 2015;10:e125448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brugman S, Klatter FA, Visser JT, et al. Antibiotic treatment partially protects against type 1 diabetes in the bio‐breeding diabetes‐prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49(9):2105‐2108. [DOI] [PubMed] [Google Scholar]
- 24. Hansen CH, Krych L, Nielsen DS, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55(8):2285‐2294. [DOI] [PubMed] [Google Scholar]
- 25. Mikkelsen KH, Knop FK, Vilsbøll T, Frost M, Hallas J, Pottegård A. Use of antibiotics in childhood and risk of type 1 diabetes: a population‐based case‐control study. Diabet Med. 2017;34(2):272‐277. [DOI] [PubMed] [Google Scholar]
- 26. Hooper LV, Gordon JI. Commensal host‐bacterial relationships in the gut. Science. 292(5519):1115‐1118. [DOI] [PubMed] [Google Scholar]
- 27. Consortium HMP. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu Y, Peng J, Tai N, et al. Maternal antibiotic treatment protects offspring from diabetes development in nonobese diabetic mice by generation of tolerogenic APCs. J Immunol. 2015;195(9):4176‐4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bibbò S, Dore MP, Pes GM, Delitala G, Delitala AP. Is there a role for gut microbiota in type 1 diabetes pathogenesis? Ann Med. 2017;49(1):11‐22. [DOI] [PubMed] [Google Scholar]
- 30. Hidalgo‐Cantabrana C, Delgado S, Ruiz L, Ruas‐Madiedo P, Sánchez B, Margolles A. Bifidobacteria and their health‐promoting effects. Microbiol Spectr. 2017;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Samantha A, Whiteside HRSD. The microbiome of the urinary tract—a role beyond infection. Nat Rev Urol. 2015;12:81‐90. [DOI] [PubMed] [Google Scholar]
- 32. Dominguez‐Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971‐11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G̈. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr. 2008;138(9):1796S‐1800S. [DOI] [PubMed] [Google Scholar]
- 34. Brown K, DeCoffe D, Molcan E, Gibson DL. Diet‐induced Dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4(8):1095‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu Y, Wong FS, Wen L. Antibiotics, gut microbiota, environment in early life and type 1 diabetes. Pharmacol Res. 2017;119:219‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Geach T. Gut microbiota: Antibiotics do not affect metabolism in obesity. Nat Rev Endocrinol. 2016;12:558. [DOI] [PubMed] [Google Scholar]
- 37. Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sofi MH, Gudi R, Karumuthil‐Melethil S, Perez N, Johnson BM, Vasu C. pH of drinking water influences the composition of gut microbiome and type 1 diabetes incidence. Diabetes. 2014;63(2):632‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wolf KJ, Daft JG, Tanner SM, Hartmann R, Khafipour E, Lorenz RG. Consumption of acidic water alters the gut microbiome and decreases the risk of diabetes in NOD mice. J Histochem Cytochem. 2014;62(4):237‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alkanani AK, Hara N, Gottlieb PA, et al. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes. 2015;64:3510‐3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455(7216):1109‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. King C, Sarvetnick N. The incidence of type‐1 diabetes in NOD mice is modulated by restricted flora not germ‐free conditions. PLoS One. 2011;6(2):e17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tai N, Peng J, Liu F, et al. Microbial antigen mimics activate diabetogenic CD8 T cells in NOD mice. J Exp Med. 2016;213(10):2129‐2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knip M, Honkanen J. Modulation of type 1 diabetes risk by the intestinal microbiome. Curr Diab Rep. 2017;17(11):105. [DOI] [PubMed] [Google Scholar]
- 45. Giongo A, Gano KA, Crabb DB, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5(1):82‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davis‐Richardson AG, Ardissone AN, Dias R, et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front Microbiol. 2014;5:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Goffau MC, Fuentes S, van den Bogert B, et al. Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia. 2014;57(8):1569‐1577. [DOI] [PubMed] [Google Scholar]
- 48. de Goffau MC, Luopajarvi K, Knip M, et al. Fecal microbiota composition differs between children with beta‐cell autoimmunity and those without. Diabetes. 2013;62(4):1238‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vaarala O. Human intestinal microbiota and type 1 diabetes. Curr Diab Rep. 2013;13(5):601‐607. [DOI] [PubMed] [Google Scholar]
- 50. Brown CT, Davis‐Richardson AG, Giongo A, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011;6(10):e25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Krych Ł, Nielsen DS, Hansen AK, Hansen CHF. Gut microbial markers are associated with diabetes onset, regulatory imbalance, and IFN‐γlevel in NOD mice. Gut Microbes. 2015;6(2):101‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hara N, Alkanani AK, Ir D, et al. The role of the intestinal microbiota in type 1 diabetes. Clin Immunol. 2013;146(2):112‐119. [DOI] [PubMed] [Google Scholar]
- 53. Mejía‐León M, Barca A. Diet, microbiota and immune system in type 1 diabetes development and evolution. Nutrients. 2015;7(11):9171‐9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kostic AD, Gevers D, Siljander H, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17(2):260‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Patterson E, Marques TM, O'Sullivan O, et al. Streptozotocin‐induced type‐1‐diabetes disease onset in Sprague‐Dawley rats is associated with an altered intestinal microbiota composition and decreased diversity. Microbiology. 2015;161(1):182‐193. [DOI] [PubMed] [Google Scholar]
- 56. Li X, Atkinson MA. The role for gut permeability in the pathogenesis of type 1 diabetes—a solid or leaky concept? Pediatr Diabetes. 2015;16(7):485‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maffeis C, Martina A, Corradi M, et al. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. Diabetes Metab Res Rev. 2016;32(7):700‐709. [DOI] [PubMed] [Google Scholar]
- 58. Ohkusa T, Okayasu I, Ogihara T, Morita K, Ogawa M, Sato N. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut. 2003;52(1):79‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Strauss J, Kaplan GG, Beck PL, et al. Invasive potential of gut mucosa‐derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis. 2011;17(9):1971‐1978. [DOI] [PubMed] [Google Scholar]
- 60. Legaria MC, Lumelsky G, Rodriguez V, Rosetti S. Clindamycin‐resistant Fusobacterium varium bacteremia and decubitus ulcer infection. J Clin Microbiol. 2005;43(8):4293‐4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hevia A, Milani C, Lopez P, et al. Intestinal dysbiosis associated with systemic lupus erythematosus. MBio. 2014;5:e1514‐e1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schickel JN, Glauzy S, Ng YS, et al. Self‐reactive VH4‐34‐expressing IgG B cells recognize commensal bacteria. J Exp Med. 2017;214(7):2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hebbandi NR, Ronchi F, Wang J, et al. A gut microbial mimic that hijacks Diabetogenic autoreactivity to suppress colitis. Cell. 2017;171(3):655‐667. [DOI] [PubMed] [Google Scholar]
- 64. Ferris ST, Zakharov PN, Wan X, et al. The islet‐resident macrophage is in an inflammatory state and senses microbial products in blood. J Exp Med. 2017;214(8):2369‐2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155(2):173‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Janeway CJ. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(0):1‐13. [DOI] [PubMed] [Google Scholar]
- 67. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783‐801. [DOI] [PubMed] [Google Scholar]
- 68. Kieser KJ, Kagan JC. Multi‐receptor detection of individual bacterial products by the innate immune system. Nat Rev Immunol. 2017;77:376‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Burrows MP, Volchkov P, Kobayashi KS, Chervonsky AV. Microbiota regulates type 1 diabetes through toll‐like receptors. Proc Natl Acad Sci U S A. 2015;112(32):9973‐9977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McCoy KD, Ronchi F, Geuking MB. Host‐microbiota interactions and adaptive immunity. Immunol Rev. 2017;279(1):63‐69. [DOI] [PubMed] [Google Scholar]
- 71. Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yu H, Gagliani N, Ishigame H, et al. Intestinal type 1 regulatory T cells migrate to periphery to suppress diabetogenic T cells and prevent diabetes development. Proc Natl Acad Sci U S A. 2017;114(39):10443‐10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Postler TS, Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 2017;26(1):110‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T‐cell generation. Nature. 2013;504(7480):451‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mariño E, Richards JL, McLeod KH, et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol. 2017;18(5):552‐562. [DOI] [PubMed] [Google Scholar]
- 77. Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T‐cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107(27):12204‐12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe‐derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446‐450. [DOI] [PubMed] [Google Scholar]
- 79. Kudoh K, Shimizu J, Wada M, et al. Effect of indigestible saccharides on B lymphocyte response of intestinal mucosa and cecal fermentation in rats. J Nutr Sci Vitaminol (Tokyo). 1998;44(1):103‐112. [DOI] [PubMed] [Google Scholar]
- 80. Kim CH. B cell‐helping functions of gut microbial metabolites. Microb Cell. 2016;3(10):529‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kim M, Qie Y, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016;20(2):202‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Calcinaro F, Dionisi S, Marinaro M, et al. Oral probiotic administration induces interleukin‐10 production and prevents spontaneous autoimmune diabetes in the non‐obese diabetic mouse. Diabetologia. 2005;48(8):1565‐1575. [DOI] [PubMed] [Google Scholar]
- 83. Zhu W, Winter MG, Byndloss MX, et al. Precision editing of the gut microbiota ameliorates colitis. Nature. 2018;553(7687):208‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee H, Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol. 2014;80(19):5935‐5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang L, Li P, Tang Z, Yan X, Feng B. Structural modulation of the gut microbiota and the relationship with body weight: compared evaluation of liraglutide and saxagliptin treatment. Sci Rep. 2016;6(1):33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kihl P, Krych L, Buschard K, et al. Oral insulin does not alter gut microbiota composition of NOD mice. Diabetes Metab Res Rev. 2018;34(6):e3010. [DOI] [PubMed] [Google Scholar]
- 87. Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. β‐Cell function in new‐onset type 1 diabetes and immunomodulation with a heat‐shock protein peptide (DiaPep277): a randomised, double‐blind, phase II trial. Lancet. 2001;358(9295):1749‐1753. [DOI] [PubMed] [Google Scholar]
- 88. Fenalti G, Rowley M. GAD65 as a prototypic autoantigen. J Autoimmun. 2008;32:228‐232. [DOI] [PubMed] [Google Scholar]
- 89. Chatenoud L, Thervet E, Primo J, Bach JF. Anti‐CD3 antibody induces long‐term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A. 1994;91(1):123‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Alegre ML, Peterson LJ, Xu D, et al. A non‐activating “humanized” anti‐CD3 monoclonal antibody retains immunosuppressive properties in vivo. Transplantation. 1994;57(11):1537‐1543. [PubMed] [Google Scholar]
- 91. Kremer JM, Genant HK, Moreland LW, et al. Effects of abatacept in patients with methotrexate‐resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144(12):865‐876. [DOI] [PubMed] [Google Scholar]
- 92. Hu CY, Rodriguezpinto D, Du W, et al. Treatment with CD20‐specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117(12):3857‐3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Waldron‐Lynch F, Henegariu O, Deng S, et al. Teplizumab induces human gut‐tropic regulatory cells in humanized mice and patients. Sci Transl Med. 2012;4:112r‐118r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6(1):7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zheng S, Zhao M, Wu Y, et al. Suppression of pancreatic beta cell apoptosis by Danzhi Jiangtang capsule contributes to the attenuation of type 1 diabetes in rats. BMC Complement Altern Med. 2016;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Li J, Casteels T, Frogne T, et al. Artemisinins target GABA A receptor signaling and impair α cell identity. Cell. 2017;168(1‐2):86‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Michael S, Lindsay K, Alessandro T, et al. Protective major histocompatibility complex allele prevents type 1 diabetes by shaping the intestinal microbiota early in ontogeny. Proc Natl Acad Sci U S A. 2017;114:9671‐9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Onengut‐Gumuscu S, Chen W, Burren O, et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47(4):381‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li AH, Morrison AC, Kovar C, et al. Analysis of loss‐of‐function variants and 20 risk factor phenotypes in 8,554 individuals identifies loci influencing chronic disease. Nat Genet. 2015;47(6):640‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]


