Abstract
Fosravuconazole L‐lysine ethanolate (F‐RVCZ) is a prodrug of ravuconazole, a novel triazole antifungal agent, exerting broad and potent antifungal activity. The efficacy and safety of F‐RVCZ, compared with a placebo, were investigated in a multicenter, double‐blind, randomized study of Japanese onychomycosis patients with 25% or more clinical involvement of the target toenail. Subjects (n = 153) were randomly assigned to receive F‐RVCZ (100 mg RVCZ, n = 101) or placebo (n = 52) p.o. once daily for 12 weeks. The primary end‐point was the rate of complete cure (clinical cure [0% clinical involvement of the target toenail] plus mycological cure [negative potassium hydroxide examination]) at week 48 (36‐week post‐treatment visit). Secondary end‐points were changes over time in the efficacy and mycological effect of F‐RVCZ. Safety was also evaluated. The complete cure rate at week 48 was significantly higher with F‐RVCZ (59.4%, 60/101) than the placebo (5.8%, 3/52) in the full analysis set (P < 0.001). The mycological cure rate at week 48 was also significantly higher with F‐RVCZ (82.0%, 73/89) than the placebo (20.0%, 10/50, P < 0.001). Regarding safety, adverse events were observed in 83.2% (84/101) and 80.8% (42/52), and adverse drug reactions (ADR) in 23.8% (24/101) and 3.8% (2/52) of F‐RVCZ and placebo subjects, respectively. ADR were mild to moderate in severity, with none being serious. F‐RVCZ (equivalent to 100 mg ravuconazole) administrated once daily for 12 weeks was more effective than placebo and tolerable in patients with onychomycosis, suggesting it to be a promising drug for onychomycosis treatment.
Keywords: fosravuconazole, onychomycosis, oral antifungal agents, randomized controlled trial, ravuconazole
Introduction
Onychomycosis is one of the most common superficial fungal infections. An epidemiological survey conducted in 2011 in Japan by the Japanese Society for Medical Mycology showed that 2980 of 36 052 outpatients who visited dermatological clinics had tinea infections, with tinea pedis being most commonly reported (1930 patients), followed by tinea unguium (780).1 In Japan, itraconazole and terbinafine hydrochloride have been clinically used as oral antifungal agents for the treatment of onychomycosis and no new oral drugs have been approved since marketing approvals for these two agents were obtained more than 20 years ago.
Ravuconazole (RVCZ) and its prodrug, fosravuconazole L‐lysine ethanolate (F‐RVCZ), are newly developed oral antifungal agents.2 In the early stages of clinical development, clinical studies using RVCZ in patients with onychomycosis were conducted mainly in the USA.3 Subsequently, F‐RVCZ, a prodrug of RVCZ was discovered to have improved hydrophilicity and oral absorbability (bioavailability), and has been examined in clinical studies of onychomycosis treatment in Japan.
The mechanism underlying the antifungal activities of RVCZ is considered to involve inhibition of ergosterol biosynthesis, as is the case with other azole antifungals, and this activity is potently exerted against a broad spectrum of dermatophytes and pathogenic fungi, including the genera Trichophyton, Candida, Aspergillus and Cryptococcus.4, 5, 6, 7, 8, 9, 10, 11, 12
Ravuconazole reportedly shows a lower inhibitory effect against CYP3A4, a typical hepatic metabolic enzyme, than itraconazole.13 It also has no clinically meaningful inhibitory effects on CYP2C8, CYP2C19, CYP2D6 and CYP1A2, as well as none against CYP2C9, and virtually no clinically relevant inhibitory effects on transporters such as P‐glycoprotein and breast cancer resistance protein, suggesting that drug interactions would be of minimal concern.14
This report provides the results of a multicenter, double‐blind, randomized study designed to investigate the efficacy and safety of F‐RVCZ, as compared with a placebo, in patients with onychomycosis.
Methods
This study was conducted in compliance with the Declaration of Helsinki and the Ministerial Ordinance on Good Clinical Practice of Japan, with approval from the institutional review board at each study site (JAPIC Clinical Trials Information no. JapicCTI‐152779).
Study design
This was a multicenter, placebo‐controlled, randomized, double‐blind, parallel‐group study conducted at 26 sites in Japan between December 2014 and June 2016. The objective of this study was to evaluate the efficacy and safety of F‐RVCZ in patients with onychomycosis.
In this study, Japanese adult patients with onychomycosis of the great toenail were randomized, within 4 weeks after providing written informed consent, to receive a capsule of either F‐RVCZ or placebo once daily for 12 weeks (treatment period), and then followed up for 36 weeks without study drug treatment (observation period). The dose of F‐RVCZ administrated was calculated to deliver 100 mg of RVCZ per dose. The number of subjects to be included in this study was calculated on the assumption of a 35% cure rate for F‐RVCZ and a 10% cure rate for placebo, with a two‐tailed significance level of 5%, with 80% statistical power. The ratio of the number of subjects to be treated with F‐RVCZ to that of those given the placebo was set at 2:1. The target number of subjects was thus set at 100 for F‐RVCZ and 50 for the placebo, taking into consideration the number of subjects who might withdraw from the study. Allocation of the study drug was entrusted to a third party not involved in the study, using the block randomization method with six subjects per block, and kept double‐blinded until the completion of the entire study.
The subjects were instructed to visit the clinics for follow‐up observations every 2 weeks for the first 16 weeks after the first administration of the study drug and every 4 weeks thereafter until week 48.
Subjects
The main inclusion criteria for patients were as follows: (i) 20 years or older but less than 75 years of age; (ii) clinical involvement in either the left or right great toenail; (iii) positive potassium hydroxide examination results under direct microscopy and confirmed detection of Trichophyton rubrum or Trichophyton mentagrophytes by loop‐mediated isothermal amplification, a method proven to have a high correlation with fungal culture in identification of T. rubrum or T. mentagrophytes, prior to this study (Dr Shinichi Watanabe, 2013, unpubl. data); (iv) clinical involvement affecting 25% or more of the toenail; and (v) the provision of written informed consent.
The main exclusion criteria applied to patients were as follows: (i) use of any oral or injectable antifungal agent or topical antifungal agent for nails within 36 weeks before the first administration of the study drug; (ii) longitudinal streaks or a spike in the toenail with clinical involvement to be evaluated (target toenail); (iii) clinical involvement reaching the proximal nail fold of the target toenail; (iv) dermatophytes detected only on the nail surface (superficial white onychomycosis); or (v) significant nail thickening or deformity due to onychogryphosis or other conditions.
During the study period, subjects were prohibited from using any oral or topical antifungal agent other than the study drug, or other therapies for onychomycosis such as surgical removal of an affected nail. No drugs were prohibited from concomitant use from the viewpoint of drug interactions. Subjects with a post‐baseline aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level exceeding 2.5 times the upper limit of the normal range of the clinical laboratory values were to be prematurely withdrawn from the study.
Efficacy
Before the first administration of the study drug, either the left or right great toenail was designated as the target toenail for the efficacy evaluation. The nail involvement ratio was calculated as the percentage of nail involvement to the total nail area of the target toenail, based on nail photographs taken at baseline and at weeks 12, 24, 36 and 48.
Primary end‐point
Complete cure rate at week 48: The rate of complete cure (clinical cure [0% clinical involvement of the target toenail] plus mycological cure [negative potassium hydroxide examination]) at week 48 was determined.
Secondary end‐points
Change over time in complete cure rate
Complete cure rates at weeks 12, 24, 36 and 48 were determined.
Efficacy rating
The efficacy of the study drug on the target toenail was rated at weeks 12, 24, 36 and 48, based on the percent decrease in the area of nail involvement, as follows: marked improvement, 60% or more; improvement, 30% or more to less than 60%; and failure, less than 30%.
Percent decrease in the area of nail involvement
The percent decreases in the area of nail involvement of the target toenail from those at screening were determined at weeks 12, 24, 36 and 48. These percent decreases in the area of nail involvement were calculated by the following formula:
Mycological cure rate
The mycological cure rate of the target toenail was determined at weeks 12, 24, 36 and 48.
Subgroup analysis of complete cure rate
The complete cure rate at week 48 was determined for each subgroup according to sex, age (<65 or ≥65 years), causative fungal species detected at screening (T. rubrum or T. mentagrophytes) and the nail involvement ratio at screening (<50% or ≥50%).
Safety
For the safety evaluation, we determined whether the subjects had any subjective symptoms/objective findings, and monitored abnormal changes in laboratory test values to identify the occurrence of any adverse events (AE) during the study period.
Statistical analysis
Analysis sets
We defined the following three analysis sets: the full analysis set (FAS) and per‐protocol set (PPS) for efficacy evaluation, and the safety analysis set (SAF) for safety evaluation. Efficacy analysis was performed primarily in the FAS, and secondarily in the PPS to examine the robustness of the analysis.
Efficacy analysis
Primary end‐point
The complete cure rate and its 95% confidence interval (CI) at week 48 were determined for each treatment group. Subjects prematurely withdrawn from the study were regarded as not having been cured. The significance of differences between groups was analyzed using Fisher's exact test at a two‐tailed significance level of 0.05.
Secondary end‐points
The complete cure rates, efficacy ratings, percent decreases in the area of nail involvement and mycological cure rates at weeks 12, 24, 36 and 48 were determined for each treatment group, and analyzed for significant differences between the groups. The significance of differences was analyzed using Fisher's exact test for the complete cure rate and the mycological cure rate, Wilcoxon rank sum test for efficacy rating and Student's t‐test for percent decrease in the area of nail involvement, at a two‐tailed significance level of 0.05. The complete cure rate was also determined for each subgroup based on age (<65 or ≥65 years), sex, the nail involvement ratio at screening (<50% or ≥50%) and causative fungal species detected at screening (T. rubrum or T. mentagrophytes), with the application of Fisher's exact test for analysis of significant differences. For analysis of the complete cure rate, subjects prematurely withdrawn from the study were regarded as not having been cured. For the analysis of other end‐points, subjects with missing data at any assessment time point due to withdrawal were excluded from the analysis.
Safety analysis
Adverse events and adverse drug reactions (ADR), for which a causal relationship to the study drug could not be ruled out, were classified according to the Medical Dictionary for Regulatory Activities Terminology/Japanese version 19.0, and the incidences of each event were summarized for each treatment group. Descriptive statistic values were calculated for each laboratory test value.
Results
Subjects
Of 213 consenting patients with onychomycosis, 153 (101 F‐RVCZ and 52 placebo) were randomized (Fig. 1). All randomized subjects were included in the FAS and SAF, while 139 subjects (89 F‐RVCZ and 50 placebo) were included in the PPS. The baseline characteristics of the randomized subjects (FAS) are shown in Table 1. Those receiving F‐RVCZ and the placebo were comparable in terms of age, sex, causative fungal species and the nail involvement ratio of the target toenail. Ninety and 51 subjects treated with F‐RVCZ and placebo, respectively, completed the 12‐week treatment with the study drug, of whom 89 and 50 completed the final follow‐up observation at week 48.
Figure 1.
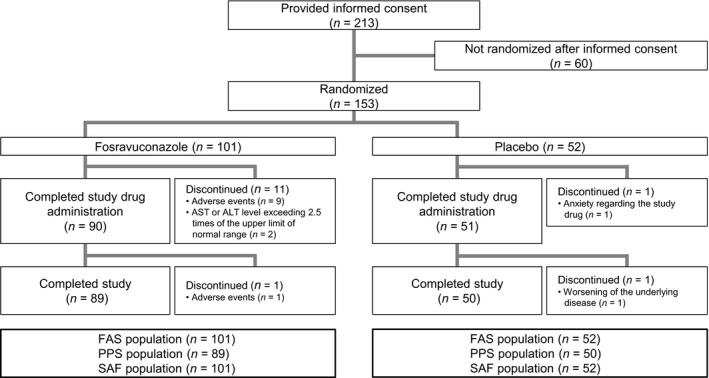
Disposition of subjects. FAS, full analysis set; PPS, per‐protocol set; SAF, safety analysis set. Completed study drug administration, subjects who completed the treatment period (12 weeks) and entered the observation period (from weeks 12 to 48 after initiation of study drug administration). Completed study, subjects who completed the observation period.
Table 1.
Baseline characteristics of subjects (full analysis set)
| Variables | Fosravuconazole (n = 101) | Placebo (n = 52) | Total (n = 153) |
|---|---|---|---|
| Age (years) | |||
| Mean | 58.4 | 58.3 | 58.4 |
| Median | 61.0 | 62.5 | 61.0 |
| Range | 23–74 | 28–74 | 23–74 |
| Sex (%) | |||
| Male | 77 (76.2) | 33 (63.5) | 110 (71.9) |
| Female | 24 (23.8) | 19 (36.5) | 43 (28.1) |
| Causative fungal species detected at screening (%)a | |||
| Trichophyton rubrum | 84 (83.2) | 41 (78.8) | 125 (81.7) |
| Trichophyton mentagrophytes | 18 (17.8) | 12 (23.1) | 30 (19.6) |
| Nail involvement ratio at screening (%) | |||
| Mean | 54.51 | 52.46 | 53.82 |
| Median | 56.05 | 53.20 | 55.25 |
| Range | 26.4–92.9 | 25.4–96.7 | 25.4–96.7 |
Subjects who tested positive for both fungal species were included in the counts of both species in loop‐mediated isothermal amplification at screening.
Efficacy
Primary end‐point
Complete cure rates in the F‐RVCZ and placebo subjects at week 48 were 59.4% and 5.8% in the FAS, and 66.3% and 6.0% in the PPS, respectively, being significantly higher with F‐RVCZ in both analysis sets (P < 0.001, Table 2).
Table 2.
Summary of primary efficacy end‐point (complete cure rate) at week 48
| Complete cure rate | Fosravuconazole | Placebo | P (Fisher's exact test) | ||
|---|---|---|---|---|---|
| % (n/n) | 95% CI | % (n/n) | 95% CI | ||
| FAS | 59.4 (60/101) | 49.2–69.1 | 5.8 (3/52) | 1.2–15.9 | <0.001 |
| PPS | 66.3 (59/89) | 55.5–76.0 | 6.0 (3/50) | 1.3–16.5 | <0.001 |
Subjects who had no data at week 48 of study drug administration were handled as “no cure”. CI, confidence interval; FAS, full analysis set; PPS, per‐protocol set.
Secondary end‐points
Change over time in complete cure rate
The complete cure rate was determined every 12 weeks after initiation of treatment with the study drug. The complete cure rate with F‐RVCZ gradually increased over time and became significantly higher than that with placebo from week 36 onward (Fig. 2a).
Figure 2.
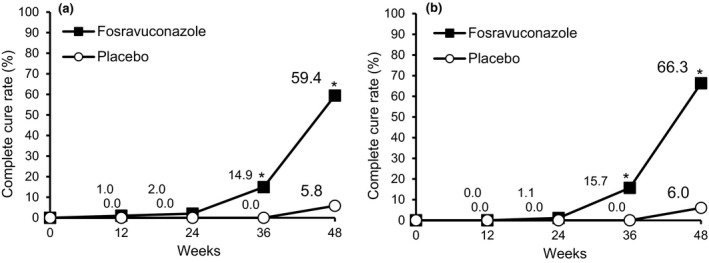
Changes in complete cure rate of toenail onychomycosis. Changes in the proportion of subjects who achieved complete cure are shown. (a) The complete cure rate in the full analysis set population receiving fosravuconazole gradually increased over time. P = 0.003 at week 36, and P < 0.001 at week 48 (fosravuconazole, n = 101; placebo, n = 52). (b) A similar tendency was observed in the per‐protocol set population. P = 0.002 at week 36, and P < 0.001 at week 48 (fosravuconazole, n = 89; placebo, n = 50). *P < 0.05.
A similar tendency was observed in the PPS, wherein the complete cure rate with F‐RVCZ gradually increased over time and became significantly higher than that with placebo from week 36 onward (Fig. 2b).
Similar results were obtained in the FAS and PPS for all other efficacy end‐points, demonstrating the robustness of this analysis. Thus, only the analysis results obtained in the FAS are presented in the following portions of this report.
The toenail photographs of representative subjects achieving complete cure are presented in Figure 3, showing changes in the appearance of the target toenails over time.
Figure 3.
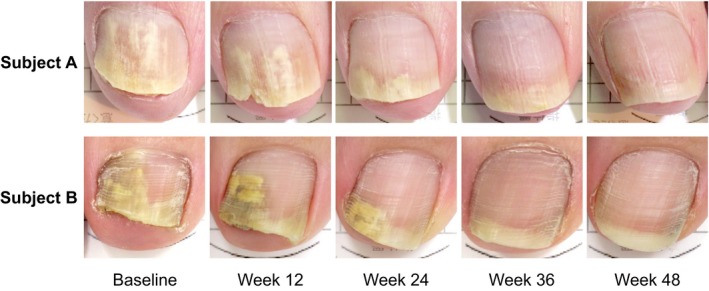
Healing process of toenail onychomycosis with 12‐week fosravuconazole treatment in representative subjects (baseline and at weeks 12, 24, 36 and 48). Toenail onychomycosis gradually improved in subjects with baseline nail involvement ratios of 67.5% (upper) and 58.7% (lower), leading to complete cure by week 48.
Efficacy rating
We also rated the efficacy of treatment based on the percent decrease in the area of nail involvement every 12 weeks on a 3‐point scale: marked improvement, improvement and failure (Fig. 4). Significant differences in efficacy ratings were observed between F‐RVCZ and placebo from week 24 onward. The proportion of subjects rated as marked improvement at week 48 was 83.1% with F‐RVCZ, 26.0% with placebo (P < 0.001 at weeks 24, 36 and 48).
Figure 4.
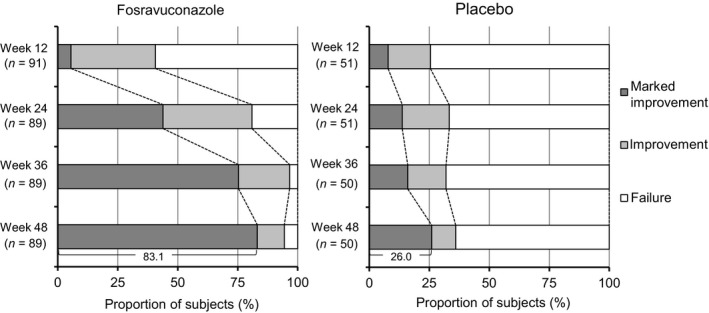
Changes in the distribution of efficacy ratings against toenail onychomycosis. Significant differences were observed from week 24 onward (P < 0.001 at weeks 24, 36 and 48; full analysis set).
Percent decrease in the area of nail involvement
Changes over time in the nail involvement ratio up to week 48 are shown in Figure 5. The difference in the percent decrease in the area of nail involvement between F‐RVCZ and placebo became statistically significant at week 12 and remained significant thereafter (P = 0.013 at week 12, P < 0.001 at weeks 24, 36 and 48). The percent decrease in the area of nail involvement at week 48 was 85.6% with F‐RVCZ and 24.2% with placebo, demonstrating a substantial decrease in the nail involvement ratio in subjects receiving F‐RVCZ.
Figure 5.
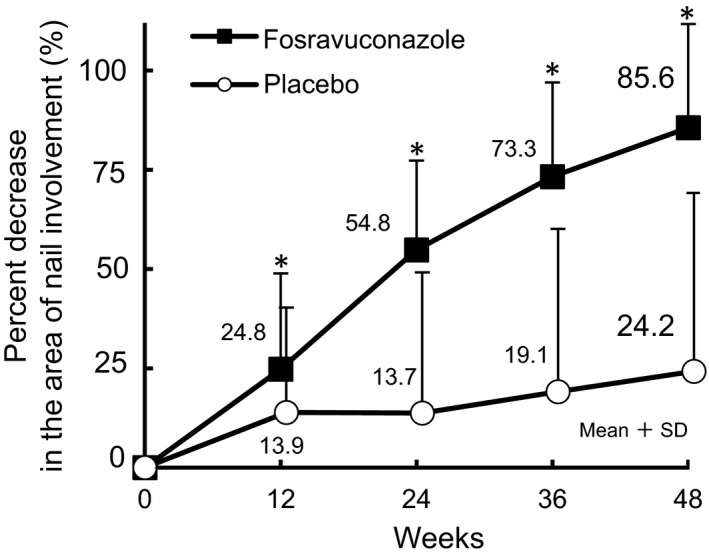
Changes in the percent decrease in the area of nail involvement of toenail onychomycosis. The mean changes in the percent decrease in the area of nail involvement are shown. P = 0.013 at week 12, and P < 0.001 at weeks 24, 36 and 48 (full analysis set population at week 48: fosravuconazole, n = 89; placebo, n = 50). *P < 0.05.
Mycological cure rate
The mycological cure rate was determined every 12 weeks (Fig. 6). The mycological cure rate with F‐RVCZ gradually increased over time and the difference was statistically significant as compared with that with placebo from week 24 onward (P = 0.002 at week 24, P < 0.001 at weeks 36 and 48). The mycological cure rates with F‐RVCZ and placebo at week 48 were 82.0% and 20.0%, respectively.
Figure 6.
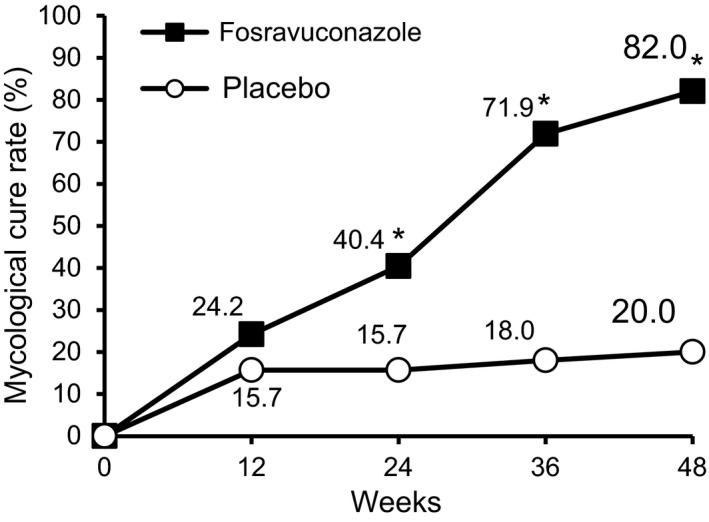
Changes in the mycological cure rate of toenail onychomycosis. Changes in the proportion of subjects who achieved mycological cure are shown. P = 0.002 at week 24 and P < 0.001 at weeks 36 and 48 (full analysis set population at week 48: fosravuconazole, n = 89; placebo, n = 50). *P < 0.05.
Subgroup analysis of complete cure rate
When the complete cure rates at week 48 were determined for each subgroup based on age, sex, causative fungal species detected at screening and the nail involvement ratio at screening, the subjects given F‐RVCZ showed significantly higher rates than those receiving the placebo, and this was true in all subgroups (Table 3).
Table 3.
Subgroup analysis of complete cure rate (full analysis set)
| Baseline characteristics | Complete cure rate | P (Fisher's exact test) | |
|---|---|---|---|
| Fosravuconazole % (n/n) | Placebo % (n/n) | ||
| Age | |||
| <65 years | 56.5 (35/62) | 3.7 (1/27) | <0.001 |
| ≥65 years | 64.1 (25/39) | 8.0 (2/25) | <0.001 |
| Sex | |||
| Male | 55.8 (43/77) | 6.1 (2/33) | <0.001 |
| Female | 70.8 (17/24) | 5.3 (1/19) | <0.001 |
| Causative fungal species detected at screeninga | |||
| Trichophyton rubrum | 58.3 (49/84) | 2.4 (1/41) | <0.001 |
| Trichophyton mentagrophytes | 66.7 (12/18) | 16.7 (2/12) | 0.011 |
| Nail involvement ratio at screening | |||
| <50% | 64.9 (24/37) | 8.3 (2/24) | <0.001 |
| ≥50% | 56.3 (36/64) | 3.6 (1/28) | <0.001 |
Subjects who tested positive for both fungal species were included in the counts of both species in loop‐mediated isothermal amplification at screening.
Safety
Safety analysis was performed in the SAF consisting of 153 subjects (101 F‐RVCZ and 52 placebo). AE occurred in 83.2% (84/101) and 80.8% (42/52) of the F‐RVCZ and placebo subjects, respectively. Serious AE were observed in 5.9% (6/101) of the F‐RVCZ subjects, but a causal relationship with the study drug was ruled out. Neither death nor serious ADR were observed.
The ADR are listed in Table 4. ADR were observed in 23.8% (24/101) and 3.8% (2/52) of the F‐RVCZ and placebo subjects, respectively. All ADR manifesting clinically in the subjects receiving F‐RVCZ and placebo were gastrointestinal disorders, with the most common events being abdominal discomfort in 4.0% of F‐RVCZ and diarrhea in 3.8% of placebo subjects. The most common laboratory test abnormalities in those given F‐RVCZ were increased liver function test values, including elevations of γ‐glutamyltransferase (γ‐GT) in 15.8%, ALT in 8.9%, AST in 7.9% and blood alkaline phosphatase in 2.0%. All ADR, including laboratory test abnormalities, were mild to moderate in severity and recovered to normal in all cases after treatment discontinuation or completion of the 12‐week treatment with the study drug. The incidence of increased γ‐GT was higher than those of other liver function test abnormalities. These subjects with increased liver function test values had bilirubin levels within the normal range during participation in the study and showed no clinical manifestations suggestive of hepatic functional disorders, such as malaise or jaundice.
Table 4.
List of adverse drug reactions (safety analysis set)
| Adverse drug reactions (ADR) | Fosravuconazole (n = 101) | Placebo (n = 52) |
|---|---|---|
| No. of subjects (%) | No. of subjects (%) | |
| Total no. of subjects with ADR | 24 (23.8) | 2 (3.8) |
| Gastrointestinal disorders | 7 (6.9) | 2 (3.8) |
| Abdominal discomfort | 4 (4.0) | 0 (0.0) |
| Constipation | 1 (1.0) | 0 (0.0) |
| Diarrhea | 0 (0.0) | 2 (3.8) |
| Dyspepsia | 1 (1.0) | 0 (0.0) |
| Gastritis erosive | 1 (1.0) | 0 (0.0) |
| Nausea | 0 (0.0) | 1 (1.9) |
| Vomiting | 0 (0.0) | 1 (1.9) |
| Investigations | 19 (18.8) | 1 (1.9) |
| Alanine aminotransferase increased | 9 (8.9) | 0 (0.0) |
| Aspartate aminotransferase increased | 8 (7.9) | 0 (0.0) |
| Blood creatinine increased | 1 (1.0) | 0 (0.0) |
| γ‐Glutamyltransferase increased | 16 (15.8) | 1 (1.9) |
| Hemoglobin decreased | 1 (1.0) | 0 (0.0) |
| Red blood cell count decreased | 1 (1.0) | 0 (0.0) |
| White blood cell count decreased | 1 (1.0) | 0 (0.0) |
| White blood cell count increased | 1 (1.0) | 0 (0.0) |
| Blood alkaline phosphatase increased | 2 (2.0) | 1 (1.9) |
Medical Dictionary for Regulatory Activities Terminology/Japanese version 19.0.
Discussion
Itraconazole and terbinafine hydrochloride are the only oral agents marketed in Japan thus far for the treatment of onychomycosis, and more than 20 years have passed since the marketing approvals for these agents were obtained. While no domestic comparative studies have been published in Japan for these two agents, to date, a large‐scale clinical study was conducted in Europe by Sigurgeirsson et al.15 in 1999. They reported that the complete cure rate was 23.4% after 72 weeks of pulse therapy consisting of three cycles of 400 mg/day of itraconazole administrated for 7 days followed by a 21‐day washout, and 45.8% at 72 weeks after initiation of treatment with 250 mg/day of terbinafine continuously administrated for 12 weeks. In phase III studies of efinaconazole and luliconazole, topical drugs indicated for onychomycosis that recently became available in Japan, conducted in patients with mild to moderate onychomycosis with a nail involvement ratio of 20–50%, the complete cure rates after 48 weeks of once‐daily topical administration were 17.8% and 15.2% for efinaconazole (at week 52, data from two different studies)16 and 14.9% for luliconazole (at week 48).17 Given these relatively low rates, demand persisted for the development of an oral antifungal agent with a high cure rate.2
Our current phase III study of F‐RVCZ included patients with onychomycosis, some of whom had a mean nail involvement ratio of 54.51% or more. Nevertheless, a complete cure rate of 59.4% at week 48 was obtained. Although the above studies were conducted under different protocols, such that simple comparisons are not feasible, the results of our current study suggest higher efficacy of F‐RVCZ for onychomycosis as compared with the complete cure rates achieved in previous clinical studies of drugs for onychomycosis.15, 16, 17 The subgroup analysis results according to age (<65 or ≥65 years), sex, fungal species and nail involvement ratio at screening (<50% or ≥50%) demonstrated significantly higher efficacy of F‐RVCZ than placebo in all subgroups, suggesting substantial efficacy of F‐RVCZ regardless of baseline patient characteristics.
In our current study, the complete cure rate with F‐RVCZ gradually increased over time and became significantly different compared with placebo from the time point of week 36 onward. Given that onychomycosis is known to improve as a nail grows,18, 19 this finding suggests that a certain period of time is required before the complete cure of onychomycosis can be achieved even with F‐RVCZ, which is a drug suggested to have high efficacy. In clinical practise, it may be difficult to judge the efficacy of F‐RVCZ soon after treatment initiation based only on the complete cure. To facilitate an early judgment on efficacy, physicians should pay attention to the percent decrease in the area of nail involvement. In our current study, the percent decrease in the area of nail involvement with F‐RVCZ demonstrated a significant difference as compared with the placebo by the end of the treatment period (week 12). Aside from the variables compared among subjects, the nail involvement ratio in those given F‐RVCZ was decreased by approximately 25% on average (week 12). This suggests that F‐RVCZ efficacy can be perceived in a relatively early stage of treatment by detecting a decrease in the area of nail involvement.
Many of the conventional oral antifungal agents are known to induce hepatic functional disorders20, 21, 22, 23 and such agents are contraindicated for patients with serious hepatic functional disorders, as indicated in their package inserts used in Japan.24, 25
Although increased liver function test values were noted in some of the F‐RVCZ subjects, all such changes were reversible and did not manifest as serious hepatic functional disorders. However, because our current study was conducted in a limited number of subjects, detailed investigation will be needed to confirm the safety of F‐RVCZ by accumulating actual data in clinical settings after administration of this drug to a larger number of patients with longer follow up. As for the ADR, abdominal discomfort and other gastrointestinal disorders were reported, but these were generally mild, suggesting overall good tolerability of F‐RVCZ.
Fosravuconazole L‐lysine ethanolate, a novel oral antifungal agent, demonstrated significantly higher efficacy than placebo and sufficient tolerability, when administrated to patients with onychomycosis in a phase III study, as a capsule (equivalent to 100 mg of RVCZ) once daily for 12 weeks. F‐RVCZ appears to have a superior dosing regimen and better efficacy than the oral agents currently available on the market, and may well be a clinically useful drug for the treatment of onychomycosis, regardless of disease severity.
Conflict of Interest
I. T. and A. O. are employees of Sato Pharmaceutical Co., Ltd, Japan.
Supporting information
Table S1. List of principal investigators.
Acknowledgments
The authors acknowledge the contributions of the principal investigators listed in Table S1. This study was funded by Sato Pharmaceutical Co., Ltd, Japan.
References
- 1. Epidemiological Investigation Committee for Human Mycoses in the Japanese Society for Medical Mycology (Chairman and Reporter Yoshihiro Sei) . 2011 Epidemiological survey of dermatomycoses in Japan. Med Mycol J 2015; 56: J129–J135. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 2. Yamaguchi H. Potential of ravuconazole and its prodrugs as the new oral therapeutics for onychomycosis. Med Mycol J 2016; 57: E93–E110. [DOI] [PubMed] [Google Scholar]
- 3. Gupta AK, Leonardi C, Stoltz RR, Pierce PF, Conetta B, Ravuconazole Onychomycosis Group . A phase I/II randomized, double‐blind, placebo‐controlled, dose‐ranging study evaluating the efficacy, safety and pharmacokinetics of ravuconazole in the treatment of onychomycosis. J Eur Acad Dermatol Venereol 2005; 19: 437–443. [DOI] [PubMed] [Google Scholar]
- 4. Yamaguchi H, Ikeda F, Iyoda T, Suzuki M, Mikawa T. In vitro antifungal activity of ravuconazole against isolates of dermatophytes and Candida species from patients with dermatomycoses. Med Mycol J 2014; 55: J157–J163. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 5. Pfaller MA, Messer SA, Hollis RJ, Jones RN, Diekema DJ. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob Agents Chemother 2002; 46: 1723–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamazumi T, Pfaller MA, Messer SA, Houston A, Hollis RJ, Jones RN. In vitro activities of ravuconazole (BMS‐207147) against 541 clinical isolates of Cryptococcus neoformans. Antimicrob Agents Chemother 2000; 44: 2883–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta AK, Kohli Y, Batra R. In vitro activities of posaconazole, ravuconazole, terbinafine, itraconazole and fluconazole against dermatophyte, yeast and non‐dermatophyte species. Med Mycol 2005; 43: 179–185. [DOI] [PubMed] [Google Scholar]
- 8. Cuenca‐Estrella M, Gomez‐Lopez A, Mellado E, Garcia‐Effron G, Monzon A, Rodriguez‐Tudela JL. In vitro activity of ravuconazole against 923 clinical isolates of nondermatophyte filamentous fungi. Antimicrob Agents Chemother 2005; 49: 5136–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hata K, Kimura J, Miki H, Toyosawa T, Nakamura T, Katsu K. In vitro and in vivo antifungal activities of ER‐30346, a novel oral triazole with a broad antifungal spectrum. Antimicrob Agents Chemother 1996; 40: 2237–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fung‐Tomc JC, Huczko E, Minassian B, Bonner DP. In vitro activity of a new oral triazole, BMS‐207147 (ER‐30346). Antimicrob Agents Chemother 1998; 42: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pfaller MA, Diekema DJ, Jones RN et al International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J Clin Microbiol 2001; 39: 3254–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cuenca‐Estrella M, Gomez‐Lopez A, Mellado E, Garcia‐Effron G, Rodriguez‐Tudela JL. In vitro activities of ravuconazole and four other antifungal agents against fluconazole‐resistant or ‐susceptible clinical yeast isolates. Antimicrob Agents Chemother 2004; 48: 3107–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mummaneni V, Geraldes M, Hadjilambris OW, Ouyang Z, Uderman H, Marino MR. Effect of ravuconazole on the pharmacokinetics of simvastatin in healthy subjects. In Abstracts of the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 2000.
- 14. Ishii Y, Ito Y, Matsuki S et al Clinical drug‐drug interaction potential of BFE1224, prodrug of antifungal ravuconazole, using two types of cocktails in healthy subjects. Clin Transl Sci 2018; 11: 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sigurgeirsson B, Billstein S, Rantanen T et al L.I.ON. Study: efficacy and tolerability of continuous terbinafine (Lamisil®) compared to intermittent itraconazole in the treatment of toenail onychomycosis. Lamisil vs. Itraconazole in Onychomycosis. Br J Dermatol 1999; 141(Suppl 56): 5–14. [DOI] [PubMed] [Google Scholar]
- 16. Elewski BE, Rich P, Pollak R et al Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double‐blind studies. J Am Acad Dermatol 2013; 68: 600–608. [DOI] [PubMed] [Google Scholar]
- 17. Watanabe S, Kishida H, Okubo A. Efficacy and safety of luliconazole 5% nail solution for the treatment of onychomycosis: a multicenter, double‐blind, randomized phase III study. J Dermatol 2017; 44: 753–759. [DOI] [PubMed] [Google Scholar]
- 18. Werschler WP, Bondar G, Armstrong D. Assessing treatment outcomes in toenail onychomycosis clinical trials. Am J Clin Dermatol 2004; 5: 145–152. [DOI] [PubMed] [Google Scholar]
- 19. Sigurgeirsson B. Prognostic factors for cure following treatment of onychomycosis. J Eur Acad Dermatol Venereol 2010; 24: 679–684. [DOI] [PubMed] [Google Scholar]
- 20. Zapata Garrido AJ, Romo AC, Padilla FB. Terbinafine hepatotoxicity. A case report and review of literature. Ann Hepatol 2003; 2: 47–51. [PubMed] [Google Scholar]
- 21. van ‘t Wout JW, Herrmann WA, de Vries RA, Stricker BH. Terbinafine‐associated hepatic injury. J Hepatol 1994; 21: 115–117. [DOI] [PubMed] [Google Scholar]
- 22. Srebrnik A, Levtov S, Ben‐Ami R, Brenner S. Liver failure and transplantation after itraconazole treatment for toenail onychomycosis. J Eur Acad Dermatol Venereol 2005; 19: 205–207. [DOI] [PubMed] [Google Scholar]
- 23. Song JC, Deresinski S. Hepatotoxicity of antifungal agents. Curr Opin Investig Drugs 2005; 6: 170–177. [PubMed] [Google Scholar]
- 24. ITRIZOLE® (itraconazole) [package insert]. Tokyo, Japan: Janssen Pharmaceutical K.K., 2017. (in Japanese).
- 25. LAMISIL® (terbinafine) [package insert]. Tokyo, Japan: Sun Pharma Japan Limited, 2017. (in Japanese).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of principal investigators.


