Abstract
A high body mass index (BMI) is associated with increased cardiovascular risk. We sought to identify whether BMI influences the choice of lipid‐lowering treatment in a large, real‐world cohort of 52 916 patients treated with statins. The Dyslipidemia International Study (DYSIS) is a cross‐sectional, observational, multicentre study in statin‐treated patients ≥45 years of age from 30 countries; 1.1% were underweight (BMI < 18.5 kg/m2), 33.1% had normal weight (BMI 18.5‐24.9 kg/m2), 41.5% were overweight (BMI 25‐29.9 kg/m2), 17.1% had class I obesity (BMI 30.0‐34.9 kg/m2), 5.0% had class II obesity (BMI 35‐39.9 kg/m2), and 2.1% had class III obesity (≥40 kg/m2). BMI correlated with high‐density lipoprotein cholesterol (HDL‐C) and triglycerides (Spearman's ρ: −0.147 and 0.170, respectively; P < 0.0001 for both); however, there was no correlation with low‐density lipoprotein cholesterol (LDL‐C; ρ: 0.003; P = 0.51). Statin intensity increased with increasing BMI (ρ: 0.13; P < 0.001), an association that held after adjustment for comorbidities (OR: 2.4; 95% CI: 2.0‐3.0) on BMI ≥ 30 kg/m2 for atorvastatin equivalent ≥40 mg/d.
Keywords: body mass index, cardiovascular risk, cholesterol, hyperlipidemia, obesity, overweight, statins
1. INTRODUCTION
A high body mass index (BMI) has been associated with increased cardiovascular risk and all‐cause mortality.1, 2, 3, 4 The relationship between BMI and individual serum lipid components has been evaluated in a number of studies.5, 6
Lowering LDL‐C is the focus of current guidelines for the treatment of dyslipidemia, with specific target levels for reducing cardiovascular risk provided for different risk groups.7, 8 Statins form the backbone of lipid‐lowering therapy (LLT)8; however, it is not clear what factors influence their prescription in clinical practice. Various cardiovascular risk factors increase the likelihood of an individual being prescribed LLT, including hypertension and diabetes mellitus.9, 10 However, there is little evidence that BMI contributes significantly to the decision‐making process. These studies, however, did not evaluate the relationship between statin intensity and BMI.
In order to clarify the association of BMI with the levels of individual lipid components, and with prescribed statin intensity, we analyzed data from the global cohort of the Dyslipidemia International Study (DYSIS).
2. MATERIALS AND METHODS
2.1. Study design and patients
DYSIS is a cross‐sectional, observational, multicentre study including statin‐treated patients from 30 countries around the world.11 Patients were enrolled between 2006 and 2013, 6 to 10 patients per centre in order to maximize representativeness. The regions of the world included Europe, Canada, Israel, the Middle East and northern Africa, South Africa and China. Patients aged 45 years or over were included if they had a full fasting blood lipid profile available 12 months before enrollment, were being treated with statin therapy at the time the test was performed, and had been receiving a statin for at least 3 months prior to the test.
For this specific analysis we included all patients with the following parameters available: body weight and height, total cholesterol, LDL‐C, HDL‐C, and triglycerides. The study adhered to the Declaration of Helsinki.
2.2. Data collection
Details of the LLT that each patient was receiving were noted. Administration was per discretion of the treating physician, and not influenced by the design of the study, which only documented the respective, administered dosages. Statin potency was normalized according to atorvastatin potency to allow comparisons among patients.12 The most recent blood test was used to construct a full lipid profile, which included levels of LDL‐C, HDL‐C, total cholesterol, and triglycerides. LDL‐C levels were measured in accordance with standard practice in the respective regions of the world. BMI was recorded on the date of the DYSIS data collection.
2.3. Statistical methods
In order to identify correlations between BMI and LDL‐C, HDL‐C, and triglycerides, non‐parametric Spearman's ρ correlation coefficients were calculated, with all variables treated as continuous variables. The Jonkheere‐Terpstra tests were used to identify trends between lipid levels and BMI categories as defined by the World Health Organization (WHO; <18.5, 18.5‐24.9, 25.0‐29.9, 30.0‐34.9, 35.0‐39.9, ≥40.0 kg/m2), following the indicated a priori ordering. We used the Cochran‐Armitage test for trends in dichotomous variables such as female gender or comorbidities; <18.5 and 18.5 to 24.9 were subsequently grouped to <25 kg/m2, and 35.0 to 39.9 and ≥40.0 kg/m2 were subsequently grouped to ≥35.0 kg/m2, in order to have representative samples in each group and avoid a group with a very small sample size. In addition, Spearman's ρ correlation was calculated between BMI, as a categorical variable, and atorvastatin‐equivalent daily statin dosage, as a continuous variable. We used atorvastatin equivalents of 10, 20, 40 and 80 mg, respectively, and also included hypothetical 5 and 160 mg doses.12 This test was chosen for its higher rigour rather than a Pearson test, given the non‐parametric distribution of variables.
Odds Ratio (OR) was calculated using a logistic regression model considering both BMI (≥30 kg/m2) and statin dosage in atorvastatin equivalents (≥40 mg) as dichotomous variables. To enhance precision, factors found to be significantly associated with BMI were entered into a further forward logistic regression in order to determine the effect of BMI on statin dosage.
3. RESULTS
3.1. Patients
A total of 52 916 statin‐treated patients were enrolled. Of these, 1.1% were underweight (BMI < 18.5 kg/m2), 33.1% had a normal weight (BMI 18.5‐24.9 kg/m2), 41.5% were classed as being overweight (BMI 25‐29.9 kg/m2), 17.1% had class I obesity (BMI 30.0‐34.9 kg/m2), 5.0% had class II obesity (BMI 35‐39.9 kg/m2), and the remaining 2.1% had class III obesity (≥40 kg/m2) (Table 1).
Table 1.
Patient characteristics
|
Total
[N = 52 916] % (n/N), mean ± SD or median (Q1, Q3) |
BMI < 25 kg/m2
[N = 18 088] N (%), mean ± SD or median (Q1/Q3) |
BMI 25 to < 30 kg/m2
[N = 21 976] N (%), mean ± SD or median (Q1/Q3) |
BMI 30 to < 35 kg/m2
[N = 9062] N (%), mean ± SD or median (Q1/Q3) |
BMI ≥ 35 kg/m2
[N = 3790] N (%), mean ± SD or median (Q1/Q3) |
P‐value | |
|---|---|---|---|---|---|---|
| Age (y) | 65.39 ± 10.18 | 66.94 ± 10.49 | 65.11 ± 10.04 | 64.19 ± 9.72 | 62.50 ± 9.31 | < 0.0001 |
| Female | 45.0 (23 771/52 792) | 49.6 (8961/18 068) | 40.5 (8869/21 915) | 42.8 (3863/9031) | 55.0 (2078/3778) | 0.007 |
| BMI (kg/m2) | 26.5 (24.0, 29.8) | 23.0 (21.6, 24.1) | 27.1 (26.0, 28.3) | 31.7 (30.8, 33.1) | 37.7 (36.14, 40.8) | < 0.0001 |
| SBP (mm Hg) | 132.84 ± 15.76 | 130.23 ± 15.79 | 133.13 ± 15.27 | 135.77 ± 15.74 | 136.65 ± 16.34 | < 0.0001 |
| DBP (mm Hg) | 78.60 ± 9.68 | 76.87 ± 9.38 | 78.92 ± 9.44 | 80.35 ± 10.05 | 80.79 ± 10.17 | < 0.0001 |
| Hypertension | 72.6 (38 419/52 892) | 63.5 (11 483/18 082) | 73.2 (16 075/21 969) | 83.3 (7543/9052) | 87.6 (3318/3789) | < 0.0001 |
| Diabetes mellitus | 37.9 (20 056/52 855) | 29.5 (5339/18 079) | 35.7 (7836/21 957) | 50.3 (4549/9038) | 61.7 (2332/3781) | < 0.0001 |
| Ischemic heart disease | 40.7 (21 547/52 900) | 39.4 (7118/18 087) | 41.5 (9110/21 970) | 43.4 (3925/9054) | 36.8 (1394/3789) | 0.063 |
| Cerebrovascular disease | 13.0 (6862/52 704) | 15.5 (2795/18 049) | 12.8 (2791/21 890) | 10.9 (981/9000) | 7.8 (295/3765) | < 0.0001 |
| Heart failure | 8.2 (4331/52 895) | 5.8 (1056/18 086) | 7.8 (1707/21 966) | 11.3 (1024/9053) | 14.4 (544/3790) | < 0.0001 |
| Peripheral artery disease | 5.7 (3018/52 694) | 3.9 (703/18 046) | 6.1 (1331/21 886) | 7.9 (713/8997) | 7.2 (271/3765) | < 0.0001 |
| Lipid profile | ||||||
| LDL‐C (mg/dL) | 98.0 (76.0, 125.0) | 97.1 (75.4, 124.1) | 99.4 (77.3, 126.1) | 98.0 (77.0, 125.0) | 93.6 (72.7, 121.4) | 0.562 |
| HDL‐C (mg/dL) | 48.0 (39.8, 58.0) | 50.3 (41.8, 60.7) | 47.6 (39.8, 57.2) | 45.6 (38.7, 54.9) | 45.0 (38.0, 54.1) | < 0.0001 |
| Total cholesterol (mg/dL) | 176. 0 (147.7, 208.8) | 174.8 (145.4, 207.7) | 177.1 (148.9, 208.8) | 177.9 (150.8, 209.0) | 173.0 (147.0, 205.0) | < 0.0001 |
| Triglycerides (mg/dL) | 130.2 (93.9, 182.5) | 117.0 (84.2, 166.5) | 132.9 (95.7, 185.0) | 144.7 (104.5, 198.0) | 149.7 (109.0, 200.0) | < 0.0001 |
| LLT | ||||||
| Statin dosage (mg/d)a | 16.88 ± 12.38 | 14.98 ± 10.15 | 16.91 ± 12.38 | 19.27 ± 14.30 | 20.08 ± 15.18 | < 0.0001 |
| Ezetimibe | 6.6 (3494/52 581) | 3.2 (575/18 034) | 6.7 (1470/21 813) | 11.7 (1054/8979) | 10.5 (395/3755) | < 0.0001 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LLT, lipid‐lowering therapy; Q1, 25th percentile; Q3, 75th percentile; SBP, systolic blood pressure; SD, standard deviation.
P‐values: Cochran‐Armitage test or Jonckheere‐Terpstra test.
Normalized to atorvastatin potency13.
The prevalence of hypertension increased with increasing BMI category, reaching 87.6% for the group of patients with a BMI of ≥35 kg/m2. Diabetes mellitus also varied with BMI, rising from 29.5% of the patients with a BMI < 25 kg/m2, to 35.7% of those with a BMI of 25 to <30 kg/m2, 50.3% of those with a BMI of 30 to <35 kg/m2, and 61.7% of those with a BMI of ≥35 kg/m2. Heart failure and peripheral artery disease increased with increasing BMI category, while ischemic heart disease did not show a trend, and cerebrovascular disease is more prevalent in patients with lower BMI.
3.2. Lipid profile
LDL‐C levels did not vary significantly between BMI categories, with values of 97.1, 99.4, 98.0, and 93.6 mg/dL for the <25, 25 to <30, 30 to <35, and ≥ 35 kg/m2 groups, respectively (P = 0.562). On the other hand, HDL‐C levels varied with BMI category, decreasing with increasing BMI (50.3, 47.6, 45.6, and 45.0 mg/dL, respectively; P < 0.0001). Triglycerides increased with increasing BMI, with values of 117.0, 132.9, 144.7 and 149.7 mg/dL (P < 0.0001).
3.3. Lipid‐lowering therapy
The mean atorvastatin equivalent statin dosage for the overall population was 16.88 ± 12.38 mg/d. The daily dosage increased with increasing BMI, rising from 14.98 mg/d for the <25 kg/m2 group to 16.91, 19.27 and 20.08 mg/d for the 25 to <30, 30 to <35, and ≥35 kg/m2 groups, respectively (P < 0.0001). The proportion of patients being treated with ezetimibe varied significantly between BMI categories, with increasing frequency in patients with increasing BMI (3.2%, 6.7%, 11.7%, 10.5% for the <25, 25 to <30, 30 to <35, and ≥35 kg/m2, respectively; P < 0.0001).
3.4. Correlation between BMI and lipid levels or statin dose
LDL‐C levels were not found to correlate with BMI (ρ = 0.003; P = 0.51). On the other hand, HDL‐C (ρ = −0.147; P < 0.0001) negatively correlated, and triglycerides (ρ = 0.170; P < 0.0001) positively correlated with BMI (Figure 1).
Figure 1.
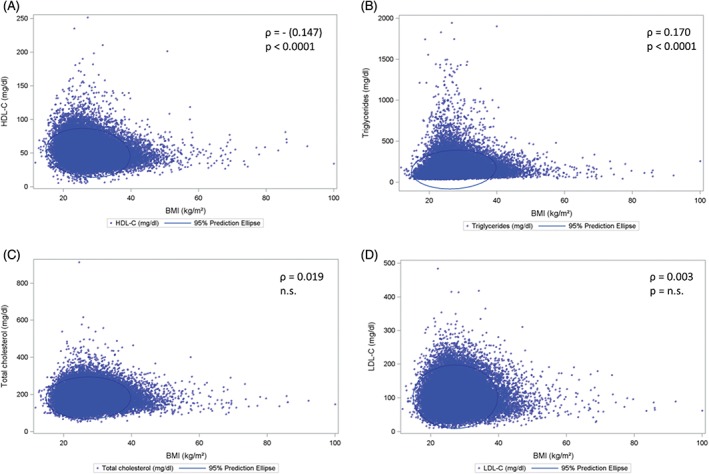
Correlations of blood lipoproteins and triglycerides with BMI. Scatter plots and prediction ellipses. Correlations between A, BMI and HDL‐C; B, BMI and triglycerides; C, BMI and total cholesterol; and D, BMI and LDL‐C; n = 52 916 for (A), (B), (C) and (D). Abbreviations: BMI, body mass index; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density‐lipoprotein cholesterol
Statin dosage also positively correlated with BMI (ρ = 0.13; P = 0.001). Furthermore, patients treated with a high statin dose were more likely to have a higher BMI. Statin intensity increased with increasing BMI (ρ: 0.13; P < 0.001). The same association was identified for the use of ezetimibe (P < 0.0001). Patients with cardio‐metabolic diseases were found to be more likely to have a high BMI, including ischemic heart disease (OR: 2.10; 95% CI: 1.89‐2.33), cerebrovascular disease (OR: 1.22; 95% CI: 1.05‐1.41), peripheral artery disease (OR: 1.38; 95% CI: 1.12‐1.71), and diabetes mellitus (OR: 1.18; 95% CI: 1.06‐1.31). This is further indicated by the incremental increase in prevalence of these diseases at higher BMI levels (Table 1). Introduction of each of these diseases into the logistic regression model demonstrated that a high statin dosage remained significantly associated with a high BMI (OR: 2.4; 95% CI: 2.0‐3.0 on BMI ≥ 30 kg/m2 for atorvastatin equivalent ≥40 mg/d).
4. DISCUSSION
BMI was found to correlate with HDL‐C and triglycerides levels, but not with LDL‐C. In addition, increased BMI was associated with the use of a higher statin dosage, independent of the presence of cardiovascular diseases. Ezetimibe use was more common in obese patients than in those patients with normal body weight.
In agreement with other studies, we found that increasing BMI correlated with decreasing HDL‐C levels and increasing triglycerides levels.5, 6, 14 On the other hand, we saw no such correlation between BMI and total cholesterol and, more importantly, LDL‐C.5, 6 We would like to warn, though, that the DYSIS dataset may not be ideal for such a conclusion. All the patients included in the DYSIS project are statin‐treated, which results in the potential of interaction between the lipid‐lowering treatment and its dose with the triglycerides, HDL‐C, LDL‐C and BMI. Based on the current data, we cannot exclude the possibility that there was no increase in LDL‐C with BMI because of the effect of statin use and dosing. We are convinced that this is not the case, but the data are not suitable for dismissing this possibility. Furthermore, we cannot exclude the potential role for concomitant drugs having an effect on BMI and/or LDL‐C levels.
The importance of lowering LDL‐C levels is undisputed, with studies demonstrating that it results in decreased rates of acute cardiovascular events.7, 8 Indeed, this particular lipid is the main focus of current guidelines for the treatment of dyslipidemias.7, 8 Statins and ezetimibe are the mainstays of LLT, having consistently been shown to reduce cardiovascular events in proportion to the absolute decrease in serum LDL‐C concentration with PCSK9 inhibitors as novel therapies.7, 15 In clinical practice, the presence of cardiovascular risk factors such as hypertension and diabetes mellitus increases the likelihood that an individual will be prescribed statin therapy.9, 10 A high BMI has also been linked to statin use.10 However, these studies only reported whether or not a patient was taking a statin; they did not evaluate the intensity of therapy.
In the global population of patients included in DYSIS, we found that BMI positively correlated with atorvastatin equivalent daily statin dosage. This association was significant even after adjustment for the presence of ischemic heart disease, cerebrovascular disease, peripheral artery disease, and diabetes mellitus, demonstrating the strength of the relationship. Furthermore, ezetimibe use was found to be more frequent in obese patients. It therefore appears that physicians may be influenced by BMI when deciding on LLT prescription, although there is no mention in the literature, or even recommendation in the prescription information, to adjust the dose with increasing BMI. As we have demonstrated that BMI does not correlate with LDL‐C level, this approach could be misleading. Even though cardiovascular risk clearly increases with increasing BMI, we propose to diagnose cardiovascular disease correctly, and further use available risk‐scoring tools to guide prescription. The Systematic COronary Risk Evaluation (SCORE) recommended by the ESC/EAS guidelines for primary prevention patients, the lifetime risk calculator used in the ACC/AHA guidelines, and the recently proposed and validated TRS2P risk score, are such examples to help identify patients who would benefit specifically from lipid‐lowering treatment.7, 8, 13
From this very large and representative cohort, it appears that physicians' decisions are directly influenced by patients' BMI when choosing statin dose or in combination with ezetimibe. Patients with higher BMI received higher doses of statins even after correcting for potential confounding factors such as diabetes or a history of ischemic heart disease. We therefore propose the use of established risk scores to guide clinical decision making in lipid‐lowering therapy.
ACKNOWLEDGMENTS
The authors thank all DYSIS investigators for their contribution to the successful completion of this study.
Conflicts of interest
JF reports personal fees from Amgen, MSD Inc., and Sanofi. DL, BA, and PB are employees of Merck & Co., Inc., Kenilworth, NJ, USA. CB is an employee of MSD Ltd. GDF reports grants and personal fees from MSD and Amgen, grants from Boston Scientific, and personal fees from LivaNova and SigmaTau. ME reports grants and personal fees from MSD; and personal fees from AstraZeneca, Pfizer, Abbott, Sanofi, Boehringer Ingelheim, Eli Lilly, GSK, Bristol‐Myers Squibb, Amgen, Novartis, Vianex and TEVA. AKG reports personal fees from Merck & Co., Inc., Kenilworth, NJ, USA. HT has served as an advisor and speaker for Novo Nordisk, Vivus and Orexigen. PPT is a member of the speakers' bureau of Amgen, Amarin, AstraZeneca, Kowa, Merck, Novartis, Regeneron, and Sanofi; he is a consultant of Amgen, AstraZeneca, Gemphire, Kowa, Merck, Regeneron. MH and HD declare no conflicts of interest.
Ferrières J, Lautsch D, Gitt AK, et al. Body mass index impacts the choice of lipid‐lowering treatment with no correlation to blood cholesterol – Findings from 52 916 patients in the Dyslipidemia International Study (DYSIS). Diabetes Obes Metab. 2018;20:2670–2674. 10.1111/dom.13415
Funding information Merck
REFERENCES
- 1. Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non‐linear dose‐response meta‐analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Angelantonio E, Bhupathiraju Sh N, Wormser D, et al. Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet. 2016;388:776‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body‐mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van‐Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875‐880. [DOI] [PubMed] [Google Scholar]
- 5. Lamon‐Fava S, Wilson PW, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women. The Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 1996;16:1509‐1515. [DOI] [PubMed] [Google Scholar]
- 6. Guardiola M, Sola R, Vallve JC, et al. Body mass index correlates with atherogenic lipoprotein profile even in nonobese, normoglycemic, and normolipidemic healthy men. J Clin Lipidol. 2015;9:824‐831. [DOI] [PubMed] [Google Scholar]
- 7. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999‐3058. [DOI] [PubMed] [Google Scholar]
- 8. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889‐2934. [DOI] [PubMed] [Google Scholar]
- 9. Fleetcroft R, Schofield P, Ashworth M. Variations in statin prescribing for primary cardiovascular disease prevention: cross‐sectional analysis. BMC Health Serv Res. 2014;14:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neutel CI, Morrison H, Campbell NR, de Groh M. Statin use in Canadians: trends, determinants and persistence. Can J Public Health. 2007;98:412‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gitt AK, Lautsch D, Ferrieres J, et al. Low‐density lipoprotein cholesterol in a global cohort of 57,885 statin‐treated patients. Atherosclerosis. 2016;255:200‐209. [DOI] [PubMed] [Google Scholar]
- 12. U.S. Food and Drug Administration . FDA drug safety communication: new restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury, 2011. https://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Accessed June 10, 2017.
- 13. Bohula EA, Morrow DA, Giugliano RP, et al. Atherothrombotic risk stratification and ezetimibe for secondary prevention. J Am Coll Cardiol. 2017;69:911‐921. [DOI] [PubMed] [Google Scholar]
- 14. Magkos F, Mohammed BS, Mittendorfer B. Effect of obesity on the plasma lipoprotein subclass profile in normoglycemic and normolipidemic men and women. Int J Obes (Lond). 2008;32:1655‐1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landmesser U, Chapman JM, Farnier M, et al. European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on protein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur Heart J. 2017;38:2245‐2255. [DOI] [PubMed] [Google Scholar]


