Abstract
Elucidating how cannabinoids affect brain function is instrumental for the development of therapeutic tools aiming to mitigate ‘on target’ side effects of cannabinoid‐based therapies. A single treatment with the cannabinoid receptor agonist, WIN 55,212‐2, disrupts recognition memory in mice. Here, we evaluate how prolonged, intermittent (30 days) exposure to WIN 55,212‐2 (1 mg/kg) alters recognition memory and impacts on brain metabolism and functional connectivity. We show that chronic, intermittent treatment with WIN 55,212‐2 disrupts recognition memory (Novel Object Recognition Test) without affecting locomotion and anxiety‐like behaviour (Open Field and Elevated Plus Maze). Through 14C‐2‐deoxyglucose functional brain imaging we show that chronic, intermittent WIN 55,212‐2 exposure induces hypometabolism in the hippocampal dorsal subiculum and in the mediodorsal nucleus of the thalamus, two brain regions directly involved in recognition memory. In addition, WIN 55,212‐2 exposure induces hypometabolism in the habenula with a contrasting hypermetabolism in the globus pallidus. Through the application of the Partial Least Squares Regression (PLSR) algorithm to the brain imaging data, we observed that prolonged WIN 55,212‐2 administration alters functional connectivity in brain networks that underlie recognition memory, including that between the hippocampus and prefrontal cortex, the thalamus and prefrontal cortex, and between the hippocampus and the perirhinal cortex. In addition, our results support disturbed lateral habenula and serotonin system functional connectivity following WIN 55,212‐2 exposure. Overall, this study provides new insight into the functional mechanisms underlying the impact of chronic cannabinoid exposure on memory and highlights the serotonin system as a particularly vulnerable target.
![]()
Keywords: cannabinoids, chronic, functional connectivity, recognition memory
Abbreviations used
- 14C‐2‐DG
14C‐2‐deoxyglucose
- AcbC
nucleus accumbens core
- AcbSh
nucleus accumbens shell
- AM
anteromedial thalamus
- aPrL
anterior prelimbic cortex
- aRT
reticular thalamus
- AV
anteroventral thalamus
- BLA
basolateral amygdala
- C57BL/6
black‐six mice
- CB1R
cannabinoid receptor 1
- Cg1
cingulate cortex
- ChVeh
chronic VEH
- ChWin
chronic WIN 55,212‐2
- CoA
central Amygdala
- DH CA1
dorsal Hippocampus, CA1
- DH CA2
dorsal Hippocampus, CA2
- DH DG
dorsal Hippocampus, Dentate Gyrus
- DH Mol
dorsal Hippocampus, Molecular Layer
- DLO
dorsolateral orbital cortex
- DLST
dorsolateral striatum
- DMSO
dimethylsulfoxide
- DR
dorsal raphe
- DSub
dorsal subiculum
- ENT
entorhinal cortex
- EPMT
elevated plus maze test
- fMRI
functional magnetic resonance imaging
- FRA
frontal association area
- GP
globus pallidus
- Hab
habenula
- HDB
horizontal limb of diagonal band of Broca
- I.P
intraperitoneal injection
- IL
infralimbic cortex
- Ins
insular cortex
- LCGU
local cerebral glucose utilization
- LO
lateral orbital cortex
- LS
lateral septum
- MB
mammillary body
- MD
medio‐dorsal thalamus
- MeA
medial amygdala
- MG
medial geniculate
- MO
medial orbital cortex
- mPrL
medial prelimbic cortex
- MR
medial raphe
- MS
medial septum
- MTL
medial temporal lobe
- NORT
novel object recognition test
- NPI
novelty preference index
- OFT
open field test
- PD
post‐natal days
- Piri
piriform cortex
- PLSR
partial least squares regression
- PRh
perirhinal cortex
- RSc
retrosplenial cortex
- S1
somatosensory cortex
- SNC
substancia nigra pars compacta
- SNR
substancia nigra pars reticulata
- UR
uptake ratio
- VDB
ventral limb of diagonal band of broca
- VH CA1
ventral hippocampus, CA1
- VH CA3
ventral hippocampus, CA3
- VH DG
ventral hippocampus, dentate gyrus
- VH LMol
ventral hippocampus, molecular layer
- VL
ventrolateral thalamus
- VMST
ventrolateral striatum
- VM
ventromedial thalamus
- VTA
ventral tegmental area
- WIN55,212‐2
(R)‐(+)‐[2,3‐dihydro‐5‐methyl‐3‐(4‐morpholinylmethyl)pyrrolo[1,2,3‐de]‐1,4‐benzoxazin‐6‐yl]‐1‐naphthyl‐methanone mesylate
Heavy or regular cannabis abuse, generally defined as daily or almost‐daily use over a prolonged period of time, has been linked to cognitive dysfunction (Abush and Akirav 2012) and increased risk of developing psychiatric symptoms, including schizophrenia spectrum disorders (Andréasson et al. 1987; Hall and Degenhardt 2009), acute psychosis and mania (Khan and Akella 2009) and an amotivational syndrome (Tunving 1987; Fujiwara 2001; Ozaki and Wada 2001). In addition, cannabis‐based medicines are increasingly being used to treat several diseases such as epilepsy (Maa and Figi 2014), chronic pain (Carter et al., 2015), multiple sclerosis (Fitzpatrick and Downer 2017) and neurodegenerative diseases (Fagan and Campbell 2014), but the potential for negative side effects has not been well characterized. Understanding the effects of chronic cannabinoid exposure upon brain and synaptic function opens a window into the development of therapeutic tools that could counteract the ‘on target’ side effects associated with chronic use of cannabis and cannabinoid‐based medicines (Copeland et al. 2013; Lovelace et al. 2015).
Cannabinoid receptor 1 (CB1R) mediates the characteristic psychoactive effects of exogenous cannabinoids and the synaptic actions of endocannabinoids (Kano et al. 2009). One immediate consequence of cannabis consumption is an impairment in memory consolidation, seen in both humans (Ranganathan and D'Souza 2006; Borgelt et al. 2013) and laboratory animals (Clarke et al. 2008; Kano et al. 2009; Sousa et al. 2011; Mouro et al. 2017). Cannabinoid‐mediated disruptions in learning and memory may be related to reported impairments in long‐term potentiation at glutamatergic synapses (Terranova et al. 1995; Stella et al. 1997; Misner and Sullivan 1999; Wang et al. 2016; Silva‐Cruz et al. 2017), detrimental modifications in fast‐/slow‐wave oscillations, known to be modulated by GABAergic interneurons (Freund et al. 2003), and altered activity in septal‐hippocampal monoaminergic and cholinergic pathways, known to regulate cortical plasticity and activity (Miller and Branconnier 1983; Gessa et al. 1998; Sullivan 2000; Redmer et al. 2003; Khakpai et al. 2012).
Studies in humans, using functional magnetic resonance imaging, have shown that chronic cannabis users display significant alterations in functional connectivity in brain networks relevant to self‐awareness (Pujol et al. 2014), working memory (Cousijn et al. 2013) and recognition memory (Riba et al. 2015) which may be linked with functional differences in structures of the medial temporal lobe and prefrontal cortex (PFC) (Riba et al. 2015). In a recent study, it was also shown that chronic marijuana use leads to increased functional connectivity in the orbitofrontal network, as well as higher functional connectivity in tracts that innervate the orbitofrontal cortex (Filbey et al. 2014). However, chronic consumption studies in humans can be contaminated by confounding variables, such as lifestyle factors or mixed drug use.
This work was designed to elucidate the impact of chronic, intermittent cannabinoid exposure on brain metabolism, functional brain connectivity and recognition memory. We carry out three different analysis: first, we evaluated recognition memory in the Novel Object Recognition Test (NORT); secondly, we studied brain metabolic activity in these animals using 14‐C‐2‐deoxyglucose (14C‐2‐DG) functional brain imaging and finally, we characterized alterations in brain network functional connectivity through the application of the Partial Least Squares Regression (PLSR) algorithm to the 14C‐2‐DG brain imaging data. We found out that adult mice chronically exposed to WIN 55,212‐2 displayed impaired recognition memory and differences in metabolic brain activity and dysfunctional connectivity in circuits that underlie memory processing, thus providing new insights into the functional mechanisms that underlie the impact of chronic cannabinoid exposure on memory.
Methods
Animals
Adult (8–12 weeks old) male C57BL/6 mice (IMSR Cat# CRL:27, RRID:IMSR_CRL:27) (Charles River, Barcelona, Spain) were used. Animals were housed in a temperature (22/24°C) and humidity (45–65%) regulated room with a 14/10‐h light/dark cycle (07:00–21:00) with ad libitum access to food and water. Animal behaviour experiments were conducted during the light phase at around the same time each day (10 : 00). All experimentation followed the European Community Guidelines (Directive 2010/63/EU) and the Portuguese law (DL 113/2013) for Animal Care for Research Purposes, and were approved by the ‘Instituto de Medicina Molecular’ Internal Committee and the Portuguese Animal Ethics Committee – Direcção Geral de Veterinária. The experimental protocol was not preregistered. Animals were habituated to the presence of the investigator and handled for 5 days before testing. A total of 40 mice were used, 20 were treated with WIN 55,212‐2 and 20 were receiving the vehicle only. All animals were used for behaviour analysis, which was performed in two series of 10 mice per drug condition, giving a total of 20 mice per condition. For connectivity analysis, 10 mice per condition were used. To determine whether an animal would be allocated to the control or experimental treatment group a pseudo‐randomization procedure was employed. Animals were tagged and distributed in groups of five animals to each housing cage (8 cages of 5 animals). Subsequently, four cages were randomly attributed to each treatment condition (either control or chronic WIN 55,212‐2). To do so, a number was attributed to each cage and randomly drawn. Drawn cage numbers were distributed sequentially to either the control or the experimental group (first drawn number allocated to control, the second drawn number attributed to experimental group, until all numbers have been drawn).
Drugs
WIN 55,212‐2 (TOCRIS Bioscience, catalogue number 1038, Bristol, Avon, United Kingdom) was suspended in dimethylsulfoxide (Sigma Aldrich, catalogue number D2650, Missouri, St. Louis, MO, USA at stock concentration of 100 mM, and stored at −20°C; appropriate dilutions of these solutions were made in saline (NaCl 0.9%) before injection. The amount of dimethylsulfoxide present in the solutions used for intraperitoneal (i.p.) injections was < 0.6 μL per mouse, and control animals were injected with equivalent amounts of vehicle (2 mL/kg).
Chronic WIN 55,212‐2 treatment protocol
WIN 55,212‐2 was administered at a dose of 1 mg/kg i.p., a dose known to affect recognition memory in the NORT without creating sedative or cataleptic effects that are associated with higher doses (Schneider and Koch 2003; Baek et al. 2009; Mouro et al. 2017), over 30 days. Behavioural tests were carried out on the last 7 days of treatment. The injections were performed around the same hour of the day (18 : 00 ± 1 h), while behavioural tests were performed during the morning (10 : 00). Doing so, we avoided withdrawal symptoms (Maldonado 2002; Lichtman and Martin 2005; Solymosi and Köfalvi 2017), while minimizing acute effects of the drug during testing. In order to replicate a pattern of chronic intermittent cannabinoid exposure (Lamarque et al. 2001; Schneider and Koch 2003) animals were treated for 5 consecutive days followed by 2 days without treatment (22 injections over 30 days). This protocol was designed to minimize the tolerance to WIN 55,212‐2 that may develop during chronic continuous administration (see Maldonado 2002; Hampson et al. 2003; Solymosi and Köfalvi 2017). For further detail on the treatment and experimental protocol see Fig. 1.
Figure 1.
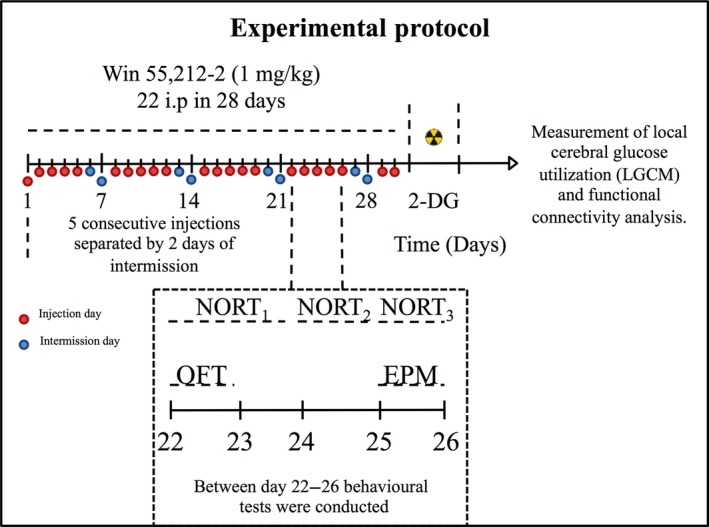
Scheme depicting the treatment schedule and overall experimental protocol. Over 30 consecutive days mice were treated with 22 intraperitoneal (i.p.) injections with WIN 55,212‐2 or vehicle control. Behavioural tests were performed between days 22 and 26; as indicated in the inset. The habituation phase of novel object recognition test (NORT) was initiated on day 22, simultaneously with the open field test (OFT). The habituation continued on the days 23 and 24. The training phase of NORT was performed on day 25. On day 26 the test phase of NORT was performed. Immediately after the NORT animals were placed in the EPM to assess anxiety‐like behaviour. Subsequently, 24 h after the final WIN 55,212‐2 treatment, animals underwent the 14C‐2‐deoxyglucose brain imaging protocol. From the initial 40 animals, one animal of the control group was excluded by post‐analysis (outlier, p < 0.05); thus, 19 control and 20 WIN 55,212‐2‐treated mice were used in the behavioural assays and 10 control and 10 WIN 55,212‐2‐treated mice were used in the 14C‐2‐deoxyglucose brain imaging study.
Novel object recognition test (NORT)
NORT was conducted in a wooden square open field arena (40 × 40 × 40 cm) as previously reported (Bailey and Crawley 2009; Antunes and Biala 2012). In brief, the test involved a habituation period (3 days), a training day and a test day. Before training, animals were habituated to the arena in the absence of any stimulus or object, under the same lighting and environmental conditions, for 20 min over 3 consecutive days. On the fourth day, after habituation, animals were placed inside the arena, facing away from the two identical objects (familiar objects) and allowed to freely explore the environment and objects for 5 min. After a retention interval of 24 h, to test long‐term memory (Clarke et al. 2008; Antunes and Biala 2012), animals were placed inside the arena with one novel and one familiar object (test day). Animals were allowed to explore the objects for 5 min. The objects used in the training and test days were wooden dolls (7 cm height × 6 cm width). The role of the object, as either familiar or novel, was randomized as was the location of their presentation. To randomize the role of the object a blind experimenter was asked to randomly select one of the objects to be the novel object for the first animal. Then, for the following animals, the novel object was always permuted, to ensure that both objects were used as the novel object the same number of times in both the control and the experimental groups (for instance, if the object A was randomly selected for the trial of animal 1, then for animal 2 the novel object would be object B, for animal 3 the object would be again object A, and so on). Between every trial the arena and the objects were cleaned with 30% ethanol to erase any olfactory clues. The objects were secured to the bottom of the arena with Velcro, which could not be seen or touched by the animals, and were placed in opposite corners of the arena. Activity was recorded using the video‐tracking software – SMART® (RRID:SCR_002852). To refine the results obtained by software measures, a post‐analysis was conducted. In the post‐analysis, the investigator was blind to the experimental condition. Exploratory behaviour was quantified as the amount of time (seconds) animals spent investigating each object (only direct approaches were considered; ≤ 1 cm distance). The number of approaches that included sniffing the object, rearing towards the object or touching the object was counted (Ennaceur and Delacour 1988; Ennaceur 2010; Antunes and Biala 2012). Exploration of each object was quantified as the novelty preference index (NPI) – calculated as (B−A)/(B+A), where B corresponds to the time spent exploring the novel object and A the time spent exploring the familiar object, during the test phase of NORT. This index ranges from −1 to 1 (−1 = exclusive exploration of the familiar object; 0 = absence of discrimination between novel and familiar objects, i.e. equal time exploring both objects; and 1 = exploration of the novel object only). We defined full immobility during the test stage of NORT as an a priori exclusion criterion, by which no animals were excluded from analysis. However, data from one animal on the control group were eliminated by post‐analysis as it corresponded to a significant outlier (p < 0.05).
Open field test (OFT) and elevated plus maze (EPM)
Anxiety‐like and locomotor behaviour were analysed in the OFT and EPM. Behaviour in the OFT was analysed before the NORT. The OFT was used to assess individual behaviour when animals were placed in a novel environment (Wilson et al. 1976), as well as anxiety (Careau et al. 2012). As locomotor activity can impact exploratory drive (Broadhurst 1957, 1958; Stanford 2007), it was important to ascertain that animals did not display significant differences in locomotor activity. The OFT took place in the same arena as that used for the NORT (square open field arena: 40 × 40 × 40 cm) and activity was recorded during the first time that animals had contact with the environment, that is, on the first 5 min of the first day of NOR habituation phase (see schematics on Fig. 1). To quantify behaviour, the percentage of time spent in the central zone of the arena was used as an indicator of anxiety, as previously described (Mouro et al. 2017). Mean velocity and distance moved were quantified to compare locomotor abilities between the experimental groups. Activity was recorded and analysed using the video‐tracking software – SMART® (RRID:SCR_002852). The reference point used by the software to determine the position of the animal was the centre of the mouse dorsum, as done previously in our Institute (Batalha et al. 2013; Coelho et al. 2014; Mouro et al. 2017 ). Environmental conditions and animal manipulation procedures were kept as similar as possible between animals.
Anxiety‐like behaviour was also assessed in the EPM. Immediately after being tested in the NORT during the test day (see schematics on Fig. 1), animals were placed in a maze shaped like a plus sign composed by two open arms with no walls (5 × 29 cm) and two closed arms (5 × 29 × 15 cm) arranged perpendicularly and elevated 50 cm above the floor (Mouro et al. 2017). Animals were placed on the centre of the maze, facing an open arm, and allowed to freely explore the maze during 5 min. The total time spent in open arms was used as a measure of anxiety‐like behaviour (Pellow et al. 1985; Coelho et al. 2014; Mouro et al. 2017).
14C‐2‐D‐deoxyglucose functional brain imaging
Local cerebral glucose utilization (LCGU) was determined after the final WIN 55,212‐2 (or vehicle) treatment (n = 10 per group, at day 31 for half of the controls and half of the test animals, at day 32 for the remaining animals), in accordance with previously published protocols (Dawson et al. 2012, 2015). In brief, mice were injected i.p. with 4.625 MBq/Kg of 2‐deoxy‐D‐[14C]glucose diluted in physiological saline (American Radiolabeled Chemicals Inc., USA, catalogue number ARC 0111A, dose volume of 2.5 mL/kg). After the injection animals were returned to their home cages. Forty‐five minutes after isotope injection, animals were killed by cervical dislocation and subsequently decapitated. A terminal blood sample was collected from the neck by torso inversion, to determine circulating glucose levels (Accu‐Chek Aviva Blood Glucose Monitor). The brain was dissected out and immediately frozen in isopentane (−40°C) and stored at −80°C until sectioning. Blood samples were centrifuged to separate the plasma for determination of plasma 2‐deoxy‐D‐[14C]‐glucose concentration by liquid scintillation analysis (Table S1).
Brains were coronally sectioned in a cryostat (−20°C) at 20 μm. Three consecutive sections were retained from every 60 μm and dried rapidly onto slide covers on a hot plate (70°C). Autoradiograms were obtained by placing these sections, along with pre‐calibrated 14C‐standards (39‐1069 nCi/g tissue equivalents; American Radiolabelled Chemicals Inc., St. Louis, MO, USA, catalogue number ARC 0146R) to high‐resolution autoradiographic film (Carestream Kodak Biomax MR, Sigma‐Aldrich, UK, catalogue number Z358460‐50EA) for 7 days, after which they were developed in accordance with the manufacturer's instructions. Autoradiographic images were analysed by computer‐based images analysis (MCID/M5) (RRID:SCR_014278). The local isotope concentration for each brain region of interest (RoI) was obtained directly from the optical density of autoradiographic images relative to that of co‐exposed 14C‐standards. Forty‐nine anatomically distinct RoI were measured with reference to a stereotactic mouse brain atlas (Paxinos and Franklin 2001) (RRID:SCR_007127). LCGU in each RoI was obtained by comparing the ratio of 14C in each RoI to the 14C concentration in the whole brain of the same animal, referred to as the 14C‐2‐DG uptake ratio (14C‐2‐DG UR). The whole brain average 14C concentration was determined as the average 14C concentration across all sections in which a RoI was measured. No animals were excluded from this data set.
Functional brain connectivity
Regional functional connectivity was analysed in control (saline treated) and WIN 55,212‐2‐treated mice. To elucidate the impact of chronic WIN 55,212‐2 exposure on regional functional connectivity, we applied the Partial Least Squares Regression (PLSR) algorithm (pls package in R, https://CRAN.R-project.org/package=pls, Mevik and Wehrens 2007) to characterize statistically significant differences in the functional connectivity of defined ‘seed regions’ to all the other RoI's analysed. In our analysis these seed regions were chosen on the basis of the overt differences in LCGU seen following chronic WIN 55,212‐2 treatment (Fig. 4). Thus, the chosen seed brain regions in our analysis were the dorsal subiculum of the ventral hippocampus (DSub), the mediodorsal thalamic nucleus (MD), the habenula (Hab) and the globus pallidus (GP). The application of the PLSR algorithm to functional 14C‐2‐DG imaging data was undertaken as previously reported (Dawson et al. 2012, 2015). In brief, functional connectivity between each seed RoI and all other RoI measured was defined by the variable importance to the projection (VIP) statistic gained from PLSR analysis. A significant functional connection between brain regions was considered to exist if the 95% confidence interval (CI) of the VIP statistic exceeded 0.8, denoting a considerable contribution of the explanatory variable (RoI) to the dependent variable (seed region) in PLSR analysis. The SD and CI of the VIP statistic were estimated by jack‐knifing. The significance of WIN 55,212‐2‐induced alterations in the VIP statistic was determined by t‐test with post hoc Bonferroni correction for multiple comparisons. WIN 55,212‐2‐induced alterations in functional connectivity on this basis were thus defined as being significant increases or reductions in functional connectivity (where the value of the VIP statistic is significantly altered between the groups but the 95% CI of the VIP statistic exceeds the 0.8 threshold in both experimental groups), or significantly lost or newly gained functional connectivity (WIN 55,212‐2‐treated animals significantly different from controls and the 95% CI of the VIP statistic exceeds the 0.8 threshold in only one of the experimental groups).
Statistical analysis
Data are expressed as means ± SEM. All data sets were tested for normality and analysed in Graphpad Prism 6 software (RRID:SCR_002798) or R (version 3.4.4, http://www.r-project.org, RRID: SCR_001905). A test for outliers was performed using the Graphpad Outlier Calculator. NORT data were analysed using two‐tailed, paired or unpaired Student's t‐tests, as appropriate for each condition and as indicated in the figure legends. Overt alterations in LCGU were statistically analysed by Student's t‐test, with discrete RoI treated as independent variables, as previously justified (Kelly and McCulloch 1987). Significance was set at p < 0.05 throughout.
Results
Chronic, intermittent WIN‐55,212‐2 administration disrupts recognition memory
Chronic, intermittent exposure to the cannabinoid agonist WIN 55,212‐2 disrupted recognition memory evaluated in the NORT. In the training phase, control animals (vehicle treated) and animals treated chronically with WIN 55,212‐2 explored the two identical objects for a similar amount of time (Fig. 2, panel a). However, on the test day, while control animals showed a significant preference for the novel as compared with the familiar object (p < 0.001, n = 19), WIN 22,515‐2‐treated animals did not (p > 0.05, n = 20). A similar effect was seen when analysing the novelty preference index (NPI) with control animals showing a significant preference for the novel object during the test phase (p < 0.05, n = 19), while WIN 55,212‐2‐treated animals did not (Fig. 2, panel b). Importantly, we found that there was no significant difference in the total time of object exploration between control and WIN 55,212‐2‐treated mice, during either the training or test phase (Fig. 2, panels c and d).
Figure 2.
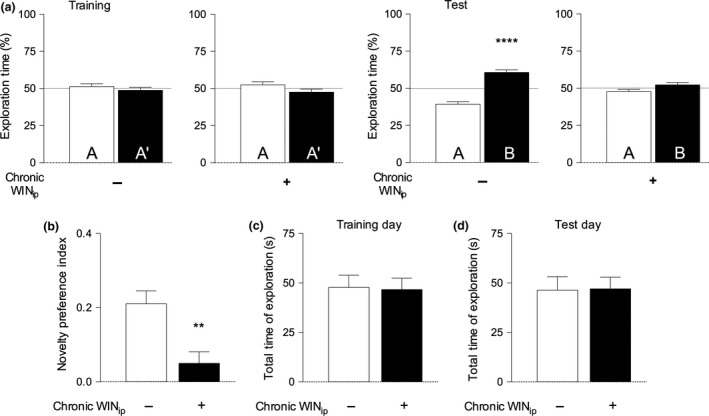
Chronic, intermittent WIN 55, 212‐2 treatment impairs recognition memory in the novel object recognition test (NORT). Panel a. Data are represented as % of exploration time. A denotes familiar object 1, A’ denotes familiar object 2, B denotes the novel object. – On the training day the % of time spent exploring each of the familiar objects did not differ between control and WIN 55,212‐2‐treated animals (p > 0.05, paired Student's t‐test). On the test day, control animals explored the novel object significantly more than the familiar object (****p < 0.0001, paired Student's t‐test). However, animals treated chronically with WIN 55,212‐2 spent a similar amount of time exploring both the familiar and novel object, (p > 0.05, paired Student's t‐test, comparing the % of time spent with a and b). Panel (b) – The Novelty Preference Index (NPI) is significantly decreased in WIN 55‐212‐2‐treated animals as compared with controls (**p < 0.01, unpaired Student's t‐test). A novelty index of zero represents absence of discrimination between objects (see Methods for details). The NPI of WIN55,212‐2‐treated mice was not significantly different from zero (p > 0.05, unpaired Student's t‐test), whereas in control mice the NPI was significantly different (p < 0.0001) from zero. Panels (c) and (d) – Total time of object exploration (TTE) during the training and test phase of novel object recognition test (NORT) was not significantly different between VEH and WIN‐treated animals (p > 0.05, unpaired Student's t‐test). An a posteriori Power Analysis for novel object recognition test (NORT) data was performed using G*power 3.1 software; considering a mean of 0.201 of preference for the novelty in control and 0.050 for drug‐treated animals (pooled SD (0.15), there is a 87.5% correct chance of correctly rejecting the null hypothesis (no differences on the t‐test), using 19 animals in the control group and 20 animals in the experimental group from a total of 39 animals.
The NPI scores obtained in this work were not significantly different from zero, being similar to those previously reported by us using the same NORT paradigm in mice of the same age during acute WIN 55,212‐2 administration (Mouro et al. 2017). This indicates that the treatment schedule used in this work does not result in tolerance with regards to recognition memory. Once confirmed this was then taken as a positive control to proceed for the assessment of the influence of WIN 55,212‐2 upon brain metabolism and functional brain connectivity, to elucidated the functional changes underlying this deficit.
Chronic, intermittent WIN‐55,212‐2 administration does not alter locomotor behaviour or anxiety‐like behaviour
Anxiety‐like behaviour and locomotor abilities were measured before, after and during the memory test, using, respectively, the OF test, the EPM and by measuring the total time of object exploration during the test phase of the NORT. We found no evidence to suggest that chronic WIN 55,212‐2 administration significantly impacted on locomotor activity or anxiety‐like behaviour (Figs 2 panel c and d and 3).
Figure 3.
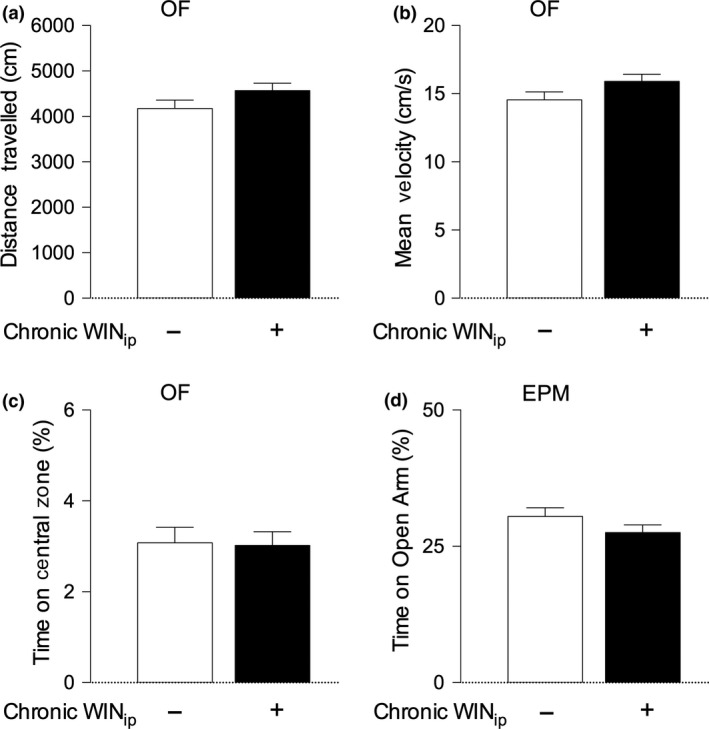
Chronic, intermittent WIN55, 212‐2 treatment does not impact on locomotor activity or anxiety‐like behaviour. Panel (a) and (b) – Locomotor abilities were assessed in the open field test (OFT) (panels (a) and (b)), performed during the first habituation day of the novel object recognition test (NORT). Chronic WIN, 55,212‐2 treatment did not significantly alter the total distance travelled (panel a) or velocity (panel b) in comparison with vehicle‐treated controls (p > 0.05, unpaired Student's t‐test). Panels (c) and (d) –Anxiety‐like behaviour was assessed at two time points: before the novel object recognition test (NORT) in the OFT, by evaluating the percentage of time spent in the central zone (panel c), and after novel object recognition test (NORT), in the EPM test (panel d) by evaluating the percentage of time spent in the open arms of the maze. In both tests, there were no significant differences between WIN 55,212‐2‐treated animals and saline‐treated controls (p > 0.05, unpaired Student's t‐test).
Chronic, intermittent WIN 55,212‐2 administration alters LCGU
Chronic, intermittent WIN 55,212‐2 administration induced both increases and decreases in LCGU on a brain region‐dependent basis. WIN 55,212‐2‐treated mice showed marked hypometabolism in the dorsal subiculum of the ventral hippocampus (DSub), the thalamic mediodorsal nucleus (MD) and in the habenula (Hab) (p < 0.05, n = 10). By contrast, WIN 55,212‐2‐treated animals showed significant hypermetabolism in only one of the brain regions analysed, the Globus Pallidus (GP) (p < 0.05, n = 10). These results support altered function in the basal ganglia–thalamic–hippocampal circuits (Fig. 4). Interestingly, when measuring LCGU on three different amygdala regions, there were no statistically significant differences between control and experimental groups. Thus, these data support the results obtained in the behaviour tests which showed no significant differences in anxiety‐like behaviour (Fig. 3) following chronic WIN 55,212‐2 administration. Full 14C‐2‐deoxyglucose brain imaging data are shown in the supplemental material (Table S2). Blood glucose and level of 14C‐2‐DG in the plasma were not significantly altered in control and WIN 55,212‐2‐treated animals (Table S1).
Figure 4.
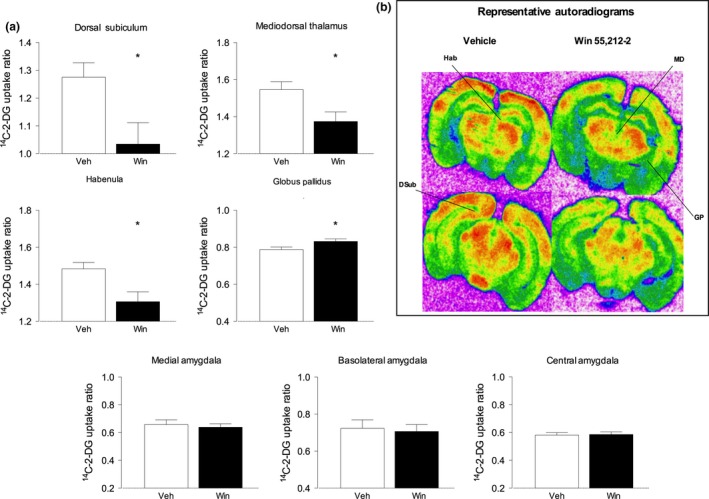
Chronic, intermittent WIN 55,212‐2 administration induces localized increases and decreases in local cerebral glucose utilization. Panel (a) – Chronic, intermittent WIN 55,212‐2 administration induced significantly decreased cerebral metabolism in the dorsal subiculum (DS), Mediodorsal thalamus (MD) and Habenula (Hab) and increased metabolism in the Globus Pallidus (GP). By contrast, cerebral metabolism in the amygdala nuclei (Basolateral, BLA; Central, CeA; Medial, MeA) was not significantly altered in WIN 55,212‐treated animals. | Data shown as the 14C‐2‐DG uptake ratio obtained for each RoI. *denotes p < 0.05 significant difference from control (unpaired Student's t‐test). Panel (b) – representative false colour autoradiograms at the level of the dorsal and ventral hippocampus showing altered cerebral metabolism in WIN 55,212‐2‐treated animals. Higher rates of metabolism are indicated by warmer colours (red/orange) and lower rates of metabolism by cooler colours (blue/green). Full data are shown in the Table S2.
Regional functional connectivity
We analysed the functional connectivity of four ‘seed’ regions, defined as those where we had identified a significant impact of WIN 55,212‐2 treatment on LCGU (DSub, MD, Hab, GP). Analysis by means of PLSR algorithm revealed significant modifications in functional connectivity in the mice that had received WIN 55,212‐2 (Fig. 5).
Figure 5.
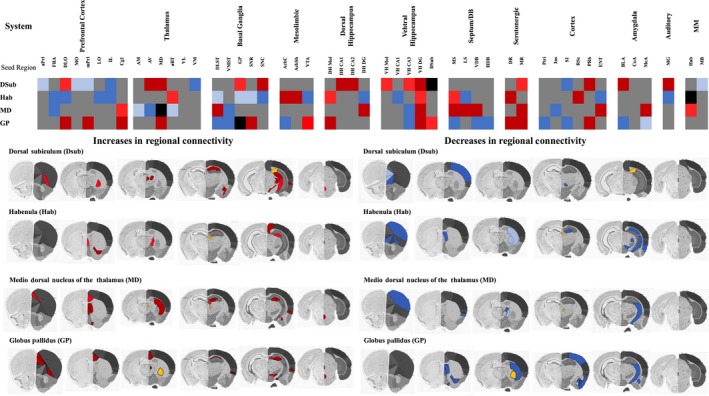
Chronic, intermittent WIN 55,212 treatment alters functional connectivity in neural circuits underpinning recognition memory. Panel (a) | Heatmap showing how chronic, intermittent treatment with WIN 55,212‐2‐induced modifications in the functional connectivity of ‘seed’ brain regions (DSub, MD, Hab and GP). Dark red denotes significantly gained connectivity, whereas light red represents significantly increased connectivity. Dark blue represents significantly lost connectivity, whereas light blue denotes significantly decreased connectivity. Significant gains, losses, increases and decreases in connectivity were determined by statistical comparison of the VIP statistic (unpaired Student's t‐test with Bonferroni post hoc correction) determined PSLR, with significance level set at p < 0.05. Full data for each ‘seed’ region are shown in the Tables S3–S6. Panel (b) | Brain images showing the anatomical localization of brain regions with significant increased/gained connectivity to the ‘seed’ regions (DSub, Hab, MD and GP). Yellow represents the anatomical localization of the ‘seed’ brain region. Brain section figures are modified from the Allen mouse brain atlas (mouse.brain‐map.org/static/atlas).
For the DSub of the ventral hippocampus, in control animals this region was significantly connected to multiple subfields of the PFC, selected other ventral hippocampal subfields and to the serotonergic raphé (dorsal raphé, DR; median raphé, MR; Table S3). In animals treated with WIN 55,212‐2, functional connectivity of the DSub was significantly altered. Specifically, significant decreases in (anterior prelimbic, aPrL; medial orbital MO and medial prelimbic, mPrL) and lost (infralimbic, IL) connectivity with multiple PFC regions were seen in animals treated chronically with WIN 55,212‐2. By contrast, chronic WIN 55,212‐2 administration resulted in new abnormal functional connectivity between the DSub of the ventral hippocampus and subfields of the dorsal hippocampus (CA1, CA2). In addition, functional connectivity of the DSub to other subfields in the ventral hippocampus was significantly enhanced (Mol, CA3) or gained (DG) in WIN 55,212‐2‐treated animals. This suggests that the local connectivity of the ventral hippocampus DSub to other hippocampal subfields was significantly increased as a result of chronic WIN 55,212‐2 administration. In addition, functional connectivity of the DSub to the perirhinal cortex was abnormally gained in response to chronic WIN 55,212‐2 treatment. Furthermore, the DSub gained new functional connectivity to discrete thalamic nuclei (anteroventral, AV; mediodorsal, MD) and to the serotonergic MR following chronic WIN‐55,212‐2 administration.
In control animals the Hab was functionally connected with several PFC subfields and several striatal, hippocampal and amygdala regions (Table S4). Following chronic WIN 55,212‐2 treatment the functional connectivity of the Hab was significantly altered. On one hand, the Hab lost functional connectivity with multiple PFC subfields (frontal association area; dorsolateral orbital cortex; lateral orbital cortex, LO and IL) and showed significantly lost (substantia nigra pars compacta, SNC) and decreased connectivity (dorsolateral striatum; GP and substantia nigra pars reticulata, SNR) to several regions of the basal ganglia. By contrast, WIN 55,212‐2 treatment provoked abnormal gains in connectivity between the Hab and subfields of the nucleus accumbens (core, AcbC; shell, AcbSh), with the serotonergic system (dorsal raphé, DR).
In control animals the MD thalamic nucleus was significantly functionally connected to multiple subfields of the prefrontal cortex (PFC), other cortical regions and other thalamic nuclei (Table S5). The functional connectivity of the MD was significantly altered in mice treated chronically with WIN 55,212‐2. This included significantly lost (anteroventral nucleus, AV) or decreased (anteromedial nucleus, AM; anterior reticular thalamus) connectivity to other thalamic nuclei, and cortical regions (frontal association cortex, frontal association area; insular cortex, Ins). By contrast, chronic WIN 55,212‐2 treatment resulted in new, abnormal functional connectivity between the MD and multiple subfields of the septum/diagonal band of Broca (medial septum, MS; lateral septum, LS; ventral limb of the diagonal band of broca, VDB).
Finally, in control animals the GP was functionally connected to numerous regions of the septum/diagonal band of Broca, and to striatal and cortical regions (Table S6). As a consequence of chronic WIN 55,212‐2 treatment the functional connectivity of the GP was significantly modified. The GP significantly lost functional connectivity with several regions of the septum/diagonal band of Broca (medial septum, MS; ventral limb diagonal band of Broca, VDB; horizontal limb diagonal band of Broca) and to the striatum (dorsolateral striatum and ventrolateral striatum). By contrast, the GP gained abnormal new connectivity with PFC (dorsolateral orbital cortex, mPrl and cingulate cortex, Cg1) and to the serotonergic raphe (MR and DR) following chronic, intermittent WIN 55,212‐2 treatment.
Discussion
In this work we demonstrated that mice chronically exposed to the non‐selective cannabinoid receptor agonist, WIN 55,212‐2, displayed disrupted cerebral metabolism and abnormal functional connectivity in the cortico–thalamic–hippocampal circuits that underlie recognition memory. This includes compromised perirhinal–hippocampus–prefrontal cortex and thalamo‐prefrontal functional connectivity. In parallel, we observed deficits in recognition memory as a consequence of chronic WIN 55,212‐2 administration without signs of altered motor abilities and anxiety‐like behaviour.
CB1Rs on synapses inhibit glutamatergic and GABAergic transmission, modulate different forms of synaptic plasticity and control neural oscillations that support behaviour and diverse cognitive functions, including learning and memory (Hajos et al. 2000; Piomelli 2003; Kano et al. 2009; Albayram et al. 2016; Araque et al. 2017; Lupica et al. 2017). Altogether, previous data also suggest that endo‐ and exo‐ cannabinoids may induce the functional reconfiguration of neuronal and brain networks to impact on memory processing, and we now specifically addressed this possibility.
Object recognition learning and memory is a process involving multiple items, the contextual clues surrounding them and the temporal order in each they are presented. Effective recognition memory depends on functional interactions within a circuit comprised by the perirhinal cortex (Bussey et al. 2000; Warburton and Brown 2010), the hippocampus (Brown and Aggleton 2001; Barker and Warburton 2011), the medial prefrontal cortex (O'Neil et al. 2012) and the mediodorsal nucleus of the thalamus (Mitchell et al. 2005; Warburton and Brown 2015). In this work we report deficits on recognition memory following chronic, intermittent WIN 55,212‐2 treatment as assessed by the NORT. Data from the behavioural test served as a positive confirmation for the results obtained from the brain imaging and functional connectivity experiments, discussed below. We found that chronic, intermittent WIN 55‐212‐2 administration significantly impacts on the function and connectivity of the hippocampal dorsal subiculum (DSub, Fig. 5), in line with previous suggestions that the subiculum is involved in recognition memory (Chang and Huerta 2012). The subiculum is a primary output structure of the hippocampus and receives direct projections from other brain regions critical for recognition memory, including the perirhinal cortex (Amaral et al. 2007). Monosynaptic and reciprocal connections between the subiculum and the perirhinal and the postrhinal cortices exist (Witter et al. 2000), implicating the subiculum in a short functional loop with cortical areas known to be crucial for recognition memory (Warburton and Brown 2010). Our study revealed that chronic, intermittent WIN 55,212‐2 treatment induced a pattern of irregular and dysfunctional connectivity between the dorsal subiculum and virtually every other subfield of the hippocampus (CA1, CA2, CA3, ML and DG; Fig. 5). Furthermore, chronic, intermittent WIN 55,212‐2 treatment impaired connectivity between the dorsal subiculum and several subfields of the prefrontal cortex, another structure directly implicated in recognition memory (Riba et al. 2015).
We also found widespread evidence for functional dysconnectivity of the mediodorsal (MD) thalamic nuclei as a consequence of WIN 55,212‐2 administration, which could also contribute to the deficits in recognition memory seen in these animals. Indeed, there is building evidence supporting a cortico–thalamic–hippocampal network, including the MD, that underlies recognition memory, in part as a result of the ability of the mediodorsal thalamic nuclei to act as a relay between the perirhinal and the medial prefrontal cortex (Parker and Gaffan 1998; Warburton and Brown 2015). The mediodorsal thalamic nucleus directly projects to the hippocampus and to the prefrontal cortex. Lesions in this thalamic nucleus result in recognition memory deficits (Parker et al. 1997), replicating deficits related with lesions of the medial temporal lobe (Warburton and Brown 2015). In addition, it has been previously shown that disconnection of the mediodorsal thalamic nucleus from the medial temporal cortex impairs object‐in‐place and temporal order performance (Cross et al. 2012).
The habenula is an important anatomical hub involved in a diverse range of behaviours including reward processing, reward prediction error, memory and the stress response (Naamboodiri et al. 2016). The habenula receives direct projections from the prefrontal cortex and the basal ganglia (globus pallidus, Hikosaka et al. 2008), regions that show reduced functional connectivity to the habenula after chronic, intermittent WIN 55,212‐2 administration (Fig. 4). The habenula also sends direct projections to dopamine‐rich brain regions including the ventral tegmental area and substantia nigra pars compacta (SNC, Hikosaka et al. 2008). Remarkably, the functional connectivity of the habenula to these regions is also lost in animals treated chronically with WIN 55,212‐2. Moreover, the functional connectivity of the habenula to other components of the mesolimbic system, including the nucleus accumbens, is also significantly altered by chronic WIN 55,212‐2‐treated animals. These effects may relate to the amotivation syndrome (Tunving 1987) and reward processing deficits (Fujiwara 2001; Friemel et al. 2014) seen as a result of cannabinoid exposure. By contrast, the functional connectivity of the habenula to the serotonergic raphe, to which the habenula directly projects, is abnormally enhanced by chronic WIN 55,212‐2 administration. This suggests that chronic cannabinoid exposure may impact both dopaminergic and serotonergic system function by impacting, in part, on the functional connectivity of the habenula.
Altered serotonin system function as a result of chronic, intermittent WIN 55,212‐2 administration, is also supported by broader evidence of altered raphé nuclei functional connectivity, being evident to each of the seed brain regions analysed. A number of previous studies have found that chronic cannabinoid exposure alters the functioning of the serotonin system, including the induction of altered serotonin levels (Sagredo et al. 2006) and serotonin receptor activity (Darmani 2001; Hill et al. 2006; Moranta et al. 2009; Franklin et al., 2013; Esteban and García‐Sevilla 2012). Moreover, the role of the serotonin system in recognition memory is firmly established (Zhang et al. 2013, 2015) suggesting that the disruption of serotonin system connectivity may be one of the key mechanisms by which chronic, intermittent WIN 55,212‐2 exposure impairs recognition memory. This possibility warrants, however, further systematic investigation. If found to be correct, targeting the serotonin system may represent a therapeutic strategy to restore memory deficits as a consequence of chronic cannabinoid exposure.
Cannabinoid intake induces more severe behavioural deficits in pubertal rats than in mature animals (Schneider and Koch 2002; Schneider et al., 2008). Indeed, short‐term recognition memory impairments (30 min retention time in NORT) can persist beyond cannabinoid exposure if the exposure occurs during the pubertal period (Schneider and Koch 2002; Schneider et al., 2008), but not if the exposure occurs only during adulthood (Schneider and Koch 2003) or even if it occurs only during the pre‐pubertal period (Schneider et al. 2005). However, as we herein show, cannabinoid exposure in adulthood does induce alterations in brain metabolism and connectivity, and these alterations are accompanied by significant recognition memory impairments over longer retention intervals (24 h). In addition, the present evidence showing that functional connectivity between the thalamus and prefrontal cortex is affected by cannabinoid exposure is in line with previous findings that cannabinoids can exacerbate deficits induced by prefrontal cortex lesions (Schneider and Koch 2005; Schneider and Koch 2007).
The exact pharmacological identification of the receptor influenced by the cannabinoid compound used in this work is outside its objective. However, there is evidence that supports the possibility of the presently described actions of WIN 55,212‐2 being mediated through CB1R. WIN 55‐212,2 is one of the most commonly used cannabinomimetics to study the role of the CB1R and the CB2R (Solymosi and Köfalvi 2017), showing similar preference for activating both receptors (Pertwee et al. 2010). On the other hand, evidence has consistently shown that WIN 55,212‐2 has no effect upon G protein‐coupled receptor 55 (GPR55) (Johns et al. 2007; Ryberg et al. 2007; Pertwee et al. 2010; Solymosi and Köfalvi 2017). We previously showed that the CB1R selective antagonist (AM 251) abolished the impact of acute WIN 55,212‐2 administration on recognition memory, which was evaluated with the same NORT paradigm and using mice of the same age as used in this study (Mouro et al. 2017), supporting involvement of CB1R. There is, in fact, a broad range of evidence supporting the involvement of CB1R in memory deficits (Clarke et al. 2008; Suenaga and Ichitani 2008; Wise et al. 2009), while CB2R agonists do not seem to affect recognition memory (Clarke et al. 2008). Regarding modifications of brain metabolism following cannabinoid administration, evidence shows that CB1R agonists lead to decreases in glucose uptake (Duarte et al. 2012; Miederer et al. 2017) and mitochondrial respiration (Bénard et al. 2012).
In conclusion, we herein demonstrated that prolonged, intermittent exposure of adult mice to the non‐selective cannabinoid receptor agonist WIN 55,212‐2 induces alterations in metabolic brain activity in selected brain regions, alters their functional connectivity and impairs recognition memory. Connectivity modifications were seen in circuits known to be directly involved in recognition memory, and for the habenula and raphé nuclei. These data give new insight into the mechanisms by which chronic cannabinoid exposure impacts on behaviour and cognition, and highlight the value of considering cannabinoid actions at the systems‐level perspective.
Author contributions
FMM conducted the experiments and data acquisition, as well as performed all habituation procedures and data analysis. ND contributed to data acquisition, to the design of the brain connectivity experiments and introduced FMM to the corresponding technical procedures and data analysis. AMS and JAR contributed to data discussion. AMS designed the project. FMM, AMS and ND wrote the manuscript. All authors contributed to the final version of the manuscript.
Acknowledgments and conflict of interest disclosure
This work was supported by LISBOA‐01‐0145‐FEDER‐007391, project co‐funded by FEDER through POR Lisboa 2020 (Programa Operacional Regional de Lisboa) from PORTUGAL 2020 and Fundação para a Ciência e Tecnologia (FCT), by an FCT project (Fundação para a Ciência e a Tecnologia, PTDC/DTP‐FTO/3346/2014) and by a Twinning action (SynaNet) from the EU H2020 Programme (project number: 692340), which covered short‐term scientific missions of FMM at ND laboratory and of ND at AMS laboratory. FMM was in receipt of SFRH/BD/89582/2012 FTC fellowship. AMS is a handling editor for the Journal of Neurochemistry.
All experiments were conducted in compliance with the ARRIVE guidelines.
Supporting information
Table S1. Body weight and blood parameters for animals undergoing the 14C‐2‐DG experiment.
Table S2. Local cerebral glucose utilization (LCGU) in WIN 55,212‐2 and control animals.
Table S3. Functional Connectivity of the Ventral Hippocampal Dorsal Subiculum.
Table S4. Functional Connectivity of the Habenula.
Table S5. Functional Connectivity of the Mediodorsal Thalamic Nucleus.
Table S6. Functional Connectivity of the Globus Pallidus.
References
- Abush H. and Akirav I. (2012) Short‐ and long‐term cognitive effects of chronic cannabinoids administration in late‐adolescence rats. PLoS ONE 7, e31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albayram O., Passlick S., Bilkei‐Gorzo A., Zimmer A. and Steinhäuser C. (2016) Physiological impact of CB1 receptor expression by hippocampal GABAergic interneurons. Eur. J. Physiol. 468, 727–737. [DOI] [PubMed] [Google Scholar]
- Amaral D. G., Scharfman H. E. and Lavenex P. (2007) The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 163, 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andréasson S., Engström A., Allebeck P. and Rydberg U. (1987) Cannabis and schizophrenia: a longitudinal study of swedish conscripts. Lancet 330, 1483–1486. [DOI] [PubMed] [Google Scholar]
- Antunes M. and Biala G. (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 13, 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A., Castillo P. E., Manzoni O. J. and Tonini R. (2017) Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology 124, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J. H., Zheng Y., Darlington C. L. and Smith P. F. (2009) The CB1 receptor agonist, WIN 55,212‐2, dose‐dependently disrupts object recognition memory in adult rats. Neurosci. Lett. 464, 71–73. [DOI] [PubMed] [Google Scholar]
- Bailey K. and Crawley J. (2009). Anxiety‐related behaviors in mice, in Methods of Behavior Analysis in Nueroscience (Buccafusco J., ed). 2nd edn. Boca Raton (FL): CRC Prss/Taylor & Francis. [PubMed] [Google Scholar]
- Barker G. R. I. and Warburton E. C. (2011) When is the hippocampus involved in recognition memory? J. Neurosci. 31, 10721–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalha V. L., Pego J. M., Fontinha B. M., Costenla A. R., Valadas J. S., Baqi Y., Radjaina H., Müller C. E., Sebastião A. M. and Lopes L. V. (2013) Adenosine A2A receptor blockade reverts hippocampal stress‐induced deficits and restores corticosterone circadian oscillation. Mol. Psychiatry 18, 320–331. [DOI] [PubMed] [Google Scholar]
- Bénard G., Massa F., Puente N. et al (2012). Mitochondrial CB₁ receptors regulate neuronal energy metabolism. Nat. Neurosci. 15:558–564. [DOI] [PubMed] [Google Scholar]
- Borgelt L. M., Franson K. L., Nussbaum A. M. and Wang G. S. (2013) The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 33, 195–209. [DOI] [PubMed] [Google Scholar]
- Broadhurst P. L. (1957) Determination of emotionality in the rat. I. Situational factors. Br. J. Psychol. 49, 12–20. [DOI] [PubMed] [Google Scholar]
- Broadhurst P. L. (1958) Determinants of emotionality in the rat. II. Antecedent factors. Br. J. Psychol. 49, 12–20. [DOI] [PubMed] [Google Scholar]
- Brown M. W. and Aggleton J. P. (2001) Recognition memory: what are the roles of theperirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2, 51–61. [DOI] [PubMed] [Google Scholar]
- Bussey T. J., Duck J., Muir J. and Aggleton J. P. (2000) Distinct patterns of behavioural impair‐ments resulting from fornix transection or neurotoxic lesions of the perirhinaland postrhinal cortices in the rat. Behav. Brain Res. 111, 187–202. [DOI] [PubMed] [Google Scholar]
- Careau V., Bininda‐Emonds O. R. P., Ordonez G. and Garland T. (2012) Are voluntary wheel running and open‐field behavior correlated in mice? different answers from comparative and artificial selection approaches. Behav. Genet. 42, 830–844. [DOI] [PubMed] [Google Scholar]
- Carter G. T., Javaher S. P., Nguyen M. H., Garret S. and Carlini B. H. (2015) Re‐branding cannabis: the next generation of chronic pain medicine? Pain Manag. 5(1), 13–21. [DOI] [PubMed] [Google Scholar]
- Chang E. H. and Huerta P. T. (2012) Neurophysiological correlates of object recognition in the dorsal subiculum. Front. Behav. Neurosci. 6, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. R., Rossato J. I., Monteiro S., Bevilaqua L. R. M., Izquierdo I. and Cammarota M. (2008) Posttraining activation of CB1 cannabinoid receptors in the CA1 region of the dorsal hippocampus impairs object recognition long‐term memory. Neurobiol. Learn. Mem. 90, 374–381. [DOI] [PubMed] [Google Scholar]
- Coelho J. E., Alves P., Canas P. M. et al (2014). Overexpression of adenosine A2A receptors in Rats: effects on depression, locomotion, and anxiety. Front. Psych. 5, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J., Rooke S. and Swift W. (2013) Changes in cannabis use among young people: impact on mental health. Curr Opin Psychiatry 26, 325–9. [DOI] [PubMed] [Google Scholar]
- Cousijn J., Goudriaan A. E., Ridderinkhof K. R., van den Brink W., Veltman D. J. and Wiers R. W. (2013) Neural responses associated with cue‐reactivity in frequent cannabis users. Addict Biol. 18, 570–80. [DOI] [PubMed] [Google Scholar]
- Cross L., Brown M. W., Aggleton J. P. and Warburton E. C. (2012) The medial dorsal thalamic nucleus and the medial prefrontal cortex of the rat function together to support associative recognition and recency but not item recognition. Learn Mem 20, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani N. A. (2001) Cannabinoids of diverse structure inhibit two DOI‐induced 5‐HT(2A) receptor mediated behaviors in mice. Pharmacol. Biochem. Behav. 68, 311–317. [DOI] [PubMed] [Google Scholar]
- Dawson N., Thompson R. J., McVie A., Thomson D. M., Morris B. J. and Pratt J. A. (2012) Modafinil reverses phencyclidine‐induced deficits in cognitive flexibility, cerebral metabolism, and functional brain connectivity. Schizophr. Bull. 38, 457–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson N., Kurihara M., Thomson D. M. et al (2015) Altered functional brain network connectivity and glutamate system function in transgenic mice expressing truncated Disrupted‐in‐Schizophrenia 1. Transl. Psychiatry 5, e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte J. M. N., Ferreira S. G., Carvalho R. A., Cunha R. A. and Köfalvi A. (2012) CB1 receptor activation inhibits neuronal and astrocytic intermediary metabolism in the rat hippocampus. Neurochem. Int. 60, 1–8. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. (2010) One‐trial object recognition in rats and mice: methodological and theoretical issues. Behav. Brain Res. 215, 244–254. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. and Delacour J. (1988) A new one‐trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav. Brain Res. 31, 47–59. [DOI] [PubMed] [Google Scholar]
- Esteban S. and García‐Sevilla J. A. (2012) Effects induced by cannabinoids on monoaminergic systems in the brain and their implications for psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 38, 78–87. [DOI] [PubMed] [Google Scholar]
- Fagan S. G. and Campbell V. A. (2014) The influence of cannabinoids on generic traits of neurodegeneration. Br. J. Pharmacol. 171, 1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F. M., Aslan S., Calhoun V. D., Spence J. S., Damaraju E., Caprihan A. and Segall J. (2014) Long‐term effects of marijuana use on the brain. Proc. Natl Acad. Sci. USA 111, 16913–16918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick J. K. and Downer E. J. (2017) Toll‐like receptor signalling as a cannabinoid target in Multiple Sclerosis. Neuropharmacology 113, 618–626. [DOI] [PubMed] [Google Scholar]
- Franklin J. M., Mathew M. and Carrasco G. A. (2013) Cannabinoid‐induced upregulation of serotonin 2A receptors in the hypothalamic paraventricular nucleus and anxiety‐like behaviors in rats. Neurosci. Lett. 548, 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund T. F., Katona I. and Piomelli D. (2003) Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 83, 1017–1066. [DOI] [PubMed] [Google Scholar]
- Friemel C. M., Zimmer A. and Schneider M. (2014) The CB1 receptor as an important mediator of hedonic reward processing. Neuropsychopharmacology 39, 2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M. (2001) Characteristics of abnormal behavior induced by delta 9‐tetrahydrocannabinol in rats. Nihon Yakurigaku Zasshi 117, 35–41. [DOI] [PubMed] [Google Scholar]
- Gessa G. L., Casu M. A., Carta G. and Mascia M. S. (1998) Cannabinoids decrease acetylcholine release in the medial‐prefrontal cortex and hippocampus, reversal by SR 141716A. Eur. J. Pharmacol. 355, 119–124. [DOI] [PubMed] [Google Scholar]
- Hajos N., Katona I., Naiem S. S., Mackie K., Ledent C., Mody I. and Freund T. F. (2000) Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur. J. Neurosci. 12, 3239–3249. [DOI] [PubMed] [Google Scholar]
- Hall W. and Degenhardt L. (2009) Adverse health effects of non‐medical cannabis use. Lancet 374, 1383–1391. [DOI] [PubMed] [Google Scholar]
- Hampson R. E., Simeral J. D., Kelly E. J. and Deadwyler S. A. (2003). Tolerance to the memory disruptive effects of cannabinoids involves adaptation by hippocampal neurons. Hippocampus 13, 543–556. [DOI] [PubMed] [Google Scholar]
- Hikosaka O., Sesack S. R., Lecourtier L. and Shepard P. D. (2008) Habenula: crossroad between the Basal Ganglia and the Limbic System. J. Neurosci. 28, 11825–11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. N., Sun J. C., Tse M. T. and Gorzalka B. B. (2006) Altered responsiveness of serotonin receptor subtypes following long‐term cannabinoid treatment. Int. J. Neuropsychopharmacol. 9, 277–286. [DOI] [PubMed] [Google Scholar]
- Johns D. G., Behm D. J., Walker D. J. et al (2007) Br. J. Pharmacol. 152, 825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M., Ohno‐shosaku T., Hashimotodani Y. and Uchigashima M. (2009) Endocannabinoid‐mediated control of synaptic transmission. Physiol. Rev. 89, 309–380. [DOI] [PubMed] [Google Scholar]
- Kelly P. A. and McCulloch J. (1987) Cerebral glucose utilization following striatal lesions: the effects of the GABA agonist, muscimol, and the dopaminergic agonist, apomorphine. Brain Res. 425, 290–300. [DOI] [PubMed] [Google Scholar]
- Khakpai F., Nasehi M., Haeri‐Rohani A., Eidi A. and Zarrindast M. R. (2012) Basic . Clin. Neurosci. 4, 5–23. [PMC free article] [PubMed] [Google Scholar]
- Khan M. A. and Akella S. (2009) Cannabis‐induced bipolar disorder with psychotic features: a case report. Psychiatry (Edgmont) 6, 44–48. [PMC free article] [PubMed] [Google Scholar]
- Lamarque S., Taghzouti K. and Simon H. (2001) Chronic treatment with D9‐tetrahydrocannabinol enhances the locomotor response to amphetamine and heroin Implications for vulnerability to drug addiction. Neuropharmacology 41, 118–129. [DOI] [PubMed] [Google Scholar]
- Lichtman A. H. and Martin B. R. (2005) Cannabinoid tolerance and dependence. Handb. Exp. Pharmacol. 168, 691–717. [DOI] [PubMed] [Google Scholar]
- Lovelace J. W., Corches A., Vieira P. A., Hiroto A. S., Mackie K. and Korzus E. (2015) An animal model of female adolescent cannabinoid exposure elicits a long‐lasting deficit in presynaptic long‐term plasticity. Neuropharmacology 99, 242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica C. R., Hu Y., Devinsky O. and Hoffman A. F. (2017) Cannabinoids as hippocampal network administrators. Neuropharmacology 124, 25–37. [DOI] [PubMed] [Google Scholar]
- Maa E. and Figi P. (2014) The case for medical marijuana in epilepsy. Epilepsia 55, 783–786. [DOI] [PubMed] [Google Scholar]
- Maldonado R. (2002) Study of cannabinoid dependence in animals. Pharmacol. Ther. 95, 153–164. [DOI] [PubMed] [Google Scholar]
- Mevik B. H. and Wehrens R. (2007) The pls package: principal component and partial least squares regression in R. J. Stat. Softw. 18, 1–24. [Google Scholar]
- Miederer I., Uebbing K., Röhrich J., Maus S., Bausbacher N., Krauter K., Weyer‐Elberich V., Lutz B., Schreckenberger M. and Urban R. (2017) Effects of tetrahydrocannabinol on glucose uptake in the rat brain. Neuropharmacology 117, 273–281. [DOI] [PubMed] [Google Scholar]
- Miller L. L. and Branconnier R. J. (1983) Cannabis: effects on memory and the cholinergic limbic system. Psychol. Bull. 93, 441–456. [PubMed] [Google Scholar]
- Misner D. L. and Sullivan J. M. (1999) Mechanism of cannabinoid effects on long‐term potentiation and depression in hippocampal CA1 neurons. J. Neurosci. 19, 6795–6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. S. and Dalrymple‐Alford J. C. (2005) Dissociable memory effects after medial thalamus lesions in the rat. Eur. J. Neurosci. 22, 973–85. [DOI] [PubMed] [Google Scholar]
- Moranta D., Esteban S. and García‐Sevilla J. A. (2009) Chronic treatment and withdrawal of the cannabinoidagonist WIN 55,212‐2 modulate the sensitivityof presynaptic receptors involved in the regulation of monoamine syntheses in rat brain. Naunyn‐Schmiedeberg's Arch. Pharmacol. 379, 61–72. [DOI] [PubMed] [Google Scholar]
- Mouro F. M., Batalha V. L., Ferreira D. G., Coelho J. E., Baqi Y., Müller C. E., Lopes L. V., Ribeiro J. A. and Sebastião A. M. (2017) Chronic and acute adenosine A 2A receptor blockade prevents long‐term episodic memory disruption caused by acute cannabinoid CB 1 receptor activation. Neuropharmacology 117, 316–327. [DOI] [PubMed] [Google Scholar]
- Naamboodiri V. M. K., Rodriguez‐Romaguera J. and Stuber G. D. (2016) Curr. Biol. 26, R865–R881. [DOI] [PubMed] [Google Scholar]
- Nudo R. J. and Masterton R. B. (1986) Stimulation‐induced [14C]2‐deoxyglucose labeling of synaptic activity in the central auditory system. J. Comp. Neurol. 245, 553–565. [DOI] [PubMed] [Google Scholar]
- Ozaki S. and Wada K. (2001) Amotivational syndrome in organic solvent abusers. Nihon Yakurigaku Zasshi 117, 42–48. [DOI] [PubMed] [Google Scholar]
- Parker A. and Gaffan D. (1998) Interaction of frontal and perirhinal cortices in visual object recognition memory in monkeys. Eur. J. Neurosci. 10, 3044–3057. [DOI] [PubMed] [Google Scholar]
- Parker A., Eacott M. J. and Gaffan D. (1997) The recognition memory deficit caused by mediodorsal thalamic lesion in non‐human primates: a comparison with rhinal cortex lesion. Eur. J. Neurosci. 9, 2423–2431. [DOI] [PubMed] [Google Scholar]
- Paxinos G. and Franklin K. (2001) Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates, 4th ed Academic Press, San Diego. [Google Scholar]
- Pellow S., Chopin P., File S. E. and Briley M. (1985) Validation of open: closed arm entries in an elevated plus‐maze as a measure of anxiety in the rat. J. Neurosci. Methods 14, 149–167. [DOI] [PubMed] [Google Scholar]
- Pertwee R. G., Howlett A. C., Abood M. E. et al (2010) International union of basic and clinical pharmacology. LXXIX. cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol. Rev. 62, 588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. (2003) The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 4, 873–884. [DOI] [PubMed] [Google Scholar]
- Pujol J., Blanco‐Hinojo L., Batalla A. et al (2014) Functional connectivity alterations in brain networks relevant to self‐awareness in chronic cannabis users. J. Psychiatr. Res. 51, 68–78. [DOI] [PubMed] [Google Scholar]
- Ranganathan M. and D'Souza D. C. (2006) The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology 188, 425–444. [DOI] [PubMed] [Google Scholar]
- Redmer A., Kathmann M. and Schlicker E. (2003) Cannabinoid CB1 receptor‐mediated inhibition of hippocampal acetylcholine release is preserved in aged mice. Br. J. Pharmacol. 138, 1425–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel L. B., Tavares R. F., Lisboa S. F., Joca S. R., Corrêa F. M. and Guimarães F. S. (2009) 5‐HT1A receptors are involved in the cannabidiol‐induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 156, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba J., Valle M., Sampedro F., Rodríguez‐Pujadas A., Martínez‐Horta S., Kulisevsky J. and Rodríguez‐Fornells A. (2015) Telling true from false: cannabis users show increased susceptibility to false memories. Mol. Psychiatry 20, 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E., Larsson N., Sjögren S., Hjorth S., Hermansson N.‐O., Leonova1 J., Elebring T., Nilsson K., Drmota T. and Greasley P. J. (2007) The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 152, 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagredo O., Ramos J. A., Fernández‐Ruiz J., Rodríguez M. L. L. and de Miguel R. (2006) Chronic Δ9‐tetrahydrocannabinol administration affects serotonin levels in the rat frontal cortex. Naunyn‐Schmiedeberg's Arch. Pharmacol. 372, 313–317. [DOI] [PubMed] [Google Scholar]
- Schneider M. and Koch M. (2003) Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 28, 1760–1769. [DOI] [PubMed] [Google Scholar]
- Schneider M. and Koch M. (2002) The cannabinoid agonist WIN 55,212‐2 reduces sensorimotor gating and recognition memory in rats. Behav. Pharmacol. 13(1), 29–37. [DOI] [PubMed] [Google Scholar]
- Schneider M., Drews E. and Koch M. (2005) Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55,212‐2. Behav. Pharmacol. 16, 447–454. [DOI] [PubMed] [Google Scholar]
- Schneider M. and Koch M. (2007) The effect of chronic peripubertal cannabinoid treatment on deficient object recognition memory in rats after neonatal mPFC lesion. Eur. Neuropsychopharmacol. 17, 180–6. [DOI] [PubMed] [Google Scholar]
- Schneider M., Schömig E. and Leweke F. M. (2008) Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict Biol. 13, 345–57. [DOI] [PubMed] [Google Scholar]
- Silva‐Cruz A., Carlström M., Ribeiro J. A. and Sebastião A. M. (2017) Dual influence of endocannabinoids on long‐term potentiation of synaptic transmission. Front Pharmacol. 8, 921. eCollection [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solymosi A. and Köfalvi A. (2017) Cannabis: a treasure trove or pandora's Box? Mini‐Rev. Med. Chem. 2017, 17. [DOI] [PubMed] [Google Scholar]
- Sousa V. C., Assaife‐Lopes N., Ribeiro J. A., Pratt J. A., Brett R. R. and Sebastião A. M. (2011) Regulation of hippocampal cannabinoid CB1 receptor actions by adenosine A1 receptors and chronic caffeine administration: implications for the effects of Δ9‐tetrahydrocannabinol on spatial memory. Neuropsychopharmacology 36, 472–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford S. C. (2007) The open field test: reinventing the wheel. J. Psychopharmacol. 21, 134–135. [DOI] [PubMed] [Google Scholar]
- Stella N., Schweitzer P. and Piomelli D. (1997) A second endogenous cannabinoid that modulates long‐term potentiation. Nature 388, 773–778. [DOI] [PubMed] [Google Scholar]
- Suenaga T. and Ichitani Y. (2008) Effects of hippocampal administration of a cannabinoid receptor agonist WIN 55,212‐2 on spontaneous object and place recognition in rats. Behav. Brain Res. 190, 248–252. [DOI] [PubMed] [Google Scholar]
- Sullivan J. M. (2000) Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learn. Mem. 7, 132–139. [DOI] [PubMed] [Google Scholar]
- Terranova J. P., Michaud J. C., Le Fur G. and Soubrié P. (1995) Inhibition of long‐term potentiation in rat hippocampal slices by anandamide and WIN 55,212‐2: reversal by SR141716 A, a selective antagonist of CB1 cannabinoid receptors. Naunyn Schmiedebergs Arch. Pharmacol. 352, 576–579. [DOI] [PubMed] [Google Scholar]
- Tunving K. (1987) Psychiatric aspects of cannabis use in adolescents and young adults. Pediatrician 14, 83–91. [PubMed] [Google Scholar]
- Wang W., Trieu B. H., Palmer L. C. et al (2016). A primary cortical input to hippocampus expresses a pathway‐specific and endocannabinoid‐dependent form of long‐term potentiation. eNeuro. 3, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton E. C. and Brown M. W. (2010) Findings from animals concerning when interac‐tions between perirhinal cortex, hippocampus and medial prefrontal cortexare necessary for recognition memory. Neuropsychologia 48, 2262–2272. [DOI] [PubMed] [Google Scholar]
- Warburton E. C. and Brown M. W. (2015) Neural circuitry for rat recognition memory. Behav. Brain Res. 285, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. C., Vacek T., Lanier D. L. and Dewsbury D. A. (1976). Open‐field behavior in muroid rodents. Behav. Biol. 17, 495–506. [DOI] [PubMed] [Google Scholar]
- Wise L. E., Thorpe A. J. and Lichtman A. H. (2009) Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)‐tetrahydrocannabinol. Neuropsychopharmacology 34, 2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter M. P., Naber P. A., van Haeften T., Machielsen W. C., Rombouts S. A., Barkhof F., Scheltens P. and Lopes da Silva F. H. (2000) Cortico‐hippocampal communication by way of parallel parahippocampal‐subicular pathways. Hippocampus 10, 398–410. [DOI] [PubMed] [Google Scholar]
- Zhang G., Ásgeirsdóttir H. N., Cohen S. J., Munchow A. H., Barrera M. P. and Stackman R. W., Jr. (2013) Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice. Neuropharmacology 64, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Cinalli D., Barrera M. P. and Stackman R. W. (2015). Activation of serotonin 5‐HT2A receptor delays the retrieval of spatial memory in a Morris water maze task. In Proceedings of the Society of Neuroscience Conference, Chicago.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Body weight and blood parameters for animals undergoing the 14C‐2‐DG experiment.
Table S2. Local cerebral glucose utilization (LCGU) in WIN 55,212‐2 and control animals.
Table S3. Functional Connectivity of the Ventral Hippocampal Dorsal Subiculum.
Table S4. Functional Connectivity of the Habenula.
Table S5. Functional Connectivity of the Mediodorsal Thalamic Nucleus.
Table S6. Functional Connectivity of the Globus Pallidus.


