Abstract
Well‐tolerated, ribavirin‐free, pangenotypic hepatitis C virus (HCV) treatments for transplant recipients remain a high priority. Once‐daily glecaprevir/pibrentasvir demonstrates high rates of sustained virologic response at 12 weeks posttreatment (SVR12) across all major HCV genotypes (GTs). This trial evaluated the safety and efficacy of glecaprevir/pibrentasvir for patients with chronic HCV GT1‐6 infection who had received a liver or kidney transplant. MAGELLAN‐2 was a phase 3, open‐label trial conducted in patients who were ≥3 months posttransplant. Patients without cirrhosis who were HCV treatment‐naive (GT1‐6) or treatment‐experienced (GT1, 2, 4‐6; with interferon‐based therapy with or without sofosbuvir, or sofosbuvir plus ribavirin) received glecaprevir/pibrentasvir (300/120 mg) once daily for 12 weeks. The primary endpoint compared the percentage of patients receiving glecaprevir/pibrentasvir with SVR12 to a historic SVR12 rate based on the standard of care. Safety of glecaprevir/pibrentasvir was assessed. In total, 80 liver transplant and 20 kidney transplant patients participated in the trial. Most patients had no or minimal fibrosis (80% had fibrosis scores F0‐F1) and were infected with HCV GT1 (57%) or GT3 (24%). The overall SVR12 was 98% (n/N = 98/100; 95% confidence interval, 95.3%–100%), which exceeded the prespecified historic standard‐of‐care SVR12 threshold of 94%. One patient experienced virologic failure. One patient discontinued because of an adverse event considered to be unrelated to treatment; this patient achieved SVR12. Adverse events were mostly mild in severity, and laboratory abnormalities were infrequent. Conclusion: Once‐daily glecaprevir/pibrentasvir for 12 weeks is a well‐tolerated and efficacious, ribavirin‐free treatment for patients with chronic HCV GT1‐6 infection who have received a liver or kidney transplant. (http://ClinicalTrials.gov NCT02692703.) (Hepatology 2018; 00:000‐000).
Hepatitis C virus (HCV) infection represents a major global health care challenge that affects an estimated 177 million people worldwide.1 Patients living with chronic HCV infections are at substantially increased risk of end‐stage liver or kidney disease, necessitating liver and kidney transplantation. Despite recent advances in HCV treatments, existing regimens may be complicated by the addition of ribavirin (RBV), may be limited in patients with renal impairment, or may not be suitable for all HCV genotypes (GTs)2; and more therapeutic options for transplant patients are needed.
End‐stage liver disease caused by chronic HCV infection is a leading indication for liver transplantation, accounting for up to 30% of all liver transplants.3 In untreated HCV‐infected transplant recipients, infection of the new liver allograft occurs almost universally4, 5 and is associated with more rapid fibrosis progression, with approximately 30% of untreated transplant recipients developing cirrhosis within 5 years.6, 7 Following liver transplantation, the rate of patient and allograft survival has been lower in HCV‐infected recipients compared with HCV‐negative recipients.8
Anti‐HCV‐positive serologic status is also associated with lower patient and graft survival after kidney transplantation.9 Within the kidney transplant population, as well as patients who are receiving hemodialysis, the prevalence of HCV infection is approximately 5%‐9%.10, 11, 12 In kidney transplant patients, HCV infection is associated with increased liver‐related mortality and fibrosis progression, and up to 28% of kidney transplant recipients will die of chronic liver disease.13, 14 HCV infections in kidney transplant recipients are also associated with increased rates of glomerulopathies and risk of cardiovascular death.15
Clearance of HCV infection in liver transplant patients can decrease the risk of HCV‐related complications and improve patient survival.16 Until recently, treatment options for HCV were limited to interferon (IFN)‐based therapies, which had low sustained virologic response (SVR) rates and were poorly tolerated by liver transplant patients or were contraindicated in kidney transplant patients.17 Newer IFN‐free, all‐oral direct‐acting antiviral (DAA)–based regimens are associated with high rates of efficacy and tolerability2 and have demonstrated promising outcomes in clinical trials and real‐world studies in transplant patients.18, 19, 20, 21, 22, 23, 24, 25, 26 However, regimens currently available for transplant patients are not equally potent across all major HCV GTs, and some patient subgroups may require RBV as part of their recommended treatment, which carries a risk of anemia adverse effects.2 In addition, currently available DAAs have the potential for clinically significant drug–drug interactions, particularly with calcineurin inhibitors.2 Options for kidney transplant patients are especially limited because of concerns over the use of sofosbuvir and RBV in patients with severe renal impairment.27
The HCV nonstructural protein 3/4A (NS3/4A) protease inhibitor glecaprevir (formerly ABT‐493) and the NS5A inhibitor pibrentasvir (formerly ABT‐530) have been developed as a once‐daily, all‐oral, RBV‐free combination therapy for chronic HCV infection of all GTs. Both DAAs have shown potent pangenotypic antiviral activity in vitro and maintain this activity against most of the common amino acid substitutions of HCV GT1‐6 that are known to confer resistance to currently approved inhibitors.28, 29 In phase 2 and 3 clinical trials, treatment with glecaprevir/pibrentasvir was well tolerated and demonstrated high SVR rates in patients across all GTs,30, 31, 32, 33 including patients with compensated cirrhosis and prior treatment failures to DAA‐containing regimens, as well as patients with severe kidney impairment, with end‐stage renal disease, and undergoing dialysis.34, 35, 36
In the MAGELLAN‐2 study, we evaluated the safety and efficacy of glecaprevir/pibrentasvir without RBV for 12 weeks in patients infected with HCV GT1‐6, without cirrhosis, who have undergone liver or kidney transplantation.
Patients and Methods
PATIENTS AND STUDY DESIGN
MAGELLAN‐2 (NCT02692703) was a phase 3, single‐arm, open‐label, multicenter trial, conducted to evaluate the safety and efficacy of glecaprevir/pibrentasvir for adult patients with chronic HCV GT1‐6 infection, without cirrhosis, who had received primary liver or kidney transplants. Patients must have had a positive anti‐HCV antibody test and a plasma HCV RNA viral load ≥1,000 IU/mL, as well as evidence of chronic HCV infection, defined as either positive for anti‐HCV antibody or HCV RNA ≥6 months before screening, liver biopsy consistent with chronic infection, or abnormal alanine aminotransferase levels ≥6 months before screening.
Enrolled patients did not have cirrhosis, as confirmed by liver biopsy within 6 months prior to screening (METAVIR, Batts‐Ludwig, Knodell, International Association for the Study of the Liver, Scheuer, or Laennec fibrosis score of ≤3 or Ishak fibrosis score ≤4), a FibroScan (Echosens, Paris, France) score of <12.5 kPa during or within 3 months of screening, or a FibroTest (BioPredictive SAS, Paris, France) score of ≤0.48 and aspartate aminotransferase‐to‐platelet ratio index <1 at screening. Patients could be HCV treatment‐naive (GT1‐6) or treatment‐experienced (GT1, 2, 4‐6), with treatment experience defined as prior treatment with IFN, pegylated IFN with or without RBV, or sofosbuvir plus RBV with or without pegylated IFN that was completed ≥2 months before screening. HCV GT3‐infected, treatment‐experienced patients were excluded.
Patients had to have received a deceased or living donor liver or kidney transplant ≥3 months before screening and must have been maintained on a stable immunosuppression regimen based on tacrolimus, sirolimus, everolimus, mycophenolic acid, azathioprine, cyclosporine (dosage of 100 mg or less per day), and/or low‐dose steroids (defined as corticosteroids such as prednisone or prednisolone at dosages of no more than 10 mg per day at the time of screening). Patients were excluded if they had evidence of hepatitis B virus or human immunodeficiency virus coinfection. Full eligibility criteria are provided in the http://onlinelibrary.wiley.com/doi/10.1002/hep.30046/suppinfo.
The trial consisted of a screening period, a 12‐week treatment period, and a 24‐week follow‐up period (Fig. 1). Eligible patients received oral glecaprevir/pibrentasvir 300/120 mg once daily (provided as three 100/40 mg tablets; AbbVie, Chicago, IL). Glecaprevir/pibrentasvir was to be taken with food at approximately the same time in the morning every day. Patients took their concomitant immunosuppression regimens, titrated per usual care as directed by their physician.
Figure 1.
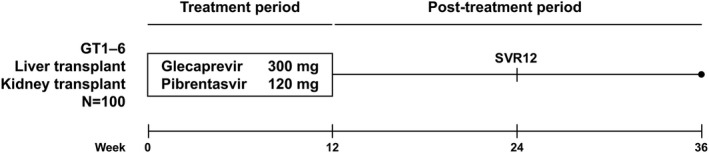
MAGELLAN‐2 trial design. Patients received 12 weeks of open‐label, coformulated, once‐daily glecaprevir/pibrentasvir 300/120 mg (administered as three tablets of glecaprevir/pibrentasvir, 100/40 mg each). All patients were followed until week 36 to monitor for safety and HCV RNA. The primary endpoint was SVR12.
The trial protocol, designed and sponsored by AbbVie, was approved by the independent ethics committee or institutional review board for each trial center. The trial was conducted in accordance with good clinical practice guidelines and the ethical principles of the Declaration of Helsinki, and all patients provided written informed consent. All authors had access to trial data and reviewed and approved the final manuscript.
EFFICACY, SAFETY, AND RESISTANCE ASSESSMENTS
Plasma samples were collected at screening, at regular intervals during the trial, and up to 24 weeks following the end of treatment for determination of HCV RNA concentrations. Viral RNA concentrations were measured at a central laboratory using the COBAS AmpliPrep/COBAS TaqMan HCV Quantitative Test, v2.0 (Roche Diagnostics, Indianapolis, IN). The lower limit of detection and the lower limit of quantitation (LLOQ) for this assay were both 15 IU/mL.
The primary endpoint was SVR at 12 weeks posttreatment (SVR12), which was defined as HCV RNA below the LLOQ 12 weeks after the last dose of glecaprevir/pibrentasvir. Secondary efficacy endpoints were the percentages of patients with on‐treatment virologic failure or posttreatment relapse. On‐treatment virologic failure was defined as a confirmed increase in HCV RNA concentration of >1 log10 IU/mL above nadir during treatment, confirmed HCV RNA ≥100 IU/mL after HCV RNA had fallen below the LLOQ during treatment, or HCV RNA that exceeded the LLOQ at the end of treatment for ≥6 weeks of treatment. Posttreatment relapse was defined as confirmed HCV RNA that exceeded the LLOQ between the end of treatment and 12 weeks after the last dose of glecaprevir/pibrentasvir among patients who had completed treatment with an HCV RNA level below the LLOQ.
Adverse events (AEs) that occurred from initiation of treatment through 30 days after the last dose of glecaprevir/pibrentasvir were monitored and recorded using the Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class and preferred term. Severity and relationship to treatment were assessed by trial investigators. Clinical laboratory tests, physical examinations, and measurements of vital signs were conducted throughout the trial. Worsening from baseline during treatment in laboratory test values was graded using the National Cancer Institute's Common Terminology Criteria for Adverse Events. Plasma samples were collected prior to dosing on day 1, as well as during the treatment and follow‐up periods for viral RNA isolation and HCV resistance testing. Next‐generation sequencing was performed using samples collected from all patients at baseline. Presence of HCV baseline polymorphisms in the NS3 and NS5A genes, relative to subtype‐specific reference sequences, was evaluated using a 15% detection threshold. For patients who experienced virologic failure, treatment‐emergent substitutions in NS3 and NS5A relative to the patient's baseline HCV sequence were analyzed.
Patients recorded their immunosuppressant dosages in diaries throughout the treatment period, and plasma samples were also collected locally at screening and at the following time points during the trial: day 1 (optional); day 3; week 1; day 10; weeks 2, 4, 6, 8, and 12 (or at any time, at the investigators' discretion) to monitor cyclosporine, tacrolimus, everolimus, and sirolimus concentrations.
Treatment adherence data were collected by recording the total number of tablets dispensed and the total number of tablets returned. Adherence was calculated as the percentage of tablets taken relative to the total number of tablets expected to be taken. A patient was considered adherent to treatment if the calculated adherence with the study regimen was between 80% and 120%.
STATISTICAL ANALYSIS
For the primary efficacy endpoint of SVR12, a sample size of 90 patients was required for this trial to have >90% power to demonstrate noninferiority to a historical SVR12 rate of 94% (assuming that 96% of the patients receiving glecaprevir/pibrentasvir would achieve SVR12). The historical SVR12 rate was derived from the SVR rates for the standard of care, 12‐week regimens of sofosbuvir/ledipasvir plus RBV or sofosbuvir plus daclatasvir and RBV in the post–liver transplant population.18, 22, 37 The percentages of patients who achieved SVR12 were summarized, and a two‐sided 95% confidence interval (CI) was calculated using the normal approximation to the binomial distribution. If the SVR12 rate was 100%, the Wilson's score method would be used to calculate the CI. The lower confidence bound of the two‐sided 95% CI for the percentage of patients achieving SVR12 must have exceeded 86% to achieve noninferiority to the standard of care (i.e., a noninferiority margin of −8%). Patients with missing data for posttreatment week 12 were counted as SVR12 failures. Efficacy analyses were conducted for the intention‐to‐treat population, which comprised all enrolled patients who received at least one dose of glecaprevir/pibrentasvir. Analysis of the primary endpoint was also performed for the modified intention‐to‐treat population, defined as the intention‐to‐treat population excluding patients who did not achieve SVR12 for reasons other than virologic failure. Safety analyses were carried out for all patients who received at least one dose of glecaprevir/pibrentasvir. Data were analyzed using SAS (SAS Institute, Inc., Cary, NC).
Results
PATIENTS
A total of 100 patients were enrolled at trial centers in Australia, Canada, Italy, New Zealand, Puerto Rico, Spain, Taiwan, the United Kingdom, and the United States. All patients received treatment with glecaprevir/pibrentasvir, and 99 patients completed treatment. One patient discontinued treatment because of an AE of cerebrovascular accident that was unrelated to glecaprevir/pibrentasvir but achieved SVR12.
Most enrolled patients were male (75%), were white (78%), and had no or minimal fibrosis (80% had fibrosis scores of F0‐F1, 14% had a fibrosis score of F3). All HCV GTs were represented, except GT5 (Table 1). Among the enrolled patients, 80 had received liver transplants and 20 had received kidney transplants. Only 11% of patients had normal renal function (estimated glomerular filtration rate [eGFR] ≥90 mL/min/1.73 m2) at baseline (11% of liver transplant patients; 10% of kidney transplant patients). Overall, 54% of liver transplant patients had an eGFR ≥60 to <90 mL/min/1.73 m2, and 55% of kidney transplant patients had an eGFR <60 mL/min/1.73 m2 (Table 1). Median time since transplantation at baseline was longer for kidney transplant patients than for liver transplant patients (132.1 versus 53.8 months, respectively). The most common immunosuppression regimen for liver and kidney transplant patients was based on tacrolimus (68%).
Table 1.
Patient Demographics and Baseline Clinical Characteristics
| Characteristic |
Liver Transplant (n = 80) |
Kidney Transplant (n = 20) |
Total (N = 100) |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 16 (20) | 9 (45) | 25 (25) |
| Male | 64 (80) | 11 (55) | 75 (75) |
| Race, n (%) | |||
| White | 67 (84) | 11 (55) | 78 (78) |
| Black or African American | 1 (1) | 7 (35) | 8 (8) |
| Asian | 8 (10) | 2 (10) | 10 (10) |
| Othera | 4 (5) | 0 | 4 (4) |
| Age (years), median (range) | 61 (42‐78) | 56 (39‐70) | 60 (39‐78) |
| Body mass index (kg/m2), mean (SD) | 27.5 (5.0) | 27.3 (6.7) | 27.4 (5.3) |
| HCV genotype, n (%) | |||
| 1a | 22 (28) | 6 (30) | 28 (28) |
| 1b | 18 (23) | 11 (55) | 29 (29) |
| 2 | 13 (16) | 0 | 13 (13) |
| 3 | 22 (28) | 2 (10) | 24 (24) |
| 4 | 3 (4) | 1 (5) | 4 (4) |
| 5 | 0 | 0 | 0 |
| 6 | 2 (3) | 0 | 2 (2) |
| HCV RNA ≥6 × 106 IU/mL, n (%) | 27 (34) | 4 (20) | 31 (31) |
| IL28B non‐CC genotype, n (%) | 45 (56) | 13 (65) | 58 (58) |
| Baseline fibrosis stage, n (%) | |||
| F0‐F1 | 62 (78) | 18 (90) | 80 (80) |
| F2 | 6 (8) | 0 | 6 (6) |
| F3 | 12 (15) | 2 (10) | 14 (14) |
| Baseline eGFR (mL/min/1.73 m2), n (%) | |||
| <60 | 28 (35) | 11 (55) | 39 (39) |
| ≥60 to <90 | 43 (54) | 7 (35) | 50 (50) |
| ≥90 | 9 (11) | 2 (10) | 11 (11) |
| Platelet count ≥90 × 109/L, n (%) | 76 (95) | 20 (100) | 96 (96) |
| Albumin ≥35 g/L, n (%) | 78 (98) | 19 (95) | 97 (97) |
| Time since transplantation (months), median (range) | 53.8 (4.2‐213.7) | 132.1 (4.6‐545.3) | 55.6 (4.2‐545.3) |
| Immunosuppressant medication, n (%) | |||
| Cyclosporine | 9 (11) | 4 (20) | 13 (13) |
| Tacrolimus | 57 (71) | 11 (55) | 68 (68) |
| Otherb | 14 (18) | 5 (25) | 19 (19) |
| HCV treatment experience, n (%) | 30 (38) | 4 (20) | 34 (34) |
| IFN‐based | 28 (35) | 4 (20) | 32 (32) |
| Sofosbuvir‐based | 1 (1) | 0 | 1 (1) |
| Other | 1 (1) | 0 | 1 (1) |
| Pretransplant HCV treatment experience, n (%) | 21 (26) | 3 (15) | 24 (24) |
| Posttransplant HCV treatment experience, n (%) | 9 (11) | 1 (5) | 10 (10) |
| Response to previous HCV treatment, n (%) | |||
| Breakthrough/nonresponder | 13 (16) | 2 (10) | 15 (15) |
| Posttreatment relapse | 10 (13) | 1 (5) | 11 (11) |
| Unknown or other | 7 (9) | 1 (5) | 8 (8) |
“Other” category includes Native American or Alaska native, native Hawaiian or other Pacific Islander, and multiple races.
“Other” category includes azathioprine, everolimus, mycophenolic acid, prednisolone, prednisone, and sirolimus.
Abbreviations: IL, interleukin; SD, standard deviation.
The majority of patients (66%) had received no previous HCV treatment. Patients who had received prior HCV treatment had most commonly received an IFN‐based regimen before their liver or kidney transplant. The most common reasons for prior treatment failure were breakthrough, on‐treatment nonresponse, or posttreatment relapse.
Baseline polymorphisms were not detected in NS3 at amino acid positions 155, 156, or 168 in any patient, and were detected in NS5A at amino acid positions 24, 28, 30, 31, 58, 92, or 93 in 33% of patients (see http://onlinelibrary.wiley.com/doi/10.1002/hep.30046/suppinfo). Two patients with GT1b infections and 3 patients with GT3a infections had NS5A‐Y93H polymorphisms at baseline.
In total, 99 patients completed ≥77 days of glecaprevir/pibrentasvir treatment. Of the 83 patients with available adherence data, 82 (99%) were >80% adherent to therapy.
EFFICACY
For the primary efficacy endpoint, 98% of patients treated with glecaprevir/pibrentasvir achieved SVR12 (n/N = 98/100; 95% CI, 95.3%‐100%) by the intention‐to‐treat analysis (Fig. 2). The lower bound of the 95% CI was >86%, indicating noninferiority to the standard of care. For the secondary endpoints, no patients had on‐treatment virologic failure. Two patients were designated as SVR12 nonresponders: 1 patient had virologic failure (relapse by posttreatment week 4) and 1 patient had nonvirologic failure as a result of missing SVR12 data. By modified intention‐to‐treat analysis, which excluded patients who failed to achieve SVR12 for nonvirologic reasons, the SVR12 rate was 99% (n/N = 98/99; 95% CI, 97%‐100%). SVR12 rates for patient subgroups are provided in the http://onlinelibrary.wiley.com/doi/10.1002/hep.30046/suppinfo.
Figure 2.
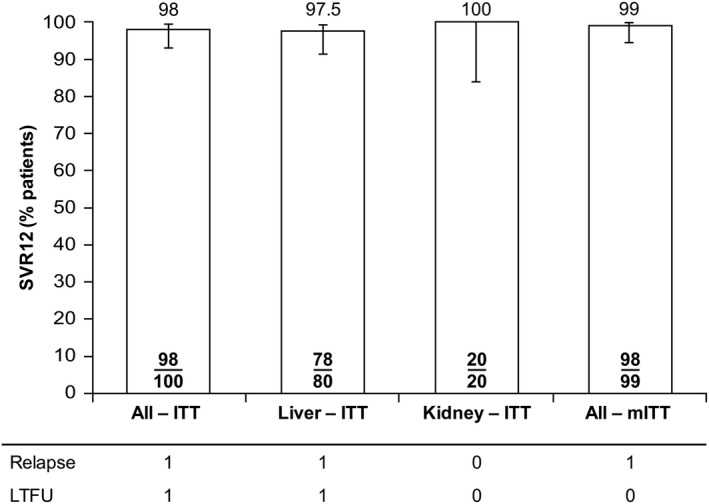
Rates of SVR12 following treatment for all patients who received at least one dose of glecaprevir/pibrentasvir. For the overall intention‐to‐treat and modified intention‐to‐treat analyses, 95% CIs were calculated using the normal approximation to the binomial distribution or the Wilson's score method for rates of 100%. For separate intention‐to‐treat analyses of liver and kidney transplant patients, 95% CIs were calculated using the Wilson's score method. Abbreviations: ITT, intention‐to‐treat; LTFU, lost to follow‐up; mITT, modified intention‐to‐treat (excluding patients with nonvirologic failure).
The 1 patient who experienced virologic relapse had a treatment duration of 88 days and was 97.7% adherent to glecaprevir/pibrentasvir treatment. This patient had GT3a infection, was HCV treatment‐naive, had F0‐F1 fibrosis, had a baseline HCV RNA level of 7.56 log10 IU/mL, and had received a liver transplant. The patient had no baseline polymorphisms in NS3 and had the treatment‐emergent substitution Y56H at the time of failure. The Y93H polymorphism in NS5A was detected at baseline and at the time of failure. Glecaprevir and pibrentasvir plasma concentrations were within the proposed therapeutic range throughout the treatment period (not shown).
SAFETY
Overall, 85 patients (85%) experienced AEs (Table 2). The most commonly experienced AEs were fatigue (22%), headache (22%), nausea (12%), pruritus (12%), and diarrhea (10%). AEs for the majority of patients (56%) were mild.
Table 2.
Summary of AEs (Safety Population)a
| Event, n (%) |
Glecaprevir/Pibrentasvir (N = 100) |
|---|---|
| Any AE | 85 (85) |
| Any AE possibly related to DAAs | 48 (48) |
| Any AE with grade 3 severity or greater | 12 (12) |
| Any DAA‐related AE with grade 3 severity or greater | 3 (3) |
| Any SAE | 8 (8) |
| Any SAE possibly related to DAAs | 2 (2) |
| Any AE leading to treatment discontinuation | 1 (1) |
| Death | 0 |
| AEs occurring in ≥10% of patients | |
| Fatigue | 22 (22) |
| Headache | 22 (22) |
| Nausea | 12 (12) |
| Pruritus | 12 (12) |
| Diarrhea | 10 (10) |
MedDRA version 19.1 was used for reporting of AEs.
Eight patients experienced serious AEs (SAEs; see http://onlinelibrary.wiley.com/doi/10.1002/hep.30046/suppinfo), 2 of which were considered to be related to glecaprevir/pibrentasvir (sinusitis and abnormal hepatic function [MedDRA preferred terms]). One patient experienced an SAE of cerebrovascular accident, which was not considered to be related to glecaprevir/pibrentasvir therapy, that led to treatment discontinuation on day 50. This patient achieved SVR12. No other patients experienced AEs that led to treatment discontinuation, and there were no deaths.
One liver transplant patient experienced a non‐SAE of transplant rejection (confirmed by biopsy). This AE was mild (grade 1), managed by titration of the patient's immunosuppression regimen (everolimus and tacrolimus), and resolved after 9 days. Glecaprevir/pibrentasvir was continued without interruption, and the patient achieved SVR12. No kidney transplant patients experienced AEs related to transplant rejection.
Six patients (6%) experienced worsening of laboratory values during treatment, relative to baseline, that were grade 3 or 4 in severity (Table 3): 1 patient had a single grade 3 alanine aminotransferase value on day 3, which steadily declined while on treatment and normalized; 1 patient had total bilirubin and creatinine elevations to grade 3 on days 43 and 46 (attributed to a drug interaction between concomitant medications tacrolimus and clarithromycin and not related to glecaprevir/pibrentasvir); 1 patient on anticoagulant therapy had grade 3 international normalized ratio values on days 8, 11, 43, and 57; 1 patient had a single grade 3 platelet value on day 8 and a grade 3 international normalized ratio (international normalized ratio value was the result of incorrect recording in the patient database); 1 patient had a grade 3 creatinine elevation on day 87, grade 3 reductions in creatinine clearance on days 4, 8, 29, 43, and 85, and a grade 4 reduction in creatinine clearance on day 87; 1 patient had grade 3 reductions in creatinine clearance on days 5 and 8. The creatinine or creatinine clearance changes were attributable to ongoing diagnosis of urinary tract infections in both patients.
Table 3.
On‐Treatment Laboratory Abnormalitiesa
| Parameter, n (%) |
Glecaprevir/Pibrentasvir (N = 100) |
|---|---|
| Platelets (<50 × 109/L) | 1 (1) |
| INR (>2.5 × ULN) | 1 (1) |
| Alanine aminotransferase (>5 × ULN) | 1 (1) |
| Total bilirubin (>3 × ULN) | 1 (1) |
| Creatinine clearance (<30 mL/min) | 2 (2) |
Numbers and percentages of patients who experienced grade 3 or 4 (Common Terminology Criteria for Adverse Events) worsening of laboratory values from baseline are shown.
Abbreviations: INR, international normalized ratio; ULN, upper limit of normal.
IMMUNOSUPPRESSANT DOSING
At any given study visit, a minority of patients had either an increase or a decrease in the dosage of their immunosuppressant medication, without any consistent pattern associated with study drug dosing (see http://onlinelibrary.wiley.com/doi/10.1002/hep.30046/suppinfo). Overall, the median total daily doses of cyclosporine (100 mg/day), everolimus (1.25 mg/day), and sirolimus (1.00 mg/day) were unchanged throughout the duration of the trial (Fig. 3). The median total daily tacrolimus dose decreased from 2.75 to 2.00 mg/day between baseline and day 7 but remained unchanged through the remainder of the trial.
Figure 3.
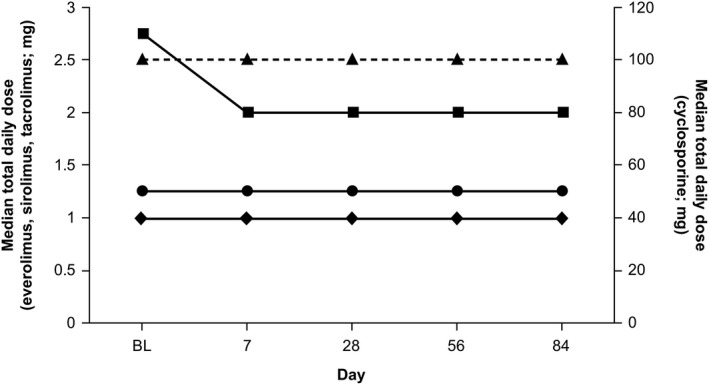
Immunosuppressant dosing from baseline to end of treatment for liver and kidney transplant patients. Median dosages of everolimus (closed circles), sirolimus (closed diamonds), and tacrolimus (closed squares) are plotted using the left y‐axis; median dosages of cyclosporine (closed triangles) are plotted using the right y‐axis. Abbreviation: BL, baseline.
Discussion
The MAGELLAN‐2 trial was conducted to evaluate the efficacy and safety of 12 weeks of glecaprevir/pibrentasvir without RBV for patients without cirrhosis but with HCV GT1‐6 infections who had received liver or kidney transplants. In this trial, liver or kidney transplant patients, most of whom had no or minimal fibrosis, achieved an SVR12 rate of 98%, demonstrating noninferiority of glecaprevir/pibrentasvir to the historic standard of care. The efficacy of glecaprevir/pibrentasvir was unaffected by patients' baseline characteristics, HCV GT, baseline polymorphisms in NS3 and/or NS5A, or previous hepatitis C treatment experience. Outside of the transplant setting, similarly high SVR12 rates have been demonstrated in HCV‐infected patients with minimal fibrosis treated with glecaprevir/pibrentasvir.31, 32
A single patient experienced virologic failure in the MAGELLAN‐2 trial. This patient had minimal fibrosis, was adherent to therapy, and had glecaprevir and pibrentasvir exposures within the therapeutic ranges. The patient had a treatment‐emergent substitution Y56H in NS3 at the time of failure, and Y93H in NS5A was detected at baseline and at the time of failure. Given the low number (n = 3) of GT3‐infected patients with baseline NS5A‐Y93H in the trial population, conclusions on the potential impact of this polymorphism on efficacy cannot be made. However, in an integrated analysis of glecaprevir/pibrentasvir phase 2 and 3 clinical trials, this polymorphism had no impact on treatment outcome.38 In addition, 100% (n/N = 5/5) of GT3‐infected patients with baseline Y93H polymorphisms achieved SVR12 following 8 weeks of treatment with glecaprevir/pibrentasvir in the ENDURANCE‐3 trial.33
Once‐daily glecaprevir/pibrentasvir was well tolerated. The majority of AEs were mild and consistent with those reported in previous studies of glecaprevir/pibrentasvir.32, 33 No patients discontinued because of treatment‐related AEs, and there were few SAEs attributed to glecaprevir/pibrentasvir. Laboratory abnormalities of grade 3 or higher were infrequent. The overall safety profile for patients treated with glecaprevir/pibrentasvir in the MAGELLAN‐2 trial was generally consistent with the safety profile expected for transplant patients and reflective of the underlying medical conditions of this population.
Interactions with concomitant immunosuppression regimens are a particular concern for posttransplant patients. In the current trial, median dosages of tacrolimus were slightly reduced within the first week of treatment but remained unchanged for the remainder of the treatment period. Adjustments to immunosuppressant dosages have been reported18, 22 and may be attributable to improving liver function and increased immunosuppressant metabolism.18 There were no overall changes to median dosages of cyclosporine, sirolimus, or everolimus. No clinically relevant safety findings were identified and attributed to a potential drug interaction between glecaprevir/pibrentasvir and any of the coadministered immunosuppressants.
The joint HCV treatment guidelines of the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America were updated in 2017 to recommend combination therapy with glecaprevir/pibrentasvir for 12 weeks for the treatment of chronic HCV GT1‐6 infections in liver transplant patients without cirrhosis on the basis of the results of the MAGELLAN‐2 trial.2 Ledipasvir/sofosbuvir plus RBV for 12 weeks (GT1, 4, 5, and 6) and daclatasvir/sofosbuvir plus RBV for 12 weeks (GT2 and 3) are recommended for liver transplant patients without cirrhosis or with compensated cirrhosis.2 In the phase 2, open‐label SOLAR‐1 trial, treatment with ledipasvir/sofosbuvir plus RBV for 12 or 24 weeks resulted in SVR12 rates of 96%‐98% in a broad spectrum of liver transplant patients with GT1 infections.18 In the phase 3, open‐label ALLY‐1 trial, daclatasvir/sofosbuvir plus RBV for 12 weeks resulted in SVR12 rates of 95% for liver transplant patients with GT1 infections and 91% for patients with GT3 infections.22 However, these studies were limited by the inclusion of very low numbers of patients with non‐GT1 infections. By contrast, enrollment of GT1 patients was limited to approximately 50% of the MAGELLAN‐2 trial population in order to enrich the trial with patients with non‐GT1 infections. In addition, currently recommended DAA regimens are complicated by the addition of weight‐based RBV dosing and the requirement to monitor for RBV‐associated AEs (particularly RBV‐induced anemia),2 whereas 12 weeks of treatment with glecaprevir/pibrentasvir achieved high SVR12 rates in the absence of RBV. The efficacy and safety profiles of glecaprevir/pibrentasvir also raise the possibility of reducing waitlist times for patients requiring transplants, by increasing the size of the pool of available organs to include those from HCV‐infected donors.
MAGELLAN‐2 represents the first trial of a pangenotypic DAA regimen to achieve SVR12 rates at or approaching 100% across multiple genotypes in kidney transplant patients. Based on these findings, the updated American Association for the Study of Liver Diseases/Infectious Diseases Society of America guidelines recommend glecaprevir/pibrentasvir for 12 weeks for kidney transplant patients with HCV GT1‐6 infections. Ledipasvir/sofosbuvir treatment for 12 weeks is also recommended for kidney transplant patients with GT1 or GT4 infections. In a randomized, phase 2, open‐label trial, 12 or 24 weeks of ledipasvir/sofosbuvir treatment resulted in 100% SVR12 for kidney transplant patients with GT1 or GT4 infections without cirrhosis or with compensated cirrhosis.23 However, a number of patients treated with ledipasvir/sofosbuvir experienced decreased eGFR values during treatment.23
A limitation of the MAGELLAN‐2 trial was that patients with cirrhosis and those who previously failed DAA therapy were excluded from the study, populations that may require a longer duration of therapy. In addition, patients with a history of fibrosing cholestatic hepatitis or coinfection with human immunodeficiency virus were excluded from the trial.
Glecaprevir/pibrentasvir is a pangenotypic, 12‐week, RBV‐free treatment option for transplant patients without cirrhosis that demonstrates high efficacy, a favorable safety profile, and minimal interaction with concomitant immunosuppression regimens.
Author names in bold designate shared co–first authorship.
Abbreviations
- AE
adverse event
- CI
confidence interval
- DAA
direct‐acting antiviral
- eGFR
estimated glomerular filtration rate
- GT
genotype
- HCV
hepatitis C virus
- IFN
interferon
- LLOQ
lower limit of quantitation
- MedDRA
Medical Dictionary for Regulatory Activities
- NS
nonstructural protein
- RBV
ribavirin
- SAE
serious AE
- SVR
sustained virologic response
- SVR12
SVR at 12 weeks posttreatment
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep.30046/suppinfo.
Supporting Information
Acknowledgment
The authors express their gratitude to the patients who participated in this study and their families, as well as all of the trial investigators and their research staff. Editorial support was provided by Neil M. Thomas, Ph.D., and Paul MacCallum, Ph.D., of the Fishawack Group Ltd.
Potential conflict of interest: Dr. Reau advises and received grants from AbbVie. She advises Merck and Gilead. Dr. Reddy advises and received grants from AbbVie, Merck, and Gilead. He received grants from Bristol‐Myers Squibb, Conatus, and Mallinckrodt. Dr. Tran consults for, advises, is on the speakers' bureau of, and received grants from Gilead, AbbVie, and Merck. She consults for and advises Janssen. Dr. Agarwal consults for, advises, is on the speakers' bureau of, and received grants from Gilead. He advises, is on the speakers' bureau of, and received grants from Merck. He advises and received grants from AbbVie. Dr. Forns consults for and received grants from AbbVie. He consults for Gilead. Dr. Kwo advises and received grants from AbbVie and Gilead. He advises Bristol‐Myers Squibb and Merck. Dr. Mantry consults for, advises, is on the speakers' bureau of, and received grants from Intercept. He consults for, advises, and received grants from Gilead. He consults for and advises Bayer. He consults for and is on the speakers' bureau of AbbVie and Bristol‐Myers Squibb. He advises and is on the speakers' bureau of Merck. He received grants from Genfit and Tobira. Dr. Brown consults for and received grants from Gilead, AbbVie, and Bristol‐Myers Squibb. Dr. Mutimer consults for, advises, is on the speakers' bureau of, received grants from, and is employed by AbbVie. Dr. Gane advises and is on the speakers' bureau of AbbVie and Gilead. He advises Alios/Janssen. Dr. Trinh is employed by and owns stock in AbbVie. Dr. Hu is employed by and owns stock in AbbVie. Dr. Samanta is employed by and owns stock in AbbVie. Dr. Shulman is employed by and owns stock in AbbVie. Dr. Gulati is employed by and owns stock in AbbVie. Dr. Porcalla is employed by and owns stock in AbbVie. Dr. Rhee is employed by and owns stock in AbbVie. Dr. Dumas is employed by and owns stock in AbbVie. Dr. Krishnan is employed by and owns stock in AbbVie. Dr. Liu is employed by and owns stock in AbbVie.
AbbVie sponsored the study; contributed to its design; and participated in the collection, analysis, and interpretation of the data and in the writing, reviewing, and approval of the publication.
Presented in part at The International Liver Congress of the European Association for the Study of the Liver, Amsterdam, The Netherlands (April 19‐23, 2017), and the American Society of Nephrology's Kidney Week, New Orleans, LA (October 31‐November 5, 2017). Glecaprevir (ABT‐493) was identified by AbbVie and Enanta.
REFERENCES
- 1. Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up‐date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol 2016;22:7824‐7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Association for the Study of Liver Diseases, Infectious Diseases Society of America . Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Published 2017. Accessed November 14, 2017. [DOI] [PMC free article] [PubMed]
- 3. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients . United States organ transplantation. OPTN/SRTR 2012 annual data report. https://srtr.transplant.hrsa.gov/annual_reports/2012/pdf/2012_SRTR_ADR_updated_full_intro.pdf. Published June 2014. Accessed June 14, 2017.
- 4. Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, et al. Long‐term outcome of hepatitis C infection after liver transplantation. N Engl J Med 1996;334:815‐820. [DOI] [PubMed] [Google Scholar]
- 5. Garcia‐Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology 2002;35:680‐687. [DOI] [PubMed] [Google Scholar]
- 6. Crespo G, Marino Z, Navasa M, Forns X. Viral hepatitis in liver transplantation. Gastroenterology 2012;142:1373‐1383. [DOI] [PubMed] [Google Scholar]
- 7. Blasco A, Forns X, Carrion JA, Garcia‐Pagan JC, Gilabert R, Rimola A, et al. Hepatic venous pressure gradient identifies patients at risk of severe hepatitis C recurrence after liver transplantation. Hepatology 2006;43:492‐499. [DOI] [PubMed] [Google Scholar]
- 8. Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology 2002;122:889‐896. [DOI] [PubMed] [Google Scholar]
- 9. Fabrizi F, Martin P, Dixit V, Messa P. Meta‐analysis of observational studies: hepatitis C and survival after renal transplant. J Viral Hepat 2014;21:314‐324. [DOI] [PubMed] [Google Scholar]
- 10. Kliem V, Michel U, Burg M, Bock A, Chapman J, Dussol B, et al. Geographical prevalence, risk factors and impact of hepatitis B and C after renal transplantation. Clin Nephrol 2009;71:423‐429. [DOI] [PubMed] [Google Scholar]
- 11. Santos L, Alves R, Macario F, Parada B, Campos M, Mota A. Impact of hepatitis B and C virus infections on kidney transplantation: a single center experience. Transplant Proc 2009;41:880‐882. [DOI] [PubMed] [Google Scholar]
- 12. Goodkin DA, Bieber B, Jadoul M, Martin P, Kanda E, Pisoni RL. Mortality, hospitalization, and quality of life among patients with hepatitis C infection on hemodialysis. Clin J Am Soc Nephrol 2017;12:287‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Oliveira Uehara SN, Emori CT, da Silva Fucuta Pereira P, Perez RM, Pestana JO, Lanzoni VP, et al. Histological evolution of hepatitis C virus infection after renal transplantation. Clin Transplant 2012;26:842‐848. [DOI] [PubMed] [Google Scholar]
- 14. Fabrizi F, Martin P, Ponticelli C. Hepatitis C virus infection and renal transplantation. Am J Kidney Dis 2001;38:919‐934. [DOI] [PubMed] [Google Scholar]
- 15. Morales JM, Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol 2015;11:172‐182. [DOI] [PubMed] [Google Scholar]
- 16. Berenguer M, Palau A, Aguilera V, Rayon JM, Juan FS, Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant 2008;8:679‐687. [DOI] [PubMed] [Google Scholar]
- 17. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver D . Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335‐1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015;149:649‐659. [DOI] [PubMed] [Google Scholar]
- 19. Fontana RJ, Brown RS Jr, Moreno‐Zamora A, Prieto M, Joshi S, Londono MC, et al. Daclatasvir combined with sofosbuvir or simeprevir in liver transplant recipients with severe recurrent hepatitis C infection. Liver Transpl 2016;22:446‐458. [DOI] [PubMed] [Google Scholar]
- 20. Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R Jr, et al. An interferon‐free antiviral regimen for HCV after liver transplantation. N Engl J Med 2014;371:2375‐2382. [DOI] [PubMed] [Google Scholar]
- 21. O'Leary JG, Fontana RJ, Brown K, Burton JR Jr, Firpi‐Morell R, Muir A, et al. Efficacy and safety of simeprevir and sofosbuvir with and without ribavirin in subjects with recurrent genotype 1 hepatitis C postorthotopic liver transplant: the randomized GALAXY study. Transpl Int 2017;30:196‐208. [DOI] [PubMed] [Google Scholar]
- 22. Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post–liver transplantation recurrence. Hepatology 2016;63:1493‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colombo M, Aghemo A, Liu H, Zhang J, Dvory‐Sobol H, Hyland R, et al. Treatment with ledipasvir‐sofosbuvir for 12 or 24 weeks in kidney transplant recipients with chronic hepatitis C virus genotype 1 or 4 infection: a randomized trial. Ann Intern Med 2017;166:109‐117. [DOI] [PubMed] [Google Scholar]
- 24. Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS Jr, Hassan MA, et al. Safety and efficacy of current direct‐acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: results from the HCV‐TARGET study. Hepatology 2017;66:1090‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamar N, Marion O, Rostaing L, Cointault O, Ribes D, Lavayssiere L, et al. Efficacy and safety of sofosbuvir‐based antiviral therapy to treat hepatitis C virus infection after kidney transplantation. Am J Transplant 2016;16:1474‐1479. [DOI] [PubMed] [Google Scholar]
- 26. Sawinski D, Kaur N, Ajeti A, Trofe‐Clark J, Lim M, Bleicher M, et al. Successful treatment of hepatitis C in renal transplant recipients with direct‐acting antiviral agents. Am J Transplant 2016;16:1588‐1595. [DOI] [PubMed] [Google Scholar]
- 27. Azmi AN, Tan SS, Mohamed R. Hepatitis C and kidney disease: an overview and approach to management. World J Hepatol 2015;7:78‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ng TI, Krishnan P, Pilot‐Matias T, Kati W, Schnell G, Beyer J, et al. In vitro antiviral activity and resistance profile of the next‐generation hepatitis C virus NS5A inhibitor pibrentasvir. Antimicrob Agents Chemother 2017;61:e02558‐02516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ng TI, Reisch T, Middleton T, McDaniel K, Kempf D, Lu L, et al. ABT‐493, a potent HCV NS3/4A protease inhibitor with broad genotype coverage. Presented at: 21st Annual Conference on Retroviruses and Opportunistic Infections (CROI); March 3‐6, 2014; Boston, MA. Poster 636.
- 30. Gane E, Poordad F, Wang S, Asatryan A, Kwo PY, Lalezari J, et al. High efficacy of ABT‐493 and ABT‐530 treatment in patients with HCV genotype 1 or 3 infection and compensated cirrhosis. Gastroenterology 2016;151:651‐659. [DOI] [PubMed] [Google Scholar]
- 31. Kwo PY, Poordad F, Asatryan A, Wang S, Wyles DL, Hassanein T, et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1‐6 without cirrhosis. J Hepatol 2017;67:263‐271. [DOI] [PubMed] [Google Scholar]
- 32. Asselah T, Kowdley KV, Zadeikis N, Wang S, Hassanein T, Horsmans Y, et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol 2017;16:417‐426. [DOI] [PubMed] [Google Scholar]
- 33. Zeuzem S, Foster GR, Wang S, Asatryan A, Gane E, Feld JJ, et al. Glecaprevir‐pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med 2018;378:354‐369. [DOI] [PubMed] [Google Scholar]
- 34. Poordad F, Felizarta F, Asatryan A, Sulkowski MS, Reindollar RW, Landis CS, et al. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct‐acting antiviral treatment. Hepatology 2017;66:389‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Forns X, Lee SS, Valdes J, Lens S, Ghalib R, Aguilar H, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION‐1): a single‐arm, open‐label, multicentre phase 3 trial. Lancet Infect Dis 2017;17:1062‐1068. [DOI] [PubMed] [Google Scholar]
- 36. Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Brau N, Brown A, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med 2017;377:1448‐1455. [DOI] [PubMed] [Google Scholar]
- 37. Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open‐label, randomised, phase 2 trial. Lancet Infect Dis 2016;16:685‐697. [DOI] [PubMed] [Google Scholar]
- 38. Krishnan P, Schnell G, Tripathi R, Ng TI, Reisch T, Beyer J, et al. Pooled resistance analysis in HCV genotype 1‐6‐infected patients treated with glecaprevir/pibrentasvir in phase 2 and 3 clinical trials. J Hepatol 2017;66(Suppl.):S500. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep.30046/suppinfo.
Supporting Information


