Abstract
The objective of the systematic review is to provide complete and updated information on efficacy and safety of sublingual immunotherapy (SLIT) formulations for the treatment of allergic respiratory diseases (ARDs). The literature search was conducted on PubMed database, involving double-blind, randomized clinical trials published between January 1992 and 2018, written in English, and performed in humans. The number of articles finally selected for review was 112. Data from the majority of properly controlled clinical trials demonstrate that SLIT is effective not only with short-term use (first year) but also with long-term use (up to the third year of active therapy), for treating ARDs in children and adults. Both continuous and discontinuous schemes of administration showed significant reductions in symptom and medication scores. Moreover, a SLIT-induced disease-modifying effect has been documented mainly with grass pollen extracts, since improvement is maintained during at least 2 years of follow-up after a 3-year treatment period. Additionally, allergen immunotherapy should also be considered a preventive strategy, especially for decreasing bronchial asthma incidence in children and adolescents with allergic rhinitis treated with SLIT. This therapy is also safe, producing only a few mainly local and mild-to-moderate adverse events, and usually self-limited in time. The registration and authorization of allergen SLIT preparations (grasses and house-dust mite tablets) as drugs by regulatory agencies, such as the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA), has represented a landmark in allergy immunotherapy research. Further long-term studies, specially designed with allergens other than grass pollen or house-dust mites, not only in allergic rhinoconjunctivitis but also on asthmatic subjects, as well as studies comparing different administration schedules and/or routes, are required in order to continue the progress in the modern development of this particularly promising therapy.
Keywords: allergen, allergic respiratory diseases, asthma, rhinoconjunctivitis, sublingual immunotherapy, systematic review
Introduction
The prevalence of allergic respiratory diseases (ARDs) has increased worldwide, becoming an important public health problem.1,2 ARDs are triggered by exposure to allergens and comprise allergic rhinitis (AR), with or without conjunctivitis, and bronchial asthma.3,4 AR affects approximately 1 in 5 individuals of the general population, whereas asthma affects between 1 and 18%.5,6 Asthma is triggered by allergic reactions (to house-dust mites [HDMs] or pollens, for instance) in half of cases, affecting up to 40% of subjects with allergic rhinoconjunctivitis (ARC).7 Children with ARC have a three-fold increased risk of developing asthma.8 The ARDs have been associated with impaired quality of life and a high economic burden.9 Allergen-specific immunotherapy is the only disease-modifying therapy preventing the evolution of AR to asthma, and its efficacy has long been known since observations by Leonard Noon in 1911.10,11 Allergen immunotherapy for AR is currently considered when showing strongly suggestive symptoms of AR which interfere with daily activities or sleep (despite pharmacotherapy and/or avoidance strategies), and having evidence of IgE sensitization to ≥1 clinically relevant allergen.12 For preventing asthma and AR symptoms and to spare medication use on a long-term basis, the European Academy of Allergy and Clinical Immunology (EAACI) recommends a minimum of 3 years of treatment with allergen immunotherapy in children or adolescents with moderate-to-severe grass or birch pollen-triggered AR.13 SLIT efficacy has been evidenced from results of controlled clinical trials and meta-analyses.14 Data for determining which administration route (subcutaneous immunotherapy [SCIT] or sublingual immunotherapy [SLIT]) is most effective are currently insufficient. Indeed, EAACI recommends both SCIT and SLIT for seasonal and perennial ARC.12 A landmark in the development of SLIT occurred in 2014 with the registration and authorization of grass pollen extract tablets as drugs by the United States Food and Drug Administration (FDA).15,16 Since then, and thanks to a huge research effort reflected by a number of controlled clinical trials involving large cohorts of subjects, the FDA together with the European Medicine Agency (EMA) and Japan regulatory authorities have also approved HDM formulations as drugs for the SLIT treatment of ARDs.17,18 The objective of the present manuscript was to provide complete and updated information on the efficacy and safety of SLIT formulations for the treatment of ARDs.
Methods
This systematic review was performed by using PubMed database. Keywords were chosen following the PICO methodology: population (pediatric or adult patients experiencing AR, rhinoconjunctivitis [RC], and/or asthma, by pollen, mites, pets, and/or molds); intervention (SLIT); comparator (placebo); and outcome (efficacy and safety).19 We used the following keywords in the search: (‘rhinitis’ or ‘allergic’ or ‘asthma’) and (‘Sublingual immunotherapy’) and (‘placebo’) and (‘pollen’ or ‘fungi’ or ‘mold’ or ‘dust’ or ‘mite’ or ‘pet’). We searched for studies published between January 1992 and 2018, written in English, and conducted in humans. The search was performed on June 21, 2018. Study selection was independently performed by two investigators (JH and CB). Duplicate articles were initially removed. Meta-analyses (n=7), systematic reviews (n=15), reviews (n=30), expert opinions (n=1), position papers (n=1), short communications (n=1), letters to editor (n=1), and protocols (n=1), together with articles written in a non-English language (n=1), were then excluded. This first selection was performed reading only the title and abstract of each study. Nonclinical studies, studies with limitations in their methodology, no SLIT, or those with no interest for both investigators were subsequently discarded. Methodological quality of studies was evaluated by using the Jadad scale.20 Differences between investigators were solved by consensus. Only double-blind, randomized studies were finally selected for review. Manuscripts not available online were requested from the authors. From each study, we extracted information regarding age, number of patients, diagnosis, allergen used, type of administration, study duration, and results from efficacy (symptom and medication scores, improvement in symptoms) and safety (severe or serious adverse events [SAEs], and development of anaphylactic reactions [Ax] related to SLIT treatment). The present study was approved by the Ethics Committee of La Princesa University Hospital, and its design was established in accordance with Equator network guidelines: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Results
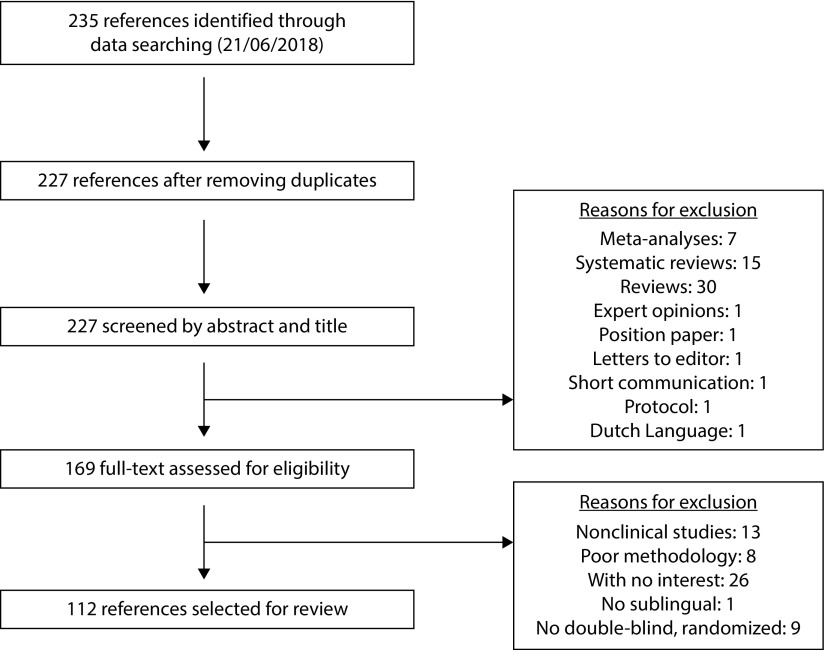
A total of 235 articles dealing with allergen SLIT therapy were initially identified. After the selection process, the number of articles finally selected for review was 112 (Figure 1).21–132 Briefly, most studies involved only adult or both pediatric and adult patients with RC with or without concomitant bronchial asthma (Table 1), although 15 were specifically performed in children and 2 in the elderly (Table 2). Most studies evaluated both efficacy, by symptom and medication scores, and safety; however, 12 of them were addressed to evaluate only SLIT safety profile (Table 3). Grass pollen was the most studied allergen for SLIT (including 5-grass pollen, MK-7243 grass pollen, 3-grass pollen, timothy grass pollen, and 6-grass pollen), followed by ragweed, birch, Japanese cedar, HDMs, Parietaria judaica, Juniperus ashei, Cupressus arizonica, and Alternaria spp. SLIT administration schedule was diverse, varying from pre-/coseasonal to coseasonal, pre-seasonal, continuous, or outside season.
Figure 1.
PRISMA flow diagram.
Table 1.
Double-blind, randomized studies (versus placebo, comparator) selected in this systematic review involving either adult or both pediatric and adult patients.
| Ref* | Age† | Cohort size | Diagnosis | Allergen | Administration type | Study duration | Efficacyα | Safety |
|---|---|---|---|---|---|---|---|---|
| Mäkelä et al.21 | 12–65 | 637 | RC | Birch pollen | Pre-/coseasonal | 16 weeks (pre) + 6 months during birch and tree seasons | 30–33% reduction in DSS for 7DU | 68 SAEs No Ax |
| Pfaar et al.33 | 19–59 | 269 | R/RC | Birch pollen | Outside season | 5 months | Stepwise improvement in SS, significant in 20,000 AUN/mL and 40,000 AUN/mL doses | - |
| Voltolini et al.73 | 44±9 | 24 | R | Birch pollen | Pre-/coseasonal | 4 months over 2 consecutive seasons | Rhinorrhea and nasal obstruction decreased | No SAEs No Ax |
| Khinchi et al.111 | 20–58 | 71 | R | Standardized birch pollen | Coseasonal | 1 baseline year + 2 years treatment | 0.36/0.29 improvement in SS/MS in first season | No SAEs No Ax |
| Didier et al.41,65 | 18–50 | 633 | RC | 300 IR 5-grass pollen | Pre-/coseasonal | 2 or 4 months (pre) until end of season 1–3 years of treatment + 2 years of follow-up |
34.5–36.0% reduction in AASS at season 3 25.3–31.1% reduction in AASS after 1 year of follow-up 28.1% reduction in AASS after 2 years of follow-up |
3 SAEs at year 1 No Ax |
| Maloney et al.47 | 5–65 | 1501 | R/RC | MK-7243 grass | Pre-/coseasonal | 12 weeks (pre) until end of season | 23/29% improvement in TCS in entire/peak season 20% improvement in DSS in entire-season 35% improvement in DMS in entire-season |
No SAEs No Ax |
| Durham et al.60 | 18–65 | 634 | RC | SQ-grass pollen | Pre-/coseasonal | 4–8 months (pre) until end of season 3 years of treatment + 2 years of follow-up |
25–36% reduction in DSS after 5 seasons 20–45% reduction in DMS for 1–4 seasons 27–41% reduction in TCS after 5 seasons |
No SAEs No Ax |
| Horak et al.81 | 19–50 | 89 | RC | 300 IR 5-grass pollen | Pre-/coseasonal | 4 months | 33% improvement in TSS Effect since first and second month of treatment |
No SAEs No Ax |
| Mösges et al.86 | 18–50 | 105 | RC | Grass and rye pollen | Out of season | 9 months | Reduced TSC | No SAEs No Ax |
| Moreno-Ancillo et al.87 | 14–55 | 105 | R ± Asthma | Grass and olive pollen | Pre-/coseasonal | 6 months (pre) until end of season | Reduction in SS and MS No differences in SS and MS between groups |
No SAEs No Ax |
| de Blay et al.88 | 12–41 | 127 | RC | Standardized 3-grass pollen | Pre-/coseasonal | 10 months (pre) until end of season | Trend of improvement in clinical score | No SAEs No Ax |
| Didier et al.89 | 18–45 | 628 | RC | Standardized 5-grass pollen | Pre-/coseasonal | 4 months (pre) until end of season | Reduction in TSS with 300 IR and 500 IR | No SAEs No Ax |
| Smith et al.107 | 18–60 | 186 | R | 5-grass pollen | Continuous | 1 baseline year + 1–2 years of treatment | Improvement in SS in years 1 and 2 6.8 and 2.4 times to show reduced nose running and sneezing |
7 SAEs No Ax |
| Clavel et al.127 | 8–55 | 136 | R | Standardized 5-grass-pollen |
Coseasonal | 6 months | Lower MS during first 6 weeks of the season | No SAEs No Ax |
| Pfaar et al.84 | 18–59 | 185 | R/RC | 6-grass pollen | Continuous | 2 years | Improvement in TCS during a 42-day period in season | No SAEs No Ax |
| Palma-Carlos et al.98 | 19–43 | 33 | R | Grass pollen | Pre-/coseasonal | 2 years | Reduction in SS between first and second year, and after 2 years of treatment | No SAEs No Ax |
| Nelson et al.66 | 18–63 | 439 | RC | Timothy grass pollen | Pre-/coseasonal | 16 weeks (pre) until end of season | 18 and 20% improvement in DSS and TCS 26% improvement in DMS |
No SAEs No Ax |
| Durham et al.103 | 18–65 | 855 | RC | Timothy grass pollen | Pre-/coseasonal | 8 weeks (pre) and season (10 weeks) | 16/28% reduction in SS/MS during season with 75,000 SQ-T 21/29% efficacy increased with pre-seasonal (≤8 weeks) |
No SAEs No Ax |
| Lima et al.116 | 18– | 56 | RC | Timothy grass pollen | Continuous | 12–18 months | No differences between groups in SS and MS | No SAEs No Ax |
| Creticos et al.48 | 18–55 | 429 | R/RC | Ragweed pollen | Pre-/coseasonal | 8–16 weeks (pre) until end of season | 43% decrease in TCS in entire season 42/41% decrease in DSS in entire/peak season |
No SAEs No Ax |
| Creticos et al.54 | 18–50 | 784 | R/RC | Ragweed pollen | Pre-/coseasonal | 12–16 weeks (pre) until end of season | 9–24% reduction in TCS in peak season (1.5, 6, 12 Amb a 1-U) 12–27% reduction in DCS in entire season (same doses) |
12 SAEs No Ax |
| Skoner et al.71 | 18–50 | 115 | RC | Ragweed pollen | Pre-/coseasonal | 8–10 weeks (pre) until end of season | 15% reduction in rhinoconjunctivitis SS in entire season DSS and DMS reduced in 48 mg Amb a 1/d (same period) |
18 SAEs No Ax |
| Bowen et al.105 | 6–58 | 83 | RC | Ragweed pollen | Pre-/coseasonal | 1–2 weeks (pre) and season (3 months) | No differences between groups in SS and MS | No SAEs No Ax |
| André et al.114 | 7–55 | 110 | R | Standardized ragweed pollen | Pre-/coseasonal | 28 days + 30 days (pre) and co-seasonal 6.5 months with maintenance treatment | Lower SS and MS during the season Highest doses showed highly response for TSS than lower ones |
No SAEs No Ax |
| Okamoto et al.42 | 12–64 | 531 | RC | Japanese cedar pollen | Continuous | 4 months (pre) until end of second consecutive season | 18 and 30% lower TNSMS in first and second seasons | No SAEs No Ax |
| Okubo et al.83 | 40±15 | 61 | RC | Japanese cedar pollen | Pre-/coseasonal | 6 weeks (pre) until end of season | Lower TSS for some days | No SAEs No Ax |
| Horiguchi et al.85 | 20–37 | 77 | RC | Japanese cedar pollen | Pre-/coseasonal | 4 months (pre) until end of season | Lower SS | No SAEs No Ax |
| Vervloet et al.93 | 19–60 | 76 | RC | Juniperus ashei pollen | Coseasonal | 2 seasons | 40–60% reduction in TMS No differences between groups in TSS |
No SAEs No Ax |
| Tonnel et al.110 | 7–45 | 120 | R | House-dust mite | Continuous | 24 months | SS decreased after 1 year and persisted | No SAEs No Ax |
| Bousquet et al.123 | 7–42 | 85 | Asthma | House-dust mite | Continuous | 25 months | Reduction in SS | No SAEs No Ax |
| Guo et al.27 | 18±9 | 48 | R | House-dust mite | Continuous | 12 months | Improvement in individual nasal SS and TNSS after 11–12 months of treatment | No SAEs No Ax |
| Okubo et al.28 | 12–64 | 946 | R | House-dust mite | Continuous | 12 months | 19 and 22% reduction in TCRS with 20,000 and 10,000 JAU 18 and 22% improvement in SS with same respective doses |
No SAEs No Ax |
| Zieglmayer et al.34 | 18–58 | 106 | R/RC ± Asthma | SQ-House-dust mite | Continuous | 12 months | Improvement of symptoms in patients with 12 SQ-HDM Reduction in 65% in TASS |
No SAEs No Ax |
| Nolte et al.35 | 12–85 | 1482 | R/RC | SQ-House-dust mite | Continuous | Up to 52 weeks | 17% improvement in TCRS 16% reduction in DSS |
No SAEs One Ax |
| Okamoto et al.29 | 12–64 | 968 | R ± Asthma | House-dust mite | Continuous | 52 weeks | 18 and 13% improvement in AASS in the weeks 44–52 for 300 IR and 500 IR | No SAEs No Ax |
| Roux et al.36 | 18–55 | 355 | R | House-dust mite | Continuous | 6 months | 33, 29, and 20% reduction in SS with 500 IR, 300 IR and 100 IR | No SAEs No Ax |
| Virchow et al.37 | 17–83 | 834 | R + Asthma | SQ-House-dust mite | Continuous | Up to 18 months | Both 6 SQ and 12 SQ doses reduced the risk of asthma exacerbation (moderate or severe, or with deterioration in asthma symptoms) | No SAEs No Ax |
| Demoly et al.39 | 18–66 | 992 | R/RC ± Asthma | SQ-House-dust mite | Continuous | 12 months | 18–22% reduction in TCS with 6 and 12 SQ Significant reduction in SS and MS with both doses |
No SAEs No Ax |
| Potter et al.40 | 18–60 | 60 | R ± Asthma | House-dust mite | Continuous | 24 months | Progressive improvement in TSS No differences between SLIT and placebo |
No SAEs No Ax |
| Nolte et al.44 | 18–58 | 124 | R/RC ± Asthma | House-dust mite | Continuous | 24 weeks | 27 and 49% reduction in TNSS at week 24 with 6 DU and 12 DU | No SAEs No Ax |
| Mosbech et al.45 | 14–73 | 604 | R + Asthma | House-dust mite | Continuous | 12 months | 29% improvement in TCRS with 6 SQ dose in the end of treatment | 4 SAEs No Ax |
| de Blay et al.49 | >14 | 108 | Asthma | House-dust mite | Continuous | 12 months | Significant reduction in ACQ at the end of study with 6 SQ | No SAEs No Ax |
| Wang et al.50 | 14–50 | 484 | Asthma | House-dust mite | Continuous | 12 months | 80.5 and 54.0% improvement in well-, or totally-controlled asthma in subjects with moderate, persistent asthma and SLIT | No SAEs No Ax |
| Bergmann et al.52 | 18–50 | 509 | R | House-dust mite | Continuous | 12 months + 12 months follow-up | 17.9 and 20.2% reduction in AASS with 300 IR and 500 IR maintained during the follow-up | 4 SAEs No Ax |
| Mosbech et al.53 | >14 | 604 | R + Asthma | SQ-House-dust mite | Continuous | 12 months | 42.0 and 50.0% relative mean and median reduction for 6 SQ | 2 SAEs No Ax |
| Wang et al.56 | 4–60 | 120 | R | House-dust mite | Continuous | 6 months | Significant reduction in TSS since week 14 | No SAEs No Ax |
| Cortellini et al.69 | 14–42 | 27 | R | Alternaria | Coseasonal | 10 months | Improvement in mean SS at the end of treatment Reduction in MS compared with run-in season and placebo |
No SAEs No Ax |
| Ariano et al.117 | 35±13 | 20 | RC | Cupressus arizonica | Coseasonal | 12 months | Lower SS and MS during the season | No SAEs No Ax |
| Passalacqua et al.120 | 19–47 | 30 | RC | Parietaria sp. | Pre-seasonal | 5 months | Decrease in SS and MS after therapy | No SAEs No Ax |
| Purello-D’Ambrosio et al.121 | 32±17 | 30 | RC ± Asthma | Parietaria judaica | Pre-/coseasonal | 1 season | Reduced SS and MS especially during the season | No SAEs No Ax |
Subanalyses (with redundant results), pooled studies, or references with not available full-text were not included in the table.
Age is shown as range (minimum–maximum) or mean ± standard deviation.
If not indicated, efficacy results are referred to the active treatment group. Comparisons are made with placebo. Only significant results are shown (p<0.05).
AASS, average adjusted symptoms score; ACQ, asthma control questionnaire; Ax, anaphylactic reaction; DMS, daily medication score; DSS, daily symptom score; DU, development units; IR, index of reactivity; JAU, Japanese allergy units; MS, medication score; R, rhinitis; RC, rhinoconjunctivitis; SAE, severe or serious adverse events (related to SLIT); SS, symptom score; TASS, total asthma symptom score; TCRS, total combined rhinitis score; TCS, total combined score; TMS, total medication score; TNSMS, total nasal symptom and medication score; TNSS, total nasal symptom score; TSS, total symptom score.
Table 2.
Double-blind, randomized studies (versus placebo, comparator) selected in this systematic review involving specific populations (either children or elderly).
| Ref* | Age† | Cohort size | Diagnosis | Allergen | Administration type | Study duration | Efficacyα | Safety |
|---|---|---|---|---|---|---|---|---|
| Children | ||||||||
| Valovirta et al.22 | 5–12 | 812 | RC | SQ-grass pollen | Continuous | 3 years of treatment + 2 years of follow-up | 22% reduction in DSS after 5 years 27% reduction in DMS after 5 years |
6 SAEs No Ax |
| Wahn et al.59 | 4–12 | 207 | R/RC | Grass pollen | Pre-/coseasonal | 8 months | Changes of −212.5 in AUC of TCS from baseline to first season Changes of −126.6/−85.9 in AUC of SS/MS (same period) |
No SAEs No Ax |
| Stelmach et al.61 | 6–18 | 60 | R | 5-grass pollen | Pre-/coseasonal versus continuous | 2 years | Reduction in TCS/TSS in pre-/coseasonal and continuous Pre-/coseasonal reduced more nasal symptoms than continuous |
No SAEs No Ax |
| Stelmach et al.76 | 6–17 | 50 | Asthma ± RC | 5-grass pollen | Pre-/coseasonal | 2 weeks (pre) until end of season 2 seasons |
25 and 41% improvement in nasal and asthma SS 10% improvement in use of rescue medication |
No SAEs No Ax |
| Bufe et al.77 | 5–16 | 253 | RC | SQ-grass pollen | Pre-/coseasonal | 8–23 weeks (pre) until end of season | 24 and 34% reduction in rhinoconjunctivitis SS and MS 64% reduction in asthma SS |
No SAEs No Ax |
| Wahn et al.80 | 5–17 | 278 | RC | 300 IR 5-grass pollen | Pre-/coseasonal | 4 months (pre) until end of season | 28.0% improvement in TSS −0.20 mean reduction in rescue MS |
17 SAEs No Ax |
| Röder et al.82 | 6–18 | 204 | RC | Grass pollen | Continuous | 2 years | No differences between groups in SS | - |
| Röder et al.92 | 6–18 | 204 | RC | 5-grass pollen | Continuous | 2 years | No differences between groups in TSS | - |
| Rolinck-Werninghaus et al.106 | 3–14 | 97 | RC | 5-grass pollen | Continuous | 32 months | TCS reduced by 77.3% of placebo group MS reduced by 67.3% of placebo group |
1 SAE No Ax |
| Wüthrich et al.113 | 4–11 | 28 | RC | 5-grass pollen | Continuous | 2 years | 70% improvement in MS in second year compared with first | No SAEs No Ax |
| Valovirta et al.99 | 5–15 | 88 | RC | Birch, alder, and hazel pollen | Continuous | Up to 18 months | Reduction in SS and MS with 24,000 and 200,000 SQ-U doses No differences between doses |
No SAEs No Ax |
| Pajno et al.118 | 8–15 | 24 | Asthma | House-dust mite | Continuous | 2 years | Reduced SS and MS after 2 years of treatment | No SAEs No Ax |
| de Bot et al.63 | 6–18 | 251 | R | House-dust mite | Continuous | 2 years | No significant effect in mean NSS after treatment | No SAEs No Ax |
| Yonekura et al.75 | 7–15 | 31 | R | House-dust mite | Continuous | 40 weeks | Reduction in mean NSS in week 32 and 35 Reduction in mean TSS in week 24 |
No SAEs No Ax |
| Pham-Thi et al.95 | 5–15 | 111 | Asthma ± R | House-dust mite | Continuous | 18 months | Decrease in rhinitis SS, but no difference with placebo | No SAEs No Ax |
| Hirsch et al.128 | 6–15 | 30 | Asthma ± R | House-dust mite | Continuous | 12 months | Reduction in mean NSS, but no difference with placebo | No SAEs No Ax |
| Pajno et al. 112 | 8–14 | 38 | Asthma ± RC | Parietaria Judaica pollen | Coseasonal | 13 months | Improvement in SS and MS in active and placebo groups | - |
| La Rosa et al.122 | 6–14 | 41 | RC | Parietaria judaica | Continuous | 2 years | Reduction in SS during the second season | No SAEs No Ax |
| Vourdas et al.125 | 7–17 | 66 | RC | Olive pollen | Pre-/coseasonal | 2 seasons | Decreased SS during first and second seasons | No SAEs No Ax |
| Elderly | ||||||||
| Bozek et al.25 | 66±5 | 47 | R | House-dust mite | Continuous | 3 years of treatment + 3 years of follow-up | 4.01 mean reduction in AASS after 3 years 3.17 mean reduction in AASS after 6 years Significant differences SLIT – placebo after 3 and 6 years |
- |
| Bozek et al.57 | 60–75 | 111 | R | House-dust mite | Continuous | 3 years | 44% decrease in TNSS at the end of treatment 51% decrease in TMS at the end of treatment |
No SAEs No Ax |
| Bozek et al.46 | 60–70 | 78 | R | 5-grass pollen | Preseasonal | 3 years | 64% decrease in nasal SS at the end of treatment 51% decrease in TMS at the end of treatment |
No SAEs No Ax |
Subanalyses (with redundant results), pooled studies, or references with not available full-text were not included in the table.
Age is shown as range (minimum–maximum) or mean ± standard deviation.
If not indicated, efficacy results are referred to the active treatment group. Comparisons are made with placebo. Only significant results are shown (p<0.05).
AASS, average adjusted symptoms score; AUC, area under the curve; Ax, anaphylactic reaction; DMS, daily medication score; DSS, daily symptom score; MS, medication score; NSS, nasal symptom score; R, rhinitis; RC, rhinoconjunctivitis; SAE, severe or serious adverse events (related to SLIT); SS, symptom score; TCS, total combined score; TMS, total medication score; TNSS, total nasal symptom score; TSS, total symptom score.
Table 3.
Double-blind, randomized studies (versus placebo, comparator) selected in this systematic review addressing only the evaluation of the safety profile.
| Ref* | Age† | Cohort size | Diagnosis | Allergen | Administration type | Study duration | Safety |
|---|---|---|---|---|---|---|---|
| Children and adults | |||||||
| Birk et al.23 | 19–61 | 70 | RC | Birch pollen | Out of season | 26–29 days | 5 SAEs with 2 and 4 DU 1 Ax with 8 DU |
| Devillier et al.38 | 14–50 | 484 | Asthma | House-dust mite | Continuous | 12 months | No SAEs No Ax |
| Nayak et al.58 | 18–50 | 80 | RC | Ragweed pollen | Out of season | 28 days | 10 SAEs No Ax |
| Sieber et al.62 | 8–65 | 209 | R | 5-grass pollen | Coseasonal | 3 consecutive seasons | No SAEs No Ax |
| Pfaar et al.67 | 18–65 | 80 | R | 12 grass pollens | Outside season | 8 weeks | No SAEs No Ax |
| Calderón et al.97 | 18–42 | 43 | R + Asthma | 5-grass pollen | Out of season | 28 days | No SAEs No Ax |
| Larsen et al.100 | 18–50 | 30 | R | 5-grass pollen | Out of season | 10 days | 8 SAEs No Ax |
| Malling et al.102 | 18–65 | 47 | RC | Grass pollen | Pre-/coseasonal | 8 weeks (pre) until end of season | No SAEs No Ax |
| Kleine-Tebbe et al.104 | 18–65 | 84 | RC | Grass pollen | Outside season | 28 days | No SAEs No Ax |
| Grosclaude et al.115 | 5–46 | 64 | RC | 5-grass pollen | Out of season | 5 months ahead season, for 8 months | 3 SAEs No Ax |
| Children | |||||||
| Maloney et al.32 | 12–17 | 195 | R/RC | 6- or 12-SQ house-dust mite | Out of season | 28 days | No SAEs No Ax |
| Mösges et al.68 | 6–14 | 54 | R | Birch pollen | Out of season | 3 months | No SAEs No Ax |
| Ibañez et al.90 | 5–12 | 60 | RC | SQ standardized grass pollen | Out of season | 28 days outside the grass pollen season | 18 SAEs No Ax |
Subanalyses (with redundant results), pooled studies, or references with not available full-text were not included in the table.
Age is shown as range (minimum–maximum).
Ax, anaphylactic reaction; R, rhinitis; RC, rhinoconjunctivitis; SAE, severe or serious adverse events (related to SLIT).
Efficacy results
The list of references involving either adult or both pediatric and adult patients is shown in Table 1. With the exception of four studies,63,87,105,116 all of them demonstrated a significant reduction in symptoms and medication scores after SLIT administration.
SLIT with grass pollen
SLIT for the treatment of grass pollen–induced AR has demonstrated its efficacy at different administration schedules (continuous and discontinuous). Data from more than 3000 patients revealed that SLIT in a pre-/coseasonal scheme reduces symptom and medication scores up to 30 and 29%, respectively, during the first 12 months of treatment, or to 36 and 45% after 3 years.41,60,65,66,103 The registration and authorization of grass pollen extract tablets was mainly based on results of these studies. Efficacy of SLIT has been evidenced since the first month of treatment.81
SLIT with HDMs
SLIT has proven its efficacy for HDM-induced AR during 6, 12, and 18 months of continuous treatment. Data from more than 5000 patients demonstrated a significant reduction in symptoms score between 16 and 42% after 12 months of treatment,29,35,36,37,39,52,53 which led to the registration and authorization of HDMs extracts as drugs.
Long-term and disease-modifying effect of SLIT
Both SLIT with grass pollen and HDMs have also demonstrated long-term effects for the treatment of AR. Studies involving 3 years of treatment with grass pollen extracts have shown significant improvements in symptoms scores at season 1, 2, and 3 (with values ranging between 25 and 35%).60,63 Furthermore, SLIT with grass pollen and HDMs showed a disease-modifying effect after a period of treatment. The reduction in symptoms scores ranged between 25 and 36% with grass pollen (for 2 years after a 3-year treatment period),41,60,65 and between 18 and 20% with HDMs (for 1 year after a 1-year treatment).52
SLIT and asthma
SLIT efficacy with HDMs has been shown in mild-to-moderate and moderate persistent asthma by a clinically and statistically significant reduction in inhaled corticosteroid (ICS) dose required to asthma control and a greater rate of well- and total-control of asthma,51,53 which allowed the inclusion of HDM tablets in the Global Initiative for Asthma (GINA) guideline for the treatment of allergic asthma induced by HDMs. Some studies have also demonstrated that SLIT treatment with HDMs improves asthma symptoms, and reduces the risk of moderate/severe exacerbations.35,37 Moreover, SLIT with HDMs prevented the risk of developing asthma in children during a 5-year period (3 years of treatment and 2-year follow-up).13,22 This preventive effect was apparently strongest in youngest children.
SLIT in children and the elderly
The reference list involving specific populations (either children or elderly) is shown in Table 2. Studies specifically designed for pediatric population involved approximately 2000 children. Most of these studies demonstrated a significant reduction in symptom (22–28%) and medication scores (27–34%) after 1, 2, or 3 years of treatment with grass pollen extracts.22,77,80 SLIT with HDM also showed significant reduction in symptom and medication scores;75,118 however, some of them showed no differences with placebo.95,128 There was a scarce number of studies regarding elderly subjects, and they involved only a few patients (about 200).25,46,57 Besides this, symptom scores significantly reduced after 3 years of treatment with HDMs (44%)57 or 5-grass pollen SLIT (64%),46 together with medication scores by 51%. A study of 3 years of treatment with HDMs and a 3-year follow-up with no treatment also revealed a reduction in symptoms after this 6-year period.25
Safety profile
In the majority of studies, AEs were local and mild or moderate in severity. Most of these AEs commonly occurred during the first weeks of treatment. The frequency of treatment-related AEs (TRAEs) ranged between 46 and 69%.133 The majority of cases (>80%) were oral reactions, including throat irritation (reported in 17–43% of subjects), oral pruritus (11–43%), ear pruritus (7–29%), mouth edema (4–11%), oral paresthesia (5–10%), tongue pruritus (5–9%), lip swelling (3–11%), swollen tongue (3–10%), glossodynia (1–9%), and dysgeusia (0.2–5%). Other TRAEs reported in more than 5% of subjects were: nausea (1–8%), and upper abdominal pain (1–6%). Asthma, cough, and dyspnea were also the most frequently reported asthma-related AEs among subjects with concomitant asthma. Approximately 5% of subjects discontinued the trials because of the TRAEs.
Scarce studies have reported serious TRAEs regarding SLIT. For instance, in a study with 80 adults with RC receiving ragweed pollen SLIT or placebo, there were reported 10 serious TRAEs (chest discomfort, chest pain, dysphagia, oral pruritus, allergic conjunctivitis, mouth edema, swollen tongue, conjunctivitis, allergic conjunctivitis, and periorbital edema).58 With only one exception,23 none of these studies, involving in total more than 4000 subjects, have reported cases of Ax during SLIT. The reference list involving studies only addressed to evaluate SLIT safety profile is shown in Table 3. Among them, Birk and colleagues23 evaluated SQ tree SLIT tablet (ALK, doses from 1 to 24 DU) tolerability for 26–29 days outside birch pollen season in 70 adults with RC with or without asthma, and reported 3 TRAEs: asthma (at 2 DU dose), eye pruritus (4 DU), and Ax (8 DU).
Discussion
Registration and authorization of allergen SLIT preparations (firstly grass pollen, and secondly HDMs) as drugs by regulatory agencies represented a landmark in the research on allergy immunotherapy.15–18 Apart from standardizing preparation content and production procedures (reproducibility), which in turn increased patient safety, its clinical development by the inclusion of a large number of patients in phase III clinical trials allowed registration and authorization of these products. The amount and quality of studies shown in the present manuscript is a consequence of such decision.
Since the first published trial in 1986, SLIT has become the most promising alternative to SCIT.132 The present systematic review clearly shows that SLIT is both effective (by reducing symptom and medication scores) and safe, at least regarding HDM and certain pollen preparations. SLIT has clearly shown to be effective for the treatment of ARDs, maintaining its effectiveness up to 2 years after a 3-year treatment period, thus demonstrating not only long-term efficacy but also a disease-modifying effect.
However, several aspects should also be taken into account. First, allergen content and dosing (either for SCIT and SLIT) is not standardized, and varies among products. Several grass pollen SLIT formulations were used in studies considered, including 5-grass pollen, MK-7243 grass pollen, 3-grass pollen, timothy grass pollen, and 6-grass pollen. In this line, a study of SLIT products from US and European manufacturers has already shown a difference from 7 to 200-fold in major allergen concentration of timothy grass, HDM, ragweed, and cat extracts.134 Given the variability in allergen content, comparisons between different studies should be made cautiously.
The registration of SLIT grass pollen and HDM preparations have contributed to reduce this allergen content variability. Currently, the FDA has recommended the use of the bioequivalent allergy unit for establishing the allergenic activity (potency) of grass pollen extracts of different origins.135 By contrast, European regulatory authorities need to adopt a standardized unit. It has been documented that high antigen doses are needed to achieve immunotherapy-induced clinical benefits, thus it is expected that studies carried out with low antigen doses may not be successful. In this regard, one of the SLIT main advantages, as compared to the more traditional SCIT route, is that you can increase the extract allergen content to a certain range without compromising safety profile.
Another aspect to consider is the administration schedule. SLIT has been shown to be effective for allergic RC, both under continuous (year-round) or discontinuous (pre-seasonal, coseasonal, or pre-/coseasonal) schemes.14 SLIT with HDMs is used under a continuous scheme. Pre- and coseasonal regimens are frequently employed for SLIT, especially with grass pollen, and have some advantages over continuous regimens.136 In fact, long treatment periods have been associated with poor adherence and, in turn, lower effectiveness.137 Diverse studies have demonstrated that discontinuous schemes are, at least, as effective and safe as continuous ones. However, pre-/coseasonal treatment, as used with grass pollen, might enhance the adherence to long treatments. If SLIT products are initiated before the pollen season, it is important for practitioners to be familiar with specific pollen seasonal patterns in their locations.
The third consideration derives from the lack of comparative studies (efficacy and safety) between SLIT and SCIT. In this context, SLIT may provide some clear advantages over SCIT, such as the comfort of receiving treatment at home, without painful injections, and as mentioned earlier a better safety profile, which together with pre-/coseasonal schemes would probably improve treatment adherence. Due to the lack of studies specifically designed to compare both administration routes we have to be cautious with conclusions.
On the other hand, clinical trials with HDM-induced asthma have demonstrated that SLIT treatment not only significantly reduces asthma symptoms and exacerbations but also can prevent asthma onset.22,37,44,45 However, the number of studies involving asthmatic subjects or designed to evaluate changes in asthma symptoms is limited. Given the increasing prevalence of asthmatic patients and the impact of SLIT over the ‘allergic march’, it seems necessary to perform additional long-term controlled clinical trials with, at least, the most prevalent allergens.
Interestingly, a recent study focused on SLIT immunological mechanisms, using a grass pollen tablet and with a 3-year treatment and 2-year follow-up protocol, has suggested that SLIT sustained effect is linked to the generation of a long-term regulatory T cell response.138,139 However, up to 2 years of therapy is needed to develop this regulatory response in many patients, thus explaining why some short-term studies have failed to demonstrate SLIT efficacy.
Most studies that have failed to demonstrate SLIT efficacy show a low allergen content, a short treatment time, and/or a small study population sample size. In consequence, when evaluating SLIT, we need to focus on studies performed not only with high-dose allergen formulations but also on a long-term basis and with a large sample size, such as those used to register as drugs, both grass pollen and HDM preparations.
Final considerations about the difficulty in assessing the evidence in these studies include the following: the severity of the disease in recruited patients (SLIT showed no significant results when analyzing patients with intermittent mild asthma or AR, whereas when analyzing by moderate persistent asthma, authors did find significant results); concomitant treatments can mask the effect of immunotherapy; and intrinsic effects of clinical trials or ‘nursing effect’ can explain the improvement achieved by the treatment with placebo.
One limitation of our systematic review is intrinsically associated with the nature of the literature search, that is, using only data from published and available clinical trials. Another limitation may derive from the heterogeneity between studies, regarding factors such as treatment, inclusion criteria, or variables evaluated. Also, our study goal was to perform a complete (from 1992) and updated (to 2018) search from PubMed database on allergen SLIT, to try to provide a clear vision of the current situation of this specific therapy.
Conclusion
Data from the majority of properly controlled clinical trials demonstrate that SLIT is an effective treatment for ARDs in children and adults, since continuous and discontinuous schemes of administration show significant reductions in symptom and medication scores, both at short (first year) and long-term (sustained effect during a 3-year treatment period). Furthermore, a disease-modifying effect of SLIT has been documented mainly with grass pollen extracts, maintaining its effect for a 2-year follow-up without immunotherapy after a 3-year SLIT treatment period. At the same time, allergen immunotherapy should also be considered a preventive strategy, especially for preventing asthma in children and adolescents with AR. SLIT treatment appears to be safe in that it produces only a few self-limiting and mainly local and mild-to-moderate AEs.
Further long-term studies, specially designed with allergens other than grass pollen or HDMs, not only in ARC but also on asthmatic subjects, as well as studies comparing different administration schedules and/or routes, are required to continue the progress in the modern development of this particular promising therapy.
Acknowledgments
The authors would like to thank to Pablo Vivanco (PhD, Meisys) for assistance in preparing the manuscript.
Footnotes
Contributions: Carlos Blanco and Jenaro Hernández contributed with the search for this systematic review. Jenaro Hernandez redacted the draft of the manuscript. All authors contributed with successive reviews of the manuscript.
Disclosure and potential conflicts of interest: Dr Hernández works at Stallergenes Greer, Spain. The authors declare that there is no conflict of interest regarding the publication of this article. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at http://www.drugsincontext.com/wp-content/uploads/2018/10/dic.212552-COI.pdf
Funding declaration: Stallergenes Greer contributed financially to cover the collaboration of Meisys as Medical Writer without any intervention in the content or development of this review.
Correct attribution: Copyright © 2018 Blanco C, Bazire R, Argiz L, Hernández-Peña J. https://doi.org/10.7573/dic.212552. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/sublingual-allergen-immunotherapy-for-respiratory-allergy-a-systematic-review
Provenance: invited; externally peer reviewed.
Peer review comments to author: 9 September 2018
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J. 2014;7:12. doi: 10.1186/1939-4551-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brozek G, Lawson J, Szumilas D, Zejda J. Increasing prevalence of asthma, respiratory symptoms, and allergic diseases: four repeated surveys from 1993–2014. Respir Med. 2015;109:982–990. doi: 10.1016/j.rmed.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–476. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 4.Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. 2011;378:2112–2122. doi: 10.1016/S0140-6736(11)60130-X. [DOI] [PubMed] [Google Scholar]
- 5.Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988–1994. J Allergy Clin Immunol. 2010;126:778. doi: 10.1016/j.jaci.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 6.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention. [Accessed July 7, 2018]. http://www.ginasthma.org/
- 7.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA (2)LEN and AllerGen) Allergy. 2008;63:8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 8.Burgess JA, Walters EH, Byrnes GB, et al. Childhood allergic rhinitis predicts asthma incidence and persistence to middle age: a longitudinal study. J Allergy Clin Immunol. 2007;120:863–869. doi: 10.1016/j.jaci.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Bousquet PJ, Demoly P, Devillier P, et al. Impact of allergic rhinitis symptoms on quality of life in primary care. Int Arch Allergy Immunol. 2013;160:393–400. doi: 10.1159/000342991. [DOI] [PubMed] [Google Scholar]
- 10.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;177:1572–1573. doi: 10.1016/S0140-6736(00)78276-6. [DOI] [Google Scholar]
- 11.Larsen JN, Broge L, Jacobi H. Allergy immunotherapy: the future of allergy treatment. Drug Discov Today. 2016;21:26–37. doi: 10.1016/j.drudis.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Roberts G, Pfaar O, Akdis CA, et al. EAACI Guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73:765–798. doi: 10.1111/all.13262. [DOI] [PubMed] [Google Scholar]
- 13.Halken S, Larenas-Linnemann D, Roberts G, et al. EAACI Guidelines on allergen immunotherapy: prevention of allergy. Pediatr Allergy Immunol. 2017;28:728–745. doi: 10.1111/pai.12807. [DOI] [PubMed] [Google Scholar]
- 14.Passalacqua G. Recommendations for appropriate sublingual immunotherapy clinical trials. World Allergy Organ J. 2014;7:21. doi: 10.1186/1939-4551-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Food & Drug Administration. Approval Letter – ORALAIR. Apr 1, 2014. [Accessed July 7, 2018]. https://www.fda.gov/biologicsbloodvaccines/allergenics/ucm391573.htm.
- 16.ALK. ALK announces FDA approval for Merck’s grass sublingual allergy immunotherapy tablet GRASTEK® (GRAZAX®) [Accessed July 7, 2018]. https://globenewswire.com/news-release/2014/04/14/627053/0/en/ALK-announces-FDA-approval-for-Merck-s-grass-sublingual-allergy-immunotherapy-tablet-GRASTEK-GRAZAX.html.
- 17.European Medicine Agency. European Medicines Agency decision P/0056/2018. [Accessed 7 July, 2018]. https://www.ema.europa.eu/docs/en_GB/document_library/PIP_decision/WC500250039.pdf.
- 18.Shionogi. Shionogi got approval of partial change application in dosage and administration in Japan of Actair® for allergen immunotherapy of pediatric allergic rhinitis caused by house dust mites. [Accessed July 7, 2018]. http://www.shionogi.co.jp/en/company/news/2018/pmrltj0000003nru-att/e180216_2.pdf.
- 19.PICO Framework. PubMed Health. [Accessed July 7, 2018]. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0029906.
- 20.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Mäkelä MJ, Gyllfors P, Valovirta E, et al. Immunotherapy with the SQ Tree SLIT–tablet in adults and adolescents with allergic rhinoconjunctivitis. Clin Ther. 2018 Mar 15; doi: 10.1016/j.clinthera.2018.02.012. pii: S0149-2918(18)30062-6. [DOI] [PubMed] [Google Scholar]
- 22.Valovirta E, Petersen TH, Piotrowska T, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol. 2018;141:529–538.e13.18. doi: 10.1016/j.jaci.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Birk AO, Andersen JS, Villesen HH, Steffensen MA, Calderon MA. Tolerability of the SQ Tree SLIT tablet in adults. Clin Ther. 2017;39:1858–1867. doi: 10.1016/j.clinthera.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Casale TB, Cox LS, Wahn U, et al. Safety review of 5-grass pollen tablet from pooled data of clinical trials. J Allergy Clin Immunol Pract. 2017;5:1717–1727.e1. doi: 10.1016/j.jaip.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Bozek A, Starczewska-Dymek L, Jarzab J. Prolonged effect of allergen sublingual immunotherapy for house dust mites in elderly patients. Ann Allergy Asthma Immunol. 2017;119:77–82. doi: 10.1016/j.anai.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Emminger W, Hernández MD, Cardona V, et al. The SQ house dust mite SLIT-tablet is well tolerated in patients with house dust mite respiratory allergic disease. Int Arch Allergy Immunol. 2017;174:35–44. doi: 10.1159/000478699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Y, Li Y, Wang D, et al. A randomized, double-blind, placebo controlled trial of sublingual immunotherapy with house-dust mite extract for allergic rhinitis. Am J Rhinol Allergy. 2017;31:42–47. doi: 10.2500/ajra.2017.31.4447. [DOI] [PubMed] [Google Scholar]
- 28.Okubo K, Masuyama K, Imai T, et al. Efficacy and safety of the SQ house dust mite sublingual immunotherapy tablet in Japanese adults and adolescents with house dust mite-induced allergic rhinitis. J Allergy Clin Immunol. 2017;139:1840–1848.e10. doi: 10.1016/j.jaci.2016.09.043. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto Y, Fujieda S, Okano M, et al. House dust mite sublingual tablet is effective and safe in patients with allergic rhinitis. Allergy. 2017;72:435–443. doi: 10.1111/all.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durham SR, Creticos PS, Nelson HS, et al. Treatment effect of sublingual immunotherapy tablets and pharmacotherapies for seasonal and perennial allergic rhinitis: pooled analyses. J Allergy Clin Immunol. 2016;138:1081–1088.e4. doi: 10.1016/j.jaci.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 31.Sakurai D, Yonekura S, Iinuma T, et al. Sublingual immunotherapy for allergic rhinitis: subjective versus objective tools to evaluate its success. Rhinology. 2016;54:221–230. doi: 10.4193/Rhin15.223. [DOI] [PubMed] [Google Scholar]
- 32.Maloney J, Prenner BM, Bernstein DI, et al. Safety of house dust mite sublingual immunotherapy standardized quality tablet in children allergic to house dust mites. Ann Allergy Asthma Immunol. 2016;116:59–65. doi: 10.1016/j.anai.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Pfaar O, van Twuijver E, Boot JD, et al. A randomized DBPC trial to determine the optimal effective and safe dose of a SLIT–birch pollen extract for the treatment of allergic rhinitis: results of a phase II study. Allergy. 2016;71:99–107. doi: 10.1111/all.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zieglmayer P, Nolte H, Nelson HS, et al. Long-term effects of a house dust mite sublingual immunotherapy tablet in an environmental exposure chamber trial. Ann Allergy Asthma Immunol. 2016;117:690–696.e1. doi: 10.1016/j.jaci.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Nolte H, Bernstein DI, Nelson HS, et al. Efficacy of house dust mite sublingual immunotherapy tablet in North American adolescents and adults in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2016;138:1631–1638. doi: 10.1016/j.jaci.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 36.Roux M, Devillier P, Yang WH, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts: results of a dose-ranging study in an environmental exposure chamber. J Allergy Clin Immunol. 2016;138:451–458.e5. doi: 10.1016/j.jaci.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 37.Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715–1725. doi: 10.1542/peds.2017-2475NNN. [DOI] [PubMed] [Google Scholar]
- 38.Devillier P, Fadel R, de Beaumont O. House dust mite sublingual immunotherapy is safe in patients with mild-to-moderate, persistent asthma: a clinical trial. Allergy. 2016;71:249–257. doi: 10.1111/all.12188. [DOI] [PubMed] [Google Scholar]
- 39.Demoly P, Emminger W, Rehm D, et al. Effective treatment of house dust mite-induced allergic rhinitis with 2 doses of the SQ HDM SLIT-tablet: results from a randomized, double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol. 2016;137:444–451.e8. doi: 10.1016/j.jaci.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 40.Potter PC, Baker S, Fenemore B, Nurse B. Clinical and cytokine responses to house dust mite sublingual immunotherapy. Ann Allergy Asthma Immunol. 2015;114:327–334. doi: 10.1016/j.anai.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Didier A, Malling HJ, Worm M, et al. Prolonged efficacy of the 300IR 5-grass pollen tablet up to 2 years after treatment cessation, as measured by a recommended daily combined score. Clin Transl Allergy. 2015;5:12. doi: 10.1186/s13601-015-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okamoto Y, Okubo K, Yonekura S, et al. Efficacy and safety of sublingual immunotherapy for two seasons in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2015;166:177–188. doi: 10.1186/s13601-015-0057-8. [DOI] [PubMed] [Google Scholar]
- 43.Maloney J, Durham S, Skoner D, et al. Safety of sublingual immunotherapy Timothy grass tablet in subjects with allergic rhinitis with or without conjunctivitis and history of asthma. Allergy. 2015;70:302–309. doi: 10.1111/all.12560. [DOI] [PubMed] [Google Scholar]
- 44.Nolte H, Maloney J, Nelson HS, et al. Onset and dose-related efficacy of house dust mite sublingual immunotherapy tablets in an environmental exposure chamber. J Allergy Clin Immunol. 2015;135:1494–1501.e6. doi: 10.1016/j.jaci.2014.12.1911. [DOI] [PubMed] [Google Scholar]
- 45.Mosbech H, Canonica GW, Backer V, et al. SQ house dust mite sublingually administered immunotherapy tablet (ALK) improves allergic rhinitis in patients with house dust mite allergic asthma and rhinitis symptoms. Ann Allergy Asthma Immunol. 2015;114:134–140. doi: 10.1016/j.anai.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 46.Bozek A, Kolodziejczyk K, Warkocka-Szoltysek B, Jarzab J. Grass pollen sublingual immunotherapy: a double-blind, placebo-controlled study in elderly patients with seasonal allergic rhinitis. Am J Rhinol Allergy. 2014;28:423–427. doi: 10.2500/ajra.2014.28.4091. [DOI] [PubMed] [Google Scholar]
- 47.Maloney J, Bernstein DI, Nelson H, et al. Efficacy and safety of grass sublingual immunotherapy tablet, MK-7243: a large randomized controlled trial. Ann Allergy Asthma Immunol. 2014;112:146–153.e2. doi: 10.1016/j.anai.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 48.Creticos PS, Esch RE, Couroux P, et al. Randomized, double-blind, placebo-controlled trial of standardized ragweed sublingual-liquid immunotherapy for allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2014;133:751–758. doi: 10.1016/j.jaci.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 49.de Blay F, Kuna P, Prieto L, et al. SQ HDM SLIT-tablet (ALK) in treatment of asthma--post hoc results from a randomised trial. Respir Med. 2014;108:1430–1437. doi: 10.1016/j.rmed.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Yin J, Fadel R, et al. House dust mite sublingual immunotherapy is safe and appears to be effective in moderate, persistent asthma. Allergy. 2014;69:1181–1188. doi: 10.1111/all.12188. [DOI] [PubMed] [Google Scholar]
- 51.Corzo JL, Carrillo T, Pedemonte C, et al. Tolerability during double-blind randomized phase I trials with the house dust mite allergy immunotherapy tablet in adults and children. J Investig Allergol Clin Immunol. 2014;24:154–161. [PubMed] [Google Scholar]
- 52.Bergmann KC, Demoly P, Worm M, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133:1608–1614.e6. doi: 10.1016/j.jaci.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568–575. doi: 10.1016/j.jaci.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 54.Creticos PS, Maloney J, Bernstein DI, et al. Randomized controlled trial of a ragweed allergy immunotherapy tablet in North American and European adults. J Allergy Clin Immunol. 2013;131:1342–1349.e6. doi: 10.1016/j.jaci.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 55.Didier A, Malling HJ, Worm M, et al. Post-treatment efficacy of discontinuous treatment with 300IR 5-grass pollen sublingual tablet in adults with grass pollen-induced allergic rhinoconjunctivitis. Clin Exp Allergy. 2013;43:568–577. doi: 10.1111/cea.12100. [DOI] [PubMed] [Google Scholar]
- 56.Wang DH, Chen L, Cheng L, et al. Fast onset of action of sublingual immunotherapy in house dust mite-induced allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial. Laryngoscope. 2013;123:1334–1340. doi: 10.1002/lary.23935. [DOI] [PubMed] [Google Scholar]
- 57.Bozek A, Ignasiak B, Filipowska B, Jarzab J. House dust mite sublingual immunotherapy: a double-blind, placebo-controlled study in elderly patients with allergic rhinitis. Clin Exp Allergy. 2013;43:242–248. doi: 10.1111/cea.12039. [DOI] [PubMed] [Google Scholar]
- 58.Nayak AS, Atiee GJ, Dige E, et al. Safety of ragweed sublingual allergy immunotherapy tablets in adults with allergic rhinoconjunctivitis. Allergy Asthma Proc. 2012;33:404–410. doi: 10.1159/000487997. [DOI] [PubMed] [Google Scholar]
- 59.Wahn U, Klimek L, Ploszczuk A, et al. High-dose sublingual immunotherapy with single-dose aqueous grass pollen extract in children is effective and safe: a double-blind, placebo-controlled study. J Allergy Clin Immunol. 2012;130:886–893.e5. doi: 10.1016/j.jaci.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 60.Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–725.e5. doi: 10.1016/j.jaci.2011.12.973. [DOI] [PubMed] [Google Scholar]
- 61.Stelmach I, Kaluzińska-Parzyszek I, Jerzynska J, et al. Comparative effect of pre–coseasonal and continuous grass sublingual immunotherapy in children. Allergy. 2012;67:312–320. doi: 10.1111/j.1398-9995.2011.02758.x. [DOI] [PubMed] [Google Scholar]
- 62.Sieber J, Neis M, Brehler R, et al. Increasing long-term safety of seasonal grass pollen sublingual immunotherapy: the ECRIT study. Expert Opin Drug Saf. 2012;11:7–13. doi: 10.1517/14740338.2012.626765. [DOI] [PubMed] [Google Scholar]
- 63.de Bot CM, Moed H, Berger MY, et al. Sublingual immunotherapy not effective in-house dust mite-allergic children in primary care. Pediatr Allergy Immunol. 2012;23:150–158. doi: 10.1111/j.1399-3038.2011.01219.x. [DOI] [PubMed] [Google Scholar]
- 64.Kuna P, Samolinski B, Worm M, et al. Sustained clinical efficacy of sublingual immunotherapy with a high-dose grass pollen extract. Eur Ann Allergy Clin Immunol. 2011;43:117–121. [PubMed] [Google Scholar]
- 65.Didier A, Worm M, Horak F, et al. Sustained 3-year efficacy of pre- and coseasonal 5-grass–pollen sublingual immunotherapy tablets in patients with grass pollen-induced rhinoconjunctivitis. J Allergy Clin Immunol. 2011;128:559–566. doi: 10.1016/j.jaci.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 66.Nelson HS, Nolte H, Creticos P, et al. Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North American adults. J Allergy Clin Immunol. 2011;127:72–80.e1–2. doi: 10.1016/j.jaci.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 67.Pfaar O, Barth C, Jaschke C, et al. Sublingual allergen-specific immunotherapy adjuvanted with monophosphoryl lipid A: a phase I/IIa study. Int Arch Allergy Immunol. 2011;154:336–344. doi: 10.1159/000321826. [DOI] [PubMed] [Google Scholar]
- 68.Mösges R, Graute V, Christ H, et al. Safety of ultra–rush titration of sublingual immunotherapy in asthmatic children with tree-pollen allergy. Pediatr Allergy Immunol. 2010;21:1135–1138. doi: 10.1111/j.1399-3038.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 69.Cortellini G, Spadolini I, Patella V, et al. Sublingual immunotherapy for Alternaria-induced allergic rhinitis: a randomized placebo-controlled trial. Ann Allergy Asthma Immunol. 2010;105:382–386. doi: 10.1016/j.anai.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 70.Halken S, Agertoft L, Seidenberg J, et al. Five-grass pollen 300IR SLIT tablets: efficacy and safety in children and adolescents. Pediatr Allergy Immunol. 2010;21:970–976. doi: 10.1111/j.1399-3038.2010.01050.x. [DOI] [PubMed] [Google Scholar]
- 71.Skoner D, Gentile D, Bush R, et al. Sublingual immunotherapy in patients with allergic rhinoconjunctivitis caused by ragweed pollen. J Allergy Clin Immunol. 2010;125:660–666.e1–4. doi: 10.1016/j.jaci.2009.12.931. [DOI] [PubMed] [Google Scholar]
- 72.Durham SR, Emminger W, Kapp A, et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol. 2010;125:131–138.e1–7. doi: 10.1016/j.jaci.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 73.Voltolini S, Troise C, Incorvaia C, et al. Effectiveness of high dose sublingual immunotherapy to induce a stepdown of seasonal asthma: a pilot study. Curr Med Res Opin. 2010;26:37–40. doi: 10.1185/03007990903431886. [DOI] [PubMed] [Google Scholar]
- 74.Nieminen K, Valovirta E, Savolainen J. Clinical outcome and IL-1, IL-2, IL-27 and FOXP3 expression in peripheral blood mononuclear cells of pollen-allergic children during sublingual immunotherapy. Pediatr Allergy Immunol. 2010;21:e174–184. doi: 10.1111/j.1399-3038.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 75.Yonekura S, Okamoto Y, Sakurai D, et al. Sublingual immunotherapy with house dust extract for house dust-mite allergic rhinitis in children. Allergol Int. 2010;59:381–388. doi: 10.2332/allergolint.10-OA-0200. [DOI] [PubMed] [Google Scholar]
- 76.Stelmach I, Kaczmarek-Woźniak J, Majak P, et al. Efficacy and safety of high-doses sublingual immunotherapy in ultra–rush scheme in children allergic to grass pollen. Clin Exp Allergy. 2009;39:401–408. doi: 10.1111/crj.12058. [DOI] [PubMed] [Google Scholar]
- 77.Bufe A, Eberle P, Franke-Beckmann E, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167–173.e7. doi: 10.1016/j.jaci.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 78.Didier A, Melac M, Montagut A, et al. Agreement of efficacy assessments for five-grass pollen sublingual tablet immunotherapy. Allergy. 2009;64:166–171. doi: 10.1111/j.1398-9995.2008.01767.x. [DOI] [PubMed] [Google Scholar]
- 79.Ott H, Sieber J, Brehler R, et al. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy. 2009;64:179–186. doi: 10.1111/j.1398-9995.2009.02194.x. [DOI] [PubMed] [Google Scholar]
- 80.Wahn U, Tabar A, Kuna P, et al. Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2009;123:160–166.e3. doi: 10.1016/j.jaci.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 81.Horak F, Zieglmayer P, Zieglmayer R, et al. Early onset of action of a 5-grass-pollen 300-IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol. 2009;124:471–477. doi: 10.1016/j.jaci.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 82.Röder E, Berger MY, de Groot H, et al. Sublingual immunotherapy in youngsters: adherence in a randomized clinical trial. Clin Exp Allergy. 2008;38:1659–1667. doi: 10.1111/j.1365-2222.2008.03060.x. [DOI] [PubMed] [Google Scholar]
- 83.Okubo K, Gotoh M, Fujieda S, et al. A randomized double–blind comparative study of sublingual immunotherapy for cedar pollinosis. Allergol Int. 2008;57:265–275. doi: 10.2332/allergolint.O-07-514. [DOI] [PubMed] [Google Scholar]
- 84.Pfaar O, Klimek L. Efficacy and safety of specific immunotherapy with a high-dose sublingual grass pollen preparation: a double-blind, placebo-controlled trial. Ann Allergy Asthma Immunol. 2008;100:256–263. doi: 10.1016/s1081-1206(10)60451-6. [DOI] [PubMed] [Google Scholar]
- 85.Horiguchi S, Okamoto Y, Yonekura S, et al. A randomized controlled trial of sublingual immunotherapy for Japanese cedar pollinosis. Int Arch Allergy Immunol. 2008;146:76–84. doi: 10.1159/000112506. [DOI] [PubMed] [Google Scholar]
- 86.Mösges R, Brüning H, Hessler HJ, et al. Sublingual immunotherapy in pollen-induced seasonal rhinitis and conjunctivitis: a randomized controlled trial. Acta Dermatovenerol Alp Pannonica Adriat. 2007;16:143–148. [PubMed] [Google Scholar]
- 87.Moreno-Ancillo A, Moreno C, Ojeda P, et al. Efficacy and quality of life with once-daily sublingual immunotherapy with grasses plus olive pollen extract without updosing. J Investig Allergol Clin Immunol. 2007;17:399–405. doi: 10.7573/dic.212309. [DOI] [PubMed] [Google Scholar]
- 88.de Blay F, Barnig C, Kanny G, et al. Sublingual-swallow immunotherapy with standardized 3-grass pollen extract: a double-blind, placebo-controlled study. Ann Allergy Asthma Immunol. 2007;99:453–461. doi: 10.4103/0972-6691.124391. [DOI] [PubMed] [Google Scholar]
- 89.Didier A, Malling HJ, Worm M, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;120:1338–1345. doi: 10.1016/j.jaci.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 90.Ibañez MD, Kaiser F, Knecht R, et al. Safety of specific sublingual immunotherapy with SQ standardized grass allergen tablets in children. Pediatr Allergy Immunol. 2007;18:516–522. doi: 10.1111/j.1399-3038.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 91.Durham SR, Riis B. Grass allergen tablet immunotherapy relieves individual seasonal eye and nasal symptoms, including nasal blockage. Allergy. 2007;62:954–957. doi: 10.1111/j.1398-9995.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- 92.Röder E, Berger MY, Hop WC, et al. Sublingual immunotherapy with grass pollen is not effective in symptomatic youngsters in primary care. J Allergy Clin Immunol. 2007;119:892–898. doi: 10.1016/j.jaci.2006.12.651. [DOI] [PubMed] [Google Scholar]
- 93.Vervloet D, Birnbaum J, Laurent P, et al. Safety and efficacy of Juniperus ashei sublingual-swallow ultra-rush pollen immunotherapy in cypress rhinoconjunctivitis. A double-blind, placebo-controlled study. Int Arch Allergy Immunol. 2007;142:239–246. doi: 10.1159/000097026. [DOI] [PubMed] [Google Scholar]
- 94.Rak S, Yang WH, Pedersen MR, Durham SR. Once-daily sublingual allergen–specific immunotherapy improves quality of life in patients with grass pollen-induced allergic rhinoconjunctivitis: a double-blind, randomised study. Qual Life Res. 2007;16:191–201. doi: 10.1007/s11136-006-9110-3. [DOI] [PubMed] [Google Scholar]
- 95.Pham-Thi N, Scheinmann P, Fadel R, et al. Assessment of sublingual immunotherapy efficacy in children with house dust mite-induced allergic asthma optimally controlled by pharmacologic treatment and mite-avoidance measures. Pediatr Allergy Immunol. 2007;18:47–57. doi: 10.1111/j.1399-3038.2006.00475.x. [DOI] [PubMed] [Google Scholar]
- 96.Worm M. Efficacy and tolerability of high dose sublingual immunotherapy in patients with rhinoconjunctivitis. Eur Ann Allergy Clin Immunol. 2006;38:355–360. [PubMed] [Google Scholar]
- 97.Calderón M, Essendrop M. Specific immunotherapy with high dose SO standardized grass allergen tablets was safe and well tolerated. J Investig Allergol Clin Immunol. 2006;16:338–344. [PubMed] [Google Scholar]
- 98.Palma-Carlos AG, Santos AS, Branco-Ferreira M, et al. Clinical efficacy and safety of preseasonal sublingual immunotherapy with grass pollen carbamylated allergoid in rhinitic patients. A double-blind, placebo-controlled study. Allergol Immunopathol (Madr) 2006;34:194–198. doi: 10.1157/13094026. [DOI] [PubMed] [Google Scholar]
- 99.Valovirta E, Jacobsen L, Ljørring C, et al. Clinical efficacy and safety of sublingual immunotherapy with tree pollen extract in children. Allergy. 2006 doi: 10.1111/j.1398-9995.2006.01190.x. [DOI] [PubMed] [Google Scholar]
- 100.Larsen TH, Poulsen LK, Melac M, et al. Safety and tolerability of grass pollen tablets in sublingual immunotherapy-a phase-1 study. Allergy. 2006;61:1173–1176. doi: 10.1111/j.1398-9995.2006.01203.x. [DOI] [PubMed] [Google Scholar]
- 101.Dahl R, Kapp A, Colombo G, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;118:434–440. doi: 10.1016/j.jaci.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 102.Malling HJ, Lund L, Ipsen H, Poulsen L. Safety and immunological changes during sublingual immunotherapy with standardized quality grass allergen tablets. J Investig Allergol Clin Immunol. 2006;16:162–168. [PubMed] [Google Scholar]
- 103.Durham SR, Yang WH, Pedersen MR, et al. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–809. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 104.Kleine-Tebbe J, Ribel M, Herold DA. Safety of a SQ-standardised grass allergen tablet for sublingual immunotherapy: a randomized, placebo-controlled trial. Allergy. 2006;61:181–184. doi: 10.1111/j.1398-9995.2006.00959.x. [DOI] [PubMed] [Google Scholar]
- 105.Bowen T, Greenbaum J, Charbonneau Y, et al. Canadian trial of sublingual swallow immunotherapy for ragweed rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2004:93425–93430. doi: 10.1016/S1081-1206(10)61408-1. [DOI] [PubMed] [Google Scholar]
- 106.Rolinck-Werninghaus C, Wolf H, Liebke C, et al. A prospective, randomized, double-blind, placebo-controlled multi-centre study on the efficacy and safety of sublingual immunotherapy (SLIT) in children with seasonal allergic rhinoconjunctivitis to grass pollen. Allergy. 2004;59:1285–1293. doi: 10.1111/j.1398-9995.2004.00627.x. [DOI] [PubMed] [Google Scholar]
- 107.Smith H, White P, Annila I, et al. Randomized controlled trial of high-dose sublingual immunotherapy to treat seasonal allergic rhinitis. J Allergy Clin Immunol. 2004;114:831–837. doi: 10.1016/j.jaci.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 108.Pajno GB, Passalacqua G, Vita D, et al. Sublingual immunotherapy abrogates seasonal bronchial hyperresponsiveness in children with Parietaria-induced respiratory allergy: a randomized controlled trial. Allergy. 2004;59:883–887. doi: 10.1111/j.1398-9995.2004.00578.x. [DOI] [PubMed] [Google Scholar]
- 109.Bufe A, Ziegler-Kirbach E, Stoeckmann E, et al. Efficacy of sublingual swallow immunotherapy in children with severe grass pollen allergic symptoms: a double-blind placebo-controlled study. Allergy. 2004;59:498–504. doi: 10.1111/j.1398-9995.2004.00457.x. [DOI] [PubMed] [Google Scholar]
- 110.Tonnel AB, Scherpereel A, Douay B, et al. Allergic rhinitis due to house dust mites: evaluation of the efficacy of specific sublingual immunotherapy. Allergy. 2004;59:491–497. doi: 10.1111/j.1398-9995.2004.00456.x. [DOI] [PubMed] [Google Scholar]
- 111.Khinchi MS, Poulsen LK, Carat F, et al. Clinical efficacy of sublingual and subcutaneous birch pollen allergen-specific immunotherapy: a randomized, placebo-controlled, double-blind, double-dummy study. Allergy. 2004;59:45–53. doi: 10.1046/j.1398-9995.2003.00387.x. [DOI] [PubMed] [Google Scholar]
- 112.Pajno GB, Vita D, Parmiani S, et al. Impact of sublingual immunotherapy on seasonal asthma and skin reactivity in children allergic to Parietaria pollen treated with inhaled fluticasone propionate. Clin Exp Allergy. 2003;33:1641–1647. doi: 10.1111/j.1365-2222.2003.01809.x. [DOI] [PubMed] [Google Scholar]
- 113.Wüthrich B, Bucher CH, Jörg W, et al. Double-blind, placebo-controlled study with sublingual immunotherapy in children with seasonal allergic rhinitis to grass pollen. J Investig Allergol Clin Immunol. 2003;13:145–148. [PubMed] [Google Scholar]
- 114.André C, Perrin-Fayolle M, Grosclaude M, et al. A double–blind placebo-controlled evaluation of sublingual immunotherapy with a standardized ragweed extract in patients with seasonal rhinitis. Evidence for a dose-response relationship. Int Arch Allergy Immunol. 2003;131:111–118. doi: 10.1159/000070926. [DOI] [PubMed] [Google Scholar]
- 115.Grosclaude M, Bouillot P, Alt R, et al. Safety of various dosage regimens during induction of sublingual immunotherapy. A preliminary study. Int Arch Allergy Immunol. 2002;129:248–253. doi: 10.1159/000066779. [DOI] [PubMed] [Google Scholar]
- 116.Lima MT, Wilson D, Pitkin L, et al. Grass pollen sublingual immunotherapy for seasonal rhinoconjunctivitis: a randomized controlled trial. Clin Exp Allergy. 2002;32:507–14. doi: 10.1046/j.0954-7894.2002.01327.x. [DOI] [PubMed] [Google Scholar]
- 117.Ariano R, Spadolini I, Panzani RC. Efficacy of sublingual specific immunotherapy in Cupressaceae allergy using an extract of Cupressus arizonica. A double blind study. Allergol Immunopathol (Madr) 2001;29:238–244. doi: 10.1046/j.0954-7894.2002.01327.x. [DOI] [PubMed] [Google Scholar]
- 118.Pajno GB, Morabito L, Barberio G, Parmiani S. Clinical and immunologic effects of long-term sublingual immunotherapy in asthmatic children sensitized to mites: a double-blind, placebo-controlled study. Allergy. 2000;55:842–849. doi: 10.1034/j.1398-9995.2000.00495.x. [DOI] [PubMed] [Google Scholar]
- 119.Yuksel H, Tanac R, Gousseinov A, Demir E. Sublingual immunotherapy and influence on urinary leukotrienes in seasonal pediatric allergy. J Investig Allergol Clin Immunol. 1999;9:305–313. [PubMed] [Google Scholar]
- 120.Passalacqua G, Albano M, Riccio A, et al. Clinical and immunologic effects of a rush sublingual immunotherapy to Parietaria species: a double-blind, placebo-controlled trial. J Allergy Clin Immunol. 1999;104:964–968. doi: 10.1016/S0091-6749(99)70076-X. [DOI] [PubMed] [Google Scholar]
- 121.Purello-D’Ambrosio F, Gangemi S, Isola S, et al. Sublingual immunotherapy: a double-blind, placebo-controlled trial with Parietaria judaica extract standardized in mass units in patients with rhinoconjunctivitis, asthma, or both. Allergy. 1999;54:968–973. doi: 10.1034/j.1398-9995.1999.00203.x. [DOI] [PubMed] [Google Scholar]
- 122.La Rosa M, Ranno C, André C, et al. Double-blind placebo-controlled evaluation of sublingual-swallow immunotherapy with standardized Parietaria judaica extract in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 1999;104:425–432. doi: 10.1016/S0091-6749(99)70388-X. [DOI] [PubMed] [Google Scholar]
- 123.Bousquet J, Scheinmann P, Guinnepain MT, et al. Sublingual-swallow immunotherapy (SLIT) in patients with asthma due to house-dust mites: a double-blind, placebo-controlled study. Allergy. 1999;54:249–260. doi: 10.1034/j.1398-9995.1999.00916.x. [DOI] [PubMed] [Google Scholar]
- 124.Hordijk GJ, Antvelink JB, Luwema RA. Sublingual immunotherapy with a standardised grass pollen extract; a double-blind placebo-controlled study. Allergol Immunopathol (Madr) 1998;26:234–240. [PubMed] [Google Scholar]
- 125.Vourdas D, Syrigou E, Potamianou P, et al. Double-blind, placebo-controlled evaluation of sublingual immunotherapy with standardized olive pollen extract in pediatric patients with allergic rhinoconjunctivitis and mild asthma due to olive pollen sensitization. Allergy. 1998;53:662–672. doi: 10.1111/j.1398-9995.1998.tb03952.x. [DOI] [PubMed] [Google Scholar]
- 126.Horak F, Stübner P, Berger UE, et al. Immunotherapy with sublingual birch pollen extract. A short-term double-blind placebo study. J Investig Allergol Clin Immunol. 1998;8:165–171. [PubMed] [Google Scholar]
- 127.Clavel R, Bousquet J, André C. Clinical efficacy of sublingual-swallow immunotherapy: a double-blind, placebo-controlled trial of a standardized five-grass-pollen extract in rhinitis. Allergy. 1998;53:493–498. doi: 10.1111/j.1398-9995.1998.tb04086.x. [DOI] [PubMed] [Google Scholar]
- 128.Hirsch T, Sähn M, Leupold W. Double-blind placebo-controlled study of sublingual immunotherapy with house dust mite extract (D.pt.) in children. Pediatr Allergy Immunol. 1997;8:21–27. doi: 10.1111/j.1399-3038.1997.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 129.Feliziani V, Lattuada G, Parmiani S, Dall’Aglio PP. Safety and efficacy of sublingual rush immunotherapy with grass allergen extracts. A double blind study. Allergol Immunopathol (Madr) 1995;23:224–230. [PubMed] [Google Scholar]
- 130.Troise C, Voltolini S, Canessa A, et al. Sublingual immunotherapy in Parietaria pollen-induced rhinitis: a double-blind study. J Investig Allergol Clin Immunol. 1995;5:25–30. [PubMed] [Google Scholar]
- 131.Sabbah A, Hassoun S, Le Sellin J, et al. A double-blind, placebo-controlled trial by the sublingual route of immunotherapy with a standardized grass pollen extract. Allergy. 1994;49:309–313. doi: 10.1111/j.1398-9995.1994.tb02273.x. [DOI] [PubMed] [Google Scholar]
- 132.Scadding GK, Brostoff J. Low dose sublingual therapy in patients with allergic rhinitis due to house dust mite. Clin Allergy. 1986;16:483–491. doi: 10.1111/j.1365-2222.1986.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 133.Bernstein DI, Bardelas JA, Jr, Svanholm Fogh B, et al. A practical guide to the sublingual immunotherapy tablet adverse event profile: implications for clinical practice. Postgrad Med. 2017;129:590–597. doi: 10.1080/00325481.2017.1302306. [DOI] [PubMed] [Google Scholar]
- 134.Larenas-Linnemann DE, Mösges R. Dosing of European sublingual immunotherapy maintenance solutions relative to monthly recommended dosing of subcutaneous immunotherapy. Allergy Asthma Proc. 2016;37:50–56. doi: 10.2500/aap.2016.37.3907. [DOI] [PubMed] [Google Scholar]
- 135.Passalacqua G, Sastre J, Pfaar O, et al. Comparison of allergenic extracts from different origins: the value of the FDA’s bioequivalent allergy unit (BAU) Expert Rev Clin Immunol. 2016;12:733–739. doi: 10.1080/1744666X.2016.1187561. [DOI] [PubMed] [Google Scholar]
- 136.Demoly P, Calderon MA, Casale TB, et al. The value of pre- and co-seasonal sublingual immunotherapy in pollen-induced allergic rhinoconjunctivitis. Clin Transl Allergy. 2015;5:18. doi: 10.1186/s13601-015-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sabate E. Adherence to long-term therapies: evidence for action. [Accessed July 7, 2018]. http://www.who.int/chp/knowledge/publications/adherence_report/en/ [PubMed]
- 138.Suárez-Fueyo A, Ramos T, Galán A, et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. J Allergy Clin Immunol. 2014;133:130–138.e1–2. doi: 10.1016/j.jaci.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 139.Varona R, Ramos T, Escribese MM, et al. Persistent regulatory T cell response 2 years after 3 years of grass tablet SLIT: links to reduced eosinophil counts, sIgE levels and clinical benefit. Allergy. 2018 doi: 10.1111/all.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]