Abstract
Race, ethnicity, sex, and age are demographic factors that can influence drug exposure and/or response, and can consequently affect treatment outcome. We evaluated demographic subgroup enrollment patterns in new therapeutic products approved by the US Food and Drug Administration (FDA) for the treatment of select cancers—breast, colorectal, lung, and prostate—that have comparative differences in morbidity and/or mortality among some demographic subgroups. In submissions of products approved between 2008 and 2013, participants (n = 22,481) were white (80%), from outside the United States (74%), between 17 and 64 years old (59%), and men (56% and 53%, including and excluding sex‐specific indications, respectively). In pivotal trials of products approved between2014 and 2017, participants (n = 3,612) were white (71%), between 17 and 64 years old (61%), and men (48% and 63%, including and excluding sex‐specific indications, respectively). The US‐relevant minority populations were under‐represented. A broader representation of patient subgroups in clinical trials may contribute to better understanding of exposure and/or response variability, and consequently help personalize drug therapy.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑Demographic factors, such as age, race, ethnicity, and sex, can affect exposure and/or response to drugs, and consequently affect treatment outcome. Some demographic subgroups are often under‐represented in clinical trials. However, limited data are publicly available on the enrollment of different demographic subgroups across various phases of clinical trials that support drug development and approval.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑This analysis characterizes the extent of demographic subgroup enrollment of participants in phase I–III trials for therapeutic products approved to treat cancers with known subgroup differences in morbidity and/or mortality—breast, colorectal, lung, and prostate cancers. Previously reported data on this topic focused on later phase trials.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑Many of the traditionally under‐represented demographic subgroups, including US‐relevant minority populations and, particularly, women from minority communities were consistently under‐represented compared to their corresponding disease morbidity and/or mortality.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑As drug development is becoming increasingly global, a broader representation of US‐relevant patient subgroups in clinical trials will help personalize drug therapy for patients who will receive the product after approval in the United States.
Demographic factors, such as race, ethnicity, sex, and age, can affect exposure and/or response to drugs, and consequently affect treatment outcome. For example, differences among racial and ethnic groups in drug metabolizing enzymes and transporters can result in variability in pharmacokinetics (PK) and consequently lead to differences in safety and/or efficacy.1, 2, 3, 4 Similarly, sex‐based differences in PK and/or pharmacodynamics (PD) can lead to differences in safety and/or efficacy.4, 5 Also, age‐related physiological changes can lead to differences in PK and/or PD leading to differences in safety and/or efficacy across the entire age spectrum.6, 7 Consequently, lack of data from diverse populations could potentially lead to the overlooked differences in disease biology or even safety signals.8
These demographic factors that affect treatment outcome are especially important to characterize for diseases, such as cancer, that disproportionately affect different demographic subgroups. Cancer is the second leading cause of death in the United States, and is a source of health disparity as the incidence of various cancers and survival rates differ among demographic subgroups.9, 10, 11, 12, 13, 14, 15, 16 Given these differences, inclusion of adequate numbers of patients from various demographic subgroups would enhance the ability to detect variations in exposure and/or response to treatment.
The US Food and Drug Administration (FDA) reviews demographic information and subgroup analyses during its regulatory review of investigational new drug (IND) applications, new drug applications (NDAs), and biologic license applications (BLAs). In order to do that, the FDA recommends that the sponsors or applicants (e.g., biopharmaceutical companies) submit: (i) data on enrollment of demographic subgroups in the annual reports for the investigational new drugs and (ii) analyses of safety and effectiveness data by demographic subgroups in the NDAs and BLAs.17, 18 The FDA's regulatory focus is to ensure that the drug provides benefits that outweigh its known and potential risks for the intended population; however, there is no statutory or regulatory requirement to include different demographic subgroups or specific numbers of subjects within each subgroup in clinical trials. Inclusion of diverse demographic subgroups in the clinical trials that support therapeutic product marketing applications aids in the evaluation of safety and efficacy in population subgroups that are likely to use the drug after its approval. It is important to understand variability among the different demographic subgroups and to communicate that information to prescribers (e.g., via product labeling).
Racial/ethnic minorities, women, and the elderly are often under‐represented in clinical studies in general as well as in clinical trials supporting therapeutic product development.8, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 The participation of demographic subgroups in clinical trials submitted to support new molecular entity (NME) applications has been periodically studied by the FDA, Government Accountability Office, and other groups and organizations.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 In the pivotal clinical trials that support a therapeutic product's approval, many demographic subgroups, including racial/ethnic minorities and the elderly, as well as women in some therapeutic areas, continue to be under‐represented compared to their corresponding disease prevalence. Although information on demographic subgroups in National Institutes of Health‐funded studies as well as information from clinical trials for FDA approval have been published by many authors, limited data are publicly available27 on the enrollment of different demographic subgroups across various phases (I–III) of clinical trials that support drug development, especially in the early phases of therapeutic product development.
In this retrospective, descriptive analysis, we evaluated data from phase I, II, and III trials included in NDAs and BLAs that were approved by the Center for Drug Evaluation and Research (CDER), FDA between 2008 and 2013 to treat patients with four cancers for which differences in morbidity and mortality has been reported for some demographic subgroups—breast, colorectal, lung, and prostate cancers. In order to evaluate the potential temporal effects, we further analyzed data from key trials for products approved for these cancers between 2014 and 2017 that are available in Drug Trials Snapshots (DTS).33 Product labeling was reviewed to identify PK, safety, and efficacy information related to the demographic subgroups. Additionally, approval letters and postmarketing study databases were reviewed to identify if any additional postmarketing studies were issued to the applicants to address demographic subgroup related concerns that existed at the time of drug approval.
Methods
Drugs approved
Twelve original NME applications (i.e., NDAs or BLAs) approved by CDER, FDA between January 2008 and December 2013 to treat patients with breast, colorectal, lung, or prostate cancers were identified using Drugs@FDA.34 Additionally, seven NMEs that were approved between May 2014 and June 2017 had data available in DTS.33 This analysis excludes imaging agents (e.g., fluciclovine F18 for prostate cancer) and supplemental applications for new indications of already approved products. Product labeling and FDA reviews were extracted from Drugs@FDA.
Demographic data collection
For the 12 NMEs approved between January 2008 and December 2013
Individual subject‐level demographic data on geographic location, race, ethnicity, sex, and age were collected. This includes data from phase I–III trials submitted by the applicant in the NDA or BLA, including trials beyond the relevant cancer indication (e.g., trials in solid tumors or ovarian cancer for drugs approved to treat breast cancer) and trials and/or subjects not reviewed by the FDA to support product approval. We excluded certain studies like rollover or extension trials and expanded access programs.
Data were extracted from applicant‐submitted final clinical study reports for trial name, trial phase, total enrollment, geographic location, and demographic subgroup enrollment by race, ethnicity, sex, and age. To analyze geographic location (i.e., United States (USA) vs. outside the United States (EX‐USA)), information on the country where the clinical trial was conducted was collected. To analyze data by phase, information on the phase of the clinical trial was collected (i.e., phases I, II, and III). For this analysis, phase I/II trials were coded as phase I trials and phase II/III trials were coded as phase II.
To analyze race, individual subject‐level data were collected. Race information was captured using categories with definitions adapted from the 2005 FDA guidance on race and ethnicity data collection—American Indian or Alaska Native (AI‐AN), Asian, Black or African American, Native Hawaiian or Other Pacific Islander (NH‐OPI), and white. Individuals who reported ≥2 races were categorized as “multiracial.” The race was marked “unknown/not reported” when information was not available. In a number of trials, AI‐AN, NH‐OPI, multiracial, other races, and Hispanics (ethnic category) were combined together by the applicants as “Others.” The following are some additional terms used to describe race if they were captured in the clinical trials differently from the racial and ethnic categories described above: whites also included Caucasians, Asians also included Orientals or Asian‐Pacific Islanders, and American Indian‐Alaska Natives also included Native Americans.
To analyze ethnicity, information on “Hispanic or Latino” and “Non‐Hispanic or Latino” categories was collected. For this analysis, if Hispanic or Latino was included as race instead of ethnicity, the individual's race was marked as unknown and ethnicity as Hispanic or Latino. In some trials, Hispanics or Latinos may have been included by the applicant as “Others” in race category.
Individual subject‐level data on sex (female or male) were collected. Individual subject level data on age were collected and participants were categorized as pediatrics (≤16 years), adults (≥17 to ≤64 years), or geriatrics (≥65 years). Data on sex and age were not available for 1 and 148 subjects, respectively (excluded from further analysis of the corresponding demographic factor).
For the seven NMEs approved between May 2014 and June 2017
The DTS33 was used to collect aggregated demographic data on sex, race, and age from pivotal clinical trials that supported the FDA approval of these NMEs. The data were analyzed separately as information was available from the website for only the pivotal trials and was not available as individual subject level data. Additionally, information on geographic location was not available. Data on sex and age were not available for 2 and 81 subjects, respectively (excluded from further analysis of the corresponding demographic factor).
Postmarketing studies
Race, ethnicity, sex, and age subgroup‐related postmarketing studies that were issued to the applicants at the time of drug approval and their current status were identified using the approval letters from Drugs@FDA34 and the Post‐marketing Study and Clinical Trial Requirements and Commitments Database (updated through May 3, 2017).35
Demographic data analysis
Analysis was performed using SAS version 9.3 and JMP version 11.1.1 (SAS Institute Inc., Cary, NC) to identify the enrollment of participants from different demographic subgroups in phase I, II, and III trials included in NDAs and BLAs that were approved by CDER, FDA between 2008 and 2013 as well as pivotal and supportive trials included in NDAs and BLAs that were approved by CDER, FDA between 2014 and 2017 to treat patients with four types of cancers. Enrollment by geographic location, race, ethnicity, sex, and age group was calculated as a percentage of the total participants for whom the corresponding demographic information was available.
Results
Individual subject‐level demographic information was available for 22,481 individuals enrolled in 158 clinical trials for 12 therapeutic products approved to treat patients with breast, colorectal, lung, or prostate cancers between January 2008 and December 2013 (Data S1 ). Aggregated demographic information was available for 3,612 individuals enrolled in 9 clinical trials for 7 approved therapeutic products between May 2014 and June 2017 that are included in the DTS (Data S1 ). Information from the approved product labeling on the demographic subgroups included in this analysis is provided in Data S2 .
For the 12 NMEs approved between January 2008 and December 2013 with individual subject‐level data available
Geographic location of clinical trials
The United States enrolled the largest number of participants for any individual country (26% overall; range among cancers, 15–31%; Figure 1 and Data S3 ). The remaining 74% (range among cancers, 69–85%) were enrolled outside the United States from about 60 countries (Data S3 ). In comparing enrollment by the phase of clinical trials, phase I trials enrolled a higher proportion of participants from the United States (range among cancers, 31–56%) relative to phase III trials that enrolled a lower proportion of participants from the United States (range among cancers, 9–24%; Data S4 ).
Figure 1.
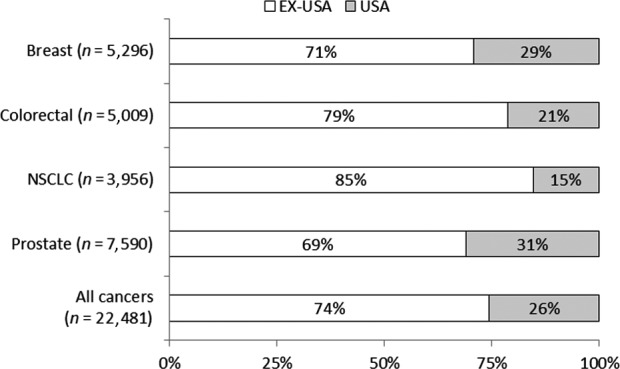
Geographic location of clinical trials for select oncology drugs approved between 2008 and 2013. Includes subject‐level data from phase I–III trials for select oncology drugs approved between 2008 and 2013. EX‐USA includes subjects enrolled in clinical trial sites outside of the United States. NSCLC, non‐small cell lung cancer.
Participation by race
Overall, the majority of the participants were white (80%), followed by Asians (12%), and Blacks/African Americans (4%), with variability across cancer types (Table 1 and Figure 2). Of the 158 trials included in this analysis, 22% of the trials enrolled only whites (34 trials exclusively enrolled 1,138 whites) and no whites were enrolled in 5% of trials (8 trials exclusively enrolled 263 Asians or Asian‐Pacific Islanders). Of note, enrollment of Asians (from outside the United States) increased over time in the drug development program targeting patients with epidermal growth factor receptor (EGFR) mutation‐positive non‐small cell lung cancer (NSCLC; Figure 3).
Table 1.
Demographic subgroup composition of clinical trial participants for select oncology drugs approved between 2008 and 2013
| Demographic subgroup | % Participants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | Colorectal cancer | NSCLC | Prostate cancer | All cancers | ||||||
| US (n = 1,731) | All (n = 5,926) | US (n = 1,065) | All (n = 5,009) | US (n = 609) | All (n = 3,956) | US (n = 2,355) | All (n = 7,590) | US (n = 5,760) | All (n = 22,481) | |
| Race | ||||||||||
| White | 82.4 | 78.7 | 79.3 | 86.6 | 82.6 | 52 | 82.3 | 90.3 | 81.8 | 79.7 |
| Black | 8.8 | 4 | 11.2 | 3.4 | 7.7 | 1.9 | 13.4 | 4.9 | 11 | 3.8 |
| Asian | 2.5 | 12.2 | 3.3 | 7.3 | 6.2 | 38.6 | 1.2 | 2.2 | 3 | 12.4 |
| AI‐AN | 0.4 | 0.7 | 0.4 | 0.1 | 0.8 | 0.5 | 0.5 | 0.9 | 0.5 | 0.6 |
| NH‐OPI | 0.4 | 0.2 | 0.1 | 0.04 | 0.2 | 0.1 | ||||
| Other | 1.5 | 0.8 | 2.4 | 0.9 | 0.7 | 1.1 | 1.3 | 1.1 | 1.5 | 1 |
| Multi | 0.1 | 0.5 | 0.1 | 0.1 | 0.2 | 0.03 | 0.04 | 0.1 | 0.1 | 0.2 |
| Ethnicitya | ||||||||||
| Hispanic | 6.4 | 5.7 | 6.5 | 1.9 | 3.9 | 1.7 | 7.7 | 4.1 | 6.7 | 3.6 |
| Non‐Hispanic | 32.7 | 30.8 | 26.1 | 20 | 27.6 | 17.9 | 71.5 | 56.8 | 46.8 | 34.9 |
| Sexb | ||||||||||
| Female | 88 | 92.8 | 45.4 | 43.3 | 46.3 | 51.4 | 1 | 2.1 | 40.2 | 43.9 |
| Male | 12 | 7.2 | 54.6 | 56.7 | 53.7 | 48.6 | 99 | 97.9 | 59.8 | 56.1 |
| Ageb | ||||||||||
| 17–64 years | 74.7 | 79.8 | 66.9 | 67.4 | 76.2 | 69.3 | 38.9 | 32.7 | 58.8 | 59.4 |
| ≥65 years | 25.3 | 20.2 | 33.1 | 32.6 | 23.8 | 30.7 | 61.1 | 67.3 | 41.2 | 40.6 |
AI‐AN, American Indian or Native Alaskan; Multi, multiracial; NH‐OPI, Native Hawaiian or Other Pacific Islander; NSCLC, non‐small cell lung cancer.
aSee METHODS for details on data collection. bSex and age information missing for 1 and 148 subjects, respectively (these subjects were excluded in respective demographic subgroup analysis).
Figure 2.
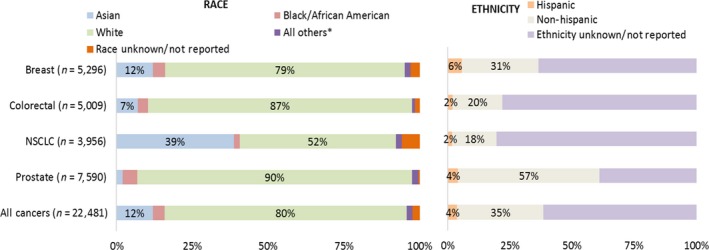
Enrollment by race and ethnicity in trials for select oncology drugs approved between 2008 and 2013. Includes subject‐level data from phase I–III trials for select oncology drugs approved between 2008 and 2013. *All Others includes individuals classified as Others, American Indian or Alaska Native, Multiracial, and Native Hawaiian or Other Pacific Islander. See METHODS for details on Ethnicity Unknown/Not reported. NSCLC, non‐small cell lung cancer.
Figure 3.
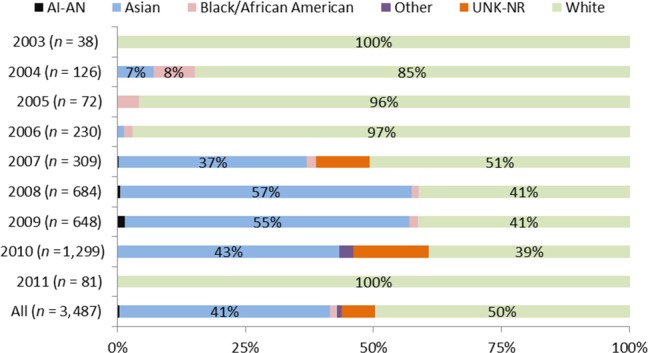
Enrollment by race in the drug targeting specific epidermal growth factor receptor mutations approved between 2008 and 2013. Includes subject level data from phase I–III trials for non‐small cell lung cancer (NSCLC) drugs approved between 2008 and 2013. AI‐AN, American Indian or Alaska Native; UNK‐NR, unknown/not reported.
Within the United States (n = 5,760), 82% were whites, and the racial minorities enrolled were Black/African American (11%), Asian‐American (3%), AI‐AN (0.5%), NH‐OPI (0.2%), and multiracial (0.1%) groups. Data S4 and S5 contain additional information on enrollment by race across trial phase and geographic location.
Participation by ethnicity
Overall, Hispanics represented 4% of the trial participants, ranging from 2% for colorectal cancer and NSCLC to 6% for breast cancer (Table 1 and Figure 2). Because of the way ethnicity information was collected and analyzed, about 62% of the participants did not have ethnicity information, thus limiting the interpretation of the results (see METHODS and DISCUSSIONS sections for additional details).
Within the United States (n = 5,760), Hispanics represented 7% of the participants (range among cancers, 4–8%). Data S4 and S6 contain additional information on enrollment by ethnicities across trial phase and geographic location.
Participation by sex
Overall, 44% of the participants were women, increasing from 2% for prostate (although prostate cancer is sex‐specific, both men and women were enrolled in exploratory early‐phase trials for other cancers), 43% for colorectal, 51% for lung, and 93% for breast cancers (although breast cancer predominantly affects women, both men and women were enrolled in exploratory early phase trials; Figure 4 and Table 1). Additional data on enrollment by sex across trial phase, race, and ethnicity are in Data S4 and S7 .
Figure 4.
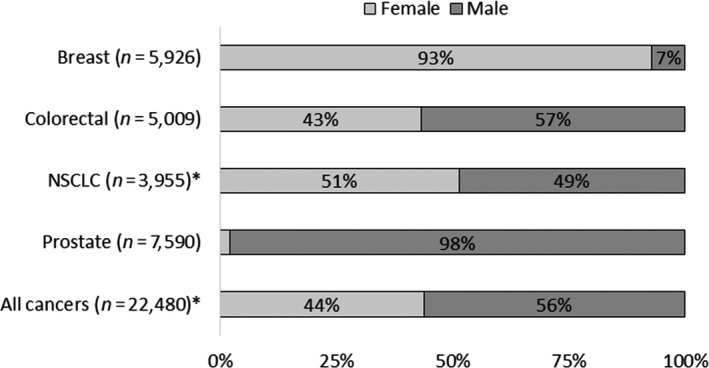
Enrollment by sex in trials for select oncology drugs approved between 2008 and 2013. Includes subject‐level data from phase I–III trials for select oncology drugs approved between 2008 and 2013. *Sex data missing for 1 subject (excluded from analysis). See METHODS for additional details. NSCLC, non‐small cell lung cancer.
Participation by age subgroups
Overall, 59% of the participants were in the 18–64 years age group and 41% in the ≥65 years age group, including 13% who were ≥75 years (Figure 5 and Table 1). Although average age was variable by cancer type and phase, phase I participants tended to be younger than phase III participants—53, 51, and 54 years vs. 60, 59, and 69 years for colorectal, NSCLC, and prostate cancers, respectively (Data S8 ). For breast cancer, the trend was in the opposite direction—on average, phase I participants tended to be older than phase III participants—61 vs. 54 years, respectively.
Figure 5.

Enrollment by age groups in trials for select oncology drugs approved between 2008 and 2013. Includes subject‐level data from phase I–III trials for select oncology drugs approved between 2008 and 2013. See METHODS for additional details. *Age information missing for a total of 148 subjects (excluded from analysis). NSCLC, non‐small cell lung cancer.
For the seven NMEs approved between May 2014 and June 2017 with data from the DTS
Aggregated results from the DTS for products approved to treat three of the four cancers (breast, colorectal, and NSCLC) between May 2014 and June 2017 are included in Table 2. Overall, 71% were white, 22% were Asian, and 1% were Black/African American. Of note, relatively high enrollment of Asians was observed in key trials for colorectal cancer (35%) and NSCLC (23%). Overall, 39% were over 65 years of age, and women represented 52% and 37% of the participants, including and excluding sex‐specific indications, respectively.
Table 2.
Demographic subgroup composition of clinical trial participants with data from Drug Trials Snapshots for select oncology drugs approved between 2014 and 2017
| Demographic subgroup | % Participants | |||
|---|---|---|---|---|
| Breast cancer (n = 833) | Colorectal cancer (n = 800) | NSCLC (n = 1,979) | All cancers (n = 3,612) | |
| Race | ||||
| White | 84 | 58 | 71 | 71 |
| Black | 2 | 1 | 1 | 1 |
| Asian | 7 | 35 | 23 | 22 |
| AI‐AN | 0.1 | 0.1 | 0.1 | |
| NH‐OPI | 0.1 | 0.1 | 0.1 | |
| Other | 3 | 5 | 3 | |
| Multi | 0.1 | 0.03 | ||
| Sexa | ||||
| Female | 100 | 39 | 37 | 52 |
| Male | 61 | 63 | 48 | |
| Agea | ||||
| 17–64 years | 56 | 56 | 65 | 61 |
| ≥65 years | 44 | 44 | 35 | 39 |
Includes data from clinical trial participants listed in Drug Trials Snapshots from pivotal and supportive trials for non‐small cell lung cancer (NSCLC), colorectal cancer, or breast cancers for select oncology drugs approved between May 2014 and June 2017. Data for race or ethnicity listed as Unknown/Not reported is not included in the Table.
AI‐AN, American Indian or Native Alaskan; Multi, multiracial; NH‐OPI, Native Hawaiian or Other Pacific Islanders.
Sex and age information missing for 2 and 81 subjects, respectively (these subjects were excluded in respective demographic subgroup analysis).
Postmarketing studies
Postmarketing studies related to specific demographic subgroups were not identified for these therapeutic products. Pediatric study requirements were waived as these studies would be impossible or impractical to conduct as the disease does not exist in children or because an orphan drug designation was granted for the indication (i.e., exemption granted).
Discussion
This analysis was undertaken to examine the enrollment of demographic subgroups in clinical trials included in new drug or biologic applications that were approved by the FDA to treat patients with breast, colorectal, lung, and prostate cancers (i.e., cancers with high morbidity and/or mortality in some demographic subgroups). Although the FDA and others periodically report on demographic subgroups included in clinical trials for products approved by the FDA,19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 this analysis is comprehensive in that it is based on subject‐level data from both early‐ and late‐phase clinical trials that were submitted by the applicants for the approval of their products to treat certain cancers between 2008 and 2013. This analysis is supplemented with data from the DTS that includes information from pivotal and supportive trials for products approved between 2014 and 2017 to treat these cancers.
Overall, in submissions of products approved between 2008 and 2013, participants (n = 22,481) were white (80%), from outside the United States (74%), between 17 and 64 years old (59%), and men (53% excluding sex‐specific indications). Some differences and similarities can be observed in data from pivotal trials of products approved between 2014 and 2017, as participants (n = 3,612) were white (71%), between 17 and 64 years old (61%), and men (63% excluding sex‐specific indications). Most notable differences between the 2008–2013 and 2014–2017 analyses include 12% vs. 22% Asians, 4% vs. 1% Blacks/African Americans, and 47% vs. 37% women in non‐sex‐specific indications, respectively. In terms of percentage participation, some United States‐relevant demographic subgroups continue to be under‐represented. For the products included in this analysis, no demography‐specific concerns were identified at the time of product approval leading to differential recommendations in the therapeutic product labeling, or needing specific postmarketing studies.
In the analysis of drugs approved between 2008 and 2013, consistent with the previous observations on globalization of clinical studies,36 a majority (74%) of participants were enrolled from outside of the United States from about 60 countries (Figure 1 and Data S3 ), most of whom were enrolled in the larger phase II and III trials. The extent of globalization varied by phase of clinical trial (Data S4 ) and across drug development programs (data not shown). The US participants represented a larger proportion of phase I trials (range among cancers, 31–56%) as compared to phase III trials (range among cancers, 9–24%). As phase I trials in oncology determine the maximum tolerated dose (MTD) and select the dose to be carried forward to future trials, this has important implications for the subsequent trials and marketed populations. The variability in geographic location between phases may be partially explained by the procedures and implications of such trials. Phase I trials often require specialized centers capable of monitoring and conducting sampling for PK analyses over several days. This level of facility may not be as available or feasible globally. Additionally, incentives may be limited to engage in complex and costly international phase I protocol agreement to enroll patients in a particular country for early candidate drugs (as these may or may not advance further in clinical development). The more extensive international participation in phase III trials supports the evaluation for safety and efficacy across a more internationally diverse population allowing for characterization of safety and efficacy in patients with the intended indication. Applicants are incentivized to include international patients at the phase III stage to support their potential approval and marketing in that country. Globalization of clinical trials also enables global regulatory authorities to assess the efficacy and safety in a diverse range of patients and treatment contexts, and to use the data to support the efficacy and safety within specific countries or regions. As drug development programs are becoming increasingly global, regional differences in clinical trial results are becoming more apparent.37, 38 These regional differences may be attributable to a number of intrinsic and extrinsic factors.1
In this analysis of drugs approved between 2008 and 2013, overall Blacks/African Americans were under‐represented (4%) compared to their disease morbidity and/or mortality. The disease burden in this population has been shown to be higher compared to white patients in several analyses.10, 11, 12, 13, 15, 16, 39 However, US enrollment of Blacks/African Americans (11%) was much higher than the enrollment of Blacks outside the United States (1.3%) (Table 1 , Figure 2 , and Data S4 and S5 ). Additionally, other US minorities, such as AI‐AN, NH‐OPI, and Hispanics/Latinos were also under‐represented (Table 1 , Figures 2 and 3 , and Data [Link] , [Link] , [Link] ) compared to their disease morbidity and/or mortality.10, 11, 12, 13 In the product labeling included in this analysis, no dose modifications were required because of safety and/or efficacy concerns in racial or ethnic subpopulation. In the US‐approved product labeling, certain adverse events were more common in Asians (Data S2 ).
Increasing racial/ethnic diversity in clinical studies would help to identify potential variability in drug exposure and/or response as environmental, genetic, or other biological factors may influence disease susceptibility or progression, as well as drug response. This is especially applicable for cancer therapies because using biomarkers to personalize cancer treatment is gaining traction. Of note, the enrollment of Asians in the NSCLC drug development program (afatinib), increased over time (Figure 3), perhaps coinciding with the discovery of EGFR mutations as an important biomarker predicting response to treatment with certain tyrosine kinase inhibitors (first association was published in 2004 and confirmed over subsequent years).40, 41, 42, 43 The frequency of certain EGFR mutations is higher in Asians (17–66%) than whites (7–17%), whereas limited data are available for Hispanics/Latinos and Blacks/African Americans.44 In drug development programs targeting EGFR, clinical trial enrichment based on molecular characteristics may improve trial sensitivity and ease enrollment without affecting generalizability to the to‐be‐indicated population.
Several decades ago, the FDA guidance had restricted women of child‐bearing potential from enrolling in phase I and early phase II trials. However, this guidance was updated in 1993 to remove this restriction and encouraged the collection of sex‐related data during research and development.45 This guidance also recommended performing PK and PD analyses to detect any sex‐based differences as well as analysis of safety and efficacy data by sex. For drugs approved between 2008 and 2013, women represented 51% and 43% of the trial participants, for non‐sex specific indications (i.e., NSCLC and colorectal cancer), respectively (Figure 4 and Data S4 ). Of note, sex is potentially a prognostic factor for these two cancers.46, 47, 48 These data show that although women were in the minority of phase I trials for the non‐sex‐specific cancers, they were well represented (range among phases 35–79%; Data S4 ). Further, women were the majority of participants in some of the phases (Data S4 ). However, the enrollment of women from minority communities (for example, Black/African American women in breast cancer trials) was low (Data S7 ). For the therapeutic products included in this analysis, no difference in dose based on sex was reported.
With respect to age, higher enrollment of participants over 65 years was observed particularly for prostate cancer but not for breast cancer (Figure 5). In the product labeling included in this analysis, no impact of age on exposure was reported. With respect to safety, several product labeling studies reported that insufficient numbers were available to determine differences based on age, however, in some instances, a higher incidence of certain adverse events were reported (Data S2 ). In clinical studies, concerns such as comorbidities and comedication, have led to lower enrollment of older patients in general.49 Because age is a major risk factor for many cancers, inclusion of older patients, particularly those over 75 years may warrant more attention to better reflect the population likely to use these products outside of typical clinical trials. Although pediatric studies were waived for the therapeutic products included in this analysis, with the passing of the FDA Reauthorization Act in 2017, when an original NDA or BLA is submitted, a pediatric investigation for an adult cancer drug may be required if that drug is directed at a molecular target that is relevant for pediatric cancers.
A number of limitations can affect the interpretation of these results. This analysis includes products approved to treat four specific cancer types and may not be representative of drugs approved for other cancers or for other diseases (e.g., human immunodeficiency virus and hepatitis C virus) with high burden to certain demographic populations. In addition, although some of these products can be considered as precision medicines, this analysis may not be representative of all approved precision medicines, in which trial geography may play an important role (e.g., greater enrollment in certain regions to increase screening efficiency for certain tumor mutations). Racial and ethnic classification are not necessarily based on a scientific definition and has evolved over time (some of the trials included here date back to 1998). In addition, race/ethnicity designations are social construct in the United States, and, therefore, caution should be exercised as data included in this analysis are from global clinical trials (which resulted in missing information for ethnicity). The previous version of the FDA guidance on collection of race and ethnicity data allowed applicants to categorize “Hispanics/Latinos” as either an ethnic or as a race subgroup. This has since been revised in the updated 2016 guidance, which specifies “Hispanics/Latinos” as an ethnic subgroup.18 Because of variability in race categories used across the different trials, accurate numbers may not be available for certain races. For example, in a number of trials, AI‐AN, NH‐OPI, multiracial, other races, were combined together by the applicants as “Others.” Moreover, race/ethnicity information is self‐reported and cannot always be collected because of regional restrictions. In spite of these limitations, it is still possible to make generalizable comparisons for at least the broad racial groups (e.g., whites, Blacks, and Asians) or ethnic groups (e.g., Hispanics and non‐Hispanics) when data are available.3
These results from the phase I–III trials for the four specific cancers are somewhat similar to the results reported for pivotal and supportive trials of FDA‐approved products.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 In clinical trial data submitted to the FDA over the past several years, some demographic subgroups, such as racial/ethnic minorities, have been under‐represented, whereas some demographic subgroups, such as women, have improved representation in general, although some exceptions, such as under‐representation of women, in drug development for cardiovascular diseases remain.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 The FDA continues to address scientific, ethical, and policy issues relating to health of the various population subgroups. Given the concerns on demographic subgroup inclusion, the FDA Safety and Innovation Act Section 907 required the FDA to report on the participation of demographic subgroups in clinical studies supporting new applications for drugs, biologics, and devices.31 The follow‐on Action Plan provided specific action items for the FDA to help address the deficiencies identified in the report through improving data quality, data transparency, and demographic subgroup participation.50 Under‐representation of patients from demographic subgroups within a drug's intended use population can limit the ability to understand and communicate the extent to which response may differ.50 This lessens the information that healthcare providers and patients have available to make decisions about the medical products they use. In addition, as an extension of the transparency effort, the DTS provides information on participation by sex, race, and age in the pivotal and supportive clinical trials (i.e., key trials) that support the approval of new drugs.33 Additionally, the FDA also updated its guidance document “Collection of Race and Ethnicity Data in Clinical Trials” to help clarify the FDA's expectations that applicants submit a plan at the earliest stages of development (no later than the end of phase II) for the inclusion of relevant demographic subgroups and that participants in clinical trials reflect the populations that will ultimately use these medical products, if approved.18
In summary, based on this analysis, with some exceptions, many of the traditionally under‐represented demographic subgroups, including racial/ethnic minorities and particularly women from racial/ethnic minorities, were consistently under‐represented compared to their disease morbidity and/or mortality. Inadequate representation of US‐demographic subgroups in clinical trials could mean that the clinical trial population may not adequately reflect the population that will use the therapeutic products once they are approved. Although drug development is becoming increasingly global, drug development programs in general should aim to recruit a patient population that resembles the population that will ultimately use the drug upon approval. This includes different racial/ethnic groups, both sexes, different age groups, individuals with a wide range of disease severity, and concomitant illnesses. This will help increase the mechanistic understanding of the factors that lead to exposure and/or response variability, and allow for generalizing findings from global clinical trials to a diverse US population, and ultimately personalize drug treatment to improve patient care.
Conflict of Interest
The authors declared no competing interests for this work.
Funding
This project was supported in part by the Office of Minority Health, FDA and an appointment to the Oak Ridge Institute for Science and Education (ORISE) Research Participation Program at the Center for Drug Evaluation and Research administered by the ORISE through an agreement between the US Department of Energy and CDER (A.R. and T.K.).
Author Contributions
A.R., M.P., L.Z., J.B., T.K., C.M., M.M., and H.M. wrote the manuscript. A.R., M.P., L.Z., J.B., and T.K. designed the research. A.R., M.P., L.Z., J.B., and T.K. performed the research. A.R., M.P., L.Z., J.B., and T.K. analyzed the data.
Supporting information
Data S1. Clinical trial database used in the analysis.
Data S2. Demographic information available in the labeling of new molecular entities approved to treat breast, prostate, lung, and colorectal cancers.
Data S3. Enrollment by country in trials for select oncology drugs approved between 2008 and 2013.
Data S4. (a) Figure and (b) table of demographic information of oncology clinical trial participants for select oncology drugs approved between 2008 and 2013.
Data S5. Enrollment by race and geographic location in trials for select oncology drugs approved between 2008 and 2013.
Data S6. Enrollment by ethnicity and geographic location for select oncology drugs approved between 2008 and 2013.
Data S7. Enrollment by sex, race, and ethnicity in clinical trials for select oncology drugs approved between 2008 and 2013.
Data S8. Average age of the participants by phase in clinical trials for select oncology drugs approved between 2008 and 2013.
Acknowledgments
The authors thank ‘Lola Fashoyin‐Aje, Junyang Wang, and John Whyte for their valuable comments. Parts of the results were presented at the 2015 American Society for Clinical Pharmacology and Therapeutics Annual Meeting. The article reflects the views of the authors and should not be construed to represent the FDA's views or policies.
References
- 1. Huang, S.M. & Temple, R. Is this the drug or dose for you? Impact and consideration of ethnic factors in global drug development, regulatory review, and clinical practice. Clin. Pharmacol. Ther. 84, 287–294 (2008). [DOI] [PubMed] [Google Scholar]
- 2. Yasuda, S.U. , Zhang, L. & Huang, S.M. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin. Pharmacol. Ther. 84, 417–423 (2008). [DOI] [PubMed] [Google Scholar]
- 3. Ramamoorthy, A. et al Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin. Pharmacol. Ther. 97, 263–273 (2015). [DOI] [PubMed] [Google Scholar]
- 4. Anderson, G.D. Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J. Womens Health (Larchmt.) 14, 19–29 (2005). [DOI] [PubMed] [Google Scholar]
- 5. Fadiran, E.O. & Zhang, L. Effects of sex differences in the pharmacokinetics of drugs and their impact on the safety of medicines in women In Medicines for Women (ed. Harrison‐Woolrych, M. ) 41–68 (Adis, Cham, 2015). [Google Scholar]
- 6. Mangoni, A.A. & Jackson, S.H. Age‐related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br. J. Clin. Pharmacol. 57, 6–14 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandez, E. et al Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics 3, 53–72 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burchard, E.G. Medical research: missing patients. Nature 513, 301–302 (2014). [DOI] [PubMed] [Google Scholar]
- 9. Torre, L.A. et al Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: converging incidence in males and females. CA Cancer J. Clin. 66, 182–202 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Cancer Society . Cancer facts & figures 2017. <https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/incidence-rates-for-selected-cancers-by-race-and-ethnicity-us-2009-2013.pdf> and <https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/death-rates-for-selected-cancers-by-race-and-ethnicity-us-2010-2014.pdf>.
- 11. National Cancer Institute , Cancer Health Disparities . <https://www.cancer.gov/about-nci/organization/crchd/cancer-health-disparities-fact-sheet>.
- 12. Centers for Disease Control and Prevention . Cancer Prevention and Control—Racial or Ethnic Variations . <http://www.cdc.gov/cancer/dcpc/data/ethnic.htm>. Accessed 22 June 2017.
- 13. National Cancer Institute . SEER fast stats. <http://seer.cancer.gov/faststats/>.
- 14. Heron, M. Deaths: leading causes for 2014. Natl. Vital Stat. Rep. 65, 1–96 (2016). [PubMed] [Google Scholar]
- 15. White, A. et al Colon cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD‐2 study. Cancer 123(suppl. 24), 5014–5036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellis, L. et al Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J. Clin. Oncol. 36, 25–33 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. US Food and Drug Administration . Good review practice: clinical review of investigational new drug applications. <http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatory%20Information/UCM377108.pdf>.
- 18. US Food and Drug Administration . Guidance for industry‐collection of race and ethnicity data in clinical trials. October 2016. <http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm126396.pdf>.
- 19. Merenda, C. Racial/ethnic composition of study participants in FDA‐approved oncology new molecular entities, 2006–2008. J. Natl. Med. Assoc. 104, 430–435 (2012). [DOI] [PubMed] [Google Scholar]
- 20. Wissing, M.D. et al Under‐representation of racial minorities in prostate cancer studies submitted to the US Food and Drug Administration to support potential marketing approval, 1993–2013. Cancer 120, 3025–3032 (2014). [DOI] [PubMed] [Google Scholar]
- 21. Downing, N.S. et al Participation of the elderly, women, and minorities in pivotal trials supporting 2011–2013 U.S. Food and Drug Administration approvals. Trials 17, 199 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poon, R. et al Participation of women and sex analyses in late‐phase clinical trials of new molecular entity drugs and biologics approved by the FDA in 2007–2009. J. Womens Health (Larchmt.) 22, 604–616 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eshera, N. et al Demographics of clinical trials participants in pivotal clinical trials for new molecular entity drugs and biologics approved by FDA from 2010 to 2012. Am. J. Ther. 22, 435–455 (2015). [DOI] [PubMed] [Google Scholar]
- 24. Whyte, J. , Woodcock, J. & Wang, J. Review of the drug trials snapshots program of the US Food and Drug Administration: women in cardiovascular drug trials. JAMA Intern. Med. 177, 724–727 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang, Y. et al Participation of women in clinical trials for new drugs approved by the food and drug administration in 2000–2002. J. Womens Health (Larchmt.) 18, 303–310 (2009). [DOI] [PubMed] [Google Scholar]
- 26. Evelyn, B. et al Participation of racial/ethnic groups in clinical trials and race‐related labeling: a review of new molecular entities approved 1995–1999. J. Natl. Med. Assoc. 93(12 suppl.), 18S–24S (2001). [PMC free article] [PubMed] [Google Scholar]
- 27. Chen, A. et al Representation of women and minorities in clinical trials for new molecular entities and original therapeutic biologics approved by FDA CDER from 2013 to 2015. J. Womens Health (Larchmt.), 27, 418–429 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. US Food and Drug Administration . 2015–2016 global participation in clinical trials report. <https://www.fda.gov/downloads/Drugs/InformationOnDrugs/UCM570195.pdf>.
- 29. Dickmann, L.J. & Schutzman, J.L. Racial and ethnic composition of cancer clinical drug trials: how diverse are we? Oncologist 23, 243–246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knepper, T.C. & McLeod, H.L. When will clinical trials finally reflect diversity? Nature 557, 157–159 (2018). [DOI] [PubMed] [Google Scholar]
- 31. US Food and Drug Administration . Collection, analysis, and availability of demographic subgroup data for FDA‐approved medical products. <http://www.fda.gov/downloads/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/UCM365544.pdf> (2014).
- 32. Oh, S.S. et al Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 12, e1001918 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. US Food and Drug Adminstration . Drug Trials Snapshots. <https://www.fda.gov/Drugs/InformationOnDrugs/ucm412998.htm>.
- 34. US Food and Drug Administration. Drugs@FDA . <https://www.accessdata.fda.gov/scripts/cder/daf/>.
- 35. US Food and Drug Administration. Postmarketing Requirements and Commitments . <https://www.accessdata.fda.gov/scripts/cder/pmc/index.cfm>.
- 36. Drain, P.K. et al Trial watch: global migration of clinical trials. Nat. Rev. Drug Discov. 13, 166–167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glickman, S.W. et al Ethical and scientific implications of the globalization of clinical research. N. Engl. J. Med. 360, 816–823 (2009). [DOI] [PubMed] [Google Scholar]
- 38. Yusuf, S. & Wittes, J. Interpreting geographic variations in results of randomized controlled trials. N. Engl. J. Med. 375, 2263–2271 (2016). [DOI] [PubMed] [Google Scholar]
- 39. Miller, J.W. et al Disparities in breast cancer survival in the United States (2001‐2009): findings from the CONCORD‐2 study. Cancer 123(Suppl 24), 5100–5118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lynch, T.J. et al Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004). [DOI] [PubMed] [Google Scholar]
- 41. Bell, D.W. et al Epidermal growth factor receptor mutations and gene amplification in non‐small‐cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J. Clin. Oncol. 23, 8081–8092 (2005). [DOI] [PubMed] [Google Scholar]
- 42. Janne, P.A. & Johnson, B.E. Effect of epidermal growth factor receptor tyrosine kinase domain mutations on the outcome of patients with non‐small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors. Clin. Cancer Res. 12, 4416s–4420s (2006). [DOI] [PubMed] [Google Scholar]
- 43. Jackman, D.M. et al Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non‐small cell lung cancer patients treated with gefitinib or erlotinib. Clin. Cancer Res. 12, 3908–3914 (2006). [DOI] [PubMed] [Google Scholar]
- 44. El‐Telbany, A. & Ma, P.C. Cancer genes in lung cancer: racial disparities: are there any? Genes Cancer 3, 467–480 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. US Food and Drug Administration . Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs; notice. Fed. Regist. 58, 39406–39416 (1993). [PubMed] [Google Scholar]
- 46. Hsu, L.H. et al Sex‐associated differences in non‐small cell lung cancer in the new era: is gender an independent prognostic factor? Lung Cancer 66, 262–267 (2009). [DOI] [PubMed] [Google Scholar]
- 47. Majek, O. et al Sex differences in colorectal cancer survival: population‐based analysis of 164,996 colorectal cancer patients in Germany. PLoS One 8, e68077 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakamura, H. et al Female gender is an independent prognostic factor in non‐small‐cell lung cancer: a meta‐analysis. Ann. Thorac. Cardiovasc. Surg. 17, 469–480 (2011). [DOI] [PubMed] [Google Scholar]
- 49. Zulman, D.M. et al Examining the evidence: a systematic review of the inclusion and analysis of older adults in randomized controlled trials. J. Gen. Intern. Med. 26, 783–790 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. US Food and Drug Administration . FDA action plan to enhance the collection and availability of demographic subgroup data. <https://www.fda.gov/downloads/regulatoryinformation/legislation/significantamendmentstothefdcact/fdasia/ucm410474.pdf>.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Clinical trial database used in the analysis.
Data S2. Demographic information available in the labeling of new molecular entities approved to treat breast, prostate, lung, and colorectal cancers.
Data S3. Enrollment by country in trials for select oncology drugs approved between 2008 and 2013.
Data S4. (a) Figure and (b) table of demographic information of oncology clinical trial participants for select oncology drugs approved between 2008 and 2013.
Data S5. Enrollment by race and geographic location in trials for select oncology drugs approved between 2008 and 2013.
Data S6. Enrollment by ethnicity and geographic location for select oncology drugs approved between 2008 and 2013.
Data S7. Enrollment by sex, race, and ethnicity in clinical trials for select oncology drugs approved between 2008 and 2013.
Data S8. Average age of the participants by phase in clinical trials for select oncology drugs approved between 2008 and 2013.


