Abstract
Primary focal segmental glomerulosclerosis (FSGS) recurs in up to 55% of patients after kidney transplantation. Herein we report the successful management of recurrent FSGS. A 5‐year‐old boy with primary FSGS received a deceased donor renal transplant. Immediate and fulminant recurrence of FSGS caused anuric graft failure that was resistant to plasmapheresis and rituximab. After exclusion of structural or immunologic damage to the kidney by repeated biopsies, the allograft was retrieved from the first recipient on day 27 and transplanted into a 52‐year‐old second recipient who had vascular nephropathy. Immediately after retransplantation, the allograft regained function with excellent graft function persistent now at 3 years after transplant. After 2 years on hemodialysis, the boy was listed for kidney retransplantation. To prevent FSGS recurrence, pretreatment with ofatumumab was performed. Nephrotic range proteinuria still occurred after the second transplantation, which responded, however, to daily plasma exchange in combination with ofatumumab. At 8 months after kidney retransplantation graft function is good. The clinical course supports the hypothesis of a circulating permeability factor in the pathogenesis of FSGS. Successful ofatumumab pretreatment implicates a key role of B cells. Herein we provide a description of successful management of kidney failure by FSGS, carefully avoiding waste of organs.
Keywords: disease, disease pathogenesis, domino transplantation, kidney disease, recurrent, retransplantation
Short abstract
Successful management of recurrent primary focal segmental glomerulosclerosis after kidney transplantation includes retransplantation of an allograft that failed in the first recipient due to disease recurrence into a second recipient and ofatumumab pretreatment before kidney retransplantation in the patient with fulminant recurrence of focal segmental glomerulosclerosis in the first graft.
Abbreviations
- CLC‐1
cardiotrophin‐like cytokine factor 1
- DCD
donation after circulatory death
- FSGS
focal segmental glomerulosclerosis
- suPAR
soluble urokinase‐type plasminogen activator receptor
1. CASE REPORT
Primary FSGS recurs in up to 55% of patients after kidney transplantation and can lead to early graft loss. The risk for recurrent FSGS is particularly high in patients with idiopathic FSGS, pediatric patients, and patients who show rapid disease progression to end‐stage renal disease. Recurrence rates may exceed 80% in patients who have lost their first transplant due to disease recurrence.1, 2 It is postulated that primary and recurrent FSGS are caused by a circulating factor affecting podocyte structure and function. This hypothesis is supported by experimental findings in isolated rat glomeruli where perfusion with patient plasma caused proteinuria.3 Clinical observations that provide evidence for a circulating permeability factor include a report on the transmission of a permeability factor from mother to child during pregnancy,4 recurrence of the disease within hours after transplantation from a donor without FSGS in about 30%,2 the effect of preemptive plasmapheresis to prevent disease recurrence,5 and the promising results of postoperative plasmapheresis to induce remission of proteinuria in recurrent FSGS.2, 6 The postulated circulating factor has not yet been identified yet. Recent findings suggest a role for cardiotrophin‐like cytokine factor 1 (CLC‐1),3 whereas the experimental findings concerning soluble urokinase‐type plasminogen activator receptor (suPAR) in the pathogenesis of FSGS in humans remain conflicting.6, 7 Here, we report on successful transplantation of a deceased donor renal allograft that failed in the first recipient due to fulminant recurrent primary FSGS and successful kidney retransplantation in the first recipient employing pretreatment with ofatumumab.
A 5‐year‐old boy with end‐stage renal disease caused by primary FSGS (truly idiopathic FSGS) received a deceased donor renal transplant from a 31‐year‐old male donor with excellent renal function. Kidney transplantation was performed through a midline incision, the renal artery and vein were anastomosed to the common iliac vessels, and the ureter was implanted into the bladder, fashioning a ureteroneocystostomy according to Lich‐Grégoire.8 Severe proteinuria required simultaneous bilateral nephrectomy of the boy's native kidneys. Cold ischemia time was 8.8 hours, and anastomosis time was 30 minutes. Immunosuppressive treatment consisted of basiliximab induction therapy and a triple regimen of cyclosporine, mycophenolate mofetil, and prednisone for maintenance therapy. Initial graft function was excellent, with a urinary output of 1000 mL/24 hours, but on posttransplant day 2 the patient developed severe proteinuria (protein/creatinine ratio 32.5 g/g) and urinary output ceased. Graft biopsy excluded acute rejection or severe damage of the graft, indicating early and fulminant recurrence of primary FSGS, electron microscopy showed complete flattening of podocyte foot processes (Figure 1). A series of plasmapheresis (7 sessions) and rituximab twice (300 mg) was not effective (Figure 2). Repeat renal biopsy on day 19 displayed only minimal interstitial fibrosis and tubular atrophy as well as minimal arteriosclerosis and excluded acute rejection and profound damage of the allograft.
Figure 1.
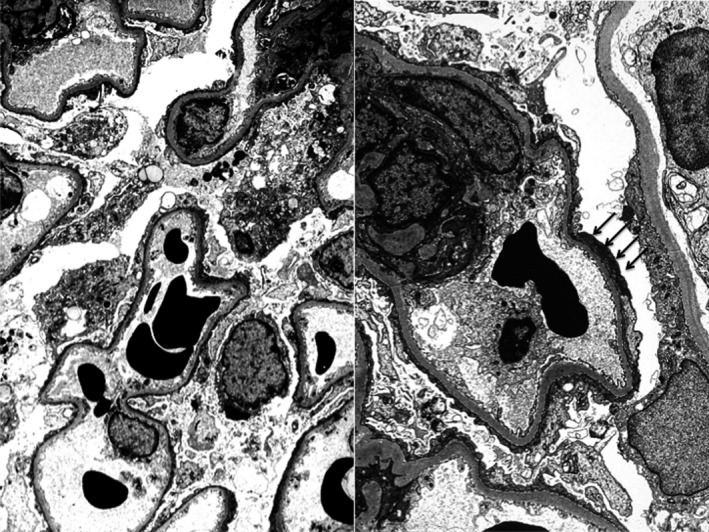
Electron microscopy (4000‐fold) of the allograft on day 19 after transplantation. Complete flattening of podocyte foot processes (black arrows). Minimal change lesion of incipient FSGS
Figure 2.
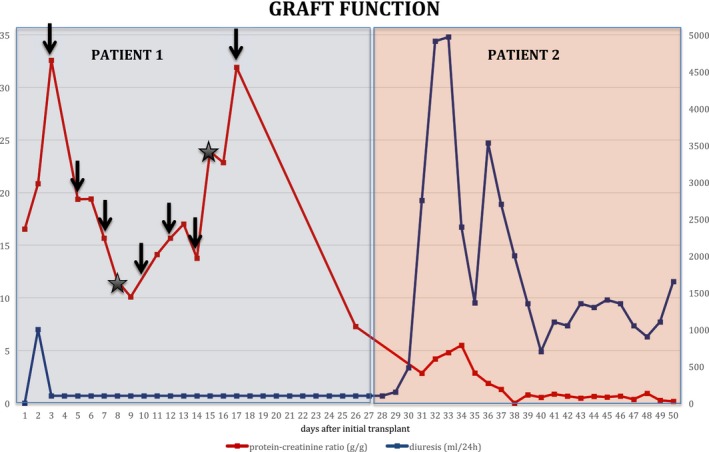
Allograft function in the first recipient and after retransplantation into a second recipient. Renal function is depicted as diuresis (mL/24 hours) and proteinuria (protein‐creatinine ratio g/g) in the first recipient (patient 1) and after retransplantation in the second recipient (patient 2). Patient 1 received 7 sessions of plasmapheresis (black arrows) and 2 sessions of rituximab 300 mg each (black asterisk)
Due to persistent protein loss and complete lack of function despite all therapeutic interventions, the graft was eventually removed. Building on a case reported by Gallon et al,9 the boy's mother was approached about donating the allograft to a second recipient from the kidney transplant waiting list in a nondirected fashion. We chose a 52‐year‐old woman with vascular nephropathy who was on dialysis treatment for 2.5 years. To reduce any confounding factors with respect to allograft function after retransplantation, allocation of the graft to this distinct patient on our center's waiting list was based on the following criteria: identical blood group, first kidney transplant, recipient age over 50 years of age, end‐stage renal disease without relevant proteinuria or risk of disease recurrence, no surgical risk factors (severe atherosclerosis of iliac vessels, obesity, previous abdominal/retroperitoneal surgery), time on dialysis, and HLA match. After obtaining written informed consent from the boy's mother and the second recipient, the graft was removed on day 27 after the original operation and transplanted into the second recipient with a cold ischemia time of 3.75 hours. The second recipient received standard immunosuppressive therapy with basiliximab induction and tacrolimus, mycophenolate mofetil, and prednisone for maintenance treatment. Immediately after retransplantation, the allograft regained function, with a urinary output of 2800 mL/24 hour on day 3. Serum creatinine levels dropped from 4.29 mg/dL to 0.8 mg/dL on day 6, and proteinuria decreased from 2.9 to 0.22 g/g at hospital discharge. The patient did not require dialysis posttransplant (Figure 2), and 3 years after retransplantation, the second recipient continues to have excellent graft function with serum creatinine levels of 0.9 mg/dL and clinically insignificant proteinuria (<0.2 g/g).
Following graft loss due to FSGS recurrence, the boy was put back on dialysis for 2 years. Patients with primary FSGS carry a risk for disease recurrence of more than 80% after repeat transplantation.2, 3 Building on recent reports about the use of ofatumumab in recurrent FSGS posttransplant,10, 11 we hypothesized that pretreatment with ofatumumab before a second kidney transplant could prevent FSGS recurrence. Ofatumumab is a human anti‐CD20 monoclonal antibody that binds to a CD20 epitope different from rituximab and induces complete B‐cell depletion. Greater binding avidity is thought to be responsible for improved complement‐dependent cytotoxicity compared to rituximab.12
Our patient received ofatumumab at a dose of 175 mg/m2 in week 1, followed by 1150 mg/m2 weekly for five infusions. Four months later, the 8‐year‐old boy received a deceased donor renal transplant from a 2‐year‐old DCD (donation after circulatory death) donor (category III). Kidney retransplantation was performed en bloc into the right iliac fossa. The donor's suprarenal and infrarenal aorta was anastomosed in an end‐to‐end fashion to the recipient's external iliac artery; the donor's vena cava was anastomosed in a side‐to‐side fashion to the recipient's external iliac vein (Figure 3). The 2 ureters were joined by a running suture and implanted into the bladder by a single ureteroneocystostomy according to Lich‐Grégoire. Cold ischemia time was 12.25 hours, anastomosis time was 50 minutes. Immunosuppressive treatment consisted of basiliximab induction and cyclosporine, mycophenolate mofetil, and prednisone for maintenance therapy. To prevent FSGS recurrence, plasma exchange was initiated immediately after transplantation.
Figure 3.
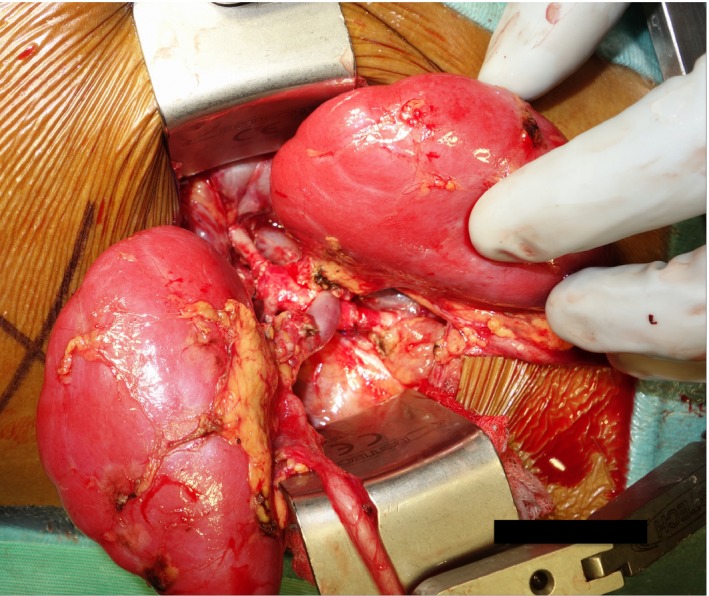
En bloc kidney retransplantation. The donor's suprarenal and infrarenal aorta were anastomosed in an end‐to‐end fashion to the recipient's external iliac artery; the donor's vena cava was anastomosed in a side‐to‐side fashion to the recipient's external iliac vein
Allograft function was excellent, with serum creatinine reaching normal values within 5 days. The patient did not require postoperative hemodialysis treatment. Nevertheless, he developed severe proteinuria (protein/creatinine ratio 58.8 g/g) within the first days, indicating FSGS recurrence. A series of 15 plasma exchanges and administration of ofatumumab at a dose of 1150 mg/m2 on day 33 posttransplant reduced proteinuria to an acceptable level (1.2 g/g). Due to an increase in B lymphocytes 6 months after transplantation and a concomitant rise in urinary protein levels (2.9 g/g), another infusion of ofatumumab at a dose of 1150 mg/m2 was administered. Ofatumumab again reduced proteinuria to subnephrotic levels (1.1 g/g). At 8 months posttransplant the patient has excellent graft function with a serum creatinine of 0.37 mg/dL (Figure 4).
Figure 4.
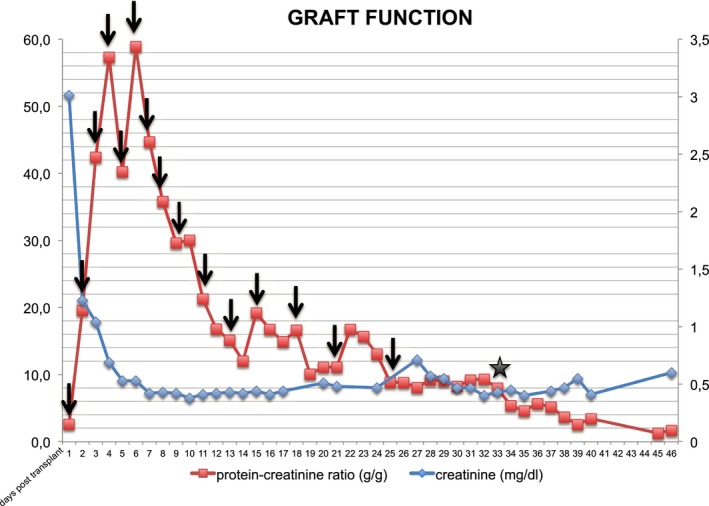
Allograft function of the en bloc kidney retransplant in the 8‐year‐old recipient. Renal function is depicted as serum creatinine (mg/dL) and proteinuria (protein‐creatinine ratio g/g). The patient received 15 sessions of plasma exchange (black arrows) and ofatumumab at a dose of 1150 mg/m2 (black asterisk) on day 33 posttransplant
This report constitutes the second case report of a successful retransplantation of an allograft that failed in the first recipient due to recurrent primary FSGS. In contrast to the case published by Gallon et al9 in 2012, the renal allograft in our case came from a deceased and not a living donor. Furthermore, retransplantation was performed almost 4 weeks after the first transplant as opposed to day 14 in their case. Therefore, our case provides evidence that retransplantation of the failed allograft—given a graft biopsy without pathologic findings—in this specific indication can be applied to organs from deceased donors with the time frame between transplantation and retransplantation of 4 weeks or possibly longer. Furthermore, in an era of organ shortage, retransplantation of an allograft nonfunctioning as a consequence of FSGS recurrence into a second recipient may be a lifeboat procedure for such a constellation and ease the decision to put a patient with a high risk for disease recurrence on the waiting list. Under the same aspect, kidney retransplantation after graft loss for FSGS recurrence is critical in the light of sensitive organ use. Our case report indicates that this may be justified under certain conditions and with according preparation.
We report here a first case of ofatumumab pretreatment for induction of profound B‐cell depletion before kidney retransplantation in a patient with fulminant recurrence of FSGS in the first graft. Ofatumumab has been reported to be effective in the treatment of refractory steroid‐resistant idiopathic nephrotic syndrome.10, 13 Bernard et al11 and Wang et al10 each report a case of successful use of ofatumumab in rituximab‐resistant posttransplant FSGS recurrence, although in these cases ofatumumab was introduced 2.5 years and 8 months posttransplant, respectively. In our case, the patient experienced an early recurrence of FSGS. Ofatumumab in combination with daily plasma exchange reduced proteinuria to subnephrotic levels resulting in excellent graft function with a serum creatinine of 0.37 mg/dL 8 months after kidney retransplantation. The fact that ofatumumab reversed FSGS recurrence supports the hypothesis that the activity and/or secretion of the postulated circulating permeability factor is B‐cell related. Although we cannot rule out that the effect of ofatumumab is transient, this treatment represents a promising strategy for the management of recurrent FSGS. All in all, this case report may extend both a novel treatment option for FSGS recurrence in kidney transplantation and a lifeboat procedure to avoid organ waste in such a high recurrence risk constellation.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Kienzl‐Wagner K, Rosales A, Scheidl S, et al. Successful management of recurrent focal segmental glomerulosclerosis. Am J Transplant. 2018;18:2818–2822. 10.1111/ajt.14998
S.S. and S.W. are senior coauthors of this work.
REFERENCES
- 1. Trachtman R, Sran SS, Trachtman H. Recurrent focal segmental glomerulosclerosis after kidney transplantation. Pediatr Nephrol. 2015;30(10):1793‐1802. [DOI] [PubMed] [Google Scholar]
- 2. Fine RN. Recurrence of nephrotic syndrome/focal segmental glomerulosclerosis following renal transplantation in children. Pediatr Nephrol. 2007;22(4):496‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5(11):2115‐2121. [DOI] [PubMed] [Google Scholar]
- 4. Kemper MJ, Wolf G, Müller‐Wiefel DE. Transmission of glomerular permeability factor from a mother to her child. N Engl J Med. 2001;344(5):386‐387. [DOI] [PubMed] [Google Scholar]
- 5. Gohh RY, Yango AF, Morrissey PE, et al. Preemptive plasmapheresis and recurrence of FSGS in high‐risk renal transplant recipients. Am J Transplant. 2005;5(12):2907‐2912. [DOI] [PubMed] [Google Scholar]
- 6. Davin JC. The glomerular permeability factors in idiopathic nephrotic syndrome. Pediatr Nephrol. 2016;31(2):207‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maas RJ, Deegens JK, Wetzels JF. Serum suPAR in patients with FSGS: trash or treasure? Pediatr Nephrol. 2013;28(7):1041‐1048. [DOI] [PubMed] [Google Scholar]
- 8. Mellin P, Eickenberg HU. Ureteral reimplantation: Lich‐Grégoire method. Urology. 1978;11(3):315. [DOI] [PubMed] [Google Scholar]
- 9. Gallon L, Leventhal J, Skaro A, et al. Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N Engl J Med. 2012;366(17):1648‐1649. [DOI] [PubMed] [Google Scholar]
- 10. Wang CS, Liverman RS, Garro R, et al. Ofatumumab for the treatment of childhood nephrotic syndrome. Pediatr Nephrol. 2017;32(5):835‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernard J, Bruel A, Allain‐Launay E, et al. Ofatumumab in posttransplantation recurrence of a pediatric steroid‐resistant idiopathic nephrotic syndrome. Pediatr Transplant. 2018;e13175. [DOI] [PubMed] [Google Scholar]
- 12. Cheson BD. Ofatumumab, a novel anti‐CD20 monoclonal antibody for the treatment of B‐cell malignancies. J Clin Oncol. 2010;28(21):3525‐3530. [DOI] [PubMed] [Google Scholar]
- 13. Basu B. Ofatumumab for rituximab‐resistant nephrotic syndrome. N Engl J Med. 2014;370(13):1268‐1270. [DOI] [PubMed] [Google Scholar]


