Abstract
Despite advances in pharmacotherapy, diabetic kidney disease (DKD) remains associated with a high burden of micro‐ and macrovascular complications often leading to premature mortality. New therapies are highly desirable to mitigate the burden of this disease. However, there are a number of barriers that hamper drug development in DKD. These include, amongst others, the lengthy and complex clinical trials required to prove drug efficacy and safety, inefficiencies in clinical trial conduct, and the high costs associated with these development programs. In this review a number of aspects are discussed, aiming to identify opportunities to transform and innovate drug development for DKD. Many clinical trials in DKD, as well as in other areas, face difficulties in timely and efficient enrolment of participants. To address this issue a network of sites should be created that are continuously recruiting individuals with DKD and collecting crucial information that can be used to understand prognosis and prognostic factors, and more importantly to serve as a pool of participants for recruitment to randomized trials. Second, the current clinical endpoints are late events in the progression of DKD. Endpoints based on lesser declines in estimated glomerular filtration rate (eGFR) or changes in albuminuria can shorten follow‐up and/or lead to smaller and cheaper trials. Enrichment by enrolling clinical trial populations based on biomarker profiles is another approach that may facilitate clinical trial efficiency and conduct. Biomarkers can be used to individualize treatment by targeting populations more likely to respond leading to smaller and more efficient trials. Finally, using new trial design such as basket, umbrella or more broadly platform trials to assess a number of therapies simultaneously offers the potential to transform the drug development process in DKD. There are a number of opportunities to transform development approaches for new therapies for DKD. Platform trials along with appropriate biomarker‐based enrichment strategies offer the possibility to foster drug development in a precision medicine era.
Keywords: chronic kidney disease, clinical trials, diabetes, drug development, personalized medicine
1. INTRODUCTION
With the global epidemic of obesity has come an epidemic of downstream manifestations, including rising rates of diabetic kidney disease (DKD).1 Roughly 450 million adults worldwide have diabetes, and more than a third of them will develop chronic kidney disease.2 The condition is relentlessly progressive, leading to significant morbidity and mortality, both from increased cardiovascular disease burden and from end‐stage renal disease (ESRD) and its complications.
The economic impact of DKD to healthcare systems is enormous as well. For example, annual per patient healthcare expenditures for hemodialysis exceed $80 000 in the United States,3 and €50 000 in Germany.4 These costs exceed the wherewithal of many developing countries, and as a result the worldwide need for renal replacement therapy exceeds capacity,5 leaving many without access to this life‐extending therapy.
Beyond the obvious need to stem the underlying obesity and diabetes epidemics, there is a need for new medicines to treat mild to moderate renal impairment, where the opportunity to halt or slow disease progression can meaningfully impact quality and quantity of life. Current strategies for delaying progression of DKD are based around risk factor reduction—primarily blood sugar and blood pressure control—and angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker therapy.6, 7 It has been decades since a new class of medicines was introduced to delay disease progression. Indeed, investment in DKD research and drug development has not kept pace with the rising prevalence of the disease, and the number of randomized controlled trials in this area lags far behind other disease areas. Currently, there are only three new drugs being tested in phase 3 trials.
The lack of a robust pipeline of new therapeutics in development is compounded by the relative homogeneity of the available mechanisms. All agents approved or in late development work in part if not entirely through alterations of renal hemodynamics and glomerular pressure. There is a gap in new medicines that take an orthogonal approach, addressing targets specific to the tubulointerstitial inflammatory and fibrotic components of the disease. A rapid increase over the past two decades in our understanding of the immune system has led to an explosion of therapies for disorders ranging from rheumatoid arthritis to lung cancer, but this growth in scientific knowledge has yet to be translated to the treatment of DKD.
The relative paucity of innovation in DKD can be traced directly to the high barrier to entry in this space. Clinical trials in DKD are lengthy and expensive. A phase 3 trial typically takes more than 5 years to conduct. Time is needed to observe patients long enough to show a separation of estimated glomerular filtration rate (eGFR) curves between study arms, and a sizable number of patients must progress to ESRD or death for a study to be considered to constitute adequate registrational evidence, a lengthy undertaking. Finally, for a trial to be sufficiently powered requires the participation of thousands of patients, a size that can take years, by standard metrics, to recruit.
Operational inefficiencies add to the timeline. Each program requires setting up a cooperative framework of sponsor, academic research organization, steering committee, independent data monitoring committee, contract research organization and multiple investigative sites across countries and continents. This amalgam must guide the trial through a number of activities that each present incremental chances for delay, including country‐specific regulatory and institutional review board approval, site selection, contracting, manufacturing of clinical supplies and obtaining necessary import licenses. After these steps, a not insignificant proportion of study sites find themselves unable to live up to overly ambitious recruitment projections, some failing to recruit a single patient over the course of a study.
All this comes at a cost of hundreds of millions of Euros. Because phase 3 data in DKD comes in a bolus at the end of large, blinded outcomes trials, this is an “all in” investment. By way of contrast, in many other disease areas evidence of efficacy accumulates over smaller, shorter trials, allowing for multiple decision points for advancing or terminating a program, and thus a lower cost to each next decision point.
In order for there to be a rejuvenation of research and development into therapies for DKD we must seek an improvement in the framework—the time, cost, and complexity—in which we conduct the clinical trials programs.
2. ADVANCES IN CLINICAL TRIAL DESIGNS TO FOSTER DRUG DEVELOPMENT
Given the size of the existing gap between development in DKD and other therapeutic areas, a multi‐pronged, transformative approach is needed—incremental change will by definition only have a marginal impact. Innovative approaches are required to address many of the issues listed above. As a community of researchers working to improve outcomes for people with diabetes, we must reimagine the research approach, and develop a new paradigm for clinical trials in DKD for the 21st century. This means carefully examining all aspects of our current approach to drug development, and being brave enough to take a leap forwards. A number of aspects are particularly important to reassess.
2.1. Populations and data collection
Currently, the design of clinical trials is finalized often with little input from the sites where the actual recruitment is to occur. Busy clinicians at sites interested in participating fill out complex forms, and estimate the number of trial participants they might be able to recruit. It is notoriously difficult to integrate the impact of various inclusion and exclusion criteria and use this information to reliably estimate how many participants might truly be recruited. As a result, actual recruitment is commonly half or less the number estimated by many sites.
A better model would be to have reliable data regarding the actual number of participants who fulfil proposed inclusion and exclusion criteria. By combining this with an estimate of the proportion of potential participants who might agree to participate, a more reliable projection can be made. Being able to do this will require effectively creating a network of sites that are continuously recruiting individuals with DKD to a large database after they have consented to participation, and collecting crucial information that can be used to assess the impact of specific criteria on recruitment capacity. Effectively, this would mean the creation of a large, international registry, albeit with limited data capture. Ideally, this data could then be used to refine the trial criteria, in an iterative approach that will help optimize design. This registry has the key additional advantage of offering a ready pool of potential trial participants for new trials.
A related aspect is collection of data. Current approaches require replication of demographic, clinical and laboratory data collection, an approach that has not been shown to be superior to the use of data that has been collected as part of routine clinical practice. Increased usage of established data sources is another way efficiency can be increased dramatically while maintaining reliability. This is particularly true where data from a range of data sources can be accessed to ascertain outcomes (eg, dialysis registries) during the course of a trial.
Achieving this goal will require regulatory authority agreement for reduced reliance on trial‐specific data collection instruments and harmonized central laboratory assessments, and upfront investment to establish a network and database. If achieved this approach has the potential to streamline and transform recruitment to clinical trials in an efficient, low cost fashion.
2.2. Endpoints
A key challenge in kidney disease, is that progression is slow, and the most clinically important endpoints take a long time to develop. This means that trial event rates may be very low. For example, the ADVANCE, EMPA‐REG and CANVAS trials recruited almost 30 000 people with diabetes at high risk of cardiovascular disease, yet despite over 100 000 patient years of follow up, fewer than 100 participants had developed kidney failure requiring dialysis or transplantation or leading to death. Endpoints based on lesser degrees of loss of kidney function are therefore of great interest. Doubling of serum creatinine (loss of 57% of kidney function) is an established endpoint, and more recent efforts led by the US National Kidney Foundation and regulatory agencies assessing lesser degrees of kidney function loss have led to the use of a 40% loss of kidney function as an endpoint for current trials.8, 9 More recently, the use of eGFR slope and albuminuria has been explored in a large workshop and offer to further improve feasibility of clinical trials in kidney disease, while maintaining rigor and reliability.
2.3. Enrichment approaches to tailor therapy
Another approach to increasing efficiency is to “enrich” the trial population. Biomarkers can be used in various ways to enrich clinical trial populations and individualize treatments. Biomarkers can be used to enrich for a population at high risk to develop the event of interest, so called prognostic enrichment. Biomarkers can also be used to enrich for a population more likely to respond to the drug of interest. These biomarkers are referred to as predictive or dynamic biomarkers (Figure 1).
Figure 1.
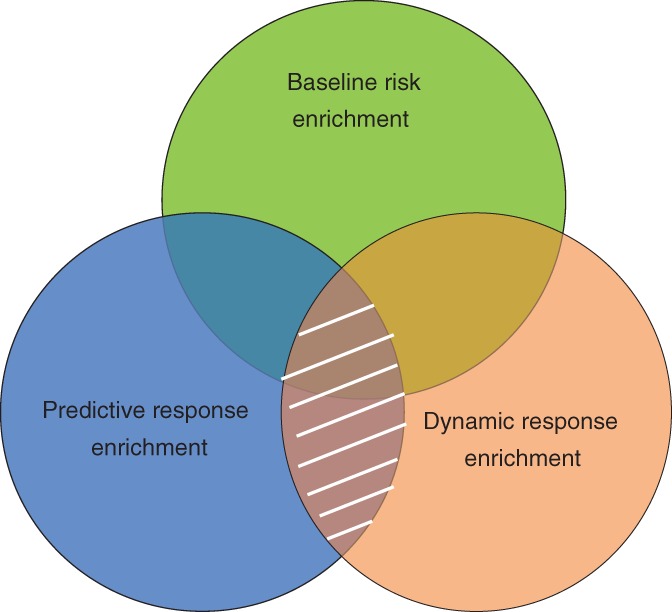
Enrichment approaches for patient selection in clinical trials. Biomarkers can be used to select patients based on risk of disease progression (baseline risk enrichment; green shaded area), based on the drug response before exposure (predictive response enrichment; blue shaded area), or based on the change in a biomarker after short‐term exposure to the drug (dynamic response enrichment; orange shaded area). The ideal biomarker or set of biomarkers would capture the three domains
Past clinical trials in DKD have enriched the population for patients at risk of reaching dialysis by including patients with low eGFR and high albuminuria. However, DKD is a multi‐factorial disease involving various pathophysiological pathways such as endothelial injury, inflammation and fibrosis that can drive eGFR progression. Novel biomarkers that capture these pathways may help to identify a clinical trial population at high risk of progressive kidney function loss which can reduce clinical trial sample sizes. Recent studies suggest that tumor necrosis factor‐1 and kidney injury molecule‐1 may be suitable for this purpose.10, 11 Another ongoing study, the PRIORITY trial (NCT02040441), uses a panel consisting of 273 peptides to identify individuals at high risk of developing microalbuminuria. These individuals are subsequently treated with spironolactone or placebo. The disadvantage of this risk‐based enrichment approach is that the biomarkers do not provide information if the individual patient is going to respond to the drug of interest. Theoretically, it is possible that a high‐risk clinical trial population could be selected at such advanced stages of disease that it would be impossible to reverse or slow disease progression by pharmacological intervention.
Biomarkers can also be used to identify a population more likely to respond before exposing the population to the drug of interest. These so called predictive biomarkers—which can be genes, proteins or metabolites—are often used in other areas in medicine in particular oncology. Various gene polymorphisms predict the response to anticancer drugs. For example, lung cancers with a mutant epidermal growth factor receptor usually respond better to epidermal growth factor inhibitors.12 Apart from one study reporting that polymorphisms in the ACE gene predict the response to angiotensin receptor blockers in patients with DKD no predictive biomarkers are yet discovered.13 The explanation for the paucity of predictive biomarkers in diabetes and kidney disease compared to oncology is that underlying molecular mechanisms are less well described in DKD compared to oncology and that specific drugs targeting these molecular processes are not (yet) available.
Perhaps a more useful approach, at least for the time being, to enrich clinical trials for populations more likely to respond is to expose the target population for a short‐term to the drug of interest. This approach has actually already been used for decades by using active run‐in periods to identify potential participants who will not tolerate the intervention soon after commencing it, and therefore are unlikely to benefit or be harmed by the intervention. The use of active run‐in periods has been employed in lipid (eg, HPS, SHARP) and blood pressure lowering (eg, ADVANCE, ONTARGET) trials.14, 15, 16
The same approach can potentially be used to identify trial subpopulations that might be particularly likely to benefit. This might include participant characteristics at entry to the trial, or might also include the effect of the intervention on post‐randomization intermediate outcomes that are expected to capture the effects of the intervention, and has been an area of interest for regulatory agencies.17 For example, a lipid lowering agent would not be expected to produce cardiovascular benefit via lipid lowering if it doesn't actually lower lipids in an individual participant, presuming the effect on the intermediate outcome can be accurately captured. Reliably measuring the effect on intermediate outcomes can be a challenge for parameters with high within‐patient day‐to‐day variability, so this needs to be considered. However, despite the day‐to‐day variation for some parameters, it is clear that the effects of at least some interventions are highly variable at a between individual level.18 This approach has been used in the recent study of diabetic nephropathy with atransentan (SONAR) trial, where potential participants were exposed to the endothelin antagonist atrasentan, and those who had a 30% reduction in albuminuria were considered responders and further randomized.19, 20 The event rate in this trial was lower than expected, highlighting the potential risk of unanticipated consequences.21 SONAR also randomized approximately 1000 “non‐responders” to the trial to assess whether responder status truly predicts effects on hard outcomes. The SONAR results are awaited with interest.
2.4. Basket and umbrella trials
A further development in clinical trials in other areas of medicine offers additional opportunities. The use of “basket” or “umbrella” trials, or more commonly called “platform” trials involves assessing the effects of multiple interventions on one or more conditions, using modern adaptive designs and statistical approaches, including Bayesian analyses (Table 1).22, 23 A common feature of basket, umbrella and platform trials is the use of a Master Trial Protocol which defines overarching clinical trial elements for the various individual trials conducted within the platform with relative minor trial design differences depending on unique drug characteristics. The master trial protocol enables sharing of trial documents and procedures across trials supporting trial consistency and efficiency.
Table 1.
Terminology for types of master protocols
| Type of trial | Objective | Examples of potential innovative approaches |
|---|---|---|
| Umbrella | To study multiple targeted therapies in the context of a single disease | Within a conventionally defined disease (eg, diabetic kidney disease [DKD]), various biomarker‐based subgroups are defined and different drugs are tested in these subgroups. This approach supports individualizing treatments and personalized medicine. |
| Basket | To study a single‐targeted therapy in the context of multiple disease or disease subtypes | Many of the potential drug targets in DKD may also be useful for other etiologies of chronic kidney disease (CKD) such as IgA nephropathy or focal segmental Glomerulo sclerosis. A basket trial enrolls patients across various CKD etiologies and characterizes the drug effect in multiple disorders. This may enhance innovation while allowing sponsors a wider range of potential indications for a given molecule. |
| Platform | To study multiple‐targeted therapies in the context of a single disease in a perpetual manner, with therapies allowed to enter or leave the platform on the basis of a decision algorithm | Platform trials may lower the hurdle to take a new drug forward into a proof of concept clinical trial because a new molecule could be plugged into an ongoing clinical trial quickly and at a lower cost. An additional benefit is that the platform enables characterizing the efficacy and safety of novel drug combinations, potentially across conditions, mechanisms and sponsors, that would otherwise not be feasible in one trial. Finally, within the platform drugs can be targeted to subgroups based on biomarker profiles to personalize treatment. |
Modified from Reference 22.
The platform trial approach would benefit from a large registry of patients with diabetes and/or kidney disease who are willing to be approached about research studies in the future and agree to the collection of a minimal set of clinically available information such as their diabetes duration, cause of their kidney disease and cardiovascular disease history. Individual trials would be nested in the registry, recruiting from the characterized registry participants. Direct access to patients from the ongoing platform offers a unique opportunity to overcome recruitment challenges often observed in clinical trials of DKD.
The platform trial approach offers the possibility to implement personalized medicine in the trial design and future clinical practice. The availability of multiple interventions within the platform can be used to successively test patients until they show a biomarker response to a treatment, at which point they would be randomized to that treatment or placebo plus standard of care. This enables the incorporation of the individual therapy response and selection of best available therapy for each patient thereby paving the way for a tailored/personalized treatment approach.
Platform trials have been established in other areas of medicine such as oncology, Alzheimer disease and community acquired pneumonia. For example, the investigation of serial studies to predict your therapeutic response with Imaging And moLecular analysis 2 platform was established for the evaluation of candidate treatments for neoadjuvant therapies for biomarker‐defined breast cancer.24 Multiple candidate therapies from individual sponsors are tested within the structure. The Biomarker‐integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE) trial is another example from the oncology area. This platform utilizes adaptive randomization schemes to assign patients to treatment with the greatest potential based on non‐small cell lung cancer tumor markers (NCT00409968). A diabetes and/or kidney disease platform should leverage the experience and knowledge gained by these initiatives.
3. FUTURE CLINICAL TRIALS IN DIABETIC KIDNEY DISEASE
The opportunity to dramatically change our approach to developing treatments in DKD exists, using most or all of these new elements. A network of sites continuously registering consenting patients with DKD, to a platform assessing a number of interventions simultaneously using an adaptive approach, and using the effects on some of the newly validated outcomes to identify effective interventions could lead to a paradigm shift in DKD management and outcomes. The additional use (where appropriate) of routinely collected data sources and enrichment approaches offer to further improve efficiency, to individualize treatment, and to drive investment in DKD research.
ACKNOWLEDGMENTS
H.J.L.H. is supported by a VIDI grant from the Netherlands Organisation for Scientific Research (917.15.306). The Precision Medicine Symposium which took place in December 2017 in Groningen was endorsed by the BEAt‐DKD project. The BEAt‐DKD project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 115974. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA.
Conflict of interest
H.J.L.H. is a consultant for Astellas, Abbvie, AstraZeneca, Boehringer Ingelheim, Fresenius, Gilead, Janssen and Merck and reports research grants from AstraZeneca, Boehringer Ingelheim and Janssen. He has a policy that all honoraria are paid to University Medical Center, Groningen, the Netherlands. J.L. is Janssen employee and may hold stock or stock options. V.P. is a steering committee member for Astellas, Abbvie, GlaxoSmithKline, Boehringer Ingelheim and Janssen; an advisory board member for Bristol‐Myers Squibb, Eli Lilly, AstraZeneca, Novo Nordisk, Pharmalink, Relypsa, Baxter, Merck, Gilead, Novartis, Durect and Janssen; reports honoraria for speaking at scientific symposia from Bayer, Pfizer, Servier, Boehringer Ingelheim, AstraZeneca, Novo Nordisk and Sanofi; reports support for clinical trials from Pfizer; reports program grants and personal fees from National Health and Medical Research Council; and reports an extramural grant for clinical trials from Baxter. All fees are paid to The George Institute for Global Health, Sydney, Australia.
Heerspink HJL, List J, Perkovic V. New clinical trial designs for establishing drug efficacy and safety in a precision medicine era. Diabetes Obes Metab. 2018;20(Suppl. 3):14–18. 10.1111/dom.13417
Funding information EFPIA; European Union's Horizon 2020 research and innovation programme; Innovative Medicines Initiative 2 Joint Undertaking, Grant/Award Number: 115974; Netherlands Organisation for Scientific Research
REFERENCES
- 1. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271‐281. [DOI] [PubMed] [Google Scholar]
- 2. Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. System, U.S.R.D . USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2017. [Google Scholar]
- 4. Icks A, Haastert B, Gandjour A, et al. Costs of dialysis‐‐a regional population‐based analysis. Nephrol Dial Transplant. 2010;25(5):1647‐1652. [DOI] [PubMed] [Google Scholar]
- 5. Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end‐stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975‐1982. [DOI] [PubMed] [Google Scholar]
- 6. Perkovic V, Agarwal R, Fioretto P, et al. Management of patients with diabetes and CKD: conclusions from a "kidney disease: improving global outcomes" (KDIGO) controversies conference. Kidney Int. 2016;90(6):1175‐1183. [DOI] [PubMed] [Google Scholar]
- 7. Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83:517‐523. [DOI] [PubMed] [Google Scholar]
- 8. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 9. Lv J, Zhang H, Wong MG, et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2017;318(5):432‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coca SG, Nadkarni GN, Huang Y, et al. Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol. 2017;28(9):2786‐2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23(3):507‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497‐1500. [DOI] [PubMed] [Google Scholar]
- 13. Parving HH, de Zeeuw D, Cooper ME, et al. ACE gene polymorphism and losartan treatment in type 2 diabetic patients with nephropathy. J Am Soc Nephrol. 2008;19(4):771‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo‐controlled trial. Lancet. 2011;377(9784):2181‐2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829‐840. [DOI] [PubMed] [Google Scholar]
- 16. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547‐1559. [DOI] [PubMed] [Google Scholar]
- 17. Temple R. Enrichment of clinical study populations. Clin Pharmacol Ther. 2010;88(6):774‐778. [DOI] [PubMed] [Google Scholar]
- 18. Petrykiv SI, de Zeeuw D, Persson F, et al. Variability in response to albuminuria lowering drugs: true or random? Br J Clin Pharmacol. 2017;83(6):1197‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heerspink HJ, Andress D, Bakris G, et al. Baseline Characteristics and Enrichment Results of the Study of Diabetic Nephropathy with AtRasentan (SONAR) Trial. Diabetes Obes Metab. 2018;20(8):1829‐1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heerspink HJ, Andress D, Bakris G, et al. Rationale and protocol of the study of diabetic nephropathy with Atrasentan (SONAR) trial: a clinical trial design novel to diabetic nephropathy. Diabetes Obes Metab. 2018;20:1369‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abbvie Statement on SONAR Study Closure. 2017. https://news.abbvie.com/news/media-statements/abbvie-statement-on-sonar-study-closure.htm. Accessed May 1, 2018. [Google Scholar]
- 22. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62‐70. [DOI] [PubMed] [Google Scholar]
- 23. Heerspink HJL, Perkovic V. Trial design innovations to accelerate therapeutic advances in chronic kidney disease: moving from single trials to an ongoing platform. Clin J Am Soc Nephrol. 2018;13(6):946‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park JW, Liu MC, Yee D, et al. Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375(1):11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]


