Abstract
Objective
The satiating effect of protein compared with other nutrients has been well described and is thought to be mediated, in part, by gut hormone release. Previously, it has been shown that oral L‐arginine acts as a GLP‐1 secretagogue both in vitro and in vivo in rodents. Here, the effect of L‐arginine on gut hormone release in humans was investigated.
Methods
The hypothesis was tested in two separate studies. The first study assessed the tolerability of oral L‐arginine in healthy human subjects. The second study assessed the effect of oral L‐arginine on gut hormone release following an ad libitum meal. Subjects were given L‐arginine, glycine (control amino acid), or vehicle control in a randomized double‐blind fashion.
Results
At a dose of 17.1 mmol, L‐arginine was well tolerated and stimulated the release of plasma GLP‐1 (P < 0.05) and PYY (P < 0.001) following an ad libitum meal. Food diaries showed a trend toward lower energy intake and particularly fat intake following L‐arginine treatment.
Conclusions
L‐arginine can significantly elevate GLP‐1 and PYY in healthy human volunteers in combination with a meal. Further work is required to investigate whether L‐arginine may have utility in the suppression of appetite and food intake.
Introduction
The satiating effect of protein is greater than that of other macronutrients 1, 2, 3, 4. High‐protein diets reduce food intake, facilitate weight loss, and improve body composition in animal models and humans 5, 6, 7, 8. These effects have been suggested to be mediated by processes including the modulation of energy expenditure 9 and hepatic gluconeogenesis 10, but the precise mechanisms involved remain unclear. There is evidence to suggest that protein influences gastrointestinal hormones to alter satiety. Protein has been reported to increase levels of specific anorectic gut hormones to a greater extent than other macronutrients 11, 12. Peptide YY (PYY) and glucagon‐like peptide‐1 (GLP‐1) are released from endocrine cells in the gut in response to food intake and reduce food intake following peripheral administration in animals and humans 13, 14. A high‐protein meal results in greater increases in circulating concentrations of both PYY and GLP‐1 in humans with normal weight when compared with isocaloric high‐fat or high‐carbohydrate meals 15. Mice lacking PYY are resistant to the body‐weight‐reducing effect of a high‐protein diet 12.
Specific nutrients are detected within the gut and by peripheral nerves to inhibit food intake in both humans and animal models 16, 17. Cells in the gut epithelial lining, which have direct contact with the intraluminal contents and include enterocytes, brush cells, and enteroendocrine cells, have chemosensory properties. Enteroendocrine cells play a specialized role in luminal nutrient sensing, although they represent less than 1% of epithelial cells within the gut. Peptide hormones are released from secretory granules located in the basal cytoplasm of this cell type 18. Specifically, following a meal, enteroendocrine L‐cells secrete the peptide hormones GLP‐1 and PYY1‐36, which is subsequently processed into the form PYY3‐36. Both GLP‐1 and PYY3‐36 reduce food intake and are involved in the regulation of energy homeostasis 19.
Evidence has suggested that the amino acid products of protein digestion may be sensed both peripherally within the gut and centrally to regulate energy intake 20. Individual amino acids have been shown to influence gastrointestinal hormone release and appetite 21, 22. G‐protein coupled receptor family class C amino‐acid‐sensing receptors are present in the gastrointestinal tract, where some are known to be expressed on enteroendocrine L cells. These receptors, which include the calcium‐sensing receptor, the T1R1/T1R3 heterodimeric receptor, and the GPRC6A receptor, are known to promiscuously bind several L‐amino acid ligands and thus appear suited to detect the varied products of protein digestion. Supplementing foods with specific amino acids selected for their appetite‐reducing effect may represent a novel approach for the prevention of weight gain or the treatment of obesity. The eventual goal would be to design foods or dietary regimens that cause an increased sense of fullness and encourage the individual to stop eating sooner, thus reducing total energy intake 23, 24. Our aim in this study was to investigate the effects of an amino acid administered at physiological levels (i.e., similar to the amount present in a high‐protein meal) on anorectic gut hormone release and the regulation of appetite.
L‐arginine appeared a good candidate for an appetite‐reducing amino acid. L‐arginine is a conditionally essential amino acid that can activate all three of the known promiscuous amino‐acid‐sensing receptors and, in particular, the T1R1/T1R3 receptor in rodents 25. Previous work has shown specific amino acids can influence food intake and gut hormone release. For example, glutamine stimulates GLP‐1 secretion from an enteroendocrine cell line 26, and studies have suggested that L‐glutamine stimulates the release of GLP‐1 in humans 22, 27, 28. The amino acid L‐arginine also reduces food intake and elevates circulating levels of GLP‐1 and PYY in rodents 29, 30, 31, while other L‐amino acids, including glycine, have no effect 21. There is evidence that oral L‐arginine acts as a GLP‐1 secretagogue both in vitro 32 and in vivo in rodents 30. However, to date, there has been no evidence of this effect in humans. We investigated the effect of L‐arginine on circulating levels of appetite‐modulating gastrointestinal hormones in humans and the consequent effect on appetite.
Methods
Study participants
Studies were conducted following ethical approval (West London Research Ethics Committee 1, London, UK) and according to the principles of the Declaration of Helsinki. All participants gave written informed consent prior to study enrollment.
Pilot study on tolerability of L‐arginine (study 1)
Healthy male (n = 1) and female (n = 6) subjects with a mean age of 39.4 (SD 11.4) years and BMI of 24.6 (SD 4.7) kg/m2 who had been weight stable for 3 months prior to study enrollment were recruited (Table 1.
Table 1.
Baseline characteristics for participants in the pilot study on the effect of L‐arginine on gut hormone release and tolerability
| All subjects | |
|---|---|
| Age (y) | 39.4 ± 11.4 |
| Female:Male | 6:1 |
| Weight (kg) | 68.9 ± 17.3 |
| Height (m) | 1.67 ± 0.08 |
| BMI (kg/m2) | 24.6 ± 4.7 |
| Blood pressure (mm Hg) | 112/68 ± 13/8 |
Data represent mean ± SD.
Subjects attended four study visits each separated by at least 1 week. The first visit was an acclimatization visit, the results of which were not included in the final analysis. The evening before each study visit, subjects were requested to consume an identical meal, supplied by the experimenters, at approximately 8 pm. Subjects then reported to the clinical research facility at 8:30 am the following day, having fasted from 9 pm the night before. On arrival, subjects were cannulated in the antecubital fossa for serial blood sampling and asked to consume hypromellose capsules containing a total of 17.1 mmol of L‐arginine hydrochloride or 17.1 mmol of glycine (Euro‐OTC‐Pharma, Horsham, UK) or empty capsules (vehicle) in a double‐blind randomized order at time (t) = 0 minutes. Glycine was chosen as a control amino acid, as this amino acid has been shown to have no effect on food intake in rodent studies 21. This dose of L‐arginine was chosen as it was similar to levels of L‐arginine found in a high‐protein meal. Hypromellose capsules, which are relatively inert and break down in the stomach, were used to deliver the amino acids 33. In addition to tolerability, we carried out a pilot analysis of gut hormone release and subjective appetite. Blood samples were taken at 15‐minute intervals commencing at t = −15 minutes for 2.5 hours after dosing for the measurement of plasma acylated ghrelin, GLP‐1, and PYY. Subjects were asked to complete visual analogue scales (VAS) at the corresponding time points. Participants rated subjective feelings of hunger (“How hungry do you feel right now?”), pleasantness to eat (“How pleasant would it be to eat right now?”), prospective food intake (“How much could you eat right now?”), fullness (“How full do you feel right now?”), and sickness (“How sick do you feel right now?”) using a 100‐mm horizontal VAS at −15, 0, 15, 30, 45, 60, 75, 90, 105, 120,135, and 150 minutes following ingestion of L‐arginine. Subjects were also asked to report any additional side effects that occurred during the visit or after the study visit was completed.
Effect of L‐arginine on gut hormone release and food intake following an ad libitum meal (study 2)
With 80% power, it was predicted that nine subjects would be required for this study based on a difference in gut hormone release of 30%. This is the approximate magnitude of increase in PYY release observed following a high‐protein meal compared with a high‐carbohydrate meal 12, 15. Healthy male (n = 1) and female (n = 8) subjects with a mean age of 36.0 (SD 10.8) years and BMI of 25.1 (SD 3.5) kg/m2 who had been weight stable for 3 months prior to study enrollment were recruited (Table 2.
Table 2.
Baseline characteristics for participants in the study of the effect of L‐arginine on gut hormone release and food intake following an ad libitum meal
| All subjects | |
|---|---|
| Age (y) | 36.0 ± 10.8 |
| Female:Male | 8:1 |
| Weight (kg) | 69.4 ± 16.4 |
| Height (m) | 1.65 ± 0.09 |
| BMI (kg/m2) | 25.1 ± 3.5 |
| Blood pressure (mm Hg) | 113/74 ± 6/5 |
Data represent mean ± SD.
The study design was as per study 1 (with administration of L‐arginine, glycine, or vehicle at t = 0 minutes), except that at t = 60 minutes, subjects were presented with an ad libitum meal, which they were asked to consume within 30 minutes. The ad libitum meal was served in excess, and participants were asked to eat until they were comfortably full. Participants were isolated during this part of the study. Food was weighed before and after the ad libitum meal, and energy intake was calculated from the manufacturer’s nutritional information. The meal given was a commercially available margherita pasta bake ready meal consisting of 1.83 kcal, 0.079 g protein, 0.196 g carbohydrate, and 0.078 g fat per gram. There were no blood samples or VAS taken at t = 75 minutes in this study. As the initial study detected no evidence of any effect on ghrelin levels, ghrelin was not measured in this second study. Subjects were asked to complete food diaries for the rest of the day and the subsequent day following their departure from the clinical research facility. Energy intake and macronutrient intake were established for each subject from analysis of completed food diaries. Food diaries were analyzed using Dietplan 6 nutritional analysis software (Forestfield Software, Horsham, UK).
Assays
Plasma acylated ghrelin was measured using a commercially available enzyme‐linked immunosorbent assay (ELISA) kit (Merck Millipore, Burlington, Massachusetts). Plasma GLP‐1 and PYY were measured using established in‐house radioimmunoassays 34, 35. The GLP‐1 antibody has 100% cross‐reactivity with all amidated forms of GLP‐1 but does not cross‐react with glycine extended forms. The PYY antibody has 100% cross‐reactivity with PYY1‐36 and PYY3‐36. The intra‐assay coefficients of variation for GLP‐1 and PYY assays were 5.6% and 5.0%, respectively.
Statistical analysis
Acute food intake and area under the curve (AUC) data are expressed as mean ± SEM and were analyzed by one‐way analysis of variance (ANOVA) and post hoc Bonferroni correction. Data from gut hormones and VAS were analyzed by repeated‐measures ANOVA and post hoc Bonferroni correction. The relationship between the variables under study was assessed using the Pearson coefficient of correlation R. P < 0.05 was considered significant. GraphPad Prism software (Prism 5.01; GraphPad Software Inc., San Diego, California) was used for all analyses.
Results
Pilot study on tolerability of L‐arginine (study 1)
L‐arginine was well tolerated.
There were no self‐reported side effects with the administration of oral L‐arginine. In addition, there were no significant changes on the VAS regarding nausea.
L‐arginine alone had no significant effect on circulating ghrelin, GLP‐1, or PYY levels.
L‐arginine had no significant effect on plasma ghrelin (Figure 1A‐1B), which was in accord with animal studies performed within our group (unpublished data). There was a trend for an increase in plasma GLP‐1 following ingestion of L‐arginine, though this effect did not achieve statistical significance (Figure 1C‐1D). There was no significant increase in plasma PYY following ingestion of L‐arginine (Figure 1E‐1F).
Figure 1.
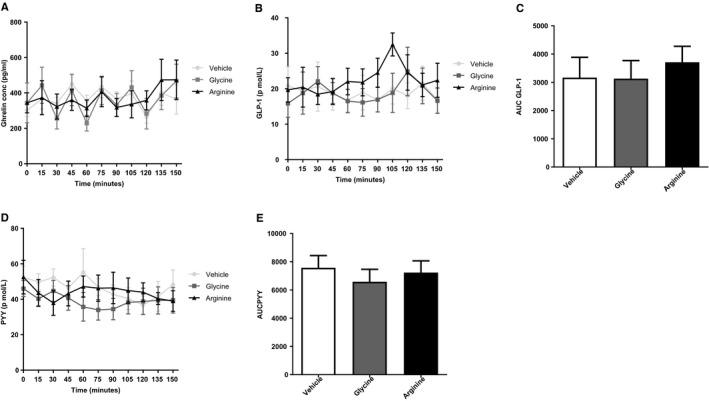
Effect of L‐arginine on gut hormone release in healthy volunteers. Results of oral ingestion of vehicle, 17.1 mmol of arginine, or 17.1 mmol of glycine (n = 7) on (A) acylated ghrelin, (B) AUC of acylated ghrelin, (C) GLP‐1, (D) AUC of GLP‐1, (E) PYY, and (F) AUC of PYY. Data are expressed as mean ± SEM.
L‐arginine had no significant effect on subjective measures of appetite.
There was no significant difference in subjective measures of appetite using VAS following ingestion of vehicle, 17.1 mmol of arginine, or 17.1 mmol of glycine.
Following observations that L‐arginine was well tolerated with no reported side effects and that it resulted in a trend for an increase in plasma GLP‐1 release, we investigated whether L‐arginine could increase gut hormone release and influence food intake.
Effect of L‐arginine on gut hormone release and food intake following an ad libitum meal (study 2)
L‐arginine stimulated plasma GLP‐1 and PYY following an ad libitum meal.
L‐arginine significantly increased plasma GLP‐1 levels following ingestion of the ad libitum meal compared with levels following vehicle treatment (P < 0.05) (Figure 2A‐2B). L‐arginine significantly increased plasma PYY levels following administration of the ad libitum meal compared with levels following vehicle treatment (P < 0.001) (Figure 2C‐2D). While it is difficult to control for food intake in this analysis, AUC for change in GLP‐1 levels following the meal correlated with food intake with both vehicle control and L‐arginine ingestion (R = 0.71 and 0.75, respectively; P < 0.05 for both) but not following glycine ingestion (P = 0.88). There was no correlation between AUC for change in PYY levels and food intake with any treatment, suggesting that this effect was independent of food intake.
Figure 2.
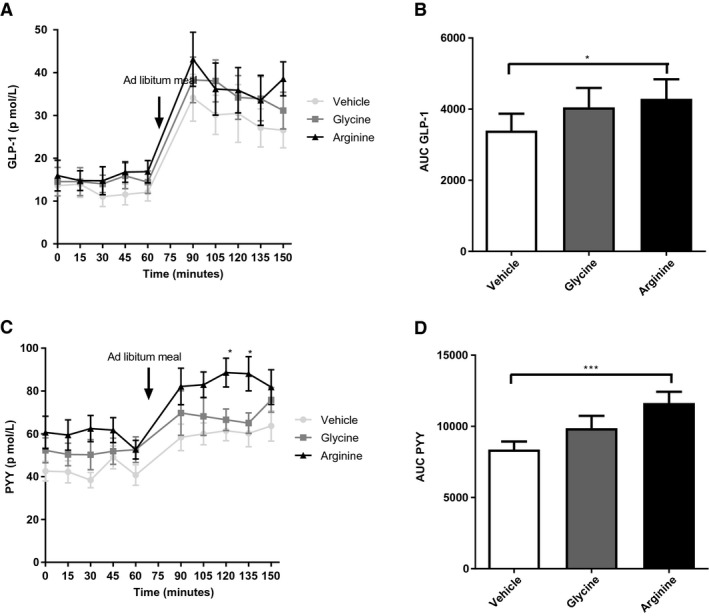
Effect of L‐arginine on gut hormone release in healthy volunteers following an ad libitum meal. Results of oral ingestion of vehicle, 17.1 mmol of arginine, or 17.1 mmol of glycine (n = 9) on (A) GLP‐1, (B) AUC of GLP‐1 (*P < 0.05), (C) PYY (*P < 0.05), and (D) AUC of PYY (***P < 0.001). Data are expressed as mean ± SEM.
L‐arginine did not reduce acute food intake or affect subjective measures of appetite.
There was no difference in energy intake or subjective measures of appetite using VAS following ingestion of vehicle, 17.1 mmol of arginine, or 17.1 mmol of glycine (n = 9). Food consumption at the ad libitum meal was 929 ± 87.6 kcal (mean ± SEM) in the vehicle group compared with 1,154 ± 156.9 kcal in the glycine group and 992 ± 93.4 kcal in the L‐arginine group (P = 0.26).
Effects of L‐arginine on subsequent energy intake and fat intake.
Assessment of subsequent energy intake by self‐reported food diaries showed a trend toward lower energy intake and particularly fat intake (Figure 3), though these effects did not achieve statistical significance.
Figure 3.
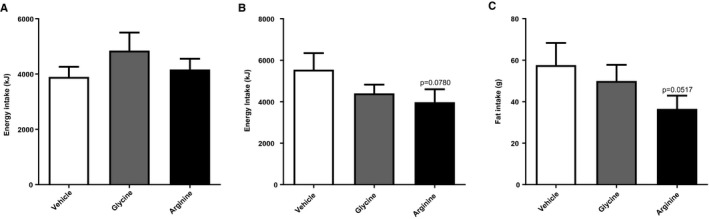
(A) Effect of L‐arginine on food intake at an ad libitum meal. (B) Twelve‐hour food intake following study visit (P = 0.0780; arginine vs. vehicle) and (C) twelve‐hour fat intake following study visit (P = 0.0517; arginine vs. vehicle) following ingestion of vehicle, 17.1 mmol of arginine, or 17.1 mmol of glycine (n = 9). Data are expressed as mean ± SEM.
Discussion
These data show that in combination with an ad libitum meal, L‐arginine significantly elevates GLP‐1 and PYY in healthy human volunteers compared with vehicle treatment. L‐arginine, at the doses administered, did not result in any serious side effects.
Appetite is regulated by complex central circuitry in response to several peripheral and central inputs. These include acute nutritional status, which is thought to be communicated to the appetite centers of the brain by signals such as changes in gut hormone release profile and vagal signaling as well as changes in circulating nutrients 36, 37. However, the precise stimuli for these signals and how they act in coordination and in the context of long‐term energy homeostasis are poorly understood. The effects observed in our study suggest L‐arginine may augment postprandial release of anorectic gut hormones. The mechanisms by which L‐arginine stimulates release of plasma GLP‐1 and PYY remain unclear 30. It is possible that L‐arginine is sensed by amino‐acid‐sensing receptors in the gut, which then mediate its effect on gut hormone release. Some of these receptors have been reported to be expressed on the same enteroendocrine cells that express GLP‐1 and PYY 38, 39, 40. These L cells are known to express a range of nutrient‐sensing receptors 19, and it may be that while L‐arginine alone does not have a significant effect on hormone release, it may potentiate the response to other nutrients ingested during the test meal. In addition, L‐arginine may possibly potentiate the effect of stomach distension on hormone release, which might also explain why differences are observed only postprandially. It is possible that the ability of L‐arginine to potentiate the effect of food intake on GLP‐1 release is reflected in the higher R value for the relationship between postprandial changes in GLP‐1 concentrations and food intake following L‐arginine ingestion, though further work would be required to confirm whether this is the case. It is interesting that there may be delayed effects of L‐arginine on appetite as suggested by the trends in the self‐reported food diaries. L‐arginine appears to potentially have effects on energy intake and fat intake subsequent to the study period. This may be a result of the postprandial effect on gut hormone release seen in the second study. Indeed, GLP‐1 analogues do suppress food intake and can do so for extended time intervals 41. L‐arginine has been shown to have a delayed and sustained anorectic effect in animals, with a reduction in food intake when food was returned 8 hours after administration in mice 31. Identifying food components that can increase postprandial GLP‐1 release may be useful in people with impaired glucose tolerance or type 2 diabetes, though further work is required to determine whether L‐arginine might have such utility, perhaps in combination with other nutrients.
A high‐protein meal consisting of 200 g of cooked chicken would be expected to contain 3 to 5 g of arginine, and hence doses of L‐arginine used (approximately 3 g) were comparable to those that might be ingested at a single high‐protein meal. However, we do acknowledge that the release profile of similar amounts of L‐arginine within the gut might be quite different from hypromellose capsules compared with that from protein ingestion. At these physiological doses, administration of L‐arginine in concert with other macronutrients (from the meal) results in the release of anorectic gut hormones. It is likely that the release of L‐arginine is a gradual process once it is in the small intestine, and it is difficult to know how far down the gastrointestinal tract significant concentrations of L‐arginine would reach before being absorbed. From our data, it is possible that L‐arginine is exerting its effect on gut hormone release at around 120 minutes; this may reflect the point at which it comes in direct contact with amino‐acid‐sensing L cells in the distal small intestine. There is a higher concentration of L cells in the distal small intestine 42, 43, and the elevation in PYY levels observed following a high‐protein meal is sustained for several hours 12, 15. It may be that this delayed effect is a result of direct sensing of amino acids in the distal small intestine, perhaps several hours after ingestion. L‐arginine is known to stimulate pancreatic hormone release when administered at higher levels to humans 44, 45, but the GLP‐1 detected should reflect the products of enteroendocrine cells rather than pancreatic alpha cells, as the assay used does not recognize glucagon or other preproglucagon products; however, it does detect all amidated forms of GLP‐1. The lack of suppression of ghrelin is in line with work carried out in our group looking at the effect of L‐arginine in rodents (unpublished data).
Further studies are required to investigate the effects of L‐arginine in combination with other macronutrients to determine whether this can result in a reduction in appetite. This study had a small number of participants as it was initially a pilot study to determine tolerability, with the second study being powered to detect a 30% change in gut hormone release. The majority of participants in these studies were women, and further work is required to determine whether similar effects occur in men. In addition, the volunteers will have had varied energy requirements, and thus there would be expected differences in their baseline food intake at an ad libitum meal; this is to some degree controlled for by the use of a crossover design, but it may be useful for future studies to measure the baseline energy expenditure of participants and to control for this in the food intake analysis. The dose of L‐arginine was chosen as it corresponded with levels found in a high‐protein meal and therefore allowed a physiological response to be observed. It is possible that higher doses of L‐arginine may have a greater effect on gut hormone release that would be sufficient to drive changes in appetite. However, 3 g of L‐arginine a day is also the maximum dose considered to be a foodstuff rather than a drug by the Medicines and Healthcare products Regulatory Agency, and effectiveness at this dose would facilitate the design of a supplement subject to food law rather than drug law. It is also possible that coadministering L‐arginine with other specific macronutrients may result in changes in gut hormone release sufficient to influence food consumption and satiety. The regulation of food intake is affected by a multitude of factors including the volume and composition of a meal, which can have synergistic effects on gut hormone release and appetite. While it seems that L‐arginine alone at the doses administered may not be useful in the suppression of appetite, further work is required to determine the effect of L‐arginine when combined with other nutrients on gut hormone release and appetite.
Funding agencies:
This paper presents independent research funded by the Biotechnology and Biological Sciences Research Council (BBSRC), Medical Research Council (MRC), and Society for Endocrinology and supported by the National Institute for Health Research (NIHR) Clinical Research Facility (CRF) and Biomedical Research Centre (BRC) at Imperial College Healthcare National Health Service (NHS) Trust. AA is supported by an MRC Clinical Training Research Fellowship. The Section of Endocrinology and Investigative Medicine is funded by grants from MRC, BBSRC, NIHR, Integrative Mammalian Biology (IMB) Capacity Building Award, and an FP7‐ HEALTH‐ 2009‐ 241592 EuroCHIP grant, and it is supported by the NIHR BRC Funding Scheme. The views expressed are those of the author(s) and not necessarily those of the funders, NHS, NIHR, or the Department of Health.
Disclosure:
The authors declared no conflict of interest.
References
- 1. Porrini M, Santangelo A, Crovetti R, Riso P, Testolin G, Blundell JE. Weight, protein, fat, and timing of preloads affect food intake. Physiol Behav 1997;62:563‐570. [DOI] [PubMed] [Google Scholar]
- 2. Reid M, Hetherington M. Relative effects of carbohydrates and protein on satiety – a review of methodology. Neurosci Biobehav Rev 1997;21:295‐308. [DOI] [PubMed] [Google Scholar]
- 3. Bensaid A, Tomé D, Gietzen D, et al. Protein is more potent than carbohydrate for reducing appetite in rats. Physiol Behav 2002;75:577‐582. [DOI] [PubMed] [Google Scholar]
- 4. Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr 2004;23:373‐385. [DOI] [PubMed] [Google Scholar]
- 5. Potier M, Darcel N, Tome D. Protein, amino acids and the control of food intake. Curr Opin Clin Nutr Metab Care 2009;12:54‐58. [DOI] [PubMed] [Google Scholar]
- 6. Jean C, Rome S, Mathé V, et al. Metabolic evidence for adaptation to a high protein diet in rats. J Nutr 2001;131:91‐98. [DOI] [PubMed] [Google Scholar]
- 7. Hannah JS, Dubey AK, Hansen BC. Postingestional effects of a high‐protein diet on the regulation of food intake in monkeys. Am J Clin Nutr 1990;52:320‐325. [DOI] [PubMed] [Google Scholar]
- 8. Kinzig KP, Hargrave SL, Hyun J, Moran TH. Energy balance and hypothalamic effects of a high‐protein/low‐carbohydrate diet. Physiol Behav 2007;92:454‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Westerterp‐Plantenga MS, Rolland V, Wilson SA, Westerterp KR. Satiety related to 24 h diet‐induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. Eur J Clin Nutr 1999;53:495‐502. [DOI] [PubMed] [Google Scholar]
- 10. Veldhorst MA, Westerterp KR, Westerterp‐Plantenga MS. Gluconeogenesis and protein‐induced satiety. Br J Nutr 2012;107:595‐600. [DOI] [PubMed] [Google Scholar]
- 11. Nilsson M, Stenberg M, Frid AH, Holst JJ, Björck IME. Glycemia and insulinemia in healthy subjects after lactose‐equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr 2004;80:1246‐1253. [DOI] [PubMed] [Google Scholar]
- 12. Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein‐mediated satiation and body‐weight regulation. Cell Metab 2006;4:223‐233. [DOI] [PubMed] [Google Scholar]
- 13. Adrian TE, Bacarese‐Hamilton AJ, Smith HA, Chohan P, Manolas KJ, Bloom SR. Distribution and postprandial release of porcine peptide YY. J Endocrinol 1987;113:11‐14. [DOI] [PubMed] [Google Scholar]
- 14. Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon‐like peptide‐1 (7–36)amide and glucose‐dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post‐prandial and 24‐h secretion patterns. J Endocrinol 1993;138:159‐166. [DOI] [PubMed] [Google Scholar]
- 15. van der Klaauw AA, Julia MK, Elana H, et al. High protein intake stimulates postprandial GLP1 and PYY release. Obesity (Silver Spring) 2013;21:1602‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenberg D, Smith GP, Gibbs J. Intraduodenal infusions of fats elicit satiety in sham‐feeding rats. Am J Physiol 1990;259 (1 Pt 2):R110‐R118. [DOI] [PubMed] [Google Scholar]
- 17. Matzinger D, Degen L, Drewe J, et al. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut 2000;46:688‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hofer D, Asan E, Drenckhahn D. Chemosensory perception in the gut. News Physiol Sci 1999;14:18‐23. [DOI] [PubMed] [Google Scholar]
- 19. Spreckley E, Murphy KG. The L‐cell in nutritional sensing and the regulation of appetite. Front Nutr 2015;2:23. doi: 10.3389/fnut.2015.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fromentin G, Darcel N, Chaumontet C, et al. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr Res Rev 2012;25:29‐39. [DOI] [PubMed] [Google Scholar]
- 21. McGavigan AK, O'Hara HC, Amin A, et al. L‐cysteine suppresses ghrelin and reduces appetite in rodents and humans. Int J Obes (Lond) 2015;39:447‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meek CL, Reimann F, Park AJ, Gribble FM. Can encapsulated glutamine increase GLP‐1 secretion, improve glucose tolerance, and reduce meal size in healthy volunteers? A randomised, placebo‐controlled, cross‐over trial. Lancet 2015;385:S68. [DOI] [PubMed] [Google Scholar]
- 23. Hill JO, Peters JC. Biomarkers and functional foods for obesity and diabetes. Br J Nutr 2002;88 (suppl 2):S213‐S218. [DOI] [PubMed] [Google Scholar]
- 24. Halford JC, Harrold JA. Satiety‐enhancing products for appetite control: science and regulation of functional foods for weight management. Proc Nutr Soc 2012;71:350‐362. [DOI] [PubMed] [Google Scholar]
- 25. Wellendorph P, Johansen LD, Brauner‐Osborne H. Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol Pharmacol 2009;76:453‐465. [DOI] [PubMed] [Google Scholar]
- 26. Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon‐like peptide‐1 secretion from GLUTag cells. Diabetologia 2004;47:1592‐1601. [DOI] [PubMed] [Google Scholar]
- 27. Samocha‐Bonet D, Chisholm D, Holst J, Greenfield J. L‐glutamine and whole protein restore first‐phase insulin response and increase glucagon‐like peptide‐1 in type 2 diabetes patients. Nutrients 2015;7:2101‐2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang J, Wu T, Greenfield JR, Samocha‐Bonet D, Horowitz M, Rayner CK. Effects of intraduodenal glutamine on incretin hormone and insulin release, the glycemic response to an intraduodenal glucose infusion, and antropyloroduodenal motility in health and type 2 diabetes. Diabetes Care 2013;36:2262‐2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jordi J, Herzog B, Camargo SMR, Boyle CN, Lutz TA, Verrey F. Specific amino acids inhibit food intake via the area postrema or vagal afferents. J Physiol 2013;591:5611‐5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clemmensen C,Smajilovic S, Smith EP, et al. Oral L‐arginine stimulates GLP‐1 secretion to improve glucose tolerance in male mice. Endocrinology 2013;154:3978‐3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alamshah A, McGavigan AK, Spreckley E, et al. L‐Arginine promotes gut hormone release and reduces food intake in rodents. Diabetes Obes Metab 2016;18:508‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mace OJ, Schindler M, Patel S. The regulation of K‐ and L‐cell activity by GLUT2 and the calcium‐sensing receptor CasR in rat small intestine. J Physiol 2012;590:2917‐2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li CL, Martini LG, Ford JL, Roberts M. The use of hypromellose in oral drug delivery. J Pharm Pharmacol 2005;57:533‐546. [DOI] [PubMed] [Google Scholar]
- 34. Kreymann B, Ghatei MA, Williams G, Bloom SR. Glucagon‐like peptide‐1 7–36: a physiological incretin in man. Lancet 1987;2:1300‐1304. [DOI] [PubMed] [Google Scholar]
- 35. Adrian TE, Ferri G‐l, Bacarese‐Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985;89:1070‐1077. [DOI] [PubMed] [Google Scholar]
- 36. Heisler LK, Lam DD. An appetite for life: brain regulation of hunger and satiety. Curr Opin Pharmacol 2017;37:100‐106. [DOI] [PubMed] [Google Scholar]
- 37. Norton M, Murphy KG. Targeting gastrointestinal nutrient sensing mechanisms to treat obesity. Curr Opin Pharmacol 2017;37:16‐23. [DOI] [PubMed] [Google Scholar]
- 38. Oya M, Kitaguchi T, Pais R, Reimann F, Gribble F, Tsuboi T. The G protein‐coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid‐induced glucagon‐like peptide‐1 secretion from GLUTag cells. J Biol Chem 2013;288:4513‐4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang H, Murthy KS, Grider JR. Expression of putative umami/L‐amino acid receptors in mouse intestine and colon, and a murine enteroendocrine cell line. FASEB J 2017;31 (suppl 1):1090.2 (abstract). [Google Scholar]
- 40. Symonds EL, Peiris M, Page AJ, et al. Mechanisms of activation of mouse and human enteroendocrine cells by nutrients. Gut 2015;64:618‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Larsen PJ, Fledelius C, Knudsen LB, Tang‐Christensen M. Systemic administration of the long‐acting GLP‐1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes 2001;50:2530‐2539. [DOI] [PubMed] [Google Scholar]
- 42. Habib AM, Richards P, Cairns LS, et al. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 2012;153:3054‐3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eissele R, Göke R, Willemer S, et al. Glucagon‐like peptide‐1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 1992;22:283‐291. [DOI] [PubMed] [Google Scholar]
- 44. Floyd JC Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest 1966;45:1487‐1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bellone J, Valetto MR, Aimaretti G, et al. Effects of phenylalanine, histidine, and leucine on basal and GHRH‐stimulated GH secretion and on PRL, insulin, and glucose levels in short children. Comparison with the effects of arginine. J Pediatr Endocrinol Metab 1996;9:523‐531. [DOI] [PubMed] [Google Scholar]


