Abstract
Objectives
To provide data on the prevalence of urinary, bowel and sexual dysfunction in Northern Ireland (NI), to act as a baseline for studies of prostate cancer outcomes and to aid service provision within the general population.
Subjects and Methods
A cross‐sectional postal survey of 10 000 men aged ≥40 years in NI was conducted and age‐matched to the distribution of men living with prostate cancer. The EuroQoL five Dimensions five Levels (EQ‐5D‐5L) and 26‐item Expanded Prostate Cancer Composite (EPIC‐26) instruments were used to enable comparisons with prostate cancer outcome studies. Whilst representative of the prostate cancer survivor population, the age‐distribution of the sample differs from the general population, thus data were generalised to the NI population by excluding those aged 40–59 years and applying survey weights. Results are presented as proportions reporting problems along with mean composite scores, with differences by respondent characteristics assessed using chi‐squared tests, analysis of variance, and multivariable log‐linear regression.
Results
Amongst men aged ≥60 years, 32.8% reported sexual dysfunction, 9.3% urinary dysfunction, and 6.5% bowel dysfunction. In all, 38.1% reported at least one problem and 2.1% all three. Worse outcome was associated with increasing number of long‐term conditions, low physical activity, and higher body mass index (BMI). Urinary incontinence, urinary irritation/obstruction, and sexual dysfunction increased with age; whilst urinary incontinence, bowel, and sexual dysfunction were more common among the unemployed.
Conclusion
These data provide an insight into sensitive issues seldom reported by elderly men, which result in poor general health, but could be addressed given adequate service provision. The relationship between these problems, raised BMI and low physical activity offers the prospect of additional health gain by addressing public health issues such as obesity. The results provide essential contemporary population data against which outcomes for those living with prostate cancer can be compared. They will facilitate greater understanding of the true impact of specific treatments such as surgical interventions, pelvic radiation or androgen‐deprivation therapy.
Keywords: urinary dysfunction, bowel dysfunction, sexual dysfunction, health‐related quality of life, prostate cancer, Life After Prostate Cancer Diagnosis
Introduction
The prevalence of prostate cancer has increased dramatically since the early 1990s 1, 2. Coupled with this there has been an increase in studies of patient‐reported outcomes and initiatives to support the morbidity burden associated with prostate cancer diagnosis and its treatment 3. However, the vast majority of studies do not have large matched control data or comparable general population data. Consequently, such studies may be overestimating the negative consequences of treatment.
Various surveys of urinary, bowel, and sexual symptoms in the general populations of the USA and Europe 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 have found these problems to be common amongst elderly men, with LUTS ranging from 48% to 72% 4, 5, 6, moderate‐to‐severe urinary incontinence from 11% to 16% 5, 6, 7, 8, severe/frequent erectile dysfunction from 5% to 10% 4, 9, 10, 11, and faecal incontinence from 6% to 15% 12, 13, 14.
However, comparing the results from these general population studies with those for current prostate cancer survivors to assess the additional impact of prostate cancer and its treatment is not straightforward. Not only are most of these studies dated, they are not specific to a particular population (e.g. they rarely report Northern Ireland [NI]/UK specific results). In addition, they typically use survey instruments not directly comparable with those used in assessments of prostate cancer outcomes, whilst the age structure of men surveyed in general population surveys rarely match those of prostate cancer survivors as more than half (54% in 2012–2014) of prostate cancer cases diagnosed in the UK are amongst males aged ≥70 years 2.
The measurement of problems of this nature is also relevant to the health of men who do not have prostate cancer. However, with significant gains in life expectancy in recent years 15, changes in lifestyle factors (such as rising obesity levels) 16, and changes in prevalence of common health conditions (e.g. reductions in hypertension, increases in diabetes) 16, contemporary older men are likely to have different health outcomes than the more historical cohorts documented by previous studies. Consequently, there is a need to update population observations of these problems to allow differentiation between the impact of prostate cancer and its treatment from the normal effects of ageing, and to provide health service planners with information on the prevalence of these conditions in the general population to ensure that the necessary support services are in place.
We report a comprehensive evaluation of self‐reported urinary, bowel, and sexual dysfunction, alongside health‐related quality of life (HRQL) and self‐assessed health rating, in a population of men aged ≥40 years in NI, a devolved nation of the UK. We utilise a sample that has been age‐matched to the prostate cancer survivor population and use survey instruments widely applied in the evaluation of prostate cancer outcomes. In addition, we generalise these data to the NI population for men aged ≥60 years to provide information necessary for public health purposes, including reporting prevalence of urinary, bowel, and sexual dysfunction; and report how sociodemographic characteristics, health‐related factors, and general health are associated with these conditions.
Subjects and Methods
Background
A cross‐sectional postal survey of the general NI population was conducted as part of the Life After Prostate Cancer Diagnosis (LAPCD) study 17. Additional surveys involved prostate cancer survivors, the results of which will be reported elsewhere.
Data Collection
An age‐stratified random sample of 10 000 men aged ≥40 years was prepared by the Health and Social Care Business Services Organisation (BSO) using the NI General Practice Register. To allow comparability with the prostate cancer survivor survey, the sampling frame was based on the age distribution of prostate cancer survivors in NI who were alive 18–42 months after diagnosis. Men identified by the NI Cancer Registry as having a previous prostate cancer diagnosis were excluded.
Each member of the sample had a unique reference number assigned, thereby protecting the identity of participants. BSO posted surveys throughout September and October 2016, with instructions to return completed surveys to an external provider (Picker Institute Europe, Oxford, UK). On completion of data entry, deprivation quintile, based on the NI multiple deprivation measure 18, and an urban/rural indicator, based upon the NI statistical classification of settlements 19, was added.
Survey
The survey (File S1) was adapted from the LAPCD survey of prostate cancer survivors and included a wide range of respondent characteristics. HRQL was evaluated using the EuroQoL five Dimensions five Levels (EQ‐5D‐5L) instrument, which included a self‐assessed health rating 20. Urinary, bowel, and sexual health were determined using the 26‐item Expanded Prostate Cancer Composite (EPIC‐26) questionnaire 21, in line with recommendations from the International Consortium for Health Outcomes Measurement (ICHOM) 22, 23. Adaptations to the survey for the general population included removing references to cancer and its treatment in the supporting text such as the introduction and completion guidance; however, changes to the actual questions asked were minimal.
Service users participated in the study design and development of the questionnaire through the User Advisory Group for the LAPCD study. Cognitive testing for user acceptability in terms of length, content and clarity of survey questions was performed with a focus group of older men from the general population accessed through a local ageing charity.
Outcome Measures
Reported prevalence of men experiencing problems was based upon the proportion of men reporting moderate/big problems in response to specific questions from the EPIC‐26 question set (urinary: q2.6, bowel: q2.8, sexual: q2.13; File S1). The individual EQ‐5D‐5L questions on mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression (q1.1–q1.5) were coded to ‘No problems’ and ‘With problems’.
Summary scores for each EPIC‐26 domain were calculated by averaging standardised scores assigned to each question's responses in that domain (urinary incontinence: q2.2–q2.5a, urinary irritation/obstruction: q2.5b–q2.5f, bowel function: q2.7a–q2.8, sexual function: q2.9a–q2.13; File S1). For each domain the possible range of scores is 0–100, with 100 corresponding to no problems. The self‐assessed health rating (q1.6 – EuroQoL visual analogue scale [EQ‐VAS]) was used as a summary score of general health, with a higher score representing better general health.
Exclusions, Weighting and Missing Data
The sample was designed to match the age structure of prostate cancer survivors thereby allowing comparability of outcomes from this cohort with prostate cancer studies. Rates of prostate cancer increase with age 1, thus the proportion of respondents to the survey aged 40–49 years is lower compared to older ages (12.1% aged 40–59, 45.0% aged 60–69, 42.9% aged ≥75 years) (Table 1). As planned this is similar to the age distribution of prostate cancer survivors; however, it is not representative of the general NI population where 59.6% of men aged ≥40 years are aged 40–59 years 24. For the purposes of making comparisons with prostate cancer survivors no further adjustments are required. When utilising these data to report on the general NI population, weights by age and deprivation need to be applied so that the sample distribution matches that of the NI population. The weights required to increase the representativeness of the men aged 40–59 years from 12.1% to 59.6% would be large and need to be applied to a small number of respondents (358 men) resulting in less robust results. Thus, respondents aged 40–59 years were excluded prior to the calculation and application of survey weights, with analysis for the general population conducted for those aged ≥60 years only.
Table 1.
Response rates and characteristics of survey respondents
| Study response rate, % | Respondents*, n or % | NI population†, n or % | Survey data generalised to NI population‡, n (%) | ||||
|---|---|---|---|---|---|---|---|
| N | Age ≥40 years | Age ≥60 years | Age ≥40 years | Age ≥60 years | |||
| Total | 29.6 | 2955 | 2955 | 2597 | 397 977 | 160 818 | 2597 (100.0) |
| Age group, years | % | % | % | % | |||
| 40–59 | 22.6 | 358 | 12.1 | 59.6 | |||
| 60–69 | 34.7 | 1331 | 45.0 | 51.3 | 21.6 | 53.3 | 1385 (53.3) |
| 70–79 | 29.9 | 1045 | 35.4 | 40.2 | 12.9 | 32.0 | 830 (32.0) |
| ≥80 | 20.3 | 221 | 7.5 | 8.5 | 5.9 | 14.7 | 382 (14.7) |
| Deprivation indicator | |||||||
| Least deprived | 40.1 | 482 | 16.3 | 18.6 | 21.6 | 22.0 | 571 (22.0) |
| Quintile 2 | 33.1 | 538 | 18.2 | 20.7 | 20.6 | 20.0 | 519 (20.0) |
| Quintile 3 | 29.2 | 592 | 20.0 | 22.8 | 19.9 | 20.3 | 527 (20.3) |
| Quintile 4 | 27.5 | 480 | 16.2 | 18.5 | 19.9 | 20.0 | 519 (20.0) |
| Most deprived | 22.9 | 505 | 17.1 | 19.4 | 18.0 | 17.8 | 461 (17.8) |
*Age distribution matched to prostate cancer survivors. †Source: Northern Ireland Statistics and Research Agency 24. ‡By excluding those aged 40–59 years and weighting to the NI population by age and deprivation.
Missing data were dealt with on a question‐by‐question basis; men with missing responses were excluded from the analysis, thus all proportions and mean values refer to the men who responded to that question.
Statistical Analysis
Pairs of proportions were compared using Z‐tests, whilst chi‐squared tests were used to compare the distribution of responses across all categories in a variable. Weighted means (with standard deviation, median and interquartile range are included as supplementary data) are reported for continuous data such as the summary EPIC‐26 domains and self‐assessed health rating, with ANOVA used to compare distributions. The Bonferroni correction was applied to compensate for multiple comparisons in all scenarios.
Multivariable analyses of the EPIC‐26 domains and the self‐assessed health rating were conducted using log‐linear regression (backwards stepwise with the cut‐off P = 0.1) of the continuous scores. Respondent's age, deprivation indicator, urban/rural indicator, marital status, employment status, carer status, number of long‐term conditions, physical activity level, and body mass index (BMI), were investigated as independent variables. Regression residuals were not normally distributed while heteroscedasticity was also evident, thus standard errors were determined using bootstrapping. Results are presented as adjusted mean ratios relative to the baseline category. To investigate the relationship between urinary, bowel, and sexual dysfunction and general health, the self‐assessed health rating was grouped into quartiles and added separately to the log‐linear models for each EPIC‐26 domain.
To investigate the relationship between the same list of covariates and the individual EQ‐5D dimensions (with the outcome as ‘With problems’), binary logistic regression with robust standard errors was utilised with results presented as odds ratios (ORs).
Analysis was conducted using the Statistical Package for the Social Sciences (SPSS® version 22; SPSS Inc., IBM Corp., Armonk, NY, USA).
Results
In total, 10 000 men aged ≥40 years were sampled, with a response rate of 29.6% (2955 men). Response rates were highest for men aged 60–69 years and those who were resident in the least deprived areas (Table 1).
Completeness of data items was high, with 100% completeness for respondent characteristics provided by BSO (age, deprivation, urban/rural), whilst completeness of the self‐reported characteristics ranged from 91.1% for both height and weight (used to create BMI) to 95.7% for employment status. Completeness of the composite EPIC‐26 scores ranged from 73.3% for urinary irritation/obstruction to 91.0% for sexual function, whilst the self‐assessed health rating was 97.8% complete.
Results for each question along with mean composite scores from the EPIC‐26 and EQ‐5D‐5L survey instruments are presented in Tables S1–S3. Presented by age group (40–59, 60–69, 70–79 and ≥80 years) these data provide a baseline against which prostate cancer outcomes in similar populations can be measured.
Urinary, Bowel and Sexual Dysfunction in the General Population
Generalising the data to the NI population by excluding men aged 40–59 years and applying survey weights, 2597 men aged ≥60 years were available for analysis (a response rate of 30.9% in this group). In all, 53.3% of the study population were aged 60–69 years (n = 1385) compared to 14.7% aged ≥80 years (n = 382). In all, 22.0% of the study population resided in the least deprived areas compared to 17.8% in the most deprived areas (Table 1).
Urinary Incontinence
Almost one‐third (31.1%) of men aged ≥60 years reported some degree of urinary leakage, with 5.6% reporting moderate/big problems. In all, 35.6% of men reported some urinary control difficulty, with 6.2% of men reporting no urinary control or frequent dribbling. One‐quarter of men reported leaking urine more than once a week (26.4%), with 14.9% reporting leaking urine daily or more. When specifically asked about urinary function, 39.8% of men reported some level of difficulty, with 9.3% reporting moderate/big difficulties (Fig. 1, Table 2).
Figure 1.
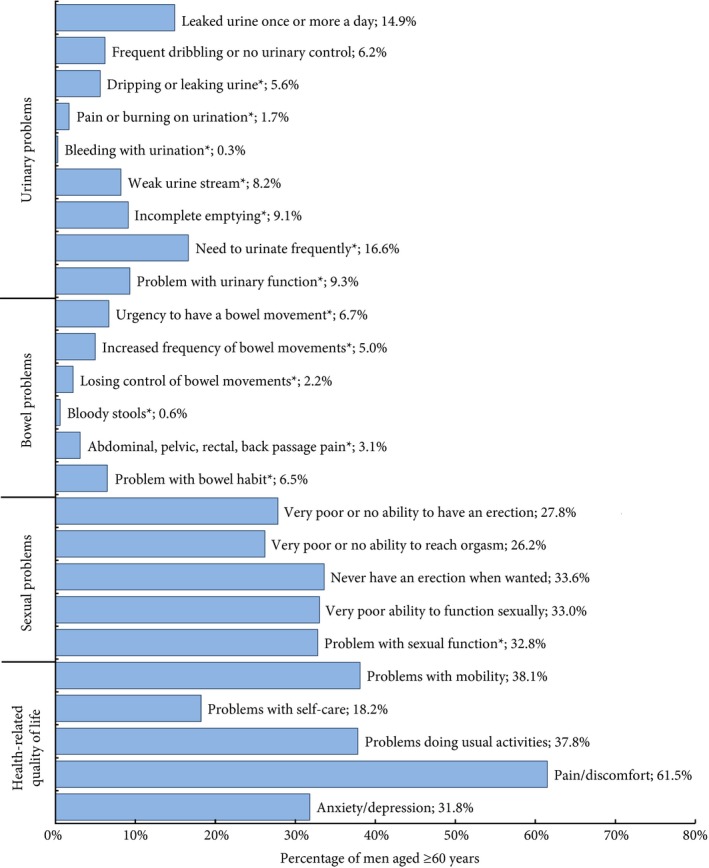
Urinary, bowel and sexual dysfunction and HRQL for men aged ≥60 years in NI. Data are weighted to the NI population by age and deprivation. Responses to individual EPIC‐26 and EQ‐5D‐5L questions, with *representing moderate/big problems. Complete responses to questions including a breakdown by age are available in Table S1.
Table 2.
Urinary, bowel and sexual dysfunction amongst men aged ≥60 years in NI by age, deprivation, number of long‐term conditions, physical activity, and BMI
| Variable | All respondents, n | Proportion of men aged ≥60 years reporting problems†, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Individual conditions | Combinations of conditions (n = 2281) | At least one of urinary, bowel or sexual dysfunction (n = 2281) | |||||||
| Urinary dysfunction (n = 2515) | Bowel dysfunction (n = 2547) | Sexual dysfunction (n = 2364) | Urinary and bowel dysfunction | Urinary and sexual dysfunction | Bowel and sexual dysfunction | Urinary, bowel and sexual dysfunction | |||
| Total | 2597 | 9.3 | 6.5 | 32.8 | 2.9 | 5.4 | 4.0 | 2.1 | 38.1 |
| Age group, years | P < 0.001* | P = 0.081 | P < 0.001* | P = 0.316 | P = 0.155 | P = 0.230 | P = 0.766 | P < 0.001* | |
| 60–69 | 1385 | 7.3 | 6.0 | 27.2 | 2.6 | 4.7 | 3.6 | 1.9 | 31.5 |
| 70–79 | 830 | 10.1 | 6.2 | 36.6 | 2.8 | 6.8 | 4.0 | 2.3 | 41.9 |
| ≥80 | 382 | 15.1 | 9.0 | 47.4 | 4.4 | 5.2 | 6.0 | 2.4 | 58.5 |
| Deprivation indicator | P = 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | |
| Least deprived | 571 | 6.7 | 3.8 | 26.4 | 0.5 | 1.8 | 2.0 | 0.0 | 32.1 |
| Quintile 2 | 519 | 8.0 | 5.2 | 28.4 | 1.4 | 4.4 | 1.9 | 0.8 | 33.5 |
| Quintile 3 | 527 | 8.4 | 4.7 | 30.6 | 1.6 | 4.6 | 2.1 | 0.9 | 36.6 |
| Quintile 4 | 519 | 10.1 | 9.0 | 38.0 | 4.7 | 8.3 | 6.4 | 4.1 | 41.8 |
| Most deprived | 461 | 14.3 | 10.6 | 42.1 | 7.1 | 9.1 | 8.5 | 5.5 | 48.2 |
| Number of long‐term conditions | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | |
| None | 747 | 5.3 | 0.9 | 19.3 | 0.4 | 1.8 | 0.5 | 0.4 | 22.7 |
| 1–2 | 1311 | 7.0 | 4.9 | 32.5 | 1.7 | 4.2 | 2.7 | 1.2 | 37.7 |
| ≥3 | 540 | 20.4 | 18.2 | 52.4 | 9.5 | 13.8 | 12.3 | 6.8 | 60.5 |
| Physical activity | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P = 0.001* | P < 0.001* | P = 0.015 | P < 0.001* | |
| None | 717 | 13.5 | 11.5 | 44.9 | 4.9 | 8.0 | 7.8 | 3.5 | 51.7 |
| 1–4 days of 30 min/day | 1164 | 6.9 | 5.3 | 28.6 | 2.0 | 4.6 | 3.0 | 1.6 | 33.2 |
| 5–7 days of 30 min/day | 486 | 7.0 | 3.5 | 27.2 | 2.2 | 3.6 | 2.3 | 1.4 | 31.5 |
| BMI, kg/m 2 | P = 0.003* | P = 0.002* | P < 0.001* | P = 0.004* | P < 0.001* | P = 0.001* | P = 0.002* | P < 0.001* | |
| Under and healthy weight (0–25) | 671 | 9.4 | 6.5 | 27.6 | 2.5 | 5.1 | 3.3 | 1.9 | 34.5 |
| Overweight (25–30) | 1060 | 7.2 | 5.1 | 31.3 | 2.3 | 4.3 | 3.2 | 1.5 | 35.4 |
| Obese (≥30) | 749 | 12.4 | 9.7 | 44.1 | 5.4 | 9.6 | 7.1 | 4.2 | 49.1 |
Data are weighted to the NI population by age and deprivation. Men can have multiple problems and thus may appear in more than one table column. †Moderate or big problems. *Significant at P < 0.05, after Bonferroni correction for multiple comparisons.
In multivariable analyses, urinary incontinence, based upon the EPIC‐26 score (mean 89.0, median 100.0), increased with increasing age (P = 0.048), deprivation (P = 0.024), number of long‐term conditions (P = 0.001), higher BMI (P = 0.045), and lower levels of physical activity (P < 0.001). Unemployed men were more likely to report urinary incontinence compared to employed men (P = 0.036) (Table 3).
Table 3.
Adjusted urinary, bowel and sexual function mean score ratios (EPIC‐26) for men aged ≥60 years in NI by demographic, socio‐economic and health‐related characteristics
| Variable | Adjusted mean ratio (95% CI) | |||
|---|---|---|---|---|
| Urinary incontinence (n = 1691) | Urinary irritation/obstructive (n = 1668) | Bowel function (n = 1821) | Sexual function (n = 2007) | |
| Age group, years | ||||
| 60–69 | 1.00 | 1.00 | 1.00 | |
| 70–79 | 0.98 (0.96,1.00) | 0.99 (0.97,1.00) | N/S | 0.78 (0.73,0.82) |
| ≥80 | 0.96 (0.92,1.00) | 0.96 (0.92,1.00) | 0.42 (0.35,0.50) | |
| Deprivation indicator | ||||
| Least deprived | 1.00 | N/S | N/S | N/S |
| Quintile 2 | 0.99 (0.96,1.01) | |||
| Quintile 3 | 0.98 (0.96,1.01) | |||
| Quintile 4 | 1.00 (0.98,1.03) | |||
| Most deprived | 0.95 (0.92,0.98) | |||
| Urban/rural indicator | ||||
| Urban | N/S | N/S | 1.00 | N/S |
| Rural | 1.01 (1.00,1.02) | |||
| Employment status | ||||
| Employed/self‐employed | 1.00 | 1.00 | 1.00 | |
| Unemployed | 0.91 (0.83,0.98) | N/S | 0.91 (0.86,0.97) | 0.76 (0.63,0.89) |
| Retired | 0.98 (0.96,1.00) | 1.00 (0.99,1.02) | 0.90 (0.86,0.95) | |
| Other | 0.98 (0.90,1.04) | 0.99 (0.94,1.04) | 0.88 (0.72,1.01) | |
| Number of long‐term conditions | ||||
| None | 1.00 | 1.00 | 1.00 | 1.00 |
| 1–2 | 0.98 (0.96,1.00) | 0.96 (0.95,0.98) | 0.98 (0.97,0.99) | 0.84 (0.79,0.88) |
| ≥3 | 0.90 (0.87,0.93) | 0.89 (0.87,0.91) | 0.90 (0.88,0.92) | 0.58 (0.52,0.64) |
| Physical activity | ||||
| None | 1.00 | 1.00 | 1.00 | 1.00 |
| 1–4 days of 30 min/day | 1.04 (1.01,1.07) | 1.02 (0.99,1.04) | 1.01 (1.00,1.03) | 1.23 (1.14,1.32) |
| 5–7 days of 30 min/day | 1.07 (1.04,1.10) | 1.03 (1.00,1.05) | 1.03 (1.01,1.04) | 1.31 (1.21,1.41) |
| BMI, kg/m 2 | ||||
| Under and healthy weight (0–25) | 1.00 | 1.00 | 1.00 | 1.00 |
| Overweight (25–30) | 1.01 (0.99,1.04) | 1.01 (0.99,1.02) | 1.01 (1.00,1.03) | 0.99 (0.95,1.04) |
| Obese (≥30) | 0.98 (0.95,1.01) | 0.98 (0.96,1.01) | 0.99 (0.97,1.01) | 0.83 (0.77,0.91) |
Data are weighted to the NI population by age and deprivation. The adjusted mean score ratio was determined using a log‐linear regression model with other significant variables as covariates. A value <1 can be interpreted as poorer functioning compared to the baseline category, whilst a value >1 can be interpreted as better functioning compared to the baseline category. N/S, not significant. Carer and marital status were not significant for any score. Unadjusted EPIC‐26 scores by sociodemographic factors along with further descriptive data are available in Tables S2 and S3 and Fig. S1.
Urinary Irritation/Obstruction/Function
In all, 16.6% of men aged ≥60 years reported needing to urinate frequently as a moderate/big problem. Incomplete emptying was reported by 9.1%, bleeding with urination by 0.3%, and pain or burning on urination by 1.7% (Fig. 1).
Based upon multivariable analysis of the EPIC‐26 score (mean 88.5, median 93.8) urinary irritation/obstruction problems were associated with increasing age (P = 0.072), higher number of long‐term conditions (P < 0.001), BMI (overweight vs obese, P = 0.047), and low physical activity (none vs 5–7 days/week, P = 0.019) (Table 3).
Bowel Function
Bowel problems were reported to some degree by 26.1% of men aged ≥60 years, with 6.5% reporting moderate/big problems. Increased urgency (6.7%) and frequency of bowel movement (5.0%) were the most common problems, with abdominal, pelvic, rectal or back passage pain noted by 3.1%, and bloody stools reported by 0.6% of men (Fig. 1, Table 2).
After multivariable adjustments poorer bowel function scores (mean 93.6, median 100.0) were more commonly reported by those resident in urban areas (P = 0.040), unemployed (P = 0.013), with three or more long‐term conditions (P < 0.001), no physical activity in the previous week (P = 0.019), and high BMI (P = 0.025) (Table 3).
Sexual Function
Three out of five (57.9%) men reported some problem with sexual function, with 32.8% of all men reporting the problem as moderate/big and a similar proportion (33.0%) reporting very poor sexual functioning (Fig. 1, Table 2).
In multivariate analyses of the EPIC‐26 score (mean 50.0, median 52.8) associations existed between sexual dysfunction and age, employment status, number of long‐term conditions, physical activity, and BMI (all P ≤ 0.001) (Table 3).
Combinations of Urinary Tract, Bowel and Sexual Dysfunction
Two out of five men (38.1%) reported at least one of urinary, bowel or sexual dysfunction, with 2.1% indicating they had all three issues (Fig. 2). Combinations of all three problems were more prevalent amongst men resident in deprived areas (P < 0.001), with increasing number of long‐term conditions (P < 0.001), and with higher BMI (P = 0.002) (Table 2).
Figure 2.
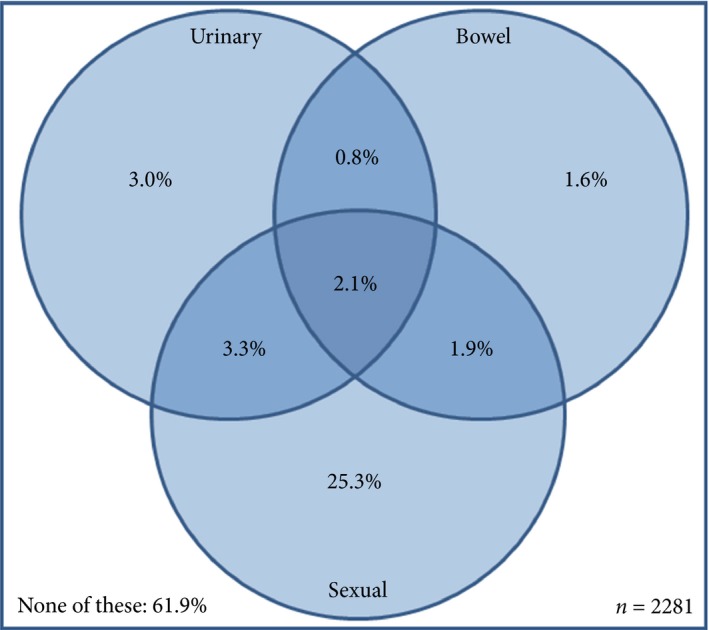
Combinations of reported urinary, bowel and sexual dysfunction amongst men aged ≥60 years in NI. Data are weighted to the NI population by age and deprivation. Venn diagram is based upon the proportion of men reporting moderate/big problems in response to specific questions from the EPIC‐26 question set (urinary: q2.6, bowel: q2.8, sexual: q2.13; File S1).
HRQL in the General Population
In all, 61.5% of men aged ≥60 years reported some degree of pain/discomfort, whilst problems with mobility were reported by 38.1%, performing usual activities by 37.8%, and anxiety/depression by 31.8%. One in five men (18.2%) had problems with self‐care (Fig. 1).
Adjusted ORs for problems in all five domains increased with increasing number of long‐term conditions, decreasing levels of physical activity and, except for anxiety/depression, with increasing BMI. Mobility problems and difficulties performing usual activities were more frequent in older men, whilst anxiety/depression levels decreased with increasing age. Reported problems in each domain increased with deprivation, with the exception of pain/discomfort, whilst living in an urban area was associated with reduced mobility and usual activities. Unemployed men reported more problems than employed or retired men. Married men reported fewer problems with mobility, self‐care, and anxiety/depression than other marital status groups, whilst having carer responsibilities was not associated with any of the five dimensions (Table 4).
Table 4.
Adjusted HRQL ORs (EQ‐5D‐5L) and adjusted self‐assessed health rating mean score ratios (EuroQoL visual analogue scale [EQ‐VAS]) for men aged ≥60 years in NI by demographic, socio‐economic and health‐related characteristics
| Variable | OR (95% CI) | Mean ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Mobility (n = 2117) | Self‐care (n = 2120) | Usual activities (n = 2153) | Pain/discomfort (n = 2153) | Anxiety/depression (n = 2278) | Self‐assessed health rating (EQ‐VAS) (n = 2120) | |
| Age group, years | ||||||
| 60–69 | 1.00 | 1.00 | N/S | 1.00 | 1.00 | |
| 70–79 | 1.37 (1.08,1.73) | N/S | 1.14 (0.91,1.43) | 0.63 (0.51,0.79) | 1.01 (0.99,1.03) | |
| ≥80 | 2.64 (1.71,4.08) | 1.98 (1.33,2.94) | 0.63 (0.43,0.92) | 0.97 (0.93,1.00) | ||
| Deprivation indicator | ||||||
| Least deprived | 1.00 | 1.00 | 1.00 | N/S | 1.00 | 1.00 |
| Quintile 2 | 1.28 (0.90,1.82) | 1.68 (0.96,2.95) | 1.51 (1.08,2.12) | 0.86 (0.61,1.19) | 0.99 (0.97,1.02) | |
| Quintile 3 | 1.52 (1.06,2.18) | 1.84 (1.05,3.23) | 1.43 (1.01,2.02) | 0.99 (0.72,1.36) | 0.97 (0.95,0.99) | |
| Quintile 4 | 1.60 (1.10,2.35) | 2.66 (1.53,4.62) | 1.62 (1.13,2.33) | 1.23 (0.88,1.71) | 0.97 (0.95,1.00) | |
| Most deprived | 1.75 (1.21,2.52) | 2.65 (1.55,4.56) | 1.62 (1.13,2.32) | 1.51 (1.10,2.08) | 0.95 (0.92,0.98) | |
| Urban/rural indicator | ||||||
| Urban | 1.00 | N/S | 1.00 | N/S | N/S | 1.00 |
| Rural | 0.68 (0.53,0.88) | 0.78 (0.62,0.99) | 1.02 (1.01,1.04) | |||
| Marital status * | ||||||
| Married | 1.00 | 1.00 | N/S | N/S | 1.00 | 1.00 |
| Separated/divorced | 1.51 (1.06,2.16) | 1.40 (0.87,2.25) | 1.37 (1.00,1.89) | 0.96 (0.93,1.00) | ||
| Widowed | 1.68 (1.10,2.57) | 1.97 (1.24,3.12) | 1.42 (0.98,2.05) | 0.98 (0.94,1.01) | ||
| Single | 1.14 (0.72,1.82) | 1.65 (0.95,2.89) | 1.28 (0.85,1.91) | 1.02 (0.98,1.06) | ||
| Employment status | ||||||
| Employed/self‐employed | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Unemployed | 7.92 (4.28,14.65) | 14.64 (7.83,27.39) | 11.18 (5.87,21.28) | 2.84 (1.53,5.30) | 6.26 (3.55,11.03) | 0.71 (0.65,0.78) |
| Retired | 1.55 (1.16,2.07) | 2.53 (1.66,3.86) | 1.68 (1.27,2.21) | 1.34 (1.07,1.67) | 1.28 (0.99,1.66) | 0.96 (0.94,0.98) |
| Other | 2.43 (1.03,5.71) | 1.87 (0.45,7.76) | 1.78 (0.69,4.59) | 1.63 (0.74,3.60) | 2.36 (1.11,5.04) | 0.88 (0.78,0.97) |
| Number of long‐term conditions | ||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1–2 | 2.41 (1.78,3.27) | 2.71 (1.56,4.71) | 2.36 (1.77,3.15) | 2.15 (1.72,2.68) | 1.44 (1.12,1.85) | 0.93 (0.92,0.95) |
| ≥3 | 7.75 (5.41,11.10) | 9.51 (5.37,16.84) | 7.36 (5.21,10.39) | 5.30 (3.84,7.33) | 3.78 (2.78,5.12) | 0.78 (0.75,0.80) |
| Physical activity | ||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1–4 days of 30 min/day | 0.38 (0.30,0.50) | 0.28 (0.20,0.38) | 0.39 (0.30,0.50) | 0.60 (0.46,0.77) | 0.57 (0.45,0.72) | 1.12 (1.09,1.15) |
| 5–7 days of 30 min/day | 0.18 (0.13,0.25) | 0.18 (0.12,0.27) | 0.22 (0.16,0.30) | 0.48 (0.37,0.63) | 0.41 (0.31,0.53) | 1.17 (1.14,1.21) |
| BMI, kg/m 2 | ||||||
| Under and healthy weight (0–25) | 1.00 | 1.00 | 1.00 | 1.00 | N/S | 1.00 |
| Overweight (25–30) | 1.11 (0.86,1.44) | 0.82 (0.58,1.16) | 1.03 (0.80,1.33) | 1.06 (0.85,1.32) | 1.01 (0.99,1.03) | |
| Obese (30+) | 1.77 (1.29,2.42) | 1.38 (0.93,2.03) | 1.55 (1.15,2.10) | 1.71 (1.29,2.28) | 0.98 (0.96,1.01) | |
Data is weighted to the NI population by age and deprivation. The adjusted ORs were determined using a logistic regression model with other significant variables as covariates. The adjusted mean score ratio was determined using a log‐linear regression model with other significant variables as covariates. A value <1 can be interpreted as poorer health compared to the baseline category, while a value >1 can be interpreted as better health compared to the baseline category. N/S, not significant. Carer status was not significant for any score. *Includes civil partnership equivalents. Unadjusted HRQL data by sociodemographic factors along with further descriptive data are available in Tables S4 and S5 and Fig. S2.
General Health
In multivariate analyses, based upon self‐assessed health rating (mean 77.2, median 80.0), poorer general health was associated with age (P = 0.074), deprivation (P = 0.001), marital status (P = 0.071), urbanity (P = 0.008), unemployment (P < 0.001), higher numbers of long‐term conditions (P < 0.001), greater BMI (P = 0.044), and lower physical activity levels (P < 0.001) (Table 4).
Relationship between General Health and Urinary, Bowel and Sexual Dysfunction
Increasing urinary, bowel and sexual dysfunction were associated with poorer general health in both univariable and multivariable analysis (all P < 0.001). The relationship was greatest for sexual dysfunction, with the mean sexual function domain score decreasing from 62.2 amongst men reporting good general health (score ≥90) to 29.7 for men reporting poorer general health (score <70). The weakest relationship was between self‐assessed health rating and bowel dysfunction (Table 5).
Table 5.
Relationship between urinary, bowel and sexual function (EPIC‐26) and general health (self‐assessed health rating) for men aged ≥60 years in NI
| Mean urinary, bowel and sexual function scores (EPIC‐26) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Urinary incontinence (n = 1949) | Urinary irritation/obstructive (n = 1847) | Bowel function (n = 2089) | Sexual function (n = 2323) | |||||
| Unadjusted mean | Adjusted mean ratio | Unadjusted mean | Adjusted mean ratio | Unadjusted mean | Adjusted mean ratio | Unadjusted mean | Adjusted mean ratio | |
| Total | 89.0 | – | 88.5 | – | 93.6 | – | 50.0 | – |
| Self‐assessed health rating (EQ‐VAS) | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* | P < 0.001* |
| ≥90 (better health) | 94.5 | 1.00 | 93.2 | 1.00 | 97.4 | 1.00 | 62.2 | 1.00 |
| 80–89.9 | 90.7 | 0.97 | 89.2 | 0.95 | 94.3 | 0.98 | 52.7 | 0.90 |
| 70–79.9 | 88.4 | 0.95 | 86.6 | 0.93 | 93.2 | 0.98 | 44.4 | 0.88 |
| <70 (poorer health) | 77.8 | 0.88 | 80.2 | 0.86 | 86.3 | 0.94 | 29.7 | 0.66 |
Data are weighted to the NI population by age and deprivation. The adjusted mean score ratio was determined using a log‐linear regression model with significant variables from Table 3 used as covariates. A value <1 can be interpreted as poorer functioning compared to the baseline category, whilst a value >1 can be interpreted as better functioning compared to the baseline category. *Significant at P < 0.05 after Bonferroni correction for multiple comparisons (correction applies to unadjusted results only).
Discussion
The present study provides the most comprehensive description of urinary, bowel and sexual function, and their relationship to general health in elderly men resident in NI to date. It is specifically designed to provide a baseline to facilitate better estimation of the effects of prostate cancer and its treatments compared to the general population.
The data also allow a detailed assessment of the prevalence of these conditions in the general population. Almost two out of five (38.1%) men reported at least one of sexual, urinary and bowel function problem to a moderate/big degree. Sexual function issues were the most common with one‐third of men reporting moderate or big problems, whilst 9.3% reported urinary dysfunction and 6.5% bowel dysfunction. A considerable proportion of additional men reported these problems to a small/very small degree, while men often experience multiple problems.
The present study adds information on sociodemographic, health‐related factors and general health, and their associations with urinary, bowel and sexual difficulties. With the exception of bowel dysfunction these problems increased with increasing age. The prevalence of these difficulties was higher amongst those with higher BMI, lower physical activity levels, greater number of long‐term conditions, and poorer general health. However, given the cross‐sectional nature of the study these relationships are likely to be interrelated and we cannot draw conclusions about cause and effect. In addition, the lack of longitudinal data means that the results do not provide any information on reporting of how problems change over time with age. Nonetheless, these findings are of public health interest in light of the increasingly sedentary lifestyle and rising levels of obesity in the population 16.
Comparison with Previous Studies
Our present findings on the prevalence of LUTS and faecal incontinence are comparable to other studies 5, 6, 12. However, we found a lower prevalence of moderate‐to‐severe urinary incontinence (5.6% vs 11–16%) 5, 6, 7, 8 than previously reported, possibly a result of using a much shorter time period for symptom reporting (1 vs 6–12 months). Conversely, we have identified a greater proportion with poor/no ability to have an erection (27.8% vs 5–10%) 4, 9, 10, 11; the difference is likely to be due to our cohort being slightly older (aged ≥60 vs 40–80 years). With the exception of the relationship to age 4, 12, 13 and some specific health conditions 25, 26, the associations with health‐related characteristics have not previously been reported. However, two North American studies specifically noted a lack of association that the present study found: One identifying no relationship between erectile dysfunction and physical activity 10, and another showing no relationship between faecal incontinence and BMI, physical activity, or number of chronic conditions 13.
Implications for Primary Care
Primary care teams are well‐placed initially to deal with problems relating to sexuality and urinary and bowel dysfunction; however, the extent of management in primary care appears limited 27. A lack of pro‐activity in relation to problems around sexual activity exists 28, with GPs having a lack of awareness, knowledge and confidence in dealing with sexual problems 29, 30. Embarrassment, negative attitudes toward sexuality in elderly people, and health professional disinterest can all inhibit discussions about these issues 29.
There is variation in the ability of GPs to deal with LUTS, and often reluctance to treat such conditions 30, 31. Combined with patient factors, such as unwillingness to acknowledge the problem 32, 33, there are numerous barriers to the appropriate management of urinary symptoms in the elderly. Primary care needs to be more pro‐active in identifying, managing and referring patients with these symptoms. If clinical contact is made, most men with LUTS, bowel and/or sexual dysfunction can potentially be managed effectively in primary care with lifestyle advice, counselling or medical therapy 34, and onward referral to urology services where necessary.
Study Limitations
The response rate of 29.6% is lower than what would normally be expected from a general postal survey, but is similar to the 30–44% response rate of other postal surveys exploring detailed personal/sexual issues 11, 35, 36, including the widely used multinational survey of the aging male 4. This is possibly a consequence of the use of a postal‐only delivery method, the inclusion of very elderly men in the cohort, the length of time needed to complete the survey, and the inclusion of highly personal sexual dysfunction questions. The less than optimal response rate could potentially result in response bias, with urinary, bowel and sexual dysfunction different amongst non‐responders than for those who completed the survey. Similarly there may be a difference between men who partially and those who fully completed the survey. The impact of these issues is difficult to quantify given the lack of information on this topic in NI. Nevertheless, a sample of almost 3000 men was obtained with an age/deprivation distribution that only deviated slightly from that of the NI population. In addition, the proportion of men classified as obese in the present study is very similar to that in the NI health survey conducted in 2016/17 37 (30.2% aged ≥60% vs 31.4% aged ≥65 years), whilst results for the EQ‐5D amongst those aged ≥75 years from the same survey conducted in 2012/13 38 compare favourably to the present results for those aged ≥80 years (mobility: 55% vs 61%; self‐care: 25% vs 27%, usual activities: 50% vs 59%, pain/discomfort: 65% vs 62%, anxiety/depression: 30% vs 25%). Both comparisons suggest that the present study, aided by weighting adjustments, accurately represents the health of the NI population.
The present study was specifically designed to provide baseline data against which prostate cancer outcomes could be compared. Using the data for purposes other than this, such as generalising the data to the general population, has some limitations. Firstly, the exclusion of men with prostate cancer may result in an underestimation of the magnitude of urinary, bowel and sexual problems across the whole population. Secondly, the EPIC‐26 question set provides respondent‐rated symptoms rather than clinical assessment; they are thus subjective in that not all reported problems may require treatment or some men may have reported a problem as being small but would still benefit from health care intervention. Finally, this question set while validated for prostate cancer survivors has not been validated in the general population.
NI is broadly similar in terms of age and healthcare provision to the rest of the UK; however, there are differences which must be recognised when generalising the data to the entire UK. In particular, NI has a lower representation of ethnic minorities 24, higher unemployment 39, and lower life expectancy than the UK average 15, meaning that reported levels of urinary, bowel and sexual dysfunction in NI may be higher than in the UK overall. Similar differences are likely to be experienced if the data are used in other countries, thus in utilising the data outside of NI it may be beneficial to weight the presented results by age (to reflect the age distribution of the country being compared to), or to make any comparisons only for specific subgroups of the population (e.g. by excluding ethnic minorities or the most affluent from data from other countries).
Conclusions
Urinary tract, bowel and sexual dysfunction are common amongst men aged ≥60 years. The high population prevalence must be considered when evaluating the impact of specific diseases and their treatments on function, otherwise inappropriate advice and therapies may be provided.
With almost two out of five men aged ≥60 years reporting moderate/big problems in at least one of these areas of function, there are clear implications for service providers and a need to encourage men experiencing difficulties to seek assistance. The reported problems are associated with the presence of long‐term conditions, lower physical activity levels, higher BMI, age, and lower socio‐economic status, with a strong relationship to general health also identified. This suggests that opportunities exist to reduce prevalence of these conditions through continued promotion of healthy lifestyles and by addressing health inequalities associated with socio‐economic status.
Funding
The LAPCD study was funded by the Movember Foundation, in partnership with Prostate Cancer UK, as part of the Prostate Cancer Outcomes programme, grant number BO26/MO.
Ethical Approval
Ethical approval was granted by The Office of Research Ethics Committees NI (ORECNI). Queen's University, Belfast was the study sponsor.
Conflicts of Interest
Eila Watson reports grants from Oxford Brookes University during the conduct of the study. All other authors declare no competing interests.
Abbreviations
- BMI
body mass index
- BSO
Health and Social Care Business Services Organisation
- EPIC‐26
26‐item Expanded Prostate Cancer Composite (questionnaire)
- EQ‐5D‐5L
EuroQoL five Dimensions five Levels (instrument)
- EQ‐VAS
EuroQoL visual analogue scale
- HRQL
health‐related quality of life
- LAPCD
Life After Prostate Cancer Diagnosis (study)
- NI
Northern Ireland
- OR
odds ratio
Supporting information
Table S1. Responses to EPIC‐26 questions by age group.
Table S2. Urinary, bowel and sexual function scores (EPIC‐26) for men aged ≥60 years by demographic, socio‐economic and health‐related characteristics.
Table S3. Urinary, bowel and sexual function scores (EPIC‐26) for men aged ≥60 years in NI by demographic, socio‐economic and health‐related characteristics – Detailed descriptive statistics.
Table S4. HRQL (EQ‐5D‐5L) and self‐assessed health rating EuroQoL visual analogue scale (EQ‐VAS) in men aged ≥60 years by demographic, socio‐economic and health‐related characteristics.
Table S5. Self‐assessed health rating (EQ‐VAS) for men aged ≥60 years in NI by demographic, socio‐economic and health‐related characteristics – Detailed descriptive statistics.
Figure S1. Urinary, bowel and sexual function scores (EPIC‐26) for men aged ≥60 years in NI.
Figure S2. Self‐assessed health rating (EQ‐VAS) for men aged ≥60 years in NI.
File S1. Men's Health & Wellbeing Survey.
Acknowledgements
The authors thank all the men who responded to the survey. We acknowledge the following people for their contribution to the development, setting up, and running of the study: Heather Kinnear, Oonagh McSorley, Victoria Cairnduff, Linda Roberts, Adrian Slater, the LAPCD User Advisory Group and Clinical and Scientific Advisory Group, Picker Institute Europe and Business Services Organisation (NI). The authors also thank Age Concern in NI for providing feedback on the survey content and layout.
A.G and A.W.G. are co‐senior authors.
References
- 1. Northern Ireland Cancer Registry . Prostate cancer. Available at: http://www.qub.ac.uk/research-centres/nicr/cancerinformation/official-statistics/bysite/prostate.html. Accessed December 2017
- 2. Cancer Research UK . Prostate cancer incidence statistics. Available at: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence. Accessed December 2017
- 3. Glaser AW, Corner JL. Prostate cancer outcomes: the three questions. Eur Urol 2015; 67: 357–8 [DOI] [PubMed] [Google Scholar]
- 4. Rosen R, Altwein J, Boyle P et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM‐7). Eur Urol 2003; 44: 637–49 [DOI] [PubMed] [Google Scholar]
- 5. Coyne KS, Sexton CC, Thompson CL. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int 2009; 104: 352–60 [DOI] [PubMed] [Google Scholar]
- 6. Irwin DE, Milson I, Hunskaar S et al. Population‐based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006; 50: 1306–15 [DOI] [PubMed] [Google Scholar]
- 7. Markland AD, Goode PS, Redden DT, Borrud LG, Burgio KL. Prevalence of urinary incontinence in men: results from the National Health and Nutrition Examination Survey. J Urol 2010; 184: 1022–7 [DOI] [PubMed] [Google Scholar]
- 8. White AJ, Reeve BB, Chen RC, Stover AM, Irwin DE. Urinary incontinence and health‐related quality of life among older Americans with and without cancer: a cross‐sectional study. BMC Cancer 2013; 13: 377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moreira ED, Glasser DB, Nicolosit A, Duarte FG, Gingell C, GSSAB Investigators’ Group . Sexual problems and help seeking behavior in adults in the United Kingdom and continental Europe. BJU Int 2008; 101: 1005–11 [DOI] [PubMed] [Google Scholar]
- 10. Laumann EO, Glasser DB, Neves RC, Moreira ED,GSSAB Investigators’ Group . A population‐based survey of sexual activity, sexual problems and associated help‐seeking behavior patterns in mature adults in the United States of America. Int J Impot Res 2009; 21: 171–8 [DOI] [PubMed] [Google Scholar]
- 11. Quilter M, Hodges L, von Hurst P, Borman B, Coad J. Male sexual function in New Zealand: a population‐based cross‐sectional survey of the prevalence of erectile dysfunction in men aged 40‐70 years. J Sex Med 2017; 14: 928–36 [DOI] [PubMed] [Google Scholar]
- 12. Perry S, Shaw C, McGrother C et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut 2002; 50: 480–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whitehead WE, Borrud L, Goode PS et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology 2009; 137: 512–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meinds RJ, van Meegdenburg MM, Trzpis M, Broens PM. On the prevalence of constipation and fecal incontinence, and their co‐occurrence, in the Netherlands. Int J Colorectal Dis 2017; 32: 475–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Office of National Statistics . National Lifetables, UK: 2013–2015. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables. Accessed July 2017
- 16. NHS Digital . Health survey for England 2015: trend tables. Available at: https://content.digital.nhs.uk/catalogue/PUB22616. Accessed August 2017
- 17. Downing A, Wright P, Wagland R et al. Life after prostate cancer diagnosis: protocol for a UK‐wide patient‐reported outcomes study. BMJ Open 2016; 6: e013555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Northern Ireland Statistics and Research Agency . Northern Ireland Multiple Deprivation Measure. Available at: https://www.nisra.gov.uk/statistics/deprivation/northern-ireland-multiple-deprivation-measure-2010-nimdm2010. Accessed July 2017
- 19. Northern Ireland Statistics and Research Agency . Urban – Rural Classification. Available at: https://www.nisra.gov.uk/support/geography/urban-rural-classification. Accessed July 2017
- 20. Herdman M, Gudex C, Lloyd A et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res 2011; 20: 1727–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health‐related quality of life among prostate cancer survivors. Urology 2010; 76: 1245–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin NE, Massey L, Stowell C et al. Defining a standard set of patient‐centered outcomes for men with localized prostate cancer. Eur Urol 2015; 67: 460–7 [DOI] [PubMed] [Google Scholar]
- 23. Morgans AK, van Bommel ACM, Stowell C et al. Development of a standardized set of patient‐centered outcomes for advanced prostate cancer: an international effort for a unified approach. Eur Urol 2015; 68: 891–8 [DOI] [PubMed] [Google Scholar]
- 24. Northern Ireland Statistics and Research Agency . 2011 Census. Available at: https://www.nisra.gov.uk/statistics/census. Accessed July 2017
- 25. Gandaglia G, Briganti A, Jackson G et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014; 65: 968–78 [DOI] [PubMed] [Google Scholar]
- 26. Kouidrat Y, Pizzol D, Cosco T et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta‐analysis of 145 studies. Diabet Med 2017; 34: 1185–92 [DOI] [PubMed] [Google Scholar]
- 27. Watson EK, O'Brien R, Campbell C et al. Views of health professionals on the role of primary care in the follow‐up of men with prostate cancer. Fam Pract 2011; 28: 647–54 [DOI] [PubMed] [Google Scholar]
- 28. Gott M, Hinchliff S. Barriers to seeking treatment for sexual problems in primary care: a qualitative study with older people. Fam Pract 2003; 20: 690–5 [DOI] [PubMed] [Google Scholar]
- 29. Bauer M, Haesler E, Fetherstonhaugh D. Let's talk about sex: older people's views on the recognition of sexuality and sexual health in the health‐care setting. Health Expect 2016; 19: 1237–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nguyen K, Hunter KF, Wagg A. Knowledge and understanding of urinary incontinence: survey of family practitioners in northern Alberta. Can Fam Physician 2013; 59: e330–7 [PMC free article] [PubMed] [Google Scholar]
- 31. Teunissen D, van den Bosch W, van Weel C, Lagro‐Janssen T. Urinary incontinence in the elderly: attitudes and experiences of general practitioners. A focus group study. Scand J Prim Health Care 2006;24:56–61 [DOI] [PubMed] [Google Scholar]
- 32. Whitaker KL, Macleod U, Winstanley K, Scott SE, Wardle J. Help seeking for cancer ‘alarm’ symptoms: a qualitative interview study of primary care patients in the UK. Br J Gen Pract 2015; 65: e96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forbes LJ, Simon AE, Warburton F et al. Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): so they contribute to cancer survival? Br J Cancer 2013; 108: 292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rees J, Bultitude M, Challacombe B. The management of lower urinary tract symptoms in men. BMJ 2014; 348: g3861 [DOI] [PubMed] [Google Scholar]
- 35. Dunn KM, Croft PR, Hackett GI. Sexual problems: a study of the prevalence and need for health care in the general population. Fam Pract 1998; 15: 519–24 [DOI] [PubMed] [Google Scholar]
- 36. Chew KK, Stuckey B, Bremner A et al. Male erectile dysfunction: its prevalence in Western Australia and associated sociodemographic factors. J Sex Med 2008; 5: 60–9 [DOI] [PubMed] [Google Scholar]
- 37. Department of Health . Health Survey (NI): First results 2015/16. Available at: https://www.health-ni.gov.uk/sites/default/files/publications/health/hsni-first-results-15-16.pdf. Accessed December 2017
- 38. Department of Health . Health Survey Northern Ireland – 2012/13. Available at: https://www.health-ni.gov.uk/sites/default/files/publications/dhssps/hsni-first-results-12-13.pdf. Accessed December 2017
- 39. Office of National Statistics . Regional labour market statistics in the UK: August 2017. Available at: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/bulletins/regionallabourmarket/august2017#unemployment. Accessed August 2017
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Responses to EPIC‐26 questions by age group.
Table S2. Urinary, bowel and sexual function scores (EPIC‐26) for men aged ≥60 years by demographic, socio‐economic and health‐related characteristics.
Table S3. Urinary, bowel and sexual function scores (EPIC‐26) for men aged ≥60 years in NI by demographic, socio‐economic and health‐related characteristics – Detailed descriptive statistics.
Table S4. HRQL (EQ‐5D‐5L) and self‐assessed health rating EuroQoL visual analogue scale (EQ‐VAS) in men aged ≥60 years by demographic, socio‐economic and health‐related characteristics.
Table S5. Self‐assessed health rating (EQ‐VAS) for men aged ≥60 years in NI by demographic, socio‐economic and health‐related characteristics – Detailed descriptive statistics.
Figure S1. Urinary, bowel and sexual function scores (EPIC‐26) for men aged ≥60 years in NI.
Figure S2. Self‐assessed health rating (EQ‐VAS) for men aged ≥60 years in NI.
File S1. Men's Health & Wellbeing Survey.


