Abstract
Species and hybrids of Eucalyptus are the world's most widely planted hardwood trees. They are cultivated across a wide range of latitudes and therefore environmental conditions. In this context, comprehensive metabolomics approaches have been used to assess how different temperature regimes may affect the metabolism of three species of Eucalyptus, E. dunnii, E. grandis and E. pellita. Young plants were grown for 53 d in the greenhouse and then transferred to growth chambers at 10°C, 20°C or 30°C for another 7 d. In all three species the leaf chlorophyll content was positively correlated to temperature, and in E. pellita the highest temperature also resulted in a significant increase in stem biomass. Comprehensive metabolomics was performed using untargeted gas chromatography mass spectrometry (GC‐MS) and liquid chromatography (LC)‐MS. This approach enabled the comparison of the relative abundance of 88 polar primary metabolites from GC‐MS and 625 semi‐polar secondary metabolites from LC‐MS. Using principal components analysis, a major effect of temperature was observed in each species which was larger than that resulting from the genetic background. Compounds mostly affected by temperature treatment were subsequently selected using partial least squares discriminant analysis and were further identified. These putative annotations indicated that soluble sugars and several polyphenols, including tannins, triterpenes and alkaloids were mostly influenced.
Edited by: Uwe Sonnewald, Friedrich‐Alexander University, Germany
INTRODUCTION
Eucalyptus is an important commercial crop and a major source of raw materials for several industries, including those for cellulose pulp and paper, charcoal, timber and others. Furthermore, its essential oil (mainly mixtures of mono‐ and sesquiterpenes) is employed for therapeutic and medicinal applications, as well as to produce cosmetic and aromatherapy products (Vuong et al. 2015; Joshi et al. 2016). Currently, it is estimated that over 700 eucalyptus species have been identified and a range of them and their hybrids are economically exploited (Turnbull et al. 2002; Zhao et al. 2007). Due to this remarkable diversity and related genetic adaptability, eucalyptus is the most widely planted hardwood forest crop in the world, representing a global renewable resource of fiber and energy (Myburg et al. 2014). It is cultivated in more than 100 countries, across all continents, predominantly in tropical and sub‐tropical areas, but plantations may be also found in temperate regions of New Zealand, Chile, Argentina, Brazil, Uruguay, South Africa, the Iberian Peninsula and the United States (Turnbull et al. 2002; Zhao et al. 2007). In countries such as Australia, Chile, Portugal, Spain and South Africa, eucalyptus became the most popular tree used in the pulp and paper industry (Gamboa et al. 2007).
Brazil has about 5 million ha (about 25% of the world's total) cultivated with eucalyptus. In 2012 this represented a trade balance of US$ 4.7 billion and was responsible for sustaining over 150,000 jobs in the country (Goncalves et al. 2013). This outstanding production has led Brazil to be currently ranked fourth and ninth places in pulp and paper production, respectively. This industry is of growing importance and over the past decade, the Brazilian pulp and paper industry has invested approximately US$12 billion in innovation, technology and industrial modernization (BRACELPA 2014; Klabin 2015).
Due to the global economic importance of eucalyptus, companies have adopted breeding strategies to monitor and improve eucalyptus species in order to obtain genotypes better adapted to a wider range of climate conditions. This will enable the expansion of productive plants into marginal areas (Klabin 2015). In this context, new advances may come from the recent genome sequencing of the species Eucalyptus grandis by an international consortium (Myburg et al. 2014). Besides the ongoing technological advances in genetics and plant biotechnology, one of the main limiting factors for high productivity in the forestry sector is a suboptimal climate, related either to seasonal fluctuations and/or geographic location with respect to the genotypes being grown.
In general, drought and low temperatures are two of the main stresses limiting the geographical distribution and seasonal growth of various plants species, affecting both the productivity and quality of crops and forest plantations (Fernández et al. 2010). On the other hand, high temperatures (usually associated with drought) can also affect several physiological plant processes. However, in eucalyptus the existence of several species and hybrids with different genetic backgrounds and characteristics have allowed cultivation under a wide range of climatic conditions. This suggests that eucalyptus species have developed mechanisms to tolerate adverse conditions through physical, cellular and molecular strategies (Knight and Knight 2001; Rivero et al. 2001).
Under the scenario of the global climatic changes related to greenhouse gas emissions, metabolic versatility may play a crucial role in plant survival and success. According to the Inter‐governmental Panel on Climate Change, global temperatures have risen by 0.74°C during the twentieth century and it is estimated that surface temperatures across the globe will increase from 1.1°C to 6.4°C by the year 2100 (Edenhofer et al. 2014). Such changes will impact not only the whole distribution of the plant kingdom across the globe but also crop success, that is, yield and product quality. Wood production and composition is also expected to be affected by elevated levels of CO2 (Kilpelainen et al. 2003). Consequently, knowledge and understanding of the effect of temperature on crop growth and metabolism, and especially genotype verus environment interactions, is crucial for the design of a sustainable crop production strategy for the longer term.
In this study, three eucalyptus species were chosen to assess the effects of different temperatures on plant growth. The subtropical E. grandis is a species which is widely cultivated around the world, mainly due to its rapid growth rate and good yield, particularly under mild climate conditions (Conroy et al. 1992). Because of these characteristics, this was also the species chosen for genome sequencing (Myburg et al. 2014). Eucalyptus dunnii is said to be adapted to chilly temperatures and, although it has a relatively limited natural distribution, this species has been planted both for its good wood quality and for the production of essential oils. As this species was found to demonstrate superior growth and survival under more adverse conditions, it has attracted extra attention as a crop option for multiple locations (Jovanovic et al. 2000; von Muhlen et al. 2008). Finally, E. pellita has good potential for delivering solid wood products due to its high density and hardness (Sun et al. 2013). It is a species well adapted to warm climate conditions and is widely utilized for reforestation of humid and sub‐humid lowland tropical regions where few other eucalyptus species can thrive. E. pellita is also renowned for its fast growth, adaptability and coppicing ability (Doran et al. 1995).
Apart from research on essential oils, there are currently only a few reports on the non‐volatile metabolite composition of eucalyptus and only one was related to the impact of environment, in this case drought stress (Warren et al. 2012). In that study, the mesic species E. pauciflora and the semi‐arid species E. dumosa were subjected to water stress after which the polar metabolite profiles were compared using gas chromatography mass spectrometry (GC‐MS) of derivatized extracts.
Herein we present a detailed study of the effect of different temperatures (10°C, 20°C and 30°C) on the leaf metabolome of young plants of E. grandis, E. dunnii and E. pellita after being grown together under controlled conditions. We applied comprehensive, untargeted metabolomics on two complementary MS‐based analytical platforms (GC‐TOF (time‐of‐flight) MS and liquid chromatography (LC)‐Orbitrap Fourier‐transform MS (FTMS), abbreviated as GC‐MS and LC‐MS) for the analyses of polar and semi‐polar compounds, respectively, as well as targeted analyses of lipid‐soluble isoprenoid compounds (high‐performance (HP)LC with both absorbance spectra and fluorescence detection). Using this combined approach, a wide range of chemically diverse compounds (primary and secondary metabolites) was detected. Profile comparisons provided an overview of the leaf metabolites which are most affected by temperature changes in these three eucalyptus species. Our results will help to obtain new insights into how eucalyptus responds physiologically to temperature variation.
RESULTS
Growth and chlorophyll content of eucalyptus species
After the initial growth for 53 d in the greenhouse, at an average temperature of 22.7°C, young plants from three eucalyptus species were transferred to climate chambers at 10°C, 20°C or 30°C, with three biological replicates. Seven days later, the effects of temperature treatments on plant growth were assessed by measuring both stem height and stem weight (Figure 1). For stem height (Figure 1A), no significant differences (at α = 0.05) were observed between samples, neither between species nor between the 7 d of temperature treatment. The weight of the complete stem, after freeze‐drying, was used as a measure of total biomass production (Figure 1B). No significant differences between treatments were observed for both E. dunnii and E. grandis, whereas in E. pellita the biomass production was enhanced at 30°C as compared to both 10°C and 20°C.
Figure 1.
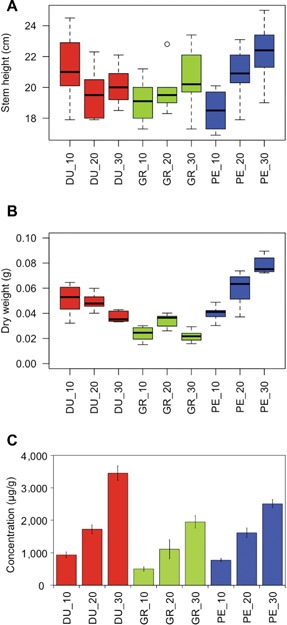
Physiological responses of eucalyptus species to temperature treatments Boxplot of stem height (A) and stem dry weight (B), and leaf chlorophyll content (C) of Eucalyptus dunnii (DU), E. grandis (GR) and E. pellita (PE) after a 7 d exposure to different growth temperatures (10°C, 20°C and 30°C). Data are means (± SD) of three replicates. Boxes correspond to a 95% confidence interval. Individual outlying data point is displayed as unfilled circle.
Nonpolar isoprenoid compounds such as chlorophylls, tocopherols and carotenoids were quantified using HPLC with both photodiode array and fluorescence detection. For the tocopherols and carotenoids, temperature effects were negligible and not statistically significant (data not shown – concentration under the limit of quantification). In contrast, levels of both chlorophyll a and b depended on temperature: the higher the temperature the higher the content of chlorophylls a and b in the leaves of each species. As the effect was similar for both chlorophylls, we calculated and depicted the levels of total chlorophyll (a + b) versus temperature for each species (Figure 1C).
Untargeted metabolomic profiling by LC‐MS and GC‐MS
Untargeted metabolomic profiling was chosen to determine the effect of the different temperature treatments on the overall metabolome of the three eucalyptus species. As an example, in Figure S1, the LC‐MS profiles of the three species at the same temperature (20°C) as acquired by LC‐Orbitrap FTMS high‐resolution mass spectrometry are provided. A clear difference between species in these LC‐MS profiles can be observed. Figure S2 illustrates as an example the metabolite profiles of a single species (E. dunnii) after the 7 d exposure to different temperatures.
The untargeted and automated processing of these raw LC‐MS data sets, including unbiased peak picking, alignment and clustering of all mass signals into so‐called reconstructed metabolites, resulted in a spreadsheet with the relative intensities of a total of 625 metabolites in all of the samples analyzed (Table S1). Corresponding to the specific extraction and chromatographic conditions applied, we mainly detected semi‐polar metabolites from plant secondary metabolism, such as phenylpropanoids, flavonoids, tannins and alkaloids. Likewise, the processing of the GC‐MS raw data, derived from derivatized polar extracts, resulted in a spreadsheet with the relative abundance of 88 reconstructed polar metabolites, mainly plant primary metabolites such as sugars, amino acids and organic acids (Table S2).
Temperature and genotype effects on the eucalyptus metabolome
An unsupervised multivariate analysis approach, that is, principal component analysis (PCA), was performed to determine differences between samples, based on their metabolite profiles. The score plots are shown in Figure 2. For the LC‐MS data, 54.9% of the variance is explained by the first two principal components; for the GC‐MS data this value is 51.5%. The close clustering of the three biological replicates per species and the clear grouping per treatment indicates a relatively high reproducibility. These PCA plots also indicate a major effect of temperature (PC1), with differences between eucalyptus species being observed in PC2, for both LC‐MS and GC‐MS data.
Figure 2.
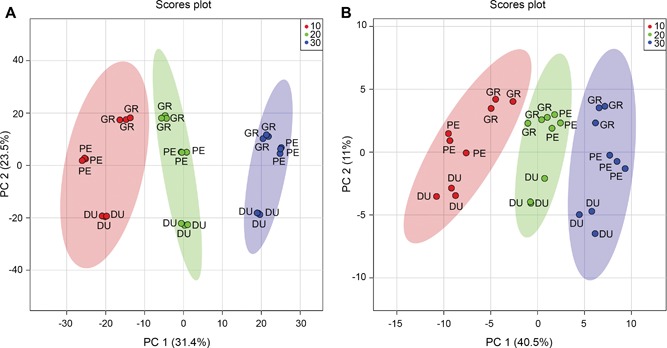
Prinicpal components analysis (PCA) score plots for liquid chromatography mass spectrometry (LC‐MS) and gas chromatography (GC)‐MS analysis PCA score plots of the LC‐MS (A) and GC‐MS (B) metabolite data for Eucalyptus dunnii (DU), E. grandis (GR) and E. pellita (PE) after a 7 d exposure to different growth temperatures (10 (red), 20 (green) and 30°C (blue)). Ellipses represent 95% confidence interval. n = 3.
The 625 reconstructed metabolites from LC‐MS and 88 from GC‐MS were concatenated and used to create Venn diagrams, to further illustrate how the 713 metabolites detected were distributed between species and/or temperature treatments (Figure 3). As criteria for uniqueness, each metabolite should be present in all three biological replicates and at least in one species and/or temperature. Most metabolites were common to all three species (575, i.e., 80.6%; Figure 3A) or all three temperatures (596, i.e., 83.6%; Figure 3B). Nevertheless, these diagrams allow us to select a number of metabolites that appear unique for each species. Thus 15, 20 and 20 metabolites were selected for E. grandis, E. pellita and E. dunnii, respectively, and may be considered as differential molecules of the individual species. Table S3 lists the peak intensities of these 55 metabolites in the samples, next to all other metabolites detected. Another 32 metabolites could also be designated as being unique for a specific temperature treatment: thus eight, nine and 15 metabolites were related to 10°C, 20°C and 30°C treatments, respectively (Figure 3B; Table S4). In both cases, all differential molecules (except one) were observed in the LC‐MS dataset and therefore are likely involved in plant secondary metabolism.
Figure 3.
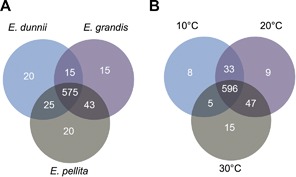
Metabolite distribution for species and temperature treatments Venn diagram representing the distribution of all 713 metabolites detected over species (A) and temperature treatments (B).
Those compounds that appeared to be discriminatory between the different species (55 metabolites) or temperature treatment (32 metabolites) were selected for manual annotation based on their MS/MS fragments and UV/Vis‐absorbance spectra information. For the lowest temperature (10°C) a flavone‐synapoylglycosyl ester (C40H44O21) and a galloyl tannin (C40H30O25) were thus putatively annotated. For the 30°C treatment, a triterpene ursolic acid derivative (C30H48O5) was putatively identified. All other compounds were “unknowns”.
We subsequently used a supervised multivariate analysis approach, that is, partial least squares discriminant analysis (PLS‐DA) to select LC‐MS metabolites most specific for either species or temperature. Figure 4 shows the scores plot used for the supervised model, with component 1 describing 31.5% of the variance and component 2 describing 4.9%. Cross‐validation was employed using the leave‐one‐out method. Based on this, according to the guidepost (Q2) and prediction residual error sum of squares (PRESS), only the first latent variable was selected to build the model, thereby avoiding overfitting. Variable importance in projection (VIP) was chosen as criteria for selecting most important variables of the PLS‐DA model. The top 25 metabolites ranked by the model were selected to build a heatmap plot. The heatmap plot (Figure 5) shows two main blocks of metabolites: the first block (upper eight rows) comprises a group of metabolites that have their highest abundance in plants grown at 10°C. The higher the temperature, the lower their abundance level. One of these eight compounds could be putatively identified as chebulagic acid (a tannin) while the other metabolites could not be identified. The metabolites of the second block show the opposite temperature effect, that is, their abundance increased as the temperature increased. Two of these metabolites could be putatively annotated: jionoside B1 (a polyphenol) and magnoflorine (an alkaloid).
Figure 4.
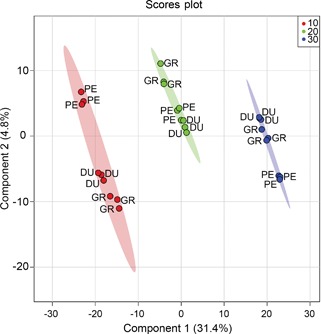
Partial least squares discriminant analysis (PLS‐DA) model for liquid chromatography mass spectrometry (LC‐MS) data Scores plot of a PLS‐DA model using LC‐MS data for Eucalyptus dunnii (DU), E. grandis (GR) and E. pellita (PE) after a 7 d exposure to different growth temperatures (10°C (red), 20°C (green) and 30°C (blue)). Ellipses represent 95% confidence intervals. n = 3.
Figure 5.
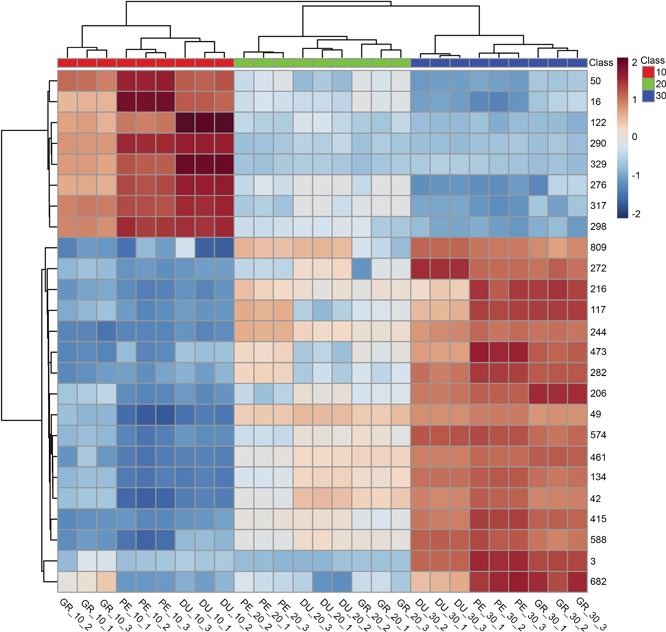
Heatmap with most significant metabolites affected by temperature treatments Heatmap plot with the 25 top‐ranking metabolites from the partial least squares discriminant analysis variable importance in projection (PLS‐DA VIP). Metabolites are represented as numbers on the right‐hand side of the plot, using the metabolite number (metabolite ID) obtained from the data processing. Eucalyptus species (DU – E. dunnii, GR – E. grandis, PE – E. pellita) and temperature treatments (10°C, 20°C and 30°C) are identified in the bottom of the plot.
All the LC‐MS compounds selected and identified in this study are listed in Table 1. For those compounds that were non‐discriminatory, the most abundant and common for all species were also manually identified and could be putatively annotated as flavonoid glycosides. Among them are quercetin‐3‐O‐rhamnoside 7‐O‐glucoside, kaempferol 3‐O‐xyloside, kaempferol‐3‐O‐galactoside and quercetin 3‐O‐glucoside. The most abundant tannins were identified as corilagin, pedunculagin, tellimagrandin I and gallotannin. Using dedicated accurate mass LC‐MS analysis at a higher mass window (m/z 500–4,000) we also detected double‐ or even triple‐charged ions at high abundance, suggesting that tannins with a molecular weight higher than 2,000 Da were also present.
Table 1.
List of annotated compounds detected in eucalyptus leaves detected by liquid chromatography mass spectrometry
| Metabolite ID | RT (min) | MW measured | KEGG ID | Annotation | Formula | MW theoretical | Mass difference (ppm) | Level of annotation |
|---|---|---|---|---|---|---|---|---|
| 24 | 2.70 | 634.08046 | C10219 | Corilagin | C27H22O18 | 634.08060 | −0.221 | 2 |
| 30 | 3.31 | 332.07451 | C01158 | 1‐O‐Galloyl‐beta‐D‐glucose | C13H16O10 | 332.07430 | 0.632 | 2 |
| 40 | 4.28 | 484.08528 | C04101 | 1‐O,6‐O‐Digalloyl‐beta‐D‐glucose | C20H20O14 | 484.08530 | −0.041 | 2 |
| 46 | 4.78 | 356.11082 | C17759 | 1‐O‐Feruloyl‐beta‐D‐glucose | C16H20O9 | 356.11070 | 0.337 | 2 |
| 48 | 4.98 | 332.07451 | C01158 | 1‐O‐Galloyl‐beta‐D‐glucose | C13H16O10 | 332.07430 | 0.632 | 2 |
| 68 | 6.24 | 312.06337 | – | Unknown | C17H12O6 | 312.06339 | −0.046 | 4 |
| 83 | 6.98 | 784.07588 | C10236 | Pedunculagin | C34H24O22 | 784.07590 | −0.026 | 2 |
| 86 | 7.13 | 484.08576 | C04101 | 1‐O,6‐O‐Digalloyl‐beta‐D‐glucose | C20H20O14 | 484.08530 | 0.950 | 2 |
| 93 | 7.51 | 786.09126 | C10241 | Tellimagrandin I | C34H26O22 | 786.09160 | −0.433 | 2 |
| 96 | 7.58 | 868.29106 | – | Unknown | C42H48N2O18 | 868.29021 | 0.981 | 4 |
| 104 | 7.98 | 354.09532 | C17147 | Neochlorogenic acid | C16H18O9 | 354.09510 | 0.621 | 2 |
| 109 | 8.18 | 338.09990 | C12208 | p‐Coumaroyl quinic acid | C16H18O8 | 338.10020 | −0.887 | 2 |
| 124 | 8.73 | 636.09627 | C17458 | Gallotannin | C27H24O18 | 636.09630 | −0.047 | 2 |
| 167 | 10.40 | 338.09993 | C10432 | 1‐Caffeoyl‐4‐deoxyquinic acid | C16H18O8 | 338.10020 | −0.799 | 2 |
| 186 | 11.03 | 936.08522 | C10212 | Casuarictin | C41H28O26 | 936.08690 | −1.795 | 2 |
| 206 | 11.73 | 814.28907 | C10473 | Jionoside B1 | C37H50O20 | 814.28950 | 0.522 | 2 |
| 217 | 12.06 | 788.10689 | C10243 | 1,2,3,4‐Tetragalloyl‐alpha‐D‐glucose | C34H28O22 | 788.10720 | −0.393 | 2 |
| 232 | 12.48 | 610.15254 | C19796 | Quercetin 3‐O‐rhamnoside 7‐O‐glucoside | C27H30O16 | 610.15340 | −1.409 | 2 |
| 241 | 12.74 | 302.00603 | C10788 | Ellagic acid | C14H6O8 | 302.00630 | −0.894 | 2 |
| 244 | 12.91 | 380.12900 | C08358 | 2‐Methoxyestrone 3‐sulfate | C19H24O6S | 380.12940 | −1.052 | 2 |
| 254 | 13.20 | 464.09541 | C05623 | Quercetin 3‐O‐glucoside | C21H20O12 | 464.09550 | −0.194 | 2 |
| 276 | 13.80 | 954.09675 | C10214 | Chebulagic acid | C41H30O27 | 954.09740 | −0.681 | 2 |
| 301 | 14.68 | 448.10005 | C12626 | Kaempferol‐3‐O‐galactoside | C21H20O11 | 448.10060 | −1.227 | 2 |
| 313 | 14.98 | 462.07960 | C03515 | Luteolin 7‐O‐glucuronide | C21H18O12 | 462.07980 | −0.433 | 2 |
| 322 | 15.37 | 418.08961 | C20727 | Kaempferol 3‐O‐beta‐D‐xyloside | C20H18O10 | 418.09000 | −0.933 | 2 |
| 329 | 15.56 | 910.10792 | – | Galloyl tannin | C40H30O25 | 910.10762 | 0.338 | 3 |
| 352 | 16.27 | 174.08954 | C08278 | Suberic acid | C8H14O4 | 174.08920 | 1.953 | 2 |
| 365 | 16.72 | 520.19459 | C17529 | (−)‐Pinoresinol glucoside | C26H32O11 | 520.19450 | 0.173 | 2 |
| 372 | 16.93 | 372.14232 | C01533 | Syringin | C17H24O9 | 372.14200 | 0.860 | 2 |
| 413 | 18.25 | 882.16286 | – | Unknown | C44H34O20 | 882.16434 | −1.677 | 4 |
| 415 | 18.43 | 342.17065 | C09581 | Magnoflorine | C20H24NO4 | 342.17050 | −0.443 | 2 |
| 462 | 20.12 | 436.10051 | C10067 | Irisxanthone | C20H20O11 | 436.10060 | −0.206 | 2 |
| 551 | 23.32 | 562.24601 | – | Unknown | C35H34N2O5 | 562.24677 | −1.349 | 4 |
| 567 | 23.89 | 654.21626 | – | Unknown | C30H38O16 | 654.21599 | 0.426 | 4 |
| 622 | 26.57 | 580.14265 | C10026 | Carlinoside | C26H28O15 | 580.14280 | −0.259 | 2 |
| 635 | 27.34 | 240.10005 | C20286 | 2‐Hydroxyamino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine | C13H12N4O | 240.10110 | −4.373 | 2 |
| 652 | 28.28 | 180.11543 | C03929 | 3‐tert‐Butyl‐5‐methylcatechol | C11H16O2 | 180.11500 | 2.387 | 2 |
| 661 | 28.87 | 324.15754 | C09560 | Tetraneurin E | C17H24O6 | 324.15730 | 0.740 | 2 |
| 664 | 29.03 | 252.10005 | C20207 | Grandinol | C13H16O5 | 252.09980 | 0.992 | 2 |
| 672 | 29.34 | 208.11036 | C10451 | Elemicin | C12H16O3 | 208.10990 | 2.210 | 2 |
| 689 | 30.74 | 488.35072 | – | Triterpene ursolic acid | C30H48O5 | 488.35017 | 1.125 | 3 |
| 695 | 31.35 | 194.13106 | C14305 | 4‐Hexyloxyphenol | C12H18O2 | 194.13070 | 1.854 | 2 |
| 719 | 33.61 | 860.23713 | – | Flavone‐synapoylglycosyl ester | C40H44O21 | 860.23751 | −0.435 | 3 |
| 744 | 35.55 | 312.09993 | C10045 | 3,5,7‐Trimethoxyflavone | C18H16O5 | 312.09980 | 0.417 | 2 |
| 811 | 40.30 | 472.35509 | C17067 | Siaresinol | C30H48O4 | 472.35530 | −0.445 | 2 |
RT = retention time; MW = molecular weight; Level of annotation according to Sumner et al. 2007
From the GC‐MS analyses, 16 polar metabolites, mostly from the plant primary metabolism, could be identified by making use of authentic standards (Table 2). Other compounds automatically annotated by the GC data processing procedure, but with retention indexes (RI) and/or mass spectra that did not match well with the available GC‐MS databases, were excluded or annotated as “Unknown”. The changes in primary metabolites indicated that the biochemical pathway most influenced by temperature was related to sugar and starch metabolism. An example is shown for E. grandis in the variation in the levels of these compounds: the disaccharide sucrose is clearly upregulated whereas the monosaccharides fructose and glucose are down‐regulated at increasing temperatures (Figure 6). The same pattern is observed for E. dunnii and E. pellita.
Table 2.
List of annotated compounds detected by gas chromatography mass spectrometry
| Metabolite ID | RT (min) | RI | Annotation | Formula | MW (g/mol) | Level of annotation |
|---|---|---|---|---|---|---|
| 555 | 6.39 | 1054 | Propanoic acid | C3H6O2 | 74.08 | 1 |
| 3093 | 7.60 | 1200 | Oxalic acid | C2H2O4 | 90.03 | 1 |
| 7990 | 9.06 | 1240 | Urea | CH4N2O | 60.06 | 1 |
| 18919 | 12.72 | 1530 | Aspartic acid | C4H7NO4 | 133.11 | 1 |
| 22292 | 13.92 | 1615 | Glutamic acid | C5H9NO4 | 147.13 | 1 |
| 23346 | 14.34 | 1642 | Xylose | C5H10O5 | 150.13 | 1 |
| 23741 | 14.52 | 1650 | Arabinose | C5H10O5 | 150.13 | 1 |
| 28924 | 16.10 | 1805 | Citric acid | C6H8O7 | 190.12 | 1 |
| 29556 | 16.47 | 1843 | Quinic acid | C7H12O6 | 192.17 | 1 |
| 30066 | 16.60 | 1860 | Sorbose | C6H12O6 | 180.16 | 1 |
| 30435 | 16.70 | 1865 | Fructose | C6H12O6 | 180.16 | 1 |
| 30711 | 16.80 | 1886 | Mannose | C6H12O6 | 180.16 | 1 |
| 30925 | 16.86 | 1890 | Glucose | C6H12O6 | 180.16 | 1 |
| 33700 | 17.52 | 2036 | Benzoic acid | C7H6O2 | 122.12 | 1 |
| 52102 | 23.25 | 2611 | Sucrose | C12H22O11 | 342.29 | 1 |
| 63027 | 26.66 | 3095 | Chlorogenic acid | C16H18O9 | 354.31 | 1 |
RT = retention time; RI = retention index; level of annotation according to Sumner et al. 2007
Figure 6.
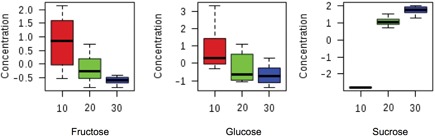
Comparison of sugar levels affected by temperature treatments Relative concentration of the most affected compounds of sucrose metabolism after a 7 d exposure to different growth temperature (10°C, 20°C and 30°C) for Eucalyptus grandis. Similar trend is observed for E. dunnii and E. pellita.
DISCUSSION
This comprehensive metabolomics study provides insight into to which extent young plants of three contrasting Eucalyptus species, which have known agronomic differences and climatic preferences, respond to a short‐term decrease or increase in the environmental temperature, with respect to leaf phytochemical composition and stem growth.
In all three species investigated, the chlorophyll content of leaves was positively correlated to the growth temperature, suggesting a potentially higher photosynthesis potential at elevated temperatures, even for E dunnii which naturally grows at lower temperatures. The ratio of chlorophyll a/b was constant for all the investigated temperatures (10°C, 20°C and 30°C), suggesting both forms are equally affected by changes in the temperature. In contrast, other photosynthetic pigments, including carotenoids, were not significantly affected. The positive association between temperature and chlorophyll supports previous data reported for E. tereticornis, as well as for other (non‐eucalyptus) plant species (Mannan et al. 1986). In another study, Warren (2008) also observed a strong positive relationship between electron transport and chlorophyll content in seedlings of E. regnans grown under different temperatures (10°C to 35°C). Apparently, chlorophyll biosynthesis and related photosynthetic potential in eucalyptus species benefits from higher temperatures, at least up to 35°C, even when this temperature exceeds that of the natural habitat of a particular species.
While in all three species the chlorophyll content was significantly increased at 30°C, as compared to 10°C and 20°C, only in E. pellita was the stem biomass significantly increased at this high temperature. E. pellita is known to thrive under warm climatic conditions. The weight of stems was highest for E. pellita grown at 30°C. This might reflect its relative high wood density as reported previously for this species (Sun et al. 2013). For the other species, no significant differences in stem weight due to temperature were observed after the 7 d treatment. Although not statistically significant in this short treatment study (at α = 0.05, n = 3), the patterns observed in the effects of temperature on height and biomass of stems may become evident after longer treatments and/or with more biological replicates.
Untargeted metabolomics has allowed us to assess constitutive differences between species and the effect of growth temperature in relation to both primary and secondary metabolism, using GC‐MS and LC‐MS based metabolomics, respectively. The PCA plots based on the several hundred metabolite features thus obtained revealed a clear effect of temperature across the metabolome. This was greater than the differences between species, for both primary and secondary metabolites.
Phenolic compounds were among the most temperature‐responsive metabolites in all three species. This class of secondary metabolite is known to be well represented in leaves of eucalyptus. These phytochemicals have frequently been related to their antiviral, antibacterial and antifungal properties and indeed are often associated with abiotic stress responses (Okamura et al. 1993; Zhou et al. 2014; Guan et al. 2015). Also, polyhydroxylated phenolic structures like ellagic acid and tannin derivatives were detected in all three species investigated here. These compounds have also previously been reported in other Eucalyptus species (Santos and Waterman 2001a, 2001b).
With regard to sugars, as a key component of primary metabolism, the plants accumulated more fructose and glucose in response to lower temperature. However, the opposite was true for sucrose. This result might reflect an inverse temperature‐dependency of the activity of the enzyme invertase. Indeed, Usadel et al. (2008) reported a clear increase in invertase transcripts in Arabidopsis upon lowering the growth temperature.
Crop plant resilience to abiotic stress facilitating a reduced impact on growth under sub‐optimal cultivation conditions is a timely topic of great importance. This is especially true for tree crops which are perennial species which have to pass through multiple seasons before they are harvested. We have shown here that a key component of the environment, temperature, already exerts an influence over plant metabolism and the biochemical composition of plant materials, even after just 1 week of exposure. Perturbations involve both primary as well as secondary/specialized metabolic pathways, suggesting that plants need to initiate a very broad metabolic response in order to cope with the altered conditions. In view of global climate change issues and the fact that the temperature treatments had here an even greater effect on the leaf metabolome than genetic background, suggests that we need to look in greater detail into the biochemical mechanisms of plant environmental responses in order to understand these better and, hence, design novel strategies for developing improved crop resilience. Our results shown here indicate that metabolomics can be a very effective tool to investigate and discriminate physiological/chemical responses of eucalyptus species to temperature changes. This broad, untargeted approach not only reveals the extent of metabolic perturbation but also identifies those phytochemical classes which appear most affected and, hence, the groups of metabolites which deserve particular attention in future research.
MATERIALS AND METHODS
Plant material and treatments
Fully documented and genetically certified seeds of Eucalyptus grandis, E. dunnii and E. pellita were purchased from a commercial supplier (Sementes Caiçara, São Paulo, Brazil – http://www.sementescaicara.com) and germinated in wet vermiculite until they presented their first pair of true leaves. These seedlings were transferred individually into cone‐shaped 250 mL plastic containers filled with 50% vermiculite and 50% commercial organic substrate mix (Genefértil, São Paulo, Brazil) where they were grown in a greenhouse with 70% natural sunlight, no control of temperature (average temperature of 22.7°C) and daily watered by an automated irrigation system. The positions of the plants in the greenhouse were randomized and re‐arranged weekly. After 53 d the seedlings were split into three groups and transferred to three growth chambers set to 10°C, 20°C and 30°C. Light was set to 300 µmol photons/m/s and the photoperiod was 8 h of light and 16 h of darkness. The plants were kept in these growth chambers for 7 d. Prior to harvesting, stem height was measured. Three independent biological replicates (individual plants) for each treatment and species were used in the metabolomics analysis. All the leaves of each plant were harvested and immediately frozen in liquid nitrogen and stored at −80°C. Before using, the frozen material was ground into a fine powder using a mortar and pestle with liquid nitrogen and then freeze‐dried before storing at −80°C. Leaf powder aliquots were extracted for pigment analysis as well as untargeted metabolomics analyses using GC‐MS and LC‐MS approaches.
HPLC‐PDA analysis of chlorophyll content
Leaf powder samples were extracted according to the protocol described by Moco et al. (2007). Briefly, 25 mg powder was extracted with 4.5 mL methanol/chloroform (5:4 v/v) + 0.1% butylated hydroxytoluene (BHT) + 0.3 mg/mL Sudan 1 as an internal standard. After sonication, 2.5 mL 50 mmol/L Tris‐buffer (pH = 7.5) containing 1 mol/L NaCl was added to the suspension. Samples were centrifuged and the chloroform phase was transferred to a fresh tube and the polar phase was re‐extracted twice with 1 mL chloroform + 0.1% BHT. Finally, the pooled chloroform fractions were dried by evaporation under a stream of N2 and then solubilized in ethyl acetate containing 0.1% BHT. Samples were analyzed using an HPLC (Waters Alliance e2695, Milford, MA, USA) coupled to a photodiode array detector (Waters 996 PDA, Milford, MA, USA). Separation was performed on a reverse‐phase C30 column (250 × 4.6 mm i.d., S‐5 µm − YMC Carotenoid, Japan) kept at 35°C at a flow rate of 1.0 mL/min. The mobile phases consisted of methanol (A), water/methanol (20:80 v/v) with 0.2% ammonium acetate (B) and tert‐methyl butyl ether (C). The gradient elution started with 95% A, 5% B isocratically for 12 min, changing to 80% A, 5% B, 15% C at 12 min, followed by a linear gradient to 30% A, 5% B, 65% C by 30 min. A conditioning phase (30–60 min) was then used to return the column to the initial concentrations of A and B. Chlorophyll a and b were identified based on comparisons of retention time and absorption spectra (240 to 700 nm) with authentic standards. External calibration curves normalized with internal standard (Sudan 1) were built to enable quantification of identified compounds. Waters Empower 3 software (Waters, Milford, MA, USA) was used for raw data processing.
LC‐MS profiling
Samples were extracted essentially according to the method proposed by De Vos et al. (2007). Leaf samples (25 mg dry weight) were transferred to 2 mL plastic safe‐lock tubes and extracted with 1 mL methanol/water (3:1 v/v) + 0.1% formic acid (FA). After vortexing (10 s) and sonication (15 min), samples were centrifuged (16,000 × g) for 10 min and the supernatant was collected. Chromatographic separation was performed on an HPLC system (Waters Acquity, Milford, MA, USA) with a C18 column (Phenomenex Luna 150 × 2 mm i.d., 3 µm – Torrance, USA) using ultra‐pure water (eluent A) and acetonitrile (eluent B) both acidified with 0.1 FA at a flow rate of 0.19 mL/min, starting with 5% B and increasing linearly to 75% B in 45 min, followed by 15 min of re‐equilibration at 5% B (Liu et al. 2014). The column was kept at 40°C and detection was with both a PDA detector (Waters) at 210–600 nm and an LTQ‐Orbitrap FTMS hybrid mass spectrometer (Thermo Scientific, Bremen, Germany) in negative ionization mode. A mass resolution of 60,000 was employed for data acquisition. Samples were analyzed using a mass range of m/z 90–1,350. MS/MS analysis was performed using data‐dependent acquisition in discovery mode (dd‐MS2), enabling the mass spectrometer to select the most intense ions and automatically fragment them.
GC‐MS profiling
Leaf samples were extracted according to the protocol described by Carreno‐Quintero et al. (2012). Briefly, 25 mg powder was extracted with 1.4 mL methanol containing 0.2 mg/mL ribitol as internal standard. After sonication and centrifugation, 500 µL supernatant was re‐extracted with 375 µL chloroform and 750 µL ultra‐pure water. Aliquots (10 µL) of the upper (polar) phase were transferred to an insert placed in a 2 mL vial. All samples were dried overnight (16 h) by vacuum centrifugation at room temperature and the vials were closed under an argon atmosphere using magnetic crimp caps. Prior to analysis, the dried samples were derivatized on‐line using a CombiPAL autosampler (CTC Analytics AG, Zwingen, Switzerland) and MSTFA (N‐methyl‐N‐trimethylsilyltrifluoroacetamide) as derivatization agent. An alkane mixture (C10–C30) was added to each sample to enable the determination of the RIs of the metabolites. The derivatized samples were analyzed by a GC‐TOF MS system comprising an Agilent 6890 gas chromatographer (Agilent Technologies, Santa Clara, CA, USA) coupled to a Pegasus III TOF MS (Leco Instruments, Saint Joseph, MI, USA) using a 1:20 split. Chromatographic separation was performed using a capillary column (Agilent DB‐5, 30 m × 0.25 mm i.d., 0.25 μm, Santa Clara, CA, USA) including a 10 m guard column with helium as carrier gas at a constant column flow rate of 1 mL/min. The column effluent was submitted to electron ionization at 70 eV.
Untargeted data processing and multivariate statistical analyses
Unbiased mass peak picking and alignment of raw data sets from both LC‐MS and GC‐MS were carried out with MetAlign software (Lommen 2009), followed by filtering out irreproducible peaks and replacing non‐detects by a random value of 40%–60% local noise as calculated by Metalign (s/n ≥ 3) using an in‐house script called MetAlign Output Transformer – METOT (Houshyani et al. 2012). Mass signals that were present in fewer than three samples were discarded. The 14,090 remaining individual mass peaks, including molecular ions, in‐source adducts and fragments and their natural isotopes, were subsequently clustered using MSClust software (Tikunov et al. 2012) into so‐called reconstructed metabolites (centrotypes) according to their corresponding retention time and peak intensity pattern across samples. The resulting spreadsheet with the relative intensity of each reconstructed metabolite in each sample was used for further statistical analyses. In the case of metabolites detected by GC‐TOF MS, the metabolite list from the automated spectral annotation was manually filtered to remove the alkanes, which were added for RI calculation, and other artifacts, such as impurities from the column and derivatization agents, prior to further data analysis.
Subsequently, for multivariate statistical analysis the on‐line tool MetaboAnalist (Xia et al. 2015) was employed. The MSClust output was uploaded into this platform and the peak intensity values of reconstructed metabolites were normalized against the group control (20°C). To compare the metabolite profiles, the matrix output was 10log‐transformed and scaled by the Pareto method (mean‐centered and divided by the square root of standard deviation of each variable). Data was assessed using analysis of variance followed by a post hoc Tukey test using a significance threshold α of 0.05. PCA was used as an unsupervised approach, making a summary review of samples and variables based on the data variance and indicating any possible outliers. Projection to latent structure discriminant analysis was employed to find significant metabolites using a discriminant model built by latent variables. Heatmaps were used to verify patterns between replicates and treatments.
Metabolite annotation
Annotation of compounds was performed following the rules described by (Sumner et al. 2007), being classified at four levels: identified metabolites by comparison with standards (level 1), putatively annotated compounds (level 2), putatively characterized compound classes (level 3), and unknown compounds (level 4).
For annotating metabolites detected by GC‐MS, the mass spectrum of each ion cluster (reconstructed metabolite) was compared with available EI‐spectral libraries, such as the NIST2008 and the Golm spectral database (http://gmd.mpimp-golm.mpg.de/), as well as an in‐house library of derivatized standards. In addition, the experimentally obtained RI was compared with reported RIs for verification of the automated spectra annotations.
For LC‐MS, the mass of the molecular ion was manually verified within the clustered mass signals of selected, reconstructed metabolites. Metabolites were then annotated using an in‐house database based on comparisons of retention time, accurate mass, isotopic composition, UV spectra and MS/MS information. On‐line available metabolite databases such as KNApSAcK, KEGG and MassBank were also employed.
ACKNOWLEDGEMENTS
This work was supported by the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) grants 2008/58035‐6 and 2011/51949‐5. P.M. thanks CNPq (Conselho Nacional de Pesquisa – Brazil) for a research fellowship; J.B.M thanks FAPESP grants 2013/21306‐0 and 2015/06987‐7. We also acknowledge Bert Schipper and Henriette van Eekelen, Bioscience, Wageningen Plant Research for their excellent help in the LC‐MS, GC‐MS and HPLC‐PDA analyses.
AUTHOR CONTRIBUTIONS
J.B.M., P.M. and A.C.H.F.S. designed the study. J.B.M. performed the experiments. J.B.M., R.M. and R.C.H.dV. analyzed the data. All authors participated in writing the manuscript.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article: http://onlinelibrary.wiley.com/doi/10.1111/jipb.12626/suppinfo
Figure S1. Chromatograms profiling of different species of eucalyptus
Comparison of the liquid chromatography mass spectrometry (LC‐MS) profiles of E. dunnii (A), E. grandis (B) and E. pellita (C) exposed to the same temperature (20°C) for 7 d.
Figure S2. Chromatograms profiling of different temperature treatments
Comparison of the liquid chromatography mass spectrometry (LC‐MS) profiles of Eucalyptus dunnii after 7 d exposure to 10°C (A), 20°C (B) or 30°C (C).
Table S1. Liquid chromatography mass spectrometry (LC‐MS) raw dataset ClusterID refers to a unique number used in the data processing, retention time (RT) in minutes and m/z information (M‐H)‐ related to the most intense ion of the cluster. In the last two columns is showed a mass list (µDA) and its relative intensity, for E. dunnii (DU), E. grandis (GR) and E. pellita (PE). Sample names are assorted according the temperature treatment and its replicate.
Table S2. Gas chromatography mass spectrometry (GC‐MS) raw dataset ClusterID refers to a unique number used in the data processing, retention time (RT) in minutes and retention index (RI) are also shown. In the last two columns is showed a mass list and its relative intensity for Eucalyptus dunnii (DU), E. grandis (GR) and E. pellita (PE). Sample names are assorted according the temperature treatment and its replicate.
Table S3. Fifty‐five metabolites that appeared to be discriminatory for the genotype
Table S4. Thirty‐two metabolites that appeared to be discriminatory for the temperature treatments
Mokochinski JB, Mazzafera P, Sawaya ACHF, Mumm R, de Vos RCH, Hall RD (2018) Metabolic responses of Eucalyptus species to different temperature regimes. J Integr Plant Biol 60: 397–411
Online on Dec. 16, 2017
REFERENCES
- BRACELPA (2014) Brazilian Pulp and Paper Industry. Available at: http://iba.org/images/shared/iba_2014_pt.pdf
- Carreno‐Quintero N, Acharjee A, Maliepaard C, Bachem CWB, Mumm R, Bouwmeester H, Visser RGF, Keurentjes JJB (2012) Untargeted metabolic quantitative trait loci analyses reveal a relationship between primary metabolism and potato tuber quality. Plant Physiol 158: 1306–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy JP, Milham PJ, Barlow EWR (1992) Effect of nitrogen and phosphorus availability on the growth response of Eucalyptus grandis to high CO2 . Plant Cell Environ 15: 843–847 [Google Scholar]
- De Vos RCH, Moco S, Lommen A, Keurentjes JJB, Bino RJ, Hall RD (2007) Untargeted large‐scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat Protoc 2: 778–791 [DOI] [PubMed] [Google Scholar]
- Doran JC, Williams ER, Brophy JJ (1995) Patterns of variation in the seedling leaf oils of Eucalyptus urophylla, E. pellita and E. scias. Austr J Bot 43: 327–336 [Google Scholar]
- Fernández M, Villarroel C, Balbotín C, Valenzuela S (2010) Validation of reference genes for real‐time qRT‐PCR normalization during cold acclimation in Eucalyptus globulus . Trees 24: 1109–1116 [Google Scholar]
- Gamboa MC, Rasmussen‐Poblete S, Valenzuela PDT, Krauskopf E (2007) Isolation and characterization of a cDNA encoding a CBF transcription factor from E. globulus . Plant Physiol Biochem 45: 1–5 [DOI] [PubMed] [Google Scholar]
- Goncalves JLD, Alvares CA, Higa AR, Silva LD, Alfenas AC, Stahl J, Ferraz SFD, Lima WDP, Brancalion PHS, Hubner A, Bouillet JPD, Laclau JP, Nouvellon Y, Epron D (2013) Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For Ecol Manage 301: 6–27 [Google Scholar]
- Guan XF, Guo QY, Huang XJ, Wang Y, Ye WC (2015) A new flavonoid glycoside from leaves of Eucalyptus robusta . J Chi Mater Med 40: 4868–4872 [PubMed] [Google Scholar]
- Houshyani B, Kabouw P, Muth D, de Vos RCH, Bino RJ, Bouwmeester HJ (2012) Characterization of the natural variation in Arabidopsis thaliana metabolome by the analysis of metabolic distance. Metabolomics 8: 131–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenhofer O, Pichs‐Madruga R, Sokona Y, Farahani E, Kadner S, Seyboth K, Adler A, Baum I, Brunner S, Eickemeier P, Kriemann B, Savolainen J, Schlömer S, von Stechow S, Zwickel T, Minx JC (2014) IPCC 2014 Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY
- Joshi A, Sharma A, Bachheti RK, Pandey DP (2016) A Comparative study of the chemical composition of the essential oil from Eucalyptus globulus growing in Dehradun (India) and around the World. Orient J Chem 32: 331–340 [Google Scholar]
- Jovanovic T, Arnold R, Booth T (2000) Determining the climatic suitability of Eucalyptus dunnii for plantations in Australia, China and Central and South America. New For 19: 215–226 [Google Scholar]
- Kilpelainen A, Peltola H, Ryyppo A, Sauvala K, Laitinen K, Kellomaki S (2003) Wood properties of Scots pines (Pinus sylvestris) grown at elevated temperature and carbon dioxide concentration. Tree Physiol 23: 889–897 [DOI] [PubMed] [Google Scholar]
- Klabin (2015) Sustentability Report Klabin in 2015. Available at: http://rs.klabin.com.br/docs/RA_Klabin_2015.pdf
- Knight H, Knight MR (2001) Abiotic stress signalling pathways specificity and cross‐talk. Trends Plant Sci 6: 262–267 [DOI] [PubMed] [Google Scholar]
- Liu Q, Manzano D, Tanic N, Pesic M, Bankovic J, Pateraki I, Ricard L, Ferrer A, de Vos R, van de Krol S, Bouwmeester H (2014) Elucidation and in planta reconstitution of the parthenolide biosynthetic pathway. Metab Eng 23: 145–153 [DOI] [PubMed] [Google Scholar]
- Lommen A (2009) MetAlign: Interface‐driven, versatile metabolomics tool for hyphenated full‐scan mass spectrometry data preprocessing. Anal Chem 81: 3079–3086 [DOI] [PubMed] [Google Scholar]
- Mannan RM, Anan S, Kulandaivelu G, Bose S (1986) Species specific chlorophyll a fluorescence‐temperature profile at high temperatures in the leaves. Photosynth Res 8: 87–92 [DOI] [PubMed] [Google Scholar]
- Moco S, Capanoglu E, Tikunov Y, Bino RJ, Boyacioglu D, Hall RD, Vervoort J, De Vos RC (2007) Tissue specialization at the metabolite level is perceived during the development of tomato fruit. J Exp Bot 58: 4131–4146 [DOI] [PubMed] [Google Scholar]
- Myburg AA, Grattapaglia D, Tuskan GA, Hellsten U, Hayes RD, Grimwood J, Jenkins J, Lindquist E, Tice H, Bauer D, Goodstein DM, Dubchak I, Poliakov A, Mizrachi E, Kullan ARK, Hussey SG, Pinard D, Van der Merwe K, Singh P, Van Jaarsveld I, Silva OB, Togawa RC, Pappas MR, Faria DA, Sansaloni CP, Petroli CD, Yang XH, Ranjan P, Tschaplinski TJ, Ye CY, Li T, Sterck L, Vanneste K, Murat F, Soler M, Clemente HS, Saidi N, Cassan‐Wang H, Dunand C, Hefer CA, Bornberg‐Bauer E, Kersting AR, Vining K, Amarasinghe V, Ranik M, Naithani S, Elser J, Boyd AE, Liston A, Spatafora JW, Dharmwardhana P, Raja R, Sullivan C, Romanel E, Alves‐Ferreira M, Lheim CK, Foley W, Carocha V, Paiva J, Kudrna D, Brommonschenkel SH, Pasquali G, Byrne M, Rigault P, Tibbits J, Spokevicius A, Jones RC, Steane DA, Vaillancourt RE, Potts BM, Joubert F, Barry K, Pappas GJ, Strauss SH, Jaiswal P, Grima‐Pettenati J, Salse J, Van de Peer Y, Rokhsar DS, Schmutz J (2014) The genome of Eucalyptus grandis . Nature 510: 356–362 [DOI] [PubMed] [Google Scholar]
- Okamura H, Mimura A, Yakou Y, Niwano M, Takahara Y (1993) Antioxidant activity of tannins and flavonoids in Eucalyptus rostrata . Phytochemistry 33: 557–561 [Google Scholar]
- Rivero RM, Ruiz JM, García PC, López‐Lefebre LR, Sánchez E, Romero L (2001) Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci 160: 315–321 [DOI] [PubMed] [Google Scholar]
- Santos SC, Waterman PG (2001a) Polyphenols from Eucalyptus consideniana and Eucalyptus viminalis . Fitoterapia 72: 95–97 [DOI] [PubMed] [Google Scholar]
- Santos SC, Waterman PG (2001b) Polyphenols from Eucalyptus ovata . Fitoterapia 72: 316–318 [DOI] [PubMed] [Google Scholar]
- Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TWM, Fiehn O, Goodacre R, Griffin JL, Hankemeier T, Hardy N, Harnly J, Higashi R, Kopka J, Lane AN, Lindon JC, Marriott P, Nicholls AW, Reily MD, Thaden JJ, Viant MR (2007) Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3: 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Wang X, Liu J (2013) Changes in dimensional stability and mechanical properties of Eucalyptus pellita by melamine–urea–formaldehyde resin impregnation and heat treatment. Eur J Wood Wood Prod 71: 557–562 [Google Scholar]
- Tikunov YM, Laptenok S, Hall RD, Bovy A, de Vos RCH (2012) MSClust: A tool for unsupervised mass spectra extraction of chromatography‐mass spectrometry ion‐wise aligned data. Metabolomics 8: 714–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull JW, Booth TH (2002) Eucalypts in cultivation: An overview In: Coppen JJW, ed. Eucalyptus. Taylor & Francis, London: pp. 50–71 [Google Scholar]
- Usadel B, Blasing OE, Gibon Y, Poree F, Hohne M, Gunter M, Trethewey R, Kamlage B, Poorter H, Stitt M (2008) Multilevel genomic analysis of the response of transcripts, enzyme activities and metabolites in Arabidopsis rosettes to a progressive decrease of temperature in the non‐freezing range. Plant Cell Environ 31: 518–547 [DOI] [PubMed] [Google Scholar]
- von Muhlen C, Zini CA, Caramao EB, Marriott PJ (2008) Comparative study of Eucalyptus dunnii volatile oil composition using retention indices and comprehensive two‐dimensional gas chromatography coupled to time‐of‐flight and quadrupole mass spectrometry. J Chromatogr A 1200: 34–42 [DOI] [PubMed] [Google Scholar]
- Vuong QV, Chalmers AC, Bhuyan DJ, Bowyer MC, Scarlett CJ (2015) Botanical, phytochemical, and anticancer properties of the Eucalyptus species. Chem Biodivers 12: 907–924 [DOI] [PubMed] [Google Scholar]
- Warren CR (2008) Does growth temperature affect the temperature responses of photosynthesis and internal conductance to CO2? A test with Eucalyptus regnans . Tree Physiol 28: 11–19 [DOI] [PubMed] [Google Scholar]
- Warren CR, Aranda I, Cano FJ (2012) Metabolomics demonstrates divergent responses of two Eucalyptus species to water stress. Metabolomics 8: 186–200 [Google Scholar]
- Xia J, Sinelnikov IV, Han B, Wishart DS (2015) MetaboAnalyst 3.0–making metabolomics more meaningful. Nucleic Acids Res 43: 251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YH, Yang YM, Yang SY, Wang JA (2007) Review of the biodiversity in Eucalyptus plantation. J Yunnan Agric Univ 22: 741–746 [Google Scholar]
- Zhou ZL, Yin WQ, Zou XP, Huang DY, Zhou CL, Li LM, Chen KC, Guo ZY, Lin SQ (2014) Flavonoid glycosides and potential antivirus activity of isolated compounds from the leaves of Eucalyptus citriodora . J Korean Soc Appl Biol Chem 57: 813–817 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article: http://onlinelibrary.wiley.com/doi/10.1111/jipb.12626/suppinfo
Figure S1. Chromatograms profiling of different species of eucalyptus
Comparison of the liquid chromatography mass spectrometry (LC‐MS) profiles of E. dunnii (A), E. grandis (B) and E. pellita (C) exposed to the same temperature (20°C) for 7 d.
Figure S2. Chromatograms profiling of different temperature treatments
Comparison of the liquid chromatography mass spectrometry (LC‐MS) profiles of Eucalyptus dunnii after 7 d exposure to 10°C (A), 20°C (B) or 30°C (C).
Table S1. Liquid chromatography mass spectrometry (LC‐MS) raw dataset ClusterID refers to a unique number used in the data processing, retention time (RT) in minutes and m/z information (M‐H)‐ related to the most intense ion of the cluster. In the last two columns is showed a mass list (µDA) and its relative intensity, for E. dunnii (DU), E. grandis (GR) and E. pellita (PE). Sample names are assorted according the temperature treatment and its replicate.
Table S2. Gas chromatography mass spectrometry (GC‐MS) raw dataset ClusterID refers to a unique number used in the data processing, retention time (RT) in minutes and retention index (RI) are also shown. In the last two columns is showed a mass list and its relative intensity for Eucalyptus dunnii (DU), E. grandis (GR) and E. pellita (PE). Sample names are assorted according the temperature treatment and its replicate.
Table S3. Fifty‐five metabolites that appeared to be discriminatory for the genotype
Table S4. Thirty‐two metabolites that appeared to be discriminatory for the temperature treatments


