Abstract
Patient‐reported outcome measures (PROMs) are important tools used to understand patient‐focused outcomes from care. Various PROMs have been developed for patients with bladder cancer (BC), although the disease's heterogeneity makes selection difficult. Accurate measurement of health‐related quality of life (HRQL) can only be achieved if the PROM chosen is ‘fit for purpose’ (i.e. psychometrically sound). Systematic reviews of psychometric properties are useful for selecting the best PROM for a specific purpose. The COnsensus‐based Standards for the selection of health Measurement INstruments (COSMIN) developed a checklist to improve the selection of health measurement instruments as part of a review process. Our aims were to undertake a systematic review, using the COSMIN criteria, to assess the quality of studies that report the psychometric properties of PROMs used with people with BC and determine the psychometric quality of these PROMs. An electronic search of seven databases including PubMed, MEDLINE and EMBASE (PROSPERO reference CRD42016051974) was undertaken to identify English language publications, published between January 1990 and September 2017 that evaluated psychometric properties of PROMs used in BC research. Two researchers independently screened abstracts and selected full‐text papers. Studies were rated on methodological quality using the COSMIN checklist. Overall, 4663 records were screened and 23 studies, reporting outcomes in 3568 patients, were evaluated using the COSMIN checklist. Most PROMs had limited information reported about their psychometric properties. Studies reporting on the Bladder Cancer Index (BCI) and Functional Assessment of Cancer Therapy Vanderbilt Cystectomy Index (FACT‐VCI) provided the most detail and these PROMs could be evaluated on the most COSMIN properties. Based on the available evidence, no existing PROM stands out as the most appropriate to measure HRQL in BC populations. This is due to two factors; (i) the heterogeneity of BC and its treatments (ii) no PROM was evaluated on all COSMIN measurement properties due to a lack of validation studies. We suggest future evaluation of generic, cancer generic and BC‐specific PROMs to better understand their application with BC populations and propose strategies to help clinicians and researchers.
Keywords: bladder cancer, COSMIN, patient reported outcome measures, psychometric properties, systematic review
Introduction
A key focus in evaluation of treatments is to have an accurate way of measuring outcomes. Survival and time to cancer progression are common primary outcomes in oncology. However, there is increased recognition of the importance of measuring what were previously regarded as softer outcomes, such as health‐related quality of life (HRQL) using patient‐reported outcome measures (PROMs) 1. The number of PROMs available has grown exponentially and now there are numerous questionnaires available for assessing a multitude of domains in patients with cancer 2. This makes it difficult to decide which PROM to use in each population. When deciding, there are a number of issues to consider: does the PROM measure what it is meant to be measuring (validity), does it do so the same way each time (reliability), and does it detect real differences or changes (sensitivity). Systematic reviews of studies that report on these measurement properties (also known as psychometric) are useful for selecting the best PROMs for a specific purpose 3. A critical appraisal checklist to improve the selection of health‐measurement instruments has been recommended: COnsensus‐based Standards for the selection of health Measurement INstruments (COSMIN). The checklist provides clear assessment criteria and standards, so that the methodological quality of studies that report and evaluate the psychometric properties of PROMs can be assessed as part of a review process 3, 4. This methodology is preferred over traditional evidence‐based reviews, as the focus is not the evaluation of data provided by, or outcomes of PROMs, but the methodological quality of studies that report the psychometric properties of PROMs.
Bladder cancer (BC) is the ninth most common cancer worldwide and one of the most expensive malignancies to manage 5, 6. Treatment of and morbidity from non‐muscle‐invasive BC (NMIBC) and muscle‐invasive BC (MIBC) markedly differ 7, 8, and contribute to differences in patient HRQL 9. Treatment choice will depend on the stage of the cancer, recommendations by clinicians, and patient preferences. This should be informed not only by survival rates but also HRQL outcomes. A review and meta‐analysis of HRQL outcomes after radical cystectomy, where a variety of generic, cancer and BC‐specific PROMs measuring HRQL were employed, found mostly low‐powered studies finding similarities in HRQL between different types of diversions 10. Comparisons with the general population showed not only poorer urinary and sexual functioning in patients after cystectomy, but also deficits in social interactions, physical activity, and emotional function. Although HRQL improved in the year after surgery, evidence was mixed about longer term outcomes 10. HRQL in patients with NMIBC has been less well researched, possibly leading to the impression that there are few differences between the HRQL of patients with NMIBC and the general population 8. However, a recent study developed a conceptual framework for patient‐reported outcomes (PROs) in NMIBC derived from the literature, patients, and clinicians. This identified concerns about symptoms, treatment side‐effects, functional problems, and experiences of care. Some of these were related to more contemporary treatments and were not included in current PROMs 11.
The heterogeneity of BC and its treatments make choosing a PROM to assess HRQL challenging 9. Accurate measurement of HRQL in BC can only be achieved if the PROM chosen is ‘fit for purpose’ (i.e. psychometrically sound) and applied to the correct population. If the PROMs used are not ‘fit for purpose’ or are inappropriate for the patient group being studied, optimum levels of useful and informative HRQL data will not be gained from research. At worst, the data reported may be misleading or unhelpful. To date, a systematic review has not been undertaken to establish the psychometric properties of PROMs used in BC. Here we undertake a systematic review, using the COSMIN criteria. This will assess the quality of studies that report the psychometric properties of PROMs used with people with BC, determine the psychometric quality of these PROMS and identify the most promising generic, cancer‐generic and BC‐specific PROMs.
Materials and Methods
Methods were informed by the University of York, Centre for Reviews and Dissemination guidance for undertaking systematic reviews and were published on their international database of prospectively registered systematic reviews, PROSPERO (reference CRD42016051974). The COSMIN approach was employed 3, 4. Guidance can be found at the COSMIN website (http://www.cosmin.nl/). The checklist has been used in other systematic reviews of oncological HRQL instruments 12, 13 and was suggested in a non‐systematic review of BC HRQL research as a way to evaluate PROMs 9. For the purposes of reporting, instruments/measures to record HRQL will be referred to as PROMs. All stages of the process following the original electronic search were undertaken by two of the research team (S.J.M., P.W.), working independently of each other and then comparing outcomes at each stage of the process. Disagreements were resolved by discussion.
Search Strategy
An electronic search of databases was carried out to identify publications evaluating psychometric properties of PROMs used in BC research. Searches were run in MEDLINE, EMBASE (both via OvidSP), CINAHL, PsycINFO, PubMed, The Cochrane Library, and Web of Science. Terms were agreed by the research team, appropriately modified for each database and limited to English language articles published between January 1990 and Current. Specific publication types were excluded from the search strategy, such as editorials and case reports, as per the search protocol developed by Terwee et al. 14. A combination of Medical Subject Headings (MeSH) and free‐text terms was used. Three groups of terms were generated describing: (i) the population; (ii) questionnaires, surveys and PROMs; and (iii) psychometric properties. Terms within each group were combined with the Boolean operator ‘AND’. Searches were run in November 2016, with an updated search in September 2017. The updated search included the names of PROMs found from the initial search. Reference lists of pertinent review articles identified in the literature search were checked for relevant articles, as was conference proceedings from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and International Society for Quality Of Life Research (ISOQOL) 14. See Data S1 for an example of one database search strategy.
Study Selection Criteria
Inclusion criteria were English language, original studies that assessed patients with BC using a questionnaire or PROM to measure HRQL, where the study was a validation study or evaluated one or more psychometric properties of the questionnaire or PROM. Excluded were studies where the patient cohort included individuals aged <18 years, where patients had bladder problems but not BC, where patients were diagnosed with another cancer (not BC), where the PROM or questionnaire was administered by interview or by proxy, and where the PROM was a clinician‐assessed instrument. Review articles, meeting abstracts, interviews, conference abstracts, editorials, and commentaries were also excluded.
Pilot testing of these criteria was undertaken to check for consensus. Testing consisted of two exercises where the reviewers (S.J.M., P.W.) independently used the inclusion and exclusion criteria to select or reject a subsample of titles and abstracts (200 in total). Following the first exercise, results were compared and interpretation of the criteria was discussed. Discrepancies were resolved through discussion. Discussions focussed on the different types of urinary diversion that may be included in BC HRQL research. Consensus was reached following the second phase of pilot testing.
Full‐text papers were retrieved for titles and abstracts that either appeared to meet inclusion criteria, or where uncertainty existed. These papers were further scrutinised independently by both reviewers to identify the final list included in the review.
Data Extraction
The following data were extracted from each paper: PROM(s) used, constructs measured, content of the PROM and number of items/domains, psychometric information, administration method, study setting, study population, number of patients, patient demographics, response rate, country, and language. Data required to complete the COSMIN checklist assessment were also extracted.
Appraising Methodological Quality
The 4‐point COSMIN checklist was used to evaluate the methodological quality of studies, this evaluation is important as low‐quality studies are considered to have a high risk of biased results. The checklist consists of nine measurement properties, each with their own quality criteria, which form three domains (‘reliability’, ‘validity’, ‘responsiveness’). Three measurement properties are part of the reliability domain: ‘internal consistency’, ‘reliability’, and ‘measurement error’. Five measurement properties comprise the validity domain: ‘content validity’, ‘structural validity’, ‘hypothesis testing’, ‘cross‐cultural validity’, and ‘criterion validity’. Responsiveness is a separate domain. An explanation of what each of the measurement properties are and COSMIN standards for reporting each measurement property is provided in Table 1.
Table 1.
Description of the COSMIN checklist measurement properties and standards for reporting each measurement property
| Measurement property | What the measurement property is | COSMIN standards for reporting the measurement property |
|---|---|---|
| Content validity |
Does the questionnaire include items relevant to the underlying outcome (construct) of interest? Does it include items covering the full scope of the outcome? The validity is assessed by examining how the items for inclusion in the questionnaire were generated. In this instance, the construct is HRQL in bladder cancer patients |
Evidence should be presented of an assessment concerning item relevance and scope Development and pilot work with experts, clinicians and patients is typically undertaken and reported |
| Structural validity |
Sometimes HRQL questionnaires comprise a number of ‘scales’ which represent different constructs of interest The items within a scale should be related to each other, all contributing in a different way to the overall scale score Tests of structural validity include factor analysis and Item Response Theory (IRT) These tests assess how well items fit the scale (unidimensionality) and whether they should be excluded |
Factor analysis should be reported for Classical Test Theory Rasch analysis should be reported for IRT If exploratory factor analysis is undertaken at least 50% of all PROM variance should be explained by the factors and if confirmatory factor analysis is undertaken, factors should match the defined PROM scales Rasch analysis should be described including estimations for parameters of the model |
| Internal consistency |
Internal consistency is closely related to structural validity in that all the items within a scale must tap into the same basic underlying construct It is measured is by looking at the correlation between the items within a scale and examining the correlation of each item to the overall scale score if that specific item was excluded Cronbach's α or Kuder–Richardson Formula 20 (KR‐20) are used |
Following initial Factor analysis to check scale unidimensionality, Cronbach's α ≥ 0.70 or KR‐20 should be reported Items within the same scale or domain should be moderately correlated with each other |
| Reliability |
For a questionnaire to be reliable it should result in the same or similar responses or scores every time, if the circumstances of the people completing the questionnaire remain the same One way of measuring reliability is using a test–retest method (using κ or ICC for scale scores) If the scale is reliable the scores will stay the same when the PROM is completed twice by patients whose health is stable |
Test–retest reliability should be calculated using ICC for continuous scores or κ for dichotomous, ordinal or nominal scores, evidence of at least two independent measurements, with an appropriate time interval during which the participants were stable should be reported |
| Measurement error | Checks if changes in PROM score are due to reasons other than genuine changes in the construct being measured (an error in the measurement) | Standard Error of Measurement (SEM), Smallest Detectable Chance (SDC) or Limits of Agreement (LoA) should be calculated |
| Hypothesis testing |
A reliable and valid questionnaire will pick up differences between groups of patients who are known to be different in terms of the construct of interest. For example, a HRQL questionnaire should be able to detect the difference between those with/without disease (disease free survivors) Questionnaire may be evaluated by testing the hypotheses |
Evidence should be presented that hypotheses were formulated a priori, with the direction of mean differences or relative magnitude of correlations stated |
| Criterion validity | Compares whether PROM scores are similar to the scores of other PROMs used to measure the same construct that is accepted in the field being studied (a ‘gold standard’ PROM) | Evidence should be presented that the criterion used was an adequate ‘gold standard’ (in the case of PROMs, the full version of a short form measure) |
| Responsiveness | Responsiveness (or sensitivity to change) measures if the PROM detects changes in scores over time that are due to the impact of treatments or interventions |
Appropriate statistical methods should be used. Reporting statistical significance with P values is not encouraged Tests should measure the change of the PROM scores, not of health status or magnitude of an event or intervention |
| Cross‐cultural validity | Measures whether the performance of the questions on a translated or culturally adapted PROM are similar or comparable to the performance of the questions in the original version of the PROM | The process of translating the PROM should be adequately described. Factor analysis should have been performed and reported |
IRT, Item Response Theory; KR‐20, Kuder–Richardson Formula 20.
Each eligible study was rated for measurement properties as ‘excellent’, ‘good’, ‘fair’ or ‘poor’. Checklist criteria are used to assess how well studies report each measurement property and whether they adhere to the COSMIN standards, e.g., providing evidence of adequate sample size, a priori hypotheses or how missing values were managed. An overall score is determined by taking the lowest rating gained on any of the checklist criteria for the evaluated measurement property: ‘the worst score counts’ 4.
Reporting of Psychometric Results
The psychometric results reported in the studies were described and categorised into the nine COSMIN measurement properties. Quality criteria proposed by Terwee et al. 15 for health‐status questionnaires was used to determine whether the results for each measurement property were ‘positive’, ‘negative’ or ‘indeterminate’. An example of these criteria is that if a study reported a Cronbach's α of <0.70 for a PROM, the internal consistency for that study would be considered a negative result.
Levels of Evidence Appraisal
A levels‐of‐evidence appraisal was undertaken to determine the overall quality of each measurement property, established in the different studies. The appraisal produced a final rating for each PROM for each of the measurement properties. All available information was synthesised, combining the results of the different studies for each PROM. PROMs were rated based on COSMIN checklist scores (reflecting the methodological quality of the studies), reported psychometric evidence, and quality of the evidence (whether results were positive, negative or indeterminate), the consistency of results between studies, and level of evidence. The levels of evidence rating could be ‘strong’ (+++ or −−−), ‘moderate’ (++ or −−), ‘limited’ (+ or −), ‘conflicting’ (+/−), or ‘unknown’ (?). For example, if two studies reported a Cronbach's α of <0.70 for a PROM, but had both scored ‘Good’ on the COSMIN checklist for internal consistency, the rating would be ‘−−−’, meaning there is strong evidence (multiple studies of good methodological quality) for low levels of internal consistency. However, if there was one study reporting a Cronbach's α of >0.70 which scored ‘Good’ on the COSMIN checklist, the rating of that PROM for internal consistency would be ‘++’, meaning there is moderate evidence in a study of good methodological quality. When there are only studies of poor methodological quality, an unknown rating is given. Levels‐of‐evidence criteria are presented in Table 2 14.
Table 2.
Levels of evidence for the quality of the measurement properties for PROMs, taken from Terwee et al. 14
| Level | Rating* | Criteria |
|---|---|---|
| Strong | +++ or −−− | Consistent findings in multiple studies of good methodological quality OR in one study of excellent methodological quality |
| Moderate | ++ or −− | Consistent findings in multiple studies of fair methodological quality OR in one study of good methodological quality |
| Limited | + or − | One study of fair methodological quality |
| Conflicting | +/− | Conflicting findings |
| Unknown | ? | Only studies of poor methodological quality |
*Positive rating, ‘+’; Indeterminate rating, ‘?’; Negative rating, ‘−’.
Results
The initial search produced 4663 results. After removal of duplicates and screening titles and abstracts, 280 full texts were agreed for further examination, of which 19 met the inclusion criteria. Three articles were included from hand‐searching the reference sections of review articles and one article was included as a result of the updated search (Fig. 1). No results were found from hand‐searching ISPOR or ISOQOL conference proceedings. Overall, 23 studies, reporting PROMs in 3568 patients, were evaluated using the COSMIN checklist. An overview of studies and PROMs are presented in Table 3 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 and Table 4 35, 36, 37, 38, respectively.
Figure 1.
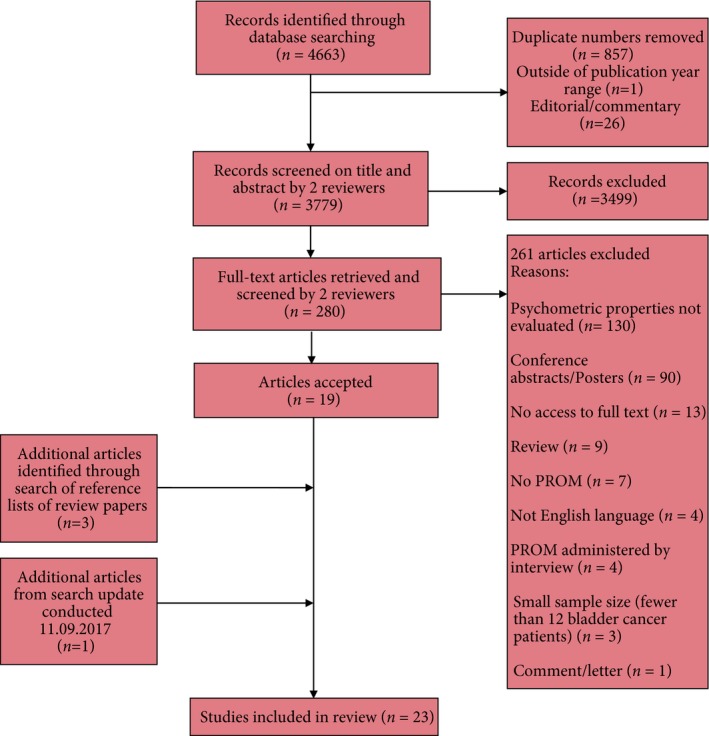
Flow chart showing identification and selection of eligible articles.
Table 3.
Overview of studies included in the review
| (a) MIBC population | |
| 17 Mak et al., 2016 | |
| Setting | Postal study in USA using English language EQ‐5D‐5L (version not reported) and EORTC QLQ‐BLM30 |
| Cohort | Patients with non‐metastatic MIBC with radical cystectomy >2 years disease free. 226 eligible, 177 returned questionnaires. 25% of 109 radical cystectomy patients (median age 73 years) and 22% of 64 trimodality therapy (median age 76 years), 100% female |
| 18 Liu et al., 2016 | |
| Setting | Recruited from Department of Urology, West China Hospital. Completed Chinese language version of WHOQOL‐BREF |
| Cohort | MIBC with ileal conduit diversion with a postoperative period of 1 month to 2 years, 188 questionnaires returned, 66% aged ≥60 years, 19% female |
| 21 Moncrief et al., 2017 | |
| Setting | Postal study in USA using English language BCI and FACT‐VCI |
| Cohort | 64 patients with MIBC with ileal conduit diversion (median age 72 years) or neobladder (median age 63 years), 25% female |
| 29 Anderson et al., 2012 | |
| Setting | Recruited from Vanderbilt University and University of Chicago, USA. Completed English language FACT‐VCI |
| Cohort | 190 patients with MIBC with radical cystectomy and urinary diversion, median (sd) age 67 (10) years, 39% female |
| 30 Cookson et al., 2003 | |
| Setting | Postal study in USA using English language FACT‐VCI |
| Cohort | 40 patients with MIBC with ileal conduit or neobladder, mean (range) age 67.5 (42–87) years, 17% female |
| 31 Stenzelius et al., 2016 | |
| Setting | Recruited ≥1 year after surgery from Skåne University Hospital, Sweden. Completed Swedish language version of FACT‐VCI, which had been translated as part of the study |
| Cohort | 63 patients with MIBC with urinary diversion, mean (sd) age 69.8 (9.1) years, 18% female |
| 32 Siracusano et al., 2014 | |
| Setting | Recruited from five Italian University clinics. Completed Italian language IONB‐PRO |
| Cohort | 171 patients with MIBC with orthotopic neobladder, mean (sd) age 64.3 (9.4) years, 9% female |
| 35 Caffo et al., 1996 | |
| Setting | Postal study in Italy using Italian language ad hoc PROM |
| Cohort | 59 patients with T2–T3 MIBC; 30 cystectomy [median (range) age 71 (49–84) years, 13% female] and 29 conservative treatment [median (range) age 72 (40–86) years, 21% female] |
| 36 Bjerre et al., 1995 | |
| Setting | Postal study and recruitment from outpatient clinics in Denmark using Danish language ad hoc PROM |
| Cohort | 67 non‐malignant patients with MIBC with urinary diversion. Excluded from study if aged ≥80 years, median (range) age 68.2 (50.8–75.7) years, 0% female |
| 37 Hart et al., 1999 | |
| Setting | Postal study in USA using English language ad hoc PROM |
| Cohort | 224 patients with MIBC with cystectomy and either ileal conduit [mean (range) age 76.2 (58–91) years], Koch pouch [mean (range) age 70.6 (49–97) years] or urethral diversion [mean (range) age 67.3 (37–86) years], 24% female |
| 38 Henningsohn et al., 2002 | |
| Setting | Postal study in Denmark using a Swedish to Danish translation of ad hoc PROM |
| Cohort | 89 patients with MIBC with urinary diversion (mean age 64 years) and a control group (mean age 65 years), 10% of controls and patients female |
| (b) NMIBC population | |
| 19 Blazeby et al., 2014 | |
| Setting | Recruited from Bladder COX‐2 Inhibition Trial (BOXIT). Completed English language EORTC QLQ‐C30 and EORTC QLQ‐NMIBC24 before treatment in UK clinic at 2, 3, 6, and 12 months |
| Cohort | 433 patients with NMIBC; 74.6% high risk, Ta 167 (41%), T1 167 (41%), Tis 45 (11%), Ta/Tis 17 (4%) and T1/Tis 14 (3%). Mean (sd) age 66.7 (9.3) years, 21% female |
| 20 Wei et al., 2014 | |
| Setting | Recruited from The People's Hospital of Guangxi Zhuang Autonomous Region, China. Completed Chinese language EORTC QLQ‐C30 before treatment and 6 weeks after treatment |
| Cohort | 106 patients with NMIBC, 33% high risk, 57% aged ≥60 years, 23% female |
| 34 Mogensen et al., 2016 | |
| Setting | Postal study in Denmark 1 week after discharge using Danish language EORTC QLQ‐NMIBC24 |
| Cohort | 121 patients with NMIBC; pTa 68 (56%), pT1a 7 (6%), carcinoma in situ 9 (7%), mean (range) age 71 (41–96) years, 31% female |
| 33 Abáigar‐Pedraza et al., 2016 | |
| Setting | Primary care in Spain using Spanish language CAVICAVENMI |
| Cohort | 180 patients with NMIBC, age and percentage of females not reported |
| (c) All patients with BC | |
| 22, 23 Gilbert et al., 2007; 2010 | |
| Setting | Postal study in USA using English language BCI |
| Cohort | 315 patients; Ta, Tis, T1 166 (53%), T2–T4 119 (38%), Unknown 30 (9%), median (range) age 69 (41–89) years, 18% female |
| 24 Heyes et al., 2016 | |
| Setting | Postal and recruitment at hospitals, private clinics and support groups in Australia. Completed English language BCI |
| Cohort | 119 patients with NMIBC and MIBC, mean (sd) age 70.7 (9.6) years, 26% female |
| 25 Schmidt et al., 2014 | |
| Setting | Multicentre prospective study using Spanish language BCI, which had been translated as part of the study |
| Cohort | 197 patients; Tx: 5 (2.5%), Ta: 58 (29.4%), Tis: 5 (2.5%), T1: 102 (51.8%), T2a: 16 (8.1%), T2b: 6 (3%), T3: 3 (1.5%), T4: 2 (1%), Missing: 11 (5.6%), mean (sd) age 69.3 (11) years, 13% female |
| 26 Li et al., 2016 | |
| Setting | Recruited from First Hospital of China Medical University. Completed Chinese language FACT‐Bl |
| Cohort | 365 patients, Stage 1: 233 (64%), Stages 2 and 3: 127 (35%), age 18–55 years, 74 (20%); 56–65 years, 125 (34%); 66–75 years, 115 (32%); >75 years, 51 (14%), 20% female |
| 27, 28 Matsuda et al., 2003; 2004 | |
| Setting | Postal survey of patients chosen from Isere registry and Tarn registry in France using French language FACT‐Bl |
| Cohort | 95 patients, 80% had a superficial tumour, pTa or pT1, and 20% survivors had pT2 or higher median (range) age 72 (33–99) years, 18% female |
| 16 Hever et al., 2015 | |
| Setting | Recruited from three hospital‐based urology centres in Hungary. Completed Hungarian language EQ‐5D (version not reported), SF‐36, BCI and FACT‐Bl |
| Cohort | 151 patients, T1: 43 (28%), T2: 14 (9%), T3: 6 (4%), T4: 1 (1%), Ta: 57 (38%), Tis: 4 (3%), Tx: 4 (3%), missing data: 22 (14%), mean (sd) age 66.3 (9.6) years, 35% female |
Table 4.
Overview of the PROMs that were evaluated
| Name of PROM | Content | |
|---|---|---|
| Generic | EQ‐5D | 5 items: mobility, self‐care, usual activities, pain, anxiety and depression, plus a visual analogue scale |
| SF‐36 | 36 items overall, mental component scale and physical component scale | |
| WHOQOL‐BREF | 26 items; one from each of the 24 facets of WHOQOL100 plus 2 items from quality of life and general health items. Scales were physical health, psychological health, social relationships, and environment | |
| Cancer generic | EORTC QLQ‐C30 | 30 items. 9 scales. Functional scales: physical, role, emotional, cognitive, social, and global health status/quality of life. Symptom scales/items: fatigue, pain, and nausea and vomiting. Single items: dyspnoea, insomnia, appetite loss, constipation, diarrhoea, and financial difficulties |
| Bladder cancer | BCI | 36 items. 3 domains: urinary (14 items), bowel (10 items), and sexual (12 items). 6 subscales as each domain has a function and bother subscale |
| FACT‐Bl | 13 items specific to bladder cancer plus 27 item FACT‐G that comprises 4 scales; functional, social/family and physical wellbeing scales (7 items), emotional wellbeing scale (6 items) | |
| MIBC | FACT‐VCI | 17 items specific to radical cystectomy patients, plus 27 item FACT‐G that comprises 4 scales; functional, social/family and physical wellbeing scales (7 items), emotional wellbeing scale (6 items) |
| IONB‐PRO | Relational, fatigue, and emotional scales. Analysis from research produced several other scales | |
| EORTC QLQ‐BLM30 | 30 items. 6 scales: urinary symptoms/problems, urostomy problems, future perspective, bloating and flatulence, body image and sexuality, plus single item about catheter use | |
| Caffo et al. 35 | Ad hoc PROM. 40 items for cystectomy version, 41 for conservative treatment version. Items about physical wellbeing, pain, bowel function, urinary function, sexual function, daily physical activities, relational and recreational activities, stoma, and general items | |
| Bjerre et al. 36 | Ad hoc PROM. 211 items including items about urine leakage, diarrhoea, and skin complications | |
| Hart et al. 37 | Ad hoc PROM. About 105 items on emotional distress, quality of life (global), sexual dissatisfaction, body image, urinary diversion problems, sexual function, physical symptomology, daily living activities | |
| Henningsohn et al. 38 | Ad hoc PROM. 137 items for patients, 125 for controls about urinary symptoms, bowel, and sexual dysfunction | |
| NMIBC | EORTC QLQ‐NMIBC24 | 24 items. 6 scales: urinary symptoms, malaise, future worries, bloating and flatulence, sexual function, male sexual problems, plus 5 single items; intravesical treatment issues, sexual intimacy, risk of contaminating partner, sexual enjoyment and female sexual problems |
| CAVICAVENMI | 21 items. 5 scales: disease, self‐esteem and emotional status, working life, daily life, and sex life |
We identified three generic PROMs (EuroQoL five Dimensions [EQ‐5D] 16, 17, 36‐item short‐form health survey [SF‐36] 16, World Health Organisation Quality of Life [WHOQOL‐BREF] 18), one cancer‐generic PROM (European Organisation for Research and Treatment of Cancer quality‐of‐life, 30 item core questionnaire [EORTC QLQ‐C30] 17, 19, 20), seven BC‐specific PROMs including two for all patients with BC (Bladder Cancer Index [BCI] 16, 21, 22, 23, 24, 25, Functional Assessment of Cancer Therapy‐Bladder [FACT‐Bl] 16, 26, 27, 28), three for MIBC (FACT Vanderbilt Cystectomy Index [FACT‐VCI] 21, 29, 30, 31, EORTC QLQ‐BLM30 17, Ileal Orthotopic Neobladder PRO [IONB‐PRO] 32), and two for NMIBC (CAVICAVENMI 33 and EORTC QLQ‐NMIBC24 19, 34). Four ad hoc MIBC PROMs were identified, which were developed specifically for the reporting study 35, 36, 37, 38. The BCI was the most frequently evaluated PROM (six studies). None of the PROMs were evaluated for all nine COSMIN measurement properties.
Methodological Quality
Table 5 presents COSMIN checklist scores, assessing methodological quality of studies that reported COSMIN measurement properties for PROMs. The most frequently reported properties were internal consistency (18 studies) and hypothesis testing (15 studies). Criterion validity was not reported in any study due to lack of a ‘gold standard’, according to the COSMIN definition. Studies reporting on the BCI and FACT‐VCI provided the most detail and these PROMs could be evaluated on the most COSMIN measurement properties (seven properties).
Table 5.
COSMIN Checklist scores evaluating methodological quality of each study per measurement property and PROM
| Measurement properties | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Content validity | Structural validity | Internal consistency | Reliability | Measurement error | Hypothesis testing | Criterion validity | Responsiveness | Cross‐cultural validity | |
| Generic | EQ‐5D | |||||||||
| 16 | Fair | |||||||||
| 17 | Fair | |||||||||
| SF‐36 | ||||||||||
| 16 | Fair | |||||||||
| WHOQOL‐BREF | ||||||||||
| 18 | Poor | |||||||||
| Cancer generic | EORTC QLQ‐C30 | |||||||||
| 19 | Good | Good | ||||||||
| 17 | Fair | |||||||||
| 20 | Fair | |||||||||
| Bladder Cancer | BCI | |||||||||
| 23 | Fair | |||||||||
| 22 | Excellent | Good | Good | Fair | Fair | |||||
| 16 | Poor | Poor | Fair | Fair | Poor | |||||
| 24 | Fair | Fair | ||||||||
| 25 | Poor | Fair | Fair | Poor | ||||||
| 21 | Poor | |||||||||
| FACT‐Bl | ||||||||||
| 16 | Fair | |||||||||
| 26 | Poor | |||||||||
| 27 | Poor | Poor | ||||||||
| 28 | Poor | |||||||||
| MIBC | FACT‐VCI | |||||||||
| 29 | Good | Good | Fair | Poor | Fair | |||||
| 30 | Poor | Poor | Fair | Fair | ||||||
| 31 | Poor | Poor | Fair | Poor | ||||||
| 21 | Poor | |||||||||
| IONB‐PRO | ||||||||||
| 32 | Excellent | Fair | Fair | Poor | Fair | |||||
| EORTC QLQ‐BLM30 | ||||||||||
| 17 | Poor | Fair | ||||||||
| Untitled | ||||||||||
| 36 | Excellent | Poor | Poor | |||||||
| 37 | Poor | |||||||||
| 38 | Excellent | Poor | ||||||||
| 35 | Excellent | Poor | Poor | Poor | ||||||
| NMIBC | EORTC QLQ‐NMIBC24 | |||||||||
| 19 | Poor | Good | Good | |||||||
| 34 | Fair | Fair | Fair | |||||||
| CAVICAVENMI | ||||||||||
| 33 | Excellent | Good | Poor | Poor | Fair | |||||
The best performing property was content validity, with six of the seven reporting studies receiving a score of ‘excellent’. None of the other properties were given this quality rating due to the COSMIN rule of ‘worst score counts’. For example, any study not reporting quantity of missing data or how missing values were managed could not receive an excellent rating on any property that included missing values as part of the assessment. The property with the worst performance was cross‐cultural validity, where all six studies scored ‘poor’ because factor analysis was not undertaken or the sample size was inadequate.
Psychometric Properties
An overview of the psychometric properties for all PROMs is presented in Table S1. When reported, response rates were between 45% and 98%, although many studies did not report response rates or percentage of missing items. Internal consistency was usually presented as Cronbach's α, although one study reported an item‐biased method but did not present results 36. Cronbach's α for scales and domains were not always presented as expected. For example, a study evaluating the BCI combined the urinary, bowel and sexual function items and urinary, bowel and sexual bother items; creating two new scales for which Cronbach's α was calculated 24 instead of reporting on the scales and domains defined in the original BCI validation work 22, 23. Test–retest reliability was the only type of reliability reported by studies; with many reporting Pearson's or Spearman's correlations. Intraclass correlation (ICC) or κ scores were less frequently reported when reporting test–retest reliability and some studies did not explicitly report the test carried out. Content validity was usually undertaken using a combination of literature searching, a working group of clinicians and patients, and cognitive or pilot testing of items. Structural validity was assessed by either exploratory or confirmatory factor analysis. Rasch analysis was less common (two studies 32, 36). Responsiveness was reported in four studies, with one study reporting effect sizes. Although aspects of hypothesis testing were presented in studies (internal relationships and correlations with other instruments) because many findings were not hypothesised a priori, these studies were considered ‘indeterminate’.
Levels of Evidence
Table 6 presents the levels of evidence for PROMs. All PROMs had at least one negative or unknown rating for COSMIN measurement properties. Despite being the most evaluated PROM, the BCI was rated negative for the most measurement properties; with reliability and hypothesis testing both rated as moderate negative.
Table 6.
Overall levels of evidence per measurement property and PROM
| PROM | Measurement properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Content validity | Structural validity | Internal consistency | Reliability | Measurement Error | Hypothesis testing | Criterion validity | Responsiveness | Cross‐cultural validity | |
| Generic | |||||||||
| EQ‐5D | 0 | 0 | 0 | 0 | 0 | −− | 0 | 0 | 0 |
| SF‐36 | 0 | 0 | 0 | 0 | 0 | − | 0 | 0 | 0 |
| WHOQOL‐BREF | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 |
| Cancer generic | |||||||||
| EORTC QLQ‐C30 | 0 | 0 | 0 | 0 | 0 | ? | 0 | ++ | 0 |
| Bladder Cancer | |||||||||
| BCI | +++ | ? | ++ | −− | 0 | −− | 0 | ? | ? |
| FACT‐Bl | 0 | ? | ? | 0 | 0 | − | 0 | 0 | ? |
| MIBC | |||||||||
| FACT‐VCI | ? | −− | ++ | ++ | 0 | ++ | 0 | + | ? |
| IONB‐PRO | +++ | + | + | 0 | ? | + | 0 | 0 | 0 |
| EORTC QLQ‐BLM30 | 0 | 0 | ? | 0 | 0 | + | 0 | 0 | 0 |
| Caffo et al. 35 | +++ | ? | ? | 0 | 0 | 0 | 0 | 0 | ? |
| Bjerre et al. 36 | +++ | ? | ? | 0 | 0 | 0 | 0 | 0 | 0 |
| Hart et al. 37 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 |
| Henningsohn et al. 38 | +++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? |
| NMIBC | |||||||||
| EORTC QLQ‐NMIBC24 | 0 | ? | + | 0 | 0 | ++ | 0 | −− | 0 |
| CAVICAVENMI | +++ | ++ | ? | ? | 0 | + | 0 | 0 | 0 |
+++, strong evidence positive result; ++ or −−, moderate evidence positive/negative result; + or −, limited evidence positive/negative result; ?, unknown rating, due to poor methodological quality; 0, not assessed.
Six PROMs received strong positive ratings for content validity. FACT‐VCI was rated as unknown because the Cookson et al. 30 paper did not assess whether all items were relevant for the study population.
Structural validity was evaluated in eight PROMs. IONB‐PRO and CAVICAVENMI received positive ratings. FACT‐VCI received a moderate negative rating, as factors generated from the factor analysis of postoperative data did not explain >50% of the variance 29. This study also presented factor analysis based on preoperative data, which was not considered appropriate as FACT‐VCI is designed for use with patients who have undergone cystectomy. Five PROMs were rated unknown as the COSMIN checklist deemed the studies reporting on these PROMs to be of poor methodological quality due to inadequate sample sizes.
Internal consistency was evaluated in eleven PROMs. The BCI and FACT‐VCI were rated moderate positive. Four PROMs and three ad hoc PROMs were rated unknown, as the reporting studies were deemed to be of poor methodological quality because factor analysis had not been performed or the sample size for the analysis was too small.
Three PROMs measured test–retest reliability. The BCI was rated moderate negative as some Pearson's r figures were <0.80. CAVICAVENMI was rated unknown as the study scored poor on the COSMIN checklist for methodological quality 33. Small sample sizes accounted for poor COSMIN checklist scores.
The IONB‐PRO, the only PROM for which measurement error was reported, received an unknown rating as the study calculated the standard error measurement based on Cronbach's α 32.
In all, 10 PROMs were evaluated for hypothesis testing. The FACT‐VCI and EORTC QLQ‐NMIBC24, were rated moderate positive. The BCI, FACT‐Bl, EQ‐5D and SF‐36 received negative ratings as <75% of the a priori hypotheses were met and correlations with related constructs were lower than with unrelated ones. EORTC QLQ‐C30 was rated unknown, as it was difficult to establish which constructs were similar between the PROM and comparator PROMs.
Responsiveness was evaluated in four PROMs. The BCI was rated unknown as effect sizes were reported rather than correlations or area‐under‐curve, as required by COSMIN. The EORTC QLQ‐NMIBC24 was moderate negative, as <75% of the results were in accordance with the stated hypotheses.
Five PROMs were evaluated for cross‐cultural validity and received unknown ratings.
Discussion
The aim of the present review was to provide robust information on the psychometric properties of PROMs used with BC cohorts, to aid selection of the most appropriate PROMs for HRQL assessment. In all, 23 studies were identified that reported measurement properties of 15 PROMs used with patients with BC over the last 27 years. None of the reviewed studies that reported psychometric properties of PROMs met all COSMIN criteria standards for methodological quality. Only two measures are applicable to all BC‐specific groups. Therefore, clinicians and researchers will have to choose the ‘best fit’ to serve their assessment objective. This may result in using a combination of generic, cancer‐generic and BC‐specific PROMs that reach the highest COSMIN standards.
Three generic PROMs were identified in the review, all had limited psychometric properties reported in studies; the EQ‐5D, SF‐36, and WHOQOL‐BREF 16, 17, 18. All three generic PROMs are established internationally with multiple non‐BC studies contributing normative and psychometric properties data. Consequently, they are accepted as being valid, reliable, sensitive, and applicable to a wide range of health problems 39. However, they may lack sensitivity in measuring cancer and BC‐specific issues, which is where more specialised PROMs, have a part to play. If being used alongside other more specific PROMs, clinicians and researchers may want to consider the following as part of their decision‐making process: the scope of the generic PROM, the number of items included, ease of administration, and permissions and costs.
Cancer‐generic PROMs, such as the EORTC QLQ‐C30 and FACT – General (FACT‐G), provide information about patient experiences of general symptoms, such as nausea, pain and fatigue, and the impact of cancer on a patient's daily life, emotional health, and relationships. They can be used to compare the HRQL of patients with BC with other cancer populations. Although used as a stand‐alone cancer‐generic PROM in BC research, FACT‐G was not evaluated separately in the present review due to its inclusion as part of the FACT‐Bl and FACT‐VCI.
As with generic PROMs, psychometric evaluation of the EORTC QLQ‐C30 was not the focal point of studies included in the present review and thus very few psychometric properties were reported. During EORTC QLQ‐C30 development, the PROM was psychometrically evaluated in a culturally diverse sample of patients with lung cancer 40 and by 2008, EORTC published reference data for a variety of cancer groups (but not for patients with BC) 41. Despite this, EORTC QLQ‐C30 is used in BC HRQL research.
BC‐specific PROMs focus on commonly reported BC problems such as with urinary, bowel and sexual function. Findings reported using these more specific PROMs may inform BC treatment regimens, policy and patient support. In the main, BC‐specific PROMs have been developed more recently than their generic and cancer‐generic counterparts and therefore have not been as widely used in research. There were 11 BC‐specific PROMs reviewed, of variable quality. On choosing which PROM to use, consideration should be given to how well it performed in the COSMIN review and the population of patients with BC being assessed.
If the target population includes the entire BC spectrum, then the choice of BC‐specific PROM must have been designed for this purpose. Only two PROMs fit this brief, the BCI and FACT‐Bl. Our present review found more support for the BCI, as it was more frequently evaluated in research and scored more favourably using COSMIN. However, the BCI is not without flaw, as evidence has suggested it may be difficult to interpret in circumstances where function and bother scores are different for a domain, under which circumstances researchers must choose whether HRQL should be determined by symptoms (function) or importance of symptoms to the patient (bother) 42. Consequently, it was recommended that a generic or cancer‐generic PROM be administered alongside the BCI 42. In comparison, FACT‐Bl scored poorly on the COSMIN checklist for all reported properties. However, a recent overview paper of BC PROMs suggests that the FACT‐Bl performs better than indicated in the present review. The authors state each new Functional Assessment of Chronic Illness Therapy (FACIT) scale undergoes an assessment of test–retest reliability, responsiveness, and convergent and divergent validity with 50 patients 42 (confirmed by personal correspondence with FACIT, by S.J.M. on 14/09/2017). These psychometric data are neither published nor available within the public domain, meaning a complete COSMIN assessment cannot be undertaken, and therefore recommendation for use is not possible.
Seven MIBC‐specific PROMs were reported. The FACT‐VCI was the most evaluated in studies and had the most positive COSMIN ratings. It is used in HRQL studies comparing types of diversion, but is unsuitable to use with conservative treatment patients. Comparatively, the EORTC QLQ‐BLM30 was assessed for two measurement properties only, but can compare radical and conservative treatment‐related HRQL. Although only evaluated by one study, the IONB‐PRO scored well using the COSMIN checklist and appears to be a viable tool to administer to MIBC patients with neobladders. Four studies reported psychometric properties for a number of unnamed, ad hoc PROMs 35, 36, 37, 38, with no traceable evidence of further use.
Two PROMs were identified that measure HRQL in patients with NMIBC; both performing well when assessed using COSMIN. Although the case could be made to use either the EORTC QLQ‐NMIBC24 or CAVICAVENMI in research, the EORTC QLQ‐NMIBC24 has been evaluated more in research and can be used alongside EORTC QLQ‐C30. However, should clinicians and researchers prefer to use a PROM that includes a combination of cancer‐generic and BC‐specific items that comprises fewer items, they may wish to use CAVICAVENMI over the EORTC QLQ‐BLM30 combined with EORTC QLQ‐C30. Findings are described in Table 7.
Table 7.
Summary of review findings
| Bladder cancer population | Best performing PROM using COSMIN | Further information | Alternatives | Further information |
|---|---|---|---|---|
| All (or a variety of stages/grades) | BCI | Suggested that BCI is used alongside generic and/or cancer‐generic PROMs as interpretation can be difficult | FACT‐Bl | FACT‐Bl can be used to collect BC‐specific and cancer‐generic HRQL information, as the PROM includes FACT‐G |
| MIBC | FACT‐VCI |
FACT‐VCI can be used to collect BC‐specific and cancer‐generic HRQL information, as the PROM includes FACT‐G FACT‐VCI is only suitable to use with MIBC patients who have had a cystectomy |
EORTC QLQ‐BLM30 |
EORTC QLQ‐BLM30 can be used to collect BC‐specific and cancer‐generic HRQL information, as the PROM can be used with EORTC QLQ‐C30 EORTC QLQ‐BLM30 is suitable to use with both cystectomy and conservative treatment patients |
| IONB‐PRO | IONB‐PRO is only suitable to use with patients who have a neobladder | |||
| NMIBC | Difficult to determine as CAVICAVENMI only evaluated in one study and both EORTC QLQ‐NMIBC24 and CAVICAVENMI scored well | EORTC QLQ‐NMIBC24 can be used to collect BC‐specific and cancer‐generic HRQL information, as the PROM can be used with EORTC QLQ‐C30 | CAVICAVENMI | CAVICAVENMI incorporates both BC‐specific and cancer‐generic questions within fewer items than the combination of EORTC QLQ‐C30 and EORTC QLQ‐NMIBC24 |
Strengths and Limitations
Although a strength of the present research is that methodological quality was assessed using the robust COSMIN checklist, carrying out the appraisal provided challenges. Many studies were not written with COSMIN criteria in mind, meaning interpretation of which COSMIN measurement properties were being assessed was sometimes difficult. For example, Blazeby et al. 19 employed multi‐trait scaling when evaluating the structural validity of the EORTC QLQ‐NMIBC24. Multi‐trait scaling is not referred to in the COSMIN guidance. Advice from COSMIN (S.J.M. personal communication with Terwee 08/05/2017) stated that this method was not appropriate to assess structural validity. The complexity of applying the checklist appears to be acknowledged by COSMIN as the checklist is under review 43 and their website has a frequently asked question (FAQ) section, regarding which measurement properties should be assessed when terminology other than that used by COSMIN is reported. Studies that received a negative or unknown rating, published following the publication of the COSMIN guidance, may have received a more favourable COSMIN rating if their guidelines had been taken into account when reporting the psychometric properties of PROMs.
Recommendations
The emergence of new questionnaires, currently published as conference abstracts only 44 and the identification of the four ad hoc MIBC PROMs, indicate existing PROMs used in BC research may not be perceived as adequate by the research and clinical community. Compared with previous COSMIN reviews 13, 45, the present review identified fewer PROM‐validation studies reporting an array of psychometric data. Unlike in other cancer populations, the psychometric properties of generic and general cancer PROMs are less well understood in BC populations. There would be a benefit in pooling data from studies that have used generic, cancer‐generic and BC‐specific PROMs to facilitate the undertaking of a more detailed psychometric analysis. This would enable organisations such as the EORTC to determine reference values for patients with BC and provide research teams with more information when choosing PROM(s) for HRQL research.
Any future psychometric evaluation of generic, cancer‐generic and BC‐specific PROMs should be reported and published, ideally using the COSMIN guidelines, so that it can be determined how useful these PROMs are with their intended BC populations.
Future HRQL research should implement the recommendation from previous research that BC‐specific PROMs be used alongside cancer‐generic PROMs in order to gain comprehensive information. Furthermore, it is recommended that generic PROMs should also be administered alongside cancer‐generic and BC‐specific PROMs to provide a robust picture of HRQL.
Conflicts of Interest
All the authors declare no conflict of interest with this work.
Funding Sources
This work was funded by Yorkshire Cancer Research (Study S385). The funder had no role in the design, analysis or collection of the data; in writing the manuscript; or in the decision to submit the manuscript for publication. We are grateful for the oversight provided by the sponsor, University of Sheffield.
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose.
Abbreviations
- BCI
Bladder Cancer Index
- COSMIN
COnsensus‐based Standards for the selection of health Measurement INstruments
- EORTC QLQ‐BLM30
European Organisation for Research and Treatment of Cancer quality of life questionnaire ‐ Muscle‐Invasive Bladder Cancer module
- EORTC QLQ‐C30
European Organisation for Research and Treatment of Cancer quality of life questionnaire – 30‐item core
- EORTC QLQ‐NMIBC24
European Organisation for Research and Treatment of Cancer quality of life questionnaire ‐ Non Muscle‐Invasive Bladder Cancer module
- EQ‐5D
EuroQoL five Dimensions
- FACIT
Functional Assessment of Chronic Illness Therapy
- FACT‐(Bl)(G)(VCI)
Functional Assessment of Cancer Therapy – (Bladder) (General) (Vanderbilt Cystectomy Index)
- HRQL
health‐related quality of life
- ICC
intraclass correlation
- IONB‐PRO
Ileal Orthotopic Neobladder PRO
- ISOQOL
International Society for Quality Of Life Research
- ISPOR
International Society for Pharmacoeconomics and Outcomes Research
- (MI)(NMI)BC
(muscle‐invasive) (non‐muscle‐invasive) bladder cancer
- PRO(M)
patient‐reported outcome (measure)
- SF‐36
36‐item short‐form health survey
- WHOQOL‐BREF
World Health Organisation Quality of Life
Supporting information
Data S1. Search strategy used for Ovid databases.
Table S1. Psychometric properties of all PROMs, categorised into the COSMIN measurement properties.
Acknowledgments
The authors thank patients and clinicians who contributed to the work reviewed within this manuscript. The authors also thank Dr Caroline Terwee for providing additional guidance and correspondence regarding the interpretation of COSMIN. This work was funded by a project grant from Yorkshire Cancer Research (S385: The Yorkshire Cancer Research Bladder Cancer Patient Reported Outcomes Survey).
References
- 1. Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013; 346: f167 [DOI] [PubMed] [Google Scholar]
- 2. Garratt A, Schmidt L, Mackintosh A, Fitzpatrick R. Quality of life measurement: bibliographic study of patient assessed health outcome measures. BMJ 2002; 324: 1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mokkink LB, Terwee CB, Stratford PW et al. Evaluation of the methodological quality of systematic reviews of health status measurement instruments. Qual Life Res 2009; 18: 313–33 [DOI] [PubMed] [Google Scholar]
- 4. Terwee CB, Mokkink LB, Knol DL, Ostelo RW, Bouter LM, de Vet HC. Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res 2012; 21: 651–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Svatek RS, Hollenbeck BK, Holmang S et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol 2014; 66: 253–62 [DOI] [PubMed] [Google Scholar]
- 6. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 2017; 71: 96–108 [DOI] [PubMed] [Google Scholar]
- 7. Barocas DA, Globe DR, Colayco DC et al. Surveillance and treatment of non‐muscle‐invasive bladder cancer in the USA. Adv Urol 2012; 2012: 421709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singer S, Ziegler C, Schwalenberg T, Hinz A, Goetze H, Schulte T. Quality of life in patients with muscle invasive and non‐muscle invasive bladder cancer. Supportive Care Cancer 2013; 21: 1383–93 [DOI] [PubMed] [Google Scholar]
- 9. Mohamed NE, Gilbert F, Lee CT et al. Pursuing quality in the application of bladder cancer quality of life research. Bladder Cancer 2016; 2: 139–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang LS, Shan BL, Shan LL et al. A systematic review and meta‐analysis of quality of life outcomes after radical cystectomy for bladder cancer. Surg Oncol 2016; 25: 281–97 [DOI] [PubMed] [Google Scholar]
- 11. Rutherford C, Costa DSJ, King MT, Smith DP, Patel MI. A conceptual framework for patient‐reported outcomes in non‐muscle invasive bladder cancer. Supportive Care Cancer 2017; 25: 3095–102 [DOI] [PubMed] [Google Scholar]
- 12. Tax C, Steenbergen ME, Zusterzeel PL, Bekkers RL, Rovers MM. Measuring health‐related quality of life in cervical cancer patients: a systematic review of the most used questionnaires and their validity. BMC Med Res Methodol 2017; 17: 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong CK, Chen J, Yu CL, Sham M, Lam CL. Systematic review recommends the European Organization for Research and Treatment of Cancer colorectal cancer‐specific module for measuring quality of life in colorectal cancer patients. J Clin Epidemiol 2015; 68: 266–78 [DOI] [PubMed] [Google Scholar]
- 14. Terwee CB, deVet HC, Prinsen CAC, Mokkink LB. Protocol for systematic reviews of measurement properties. COSMIN, 2011. Available at: http://www.cosmin.nl/images/upload/files/Protocol%20klinimetrische%20review%20version%20nov%202011.pdf. Accessed September 2017
- 15. Terwee CB, Bot SDM, de Boer MR et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007; 60: 34–42 [DOI] [PubMed] [Google Scholar]
- 16. Hevér NV, Péntek M, Balló A et al. Health related quality of life in patients with bladder cancer: a cross‐sectional survey and validation study of the Hungarian version of the Bladder Cancer Index. Pathol Oncol Res 2015; 21: 619–27 [DOI] [PubMed] [Google Scholar]
- 17. Mak KS, Smith AB, Eidelman A et al. Quality of life in long‐term survivors of muscle‐invasive bladder cancer. Int J Radiat Oncol Biol Phys 2016; 96: 1028–36 [DOI] [PubMed] [Google Scholar]
- 18. Liu CQ, Ren HF, Li JP et al. Predictors for quality of life of bladder cancer patients with ileal conduit: a cross‐sectional survey. Eur J Oncol Nurs 2016; 21: 168–73 [DOI] [PubMed] [Google Scholar]
- 19. Blazeby JM, Hall E, Aaronson NK et al. Validation and reliability testing of the EORTC QLQ‐NMIBC24 questionnaire module to assess patient‐reported outcomes in non‐muscle‐invasive bladder cancer. Eur Urol 2014; 66: 1148–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei L, Li Q, Liang H, Jianbo L. The quality of life in patients during intravesical treatment and correlation with local symptoms. J Chemother 2014; 26: 165–8 [DOI] [PubMed] [Google Scholar]
- 21. Moncrief TJ, Balaji P, Lindgren BB, Weight CJ, Konety BR. Comparative evaluation of bladder‐specific health‐related quality of life instruments for bladder cancer. Urology 2017; 108: 76–81 [DOI] [PubMed] [Google Scholar]
- 22. Gilbert SM, Dunn RL, Hollenbeck BK et al. Development and validation of the Bladder Cancer Index: a comprehensive, disease specific measure of health related quality of life in patients with localized bladder cancer. J Urol 2010; 183: 1764–9 [DOI] [PubMed] [Google Scholar]
- 23. Gilbert SM, Wood DP, Dunn RL et al. Measuring health‐related quality of life outcomes in bladder cancer patients using the Bladder Cancer Index (BCI). Cancer 2007; 109: 1756–62 [DOI] [PubMed] [Google Scholar]
- 24. Heyes SM, Bond MJ, Harrington A, Belan I. The relative contributions of function, perceived psychological burden and partner support to cognitive distress in bladder cancer. Psychooncology 2016; 25: 1043–9 [DOI] [PubMed] [Google Scholar]
- 25. Schmidt S, Riel R, Frances A et al. Bladder cancer index: cross‐cultural adaptation into Spanish and psychometric evaluation. Health Qual Life Outcomes 2014; 12: 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li MY, Yang YL, Liu L, Wang L. Effects of social support, hope and resilience on quality of life among Chinese bladder cancer patients: a cross‐sectional study. Health Qual Life Outcomes 2016; 14: 73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsuda T, Aptel I, Exbrayat C, Grosclaude P. Determinants of quality of life of bladder cancer survivors five years after treatment in France. Int J Urol 2003; 10: 423–9 [DOI] [PubMed] [Google Scholar]
- 28. Matsuda T, Marche H, Grosclaude P, Clement S. Participation behavior of bladder cancer survivors in a medical follow‐up survey on quality of life in France. Eur J Epidemiol 2004; 19: 313–21 [DOI] [PubMed] [Google Scholar]
- 29. Anderson CB, Feurer ID, Large MC et al. Psychometric characteristics of a condition‐specific, health‐related quality‐of‐life survey: the FACT‐Vanderbilt Cystectomy Index. Urology 2012; 80: 77–83 [DOI] [PubMed] [Google Scholar]
- 30. Cookson MS, Dutta SC, Chang SS, Clark T, Smith JA, Wells N. Health related quality of life in patients treated with radical cystectomy and urinary diversion for urothelial carcinoma of the bladder: development and validation of a new disease specific questionnaire. J Urol 2003; 170: 1926–30 [DOI] [PubMed] [Google Scholar]
- 31. Stenzelius K, Lind AK, Wanegard J, Liedberg F. Patient‐reported outcome after radical cystectomy: translation and psychometric validation of the Swedish version of the Functional Assessment of Cancer Therapy Scale Vanderbilt Cystectomy Index. Scand J Urol 2016; 50: 374–9 [DOI] [PubMed] [Google Scholar]
- 32. Siracusano S, Niero M, Lonardi C et al. Development of a questionnaire specifically for patients with Ileal Orthotopic Neobladder (IONB). Health Qual Life Outcomes 2014; 12: 135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abáigar‐Pedraza I, Megías‐Garrigós J, Sánchez‐Payá J. Quality‐of‐life survey for patients diagnosed with nonmuscle‐invasive bladder cancer. Actas Urol Esp 2016; 40: 251–7 [DOI] [PubMed] [Google Scholar]
- 34. Mogensen K, Christensen KB, Vrang ML, Hermann GG. Hospitalization for transurethral bladder resection reduces quality of life in Danish patients with non‐muscle‐invasive bladder tumour. Scand J Urol 2016; 50: 170–4 [DOI] [PubMed] [Google Scholar]
- 35. Caffo O, Fellin G, Graffer U, Luciani L. Assessment of quality of life after cystectomy or conservative therapy for patients with infiltrating bladder carcinoma. A survey by a self‐administered questionnaire. Cancer 1996; 78: 1089–97 [DOI] [PubMed] [Google Scholar]
- 36. Bjerre BD, Johansen C, Steven K. Health‐related quality‐of‐life after cystectomy – bladder substitution compared with ileal conduit diversion – a questionnaire survey. Br J Urol 1995; 75: 200–5 [DOI] [PubMed] [Google Scholar]
- 37. Hart S, Skinner EC, Meyerowitz BE, Boyd S, Lieskovsky G, Skinner DG. Quality of life after radical cystectomy for bladder cancer in patients with an ileal conduit, cutaneous or urethral Kock pouch. J Urol 1999; 162: 77–81 [DOI] [PubMed] [Google Scholar]
- 38. Henningsohn L, Wijkstrom H, Dickman PW, Bergmark K, Steineck G. Distressful symptoms after radical radiotherapy for urinary bladder cancer. Radiother Oncol 2002; 62: 215–25 [DOI] [PubMed] [Google Scholar]
- 39. Devlin NJ, Applebly J. Getting the Most Out of PROMs. Putting Health Outcomes at the Heart of NHS Decision‐Making. London: The King's Fund, 2010. Available at: https://www.kingsfund.org.uk/sites/default/files/Getting-the-most-out-of-PROMs-Nancy-Devlin-John-Appleby-Kings-Fund-March-2010.pdf. Accessed August 2017 [Google Scholar]
- 40. Aaronson NK, Ahmedzai S, Bergman B et al. The European Organisation for research and treatment of cancer QLQ‐C30. A quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–76 [DOI] [PubMed] [Google Scholar]
- 41. Scott NW, Fayers PM, Aaronson NK et al. EORTC QLQ‐C30 Reference Values 2008. Available at: http://groups.eortc.be/qol/sites/default/files/img/newsletter/reference_values_manual2008.pdf. Accessed July 2017
- 42. Danna BJ, Metcalfe MJ, Wood EL, Shah JB. Assessing symptom burden in bladder cancer: an overview of bladder cancer specific health‐related quality of life instruments. Bladder Cancer 2016; 2: 329–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mokkink LB, de Vet HC, Prinsen CA et al. COSMIN Risk of Bias checklist for systematic reviews of Patient‐Reported Outcome Measures. Qual Life Res 2018; 27: 1171–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perlis N, Krahn MD, Boehme K et al. Validating the Bladder Utility Symptom Scale (BUSS): a novel patient reported outcome quality of life measure for all patients with bladder cancer. J Urol 2018, pii: S0022‐5347(18)42498‐6; [Epub ahead of print]. 10.1016/j.juro.2018.03.006 [DOI] [Google Scholar]
- 45. Ojo B, Genden EM, Teng MS, Milbury K, Misiukiewicz KJ, Badr H. A systematic review of head and neck cancer quality of life assessment instruments. Oral Oncol 2012; 48: 923–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Search strategy used for Ovid databases.
Table S1. Psychometric properties of all PROMs, categorised into the COSMIN measurement properties.


