Abstract
Aim
This randomized placebo‐controlled clinical trial evaluated the effect of Bifidobacterium animalis subsp. lactis (B. lactis) HN019‐containing probiotic lozenges as adjuvant to scaling and root planing (SRP) in patients with generalized chronic periodontitis.
Materials and Methods
Forty‐one chronic periodontitis patients were recruited and monitored clinically, immunologically, and microbiologically at baseline (before SRP) and 30 and 90 days after SRP. All patients were randomly assigned to a Test (SRP + Probiotic, n = 20) or Control (SRP + Placebo, n = 21) group. The probiotic lozenges were used twice a day for 30 days. The data were statistically analysed.
Results
The Test group presented a decrease in probing pocket depth and a clinical attachment gain significantly higher than those of the Control group at 90 days. The Test group also demonstrated significantly fewer periodontal pathogens of red and orange complexes, as well as lower proinflammatory cytokine levels when compared to the Control group. Only the Test group showed an increase in the number of B. lactis HN019 DNA copies on subgingival biofilm at 30 and 90 days.
Conclusion
The use of B. lactis HN019 as an adjunct to SRP promotes additional clinical, microbiological, and immunological benefits in the treatment of chronic periodontitis (NCT03408548).
Keywords: Bifidobacterium lactis, periodontitis, probiotics, root planing
1. INTRODUCTION
Scaling and root planing (SRP) is the gold standard for the treatment of periodontitis (Berezow & Darveau, 2011). However, treated sites are subject to recolonization with a microbiota similar to that present before therapy (Mombelli, 2018). Given the limitations of SRP in the treatment of periodontitis (Berezow & Darveau, 2011), adjuvant therapies—antibiotic therapy, antimicrobial photodynamic therapy, and probiotic therapy—have been proposed (Feres et al., 2012; Petelin, Perkic, Seme, & Gaspirc, 2015 Teughels et al., 2013).
The Food and Agriculture Organization (FAO) and the World Health Organization (WHO) defined probiotics as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (Joint FAO/WHO Working Group, 2002). The studies that evaluated the effects of probiotics on the treatment of periodontal disease demonstrated that they can reduce periodontopathogens, improve periodontal clinical parameters, decrease the levels of proinflammatory cytokines, and potentiate the effects of SRP (Ince et al., 2015; Shah, Gujjari, & Chandrasekhar, 2013; Tekce et al., 2015; Teughels et al., 2013; Vivekananda, Vandana, & Bhat, 2010). In fact, two meta‐analyses seem to support the adjunctive use of probiotics in the treatment of chronic periodontitis (Ikram, Hassan, Raffat, Mirza, & Akram, 2018; Martin‐Cabezas, Davideau, Tenenbaum, & Huck, 2016). Nevertheless, further high‐quality randomized clinical trials with microbiological outcomes are warranted to obtain strong conclusions in this regard (Ikram et al., 2018).
To date, studies on the effects of probiotics on periodontal diseases have used mainly microorganisms of the genus Lactobacillus. However, other potential probiotics should be investigated. Hojo et al. (2007) observed that Bifidobacterium species and their counts could be associated with periodontal health and with treatment success in patients with periodontitis. In an in vitro study, Jasberg, Soderling, Endo, Beighton, and Haukioja (2016) demonstrated that species of the genus Bifidobacterium can adhere strongly to the subgingival biofilm and significantly reduce the count of Porphyromonas gingivalis.
Only two pre‐clinical studies evaluated the effect of bacteria of the genus Bifidobacterium on periodontal disease (Oliveira et al., 2017; Ricoldi et al., 2017). Oliveira et al. (2017) demonstrated that the topical use of Bifidobacterium animalis subsp. lactis HN019 promotes a protective effect against alveolar bone loss and connective tissue attachment loss attributable to experimental periodontitis in rats. Ricoldi et al. (2017) concluded that the oral administration of B. lactis HN019 potentiates the effects of SRP on experimental periodontitis in rats. Considering the clinical use of Bifidobacterium on periodontal diseases, there is only one clinical trial that investigated the effects of 4‐week use of yogurt supplemented with Bifidobacterium animalis subsp. lactis DN‐173010 versus a placebo yogurt, followed by a 5‐day non‐brushing period, on gingivitis (Kuru, Laleman, Yalnizoglu, Kuru, & Teughels, 2017). The authors concluded that the probiotic can have a positive effect on plaque accumulation and gingival inflammatory parameters after oral hygiene abstinence.
So far, no clinical study has investigated the effects of bacteria of the genus Bifidobacterium on non‐surgical treatment of periodontitis. This randomized placebo‐controlled clinical trial evaluated the effects of B. lactis HN019‐containing probiotic lozenges as adjuvant to SRP in patients with generalized chronic periodontitis.
2. MATERIALS AND METHODS
2.1. Patient population
Forty‐one patients were selected from the population referred to the Periodontal Clinic at the School of Dentistry of Ribeirao Preto—University of Sao Paulo (FORP‐USP, Ribeirao Preto, SP, Brazil). All eligible patients (ClinicalTrials.gov ID: NCT03408548) were thoroughly informed of the nature and potential risks and benefits of their participation in the study and signed an informed consent form. The study protocol was reviewed and approved by the Research Ethics Committee of FORP‐USP (protocol number: 06278012.1.0000.5419). The research was conducted in full accordance with ethical principles, including the World Medical Association Declaration of Helsinki (version 2008) and additional requirements.
The sample size was determined using Graphpad Statemate 2.0 (GraphPad Software, Inc., San Diego, CA, USA). An 80% power was adopted to recognize a significant difference of 1 mm (d) between groups with a 95% confidence interval (α = 0.05) and standard deviation (SD) of 1.13 mm (Vivekananda et al., 2010).
Considering changes in mean clinical attachment level (CAL) as the primary outcome variable and (Zα + Zß)2 = 7.84 (Zα = 1.96 for 2‐tailed 0.05 hypothesis test; Zß = 0.842 for power = 0.8), sample size was calculated using the following formula: n = {2[(SD)2/(d)2]} × (Zα + Zß)2. Therefore, a total of 36 patients were required. However, considering that some patients could be lost to follow‐up, 41 patients were enrolled in this study.
2.2. Inclusion and exclusion criteria
All patients were diagnosed with generalized chronic periodontitis according to the classification of the American Academy of Periodontology (Armitage, 1999). The inclusion criteria were (a) age over 30 years, (b) 30% or more of the sites with probing pocket depth (PPD) ≥ 4 mm and CAL ≥ 4 mm, (c) presence of bleeding on probing (BOP) and (d) a minimum of five teeth with at least one site with CAL and PPD ≥ 5 mm. All patients had to be in good general health.
The exclusion criteria were (a) cause‐related periodontal therapy or antimicrobial therapy in the previous 6 months, (b) systemic conditions that could influence the progression of periodontitis or the treatment response (e.g. Down Syndrome, HIV, Diabetes Mellitus types 1 and 2), (c) smoking, (d) need of prophylactic antibiotic therapy for routine dental procedures, (e) long‐term administration of anti‐inflammatory medications, and (f) usage of probiotics 6 months prior to the study.
2.3. Experimental design, allocation concealment, and treatment protocol
According to a random numeric table generated by computer software, the study coordinator (M.R.M.) allocated each patient to one of the following groups: Control (SRP + Placebo; 21 patients) or Test (SRP + Probiotic therapy, 20 patients). Before the study began, the selected individuals were identified by a numeric code that designated the experimental group to which they belonged. The study coordinator (M.R.M.) revealed the meaning of each code only after conducting the statistical analysis of the experimental data.
The patients received lozenges containing 10 mg of probiotic (Test group) or placebo (Control group). In the Test group, the lozenges had 109 colony‐forming units (CFUs) of B. lactis HN019 (HOWARU® Bifido LYO 40 DCU‐S, DuPont™ Danisco® Sweeteners Oy, Kantvik, Finland). In general, products containing probiotic microorganisms must have a minimum number of viable cells, with proven efficacy established on human clinical studies, estimated between 106 and 108 CFUs per gram (CFUs/g) of the final product or 108 to 1010 CFUs/day (considering 100 g or 100 ml of ingested food) (Champagne, Ross, Saarela, Hansen, & Charalampopoulos, 2011). A compounding pharmacy prepared identical probiotic and placebo lozenges. Identical plastic bottles containing the probiotic/placebos were sent to the study coordinator (M.R.M.), who wrote the number code of each patient on each bottle, according to the therapy they were assigned to. The coded bottles were given to the examiner (M.S.M.S.), who distributed them to the patients and did not have any access to information about the content of the lozenges. In addition, the patients were blinded to the content of the lozenges and treatment assignment during the study.
Seven days prior to the non‐surgical periodontal therapy, all patients received supragingival plaque control and oral hygiene instructions. Within 24 hr, a specialist in periodontics (M.M.I.), who was blinded to the experimental groups, performed supragingival and subgingival SRP on all teeth with periodontal involvement, using hand (Gracey Curettes; Hu‐Friedy, Chicago, IL, USA) and ultrasonic instruments. The patients were instructed (immediately after SRP) to take one lozenge twice a day (after waking up and before bedtime) for 30 days. They were also instructed not to take any other probiotic product during the study. The patients received 14 lozenges (placebo or probiotic) per week. At the end of each week, they had to be seen at the FORP‐USP Periodontal Clinic. During their visit, they should bring the packs of lozenges taken during the week and then they received new lozenges for the subsequent week. During the visit, the patients answered a questionnaire about their perception of any side effect observed during the consumption of the dietary supplement. The patients were not subjected to any clinical procedure during these visits. One research assistant (P.H.F.S.) conducted these procedures and monitored the patient's compliance with medication dosage. This assistant was not the examiner or operator in this study.
All patients received microbiological, immunological, and clinical monitoring at baseline, at 30 days, and at 90 days. The evaluations (pre‐ and post‐intervention) were conducted by a single trained and calibrated examiner (M.S.M.S.), who was blinded to the experimental groups.
2.4. Examiner calibration
The Kappa coefficient greater than or equal to 0.85 was used for examiner calibration. Ten patients with at least five teeth with PPD and CAL ≥ 5 mm on proximal sites were selected. Each patient was examined twice by a universal North Carolina‐15 periodontal probe (PCPUNC156; Hu‐Friedy), at a 48‐hour interval between the first and second assessments.
2.5. Clinical measurements
Plaque index (PI) was employed to assess the patients’ oral hygiene status. BOP was recorded based on the presence or absence of bleeding up to 20 s after probing at the experimental sites. PI and BOP were scored as plaque and bleeding being absent or present (0 or 1, respectively). PPD was measured from the free gingival margin to the bottom of the periodontal pocket. CAL was measured from the cementoenamel junction to the base of the periodontal pocket. Gingival recession (GR) was measured from the cementoenamel junction to the free gingival margin. BOP, PPD, CAL, and GR were measured at six sites per tooth. All probing measurements were performed using a manual periodontal probe (PCPUNC156; Hu‐Friedy).
2.6. Immunological monitoring
Gingival crevicular fluid (GCF) was collected from four non‐contiguous interproximal sites per patient using absorbent paper strips (Periopaper®; Oralflow Inc., Amityville, NY, USA), as previously described by Luchesi et al. (2013). Conventional enzyme immunoassays (ELISA) using commercially available kits (DCTM Protein Assay; Bio‐Rad Laboratories, Inc. Berkeley, CA, USA) were performed to determine the amount of total protein in each sample. IL‐1β, IL‐10, and IL‐8 levels in GCF samples were determined according to Moreira et al. (2015) using high sensitivity kits (LXSAHM‐03—Techne Corporation, R&D Systems, Minneapolis, MN, USA) and the multiplexing instrument (MAGPIX® analyser; Luminex Corporation, Austin, TX, USA). These cytokines were selected considering the study by Ricoldi et al. (2017), which demonstrated the effects of B. lactis HN019 on these immunoinflammatory markers in periodontal tissues of rats. The concentrations of each cytokine were estimated from the standard curve using a five‐parameter polynomial equation with specific software (Xponent® software; Luminex Corporation).
2.7. Microbiological monitoring
According to Moreira et al. (2015), subgingival plaque samples were obtained from four non‐contiguous interproximal sites from each patient, grouped according to the following PPD categories: moderate pockets (PPD = 4–6 mm) and deep pockets (PPD ≥ 7 mm). The samples were individually analysed for the presence of 40 subgingival bacterial species using the checkerboard DNA‐DNA hybridization technique (Socransky et al., 2004) at the Microbiology Laboratory of the Guarulhos University (UNG, Guarulhos, SP, Brazil).
Bifidobacterium animalis subsp. lactis HN019 was detected and quantified by real‐time polymerase chain reaction (qPCR) using specific primers, as proposed by Junick and Blaut (2012). Genomic DNA was extracted, and the qPCR technique was performed using the SYBRGreen system (Invitrogen Corp., Carlsbad, CA, USA) in StepOne Plus™ Real‐Time (Thermo Fisher Scientific).
2.8. Outcome variables
Changes in the mean CAL at 90 days post‐SRP were defined as the primary outcome variable. All other parameters were considered secondary outcomes. Sub‐analyses were made considering PPD at baseline for clinical (PPD, CAL, and GR) and microbiological parameters. Periodontal pockets were classified at baseline as moderate (PPD = 4–6 mm) or deep (PPD ≥ 7 mm).
The “need for additional periodontal treatment” was determined according to Cionca, Giannopoulou, Ugolotti, and Mombelli (2009). “Risk for periodontal disease progression” was defined at the patient level according to Lang and Tonetti (2003).
2.9. Statistical analysis
Each outcome was computed per participant and then averaged across patients in both groups. The significance level was set at 5%.
Within‐group and between‐group differences in PPD, CAL, GR, PI, BOP, and residual periodontal pockets were assessed by repeated measures analysis of variance (ANOVA) followed by Bonferroni post hoc test and by Student's t test, respectively. The differences between groups considering the outcomes “need for additional periodontal treatment” and “risk for periodontal disease progression” were assessed by the chi‐square test.
Microbiological data were presented as mean counts (x105) of individual bacterial species in both groups. Bacterial species were also grouped into complexes, according to Socransky, Haffajee, Cugini, Smith, and Kent (1998). Friedman's test was used to detect significant differences within each group. The analyses were performed after adjustments for multiple comparisons (Socransky, Haffajee, Smith, & Dibart, 1991) The between‐group differences in the changes observed at 90 days regarding the mean counts of each bacterial species compared to baseline values were assessed by the Mann–Whitney test. The within‐group and between‐group differences in the number of copies of B. lactis HN019 DNA were also assessed by the Mann–Whitney test.
Total protein values were converted to pg/mL. Final cytokine levels were obtained by dividing the initial values provided by the MAGPIX® system by the total protein content in GCF samples (pg/mL). Within‐group and between‐group differences in the mean levels of IL‐1β, IL‐8, and IL‐10 were assessed by ANOVA followed by Bonferroni post hoc test and Student's t test, respectively.
3. RESULTS
This study started in December 2015 and ended in August 2016. Figure 1 shows the study design. All patients successfully completed the study. The demographic characteristics did not show any difference between groups (for additional details, see online supplementary material). No adverse effects were observed after the use of probiotics.
Figure 1.
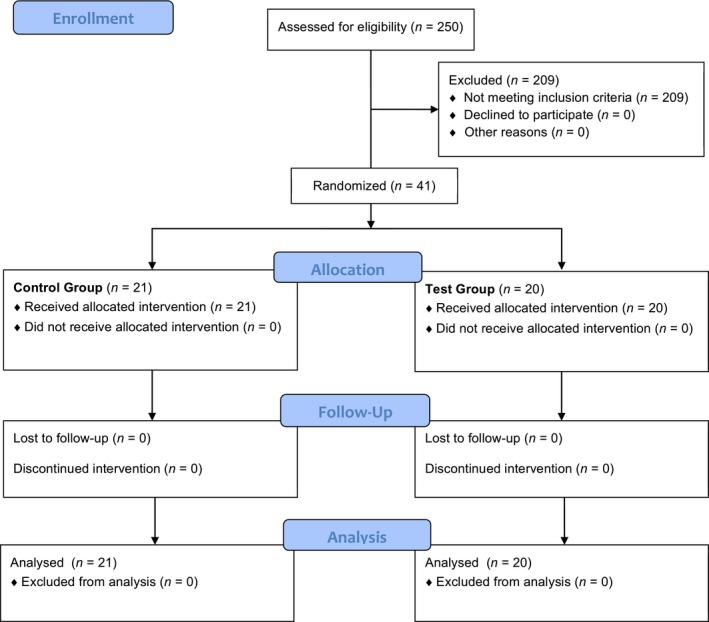
Flowchart of the study design
3.1. Clinical monitoring
Table 1 shows the PI, BOP, PPD, CAL, and GR values for the Control and Test groups. In moderate and deep pockets, the Test group had larger clinical attachment gain and lower PPD than the Control group at 90 days (p < 0.05).
Table 1.
Means and SD of clinical parameters (PPD, CAL, GR, PI, and BOP), number of moderate and deep pockets and follow‐up of pockets detected at baseline in groups Test and Control
| Variable | Time point | Experimental groups | Intergroup comparisons | |||||
|---|---|---|---|---|---|---|---|---|
| Test N = 20 | Control N = 21 | Mean difference | Student's t test p value | |||||
| Mean ± SD | Delta ± SD Δ 0–30/0–90 | Mean ± SD | Delta ± SD Δ 0–30/0–90 | Mean | Delta | |||
| PPD (mm) | ||||||||
| Overall | Baseline | 3.01 ± 0.27 | 3.10 ± 0.43 | 0.09 | NS | |||
| 30 days | 2.53 ± 0.25a | −0.48 ± 0.30 | 2.78 ± 0.37a | −0.32 ± 0.27 | 0.25 | ≤0.05 | 0.1579 | |
| 90 days | 2.49 ± 0.27a | −0.52 ± 0.32 | 2.85 ± 0.34a | −0.25 ± 0.22 | 0.36 | ≤0.05 | 0.0048 | |
| Moderate pockets | Baseline | 4.47 ± 0.20 | – | 4.44 ± 0.27 | – | 0.02 | NS | – |
| 30 days | 3.29 ± 0.47a | −1.18 ± 0.37 | 3.33 ± 0.42a | −1.11 ± 0.41 | 0.04 | NS | NS | |
| 90 days | 3.19 ± 0.52a | −1.28 ± 0.41 | 3.50 ± 0.45a | −0.94 ± 0.42 | 0.31 | ≤0.05 | 0.01 | |
| Deep pockets | Baseline | 7.27 ± 0.29 | – | 7.26 ± 0.35 | – | 0.14 | NS | – |
| 30 days | 4.07 ± 1.14a | −3.20 ± 1.00 | 4.47 ± 1.23a | −2.79 ± 1.11 | 0.39 | NS | NS | |
| 90 days | 3.75 ± 1.32a | −3.52 ± 1.19 | 4.64 ± 1.00a | −2.62 ± 0.88 | 0.90 | 0.019 | 0.0103 | |
| CAL (mm) | ||||||||
| Overall | Baseline | 3.26 ± 0.39 | 3.42 ± 0.54 | 0.16 | NS | |||
| 30 days | 2.77 ± 0.44a | −0.49 ± 0.37 | 3.13 ± 0.50a | −0.29 ± 0.26 | 0.36 | ≤0.05 | 0.0855 | |
| 90 days | 2.77 ± 0.38a | −0.49 ± 0.37 | 3.24 ± 0.51a | −0.18 ± 0.23 | 0.47 | ≤0.05 | 0.0025 | |
| Moderate pockets | Baseline | 4.63 ± 0.42 | – | 4.70 ± 0.45 | – | 0.07 | NS | – |
| 30 days | 3.51 ± 0.65a | −1.12 ± 0.44 | 3.69 ± 0.56a | −1.01 ± 0.44 | 0.18 | NS | NS | |
| 90 days | 3.48 ± 0.59a | −1.15 ± 0.45 | 3.94 ± 0.63a | −0.76 ± 0.41 | 0.45 | 0.022 | 0.0082 | |
| Deep pockets | Baseline | 7.48 ± 0.53 | – | 7.62 ± 0.72 | – | 0.13 | NS | – |
| 30 days | 4.36 ± 1.45a | −3.12 ± 1.27 | 5.08 ± 1.49a | −2.54 ± 1.33 | 0.72 | NS | NS | |
| 90 days | 4.03 ± 1.44a | −3.45 ± 1.21 | 5.55 ± 1.37a | −2.07 ± 0.97 | 1.51 | 0.001 | 0.0004 | |
| GR (mm) | ||||||||
| Overall | Baseline | 0.25 ± 0.35 | – | 0.32 ± 0.33 | – | 0.07 | NS | NS |
| 30 days | 0.23 ± 0.32 | −0.02 ± 0.16 | 0.35 ± 0.33 | 0.03 ± 0.13 | 0.12 | NS | NS | |
| 90 days | 0.27 ± 0.87 | 0.02 ± 0.12 | 0.38 ± 0.97 | 0.06 ± 0.19 | 0.11 | NS | NS | |
| Moderate pockets | Baseline | 0.15 ± 0.27 | – | 0.26 ± 0.25 | – | 0.10 | NS | – |
| 30 days | 0.21 ± 0.33 | 0.06 ± 0.16 | 0.36 ± 0.34 | 0.10 ± 0.24 | 0.14 | NS | NS | |
| 90 days | 0.28 ± 0.34 | 0.13 ± 0.27 | 0.43 ± 0.48 | 0.17 ± 0.31 | 0.15 | NS | NS | |
| Deep pockets | Baseline | 0.26 ± 0.45 | – | 0.50 ± 0.77 | – | 0.24 | NS | – |
| 30 days | 0.28 ± 0.47 | 0.02 ± 0.37 | 0.61 ± 0.91 | 0.11 ± 0.47 | 0.33 | NS | NS | |
| 90 days | 0.28 ± 0.66 | 0.02 ± 062 | 0.90 ± 1.09 | 0.40 ± 0.47 | 0.61 | 0.038 | 0.0360 | |
| PI after OHI | ||||||||
| Mean Number ± SD (%) | Baseline | 23.85 ± 15.33 (25.90) | – | 26.71 ± 16.60 (27.40) | – | 2.71 (1.50) | NS | – |
| 30 days | 14.20 ± 12.73a (15.40) | −9.65 ± 10.52 | 20.24 ± 17.53a (20.80) | −6.47 ± 11.59 | 6.51 (5.40) | ≤0.05 | NS | |
| 90 days | 21.65 ± 13.13 (23.50) | −2.20 ± 12.30 | 27.14 ± 18.64 (27.90) | 0.47 ± 12.07 | 5.49 (4.40) | NS | NS | |
| BOP after OHI | ||||||||
| Mean Number ± SD (%) | Baseline | 30.80 ± 22.07 (22.10) | – | 35.00 ± 25.84 (24.10) | – | 4.20 (2.00) | NS | – |
| 30 days | 17.05 ± 14.60a (12.20) | −13.75 ± 14.16 | 24.05 ± 19.83a (16.50) | −10.50 ± 16.17 | 7.00 (4.30) | ≤0.05 | NS | |
| 90 days | 18.80 ± 16.14a (13.50) | −12.00 ± 15.49 | 30.71 ± 27.86 (21.10) | −4.29 ± 18.61 | 11.91 (7.60) | ≤0.05 | NS | |
| Moderate pockets | ||||||||
| Mean Number ± SD (%) | Baseline | 28.45 ± 9.72 (20.40) | – | 33.90 ± 13.12 (23.30) | – | 5.45 (2.90) | NS | – |
| 30 days | 13.90 ± 7.64a (10.00) | −14.55 ± 8.63 | 22.86 ± 14.1a (15.70) | −11.04 ± 10.35 | 8.96 (5.70) | ≤0.05 | NS | |
| 90 days | 12.50 ± 6.95a (9.00) | −15.95 ± 10.58 | 23.67 ± 11.6a (16.30) | −10.23 ± 8.82 | 11.17 (7.30) | ≤0.05 | 0.0674 | |
| Deep pockets | ||||||||
| Mean Number ± SD (%) | Baseline | 5.60 ± 3.69 (4.00) | – | 6.00 ± 5.25 (4.10) | – | 0.40 (0.10) | NS | – |
| 30 days | 1.25 ± 2.26a (0.90) | −4.35 ± 3.66 | 1.90 ± 3.41a (1.30) | −4.10 ± 4.27 | 0.65 (0.40) | NS | 0.8391 | |
| 90 days | 1.25 ± 2.42a (0.90) | −4.35 ± 3.95 | 2.90 ± 4.14a (2.00) | −3.10 ± 2.40 | 1.65 (1.10) | ≤0.05 | 0.2242 | |
|
Follow‐up of moderate pockets detected at baseline Mean Number ± SD (%) | ||||||||
| PPD ≤ 3 mm | 90 days | 19.60 ± 9.33 (68.90) | – | 18.38 ± 8.80 (54.20) | – | 1.22 (14.70) | ≤0.05 | – |
| PPD ≥ 4 ≤ 6 mm | 90 days | 8.40 ± 5.39 (29.10) | – | 14.81 ± 10.54 (43.70) | – | 6.41 (14.60) | ≤0.05 | – |
| PD ≥ 7 mm | 90 days | 0.45 ± 1.23 (1.60) | – | 0.71 ± 1.30 (2.10) | – | 0.26 (0.40) | NS | – |
|
Follow‐up of deep pockets detected at baseline Mean Number ± SD (%) | ||||||||
| PPD ≤ 3 mm | 90 days | 3.25 ± 2.50 (58.00) | – | 1.33 ± 1.20 (22.20) | – | 1.92 (35.80) | ≤0.05 | – |
| PPD ≥ 4 ≤ 6 mm | 90 days | 1.65 ± 1.53 (29.50) | – | 2.8 ± 2.56 (46.80) | – | 1.15 (17.30) | ≤0.05 | – |
| PPD ≥ 7 mm | 90 days | 0.7 ± 1.42 (12.50) | – | 1.86 ± 2.85 (31.00) | – | 1.16 (18.50) | ≤0.05 | – |
PPD: probing pocket depth; CAL: clinical attachment level; GR: gingival recession; PI: plaque index; BOP: bleeding on probing; SD: standard deviation; OHI: oral hygiene instructions; Moderate pockets = PPD ≥ 4 ≤ 6 mm; Deep Pockets = PPD ≥ 7 mm; NS = no significant difference.
Significant difference when compared to baseline (repeated measures ANOVA, Bonferroni post hoc test, p < 0.05).
Regarding the number of residual pockets (Table 1), the Control group had a higher number of moderate (at 30 and 90 days) and deep (at 90 days) pockets than the Test group (p < 0.05).
As to the risk of progression of periodontal disease (Table 2), while 55% of the patients in the Test group were at a low risk, only 30% of the patients in the Control group were classified as such. The Test group had a lower rate of patients in need for additional periodontal treatment on more than three sites when compared to the Control group at 90 days (p < 0.05).
Table 2.
Number and percentage of subjects presenting low (≤4 sites with probing pocket depth (PPD) ≥ 5 mm), moderate (5–8 sites with PPD ≥ 5 mm) or high (≥9 sites with PPD ≥ 5 mm) risk for disease progression, as well as presenting 0, 1–2 or ≥3 sites in need for additional therapy
| Variable | Time point | Experimental groups | Intergroup comparisons | |
|---|---|---|---|---|
| Test N = 20 Number (%) | Control N = 21 Number (%) | Chi‐square for trend p value | ||
| Risk for disease progression | ||||
| Low | 90 days | 11 (55.00) | 6 (28.60) | 0.0863 |
| Moderate | 90 days | 3 (15.00) | 4 (19.00) | |
| High | 90 days | 6 (30.00) | 11 (52.40) | |
| Need for additional therapy | ||||
| 0 sites | 90 days | 4 (20.00) | 1 (12.20) | 0.0306 |
| 1–2 sites | 90 days | 9 (45.00) | 6 (36.60) | |
| ≥3 sites | 90 days | 7 (35.00) | 14 (51.20) | |
3.2. Microbiological monitoring
Figure 2 shows the mean total count (×105) of 40 subgingival species in deep and moderate periodontal pockets for the Control and Test groups, as well as changes in the mean counts of each bacterial species at 90 days relative to baseline values. The Test group exhibited a larger count of Actinomyces naeslundii and Streptococcus mitis and a more pronounced reduction in the count of P. gingivalis, Treponema denticola, Fusobacterium nucleatum vincentii, Campylobacter showae, and Eubacterium nodatum than the Control group (p < 0.05) for deep periodontal pockets.
Figure 2.
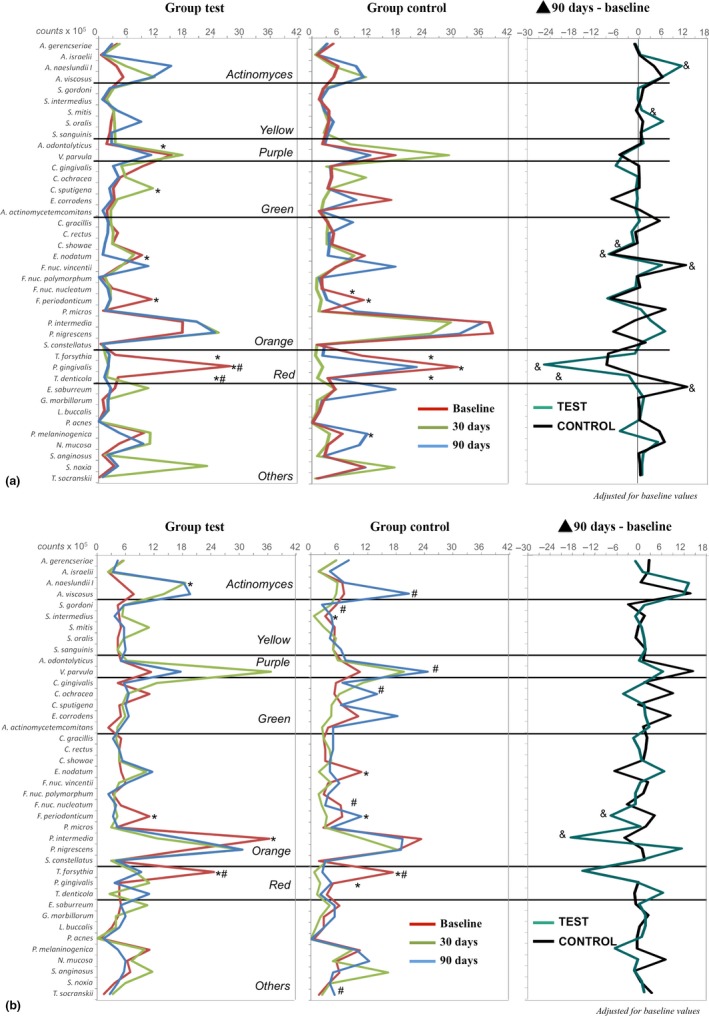
Profiles of the mean counts (×105) of 40 taxa in subgingival biofilm samples at baseline and at 30 and 90 days post‐treatment. The panel at the far right presents the changes in the mean counts of each species between baseline and 90 days post‐treatment. The species were ordered according to the microbial complexes described by Socransky et al. (1998). The significance of differences among time points was determined using Friedman test (*p < 0.05 at 30 days and # p < 0.05 at 90 days) and between groups at 90 days using Mann–Whitney test (& p < 0.05). All the analyses were adjusted for multiple comparisons (Socransky et al., 1991). (a) deep pockets, (b) moderate pocktes
Figure 3 shows the mean cumulative proportions of microbial complexes observed in both groups in different experimental periods. Test group showed mean proportions of orange (at 30 days) and red (at 90 days) complexes significantly lower than those of the Control group (p < 0.05). A significantly larger proportion of blue complex bacteria was also observed in the Test group when compared to the Control group at 90 days (p < 0.05).
Figure 3.
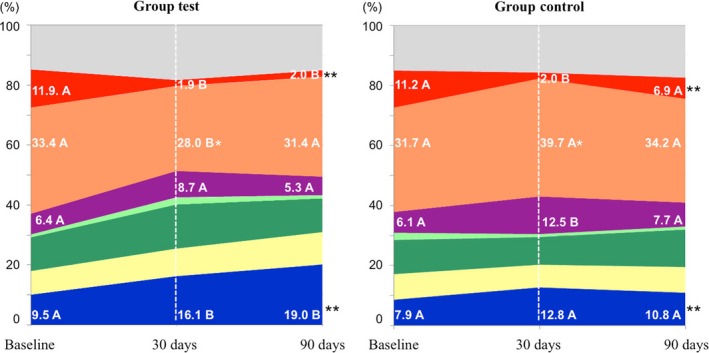
Cumulative mean proportions of microbial complexes in subgingival biofilm samples taken from subjects at baseline and at 30 and 90 days post‐treatment. The colours represent different microbial complexes (Socransky et al., 1998). The light green colour represents the proportion of Aa. The grey colour represents species that did not fall into any complex, and Actinomyces species are represented in blue. The significance of differences among time points was determined using Friedman and Dunn multiple‐comparison tests (different letters represent significant differences between time points, p < 0.05). The significance of differences between the two groups at 30 days and 90 days was determined by using Mann–Whitney test (*p < 0.05 at 30 days and ** p < 0.05 at 90 days)
The qPCR analysis (Figure 4) demonstrated that only the Test group had an increase in the number of copies/μL of the DNA of B. lactis HN019 in subgingival biofilm samples at 30 and 90 days (p < 0.05).
Figure 4.
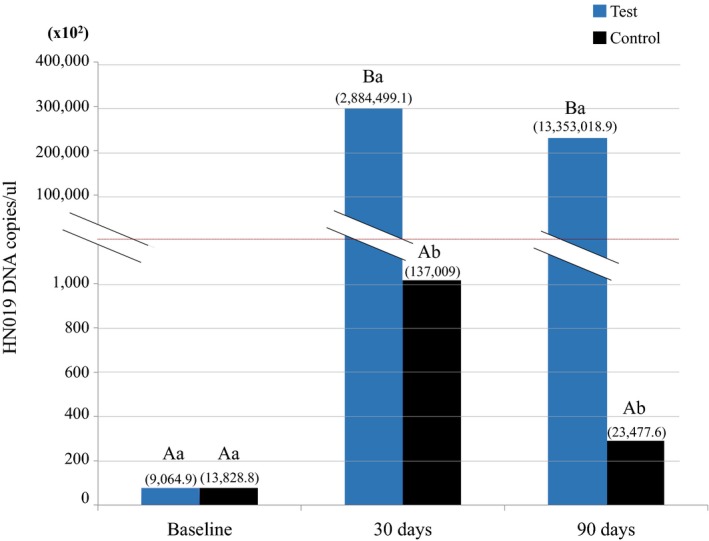
Number of copies/μL of Bifidobacterium animalis subsp. lactis HN019 DNA and standard deviations (numbers above the columns) in samples of subgingival biofilm of Test and Control groups at baseline, 30 days, and 90 days, as well as intra‐ and inter‐group comparisons. Different majuscule letters indicate significant differences in each group over experimental time (Friedman test, Dunn, p < 0.05). Different minuscule letters indicate inter group differences within the same time point (Mann–Whitney test, p < 0.05)
3.3. Immunological monitoring
IL‐8, IL‐1β, and IL‐10 levels are shown in Figure 5(a–c). Only the Test group had higher levels of IL‐10 than those at baseline at 30 days (p < 0.05). Figure 5(d–f) shows the mean ratios between the levels of each cytokine and those at baseline. The Control group had a higher ratio of IL‐1β (at 30 and 90 days) and of IL‐8 (at 30 days) when compared to the Test group (p < 0.05).
Figure 5.
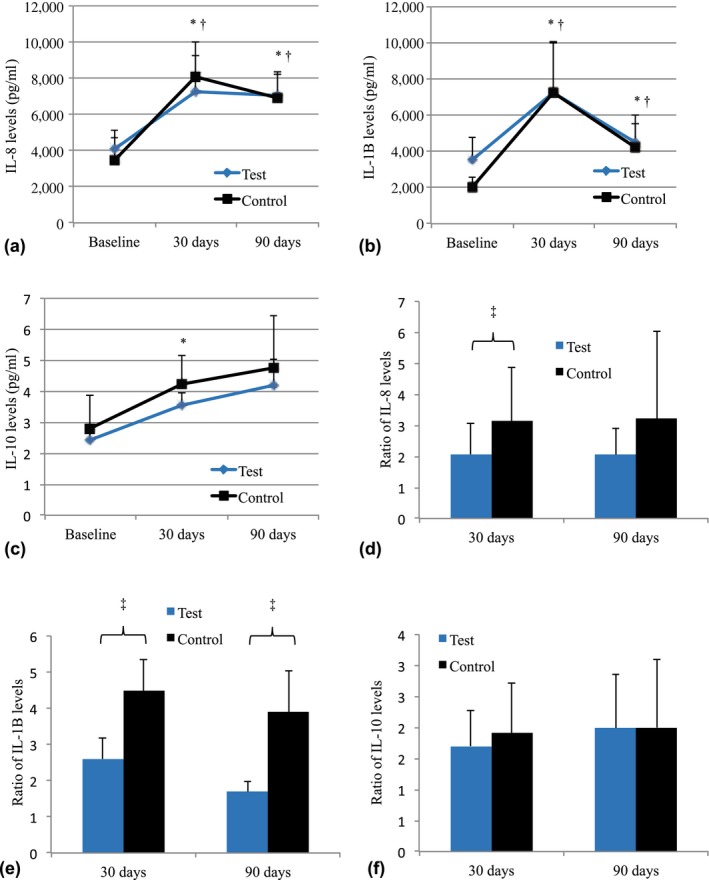
Levels (pg/mL) of IL‐8 (a), IL‐1β (b), and IL‐10 (c) for each subject of groups Test and Control at baseline and at 30 and 90 days post‐treatment. The mean changes in cytokines levels in relation to baseline values for Control and Test groups can be observed in d–f. *Significant difference in Test group when compared with baseline values (Repeated Measures ANOVA, Bonferroni, p < 0.05). †Significant difference in Control group when compared with baseline values (Repeated Measures ANOVA, Bonferroni, p < 0.05). ‡Significant difference among groups in the same time point (Student t test, p < 0.05)
4. DISCUSSION
This double‐blind, randomized controlled trial assessed the effects of B. lactis HN019 on the non‐surgical treatment of individuals with advanced generalized chronic periodontitis by analysing clinical, immunological, and microbiological parameters. The results show that probiotic therapy as an adjunct to SRP promoted additional benefits in assessments at 30 and 90 postoperative days.
The growing prevalence of antimicrobial resistance has prompted the development of new antimicrobial therapeutic approaches for the treatment of biofilm‐related oral diseases. In the present study, the results obtained for PPD and CAL in deep pockets in the Test group at 90 postoperative days were similar to or higher to those obtained in some studies that used antibiotics as adjuvants to SRP for the treatment of chronic periodontitis (Feres et al., 2012; Saleh, Rincon, Tan, & Firth, 2016; Silva et al., 2011).
In the present study, whereas 58% of pockets diagnosed at baseline with PPD ≥ 7 mm decreased to PPD ≤ 3 mm at 90 days in the Test group, only 22% of deep pockets in the Control group were reduced. Teughels et al. (2013) also verified that the use of probiotics as adjuvants to SRP reduced the risk of progression of periodontitis and the number of patients, teeth, and sites with indication of surgical periodontal treatment. These findings demonstrate the clinical significance of periodontal treatment.
It should be emphasized that the outcomes obtained with probiotics cannot be generalized (Teughels, Loozen, & Quirynen, 2011), as they depend on the strain, dosage, frequency, and mode of administration. In the present study, probiotic therapy with bacteria of the genus Bifidobacterium promoted a reduction of PPD (3.5 mm) in deep pockets larger than those obtained by Teughels et al. (2013) and Laleman et al. (2015) (2.88 and 2.37 mm, respectively) at 90 days, with the administration of probiotics of the genera Lactobacillus and Streptococcus, respectively.
Modulation of the host's microbiota is one of the basic mechanisms that may explain the beneficial effects of probiotics on periodontal health. The Test group presented higher rates of commensal bacteria on subgingival biofilm when compared with the Control group at 90 days. These commensal bacteria are associated with periodontal health (Aas, Paster, Stokes, Olsen, & Dewhirst, 2005; Abusleme et al., 2013; Aruni, Dou, Mishra, & Fletcher, 2015; Hajishengallis, Darveau, & Curtis, 2012; Lucas, Beighton, & Roberts, 2000), and they can help control inflammation and infection of periodontal tissues (Devine, Marsh, & Meade, 2015; Kumar & Mason, 2015). Compared with the Control group, the Test group presented lower proportions of some Gram‐negative anaerobic bacteria species involved in the pathogenesis of periodontal diseases. In a preclinical study, Oliveira et al. (2017) demonstrated that B. lactis HN019 reduces the amount of periodontopathogens on biofilm. The possible coaggregation of Bifidobacterium with Fusobacterium nucleatum can reduce the amount of binding sites for periodontopathogens on biofilm (Haukioja et al., 2006). Furthermore, probiotic bacteria can produce several components that act as antimicrobial agents (Gillor, Etzion, & Riley, 2008; Gordon, 2009). Some in vitro studies have shown that Bifidobacterium can inhibit the growth of periodontopathogens such as P. gingivalis (Jasberg et al., 2016; Zhu, Xiao, Shen, & Hao, 2010).
In the present study, whereas no differences were observed between the Control and Test groups at 30 postoperative days regarding the proportions of red complex bacteria, the Control Group had higher percentual of these bacteria at 90 days. It could suggest that probiotic might have acted delaying the recolonization of periodontal pockets by these bacteria in the Test Group. Tekce et al. (2015) observed that patients treated with SRP and probiotics presented improvements in the rates of strict anaerobic microorganisms on subgingival biofilm and that these rates became similar to those of the patients treated exclusively with SRP only at 360 postoperative days. Differently from these results with probiotics, the effects of antibiotics on delayed recolonization of periodontal pockets seem to occur in the short term. Mdala et al. (2013) and Bizzarro et al. (2016) have shown that no differences were seen in the number of red complex pathogens between patients treated with SRP and those treated with SRP combined with antibiotic therapy after 3 months.
The Test group had a greater number of copies/μL of the DNA of B. lactis HN019 in subgingival biofilm samples than the Control group at 30 and 90 days, which suggests exogenous translocation of the probiotic to the subgingival biofilm. B. lactis HN019 was detected in the Test group 60 days after discontinuation of probiotic therapy. This fact suggests that B. lactis HN019 might have temporarily integrated into the subgingival biofilm. Meurman, Antila, and Salminen (1994), Iniesta et al. (2012), and Tekce et al. (2015) also noted the persistence of bacteria of the genus Lactobacillus in the oral cavity at 2, 8, and 9 weeks, respectively, after discontinuation of probiotic therapy.
The Control group presented higher levels of IL‐1β (30 and 90 days) and of IL‐8 (30 days) when compared with the Test group. This finding demonstrates the possible effect of probiotic therapy on modulation of the periodontal inflammatory process. In a recent clinical study, administration of probiotic lactic acid bacteria to patients with chronic periodontitis decreased IL‐1β levels in GCF (Szkaradkiewicz, Stopa, & Karpinski, 2014). Kuru et al. (2017) demonstrated that patients with experimental gingivitis previously treated with B. lactis DN‐173010 presented lower IL‐1β levels in GCF when compared to untreated patients. With respect to IL‐10, only the Test group had higher levels of this cytokine when compared with baseline at 30 days. Ricoldi et al. (2017) also demonstrated that B. lactis HN019 can increase IL‐10 levels at periodontal sites in rats.
The present study is the first one to demonstrate the potential effect of a probiotic bacterium of the genus Bifidobacterium on the non‐surgical treatment of chronic periodontitis. The short period of assessment was a limitation of this study. A long‐term follow‐up of these patients would be important to evaluate whether the additional effects obtained with probiotic therapy as an adjunct to SRP are sustained over time. It is also important that future investigations evaluate the persistence of B. lactis HN019 in the oral cavity after discontinuation of probiotic therapy, new modes of administration, and other therapeutic regimens.
5. CONCLUSION
The use of B. lactis HN019 as an adjunct to SRP offers additional clinical, microbiological, and immunological benefits in the treatment of periodontal pockets in patients with generalized chronic periodontitis.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflict of interests in connection with this article.
6.
Clinical Relevance.
Scientific rationale for the study: Lactobacillus probiotics have been investigated for the treatment of periodontitis. However, the effects of the genus Bifidobacterium on periodontitis are poorly known.
Principal findings: Bifidobacterium animalis subsp. lactis (B. lactis) HN019 probiotic significantly improved clinical periodontal parameters, reduced periodontal pathogens more effectively, and reduced the levels of proinflammatory cytokines in the gingival crevicular fluid.
Practical implications: The use of B. lactis HN019 as an adjunct to scaling and root planing may reduce the need for additional periodontal therapies after non‐surgical treatment of chronic periodontitis.
Invernici MM, Salvador SL, Silva PHF, et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J Clin Periodontol. 2018;45:1198–1210. 10.1111/jcpe.12995
Funding information
The study was supported by the National Council for Scientific and Technological Development (CNPq – process #480982/2013‐9) awarded to Michel R. Messora. DuPont de Nemours & Co. Danisco Sweeteners Oy (Kantvik, Finland) donated the probiotics.
REFERENCES
- Aas, J. A. , Paster, B. J. , Stokes, L. N. , Olsen, I. , & Dewhirst, F. E. (2005). Defining the normal bacterial flora of the oral cavity. Journal of Clinical Microbiology, 43, 5721–5732. 10.1128/JCM.43.11.5721-5732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abusleme, L. , Dupuy, A. K. , Dutzan, N. , Silva, N. , Burleson, J. A. , Strausbaugh, L. D. , … Diaz, P. I. (2013). The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME Journal, 7, 1016–1025. 10.1038/ismej.2012.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage, G. C. (1999). Development of a classification system for periodontal diseases and conditions. Annals of Periodontology, 4, 1–6. 10.1902/annals.1999.4.1.1 [DOI] [PubMed] [Google Scholar]
- Aruni, A. W. , Dou, Y. , Mishra, A. , & Fletcher, H. M. (2015). The biofilm community‐rebels with a cause. Current Oral Health Reports, 2, 48–56. 10.1007/s40496-014-0044-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezow, A. B. , & Darveau, R. P. (2011). Microbial shift and periodontitis. Periodontology 2000, 55, 36–47. 10.1111/j.1600-0757.2010.00350.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzarro, S. , Laine, M. L. , Buijs, M. J. , Brandt, B. W. , Crielaard, W. , Loos, B. G. , & Zaura, E. (2016). Microbial profiles at baseline and not the use of antibiotics determine the clinical outcome of the treatment of chronic periodontitis. Scientific Reports, 6, 20205 10.1038/srep20205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne, C. P. , Ross, R. P. , Saarela, M. , Hansen, K. F. , & Charalampopoulos, D. (2011). Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. International Journal of Food Microbiology, 149, 185–193. 10.1016/j.ijfoodmicro.2011.07.005 [DOI] [PubMed] [Google Scholar]
- Cionca, N. , Giannopoulou, C. , Ugolotti, G. , & Mombelli, A. (2009). Amoxicillin and metronidazole as an adjunct to full‐mouth scaling and root planing of chronic periodontitis. Journal of Periodontology, 80, 364–371. 10.1902/jop.2009.080540 [DOI] [PubMed] [Google Scholar]
- Devine, D. A. , Marsh, P. D. , & Meade, J. (2015). Modulation of host responses by oral commensal bacteria. Journal of Oral Microbiology, 7, 26941 10.3402/jom.v7.26941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feres, M. , Soares, G. M. , Mendes, J. A. , Silva, M. P. , Faveri, M. , Teles, R. , … Figueiredo, L. C. (2012). Metronidazole alone or with amoxicillin as adjuncts to non‐surgical treatment of chronic periodontitis: A 1‐year double‐blinded, placebo‐controlled, randomized clinical trial. Journal of Clinical Periodontology, 39, 1149–1158. 10.1111/jcpe.12004 [DOI] [PubMed] [Google Scholar]
- Gillor, O. , Etzion, A. , & Riley, M. A. (2008). The dual role of bacteriocins as anti‐ and probiotics. Applied Microbiology and Biotechnology, 81, 591–606. 10.1007/s00253-008-1726-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, D. M. (2009). The potential of bacteriocin‐producing probiotics and associated caveats. Future Microbiology, 4, 941–943. 10.2217/fmb.09.78 [DOI] [PubMed] [Google Scholar]
- Hajishengallis, G. , Darveau, R. P. , & Curtis, M. A. (2012). The keystone‐pathogen hypothesis. Nature Reviews Microbiology, 10, 717–725. 10.1038/nrmicro2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukioja, A. , Yli‐Knuuttila, H. , Loimaranta, V. , Kari, K. , Ouwehand, A. C. , Meurman, J. H. , & Tenovuo, J. (2006). Oral adhesion and survival of probiotic and other lactobacilli and bifidobacteria in vitro. Oral Microbiology and Immunology, 21, 326–332. 10.1111/j.1399-302X.2006.00299.x [DOI] [PubMed] [Google Scholar]
- Hojo, K. , Mizoguchi, C. , Taketomo, N. , Ohshima, T. , Gomi, K. , Arai, T. , & Maeda, N. (2007). Distribution of salivary Lactobacillus and Bifidobacterium species in periodontal health and disease. Bioscience, Biotechnology, and Biochemistry, 71, 152–157. 10.1271/bbb.60420 [DOI] [PubMed] [Google Scholar]
- Ikram, S. , Hassan, N. , Raffat, M. A. , Mirza, S. , & Akram, Z. (2018). Systematic review and meta‐analysis of double‐blind, placebo‐controlled, randomized clinical trials using probiotics in chronic periodontitis. Journal of Investigative and Clinical Dentistry, e12338 10.1111/jicd.12338 [DOI] [PubMed] [Google Scholar]
- Ince, G. , Gursoy, H. , Ipci, S. D. , Cakar, G. , Emekli‐Alturfan, E. , & Yilmaz, S. (2015). Clinical and biochemical evaluation of lozenges containing Lactobacillus reuteri as an adjunct to non‐surgical periodontal therapy in chronic periodontitis. Journal of Periodontology, 86, 746–754. 10.1902/jop.2015.140612 [DOI] [PubMed] [Google Scholar]
- Iniesta, M. , Herrera, D. , Montero, E. , Zurbriggen, M. , Matos, A. R. , Marin, M. J. , … Sanz, M. (2012). Probiotic effects of orally administered Lactobacillus reuteri‐containing tablets on the subgingival and salivary microbiota in patients with gingivitis. A randomized clinical trial. Journal of Clinical Periodontology, 39, 736–744. 10.1111/j.1600-051X.2012.01914.x [DOI] [PubMed] [Google Scholar]
- Jasberg, H. , Soderling, E. , Endo, A. , Beighton, D. , & Haukioja, A. (2016). Bifidobacteria inhibit the growth of Porphyromonas gingivalis but not of Streptococcus mutans in an in vitro biofilm model. J Investig Clin Dent. 10.1111/jicd.12338.[Epubaheadofprint] [DOI] [PubMed] [Google Scholar]
- Joint FAO/WHO Working Group (2002) Working group report on drafting guidelines for the evaluation of probiotics in food. London, ON, Canada.
- Junick, J. , & Blaut, M. (2012). Quantification of human fecal bifidobacterium species by use of quantitative real‐time PCR analysis targeting the groEL gene. Applied and Environment Microbiology, 78, 2613–2622. 10.1128/AEM.07749-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, P. S. , & Mason, M. R. (2015). Mouthguards: Does the indigenous microbiome play a role in maintaining oral health? Frontiers in Cellular and Infection Microbiology, 5, 35 10.3389/fcimb.2015.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru, B. E. , Laleman, I. , Yalnizoglu, T. , Kuru, L. , & Teughels, W. (2017). The influence of a bifidobacterium animalis probiotic on gingival health: A randomized controlled clinical trial. Journal of Periodontology, 88, 1115–1123. 10.1902/jop.2017.170213 [DOI] [PubMed] [Google Scholar]
- Laleman, I. , Yilmaz, E. , Ozcelik, O. , Haytac, C. , Pauwels, M. , Herrero, E. R. , … Teughels, W. (2015). The effect of a streptococci containing probiotic in periodontal therapy: A randomized controlled trial. Journal of Clinical Periodontology, 42, 1032–1041. 10.1111/jcpe.12464 [DOI] [PubMed] [Google Scholar]
- Lang, N. P. , & Tonetti, M. S. (2003). Periodontal risk assessment (PRA) for patients in supportive periodontal therapy (SPT). Oral Health & Preventive Dentistry, 1, 7–16. [PubMed] [Google Scholar]
- Lucas, V. S. , Beighton, D. , & Roberts, G. J. (2000). Composition of the oral streptococcal flora in healthy children. Journal of Dentistry, 28, 45–50. 10.1016/S0300-5712(99)00048-2 [DOI] [PubMed] [Google Scholar]
- Luchesi, V. H. , Pimentel, S. P. , Kolbe, M. F. , Ribeiro, F. V. , Casarin, R. C. , Nociti Jr, F. H. , … Casati, M. Z. (2013). Photodynamic therapy in the treatment of class II furcation: A randomized controlled clinical trial. Journal of Clinical Periodontology, 40, 781–788. 10.1111/jcpe.12121 [DOI] [PubMed] [Google Scholar]
- Martin‐Cabezas, R. , Davideau, J. L. , Tenenbaum, H. , & Huck, O. (2016). Clinical efficacy of probiotics as an adjunctive therapy to non‐surgical periodontal treatment of chronic periodontitis: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 43, 520–530. 10.1111/jcpe.12545 [DOI] [PubMed] [Google Scholar]
- Mdala, I. , Olsen, I. , Haffajee, A. D. , Socransky, S. S. , de Blasio, B. F. , & Thoresen, M. (2013). Multilevel analysis of bacterial counts from chronic periodontitis after root planing/scaling, surgery, and systemic and local antibiotics: 2‐year results. Journal of Oral Microbiology, 5, 10.3402/jom.v5i0.20939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurman, J. H. , Antila, H. , & Salminen, S. (1994). Recovery of Lactobacillus strain GG (ATCC 53103) from saliva of healthy volunteers after consumption of yoghurt prepared with the bacterium. Microbial Ecology in Health and Disease, 7, 295–298. 10.3109/08910609409141368 [DOI] [Google Scholar]
- Mombelli, A. (2018). Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000, 76, 85–96. 10.1111/prd.12147 [DOI] [PubMed] [Google Scholar]
- Moreira, A. L. , Novaes Jr, A. B. , Grisi, M. F. , Taba Jr, M. , Souza, S. L. , Palioto, D. B. , … Messora, M. R. (2015). Antimicrobial photodynamic therapy as an adjunct to non‐surgical treatment of aggressive periodontitis: A split‐mouth randomized controlled trial. J Oral Microbiol. 2013 10.3402/jom.v5i0.20939.Print2013 [DOI] [PubMed] [Google Scholar]
- Oliveira, L. F. , Salvador, S. L. , Silva, P. H. , Furlaneto, F. A. , Figueiredo, L. , Casarin, R. , … Messora, M. R. (2017). Benefits of Bifidobacterium animalis subsp. lactis probiotic in experimental periodontitis. Journal of Periodontology, 88, 197–208. 10.1902/jop.2016.160217 [DOI] [PubMed] [Google Scholar]
- Petelin, M. , Perkic, K. , Seme, K. , & Gaspirc, B. (2015). Effect of repeated adjunctive antimicrobial photodynamic therapy on subgingival periodontal pathogens in the treatment of chronic periodontitis. Lasers in Medical Science, 30, 1647–1656. 10.1007/s10103-014-1632-2 [DOI] [PubMed] [Google Scholar]
- Ricoldi, M. S. T. , Furlaneto, F. A. C. , Oliveira, L. F. F. , Teixeira, G. C. , Pischiotini, J. P. , Moreira, A. L. G. , … Messora, M. R. (2017). Effects of the probiotic Bifidobacterium animalis subsp. lactis on the non‐surgical treatment of periodontitis. A histomorphometric, microtomographic and immunohistochemical study in rats. PLoS ONE, 12, e0179946 10.1371/journal.pone.0179946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh, A. , Rincon, J. , Tan, A. , & Firth, M. (2016). Comparison of adjunctive azithromycin and amoxicillin/metronidazole for patients with chronic periodontitis: Preliminary randomized control trial. Australian Dental Journal, 61, 469–481. 10.1111/adj.12415 [DOI] [PubMed] [Google Scholar]
- Shah, M. P. , Gujjari, S. K. , & Chandrasekhar, V. S. (2013). Evaluation of the effect of probiotic (inersan(R)) alone, combination of probiotic with doxycycline and doxycycline alone on aggressive periodontitis – A clinical and microbiological study. Journal of Clinical and Diagnostic Research, 7, 595–600. 10.7860/JCDR/2013/5225.2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, M. P. , Feres, M. , Sirotto, T. A. , Soares, G. M. , Mendes, J. A. , Faveri, M. , & Figueiredo, L. C. (2011). Clinical and microbiological benefits of metronidazole alone or with amoxicillin as adjuncts in the treatment of chronic periodontitis: A randomized placebo‐controlled clinical trial. Journal of Clinical Periodontology, 38, 828–837. 10.1111/j.1600-051X.2011.01763.x [DOI] [PubMed] [Google Scholar]
- Socransky, S. S. , Haffajee, A. D. , Cugini, M. A. , Smith, C. , & Kent Jr, R. L. (1998). Microbial complexes in subgingival plaque. Journal of Clinical Periodontology, 25, 134–144. 10.1111/j.1600-051X.1998.tb02419.x [DOI] [PubMed] [Google Scholar]
- Socransky, S. S. , Haffajee, A. D. , Smith, C. , & Dibart, S. (1991). Relation of counts of microbial species to clinical status at the sampled site. Journal of Clinical Periodontology, 18, 766–775. 10.1111/j.1600-051X.1991.tb00070.x [DOI] [PubMed] [Google Scholar]
- Socransky, S. S. , Haffajee, A. D. , Smith, C. , Martin, L. , Haffajee, J. A. , Uzel, N. G. , & Goodson, J. M. (2004). Use of checkerboard DNA‐DNA hybridization to study complex microbial ecosystems. Oral Microbiology and Immunology, 19, 352–362. 10.1111/j.1399-302x.2004.00168.x [DOI] [PubMed] [Google Scholar]
- Szkaradkiewicz, A. K. , Stopa, J. , & Karpinski, T. M. (2014). Effect of oral administration involving a probiotic strain of Lactobacillus reuteri on pro‐inflammatory cytokine response in patients with chronic periodontitis. Archivum immunolgiae et therapiae experimentalis, 62, 495–500. 10.1007/s00005-014-0277-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekce, M. , Ince, G. , Gursoy, H. , Dirikan Ipci, S. , Cakar, G. , Kadir, T. , & Yilmaz, S. (2015). Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: A 1‐year follow‐up study. Journal of Clinical Periodontology, 42, 363–372. 10.1111/jcpe.12387 [DOI] [PubMed] [Google Scholar]
- Teughels, W. , Durukan, A. , Ozcelik, O. , Pauwels, M. , Quirynen, M. , & Haytac, M. C. (2013). Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo‐controlled study. Journal of Clinical Periodontology, 40, 1025–1035. 10.1111/jcpe.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teughels, W. , Loozen, G. , & Quirynen, M. (2011). Do probiotics offer opportunities to manipulate the periodontal oral microbiota? Journal of Clinical Periodontology, 38(Suppl 11), 159–177. 10.1111/j.1600-051X.2010.01665.x [DOI] [PubMed] [Google Scholar]
- Vivekananda, M. R. , Vandana, K. L. , & Bhat, K. G. (2010). Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: A preliminary randomized clinical trial. Journal of Oral Microbiology, 2, 5344 10.3402/jom.v2i0.5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. , Xiao, L. , Shen, D. , & Hao, Y. (2010). Competition between yogurt probiotics and periodontal pathogens in vitro. Acta Odontologica Scandinavica, 68, 261–268. 10.3109/00016357.2010.492235 [DOI] [PubMed] [Google Scholar]


