Abstract
Purpose
To study newly diagnosed glaucoma patients given mono‐ or multi‐therapy regarding differences in initial intraocular pressure (IOP) reduction, target IOP levels reached and influence of untreated baseline IOP on IOP reduction.
Methods
Patients newly diagnosed with manifest primary open‐angle glaucoma and included in the Glaucoma Intensive Treatment Study (GITS) were randomized to immediate intensive treatment with any of three different IOP‐lowering substances supplied in two bottles plus 360° laser trabeculoplasty or to conventional stepwise treatment starting with a single‐drug. Intraocular pressure reduction was analysed 1 month after initiation of treatment.
Results
One hundred eighteen patients (143 eyes) received mono‐therapy and 122 patients (152 eyes) multi‐therapy. Median baseline IOP was 24.0 (min: 9.7, max: 56.0) mmHg in mono‐therapy eyes and 24.0 (min: 12.3, max: 48.5) mmHg in multi‐therapy eyes (p = 0.56). After 1 month in the two groups, respectively, values for median IOP reduction were 6.3 (range: −5.3–31.0) and 11.0 (range: 0.7–34.5) mmHg, and for mean relative decline 26.8 (range: −32.0–55.4) and 46.0 (range: 4.6–81.6) % (p = 0.000). A larger proportion of the multi‐therapy patients reached each target IOP level (p = 0.000). The higher the baseline IOP, the larger the observed pressure reduction, considering both absolute and relative figures. The effect was more pronounced in eyes with multi‐therapy than in those with mono‐therapy (p = 0.000). For every mmHg higher IOP at baseline, the IOP was reduced by an additional 0.56 (mono‐therapy) or 0.84 (multi‐therapy) mmHg.
Conclusion
Intensive treatment led to considerably greater IOP reduction than mono‐therapy. Among patients with IOP ≥30 mmHg at diagnosis an IOP of <16 was reached in 2/3 of those with multi‐therapy but in none with mono‐therapy. The IOP reduction was highly dependent on the untreated IOP level.
Keywords: baseline pressure, intraocular pressure, multi‐therapy, open‐angle glaucoma, target pressure, therapy
Introduction
The Glaucoma Intensive Treatment Study (GITS) is an ongoing project focused on treatment of open‐angle glaucoma (OAG). The primary aim of the GITS is to investigate whether visual function and vision‐related quality of life (QoL) can be better preserved by intensive initial treatment started at the time of diagnosis than by the more conventional stepwise approaches that are commonly recommended in the glaucoma guidelines, such as those established by the European and the Swedish Glaucoma Societies (Heijl et al. 2012; European Glaucoma Society 2014).
The most common strategy in modern glaucoma management is to set a target intraocular pressure (IOP) for each eye at the time of diagnosis. The individual target IOP depends on several factors, including life expectancy, stage of the glaucoma (damage), untreated IOP levels and overall risk profile (European Glaucoma Society 2014). To reach the target IOP, current glaucoma management generally recommends to start with medical mono‐therapy. Initially, an IOP of <21 mmHg and a reduction of 20% may be sufficient for patients with early stages of glaucoma, whereas patients with moderate damage may require an IOP of <18 mmHg and a 30% decrease in IOP (European Glaucoma Society 2014). Even lower levels may be necessary in advanced stage glaucoma (European Glaucoma Society 2014), for which a reduction of 40–50% is suggested (Konstas & Hollo 2016). More than one drug is often needed to reach even a fairly modest reduction (Kass et al. 2002). If mono‐therapy fails, treatment is changed until the target IOP is met, and this often entails switching drug class, combining drugs, or adding laser trabeculoplasty (LTP). When the goal is achieved, the patient follow‐up includes relatively frequent visits for 2–3 years, and the amount of data collected during that period should be sufficient to assess the rate of progression (Chauhan et al. 2008a). If the rate of progression is unacceptable, the target IOP is adjusted downwards and the treatment is increased. Thereafter, the patient is again followed to determine whether the rate of progression has been reduced to an acceptable level.
We hypothesize that this conventional approach might result in unnecessary loss of visual function, simply because it takes several years to determine the rate of progression. If modern recommendations of frequent follow‐up during the first few years after diagnosis are not complied with (Friedman et al. 2005; Quigley et al. 2007; Linden et al. 2013), the time interval needed to determine progression status might easily exceed 2–3 years. Accordingly, it might be wiser to treat all glaucoma patients more intensively as the first step. This issue is being investigated in the GITS by randomizing patients either to the conventional stepwise therapeutic approach or to more intensive treatment from the time of diagnosis. All eyes in the GITS intensive treatment arm are immediately prescribed three IOP‐reducing agents from three different drug classes (provided in two bottles) and also receive LTP 1 week later (B. Bengtsson, A. Heijl, G. Jóhanesson, S. Andersson‐Geimer, J. Aspberg, C. Lindén, in prep).
A prospective follow‐up must be conducted to determine whether a more intensive approach such as the method described here is preferable. Therefore, the GITS protocol specifies that each patient should be followed up for 5 years. In this early analysis, it is possible to establish whether there are any clear differences in IOP reduction between the two treatment approaches, and it also provides the opportunity to study the influence of pretreatment IOP on the absolute and relative IOP reduction for each treatment strategy.
Several large prospective randomized studies have found that the risk of progression from ocular hypertension to manifest glaucoma, or of damage in eyes with manifest glaucoma, is markedly decreased by as much as 0–19% for each mmHg reduction in IOP (Gordon et al. 2002; Heijl et al. 2002; Leske et al. 2003, 2007; Miglior et al. 2007; Chauhan et al. 2008a). A lower IOP is likely to reduce the rate of progression and therefore also decrease the risk of lifetime blindness or loss of QoL.
Numerous comparisons of different types and different combinations or modalities of medical treatments for OAG have been reported in the literature (Rolim de Moura et al. 2007; Vass et al. 2007; Burr et al. 2012; Li et al. 2015, 2016). There is some evidence that surgical intervention lowers IOP more effectively, but surgery is also associated with more complications (Burr et al. 2012), and thus it is relevant to search for more efficient strategies for reducing IOP that can help avoid having to resort to surgery. To our knowledge, no studies focused on IOP reduction have compared the conventional approach of stepwise treatment escalation with a more radical non‐surgical approach such as that used in the GITS. It is of interest to get an idea of the IOP reduction achieved with the conventional approach and the more intensive approach. This early report provides data on the initial IOP response, target IOP levels reached, and the influence of the untreated pressure levels in eyes included in the two treatment arms of the GITS.
Materials and Methods
Detailed information about the study design and procedures applied in the GITS is provided in an accompanying paper (Bengtsson et al. in prep.), which also describes the baseline characteristics of the patients. The present investigation is registered in the European Clinical Trials Database (EudraCT Ref. no. 2013‐002895‐42). The GITS protocol follows the tenets of the Declaration of Helsinki and has been reviewed and approved by the Regional Ethical Review Board at Lund University and by the Swedish Medical Product Agency (Ref no. 5.1‐2013‐64667). All patients signed written informed consent after receiving oral and written information about the study.
Patients
Patients with newly diagnosed, untreated glaucoma were recruited from the primary care area of the hospitals in the cities of Umeå and Malmö, including the surrounding towns. Glaucoma was defined as the presence of repeated glaucomatous visual field defects (Humphrey Visual Field Analyzer 30‐2 SITA standard programme) with corresponding disc and/or nerve fibre layer defects. Having undergone any previous eye surgery, with the exception of uncomplicated cataract surgery, was an exclusion criterion. Patients with advanced field loss were not eligible; the visual field index had to be ≥65% in both eyes. All levels of untreated IOPs were allowed for inclusion.
Between March 2013 and March 2017, 242 patients aged 40–78 years and with newly detected manifest primary OAG (n = 173, 72%) or pseudoexfoliation glaucoma (n = 69, 28%) in one or both eyes were enroled in the GITS. Fifty‐six patients (23%) contributed with both eyes that is a total of 298 eyes were included.
Treatment
Patients were randomized to either immediate intensive treatment (multi‐therapy, 124 patients) or conventional stepwise treatment starting with a single‐drug (mono‐therapy, 118 patients). In the mono‐therapy group, any IOP‐lowering single‐drug eye drop registered in Sweden could be used. Multi‐therapy patients were given three different IOP‐lowering substances as two medications at the time of inclusion and underwent 360° selective or argon LTP 1 week later; any combination of three IOP‐lowering agents from three different drug classes was allowed. Fixed dual combination was counted as two different drugs. Treatments could be adjusted at any time if deemed necessary by the ophthalmologist in charge.
Tonometry and visits
Intraocular pressure was measured once in each eye at every visit by certified technicians. Tonometry was performed in the sitting position using a calibrated Goldmann applanation tonometer. Patients made three visits before randomization; two prestudy visits and one baseline visit. The mean of these three untreated readings was used as the baseline IOP. Multi‐therapy patients were scheduled for LTP 1 week after randomization. In both treatment groups, the first follow‐up visit was scheduled 1 month after the last visit that is 1 month after baseline for mono‐therapy patients and 1 month after the LTP visit for multi‐therapy patients.
Statistical analyses
Analyses were based on eligible study eyes. Descriptive data are presented as mean and standard deviation (SD), or median and range, as appropriate. In statistical comparisons of the difference between the two treatment groups, mixed model analysis was used with subjects as a random factor to adjust for possible dependence between the two eyes in the same subject. A generalized linear model was applied to test the significance of the effect of untreated initial IOP on the IOP reduction, adjusting for possible dependence between the two eyes in the same patient. A p value of <0.05 was considered statistically significant. IBM spss version 24.0 IBM (New York, NY, USA) was used for data analysis.
Results
Patients
Two patients in the multi‐therapy group withdrew from the study immediately after randomization. Thus the analyses in this report are based on 240 individuals: 118 patients (143 eyes) in the mono‐therapy group and 122 patients (152 eyes) in the multi‐therapy group, with median ages of 68 (range: 46–78) and 69 (range: 52–78) years, respectively. Forty‐five per cent in each group were women, representing 53 and 55 individuals in the mono‐therapy and the multi‐therapy group, respectively.
Treatment
As reported in the accompanying paper, the following treatments were applied in the mono‐therapy group: most (81%) of patients received a prostaglandin analogue, 19% received timolol, and only one patient was given dorzolamide. In the multi‐therapy group, the majority of the patients were prescribed drug combinations comprising timolol + bimatoprost and brinzolamide, or timolol + dorzolamide and latanoprost; all other possible drug combinations were used by fewer than five patients each.
IOP
Median baseline IOP was 24.0 (min: 9.7, max: 56.0) mmHg in the eyes with mono‐therapy, and the mean for the same group was 26.2 mmHg. Corresponding baseline values for the eyes with multi‐therapy were 24.0 (min: 12.3, max: 48.5) mmHg and 25.3 mmHg. There was no significant difference between the two groups (p = 0.56). In nine patients randomized to mono‐therapy, the treatment was intensified at an extra visit attended before the 1‐month visit; for these nine subjects, the IOP value from the extra visit (i.e. when the patient was still on mono‐therapy) was used in the response assessment.
At the first follow‐up, the median IOP was 18.0 (min: 8.0, max: 34.0) mmHg in the eyes with mono‐therapy (mean 18.2 mmHg) and 12.0 (min: 6.0, max: 28.0) mmHg (mean 12.8 mmHg) in those with multi‐therapy, and the difference between the two groups was highly significant (p = 0.000).
The IOP reduction from baseline to first follow‐up ranged from −5.3 to 31.0 mmHg in the eyes with mono‐therapy, with a median of 6.3 mmHg and a mean of 7.7 (SD: 5.9) mmHg (Fig. 1A). Corresponding changes in the multi‐therapy eyes ranged between 0.7 and 34.5 mmHg, with a median of 11.0 mmHg and a mean of 12.5 (SD: 7.4) mmHg (Fig. 1B). In the mono‐therapy group, the mean relative IOP reduction from baseline to first follow‐up visit was 26.8% (range: −32.0–55.4%; median 27.6%), and the corresponding value for the multi‐therapy patients was 46.0% (range 4.6–81.6%; median 45.8%); the difference between the two groups was highly significant (p = 0.000).
Figure 1.
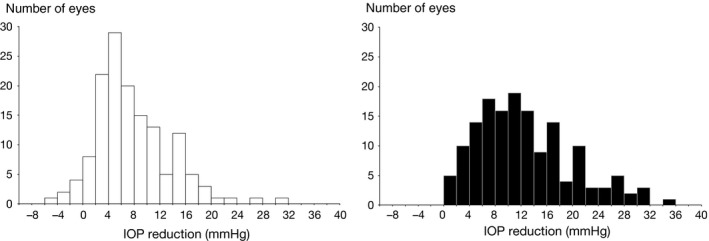
Change in intraocular pressure between baseline and 1‐month visit in eyes randomized to mono‐therapy (n = 143, A) or multi‐therapy (n = 152, B).
The absolute number and the proportion of patients reaching different target IOPs in the two treatment arms are presented in Table 1. A significantly larger proportion of the multi‐therapy patients reached each target level (p = 0.000). Results were similar when the patients were divided into three groups with respect to their untreated IOP level: low, intermediate or high baseline IOP. In addition, even when untreated baseline IOP was taken into account, the proportion of patients reaching each target level was always significantly greater for the multi‐therapy subjects.
Table 1.
Absolute numbers and proportions of eyes reaching different target IOPs in the two treatment arms
| Target IOP (mmHg) | Mono‐therapy n (%) | Multi‐therapy n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| All mono | Untreated IOP level | All multi | Untreated IOP level | |||||
| <21 | 21 ≤ IOP ≤ 30 | ≥30 | <21 | 21 ≤ IOP < 30 | ≥30 | |||
| n = 143 | n = 44 | n = 64 | n = 35 | n = 152 | n = 51 | n = 64 | n = 37 | |
| <21 | 104 (73) | 40 (91) | 53 (83) | 11 (31) | 147 (97) | 51 (100) | 61 (95) | 35 (95) |
| <18 | 70 (49) | 36 (82) | 29 (45) | 5 (14) | 140 (92) | 51 (100) | 58 (91) | 31 (84) |
| <16 | 37 (26) | 24 (55) | 13 (20) | 0 | 120 (79) | 49 (96) | 47 (73) | 24 (65) |
| <14 | 18 (13) | 15 (34) | 3 (5) | 0 | 102 (67) | 47 (92) | 36 (56) | 19 (51) |
| <12 | 5 (3) | 5 (11) | 0 | 0 | 58 (38) | 26 (51) | 22 (34) | 10 (27) |
The eyes in both treatment arms were divided into three groups with respect to untreated IOP level.
IOP = intraocular pressure.
As illustrated in Fig. 2 the IOP reduction was strongly dependent on the baseline IOP. Pressure reduction was larger in eyes with a higher baseline IOP than in those with a lower baseline IOP. This was noted in both treatment arms, although the effect was more pronounced in eyes on multi‐therapy (p = 0.000). For example, it is expected that a pretreatment IOP of 15 mmHg will be lowered by 1.4 mmHg (9%) by mono‐therapy and by 3.7 mmHg (25%) by multi‐therapy. The corresponding figures for a pretreatment IOP of 30 mmHg are 9.8 mmHg (33%) and 16.3 mmHg (54%), and for a high pretreatment IOP (50 mmHg) the reduction is estimated to 21 mmHg (42%) and 33.1 mmHg (66%). The slope of pressure‐lowering versus baseline IOP was 0.56 (R 2 = 0.66; p = 0.000) in the mono‐therapy group and 0.84 (R 2 = 0.80; p = 0.000) in the multi‐therapy group, which means that for every mmHg higher IOP at baseline, the IOP was reduced by an additional 0.56 or 0.84 mmHg, respectively.
Figure 2.
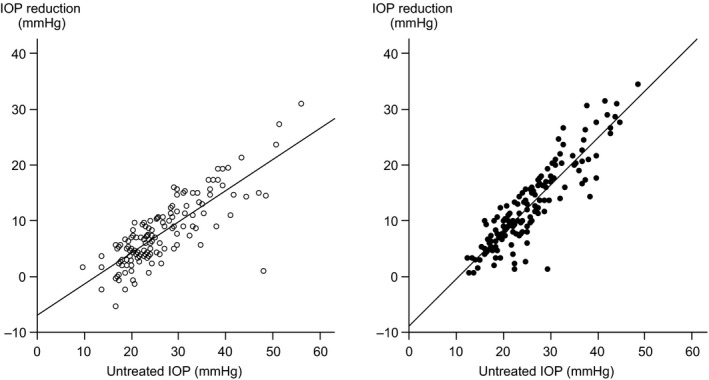
Relationship between untreated intraocular pressure (IOP) and the reduction in IOP after 1 month of treatment in eyes receiving mono‐therapy (open circles) or multi‐therapy (filled circles).
Discussion
Our results demonstrate that, compared to the mono‐therapy, the initial intensive treatment was considerably and significantly more effective in achieving overall IOP reduction in OAG patients. Short‐term mean IOP reduction among multi‐therapy patients was about 5 mmHg greater than in patients who received conventional treatment. The mean relative reduction in IOP was 46% in the experimental arm versus nearly 27% in the conventional arm. In other words, in the present study, the initial target IOP level recommended for early to moderate glaucoma was already reached by mono‐therapy, whereas the advanced stage recommendation was only fulfilled in the multi‐therapy arm (Konstas & Hollo 2016).
Prostaglandin analogues are the most efficacious topical glaucoma drugs (van der Valk et al. 2009; Li et al. 2016), with an estimated effect of approximately 30% when used as mono‐therapy (van der Valk et al. 2005). These agents are closely followed by beta‐blockers, which result in a predicted decline in IOP of about 27% (van der Valk et al. 2005). Considering that four‐fifths of the patients in the mono‐therapy arm in our study received a prostaglandin, and the remaining one‐fifth was given timolol, the mean IOP reduction of 27% was not unexpected.
The nearly 50% reduction in IOP in the intensively treated patient group was impressive and perhaps also surprising. No previous studies have investigated the combination of multiple drugs and LTP. The Early Manifest Glaucoma Trial applied a fixed treatment protocol comprising only one drug (betaxolol) and 360° LTP, which led to an IOP reduction of 25% after 3 months (Heijl et al. 2002). Many studies have assessed IOP reduction after increasing drug treatment, especially regarding unfixed or fixed combinations of two drugs. A review of additivity to the IOP‐reducing effect of prostaglandin analogues showed additional IOP reduction of less than 15%, regardless of the type of agent (beta‐blocker, topical carbonic anhydrase inhibitor or alpha‐agonist) that was added (Tabet et al. 2008). Substantial data on fixed combination drug therapy indicate responses in the order of 28–35% (Cheng et al. 2012). Few randomized investigations have evaluated the effect of more than two substances. In a 3‐month study of fixed combinations in a population with untreated IOP slightly above 22 mmHg, the combination timolol 0.5%/brimonidine 0.2% lowered the IOP 18%, whereas the triple therapy timolol 0.5%/brimonidine 0.2%/dorzolamid 2% reached a reduction of 27% from baseline (Baiza‐Duran et al. 2012). In another assessment in which the fixed combination brinzolamide 1%/brimonidine 0.2% was given together with a prostaglandin analogue, the additional IOP reduction was 25% after 6 weeks (Fechtner et al. 2016). A similar study evaluated tafluprost plus the fixed combination dorzolamid/timolol versus tafluprost alone in patients insufficiently controlled with latanoprost, and the results showed that the triple treatment provided a 22% greater reduction in IOP compared to latanoprost‐treated baseline values (Konstas et al. 2017). Thus it seems that the IOP reduction in our multi‐therapy patients was at least as efficient as in previously published reports.
We also found that the absolute decrease in IOP was highly dependent on the baseline IOP, and this was more pronounced in the intensive treatment arm, where every mmHg of higher untreated IOP was associated with 0.84 mmHg greater IOP reduction. The corresponding estimate for the mono‐therapy patients was 0.56 mmHg per mmHg higher baseline IOP. A strong relationship between decreased IOP and baseline IOP has been reported for both LTP (Mao et al. 2008; Pillunat et al. 2016) and medical treatment (Heijl et al. 2011; Konstas et al. 2017). In our study, a similar pattern was detected, and the slope was considerably steeper in the intensive treatment arm, indicating a larger IOP‐reducing effect per mmHg untreated IOP than in the conventional arm. Notably, both the absolute IOP reduction and the relative (%) reduction increased with higher baseline IOP. One reason why this strong dependence was so obvious is probably that we used wide inclusion criteria that allowed patients with a very wide range of baseline IOPs to be enroled in the trial.
Table 1 shows the number and proportion of eyes reaching different target IOPs. We assessed five arbitrarily chosen target IOP levels that could satisfactorily represent realistic, individually determined target IOPs. In eyes with an untreated IOP of ≥30 mmHg in our study, 95% of those assigned to multi‐therapy reached a level of <21 mmHg, whereas only 31% of those randomized to mono‐therapy attained that level. Again considering eyes with an untreated IOP of ≥30 mmHg but at a target IOP of <16 mmHg, that level was reached in 65% of the multi‐therapy eyes but in none of the eyes in the mono‐therapy group. Hence even very ambitious goals for IOP were met by a large proportion of eyes given the intensive treatment. Furthermore, only a small proportion of patients with high IOPs will reach a reasonable target IOP with mono‐therapy.
One strength of the current study was that it included only previously untreated patients with manifest OAG. All the patients were naive to IOP‐lowering agents, and therefore no biases in terms of responder or non‐responder were introduced. Another advantage was the inclusion of the whole spectrum of untreated IOP levels, with no upper or lower limit.
This report confirms that, compared to mono‐therapy, the described intensive multi‐therapy leads to a considerably larger IOP reduction in treatment‐naïve patients with OAG. It seems apparent that a larger proportion of patients will reach their target IOP more rapidly with initial intensive treatment than with conventional treatment. This observation is a prerequisite for the GITS to be able to corroborate or disprove the primary hypothesis of the present investigation.
This study was presented in part at the 2nd Nordic Glaucoma Congress in Roskilde, Denmark, 28–29 September 2017. Financial support was provided through regional agreements between Umeå University and Västerbotten County Council and between Lund University and Skåne Regional Council (ALF), and also by grants from Crown Princess Margareta's Foundation, Ögonfonden, Insamlingsstiftelserna vid Umeå universitet, the Swedish Medical Society Foundation, the Cronqvist Foundation, the Herman Järnhardt Foundation, the Foundation for Visually Impaired in Former Malmöhus County, Ingrid Nordmark's Foundation, the Margit and Kjell Stolz Foundation, foundations and donations administered by Skåne University Hospital, and King Gustav V and Queen Victoria's Freemason Foundation and Swedish Society for Medical Research. The funding organizations had no role in the design or conduct of this research.
Dr Lindén has received consultant fee from Allergan Nordic AB and speaking honoraria from Santen Pharma. Dr Heijl is a consultant to Carl Zeiss Meditec and Allergan and has received speaking honoraria from Allergan, Zeiss and Santen. Dr Johannesson has received speaking honoraria and/or consulting fees from Topcon, Thea, Santen, Allergan, Alcon/Novartis. Dr Aspberg has received speaking honararia from Allergan. Dr Andersson‐Geimer has nothing to disclose. Dr Bengtsson is a consultant to Carl Zeiss Meditec.
References
- Baiza‐Duran M, Llamas‐Moreno JF & Ayala‐Barajas C (2012): Comparison of timolol 0.5% + brimonidine 0.2% + dorzolamide 2% versus timolol 0.5% + brimonidine 0.2% in a Mexican population with primary open‐angle glaucoma or ocular hypertension. Clin Ophthalmol 6: 1051–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr J, Azuara‐Blanco A, Avenell A & Tuulonen A (2012): Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev: CD004399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BC, Garway‐Heath DF, Goni FJ, Rossetti L, Bengtsson B, Viswanathan AC & Heijl A (2008a): Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol 92: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BC, Mikelberg FS, Balaszi AG, LeBlanc RP, Lesk MR, Trope GE; Canadian Glaucoma Study Group (2008b): Canadian Glaucoma Study: 2. Risk factors for the progression of open‐angle glaucoma. Arch Ophthalmol 126: 1030–1036. [DOI] [PubMed] [Google Scholar]
- Cheng JW, Cheng SW, Gao LD, Lu GC & Wei RL (2012): Intraocular pressure‐lowering effects of commonly used fixed‐combination drugs with timolol: a systematic review and meta‐analysis. PLoS ONE 7: e45079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Glaucoma Society (2014): Terminology and guidelines for glaucoma. Savona, Italy: PubliComm. [Google Scholar]
- Fechtner RD, Myers JS, Hubatsch DA, Budenz DL & DuBiner HB (2016): Ocular hypotensive effect of fixed‐combination brinzolamide/brimonidine adjunctive to a prostaglandin analog: a randomized clinical trial. Eye (Lond) 30: 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DS, Nordstrom B, Mozaffari E & Quigley HA (2005): Glaucoma management among individuals enrolled in a single comprehensive insurance plan. Ophthalmology 112: 1500–1504. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD et al. (2002): The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open‐angle glaucoma. Arch Ophthalmol 120: 714–720; discussion 829‐730. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M; Early Manifest Glaucoma Trial Group (2002): Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 120: 1268–1279. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Hyman L, Yang Z, Bengtsson B; EMGT Group (2011): Intraocular pressure reduction with a fixed treatment protocol in the Early Manifest Glaucoma Trial. Acta Ophthalmol 89: 749–754. [DOI] [PubMed] [Google Scholar]
- Heijl A, Alm A, Bengtsson B, Bergstrom A, Calissendorff B, Lindblom B, Linden C; Swedish Ophthalmological Society (2012): The glaucoma guidelines of the Swedish ophthalmological society. Acta Ophthalmol Suppl (Oxf) 251: 1–40. [DOI] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ et al. (2002): The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open‐angle glaucoma. Arch Ophthalmol 120: 701–713; discussion 829‐730. [DOI] [PubMed] [Google Scholar]
- Konstas AG & Hollo G (2016): Preservative‐free tafluprost/timolol fixed combination: a new opportunity in the treatment of glaucoma. Expert Opin Pharmacother 17: 1271–1283. [DOI] [PubMed] [Google Scholar]
- Konstas AG, Boboridis KG, Kapis P et al. (2017): 24‐Hour efficacy and ocular surface health with preservative‐free tafluprost alone and in conjunction with preservative‐free dorzolamide/timolol fixed combination in open‐angle glaucoma patients insufficiently controlled with preserved latanoprost monotherapy. Adv Ther 34: 221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E; Early Manifest Glaucoma Trial Group (2003): Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 121: 48–56. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z; EMGT Group (2007): Predictors of long‐term progression in the early manifest glaucoma trial. Ophthalmology 114: 1965–1972. [DOI] [PubMed] [Google Scholar]
- Li X, Wang W & Zhang X (2015): Meta‐analysis of selective laser trabeculoplasty versus topical medication in the treatment of open‐angle glaucoma. BMC Ophthalmol 15: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lindsley K, Rouse B, Hong H, Shi Q, Friedman DS, Wormald R & Dickersin K (2016): Comparative effectiveness of first‐line medications for primary open‐angle glaucoma: a systematic review and network meta‐analysis. Ophthalmology 123: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden C, Bengtsson B, Alm A, Calissendorff B, Eckerlund I & Heijl A (2013): Glaucoma management in Sweden – results from a nationwide survey. Acta Ophthalmol 91: 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao AJ, Pan XJ, McIlraith I, Strasfeld M, Colev G & Hutnik C (2008): Development of a prediction rule to estimate the probability of acceptable intraocular pressure reduction after selective laser trabeculoplasty in open‐angle glaucoma and ocular hypertension. J Glaucoma 17: 449–454. [DOI] [PubMed] [Google Scholar]
- Miglior S, Torri V, Zeyen T, Pfeiffer N, Vaz JC, Adamsons I; EGPS Group (2007): Intercurrent factors associated with the development of open‐angle glaucoma in the European glaucoma prevention study. Am J Ophthalmol 144: 266–275. [DOI] [PubMed] [Google Scholar]
- Pillunat KR, Spoerl E, Elfes G & Pillunat LE (2016): Preoperative intraocular pressure as a predictor of selective laser trabeculoplasty efficacy. Acta Ophthalmol 94: 692–696. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Friedman DS & Hahn SR (2007): Evaluation of practice patterns for the care of open‐angle glaucoma compared with claims data: the Glaucoma Adherence and Persistency Study. Ophthalmology 114: 1599–1606. [DOI] [PubMed] [Google Scholar]
- Rolim de Moura C, Paranhos A Jr& Wormald R (2007): Laser trabeculoplasty for open angle glaucoma. Cochrane Database Syst Rev 4: CD003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabet R, Stewart WC, Feldman R & Konstas AG (2008): A review of additivity to prostaglandin analogs: fixed and unfixed combinations. Surv Ophthalmol 53(Suppl1): S85–S92. [DOI] [PubMed] [Google Scholar]
- van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F & Prins MH (2005): Intraocular pressure‐lowering effects of all commonly used glaucoma drugs: a meta‐analysis of randomized clinical trials. Ophthalmology 112: 1177–1185. [DOI] [PubMed] [Google Scholar]
- van der Valk R, Webers CA, Lumley T, Hendrikse F, Prins MH & Schouten JS (2009): A network meta‐analysis combined direct and indirect comparisons between glaucoma drugs to rank effectiveness in lowering intraocular pressure. J Clin Epidemiol 62: 1279–1283. [DOI] [PubMed] [Google Scholar]
- Vass C, Hirn C, Sycha T, Findl O, Bauer P & Schmetterer L (2007): Medical interventions for primary open angle glaucoma and ocular hypertension. Cochrane Database Syst Rev 4: CD003167. [DOI] [PMC free article] [PubMed] [Google Scholar]


