Abstract
Background and Objectives
To evaluate feasibility of intraoperative visualization of embryologically defined organ compartments and their drainage by ICG in uterine cancer.
Methods
Total of 2.5 mg of ICG have been injected into cervix or corpus in uterine cancer patients immediately prior to surgery. Green fluorescence was intermittently detected during robotically assisted laparoscopic surgery (Firefly System®, Intuitve Surgical Inc.). Total of 36 patients with uterine cancer without macroscopically suspicious nodes were evaluated with respect to their compartmental lymphatic network, collecting lymphatic vessels, and the connection to the postponed lymph basins.
Results
Müllerian (sub) compartment and transport of lymph fluid along the lymphatic collectors and connecting vessels to the postponed lymph basins could be visualized invariably in all patients. Cervix drained along the ligamentous and caudal part of vascular mesometria, whereas midcorporal and fundal drainage occurred along the upper part of vascular mesometria and along the mesonephric pathway along the ovarian vessels.
Conclusions
Visualization of lymphatic network and downstream flow of lymphatic fluid to the postponed lymph basins by ICG is feasible; it can be used to navigate along compartment boarders for education, intraoperative orientation, and quality control. It seems to confirm the compartmental order of pelvic organ systems and postponed lymph basins. J. Surg. Oncol. 2016;113:554–559. © 2016 Wiley Periodicals, Inc.
Keywords: endometrial cancer, cervical cancer, robotic surgery, radical hysterectomy, intraoperative procedures
INTRODUCTION
Embryologically organ compartments derive from their developmental precursors and are arranged topologically in defined tissue domains—that is, morphogenetic fields 1. In addition, there is determined and well controlled boundary formation between the organ compartments 2. The ontogenetic theory suggests clinical cancer as the result of pathological reactivation of normally blocked developmental programs during embryogenesis in retrograde order 3; tumor progression is thus confined for a very long time to the mature tissue embryologically derived from the same organ compartment as could be demonstrated convincingly for cervical cancer 4. The embryonic origin of lymphatic vessels has recently been summarized 5; in mammals, embryologic development is suggested as a stepwise process starting from the embryonic veins, where lymphatic endothelial cells (LEC) are initially specified 6. The lymphatic system develops by budding from “cardinal veins” and thus, parallels the venous system, although no open connection remains between the two systems except for the jugular lymph sacs to the subclavian veins which enable lymph drainage to the blood circulation. The origin of the very first draining lymphatic capillaries may also arise not only by sprouting but also by scattered local mesenchymal cells expressing lympho‐endothelial markers 5. Collecting as well as transporting lymph vessels develop lymphatic valves which guarantee a directed “downstream” lymph flow in one direction 6. Thus, visualization of the lymphatic vessel network should be able to mark the respective organ compartment and the compartments of its specific lymphatic drainage “downstream”. This should have dramatic clinical impact on surgical oncology with respect on individual radical compartmental surgery in cancer due to the compartmental order of tumor progression 7 and the impact of the lymphatic system on local, regional, and distant tumor progression. ICG may safely indicate “sentinel nodes” in various organ cancers 8, 9 and in cervical and endometrial cancer in particular 10, 11, 12. However, there may be much more potential with respect to optical guidance of the surgeon by ICG all the way along the “tissue at risk” for seeding of tumor cells in organ and lymph compartments. Thus, lymphatic network of the Müllerian compartment and its connections to the draining lymphatic compartments has been studied in detail in patients with cervical and endometrial cancer.
MATERIALS AND METHODS
Patients (n = 36) with either endometrial or cervical cancer were treated by radical hysterectomy and therapeutic +/− pelvic/paraaortic lymphadenectomy either as total mesometrial or peritoneal mesometrial resection (TMMR or PMMR 13, 14, 15). The procedures were performed by robotically assisted laparoscopy using the “da Vinci” SI or Xi System of Intuitive Surgical Inc® (Sunnyvale, CA) and using infrared laser excitation and fluorescence detection with the Firefly‐System®. The procedures were video documented to facilitate thorough analysis.
Cervical cancer patients (n = 20, mean age 44 years, FIGO stages Ib to IIb) received TMMR and therapeutic pelvic lymphadenectomy 13, 14. All resections were confirmed R0 (mean lymph node count, n = 57).
Endometrial cancer patients (n = 16, mean age 61 years, FIGO stage I–III) received PMMR with or without pelvic/para‐aortic lymphadenectomy depending on tumor stage 13, 15, 16. All patients gave their informed consent. All resections were histologically confirmed R0. Mean lymph node count following systematic lymphadenectomy was n = 56.
In all patients immediately prior to the surgery 1.2–2 ml of ICG solution at concentration of 125 mg/mL (ICG‐Pulsion®, Pulsion Medical Systems SE, Feldkirchen, Germany) was injected divided in four portions of 0.3–0.5 mL each. The injections were given either directly into the cervix at the four quadrants (cervical cancer, 5–10 mm depth) or into the uterine corpus bilaterally mid‐corporal and at the fundus at 3 and 9 o'clock (endometrial cancer, 5–10 mm depth) using a pudendal anaesthesia needle guided by a so called “Iowa trumpet”. All patients were informed about the off label use of ICG and gave their informed consent. Principles of ICG administration for sentinel node detection are excellently video‐reported by Crane et al. 17, although not in the robotic setting.
Following application of ICG the Cup of the uterine manipulator (Hohl‐manipulator Storz®) was fixed by suture which had been used to close and cover the cervix with an alcohol sponge to prevent tumor exposure and cell spillage into the surgical field. Green fluorescence was intermittently detected during the whole procedure. Fluorescence distribution and transport along the lymphatic system was studied with respect to application site and none of them had macroscopic involvement of lymph nodes suggesting potentially altered lymphatic drainage. ICG fluorescence was determined intraoperatively and on the basis of the recorded video documentation.
RESULTS
In all 36 patients ICG‐fluorescence was already detected as soon as laparoscopy was started. In some patients ICG dye was found in the recto‐uterine pouch (patent fallopian tubes) and was removed by suction/irrigation to avoid further contamination. The fluorescent fluid was found in the (sub‐) compartment of injection and followed the lymphatic network of the Müllerian compartment downstream from medial to lateral. Dependent of the sub‐compartment the drainage occurred along the ligamentous mesometria (sacrouterine and rectovaginal part) and/or along the lymphatic channel network of the vascular mesometrium caudally of the uterine artery in cervical application (cervical cancer). In endometrial cancer with corporal application of ICG no drainage along the ligamentous mesometria was observed. In all cases, however, there was predominant flow along the lymphatic network in the upper part of the vascular mesometrium directed to the interiliac region. In addition, lymphatic drainage occurred following fundal application along the ovarian vessels (“mesonephric pathway”) to the paraaortic region. Thus, the lymphatic network of the different organ sub‐compartments could be easily followed to the first draining lymph node region: in cervical cancer these corresponded to the nodes along the external and internal iliac vessels via the inferior part of the vascular mesometrium, and the deep prespinal and preischiadic nodes via the ligamentous mesometrium. In endometrial cancer the pathway along the superior part of the vascular mesometrium ended up in the same region of internal and external iliac lymph nodes, but did not drain into the prespinal and preischiadic nodes. Following fundal application, the mesonephric lymphatic drainage along the ovarian vessels drained into the infra‐ and supramesenteric, infrarenal paraaortic nodes.
Application to different sites lead to reproducible, distinct visualization of the draining lymphatic network in the Müllerian organ compartment (Table I).
Table I.
Lymphatic ICG Fluorescence of the Müllerian Compartment Depending on the Application Site
| Tumor entity | Visualization of the Müllerian (sub‐) compartment | Visualization of the pelvic vascular +/− ligamentous lymphatic drainage | Visualization of the pelvic vascular and mesonephric paraaortic lymphatic drainage | ICG drainage visible crossing compartment borders |
|---|---|---|---|---|
| Cervical cancer | 20/20 | 20/20 | 0/20 | 0/20 |
| Endometrial cancer | 16/16 | Ligamentous drainage 0/16 | 16/16 | 0/16 |
Summary of the results of 36 patients with respect to the visualization of ICG fluorescence during robotic surgery dependent on application site (corpus or cervix) visualization was categorized in local, Müller compartment, connecting vessels to the pelvic and paraaortic nodes, and connecting vessels to other compartments crossing compartment borders.
The findings of this study are exemplified in Figures 1, 2, 3, 4, 5, 6, 7; lymphatic drainage in fact differs from cervical, mid‐corporal, and fundal application. In fundal application the fluorescence could be found in the lymphatic network around the application site draining into the ipsilateral collecting vessels of the meso‐tube (Fig. 1).
Figure 1.

High fundal application of ICG (isocyanine green) at each side: endo/myometrial collecting lymph vessels are visualized; they show a directed lymph flow from medially to laterally on each side, draining predominantly into the connecting vessels to the “mesonephric” salpingo‐ovarian network.
Figure 2.

Right‐sided mid‐corporal and fundal application of ICG in endometrial cancer: the lymphatic vessels drain again via the “mesonephric” ovarian and additionally mainly along the supraureteral “Müllerian” uterine network (upper part of vascular mesometrium); the cervix and the ligamentous mesometrium (sacrouterine ligament) is not colored at all.
Figure 3.

Bilateral cervical application in cervical cancer: the lymphatic vessels drain again along the “Müllerian” uterine network, but now following the lower, infraureteral part of the vascular mesometrium; in addition drainage along the ligamentous mesometrium (sacrouterine ligament) can be demonstrated, but none at all via the mesonephric ovarian pathway.
Figure 6.
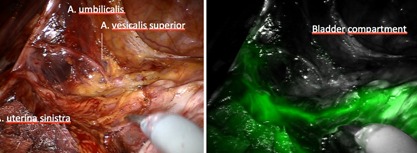
Ventral aspect of left connecting lymphatic vessels along the Müllerian uterine pathway: no crossing of vessels can be identified, neither to the bladder organ compartment, nor to the lymphatics of the superior vesical vessels.
Figure 7.
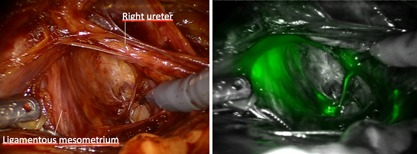
Posterior aspect of left vascular and ligamentous mesometrium: uterine (Müllerian) and rectal compartment are strictly separated—with no crossing or connecting of fluorescent lymphatic vessels.
Additional mid‐corporal injection lead to fluorescence of collecting vessels towards the uterine vessel system in all patients, but mainly cranially to the uterine vessels and the ureter along the vascular mesometrium (Fig. 2). In none of the 16 cases ligamentous mesometrium has been labelled by ICG .
Injection into the uterine cervix resulted in fluorescence of the cervical sub‐compartment and of collecting lymph vessels directed to the vascular in all patients and the ligamentous mesometria dependent on site of injection. In contrast to mid‐corporal application; however, the vessels were visualized predominantly caudally of the level of the uterine artery and the ureter as shown in Figure 3.
Following the collecting vessels of the organ compartment to the connecting channels to the draining lymph basins three different distinct pathways were observed:
-
First, the ICG of the fundal injection was predominantly transported via the ipsilateral tubo‐ovarian lymphatic system into the two main lymph channels along the ovarian artery and vein (Fig. 4). Although not object of this investigation the nodes draining this pathway were the ventrally located mesenteric and infrarenal paraaortic nodes.
Figure 4.
 Collecting lymphatic vessels from upper uterine compartment. They drain via the mesovarian network (A,B) to the infundibulopelvic vessels (C) dividing in a medial (along ovarian artery) and a lateral channel (along ovarian vein).
Collecting lymphatic vessels from upper uterine compartment. They drain via the mesovarian network (A,B) to the infundibulopelvic vessels (C) dividing in a medial (along ovarian artery) and a lateral channel (along ovarian vein). Second, from the mid‐corporal Müllerian sub‐compartment lymphatics were collected to the connecting lymph vessels running along the uterine artery and crossing the anterior branch of the internal iliac artery predominantly ventrally. These vessels drained preferentially into the proximal internal and external iliac nodes around superior to the branching of the uterine artery and to the common iliac bifurcation.
-
Finally, the lymphatic sub‐compartment of the cervix drained exclusively via the Müllerian uterine system. The connecting vessels were mainly found along the mid and lower vascular mesometrium below the uterine artery and the ureter and along the uterine veins. Additionally, lymphatic drainage of the posterior cervix could be observed also along the ligamentous mesometrium as shown in Figure 5. Thus, the lymph node basins appeared to be entered mainly at the level of the deep internal iliac branches in the obturatoric, prespinal, and preischiadic region, and the posterior region of the external iliac vessels. Secondarily, these nodes were connected to the posterior chains of the common iliac and subaortic region.
Figure 5.
 Ligamentous mesometrium and caudal vascular mesometrium on the right: following cervical application of ICG drains into the deep paravisceral, prespinal, and preischiadic nodes.
Ligamentous mesometrium and caudal vascular mesometrium on the right: following cervical application of ICG drains into the deep paravisceral, prespinal, and preischiadic nodes.
However, apart from these three connecting pathways on each side, no other connections have been observed in any of the 36 cases. As shown in Figures 6 and 7 there appeared to be a sharp border between the organ compartments never crossed by fluorescent lymphatic vessels. This is particularly important to mention: not a single fluorescent connecting vessel could be demonstrated along the vesico‐uterine or the recto‐uterine vessel anastomoses in any of these cases. However, this may be rather due to an intact valve system of the lymphatic vessels directing the lymph fluid “downstream” than due to lack of lymphatic connections.
None of the cases investigated showed lymphatic drainage to neighboring organ compartments neither via vesico‐uterine nor recto‐uterine junctions. Autonomous nerve plexus and ureter did neither show any fluorescence as long as the lymph vessel system was kept intact. Furthermore, the fluorescence remained stable during up to at least 5–6 hr.
DISCUSSION
Increasing knowledge of embryological development of organ compartments 1, tissue boundary control 2, and interaction with tumor progression and tumor spread lead to a new concept of compartmental surgery in cancer and in cervical cancer in particular 4. The adoption of this concept for endometrial cancer resulted in the definition of the “Peritoneal MesoMetrial Resection (PMMR)” combined with the pelvic and periaortic “therapeutic LymphadeNEctomy (tLNE)” according to M. Höckel 13, 15.
The lymphatic system plays an important role in cancer progression and dissemination.
Lymph capillaries may transport malignant cells via collecting vessels to intercalated lymph nodes and to their related lymphatic compartment, where they may be destroyed, persist or grow; Moreover, they could also reach jugular veins via lymphatic drainage entering circulatory system and causing blood‐borne metastases. In addition, the density of peritumoral lymph vessels and tumor neoangiogenesis of lymph vessels is not only a negative predictor of clinical course but may also contribute itself to tumor progression and dissemination 18. Since, the predominant route of dissemination of cancer cells in uterine cancer is lymphatic spread, these mechanisms may be of major importance in cervical and endometrial cancer. The complete removal of the tissue at risk might minimize the risk for loco‐regional recurrence and—considering the involved tissue an origin for further tumor spread—it may also reduce the tumor burden possibly reaching blood circulation via the lymphatic system. There is evidence for different solid tumors that compartment resection including the regional lymph compartment might dramatically reduce loco‐regional recurrence even sparing adjuvant radiotherapy 19, 20, 21, 22. At present a multicenter study is performed in Germany and Switzerland to confirm the convincing monocentric data of M. Höckel for cervical cancer (study number Germany: DRKS00004793). In addition, there is first evidence, that the compartmental concept may work equally well in endometrial cancer treated by PMMR and therapeutic lymphadenectomy 16.
As a consequence of these findings, it should be possible to reduce risk of recurrence markedly by removing compartments of a malignant tumors origin and its “loco‐regional continuous and lymphatic spread” entirely. These may include the sub‐compartment of tumor origin, the Müllerian compartment, late and early meta‐compartments together with the draining lymph compartments as outlined for uterine cancer in 3. We could show by intraoperative dynamic lymphography that uterine sub‐compartments are drained via different pathways being controlled by different lymph node compartments: deep cervix is drained along inferior vascular mesometrium and ligamentous mesometrium to the deep para‐visceral nodes, including pre‐spinal and pre‐ischiadic nodes; uterine isthmus and mid‐corporal region mainly along the upper part of vascular mesometrium, to the anterior para‐visceral, but not along the ligamentous mesometrium to the preischiadic region. The fundal region drains in addition predominantly along the “mesonephric pathway” along the infundibulopelvic ligament to the mesenteric and anterior infrarenal node compartments.
Due to the partially identical lymphatic drainage of cervical and corporal uterine subcompartments to the paravisceral nodes, use of cervical application in endometrial cancer may also work ‐in part‐ for diagnostic purposes in sentinel node detection 23, although being incorrect. In therapeutic intention, however, the “mesonephric” ovarian system has to be removed additionally—consisting of the adnexa, the infundibulopelvic ligaments and draining para‐aortic lymph node compartments—if the complete lymphatic system of risk should be removed, whereas the preischiadic and prespinal nodes may be preserved. Also, if the cancer field is the Müllerian compartment, the intercalated lymph nodes of the mesorectum, mesobladder, and mesureter are not involved in the lymphatic drainage and therefore are not at risk to be colonized by metastases, which seems to be confirmed by our findings of functional lymphatic drainage of the Müllerian compartment. Our data appear to confirm the functional order of the Müllerian organ compartment with strictly downstream dependent regional lymph node compartments and no drainage to neighboring organ systems with different embryological origin as proposed by findings of basic and clinical research 1, 3.
However, although there is evidence that resection of embryologically determined organ compartments may control for loco‐regional disease in different tumors 16, 19, 20, 21, 22, this has to be confirmed in adequate prospective surgical studies. Visualization of the compartment and its lymphatic drainage system, however, may support surgeons markedly to understand compartments structure and their order. The ICG fluorescence labels the lymphatic network 6 of a distinct compartment as a function of directed lymph flow in vivo. This may help also to individually adapt surgery to an objective anatomical basis, rather than to a subjective decision on radicalness individually required. Since compartment borders are clearly delineated, morbidity to the neighboring compartments of bladder, ureter, bowel, and nerves may be dramatically reduced. However, in addition to local resection of the organ compartment and the connecting structures the draining lymph basins have to be removed also entirely if locoregional control should be achieved. The visualization of connecting lymph vessels to the regional lymph basins supports the surgeon in removing also these compartments of risk depending on the subcompartment of primary tumor growth.
It is important to understand that ICG lymphography in compartmental surgery is conceptually different from its use in sentinel node detection. The sentinel node concept focusses exclusively on diagnostic purposes aiming on the reduction of the surgical morbidity in low risk situations 24. ICG support in compartmental surgery, however, is aimed on optimizing complete and radical resection of compartments in a therapeutic intention. Ideally, the adoption of ontogenetically based compartment resection renders additional adjuvant loco‐regional treatment (e.g., radio[chemo]therapy) unnecessary, whereas adjuvant therapy for distant tumor control may still remain important.
CONCLUSION
In summary, our data show that fluorescence guidance with ICG is feasible in ontogenetically based compartmental surgery of uterine cancer. It “highlights” the embryologically derived lymphatic network of the individual uterine sub‐compartment. Thus, it helps to understand the compartmental order of the Müllerian system and its borders to the neighboring organ systems, which never were crossed by ICG containing lymph vessels. It facilitates the surgical approach by intraoperative navigation. Furthermore, our findings add further evidence to the concept of embryologically based compartmental surgery as they demonstrate the presence of the functional and anatomical units of organ compartments and their borders visualized by their lymphatic networks in vivo.
Conflicts of interest: Rainer Kimmig received honoraria/travel reimbursement from Intuitive Surgical Inc. for presentations, proctoring with respect to robotic surgery in Europe. BA, PB, PR, MH no disclosures.
REFERENCES
- 1. Wolpert L: Positional information and spatial pattern of cellular differentiation. J Theoret Biol 1969; 25:1–47. [DOI] [PubMed] [Google Scholar]
- 2. Dahmann C, Oates AC, Brand M: Boundary formation and maintenance in tissue development. Nat Rev Genet 2011; 12:43–55. [DOI] [PubMed] [Google Scholar]
- 3. Höckel M: Morphogenetic fields of embryonic development in locoregional cancer spread. Lancet Oncol 2015; 16:e148–e151. [DOI] [PubMed] [Google Scholar]
- 4. Höckel M, Hentschel B, Horn LC: Association between developmental steps in the organogenesis of the uterine cervix and locoregional progression of cervical cancer: A prospective clinicopathological analysis. Lancet Oncol 2014; 15:445–456. [DOI] [PubMed] [Google Scholar]
- 5. Ribatti D, Crivellato E: The embryonic origins of lymphatic vessels: An historical review. Br J Haematol 2010; 149:669–674. [DOI] [PubMed] [Google Scholar]
- 6. Yang Y, Oliver G: Development of the mammalian lymphatic vasculature. J Clin Invest 2015; 124:888–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Höckel M: Cancer permeates locally within ontogenetic compartments: Clinical evidence and implications for cancer surgery. Future Oncol 2012; 8:29–36. [DOI] [PubMed] [Google Scholar]
- 8. Xiong L, Gazyakan E, Yang W, et al.: Indocyanine green fluorescence‐guided sentinel node biopsy: A meta‐analysis on detection rate and diagnostic performance. Eur J Surg Oncol 2014; 40:843–849. [DOI] [PubMed] [Google Scholar]
- 9. Verbeek FP, Troyan SL, Mieog JS, et al.: Near‐infrared fluorescence sentinel lymph node mapping in breast cancer: A multicentre experience. Breast Cancer Res Treat 2014; 143:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rossi EC, Ivanova A, Boggess JF: Robotically assisted fluorescence‐guided lymph node mapping with ICG for gynecologic malignancies: A feasibility study. Gynecol Oncol 2012; 124:78–82. [DOI] [PubMed] [Google Scholar]
- 11. Jewell EL, Huang JJ, Abu‐Rustum NR, et al.: Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near‐infrared fluorescence imaging for uterine and cervical malignancies. Gynecol Oncol 2014; 133:274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plante M, Touhami O, Trinh XB, et al. Sentinel node mapping with indocyanine green and endoscopic near‐infrared fluorescence imaging in endometrial cancer. A pilot study and review of the literature. Gynecol Oncol 2015;pii: S0090–8258;00679–4; DOI: 10.1016/j.ygyno.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 13. Kimmig R, Iannaccone A, Buderath P, et al.: Definition of compartment based radical surgery in uterine cancer‐part I: Therapeutic pelvic and periaortic lymphadenectomy by michael höckel translated to robotic surgery. ISRN Obstet Gynecol 2013; 297921; DOI: 10.1155/2013/297921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kimmig R, Wimberger P, Buderath P, et al.: Definition of compartment‐based radical surgery in uterine cancer: Radical hysterectomy in cervical cancer as ‘total mesometrial resection (TMMR)’ by Michael Höckel translated to robotic surgery (rTMMR). World J Surg Oncol 2013; 11:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kimmig R, Aktas B, Buderath P, et al.: Definition of compartment‐based radical surgery in uterine cancer: Modified radical hysterectomy in intermediate/high‐risk endometrial cancer using peritoneal mesometrial resection (PMMR) by M Höckel translated to robotic surgery. World J Surg Oncol 2013; 11:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kimmig R, Iannaccone A, Aktas B, et al.: Embryologically based radical hysterectomy as peritoneal mesometrial resection (PMMR) with pelvic/paraaortic lymphadenectomy for loco‐regional tumour control in endometrial cancer—First evidence for efficacy. Arch Gynecol Obstet 2015; DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crane LM, Themelis G, Buddingh KT, et al.: Multispectral real‐time fluorescence imaging for intraoperative detection of the sentinel lymph node in gynecologic oncology. J Vis Exp 2010; pii 2225; DOI: 10.3791/2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alitalo A, Detmar M: Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene 2012; 31:4499–4508. [DOI] [PubMed] [Google Scholar]
- 19. Quirke P, Dixon MF, Durdey P, et al.: Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Lancet 1986; 2:996–998. [DOI] [PubMed] [Google Scholar]
- 20. Höckel M, Horn L‐C, Manthey N, et al.: Resection of the embryologically defined uterovaginal (Möllerian) compartment and pelvic control in patients with cervical cancer: A prospective analysis. Lancet Oncol 2009; 10:683–692. [DOI] [PubMed] [Google Scholar]
- 21. Höckel M, Schmidt K, Bornmann, et al.: Vulvar field resection: Novel approach to the surgical treatment of vulvar cancer based on ontogenetic anatomy. Gynecol Oncol 2010; 119:106–113. [DOI] [PubMed] [Google Scholar]
- 22. Höckel M, Horn LC, Illig R, et al.: Ontogenetic anatomy of the distal vagina: Relevance for local tumor spread and implications for cancer surgery. Gynecol Oncol 2011; 122:313–318. [DOI] [PubMed] [Google Scholar]
- 23. Rossi EC, Jackson A, Ivanova A, et al.: Detection of sentinel nodes for endometrial cancer with robotic assisted fluorescence imaging: Cervical versus hysteroscopic injection. Int J Gynecol Cancer 2013; 23:1704–1711. [DOI] [PubMed] [Google Scholar]
- 24. Cibula D, Oonk MH, Abu‐Rustum NR: Sentinel lymph node biopsy in the management of gynecologic cancer. Curr Opin Obstet Gynecol 2015; 27:66–72. [DOI] [PubMed] [Google Scholar]


