Summary
Background
Misbalances in extracellular matrix turnover are key factors in the development of stricturing (Montreal B2) and penetrating (Montreal B3) Crohn's disease.
Aim
To determine whether serological markers for collagen formation and degradation could serve as biomarkers for complications of Crohn's disease.
Methods
Serum biomarkers for type I, III, V and VI collagen formation (P1NP, Pro‐C3, Pro‐C5, Pro‐C6) and matrix metalloproteinase mediated degradation (C1M, C3M, C5M and C6M) were measured in a retrospective, single centre cohort of 112 patients with Crohn's disease in the terminal ileum (nonstricturing/nonpenetrating: n=40, stricturing: n=55, penetrating: n=17) and 24 healthy controls. Active inflammation was defined as CRP >5 mg/L.
Results
C3M and Pro‐C5 levels were higher in penetrating vs nonpenetrating/nonstricturing and stricturing disease (33.6±5 vs 25.8±2.2 [P=.004] and 27.2±2.3 [P=.018] nmol/L C3M, 1262.7±259.4 vs 902.9±109.9 [P=.005] and 953.0±106.4 [P=.015] nmol/L Pro‐C5). C1M (71.2±26.1 vs 46.2±6.2 nmol/L [P<.001]), C3M (31.6±3.9 vs 26.1±1.6 nmol/L [P=.002] and Pro‐C5 levels (1171.7±171.5 vs 909.6±80.4 nmol/L [P=.002]) were higher in patients with active inflammation vs without active inflammation. Pro‐C3/C3M‐ratios were best to differentiate between penetrating vs nonstricturing/nonpenetrating and stricturing disease with area under the curves of 0.815±0.109 (P<.001) and 0.746±0.114 (P=.002) respectively.
Conclusions
Serological biomarkers show that penetrating Crohn's disease is characterised by increased matrix metalloproteinase‐9 degraded type III collagen and formation of type V collagen. Active inflammation in Crohn's disease is characterised by increased formation of type V collagen and increased matrix metalloproteinase mediated breakdown of type I, III collagen. Pro‐C3/C3M ratios are superior in differentiating between penetrating Crohn's disease vs inflammatory and stricturing Crohn's disease.
1. Introduction
Crohn's Disease is an inflammatory bowel disease showing a heterogeneous phenotype. The disease behavior can be classified according to the Montreal classification in nonstricturing/nonpenetrating (B1), stricturing (B2), and penetrating disease (B3).1, 2 This phenotype can change over time.3, 4, 5 It is thought that chronic inflammation and damage drives fibrosis and that a disturbed balance between extracellular matrix formation and degradation is responsible for the different phenotypes. Up to one‐third of patients with Crohn's disease show signs of stricturing (ie, thickening of the wall of the intestinal lumen that causes stenosis) or penetrating (ie, formation of intra‐abdominal fistulae, perforations or abscesses) disease at diagnosis.6, 7 In up to 70% of the patients with Crohn's disease, chronic and long‐standing disease results in complications when the disease shifts towards a stricturing or penetrating phenotype.6, 8, 9 Penetrating vs stricturing phenotypes are proposed to reflect two opposing directions.10 In the stricturing phenotype, excessive deposition of extracellular matrix by increased maturation of (myo)fibroblasts contributes to intestinal fibrogenesis and stenosis.11 Penetrating disease on the other hand, results among others from excessive matrix metalloproteinase mediated destruction of extracellular matrix. Fistulae might occur due to re‐epithelialisation of penetrating ulcers that healed insufficiently because of increased matrix metalloproteinase activity.12, 13 Extracellular matrix formation and degradation products can be measured in blood and could serve as biomarkers for stricturing or penetrating Crohn's disease.2
The healthy intestine mainly consists of type I (68%), III (20%) and V (12%) collagen.14 In the strictured intestine, both collagen content and the relative amount of type V collagen increases compared to control intestine.14 During the early onset of fibrosis in the intestine, the ratio of type III collagen to type I collagen is increased. In contrast, during the late stage of fibrosis, when active collagen deposition diminishes, the ratio of type III collagen to type I collagen decreases.15 Type VI collagen is known to be both a component of the interstitial membrane, the basal lamina and of the basement membrane of the intestine. Furthermore, it is proposed to be a regulator of epithelial cell‐fibronectin interactions.16 Remodelling of extracellular matrix occurs mainly via matrix metalloproteinases.17 Several subtypes of matrix metalloproteinases showed to be significantly upregulated in Crohn's disease and especially in penetrating disease.17, 18 Among others matrix metalloproteinase‐9, produced by neutrophils and macrophages, is upregulated in intestinal fistulae of patients with and without Crohn's disease.13 Matrix metalloproteinase‐9 inhibits wound healing and correlates with disease activity in Crohn's disease.10, 19 Furthermore, matrix metaloproteinase‐3 is markedly upregulated in fistulae of patients with Crohn's disease, independent of the inflammatory activity.13 Biomarker assays measuring specific, neo‐epitopes of degradation products of type I (matrix metalloproteinase‐2, 9 and 13 degraded [C1M]),20 type III (matrix metalloproteinase‐9 degraded [C3M]),21 type V (matrix metalloproteinase‐2 and ‐9 degraded [C5M])22 and type VI (matrix metalloproteinase‐2 and 9 degraded [C6M])23 collagen, and formation products of type I (P1NP),24 III (Pro‐C3),25 V (Pro‐C5)26 and VI (Pro‐C6)27 collagen can be used as diagnostic tools for auto immune diseases (eg, ankylosing spondylitis) and in liver fibrosis (Table 1).22, 28 Neo‐epitopes of degradation of extracellular matrix were able to differentiate between Crohn's disease, ulcerative colitis and non‐Inflammatory Bowel Disease (IBD).29
Table 1.
Biomarkers to assess extracellular matrix turnover in serum
| Biomarker | Target | Detection range (nmol/L) | Intra‐assay and inter‐assay variability in human serum (%) | Corresponding pathology | Reference |
|---|---|---|---|---|---|
| P1NP | Internal epitope in the N‐terminal pro‐peptide of type I collagen | 12‐516 | 4.4 and 10.3 | Wound healing/fibrogenesis | 24 |
| C1M | Matrix metalloproteinase‐2/9/13 degraded type I collagen | 15‐460 | 10.1 and 6.7 | Inflammation/collagen degradation | 20 |
| Pro‐C3 | Released N‐terminal pro‐peptide of type III collagen | 2.6‐116 | 4.11 and 11.03 | Wound healing/fibrogenesis | 25 |
| C3M | Matrix metalloproteinase‐9 degraded type III collagen | 4‐88 | 2.2 and 2.15 | Inflammation/collagen degradation | 21 |
| Pro‐C5 | Released C‐terminal pro‐peptide of type V collagen | 130‐3120 | 3.46 and 5.09 | Wound healing/fibrogenesis | 26 |
| C5M | Matrix metalloproteinase‐2/9 degraded type V collagen | 1‐28 | 4.3 and 9.13 | Inflammation/collagen degradation | 22 |
| Pro‐C6 | C‐terminal of type VI collagen | 0.15‐58.39 | 4.8 and 15.2 | Wound healing/fibrogenesis | 27 |
| C6M | Matrix metalloproteinase‐2/9 degraded type VI collagen | 3‐210 | 4.1 and 10.11 | Inflammation/collagen degradation | 23 |
The aim of this study was to establish whether formation and degradation products of collagens type I, III, V and VI can serve as biomarkers to distinguish between nonstricturing/nonpenetrating, stricturing and penetrating Crohn's disease. Furthermore, we aimed to provide insight in collagen turnover in Crohn's disease with (CRP >5 mg/L) and without active inflammation.
2. Materials and Methods
2.1. Study design and population
Serum from 112 patients with Crohn's disease was retrospectively collected from the database of the IBD centre of the University Medical Center Groningen (single centre) (Tables 2 and 4). The database contains serum samples at time of admission to the database, independent of the state of the disease at that moment. Inclusion criteria for this study were: Biopsy confirmed Crohn's disease in the terminal ileum (Montreal classification L1) when the serum sample was drawn. Exclusion criteria were: Any kind of surgery within 6 months before the sample serum sample was taken, a change in disease behavior to a more severe phenotype (ie, towards B2 or B3) during follow‐up, other fibrotic disease (eg, liver fibrosis/cirrhosis), autoimmune diseases not associated with Crohn's disease, and malignancy (except for all types of skin cancer and haematologic disease).
Table 2.
Cohort demographics separated by Montreal Behavior classification
| Crohn's disease N=112 | Nonstricturing/nonpenetrating (B1) N=40 | Stricturing (B2) N=55 | Penetrating (B3) N=17 | P value | Healthy controls N=24 | P value | |
|---|---|---|---|---|---|---|---|
| General | |||||||
| Gender n (%) female | 42 (37.5%) | 12 (30.0%) | 31 (56.4%) | 6 (35.3%) | .391 | 3 (12.5%) | .056 |
| Age at sample years, mean (minimum‐maximum) | 41.9 (18‐74) | 38.4 (18‐67) | 43.8 (19‐73) | 43.8 (23‐74) | .162 | 41.3 (19‐60) | .271 |
| Disease duration at sample years, mean (minimum‐maximum) | 11.6 (0‐35) | 8.1 (1‐34) | 14.1 (1‐35) | 11.7 (0‐20) | .002 | NA | NA |
| Age at diagnosis n (%) | |||||||
| A1, <16 years | 12 (10.7%) | 4 (10.0%) | 7 (12.7%) | 1 (5.9%) | .772 | NA | NA |
| A2, 17‐40 years | 78 (69.6%) | 28 (70.0%) | 39 (70.9%) | 11 (64.7%) | |||
| A3, >40 years | 22 (19.6%) | 8 (20.0%) | 9 (16.4%) | 5 (29.4%) | |||
| Disease activity | |||||||
| Clinical disease activity (HBI, % active disease [HBI ≥5]) | 45 (40.2%) | 15 (37.5%) | 26 (47.3%) | 4 (23.5%) | .199 | NA | NA |
| C‐reactive protein (n (%) CRP >5 mg/L) | 31 (27.7%) | 14 (35.0%) | 13 (23.6%) | 4 (23.5%) | .435 | NA | NA |
| Perianal disease n (%) | 28 (25%) | 5 (12.5%) | 17 (30.9%) | 6 (35.3%) | .070 | NA | NA |
| Smoking history n (%) | |||||||
| Current | 38 (33.9%) | 12 (30.0%) | 21 (38.2%) | 5 (29.4%) | .521 | NA | NA |
| Used to smoke | 31 (27.7%) | 12 (30.0%) | 12 (21.8%) | 7 (41.2%) | |||
| Never | 39 (34.8%) | 15 (37.5%) | 20 (36.4) | 4 (23.5%) | |||
| Missing | 2 (1.8%) | 1 (2.5%) | 1 (1.8%) | 1 (5.9%) | |||
| Medication use n (%) | |||||||
| 5‐aminosalycilates | 8 (7.1%) | 4 (10.0%) | 3 (5.5%) | 1 (5.9%) | .681 | NA | NA |
| Corticosteroids | 21 (18.8%) | 10 (25.0%) | 9 (16.4%) | 2 (11.8%) | .412 | NA | NA |
| Immunosuppressants | 65 (58.0%) | 20 (50.0%) | 35 (63.6%) | 10 (58.8%) | .412 | NA | NA |
| Anti‐Tumour Necrosis Factor alpha | 36 (32.1%) | 14 (35.0%) | 14 (25.5%) | 8 (47.1%) | .222 | NA | NA |
| Surgery n (%) | |||||||
| Resection | 73 (65.2%) | 9 (22.5%) | 47 (85.5%) | 17 (100%) | <.001 | NA | NA |
| Cause of resection | |||||||
| Therapeutic resistance | 7 (6.3%) | 7 (17.5%) | 0 (0%) | 0 (0%) | <.001 | NA | NA |
| Stenosis | 45 (40.2%) | 0 (0%) | 46 (83.6%) | 0 (0%) | |||
| Intra‐abdominal fistula/abscess | 12 (10.7%) | 0 (0%) | 0 (0%) | 11 (64.7%) | |||
| Stenosis and abscess/fistula | 5 (4.5%) | 0 (0%) | 0 (0%) | 5 (29.4%) | |||
| Other | 2 (1.8%) | 2 (5.0%) | 0 (0%) | 0 (0%) | |||
| Missing | 2 (1.8%) | 0 (0%) | 1 (1.8%) | 1 (5.9%) | |||
| Moment of resection | |||||||
| Resection before sample | 59 (52.7%) | 8 (20.0%) | 35 (63.6%) | 16 (94.1%) | <.001 | NA | NA |
| Resection after sample | 7 (6.3%) | 1 (2.5%) | 5 (9.1%) | 1 (5.9%) | |||
| Resection before and after sample | 7 (6.3%) | 0 (0%) | 7 (12.7%) | 0 (0%) | |||
| Re‐resection because of stenosis/intra‐abdominal fistula/abscess n (%) | 19 (17.0%) | 0 (0%) | 16 (29.1%) | 3 (17.6%) | .001 | NA | NA |
HBI, Harvey‐Bradshaw Index; NA, not applicable.
Nominal and continuous were data compared using ANOVA. P values depict statistical differences between patients with Crohn's disease and comparing patients with Crohn's disease to controls.
Table 4.
Cohort demographics separated by CD with active inflammation and CD without active inflammation
| Crohn's disease with CRP <5 mg/L N=81 | Crohn's disease with CRP >5 mg/L N=31 | P value | Healthy controls N=24 | P value | |
|---|---|---|---|---|---|
| General | |||||
| Gender n (%) female | 32 (39.5%) | 10 (32.3%) | .478 | 3 (12.5%) | .047 |
| Age at sample years, mean (minimum‐maximum) | 41.9 (18‐74) | 41.7 (19‐73) | .881 | 41.3 (19‐60) | .982 |
| Disease duration at sample years, mean (minimum‐ maximum) | 11.9 (0‐35) | 10.8 (1‐32) | .553 | NA | NA |
| Age at diagnosis n (%) | |||||
| A1, <16 years | 9 (11.1%) | 3 (9.7%) | .881 | NA | NA |
| A2, 17‐40 years | 57 (70.4%) | 21 (67.7%) | |||
| A3, >40 years | 15 (18.5% | 7 (22.6%) | |||
| Disease behavior n (%) | |||||
| Nonstricturing/nonpenetrating | 26 (32.1%) | 14 (45.2%) | .435 | NA | NA |
| Stricturing | 42 (51.9%) | 12 (41.9%) | |||
| Penetrating | 13 (16.0%) | 4 (12.9%) | |||
| Disease activity | |||||
| Clinical disease activity (HBI, % active disease (HBI≥5)) | 30 (37.0%) | 15 (48.4%) | .273 | NA | NA |
| Perianal disease n (%) | 19 (23.5%) | 9 (29.0%) | .542 | NA | NA |
| Smoking history n (%) | NA | NA | |||
| Current | 30 (37.0%) | 8 (25.8%) | .563 | NA | NA |
| Used to smoke | 21 (25.9%) | 10 (32.3%) | |||
| Never | 28 (34.6%) | 11 (35.5%) | |||
| Missing | 1 (1.2%) | 2 (6.4%) | |||
| Medication use n (%) | |||||
| 5‐aminosalycilates | 6 (7.4%) | 2 (6.5%) | .861 | NA | NA |
| Corticosteroids | 14 (17.3%) | 7 (22.6%) | .521 | NA | NA |
| Immunosuppressants | 51 (63.0%) | 14 (45.2%) | .088 | NA | NA |
| Anti‐Tumour Necrosis Factor alpha | 27 (33.3%) | 9 (29.0%) | .663 | NA | NA |
| Surgery n (%) | |||||
| Resection | 56 (69.1%) | 17 (54.8%) | .115 | NA | NA |
| Cause of resection | |||||
| Therapeutic resistance | 6 (7.4%) | 1 (3.2%) | .689 | NA | NA |
| Stenosis | 35 (43.2%) | 11 (35.5%) | |||
| Intra‐abdominal fistula/abscess | 9 (11.1%) | 2 (6.5%) | |||
| Stenosis and abscess/fistula | 4 (4.9%) | 1 (3.2%) | |||
| Other | 0 (0%) | 1 (3.2%) | |||
| Missing | 1 (1.2%) | 1 (3.2%) | |||
| Moment of resection | |||||
| Resection before sample | 45 (55.6%) | 14 (45.2%) | .154 | NA | NA |
| Resection after sample | 4 (4.9%) | 3 (9.7%) | |||
| Resection before and after sample | 7 (8.6%) | 0 (0%) | |||
| Re‐resection because of stenosis/intra‐abdominal fistula/abscess | 18 (22.2%) | 1 (3.2%) | .017 | NA | NA |
HBI, Harvey‐Bradshaw Index; NA, not applicable.
Nominal and continuous data were compared using ANOVA. P values depict statistical differences between patients with Crohn's disease and comparing patients with Crohn's disease to controls.
Classification into disease behavior was based on clinical patient data, and was confirmed by retrospectively available imaging (MRI, CT, X‐ray with barium contrast), endoscopic images and reports, and pathology reports of intestinal resections. Disease behavior was defined as follows: nonstricturing/nonpenetrating: Inflammatory Crohn's disease without prior stricturing or penetrating complications when the serum sample was drawn. Stricturing: Crohn's disease complicated by (symptomatic) stenosis (in‐patient history or present when the serum sample was taken), previous surgery because of stenosis/stricture or postoperative stenosis in anastomosis. Penetrating: Crohn's disease with intra‐abdominal fistulae (enteroenteric, enterocutaneous, enterovaginal, enterovesical), perforations or abscesses in‐patient history or currently present when the serum sample was collected.2 Here described Montreal disease behavior represents the patients’ most severe disease phenotype (nonstricturing/nonpenetrating<stricturing<penetrating disease), irrespective of the current presence of stenosis or fistulae in the intestine. Presence of perianal disease was separately classified from Montreal classification behavior as perianal disease modifier.2
Furthermore, patients with Crohn's disease were classified into disease with active inflammation (CRP >5 mg/L) or without active inflammation (CRP <5 mg/L) based on the C‐reactive protein (CRP) level that was determined when the serum samples were taken (Table 3).30 All patients underwent standard work up including medical history, Harvey‐Bradshaw (≤4 disease in remission, ≥5 active disease) index for assessing clinical disease activity, physical examination, ileocolonoscopy and pharmacological treatment.31 Blood samples were drawn between October 2010 and July 2014.
Table 3.
Area under the curve analysis of the biomarkers
| Biomarker | AUC univariate logistic regression (95%CI) | P value AUC | AUC multivariate (marker + residual value) logistic regression (95%CI) | P value AUC | AUC multivariate (marker + confounders) logistic regression (95%CI) | P value AUC | Confounders in final model |
|---|---|---|---|---|---|---|---|
| Nonstricturing/nonpenetrating (B1) vs penetrating (B3) Crohn's disease | |||||||
| P1NP | 0.637 (0.487‐0.786) | .105 | |||||
| C1M | 0.582 (0.433‐0.732) | .329 | |||||
| P1NP/C1M ratio | 0.639 (0.491‐0.787) | .099 | |||||
| Pro‐C3 | 0.693 (0.557‐0.828) | .022a | 0.797 (0.669‐0.925) | <.001a | Age at sample (NS) Anti‐TNFα (NS) | ||
| C3M | 0.738 (0.598‐0.878) | .005b | 0.738 (0.598‐0.878) | .005a | 0.794 (0.669‐0.919) | <.001b | Disease duration (NS) |
| Pro‐C3/C3M ratio | 0.815 (0.706‐0.923) | .000b | 0.882 (0.793‐0.972) | <.001c | Age at samplea Immunosuppression (NS) | ||
| Pro‐C5 | 0.724 (0.586‐0.861) | .008a | 0.724 (0.586‐0.861) | .008a | 0.724 (0.586‐0.861) | .008a | |
| C5M | 0.610 (0.463‐0.758) | .191 | |||||
| Pro‐C5/C5M ratio | 0.603 (0.444‐0.762) | .222 | |||||
| Pro‐C6 | 0.484 (0.329‐0.639) | .848 | |||||
| C6M | 0.632 (0.490‐0.774) | .116 | |||||
| Pro‐C6/C6M ratio | 0.649 (0.503‐0.794) | .078 | |||||
| Stricturing (B2) vs penetrating (B3) Crohn's disease | |||||||
| P1NP | 0.633 (0.490‐0.777) | .099 | |||||
| C1M | 0.642 (0.495‐0.789) | .079 | |||||
| P1NP/C1M ratio | 0.689 (0.552‐0.827) | .019a | 0.689 (0.552‐0.827) | .019a | |||
| Pro‐C3 | 0.583 (0.445‐0.722) | .301 | |||||
| C3M | 0.709 (0.575‐0.843) | .010a | 0.709 (0.575‐0.843) | .010a | 0.709 (0.575‐0.843) | .010a | |
| Pro‐C3/C3M ratio | 0.746 (0.632‐0.860) | .002a | 0.760 (0.650‐0.870) | .001a | Steroids (NS) | ||
| Pro‐C5 | 0.712 (0.580‐0.844) | .008a | 0.712 (0.580‐0.844) | .008a | 0.712 (0.580‐0.844) | .008a | |
| C5M | 0.577 (0.432‐0.722) | .340 | |||||
| Pro‐C5/C5M ratio | 0.572 (0.422‐0.722) | .371 | |||||
| Pro‐C6 | 0.451 (0.307‐0.596) | .546 | |||||
| C6M | 0.716 (0.593‐0.838) | .008 | |||||
| Pro‐C6/C6M ratio | 0.714 (0.590‐0.839) | .008a | 0.714 (0.590‐0.839) | .008a | |||
AUC, area under the curve; CI, confidence interval; NS, not significant.
No asterisk is shown when the logistic regression model was not significant or when the confounder was not significant in the final model.
Significance in the logistic regression model is depicted as: a P<.05,b P<.01, c P<.001.
Serum samples of 24 healthy persons were obtained from Valley Biomedical, Winchester, Virginia, United States of America. These healthy donors were tested and found negative for: hepatitis B surface antigen, hepatitis C virus, human immunodeficiency virus‐1, ‐2 and ‐1 antigen, n‐acetyltransferase, alanine aminotransferase, and syphilis by Food and Drug Administration‐approved Methods.
2.2. Biomarker assays
At Nordic Bioscience (Herlev, Denmark) neo‐epitope fragments of extracellular matrix synthesis and degradation were assessed by solid phase competitive enzyme linked immunosorbent assays (ELISAs). The markers included in this study are C1M, C3M, C5M, C6M, PINP, Pro‐C3, Pro‐C5 and Pro‐C6 (Table 1).
Ninety‐six well plates pre‐coated with streptavidin (Roche Diagnostic's cat. No. 11940279, Hvidovre, Denmark) were coated with a biotinylated antigen for 30 minutes at room temperature. All samples were diluted in incubation buffer containing 1% bovine serum albumin (Sigma Aldrich, cat. No. a‐7906, St. Louis, MO, USA, ≥98 purity) to maintain protein stability and for blocking. Samples and controls were incubated with horseradish peroxidase‐conjugated monoclonal antibodies. Depending on the specific assay, they were incubated for 1‐3 hours at 4°C/20°C or for 20 hours at 4°C, and agitated at 300 rpm. Subsequently, Tetramethylbenzidine (TMB, Kem‐En‐Tec cat. No. 438OH, Taastrup, Denmark) was added (100 μL/well), plates were incubated for 15 minutes at room temperature, and agitated at 300 rpm. Buffer (1% H2SO4) was added to stop the TMB reaction. After each incubation step, wells were washed with washing buffer (25 mM TRIZMA, 50 mM NaCl, 0.036% Bronidox L5, 0.1% Tween 20) using a standardised ELISA plate washing machine (BioTek Instruments, Microplate washer, ELx405 Select CW, Winooski, VT, USA). An ELISA reader (VersaMAX; Molecular Devices, Wokingham Berkshire, UK) was used to read optical densities at 450 nm and 650 nm. A standard curve was plotted using a four‐parametric mathematical fit model.
2.3. Statistical analysis
One‐way analysis of variance (ANOVA) was applied to compare differences in nominal and continuous data in Tables 2 and 4. Since we assume that marker levels are normally distributed in the general population, marker levels were compared using one‐way (ANOVA) with post hoc Fisher's least significant difference test (not correcting for multiple comparisons) and with Bonferroni correction for multiple comparisons. Marker levels are presented as Tukey box plots.
To evaluate the predictive power of the markers to differentiate between the different Montreal classes, univariate binary logistic regression analysis (method: enter) was conducted and receiver operator characteristics (ROC) curves were calculated for the markers alone (Table 3). When P values of both univariate logistic regression and ROC curves were <.05, multivariate analysis was performed: 1. Using linear regression, unstandardised residual values corrected disease duration, perianal disease and medication use (5‐aminosalicylates, corticosteroids, anti‐Tumour Necrosis‐Alpha) were created. Subsequently, multivariate backward (conditional) binary logistic regression was conducted by adding the marker and its corrected unstandardised residual value to the model, thereby determining the prognostic value of solely the marker. 2. Multivariate backward (conditional) binary logistic regression was conducted by adding the same confounders to the model, to determine the prognostic value of the marker in combination with the confounders. Correlations between marker ratios/levels and CRP were determined using a Pearson product‐moment correlation coefficient. A P≤.05 was considered to be statistical significant. Marker levels and AUCs are presented as average±95% confidence interval. Statistical analysis was performed using Statistical Package for Social Sciences 22.0 (SPSS Inc., Chicago, Ill, USA) Figures were designed using GraphPad Prism version 6.0 (La Jolla, CA, USA).
2.4. Ethical considerations
Patients gave written informed consent when participating in the University Medical Center Groningen IBD database for the use of patient data and serum.
3. Results
3.1. Patient and controls demographics
This cohort contains 112 patients suffering from Crohn's disease and 24 healthy controls (Table 2). Patients with Crohn's disease were divided into nonstricturing/nonpenetrating (n=40), stricturing (n=55) and penetrating (n=17). Disease duration was significantly different between nonstricturing/nonpenetrating and stricturing disease. As to be expected, patients with stricturing and penetrating disease, showed higher incidences of surgery. Patients with stricturing disease underwent surgery because of stenosis, while patients with penetrating disease mainly underwent surgery because of fistulae or abscesses. Furthermore, patients with stricturing and penetrating disease more often underwent surgery before the serum sample was retrieved. Moreover, the prevalence of re‐resection because of stenosis or intra‐abdominal fistulae/abscesses/perforations was higher in stricturing and penetrating disease compared to nonstricturing/nonpenetrating disease.
Furthermore, patients were divided into two groups based on the biochemical activity of the disease (Active inflammation: CRP >5 mg/L, without active inflammation: CRP <5 mg/L) (Table 4). Importantly, distribution of disease behavior was not different between the groups.
3.2. Penetrating Crohn's disease is characterised by increased matrix metalloproteinase mediated breakdown of type III collagen and increased formation of type V collagen
Levels of P1NP were equal among the groups. C1M levels were higher in all patients with Crohn's disease compared to controls (64.8±10.0 vs 16.3±2.9 nmol/L [P<.001]). The balance between formation and degradation of type I collagen (P1NP/C1M ratio) was higher in controls compared to patients with Crohn's disease (7.9±2.8 vs 3.0±0.6 nmol/L [P<.001]), indicating net decreased type I collagen formation in Crohn's disease (Figure 1A). Pro‐C3 serum concentrations were equal among patients with Crohn's disease and controls. C3M levels were higher in penetrating disease (33.6±5 nmol/L) compared to nonpenetrating/nonstricturing and stricturing disease (25.8±2.2 [P=.004] and 27.2±2.3 nmol/L [P=.018]), which indicates increased relative (matrix metalloproteinase‐9 mediated) breakdown of type III collagen in penetrating Crohn's disease. Moreover, C3M concentrations in patients with Crohn's disease were higher compared to controls (27.7±1.6 vs 9.8±1.7 nmol/L [P<.001]). Collagen type III formation/degradation (Pro‐C3/C3M) balance was lower in Crohn's disease patients compared to controls (Pro‐C3/C3M 1.0±0.2 vs 0.5±0.1 [P<.001], Figure 1B). Levels of Pro‐C5 were higher in penetrating disease (1262.7±259.4 nmol/L) compared to nonpenetrating/nonstricturing and stricturing disease (902.9±109.9 [P=.005] and 953.0±106.4 nmol/L [P=.015]). Moreover, all patients with Crohn's disease had higher serum concentrations of Pro‐C5 compared to controls (982.2±76.8 vs 311.9±50.2 nmol/L [P<.001]). For C5M, no significant differences were found between the groups. Equal levels of type V collagen degradation markers in patients with Crohn's disease and controls, but increasing formation markers in Crohn's disease and especially in penetrating disease results in a higher type V collagen formation/degradation balance (Pro‐C5/C5M) in patients with Crohn's disease compared to controls (179.8±14.8 vs 55.4±11.2 [P<.001], Figure 2A). For Pro‐C6, C6M and their ratio, no significant differences were observed between patients with Crohn's disease and controls (Figure 2B).
Figure 1.
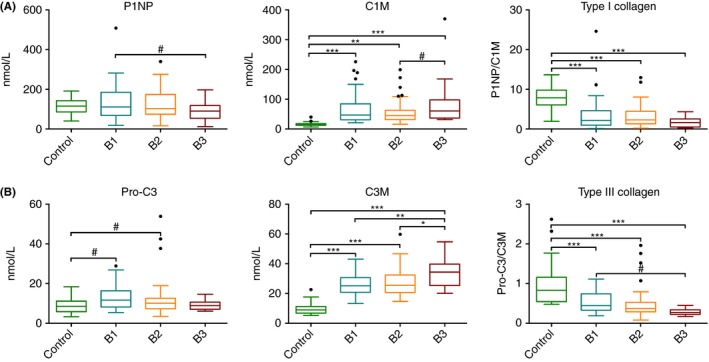
Serum biomarker levels and ratios for Montreal classes B1 (non‐stricturing/nonpenetrating), B2 (stricturing), B3 (penetrating) and healthy controls. A) P1NP, C1M, P1NP/C1M, B) Pro‐C3, C3M, Pro‐C3/C3M. Significant differences using ANOVA with post hoc Bonferroni correction for multiple comparisonsare depicted as: *P<.05, **P<.01, ***P<.001. Non‐Bonferroni corrected significant differences aredepicted as: #P<.05. Marker levels are presented as Tukey box plots
Figure 2.
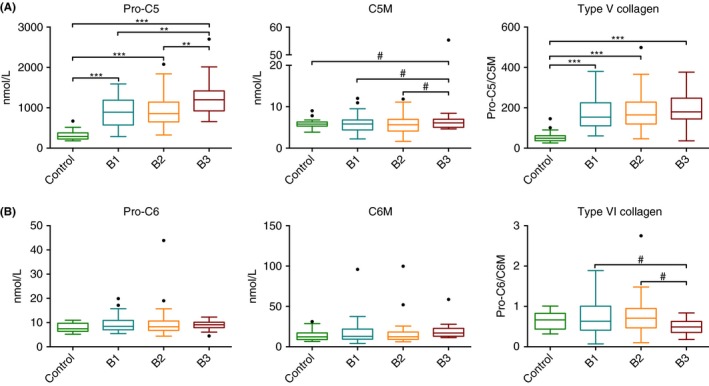
Serum biomarker levels and ratios for Montreal classes B1 (nonstricturing/nonpenetrating), B2 (stricturing), B3 (penetrating) and healthy controls. A) Pro‐C5, C5M, Pro‐C5/C5M, B) Pro‐C6, C6M, Pro‐C6/C6M. Significant differences after Bonferroni correction for multiple comparisons are depicted as: **P<.01, ***P<.001. Non‐Bonferroni correction significant differences are depicted as: #P<.05. Marker levels are presented as Tukey box plots
No statistical differences in any of the marker levels were found between patients with or without perianal disease within the same Montreal B class.
3.3. Pro‐C3/C3M ratios are superior in differentiating between Montreal nonstricturing/nonpenetrating vs stricturing and penetrating
Logistic regression was used to determine the diagnostic power for the markers alone and for the formation/degradation ratios to differentiate between the Montreal behavior classes (Table 3). Without correcting or adjusting the model for confounders, the ratio between Pro‐C3 and C3M (AUC: 0.815±0.109 [P<.001], Figure 3), and C3M itself (AUC: 0.738±0.140 [P=.005]) were best to differentiate between nonstricturing/nonpenetrating and penetrating disease. To differentiate between stricturing and penetrating disease, Pro‐C3/C3M (AUC: 0.746±0.114 [P=.002], Figure 3) and Pro‐C6/C6M ratios (AUC: 0.714±0.125 [P=.008]) were superior. Using multivariate logistic regression and correcting for the impact of disease duration, age and medication use on the marker, C3M (AUC: 0.738±0.140 [P=.005]) for nonstricturing/nonpenetrating vs penetrating, AUC: 0.709±0.134 [P=.010] for stricturing vs penetrating) and Pro‐C5 (AUC: 0.724±0.138, [P=.008] for nonstricturing/nonpenetrating vs penetrating, AUC: 0.712±0.132 [P=.008] for stricturing vs penetrating) were superior in differentiating between these Montreal behavior classes. Multivariate logistic regression for Pro‐C3/C3M ratio with confounders was superior to differentiate between nonstricturing/nonpenetrating and penetrating disease (AUC: 0.882±0.089 [P<.001]), and to differentiate between stricturing and penetrating disease (AUC: 0.760±0.110 [P=.001]). None of the markers or ratios was able to differentiate between nonstricturing/nonpenetrating and stricturing disease. Only combining Pro‐C3/C3M and Pro‐C6/C6M rations slightly increased the power to differentiate between stricturing and penetrating disease (AUC: 0.754±0.113 [P=.002]).
Figure 3.
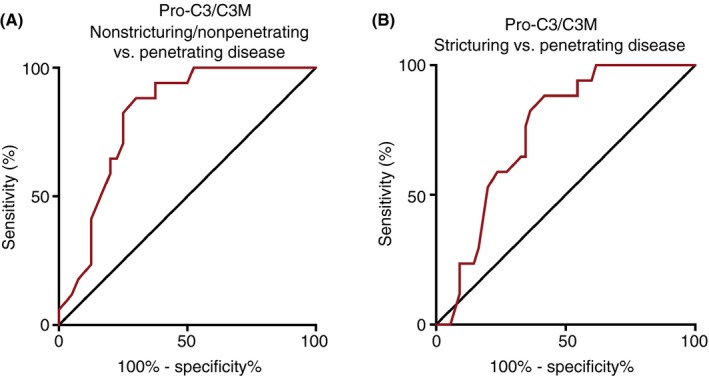
Receiver operating characteristic curve based on the univariate logistic regression model showing the diagnostic power of Pro‐C3/C3M to differentiate between nonstricturing/nonpenetrating (B1) and stricturing (B2) vs penetrating (B3) disease
3.4. Active inflammation in Crohn's disease is characterised by increased matrix metalloproteinase mediated breakdown of type I, III and VI collagen
P1NP levels were equal in patients with Crohn's disease without and with active inflammation as defined by CRP >5 mg/L. On the other hand, higher levels of C1M were observed in Crohn's disease with active inflammation (113.2±26.1) compared to Crohn's disease without active inflammation and controls (46.2±6.2 [P<.001] and 16.3±2.9 nmol/L [P<.001]). Type I collagen balance (P1NP/C1M) was lower in Crohn's disease with active inflammation compared to Crohn's disease without active inflammation and controls (P1NP/C1M 1.3±0.5 vs 3.7±0.8 [P=.001] and 7.9±1.2 nmol/L [P<.001), Figure 4A]. Pro‐C3 levels were higher in patients with inactive Crohn's disease compared to controls (12.6±1.8 vs 8.6±1.5 nmol/L [P=.030]). In line with C1M, higher levels of C3M were observed in Crohn's disease with active inflammation (31.6±3.9 nmol/L), compared to Crohn's disease without active inflammation and controls (26.1±1.6 [P=.002] and 9.8±1.7 nmol/L [P<.001]). Pro‐C3/C3M ratios were lower in patients with Crohn's disease, compared to controls (1.0±0.2 vs 0.5±0.1 [P<.001], Figure 4B). Contrary to P1NP and Pro‐C3, higher levels of Pro‐C5 were found in Crohn's disease with active inflammation (1171.7±171.5 nmol/L) compared to Crohn's disease without active inflammation and controls (909.6±80.4 [P=.002] and. 311.9±50.2 [P<=.001]). For type V collagen degradation marker C5M, no differences were observed between the groups (Figure 4C). Pro‐C5/C5M ratios were higher in patients with Crohn's disease compared to controls (179.8±14.8 vs 55.4±11.2 [P<.001], Figure 4C). Pro‐C6 levels were equal among the groups, whereas C6M was higher in Crohn's disease with active inflammation (28.2±7.9 nmol/L), compared to Crohn's disease without active inflammation and controls (12.8±1.1 [P<.001] and 13.8±2.8 [P<.001]). Pro‐C6/C6M ratios were lower in patients with active inflammation compared to patients without active inflammation (0.4±0.1 vs 0.8±0.1 [P<.001], Figure 4D). Descriptive statistics of marker levels separated by Montreal behavior, active and inactive disease and controls are presented in table [Link], [Link], [Link].
Figure 4.
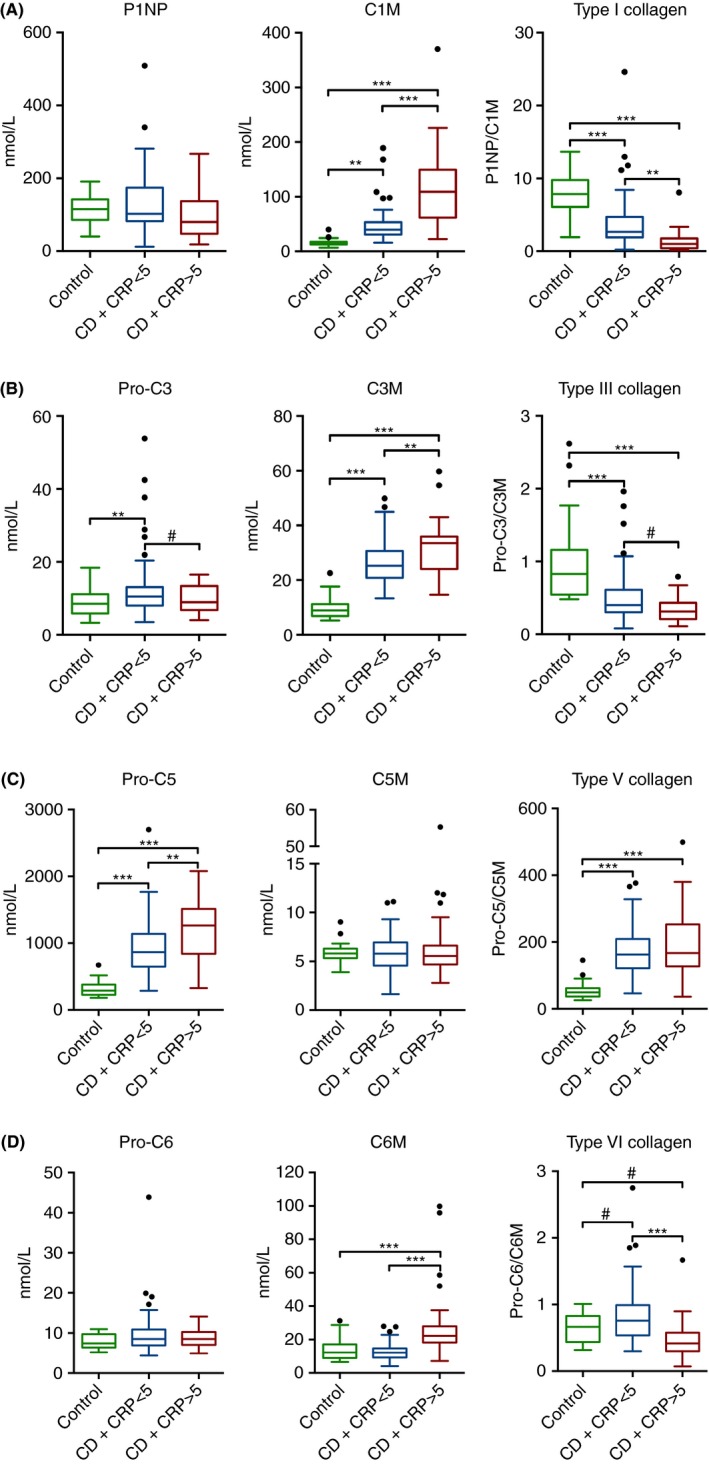
Serum biomarker levels in CD patients with active inflammation (CRP >5 mg/L) compared to CD without active inflammation (CRP <5 mg/L) and healthy controls. A) P1NP, C1M, P1NP/C1M, B) Pro‐C3, C3M, Pro‐C3/C3M, C) Pro‐C5, C5M, Pro‐C5/C5M, D) Pro‐C6, C6M, Pro‐C6/C6M. Significant differences using ANOVA with post hoc Bonferroni correction for multiple comparisons are depicted as: **P<.01, ***P<.001. Non‐Bonferroni corrected significant differences are depicted as: #P<.05. Marker levels are presented as Tukey box plots
C1M (r 2=.684, P<.001), C3M (r 2=.390, P<.001), Pro‐C5 (r 2=.288, P=.002), C5M (r 2=.519, P<.001) and C6M (r 2=.484, P<.001) correlated (independently from the underlying disease behavior) to CRP levels. P1NP/C1M ratios (r 2=−.226, P=.017), Pro‐C3/C3M ratios (r 2=−.198, P=.036) and Pro‐C6/C6M ratios (r 2=−.302, P=.001) ratios correlated negatively to CRP levels (Figure S1). No correlation between clinical disease activity (HBI) and the markers was found. Furthermore, the differences in marker levels within the Montreal behavior classification were independent of the presence of inflammation since CRP (r 2=.029, P=.762), and Harvey‐Bradshaw Index (r 2=−.087, P=.361) did not correlate to disease behavior according to the Montreal classification.
4. Discussion
The present study is the first to show that serum levels of formation and degradation products of collagens can be used to differentiate between penetrating vs nonstricturing/nonpenetrating and stricturing Crohn's disease in the terminal ileum using a competitive ELISA system based on a extracellular matrix neo‐epitope biomarker platform.32 Furthermore, it is the first study that investigates serum markers for turnover of type V and VI collagen in Crohn's disease combined to the previously studied collagen type I and III.33, 34, 35 Our study provides insight into the differences in turnover of extracellular matrix in the different phenotypes of Crohn's disease and compared this to healthy controls. Furthermore, by comparing Crohn's disease with and without elevated CRP, we provide insight into extracellular matrix turnover during active inflammation.
Our results show that penetrating Crohn's disease in the terminal ileum is characterised by increased matrix metalloproteinase‐9 mediated breakdown of type III collagen (C3M) and that this is reflected in the serum of these patients (Figure 1). A trend for increased matrix metalloproteinase‐2/9/13 mediated type I collagen degradation was observed. Furthermore, our results show that active inflammation, most likely contributes to the development of penetrating Crohn's disease (which is characterised by increased matrix metalloproteinase mediated breakdown of collagens12, 13, 36) among others by causing a disturbed collagen formation/degradation balance (Figure 4). Relative overexpression of matrix metalloproteinase‐3 and ‐9 in acutely inflamed fistulae of patients with and without Crohn's disease compared to healthy colon, was previously reported by Kirkegaard et al.13 Using gelatin zymography, they showed increased amounts of active matrix metalloproteinase‐2 in Crohn's disease fistulae specimens compared to healthy colon.13 Furthermore, Efsen et al. reported increased matrix metalloproteinase‐3 and ‐9 activity in Crohn's disease fistulae compared to non‐Crohn's disease fistulae.36 Moreover, they reported that up to 50% of the total protease activity in Crohn's disease's fistulae is attributable to matrix metalloproteinases.36 Increased C3M serum levels in patients with penetrating (Montreal B3) Crohn's disease, compared to nonstricturing/nonpenetrating and stricturing disease using the same markers was recently found in a Swiss IBD cohort as well.37 Next to show a formation/degradation misbalance in penetrating Crohn's disease, we could show that active inflammation (defined by CRP >5 mg/L) leads to an altered collagen formation/degradation balance (Figure 4). During active inflammation, CRP is almost exclusively excreted by hepatocytes as part of the acute phase response upon stimulation by IL‐6, TNFα and IL‐1β originating at the site of inflammation.30, 38 Production of pro‐inflammatory cytokines by innate immune cells like monocytes, neutrophils, mast cells, eosinophils and basophils goes along with production of matrix metalloproteinases at the site of inflammation and explains the increased levels of matrix metalloproteinase mediated degradation markers during active inflammation.39, 40 CRP is a widely used marker to monitor disease activity in Crohn's disease, which correlates well with disease activity as well as with faecal calprotectin.30 The association between a high number of flares and penetrating Crohn's disease has been reported before and is in line with the hypothesis that penetrating Crohn's disease is partly caused by matrix metalloproteinase‐over activity.5, 12 The association between marker levels and disease phenotype reported in this study was independent of the presence of active inflammation. The overlap in increased matrix metalloproteinase activity in penetrating (reported in this study and previously) as well as in active Crohn's Disease (this study) further confirms the importance to strive for mucosal healing (treat to target principle) in Crohn's disease patients to prevent complications.
We aimed to establish whether these collagen formation and degradation markers are able to distinguish between the subclasses of the Montreal disease behavior classification. According to the Montreal Behavior classification, patients are categorised into the class of the most severe phenotype (nonstricturing/nonpenetrating<stricturing<penetrating) they have had or have since they were diagnosed with Crohn's disease.2 This comprises that patients in this study not necessarily had a stenosis or fistula when the serum sample was taken. Furthermore, this means that patients that were classified as penetrating could have had a history of stricturing disease. To affirm that the differences in marker levels were due to the disease, patients were strictly selected. By only selecting patients with Crohn's disease in the terminal ileum, the bulk of disease was limited to this anatomic region. The differences we observed are thereby due to differences in extracellular matrix turnover within this region and not due to large differences in disease affected intestine between, for example, patients with ileocolonic disease or only ileal disease. Furthermore, the included patients’ disease behavior was stable during follow‐up, which substantially confirms the Montreal behavior class in which they were classified. To rule out the effects of surgery, patients that had any kind of surgery 6 months before the sample was taken were not included. De Simone et al. previously showed that intestinal resection in Crohn's disease patients with active disease reduces serum levels of type III collagen formation markers 6 months after surgery compared to levels from serum taken just before surgery.33 Furthermore, non‐Crohn's disease related fibrotic and autoimmune disease, and all forms of malignancy (except for skin cancer and haematological disease) were excluded.
Interestingly, levels of formation markers for type I, III and VI collagen were hardly influenced by disease activity or in the penetrating phenotype, whereas Pro‐C5 levels increased along with increasing CRP in patients with Crohn's disease. On the other hand, degradation markers of type I, III and VI collagen increased along with increasing CRP in patients with Crohn's disease, whereas C5M is not influenced by active inflammation or penetrating Crohn's disease. This suggests a net decrease in type I, III and VI collagen and a net increase in type V collagen in the extracellular matrix of the ileum during long‐standing and often flaring Crohn's disease. These results are in line with those of Graham et al., who showed a relative decrease in type I collagen and a relative increase in type V collagen in intestinal strictures compared to normal ileum.14 An interesting but unexpected finding is that the measured formation and degradation levels and ratios are not different between patients with nonpenetrating/nonstricturing (inflammatory) and stricturing disease. Their levels of C1M, C3M and Pro‐C5 are, however, different from the healthy controls. This implicates that turnover balance of type I, III, V and VI collagen is equal between nonstricturing/nonpenetrating and stricturing disease and suggests that the final development of stenosis (the majority of patients with Crohn's disease progress towards a penetrating or stricturing phenotype during the disease course (48% within 5 years after diagnosis and up to 70% 10 years after diagnosis3)) may depend on other factors. These factors could be other components of extracellular matrix, namely (other types of) collagens, laminin or fibronectin.17 Stricturing of the intestine depends not only on excessive deposition of extracellular matrix but also on enhanced matrix stiffness by increased crosslinking in the extracellular matrix, which may contribute to the stricture becoming symptomatic.41
Intra‐abdominal fistulae are very often surrounded by fibrosis. Therefore, patients with penetrating disease most likely partly overlap in biomarker profile with patients having stricturing disease. However, these patients did develop penetrating disease, among others due to matrix metalloproteinase over activity. These fistulae, abscesses or perforations arose despite the thickened intestinal wall and therefore might have required even more inflammation‐mediated activity of matrix metalloproteinases compared to fistulae, abscesses or perforations that arose in a nonfibrosis affected intestinal wall. This hypothesis can only be validated in a prospective longitudinal cohort in which marker levels from before and after surgery can be correlated with the resected specimen.
Patients with perianal disease were not separately grouped in the final analysis since no differences in marker levels between patients with and without perianal disease within one Montreal behavior class were found. Furthermore, when comparing patients classified as nonstricturing/nonpenetrating to patients with nonstricturing/nonpenetrating, stricturing or penetrating disease with perianal disease, no differences were found. As previously reported, intra‐abdominal fistulae are very often surrounded by fibrosis and the wall of the terminal ileum contains relatively more extracellular matrix compared to the wall of the anus.42, 43 We therefore hypothesise that the quantity of matrix metalloproteinase‐over activity (due to inflammation) needed to penetrate the (fibrotic) wall of the intestine is higher compared to the matrix metalloproteinase activity needed to penetrate the wall of the perianal region. Furthermore, matrix metalloproteinase degraded fragments released in the perianal region might not reach the systemic circulation as easily as degraded fragments from intra‐abdominal fistulae. These reasons could explain why the existence of perianal disease cannot be observed using these markers. Smith et al. who studied the Vienna classification based on serological and genetic markers in 231 well‐characterised Crohn's disease patients found that patients with perianal fistulae or abscesses were different from those with intra‐abdominal penetrating disease.2, 4 However, they did not check for differences in formation or degradation products of collagen, or for levels of matrix metalloproteinases.
Previously hypothesised differences in pathophysiology between possibly ischaemic anastomotic strictures and more fibrotic primary intestinal strictures might appear in differences in marker levels in our study. In our cohort, 16 of the 55 patients with stricturing disease underwent one or more re‐resections because of recurrent stenosis. No differences in marker levels between patients with stricturing disease with and without re‐resection were observed. Furthermore, pathology reports of the re‐resections were checked on the occurrence of ischaemia in the resected stenosis. In none of the 11 pathology reports that were retrospectively available, pathologists reported signs of ischaemia. Pathologists reported mainly active inflammation, granulomatous and fibrosis in the resected specimens. The literature describes reduced blood flow in active Crohn's disease, compared to inactive Crohn's disease and controls.44 However, Angerson et al. showed that patients with Crohn's disease that developed higher grades of neo‐terminal ileal recurrence (at least five aphthous ulcers) had similar levels of blood flow as patients with active Crohn's ileitis. These levels were lower than controls.45 Furthermore, literature describing histopathology of intestinal re‐resection specimens or the role of ischaemia on IBD pathogenesis, does not report a role for ischaemia specifically in recurrent Crohn's disease.45, 46 On the basis of our observations and the literature, we are not convinced that ischaemia plays a specific role in recurrence of stenosis in Crohn's disease.
Since the study is retrospective, disease behavior was classified according to retrospectively available imaging (MRI, CT, X‐ray with barium contrast), endoscopic images and reports and pathology reports of intestinal resections. Although we do believe that the current sub‐classification is sound enough to establish whether these markers are able to differentiate between these phenotypes, it would be superior to have data from cross‐sectional imaging to confirm the current state of the disease. Another limitation to this study is the relatively small sample size, especially the group with penetrating disease. This causes lack of power to detect a sound effect of medication use, smoking behavior, age and disease duration on biomarker levels. Ideally this would be examined in a prospective longitudinal cohort. Furthermore, one cannot confirm whether these markers are only produced in the fistulating parts of the intestine, in the inflamed intestinal tissue of Crohn's disease patients with penetrating disease or if they derive from a secondary source. Patients with penetrating Crohn's disease generally suffer from more complicated and severe intestinal (but also extraintestinal) disease.5 The combination of more severe intestinal and extraintestinal disease activity in penetrating Crohn's disease could also explain the increased matrix metalloproteinase mediated breakdown of collagens.8 The collagen degradation markers used in this study are designed to target specific matrix metalloproteinase degraded protein peptides that derive from those parts of the body in which these matrix metalloproteinases are active because of the disease.47
In conclusion, based on serological collagen formation and degradation makers, penetrating Crohn's disease is associated with increased matrix metalloproteinase‐9 mediated breakdown of type III collagen. A trend for increased matrix metalloproteinase‐2/9/13 mediated type I collagen degradation was observed. Active inflammation in Crohn's disease is also associated with increased breakdown of type I, III and VI collagen. The overlap in increased matrix metalloproteinase mediated degradation in penetrating as well as in active Crohn's confirms the importance to strive for mucosal healing to prevent complications as fistulae. A disturbed collagen type III balance mainly characterises penetrating Crohn's disease; therefore, Pro‐C3/C3M ratios are superior in differentiating between penetrating Crohn's disease vs inflammatory and stricturing Crohn's disease. Prospective longitudinal studies are warranted to validate this study and to investigate if these biomarkers could predict the disease course and guide therapy to prevent complications in Crohn's disease.48, 49
Authorship
Guarantor of the article: PO.
Author contribution: WTVH: concept and design of the study, acquisition of data, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content; GD: collecting patient samples; JHM, MAK, ACBJ, GD and PO:concept and design of the study, interpretation of data, revising critically for important intellectual content.
All authors approved to the final graft before the manuscript was submitted.
Supporting information
Acknowledgements
The UMCG would like to thank the Parelsnoer Institute for providing the Biobank Infrastructure to contribute to this study. Furthermore, the authors wish to thank professor R.A. Bank PhD (department of matrix biology, University Medical Center Groningen), I.M. Nolte PhD (department of Epidemiology, University Medical Center Groningen) and professor G. Rogler MD, PhD (department of Gastroenterology and Hepatology, UniversitätsSpital Zurich) for helpful discussions concerning the obtained results.
Declaration of personal interests: JHM, MAK, and ACB‐J are employees of Nordic Bioscience. MAK and ACB‐J own stocks in Nordic Bioscience.
van Haaften WT, Mortensen JH, Karsdal MA, Bay‐Jensen AC, Dijkstra G, Olinga P. Misbalance in type III collagen formation/degradation as a novel serological biomarker for penetrating (Montreal B3) Crohn's disease. Aliment Pharmacol Ther. 2017;46:26–39. 10.1111/apt.14092
Funding information
This study was funded in part by ZonMW (obtained by PO), grant number 114021010. The concept and design of the study, acquisition of data, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content was funded by the University Medical Center Graduate School for medical sciences who provided the MD/PhD‐student grant of WTvH.
The Handling Editor for this article was Dr Ashwin Ananthakrishnan, and it was accepted for publication after full peer‐review.
G. Dijkstra and P. Olinga contributed equally to this work.
References
- 1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066‐2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(suppl A):5A‐36A. [DOI] [PubMed] [Google Scholar]
- 3. Louis E, Collard A, Oger AF, et al. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith BRK, Arnott IDR, Drummond HE, et al. Disease location, anti‐saccharomyces cerevisiae antibody, and NOD2/CARD15 genotype influence the progression of disease behavior in Crohn‘s disease. Inflamm Bowel Dis. 2004;10:521‐528. [DOI] [PubMed] [Google Scholar]
- 5. Louis E, Michel V, Hugot JP, et al. Early development of stricturing or penetrating pattern in Crohn's disease is influenced by disease location, number of flares, and smoking but not by NOD2/CARD15 genotype. Gut. 2003;52:552‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartz DA, Loftus EV, Tremaine WJ, et al. The natural history of fistulizing Crohn's disease in Olmsted County Minnesota. Gastroenterology. 2002;122:875‐880. [DOI] [PubMed] [Google Scholar]
- 7. Siegmund B, Feakins RM, Bamias G, et al. Results of the 5th scientific workshop of the ECCO (II): pathophysiology of perianal fistulising disease. J Crohn's Colitis. 2016;10:377‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peyrin‐Biroulet L, Loftus EV, Colombel J‐F, et al. The natural history of adult Crohn's disease in population‐based cohorts. Am J Gastroenterol. 2010;105:289‐297. [DOI] [PubMed] [Google Scholar]
- 9. Bataille F, Klebl F, Rummele P, et al. Morphological characterisation of Crohn's disease fistulae. Gut. 2004;53:1314‐1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimshoni E, Yablecovitch D, Baram L, et al. ECM remodelling in IBD: innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut. 2014;64:367‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rieder F, Fiocchi C. Intestinal fibrosis in IBD–a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228‐235. [DOI] [PubMed] [Google Scholar]
- 12. Schuppan D, Freitag T. Fistulising Crohn's disease: MMPs gone awry. Gut. 2004;53:620‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirkegaard T, Hansen A, Bruun E, et al. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn's disease. Gut. 2004;53:701‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graham MF, Diegelmann RF, Elson CO, et al. Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology. 1988;94:257‐265. [DOI] [PubMed] [Google Scholar]
- 15. Lawrance IC, Maxwell L, Doe W. Inflammation location, but not type, determines the increase in TGF‐beta1 and IGF‐1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis. 2001;7:16‐26. [DOI] [PubMed] [Google Scholar]
- 16. Groulx JF, Gagné D, Benoit YD, et al. Collagen VI is a basement membrane component that regulates epithelial cell‐fibronectin interactions. Matrix Biol. 2011;30:195‐206. [DOI] [PubMed] [Google Scholar]
- 17. Ravi A, Garg P, Sitaraman SV. Matrix metalloproteinases in inflammatory bowel disease: boon or a bane? Inflamm Bowel Dis. 2007;13:97‐107. [DOI] [PubMed] [Google Scholar]
- 18. Warnaar N, Hofker HS, Maathuis MHJ, et al. Matrix metalloproteinases as profibrotic factors in terminal ileum in Crohn's disease. Inflamm Bowel Dis. 2006;12:863‐869. [DOI] [PubMed] [Google Scholar]
- 19. Baugh MD, Perry MJ, Hollander AP, et al. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814‐822. [DOI] [PubMed] [Google Scholar]
- 20. Leeming D, He Y, Veidal S, et al. A novel marker for assessment of liver matrix remodeling: an enzyme‐linked immunosorbent assay (ELISA) detecting a MMP generated type I collagen neo‐epitope (C1M). Biomarkers. 2011;16:616‐628. [DOI] [PubMed] [Google Scholar]
- 21. Barascuk N, Veidal SS, Larsen L, et al. A novel assay for extracellular matrix remodeling associated with liver fi brosis : an enzyme‐linked immunosorbent assay (ELISA) for a MMP‐9 proteolytically revealed neo‐epitope of type III collagen. Clin Biochem. 2010;43:899‐904. [DOI] [PubMed] [Google Scholar]
- 22. Veidal SS, Larsen DV, Chen X, et al. MMP mediated type V collagen degradation (C5M) is elevated in ankylosing spondylitis. Clin Biochem. 2012;45:541‐546. [DOI] [PubMed] [Google Scholar]
- 23. Veidal SS, Karsdal MA, Vassiliadis E, et al. MMP mediated degradation of type VI collagen is highly associated with liver Fibrosis ‐ Identification and validation of a novel biochemical marker assay. PLoS ONE. 2011;6:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leeming DJ, Larsen DV, Zhang C, et al. Enzyme‐linked immunosorbent serum assays (ELISAs) for rat and human N‐terminal pro‐peptide of collagen type I (PINP)–assessment of corresponding epitopes. Clin Biochem. 2010;43:1249‐1256. [DOI] [PubMed] [Google Scholar]
- 25. Nielsen MJ, Nedergaard AF, Sun S, et al. The neo‐epitope specific PRO‐C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res. 2013;5:303‐315. [PMC free article] [PubMed] [Google Scholar]
- 26. Vassiliadis E, Veidal SS, Simonsen H, et al. Immunological detection of the type V collagen propeptide fragment, PVCP‐1230, in connective tissue remodeling associated with liver fibrosis. Biomarkers. 2011;16:426‐433. [DOI] [PubMed] [Google Scholar]
- 27. Sun S, Henriksen K, Karsdal MA, et al. Collagen type III and VI turnover in response to long‐term immobilization. PLoS ONE. 2015;10:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leeming DJ, Nielsen MJ, Dai Y, et al. Enzyme‐linked immunosorbent serum assay specific for the 7S domain of Collagen Type IV (P4NP 7S): a marker related to the extracellular matrix remodeling during liver fibrogenesis. Hepatol Res. 2012;42:482‐493. [DOI] [PubMed] [Google Scholar]
- 29. Mortensen JH, Godskesen LE, Jensen MD, et al. Fragments of citrullinated and MMP‐degraded vimentin and MMP‐degraded type III collagen are novel serological biomarkers to differentiate Crohn's disease from ulcerative colitis. J Crohn's Colitis. 2015;9:863‐872. [DOI] [PubMed] [Google Scholar]
- 30. Vermeire S, Van Assche G, Rutgeerts P. C‐reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:661‐665. [DOI] [PubMed] [Google Scholar]
- 31. Harvey RF, Bradshaw JM. A simple index of Crohn's‐disease activity. Lancet. 1980;315:514. [DOI] [PubMed] [Google Scholar]
- 32. Karsdal MA, Genovese F, Madsen EA, et al. Collagen and tissue turnover as function of age ‐ implications for fibrosis. J Hepatol. 2016;64:103‐109. [DOI] [PubMed] [Google Scholar]
- 33. De Simone M, Ciulla MM, Cioffi U, et al. Effects of surgery on peripheral N‐terminal propeptide of type III procollagen in patients with Crohn's disease. J Gastrointest Surg. 2007;11:1361‐1364. [DOI] [PubMed] [Google Scholar]
- 34. De Simone M, Cioffi U, Contessini‐Avesani E, et al. Elevated serum procollagen type III peptide in splanchnic and peripheral circulation of patients with inflammatory bowel disease submitted to surgery. BMC Gastroenterol 2004;4(Cd):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kjeldsen J, Schaffalitzky de Muckadell OB, Junker P. Seromarkers of collagen I and III metabolism in active Crohn's disease. Relation to disease activity and response to therapy. Gut. 1995;37:805‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Efsen E, Saermark T, Hansen A, et al. Ramiprilate inhibits functional matrix metalloproteinase activity in Crohn's disease fistulas. Basic Clin Pharmacol Toxicol. 2011;109:208‐216. [DOI] [PubMed] [Google Scholar]
- 37. Goffin L, Fagagnini S, Vicari A, et al. Anti‐MMP‐9 antibody: a promising therapeutic strategy for treatment of inflammatory bowel disease complications with fibrosis. Inflamm Bowel Dis. 2016;22:2041‐2057. [DOI] [PubMed] [Google Scholar]
- 38. Nijsten MW, Olinga P, The TH, et al. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit Care Med. 2000;28:458‐461. [DOI] [PubMed] [Google Scholar]
- 39. Speca S, Giusti I, Rieder F, et al. Cellular and molecular mechanisms of intestinal fibrosis. World J Gastroenterol. 2012;18:3635‐3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lawrance IC, Rogler G, Bamias G, et al. Cellular and molecular mediators of intestinal fibrosis. J Crohns Colitis. 2014;9:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johnson LA, Rodansky ES, Sauder KL, et al. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm Bowel Dis. 2013;19:891‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scharl M, Rogler G. Pathophysiology of fistula formation in Crohn's disease. World J Gastrointest Pathophysiol. 2014;5:205‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scharl M, Bruckner RS, Rogler G. The two sides of the coin: similarities and differences in the pathomechanisms of fistulas and stricture formations in irritable bowel disease. United Eur Gastroenterol J. 2016;4:506‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Angerson WJ, Allison MC, Baxter JN, et al. Neoterminal ileal blood flow after ileocolonic resection for Crohn's disease. Gut. 1993;34:1531‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ibrahim CB, Aroniadis OC, Brandt LJ. On the role of ischemia in the pathogenesis of IBD: a review. Inflamm Bowel Dis. 2010;16:696‐702. [DOI] [PubMed] [Google Scholar]
- 46. Bressenot A, Peyrin‐Biroulet L. Histologic features predicting postoperative Crohnʼs disease recurrence. Inflamm Bowel Dis. 2015;21:468‐475. [DOI] [PubMed] [Google Scholar]
- 47. Karsdal MA, Nielsen MJ, Sand JM, et al. Extracellular matrix remodeling: The common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev Technol. 2013;11:70‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ryan JD, Silverberg MS, Xu W, et al. Predicting complicated Crohn's disease and surgery: phenotypes, genetics, serology and psychological characteristics of a population‐based cohort. Aliment Pharmacol Ther. 2013;38:274‐283. [DOI] [PubMed] [Google Scholar]
- 49. Choung RS, Princen F, Stockfisch TP, et al. Serologic microbial associated markers can predict Crohn's disease behaviour years before disease diagnosis. Aliment Pharmacol Ther. 2016;43:1300‐1310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


