Abstract
There is uncertainty about whether hypoxic injury accompanying donor death from ligature asphyxiation influences renal transplant outcomes, particularly for recipients of kidneys donated after circulatory death (DCD). The UK Registry analysis was undertaken to determine transplant outcomes in recipients of kidneys from donors who died following ligature asphyxiation. From 2003 to 2016, 2.7% (n = 521) of potential organ donors died following ligature asphyxiation (mostly suicide by hanging). Of these, 409 (78.5%) donated kidneys for transplantation (46.9% donation after brain death [DBD] and 53.1% DCD donors) resulting in 650 kidney transplants. Compared to other deceased donors, those dying from ligature asphyxiation were younger, more often male, and had less hypertension. Unadjusted patient and graft survival were superior for recipients of both DBD and DCD kidneys from donors dying after ligature asphyxiation, although after adjustment for donor/recipient variables, transplant outcomes were similar. A case–control matched analysis confirmed transplant outcomes for those who received kidneys from donors dying after ligature asphyxiation were similar to controls. Although caution is required in interpreting these findings because of potential selection bias, kidneys from donors dying of ligature asphyxiation suffer an additional warm ischemic insult that does not apparently adversely influence transplant outcomes, even for kidneys from DCD donors.
Keywords: clinical research/practice, donors and donation, donors and donation: deceased, ischemia reperfusion injury (IRI), kidney (allograft) function/dysfunction, kidney transplantation/nephrology, organ procurement and allocation
Short abstract
This retrospective UK registry analysis identifies all kidney donors who died following ligature asphyxiation and their transplant recipients, and demonstrates that kidney transplant outcomes are not negatively impacted by this donor mode of death.
Abbreviations
- CIT
cold ischemic time
- DBD
donation after brain death
- DCD
donation after circulatory death
- DGF
delayed graft function
- eGFR
estimated glomerular filtration rate
- HLA
human leukocyte antigen
- IQR
interquartile range
- NHSBT
National Health Service Blood and Transplant
- PNF
primary nonfunction
- UK
United Kingdom
- UKTR
UK Transplant Registry
- US
United States
1. INTRODUCTION
Kidneys from deceased donors who have undergone ligature asphyxiation are often used for transplantation, although there is little information on whether this mode of death influences transplant outcomes. Ligature asphyxiation is usually the result of attempted suicide by hanging and is one of the most common methods of suicide. Moreover, suicide remains a common cause of death worldwide, especially in the younger population.1, 2, 3 In situations where attempted resuscitation and hospitalization have occurred following ligature asphyxiation, individuals may become potential brain death (DBD) or circulatory death (DCD) organ donors. However, ligature asphyxiation in these circumstances is associated with a period of global tissue hypoxia, often of an unknown duration, which may cause warm ischemic injury of the transplantable organs.4, 5, 6 During ligature asphyxiation there is compression of the carotid arteries, the jugular veins, and the trachea, resulting in raised intracranial pressure, cerebral edema, and catastrophic brain injury.7 In addition to the above, the victims of ligature asphyxiation may also develop pulmonary edema and multiorgan failure secondary to global tissue hypoxia.7 While hypoxic tissue injury following ligature asphyxiation is a concern in DBD donors, it may have an even greater impact on organs from DCD donors where the organs are also subjected to a second period of warm ischemic injury between cardiac arrest and cold perfusion of the organs.8 However, many potential donors who die following ligature asphyxiation are relatively young and previously healthy, and therefore might be a source of good quality kidneys that can be used safely for transplantation.3
However, the evidence on which to base decisions regarding the use of organs from deceased donors following ligature asphyxiation is limited and comprises case reports and single‐center experiences.4, 5, 6 Moreover, the published experience relates almost exclusively to DBD donors, with little or no published evidence for DCD donors who are becoming an increasingly important source of organs for transplantation.4, 5, 6
To improve the evidence base and aid decision making on the use of organs from donors who die following ligature asphyxiation, we undertook a retrospective national (the United Kingdom [UK]) cohort study of all kidney transplants performed using organs from DBD and DCD donors.
2. METHODS
2.1. Identification of deceased donors who died secondary to ligature asphyxiation
The UK Transplant Registry (UKTR) was examined to identify deceased organ donors in the UK between January 1, 2003 and December 31, 2016 who died secondary to ligature asphyxiation. Death from ligature asphyxiation (including suicide by hanging and strangulation) is not currently one of the 65 designated causes of death in the UKTR and so to identify organ donors who may have died from ligature asphyxiation, the free text entries for all deceased organ donors were searched using the search terms “strangled”, “strangulation”, “hanging”, “ligature”, “suicide”, “hung”, “noose”, “asphyxiation”, and any abbreviations or common misspellings of these terms. The free text entries of the donors identified were then manually reviewed. The free text entries of all organ donors whose cause of death was stated as “other trauma‐suicide” and “other trauma‐unknown causes” were also manually reviewed to identify a further cohort of donors who died secondary to ligature asphyxiation. Information on whether a donor had a previous history of intravenous drug use (IVDU) or imprisonment was collected as described previously.9
For the purposes of this study, “potential donors” were defined as deceased donors for whom consent/authorization for organ donation had been obtained, “proceeding organ donors” as deceased donors who had one or more solid organs removed for transplantation on the basis that recipient centers had provisionally agreed to use them for transplantation, and “utilised organ donors” as proceeding organ donors whose organs where eventually transplanted. Only Maastricht category, three DCD donors and all DBD donors were included in the analysis.8
2.2. Identification of recipients who received organs from donors who died from ligature asphyxiation
The UKTR was examined to identify the recipients of kidneys (both single and dual kidney transplant recipients) from donors who died secondary to ligature asphyxiation in the UK between January 1, 2003 and December 31, 2016 and information on death censored graft survival and patient survival was collected. In recipients of renal allografts, one year eGFR was calculated.10
All cause graft failure was taken as time from transplantation to graft nephrectomy or return to permanent dialysis, whichever was earlier, or to death of the patient with a functioning graft. Survival of the patient was defined as time from transplantation until death. We defined PNF as failure of a graft to ever function. DGF was defined as the need for dialysis within the first 7 days after transplantation (excluding recipients with PNF). Graft survival was censored at 5 years. Warm ischemic time was defined as the time from circulatory arrest to cold perfusion of the kidneys. Downtime was defined as either time from discovery of cardiac arrest until return of circulation following resuscitation or when the free text entries in the registry referred to the time as downtime.
2.3. Statistical analysis
Univariate analysis was carried out using the Student's t‐test for parametric continuous data and the Mann–Whitney U test for nonparametric continuous data. Comparisons between groups were made using the χ2 test for categorical data. Kaplan–Meier tables were used to compare death‐censored graft survival and patient survival. The univariate logrank test was used to test differences in survival.
Cox proportional hazards regression model was fitted with factors known to have impact on patient and graft survival. Patient and graft survival were censored at 1 year to determine factors associated with 1‐year survival and at 5 years to determine the factors associated with 5‐year survival. This was performed as a large proportion of donors who died following ligature asphyxiation had kidneys used for transplantation in the last 3 years. Patients without graft or patient follow‐up (n = 79 [0.4%]) were not included in the analysis. Log cumulative hazard plots were drawn and proportionality of hazards were checked using log–log plots.
Multivariable linear regression modeling was carried out to assess the impact that donor cause of death from ligature asphyxiation had on 1 year eGFR and creatinine. Logistic regression analysis was performed to assess the impact of donor cause of death by ligature asphyxiation on potential donors proceeding to kidney donation and the impact of this cause of death on DGF and PNF rates.
Multiple imputations were used to account for missing donor and recipient variables. There were no missing data for donor type, ethnicity, and whether cause of death was by ligature asphyxiation. Missing information about past medical history of hypertension and/or diabetes was 7.1%. For past medical history of cardiac disease and smoking there were 0.98% and 2.3% missing data, respectively. In terms of recipient characteristics, there were <1% missing data for recipient gender, HLA mismatch level, ethnicity, and recipient sex, <2% for CIT and 37% for warm ischemic time in DCD donors. Missing data were assumed to be missing at random and the missing variables had an arbitrary missing pattern. The imputed variables were all independent variables. Missing data were estimated by a discriminant function approach for categorical variables, a logistic regression approach for ordinal variables and linear models for cumulative variables. The FCS method was used to impute missing values of both continuous and class variables in the dataset with an arbitrary missing pattern. For each analysis requiring multiple imputations, 20 imputed datasets were created.
Donor‐related variables considered for inclusion in the multivariable models were donor age, donor type, ethnic group, sex, cause of death, past medical history of diabetes, hypertension, and cardiac disease, previous drug abuse and smoking history, and blood group. Recipient factors included were recipient age, ethnicity, sex, sensitization, primary renal disease (five categories), blood group (O, A, B, AB), HLA mismatch, and CIT. Other factors considered for inclusion were renal transplant unit, which was included as a random effect, and year of transplant (as an ordinal variable).
An addition to the above multivariable analyses, a case‐control propensity score matched analysis was also performed to examine transplant outcomes in recipients of kidneys from donors who died following ligature asphyxiation. Propensity scores were calculated using logistic regression on the probability of a recipient receiving a kidney from a donor who died following ligature asphyxiation. The scores were then used to match recipients of kidneys from donors who died following ligature asphyxiation to recipients of kidneys from all other deceased donors with similar propensity scores. This was accomplished with 1:1 matching. The following covariates were included in the estimation of propensity scores since they have been shown in previous analyses of the UK dataset to impact on transplant outcomes: donor age, recipient age, donor past medical history of hypertension, primary renal disease, HLA mismatch grade, cold ischemic time (CIT), donor weight, donor type (DBD and DCD), and transplant year.11, 12
All analyses were performed using Statistical Analysis System (SAS) (version 9.3; SAS Institute Inc., Cary, NC) and P < .05 were deemed to be statistically significant.13
3. RESULTS
3.1. Potential and proceeding kidney donors who died secondary to ligature asphyxiation
Over the 14‐year study period (January 1, 2003 to December 31, 2016), 2.7% (n = 521) of all the potential UK organ donors died secondary to ligature asphyxiation. Nearly all (98.7%) were a result of attempted suicide, but a small proportion (1.3%) was accidental. From this pool of potential donors, 409 (78.5%) subsequently proceeded to donate one or more kidneys for transplantation. By comparison, only 69.9% of potential donors who died from all causes other than ligature asphyxiation proceeded to donate kidneys for transplantation (P < .001). Of the potential donors who died from ligature asphyxiation and proceeded to kidney donation, 192 (46.9%) were DBD donors and 217 (53.1%) were controlled DCD donors.8
The proportion of potential DBD donors who died after ligature asphyxiation and proceeded to donate kidneys was similar to that for all other types of potential DBD donors (91.4% vs. 87.6%, respectively, P = .092). Compared to potential DBD donors, a lower proportion of all potential DCD donors proceeded to kidney donation, irrespective of whether the cause of death was from ligature asphyxiation (50.1% vs. 87.7%, P < .001). However, more potential DCD donors proceeded to donate organs after ligature asphyxiation than after causes of death other than ligature asphyxiation (69.8% vs. 49.4%, P < .001).
Relatively little information was available in the transplant registry regarding the physiological events occurring around the time of ligature asphyxiation. A total of 203 (39%) potential donors who died following ligature asphyxiation were reported to have had a cardiac arrest at the time of ligature asphyxiation and had a recorded “downtime” (ie, the length of time following cardiac arrest until return of circulation at the time of resuscitation). Of these, 73.8% proceeded to donate kidneys for transplantation compared to 80.7% of potential donors with no stated downtime (P = .125). The median recorded downtime was 25 minutes (interquartile range [IQR] 15‐40 minutes). Of donors who died from ligature asphyxiation, DCD donors had significantly shorter recorded downtimes compared to DBD donors (median 22 minutes [IQR 15‐34.5 minutes] vs. median 33 minutes [IQR 19‐45.5 minutes)], P = .0151).
3.2. Factors associated with potential deceased donors proceeding to donate kidneys for transplantation
A multivariable analysis was undertaken on all potential donors (n = 19 310) to determine whether death from ligature asphyxiation was independently associated with a potential donor proceeding to donate one or more kidneys for transplantation. As shown in Table 1, the following donor factors were associated with proceeding to donate kidneys: donor age; DBD donor type; no past medical history of diabetes, hypertension or cardiac disease; and white ethnicity. Following adjustment for the above donor variables, ligature asphyxiation in potential donors remained strongly associated with an increased likelihood of kidney donation for transplantation (odds ratio 1.211 [95% confidence interval 1.080‐1.357], P < .001). A further multivariable analysis was performed to assess what factors influenced donors who died following ligature asphyxiation to proceed to kidney donation. Table 2 demonstrates that younger donor age; DBD donor type; no history of smoking or liver disease; no history of intravenous drug use; and not having been imprisoned were all independently associated with a donor who died following ligature asphyxiation proceeding to donate a kidney for transplantation.
Table 1.
Factors associated with potential deceased donors proceeding to donate 1 or more kidneys for transplantation. 19 310 potential deceased donors were analyzed by logistic regression
| Donor characteristics (n = 19 310) | Odds ratio (95% confidence interval) | P value |
|---|---|---|
| Donor age | 0.983 (0.981‐0.986) | <.001 |
| Donor ethnicity | ||
| White | 1.00 | — |
| Non‐white | 0.794 (0.731‐0.863) | <.001 |
| Donor type | ||
| DCD | 1.00 | — |
| DBD | 2.536 (2.444‐2.631) | <.001 |
| Past medical history of diabetes | ||
| No | 1.00 | — |
| Yes | 0.753 (0.707‐0.803) | <.001 |
| Past medical history of hypertension | ||
| No | 1.00 | — |
| Yes | 0.902 (0.863‐0.942) | <.001 |
| Past medical history of cardiac disease | ||
| No | 1.00 | — |
| Yes | 0.863 (0.819‐0.908) | <.001 |
| Past medical history of smoking | ||
| No | 1.00 | — |
| Yes | 0.970 (0.933‐1.01) | .115 |
| Donor cause of death | ||
| No ligature asphyxiation | 1.00 | — |
| Ligature asphyxiation | 1.211 (1.080‐1.357) | .001 |
DBD, donation after brain death; DCD, donation after circulatory death.
Table 2.
Factors associated with potential donors who died following ligature asphyxiation proceeding to donate 1 or more kidneys for transplantation
| Donor characteristics (n = 521) | Odds ratio (95% confidence interval) | P value |
|---|---|---|
| Donor age (y) | 0.975 (0.965‐0.985) | <.001 |
| Donor ethnicity | ||
| White | 1.00 | — |
| Non‐white | 1.259 (0.829‐1.911) | .279 |
| Donor type | ||
| DCD | 1.00 | — |
| DBD | 1.973 (1.664‐2.340) | <.001 |
| Past medical history of diabetes | ||
| No | 1.00 | — |
| Yes | 0.951 (0.630‐1.436) | .811 |
| Past medical history of hypertension | ||
| No | 1.00 | — |
| Yes | 1.223 (0.850‐1.758) | .278 |
| Past medical history of cardiac disease | ||
| No | 1.00 | — |
| Yes | 0.901 (0.514‐1.580) | .716 |
| Past medical history of smoking | ||
| No | 1.00 | — |
| Yes | 0.829 (0.706‐0.973) | .022 |
| History of intravenous drug use | ||
| No | 1.00 | — |
| Yes | 0.543 (0.412‐0.715) | <.001 |
| History of imprisonment | ||
| No | 1.00 | |
| Yes | 1.438 (1.055‐1.961) | .022 |
| History of liver disease | ||
| No | 1.00 | |
| Yes | 0.479 (0.324‐0.709) | <.001 |
| Downtime (min) | 1.005 (0.994‐1.016) | .387 |
| Predonation creatinine (umol/L) | 1.000 (0.999‐1.002) | .911 |
3.3. Clinical characteristics of proceeding kidney donors (DBD and DCD) who died from ligature asphyxiation
The clinical characteristics of proceeding kidney donors who died after ligature asphyxiation and those who died from all other causes are shown in Table 3; the data are shown separately for DBD and DCD. Both DBD and DCD kidney donors who died from ligature asphyxiation were significantly younger and a greater proportion were male than those DBD and DCD donors who died from other causes.
Table 3.
Clinical characteristics of proceeding kidney donors who died from ligature asphyxiation compared to all other deceased donors
| DBD donors who died following ligature asphyxiation (n = 192) | DBD donors who died from all other causes (n = 8846) | P value | DCD donors who died following ligature asphyxiation (n = 217) | DCD donors who died from all other causes (n = 4291) | P value | |
|---|---|---|---|---|---|---|
| Age (y) | 32 (22‐43) | 49 (37‐59) | <.001 | 34 (24‐47) | 54 (43‐65) | <.001 |
| Male/female | 113 (58.9%)/79 (41.1%) | 4448 (50.3%)/4394 (49.7%) | .074 | 160 (73.7%)/57 (26.3%) | 2583 (60.2%)/1708 (39.8%) | <.001 |
| Unknown/unstated | 0 | 4 (<0.5%) | 0 | 0 | ||
| White ethnicity | 181 (94.3%) | 8404 (95.0%) | .658 | 208 (95.9%) | 4145 (96.6%) | .557 |
| History of hypertension | ||||||
| Yes/no | 5 (2.6%)/187 (97.4%) | 2172 (24.6%)/6480 (73.3%) | <.001 | 14 (6.5%)/197 (90.8%) | 1222 (28.5%)/2780 (64.8%) | <.001 |
| Unknown/unstated | 0‐ | 194 (2.2%) | 6 (2.8%) | 289 (6.7%) | ||
| History of cardiac disease | ||||||
| Yes/No | 1 (0.5%)/189 (98.4%) | 746 (8.4%)/7900(89.3%) | <.001 | 5 (2.3%)/199 (91.7%) | 598 (13.9%)/3359 (78.3%) | <.001 |
| Unknown/unstated | 2 (1.0%) | 200 (2.3%) | 13 (5.9%) | 334 (7.8%) | ||
| History of diabetes | ||||||
| Yes | 8 (4.2%)/183 (95.3%) | 480 (5.4%)/8272(93.5%) | .565 | 5 (2.3%)/201 (92.6%) | 325 (7.6%)/3696 (86.1%) | .007 |
| Unknown/unstated | 1 (0.5%) | 94 (1.1%) | 11 (4.9%) | 270 (6.3%) | ||
| Smoking history | ||||||
| Yes/no | 125 (65.1%)/65(33.9%) | 4231 (47.8%)/4522 (51.1%) | <.001 | 140 (64.5%)/74 (34.1%) | 1802 (42.0%)/2233 (52.1%) | <.001 |
| Unknown/unstated | 2 (1.0%) | 93 (1.1%) | 3 (1.4%) | 256 (6.0%) | ||
| Predonation serum creatinine (umol/L) | 121 (93‐162) | 84 (67‐107) | <.001 | 101 (80‐129) | 84 (65‐108) | <.001 |
| Missing | 0 | 9 (<0.5%) | 3 (1.4%) | 223 (5.2%) | ||
DBD, donation after brain stem death; DCD, donation after circulatory death; IQR, interquartile range.
Data shown as median and IQR.
Donors who died following ligature asphyxiation (both DBD and DCD donors) had a markedly lower incidence of hypertension and cardiac disease than donors who died from causes other than ligature asphyxiation. The proportion of kidney donors who died following ligature asphyxiation who had diabetes mellitus was numerically lower than that of donors who died from all other causes, but the difference was only significant in the case of DCD donors. More kidney donors (both DBD and DCD) who died following ligature asphyxiation had a history of smoking compared to all other deceased donors. DBD and DCD donors who died following ligature asphyxiation had significantly higher predonation serum creatinine levels.
3.4. Clinical characteristics of recipients of kidneys from donors who died following ligature asphyxiation
Donors who died following ligature asphyxiation provided kidneys for 650 kidney only transplants. The clinical characteristics of recipients of kidneys from DBD and DCD donors who died following ligature asphyxiation and those who died from all other causes are shown in Table 4. Recipients of kidneys from donors who died following ligature asphyxiation were significantly younger than those receiving kidneys from donors who died from other causes, but were of similar gender and ethnicity. There was no difference in the calculated reaction frequency (analogous to calculated panel reactivity) or human leukocyte antigen (HLA) mismatch between recipients of kidneys from deceased donors who died following ligature asphyxiation and those who died from all other causes. The primary renal disease in recipients of kidneys from DBD and DCD donors who died following ligature asphyxiation was broadly similar to that for recipients of kidneys from other DBD and DCD donors. The CITs of kidneys from DBD donors who died following ligature asphyxiation were significantly shorter than those for kidneys from all other DBD donors. CITs were similar for kidneys from DCD donors who died following ligature asphyxiation and all other DCD donors. Over the 14‐year study period there was a marked increase in the number of deceased donor kidney transplants performed in the UK, predominantly because of an increase in transplants using kidneys from DCD donors (Figure 1A). The number of kidney transplants performed from both DBD and DCD donors who died following ligature asphyxiation increased progressively over the study period (Figure 1B), such that half of the transplants using kidneys from such donors were performed in the last 4 of the 14‐year study period.
Table 4.
Clinical characteristics of renal transplant recipients from organ donors who died from ligature asphyxiation compared to those who died of all other causes
| DBD donors following ligature asphyxiation (n = 294) | All other DBD donors (n = 14 382) | P value | DCD donors following ligature asphyxiation (n = 356) | All other DCD donors (n = 6662) | P value | |
|---|---|---|---|---|---|---|
| Age (y) | 43 (29‐53) | 48 (37‐58) | <.001 | 47 (38‐57) | 55 (46‐63) | <.001 |
| Male/female (%) | 182 (61.9%)/112(38.1%) | 8774 (61.0%)/5599 (39.0%) | .879 | 211 (59.3%)/143(40.2%) | 4406 (66.2%)/2250 (33.8%) | .001 |
| White ethnicity (%) | 203 (69.1%) | 11006 (76.5%) | .003 | 284 (79.8%) | 5167 (77.6%) | .396 |
| Dual kidney transplant (%) | 0 (0) | 101(0.7%) | .352 | 3 (0.8%) | 318 (4.7%) | .01 |
| cRF > 85% | 44 (15.0%) | 1805 (12.6%) | .217 | 20 (5.6%) | 293 (4.4%) | .304 |
| HLA mismatch grade | .638 | .168 | ||||
| 1 | 58 (19.7%) | 2728 (19.0%) | 9 (2.5%) | 226 (3.4%) | ||
| 2 | 124 (42.2%) | 5841 (40.6%) | 96 (26.7%) | 1581 (23.7%) | ||
| 3 | 106 (36.1%) | 5327 (37.1%) | 216 (59.8%) | 3889 (58.4%) | ||
| 4 | 6 (2.0%) | 481 (3.3%) | 40 (11.0%) | 965 (14.5%) | ||
| Cold ischaemic time | <.001 | .248 | ||||
| <12 h | 78 (27.1%) | 2662 (18.5%) | 110 (31.3%) | 1977 (30.0%) | ||
| 12‐18 h | 129 (44.8%) | 6820 (47.5%) | 162 (46.4%) | 2973 (45.1%) | ||
| 18‐24 h | 65 (22.6%) | 3354 (23.3%) | 68 (19.5%) | 1320 (20.0%) | ||
| >24 h | 16 (5.6%) | 1395 (9.7%) | 9 (2.6%) | 322 (4.9%) | ||
| Warm ischaemic time (min) | ‐ | ‐ | 7 (6‐10) | 8 (6‐10) | .796 | |
| Primary renal disease | .04 | .192 | ||||
| Diabetic nephropathy | 19 (6.4%) | 1004 (7.0%) | 26 (7.5%) | 637 (9.5%) | ||
| Glomerulonephritis | 38 (12.8%) | 2497 (17.4%) | 73 (20.8%) | 1256 (18.8%) | ||
| Pyelonephritis | 19 (6.4%) | 1218 (8.5%) | 24 (6.7%) | 462 (7.0%) | ||
| Polycystic kidney disease | 25 (8.5%) | 1515 (10.5%) | 42 (11.6%) | 1011 (15.2%) | ||
| Other | 193 (65.7%) | 8147 (56.6%) | 191 (53.5%) | 3295 (49.5%) |
cRF, calculated reaction frequency; DBD, donation after brain death; DCD, donation after circulatory death; HLA, Human Leucocyte Antigen.
HLA mismatch level (levels 1‐4) was defined according to the UK allocation policy for kidneys from brain‐death donors and was based on the mismatch between donor and recipient.14
aMissing data were <1% gender and HLA Mismacth level ethnicity and recipient sex and <2% for cold ischaemic time, 37% for warm ischaemic time.
Figure 1.
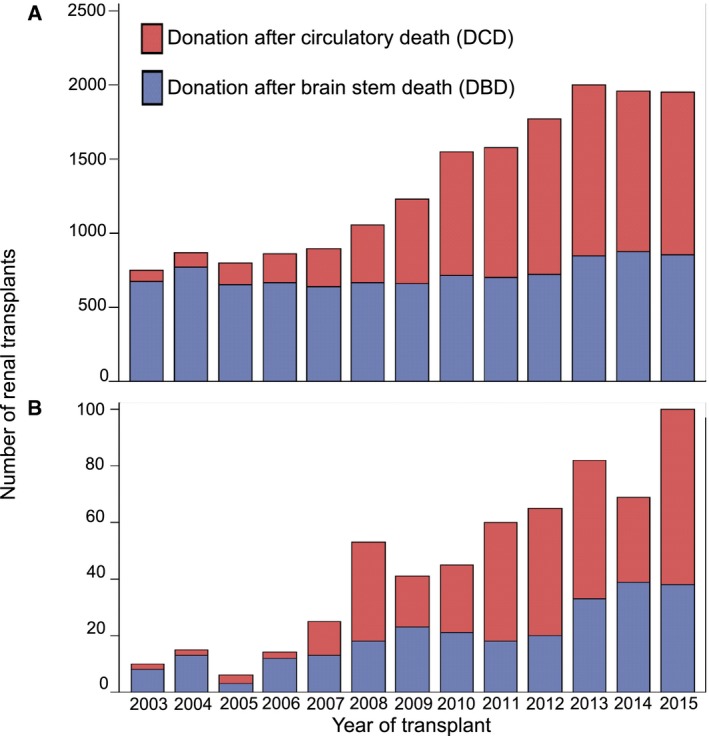
Number of renal transplants carried out from 2003‐2015 from (A) all deceased donors by donor type (DBD and DCD) and (B) donors who died following ligature asphyxiation and donor type (DBD and DCD). DBD, donation after brain death; DCD, donation after circulatory death
3.5. Outcomes in recipients of kidneys from donors who died following ligature asphyxiation
The results of survival analyses of patient and graft survival are shown in Figures 1, 2, 3, 4. For these and the multivariable analyses the median follow‐up of kidney transplant recipients was 48 months (IQR 24‐96 months). For kidney transplant recipients transplanted in 2016, the median follow‐up was 96 days (IQR 88‐356 days).
Figure 2.
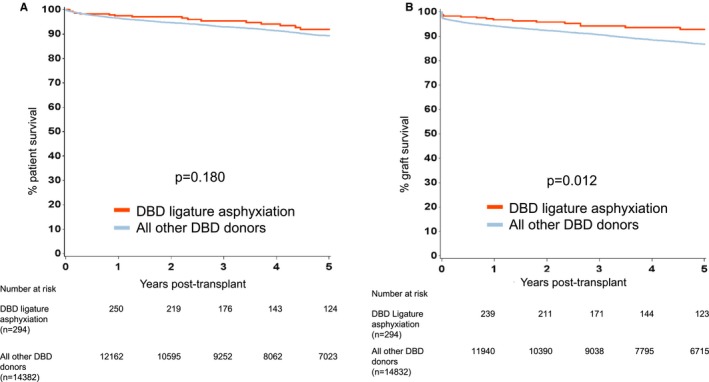
Kaplan‐Meier estimates of (A) patient survival from renal transplantation from DBD donors who died following ligature asphyxiation and all other DBD donors, and (B) death censored graft survival from renal transplantation from DBD donors who died following ligature asphyxiation and all other DBD donors
Figure 3.
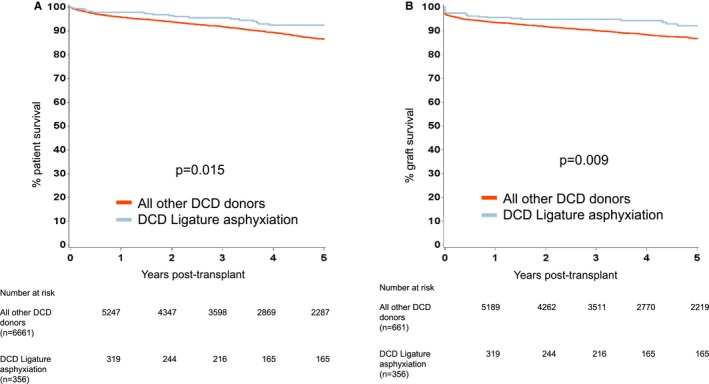
Kaplan‐Meier estimates of (A) patient survival from renal transplantation from DCD donors who died following ligature asphyxiation and all other DCD donors, and (B) death censored graft survival from renal transplantation from DCD donors who died following ligature asphyxiation and all other DCD donors. DBD, donation after brain death; DCD, donation after circulatory death
Figure 4.
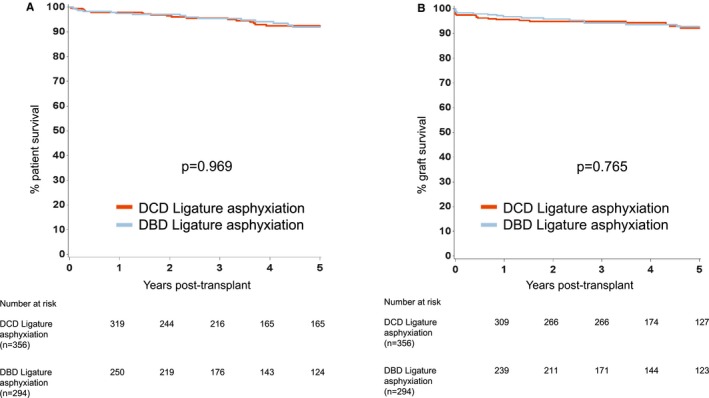
Kaplan‐Meier estimates of (A) patient survival from renal transplantation from DCD and DBD donors who died following ligature asphyxiation, and (B) death censored graft survival from renal transplantation from DCD and DBD donors who died following ligature asphyxiation. DBD, donation after brain death; DCD, donation after circulatory death
For transplant outcomes when comparing recipients of kidneys from donors who died following ligature asphyxiation with those who did not, we chose to analyze recipients of kidneys from DBD and DCD donors separately. For recipients of kidneys from DBD donors, patient survival was superior for those who received kidneys from donors who died following ligature asphyxiation, but there was no difference in graft survival (Figure 2). For recipients of kidneys from DCD donors, both patient and graft survival were better for those who received kidneys from donors who died following ligature asphyxiation (Figure 3). A comparison of recipients of kidneys from DBD and DCD donors who died following ligature asphyxiation showed similar patient and graft survival (Figure 4). Finally, a comparison of recipients of kidneys from all kidney donors (DBD and DCD) who died following ligature asphyxiation and those who received kidneys from all other deceased kidney donors demonstrated better patient and graft survival for those who received kidneys from donors who died following ligature asphyxiation (Figure 5).
Figure 5.
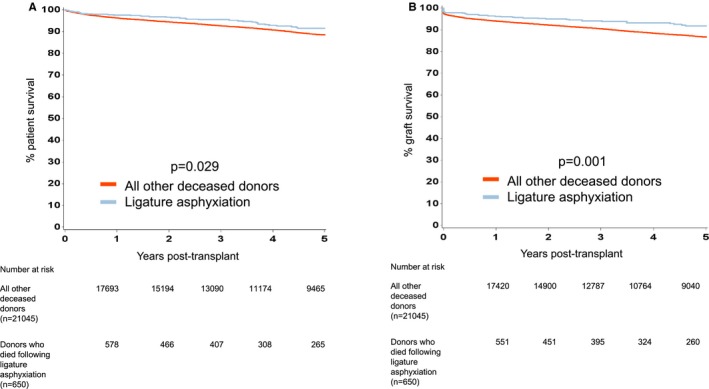
Kaplan‐Meier estimates of (A) patient survival from renal transplantation from donors who died following ligature asphyxiation (both DBD and DCD) and all other deceased donors, and (B) death censored graft survival from renal transplantation from donors who died following ligature asphyxiation (both DBD and DCD) and all other deceased donors. DBD, donation after brain death; DCD, donation after circulatory death
As already shown, significant differences were identified in donor and recipient demographics between recipients of kidneys from donors who died following ligature asphyxiation and all other deceased kidney donors. The factors considered in the analyses included donor and recipient age, CIT, donor type (DBD or DCD), HLA mismatch level, recipient primary renal disease, and donor comorbid diseases.
The results for the multivariable analyses (both unadjusted and adjusted) are shown in Table 5. Numerically, 1‐year and 5‐year patient and graft survival were superior when the donor's cause of death was by ligature asphyxiation than by other causes, both before and after confounder adjustment (Table 5). Delayed graft function (DGF) and primary nonfunction (PNF) rates were comparable between recipients of kidneys from donors who died of ligature asphyxiation and those who received kidneys from all other deceased donors after adjustment for donor and recipient characteristics.
Table 5.
Comparison of kidney transplant outcomes by donor type (DBD and DCD) and donor death secondary to ligature asphyxiation
| DBD donors following ligature asphyxiation (n = 294) | All other DBD donors (n = 14 382) | DCD donors following ligature asphyxiation (n = 356) | All other DCD donors (n = 6661) | Unadjusted ratio (95% CI) ligature asphyxiation vs. nonligature asphyxiation | Adjusted ratio (95% CI) ligature asphyxiation vs. nonligature asphyxiation | Adjusted P value | |
|---|---|---|---|---|---|---|---|
| Primary‐nonfunction | 1/262 (0.4%) | 275 /12741 (2.2%) | 8/320 (2.5%) | 157/5899 (2.7%) | OR 0.694 (0.357‐1.351) | OR 0.989 (0.704‐1.388) | .948 |
| Delayed graft function | 40/262 (15.2%) | 2778/12741 (21.8%) | 116/320 (36.3%) | 2377/5899 (40.3%) | OR 0.946 (0.783‐1.142) | OR 1.050 (0.952‐1.160) | .325 |
| 1‐y death censored graft survival | 96.8% | 94.3% | 95.6% | 93.6% | HR 0.638 (0.426‐0.955) | HR 0.851 (0.565‐1.283) | .442 |
| 5‐y death censored graft survival | 92.8% | 86.8% | 92.2% | 86.8% | HR 0.557 (0.403‐0.771) | HR 0.775 (0.564‐1.06) | .115 |
| 1‐y patient survival from transplantation | 97.5% | 96.5% | 97.9% | 95.8% | HR 0.613 (0.361‐1.041) | HR 0.984 (0.585‐1.655) | .951 |
| 5‐y patient survival from transplantation | 91.9% | 89.3% | 92.4% | 86.6% | HR 0.667 (0.478‐0.932) | HR 1.05 (0.757‐1.456) | .772 |
| 1‐y first kidney death censored graft survival (n = 18 059) | 97.8% | 96.5% | 98.0% | 95.7% | HR 0.619 (0.388‐0.987) | HR 0.814 (0.506 1.307) | .269 |
| 5‐y first kidney death censored graft survival (n = 18 059) | 95.2% | 88.4% | 93.5% | 87.3% | HR 0.506 (0.343‐0.745) | HR 0.705 (0483‐1.03) | .07 |
| 12‐mo eGFR (n = 17 846) | 61.4 (46.7‐ 76.6) (n = 238) | 50.4 (38.3‐ 64.8) (n = 11953) | 57.9 (46.2‐ 68.7) (n = 299) | 46.6 (34.9‐59.6) (n = 5350) | PE 9.525 (7.482‐11.567) | PE 0.686 (−1.103‐ 2.476) | .452 |
CI, confidence interval; DBD, donation after brain death; DCD, donation after circulatory death; HR, hazard ratio; OR, odds ratio; PE, parameter estimate.
Of the 21 682 deceased donor kidney transplants performed, 18 059 (83.4%) were first‐time kidney transplant recipients. In a sensitivity analysis of first kidney–only transplants, patient and graft survival were similar for those who received their first kidney transplant from donors who died following ligature asphyxiation and for those who received kidneys from all other deceased donors.
Of the 21 682 deceased donor kidney‐only transplant recipients, 18 258 (82.3%) had 12‐month posttransplant serum creatinine recorded and, of these, data were available to calculate eGFR for 18 216. Twelve‐month eGFR were significantly higher for those who received kidneys from donors who died from ligature asphyxiation (both DCD and DBD) (Figure 6).
Figure 6.
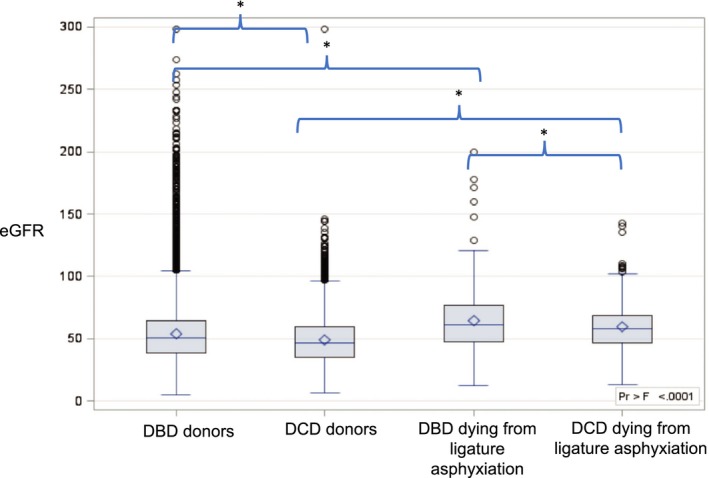
Twelve‐month eGFR by donor type and whether the donor died secondary to ligature asphyxiation. DBD, donation after brain death; DCD, donation after circulatory death; eGFR, estimated glomerular filtration rate
To examine the impact that donor death by ligature asphyxiation had on 12‐month posttransplant eGFR, a multivariable linear regression model was fitted. Following adjustment for donor and recipient factors, death by ligature asphyxiation was not an independent predictor of 12‐month eGFR (P = .452).
To assess whether the additional warm ischemic insult from ligature asphyxiation in DCD donors impacted on transplant outcomes, a separate multivariable analysis of such donors was performed. This revealed that even after adjusting for warm ischemic time in DCD donors there was no difference between transplant outcomes for recipients of kidneys from DCD donors who died following ligature asphyxiation and all other DCD donors (Table S1).
To reduce the impact of potential bias from confounding variables, a case‐control propensity score matched analysis was also performed. Recipients of kidneys from donors who died following ligature asphyxiation (n = 622) were matched to controls based on propensity scores generated using selected donor and recipient variables (see Methods). This analysis showed all transplant outcomes of recipients of kidneys from donors who died following ligature asphyxiation were similar to those in the matched control group (Table 6). An additional case–control propensity score matched analysis was performed comparing outcomes in recipients of kidneys from DCD donors who died following ligature asphyxiation and controls who received DCD donor kidneys. This also confirmed that kidneys from donors who died following ligature asphyxiation had similar outcomes to matched controls (data not shown).
Table 6.
Transplant outcomes in a 1‐1 case‐control propensity score matched analysis of recipients of kidneys from donors who died following ligature asphyxiation and their matched controls. Propensity scores were estimated using the following donor and recipient variables: donor age, recipient age, donor past medical history of hypertension, primary renal disease, HLA mismatch grade, cold ischaemic time, donor weight, donor type (DBD and DCD), and transplant year
| Recipients of kidneys from donors dying after ligature asphyxiation (n = 622) median propensity score, 0.059 (0.031‐0.108) | Matched control recipients (n = 622) median propensity score, 0.060 (0.030‐ 0.105) | Propensity score matched ligature asphyxiation vs. nonligature asphyxiation | P value | |
|---|---|---|---|---|
| Primary‐nonfunction | 9/570 (1.6%) | 11 /580 (1.9%) | OR 1.083 (0.456‐2.569) | .897 |
| Delayed graft function | 152/570 (26.7%) | 132/580 (22.8%) | OR 0.810 (0.619‐1.060) | .687 |
| 1‐y death censored graft survival | 96.1% | 96.3% | HR 1.051 (0.593‐1.862) | .865 |
| 5‐y death censored graft survival | 91.3% | 89.2% | HR 0.805 (0.537‐1.208) | .295 |
| 1‐y patient survival from transplantation | 97.5% | 97.8% | HR 0.664 (0.347‐1.273) | .218 |
| 5‐y patient survival from transplantation | 90.7% | 92.5% | HR 0.985 (0.640‐1.515) | .945 |
| 1‐y first kidney, death censored graft survival (n = 1077) | 96.6% | 96.8% | HR 1.06 (0.544‐2.046) | .875 |
| 5‐y first kidney, death censored graft survival (n = 1077) | 92.9% | 90.9% | HR 0.800 (0.499‐1.282) | .353 |
| 12‐mo eGFR (n = 1104) | 61 (47‐74) (n = 535) | 59 (47‐74) (n = 557) | PE 1.575 (−3.392‐2.788) | .848 |
HR, hazard ratio; OR, odds ratio; PE, parameter estimate.
4. DISCUSSION
Organ donors who die following ligature asphyxiation represent a relatively small but important proportion of the overall deceased donor population (~3% in the present study). Most of these deaths result from attempted suicide by hanging and tragically the incidence of this continues to increase, predominantly among younger males where suicide is the second most common cause of death.1, 15 The mode of death following ligature asphyxiation results in global tissue hypoxia and the effect that this has on end organ function following kidney transplantation has never been fully assessed. The results of the present national cohort analysis clearly demonstrate that the outcomes for recipients of kidneys from both DBD and DCD donors who have died following ligature asphyxiation are comparable to those for recipients of kidneys from donors who have died from all other causes.
In the present analysis, approximately half of the donors who died following ligature asphyxiation were DBD donors. Recipients of kidneys from such donors had similar patient survival and significantly better graft survival to those of recipients of kidneys from all other DBD donors up to 5 years. Moreover, DGF and 12‐month eGFR were significantly better in recipients of kidneys from DBD donors who died following ligature asphyxiation. The superior outcomes seen in recipients of kidneys from DBD donors who died following ligature asphyxiation is likely attributable to the fact that such donors were younger and had less comorbid disease than all other DBD donors. Indeed, after case mix adjustment in a multivariable analysis, 12‐month eGFR outcomes were similar. The case‐control propensity score matched analysis also confirmed that transplant outcomes were comparable in recipients of kidneys from donors dying from ligature asphyxiation and their matched controls.
It is now widely accepted that while recipients of kidneys from DCD donors have increased rates of PNF and DGF, the long‐term clinical outcomes are comparable to those observed in recipients of kidneys from DBD donors.11, 16 As observed with DBD donors who died following ligature asphyxiation, recipients of kidneys from DCD donors who died following ligature asphyxiation had superior transplant outcomes compared to those seen in recipients of kidneys from all other DCD donors. The additional warm ischemic insult from ligature asphyxiation was not associated with an increase in either PNF or DGF. The additional analyses of DCD donors who died following ligature asphyxiation demonstrated that even after adjustment for warm ischemic time, kidneys from such donors were not associated with poorer transplant outcomes than recipients of kidneys from all other DCD donors. This conclusion was confirmed by a case–control propensity score matched analysis of recipients of kidneys from DCD donors who died following ligature asphyxiation and their matched controls.
There is little information in the literature concerning the outcome following transplantation with kidneys from either DBD or DCD donors who died following ligature asphyxiation. A major strength of the present registry analysis is that it provides the first comprehensive analysis of transplant outcome in recipients of kidneys from donors who died following ligature asphyxiation. The analysis included a relatively large national cohort of kidney donors who died following ligature asphyxiation with a large proportion of DCD donors.
As for all retrospective transplant registry analyses, some degree of caution is required in the interpretation of the results because residual confounding factors not included in the analysis may have influenced the findings, such as significant recipient comorbidity. In the present analysis, some degree of selection bias is likely to have occurred. For example, only kidneys from younger previously healthy donors who died following ligature asphyxiation may have been preferentially selected for procurement and transplantation, thereby limiting the general applicability of the present findings. If the selection criteria for use of kidneys from potential donors following ligature asphyxiation were to be made less stringent, it cannot be assumed that the clinical outcomes would be equally favorable. Interestingly, the present analysis showed that potential organ donors who died following ligature asphyxiation were more likely to donate one or more kidneys for transplantation than all other potential deceased donors, even after adjustment for key favorable donor factors including donor age. For those donors who died following ligature asphyxiation, who had a cardiac arrest and an estimated “downtime” before restoration of circulation, the data available suggested that this did not influence whether or not a potential donor proceeded to kidney donation. Donors who died following ligature asphyxiation were more likely to proceed to kidney donation if they had a history of imprisonment (7.4% of such donors had a history of imprisonment). This may be because donors who died by hanging while incarcerated had a shorter time to resuscitation but this is speculation.
Another potential weakness of the study is that donor cause of death from ligature asphyxiation was not one of the 65 reportable causes of death recorded in the transplant registry and so identification of such donors relied on manual review of the free text entries for all deceased organ donors using specific search term variables. It is unlikely that a significant number of donors who died following ligature asphyxiation were not identified, but it is possible that the numbers presented represent an underestimate of potential donors who died following ligature asphyxiation. The dataset used in the present study had very little missing data for most of the key variables. However, a further limitation of the analysis is that for some variables missing data may impact on the results and their interpretation. There were very few missing data on graft and patient survival (<0.5% overall and 0% for recipients of kidneys from donors who died from ligature asphyxiation). In the case of 12‐month eGFR, data were missing in 17.8% of the entire study cohort, but this was distributed equally between recipients who received kidneys from donors who died following ligature asphyxiation and those who did not, making bias less likely.
In conclusion, the findings from the present analysis show that use of kidneys from both DBD and DCD donors who died following ligature asphyxiation result in excellent transplant outcomes. In view of this, increasing consideration should be given to the use of kidneys from potential donors who die following ligature asphyxiation and whose kidneys are currently declined for transplantation. To inform the increased use of such kidneys, the concept of total global tissue hypoxia from initiation of ligature asphyxiation to cold perfusion of the kidney with preservation solution may be helpful. Global hypoxia begins shortly after hanging is initiated and extends until discovery and initiation of resuscitation: its duration is highly variable and in many cases unknown. In most patients in the present cohort, this was followed by a period of “downtime” extending from discovery of a patient with no cardiac output until cardiac output is successfully reestablished and the patient is transferred to a critical care unit. Currently, a minority of patients has recorded “downtimes” and there is a need for improved documentation. These two periods of global tissue hypoxia are, in the case of DCD donors, followed by a third period of tissue hypoxia from the time of withdrawal of life supporting treatment to cold perfusion of the kidneys, the duration of which is usually well documented. Although making an assessment of the total duration of global tissue hypoxia is often problematic, incorporating this concept into decision‐making on the use of kidneys from donors who die following ligature asphyxiation may provide a basis for the safe utilization of kidney from selected donors currently being declined for transplantation.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
ACKNOWLEDGMENTS
This research was supported by the National Institute for Health Research Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT), and the NIHR Cambridge Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or NHSBT.
Trotter PB, Jochmans I, Hulme W, et al. Transplantation of kidneys from DCD and DBD donors who died after ligature asphyxiation: The UK experience. Am J Transplant. 2018;18:2739–2751. 10.1111/ajt.14989
REFERENCES
- 1. Office for National Statistics . Suicides in the UK: 2015 registrations. 2016;1–23.
- 2. Ajdacic‐Gross V. Methods of suicide: international suicide patters derived from the WHO mortality database. Bull World Health Organ. 2008;86(9):726‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varnik A, Kolves K, van der Feltz‐Cornelis CM, et al. Suicide methods in Europe: a gender‐specific analysis of countries participating in the “European Alliance Against Depression”. J Epidemiol Community Health. 2008;62(6):545‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohite PN, Patil NP, Sabashnikov A, et al. Hanging donors: are we still sceptical about the lungs? Transpl Proc. 2015;47(2):261‐266. [DOI] [PubMed] [Google Scholar]
- 5. Hoti E, Levesque E, Sebagh M, et al. Liver transplantation with grafts from donors who die from suicide by hanging. Transplantation. 2014;98(11):1236‐1243. [DOI] [PubMed] [Google Scholar]
- 6. Yaprak M, Turan MN, Sezer T, et al. Use of suicidal deaths as kidney donors: a single‐center experience. Transpl Proc. 2013;45(3):872‐874. [DOI] [PubMed] [Google Scholar]
- 7. Taniguchi T, Saito S, Mizukoshi Y, Goto Y, Inaba H. Multiple organ failure after strangulation injury. Intensive Care Med. 2002;28(8):1193‐1203. [DOI] [PubMed] [Google Scholar]
- 8. Summers DM, Watson C, Pettigrew GJ, et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int. 2015;88(2):241‐249. [DOI] [PubMed] [Google Scholar]
- 9. Trotter PB, Summers DM, Robb M, et al. Deceased organ donors with a history of increased risk behavior for the transmission of blood‐borne viral infection: the UK experience. Transplantation. 2017;101(7):1679‐1689. [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247‐254. [DOI] [PubMed] [Google Scholar]
- 11. Summers DM, Johnson RJ, Allen J, et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet. 2010;376(9749):1303‐1311. [DOI] [PubMed] [Google Scholar]
- 12. Watson C, Johnson RJ, Birch R, Collett D, Bradley JA. A simplified donor risk index for predicting outcome after deceased donor kidney transplantation. Transplantation. 2012;93(3):314‐318. [DOI] [PubMed] [Google Scholar]
- 13. SAS Institute Inc . SAS 9.3 System Options Reference, 2nd edn; 2011. [Google Scholar]
- 14. NHS Blood and Transplant: Organ Donation and Transplantation . Kidney Transplantation: Deceased Donor Organ Allocation. 2008;1–11. https://www.odt.nhs.uk/pdf/kidney_allocation_policy.pdf. Accessed December 23, 2017.
- 15. Office for National Statistics . Deaths registered in England and Wales (Series DR):2015. 2015. https://www.ons.gov.uk/peoplepopulationandcommunity/birthdeathsandmarriages/deaths/bulletins/deathsregisteredinenglandandwalesseriesdr/2015
- 16. Summers DM, Johnson RJ, Hudson A, Collett D, Watson CJ, Bradley JA. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet. 2013;381(9868):727‐734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


