Abstract
This post hoc analysis of gastrointestinal (GI) adverse events (AEs) from the phase 3 LixiLan‐L (NCT02058160) and LixiLan‐O (NCT02058147) trials aimed to determine the frequency and timing of nausea, vomiting, and diarrhoea for iGlarLixi, a titratable, fixed‐ratio combination of insulin glargine 100 units/mL (iGlar) and lixisenatide, versus iGlar alone or iGlar and lixisenatide alone, in patients with type 2 diabetes uncontrolled with oral antidiabetes drugs (OADs) or basal insulin ± OADs. In iGlarLixi‐treated patients, the rate of GI AEs during the initial weeks of treatment was lower versus patients treated with lixisenatide alone (9.6% and 11.7% of iGlarLixi‐treated patients in LixiLan‐L and LixiLan‐O, respectively, vs. 27.5% of lixisenatide‐treated patients in LixiLan‐O). Beyond day 60, these rates were generally low and similar to those of lixisenatide. These lower rates are likely due to the gradual titration of lixisenatide in iGlarLixi. Median durations of intermittent GI AEs in the iGlarLixi arms were 6.0, 2.0 and 2.5 days (LixiLan‐L), and 5.0, 1.0 and 3.5 days (LixiLan‐O), respectively. iGlarLixi‐associated GI AEs were transient, mostly mild or moderate in severity, and occurred mainly during initial titration.
Keywords: fixed‐ratio combination, gastrointestinal adverse events, insulin glargine, lixisenatide
1. INTRODUCTION
For many patients with type 2 diabetes, treatment with basal insulin ± oral antidiabetes drugs (OADs) will eventually be insufficient to maintain glycated HbA1c targets, and additional treatment will be required to control residual hyperglycaemia.1
A recent guideline's recommended approach for intensifying basal insulin is the addition of glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs),1, 2 which act on both fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) by enhancing glucose‐dependent insulin secretion and decreasing glucagon secretion while slowing gastric emptying and increasing satiety.3 Shorter‐acting GLP‐1RAs show greater reductions in PPG versus longer‐acting agents.3
Coadministration of basal insulin and GLP‐1RAs results in equivalent or improved HbA1c levels, weight loss or no weight gain, and decreased or no increased hypoglycaemia risk, compared with basal insulin alone4, 5, 6, 7, 8, 9 or in combination with rapid‐acting insulins (basal‐plus and basal‐bolus).10, 11, 12 However, gastrointestinal (GI) adverse events (AEs) are common side effects of GLP‐1RAs.1, 2
iGlarLixi (SOLIQUA 100/33), a titratable, fixed‐ratio combination of insulin glargine 100 units/mL (iGlar) and GLP‐1RA lixisenatide 33 μg/mL, is indicated in the USA as a once‐daily injection for patients with type 2 diabetes inadequately controlled with basal insulin (<60 units/day) or lixisenatide.13 In the LixiLan phase 3 trials, iGlarLixi led to greater reductions in HbA1c than either iGlar or lixisenatide alone, with the benefit of weight loss or neutrality, and without increased risk of hypoglycaemia versus iGlar alone. iGlarLixi was associated with low rates of nausea, vomiting, and diarrhoea compared with lixisenatide alone, but with higher rates of these events compared with iGlar alone.5, 6
Discontinuation rates due to GI AEs associated with iGlarLixi were very low (<1.5%), and fewer patients receiving iGlarLixi in LixiLan‐O withdrew versus those receiving lixisenatide alone. No patients treated with iGlar discontinued treatment due to GI AEs in either trial.5, 6
GI AEs associated with iGlarLixi and lixisenatide appeared to subside over time in the LixiLan trials. This post hoc analysis aimed to assess the frequency and timing of GI AEs in these trials evaluating iGlarLixi in patients with type 2 diabetes.
2. MATERIALS AND METHODS
2.1. Study design
This was a post hoc analysis of data from two open‐label, randomized, parallel‐group, multinational, multicentre phase 3 clinical trials.5, 6
LixiLan‐L (NCT02058160) compared iGlarLixi (n = 367) with iGlar (n = 369) in patients showing suboptimal glycaemic control on basal insulin ± 0–2 OADs. All patients continued/switched to iGlar (any other insulin or OAD other than metformin was stopped), which was titrated over 6 weeks. Patients with FPG ≤140 mg/dL and HbA1c 7.0%–10.0% (53–86 mmol/mol) receiving an iGlar dose of 20‐50 units/day were then randomized to either iGlarLixi or iGlar for 30 weeks. The initial dose of iGlarLixi was determined based on the last iGlar dose received before randomization (20 units/10 μg or 30 units/10 μg for iGlar <30 or ≥30 units, respectively) and remained stable for 2 weeks; the initial dose of iGlar after randomization was the one before randomization. iGlarLixi and iGlar doses were titrated by 2–4 units once‐weekly to reach and maintain the FPG target of 80–100 mg/dL, based on a 3‐day average of fasting self‐measured plasma glucose levels, and capped at 60 units/day of iGlar in both arms.6
LixiLan‐O (NCT02058147) compared iGlarLixi (n = 469) with iGlar (n = 467) and lixisenatide (n = 234) in patients with type 2 diabetes not adequately controlled with metformin monotherapy or metformin plus a second OAD. Patients discontinued the second OAD during the run‐in phase, and metformin dose was optimized (1500 or 2000 mg) as tolerated. Patients with an HbA1c of 7.0%–10.0% (53–86 mmol/mol) and FPG ≤250 mg/dL were randomly assigned to receive iGlarLixi, iGlar or lixisenatide for 30 weeks. iGlarLixi and iGlar doses were titrated and capped as described for LixiLan‐L. iGlarLixi was administered within 60 min before breakfast, iGlar at any time of the day but at roughly the same time each day, and lixisenatide within 60 min before breakfast or dinner. Ten μg of lixisenatide was administered during the first 2 weeks, rising to 20 μg over 30 weeks.5
In both trials, the safety population included all randomized patients who received at least one dose of iGlarLixi, iGlar or lixisenatide.5, 6
2.2. Statistical analysis
The incidence, timing, and duration of selected GI AEs (nausea, vomiting and diarrhoea) arising from the start to the end of the trials (~210 days) were evaluated. Results were summarized by treatment group using descriptive statistics. P values were calculated from a two‐sample t‐test comparing patients who did and did not experience GI AEs.
The severity of the GI AEs was determined by the investigators. Mild events were generally transient, requiring only minimal treatment/intervention and not generally interfering with daily activities; moderate events could be alleviated with additional treatment/intervention and interfered with daily activities, but did not pose significant/permanent risk of harm; severe events required intensive treatment/intervention and interrupted common daily activities or affected clinical status, posing a significant risk of harm. For intermittent/periodic events, the highest intensity for the overall duration of the event was recorded; GI AEs were reported only once using the overall duration of the event as the time between the start and the stop date for the event.
3. RESULTS
3.1. Baseline patient characteristics
All patients in the safety population were included in the analysis: 736 patients from LixiLan‐L and 1170 patients from LixiLan‐O. No significant differences in age, HbA1c levels, body mass index or disease duration were seen between patients experiencing GI AEs versus those not reporting GI AEs in both trials (see the supporting information for this article, Table S1 in File S1).
3.2. Incidence of GI AEs over time
The number of patients with a first complaint of nausea, vomiting or diarrhoea peaked within the first 60 days (8 weeks) and subsequently decreased over time in the iGlarLixi and lixisenatide groups (Figure 1). In LixiLan‐L, the incidences of nausea and vomiting were low throughout the trial for iGlarLixi, and almost null for the iGlar arm (Figure 1A). Nausea and vomiting were marked during the initial weeks of treatment with lixisenatide in LixiLan‐O (Figure 1B).
Figure 1.
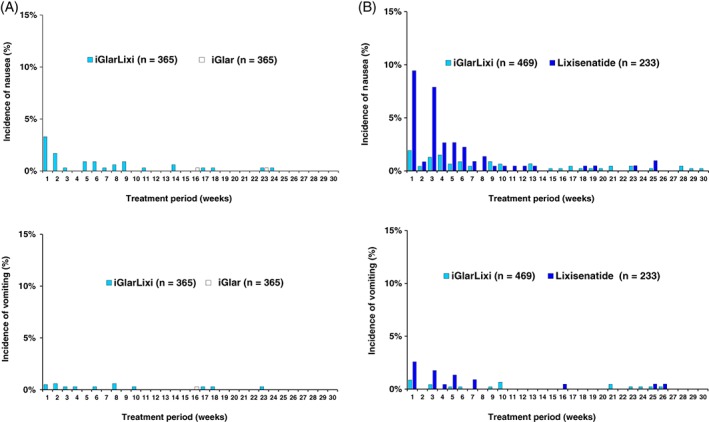
Incidence of nausea and vomiting per week with iGlarLixi A, versus iGlar in Lixilan‐L and B, versus lixisenatide in Lixilan‐O. Each subject could contribute with multiple events over time, but only the first event was counted each week
3.3. Severity of GI AEs
Most GI AEs in the LixiLan trials were mild to moderate in severity for all treatment groups (Figure 2). In the group of iGlarLixi‐treated patients with reported GI AEs (62/365 patients [17.0%] and 102/469 patients [21.7%] overall in LixiLan‐L and LixiLan‐O, respectively),5, 6 53%–78% of the events were classified as mild and 22%–44% as moderate (Figure 2), with only one reported severe event (1/47 [2.13%]) of nausea in LixiLan‐L (Figure 2A). In LixiLan‐O, however, there were five reported severe nausea events (5/77 [6.49%]) with lixisenatide.
Figure 2.
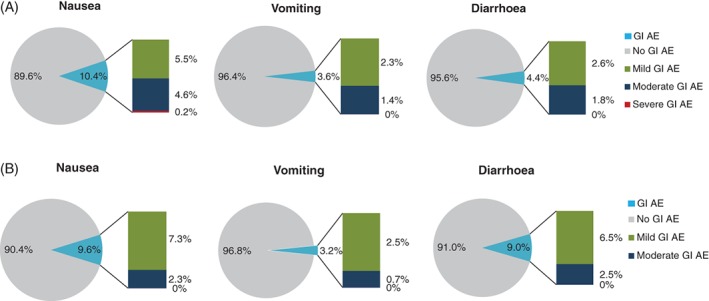
Severity of nausea, vomiting and diarrhoea with iGlarLixi in A, LixiLan‐L and B, LixiLan‐O. AE, adverse event; GI, gastrointestinal. The pie charts show the percentage of patients with/without a GI AE; the bars show the percentage of events (i.e. one patient can contribute with multiple events)
3.4. Duration of GI AEs
Median durations of nausea and vomiting were lower with iGlarLixi versus lixisenatide in LixiLan‐O, while the shortest durations of GI AEs were seen with iGlar treatment in both trials (Table S2 in File S1).
3.5. Daily doses of iGlar and lixisenatide
The mean daily doses of iGlar and lixisenatide administered as part of the combination, as well as iGlar administered individually, increased throughout the trials. There were no statistically significant differences in daily iGlar doses between patients experiencing GI AEs versus those not reporting them in the iGlarLixi and the iGlar arms in either of the trials (Table S3 in File S1).
4. DISCUSSION
GI AEs are expected side effects associated with the use of GLP‐1RAs and among the main reasons given by patients for discontinuing treatment. In this post hoc analysis of the LixiLan trials, patients with type 2 diabetes treated with iGlarLixi showed a higher rate of GI AEs compared with those treated with iGlar only, but lower compared with lixisenatide alone, which may be due to the gradual titration of lixisenatide in the combination arm. This gradual increase in the lixisenatide dose, paralleling the iGlar titration, may mitigate the risk of GI AEs seen when lixisenatide is administered separately as a fixed dose of 10 μg and increased after 2 weeks to the maintenance dose of 20 μg. Similar findings were obtained in the open‐label, phase 3 trial (DUAL‐I) comparing a combination of insulin degludec with liraglutide versus the individual components.14
Rates of GI AEs with iGlarLixi were also lower than those reported for other GLP‐1 agents administered alone, which can exceed 20% and are mainly related to nausea.15 The rates for GI AEs reported with the iGlarLixi combination in LixiLan‐L were lower than those observed in the lixisenatide plus basal insulin arm in the GetGoal‐L trial.7 However, the incidences of GI AEs in the iGlar arms in both LixiLan‐L and ‐O trials were consistent with those reported for the insulin arms in other clinical trials in insulin‐experienced7, 9 and ‐naive patients,8 suggesting that variability in patient populations across trials did not influence GI outcomes.
In this analysis, GI AEs were transient, predominantly arising during the initial 8 weeks of treatment; similarly, in DUAL‐I, nausea was most prevalent in the first 10 weeks of treatment.14 Beyond this period, the incidences of GI AEs with iGlarLixi and lixisenatide were similar and low.
Overall, the majority of GI AEs reported for all treatment groups in the current analysis were mild or moderate in severity in both trials, with only one reported severe nausea event with iGlarLixi. The median durations of nausea and vomiting were lower with iGlarLixi versus lixisenatide in LixiLan‐O.
Treatment with iGlarLixi has been shown to achieve significantly greater reductions in HbA1c levels than iGlar alone.5, 6 Although adherence to medication is a major determinant of glycaemic control in type 2 diabetes,16, 17 real‐world data show that approximately 35% of patients discontinue treatment with GLP‐1RAs after 6 months.18 Therefore, the improved glycaemic control and reduced frequency/severity of GI AEs with iGlarLixi compared with alternatives showing higher rates of GI AEs may improve treatment adherence and outcomes.
This study presents the usual limitations associated with post hoc analyses. Since this was an analysis of patients in highly controlled, randomized clinical trials, it is unclear whether the data are fully generalizable to the wider population with type 2 diabetes managed in routine care. The open‐label design of the LixiLan trials and the fact that the severity of the GI AEs was categorized at the investigator's discretion may have introduced bias in the evaluation of AEs. The individual components were compared separately, not concomitantly. The authors' analysis is descriptive only and does not allow for testing for inferiority/superiority of the combination versus the single agents. Additionally, non‐iGlarLixi/lixisenatide‐related events that could potentially cause GI AEs were not captured in this analysis. It must also be taken into consideration that the LixiLan studies evaluated different patient populations with distinct baseline characteristics.
In summary, patients with type 2 diabetes treated with iGlarLixi in the LixiLan trials showed low rates of GI AEs versus lixisenatide alone. GI AEs associated with iGlarLixi tended to be transient and mild/moderate in severity; the majority occurred early in the course of treatment, with most patients no longer experiencing them beyond 8 weeks. iGlarLixi thus offers an alternative to treatment progression for patients not at target after lifestyle changes and treatment with oral agents by adding a GLP‐1RA or basal insulin alone. Titration of iGlarLixi is similar to basal insulin titration and shows greater potential to achieve glycaemic targets with fewer GI AEs than either a GLP‐1RA alone or a GLP‐1RA plus basal insulin as a free combination.
Supporting information
File S1.
Table S1. Baseline characteristics for patients experiencing nausea, vomiting or diarrhea vs. patients not reporting nausea, vomiting or diarrhea in the Lixilan‐L and Lixilan‐O clinical trials.
Table S2. Descriptive data on duration of nausea, vomiting or diarrhea in the Lixilan‐L and Lixilan‐O clinical trials.
Table S3. Total daily doses of insulin glargine and lixisenatide for patients experiencing nausea, vomiting, or diarrhea vs patients not reporting nausea, vomiting, or diarrhea in the Lixilan‐L and Lixilan‐O clinical trials.
ACKNOWLEDGMENTS
The authors received writing/editorial support in the preparation of this manuscript provided by Patricia Fonseca, PhD, of Excerpta Medica, funded by Sanofi US, Inc. We thank Yan Yan of TechData Service Company, LLC for assisting with the statistical analysis.
Conflict of interest
J. M. T. reports past participation in advisory boards and being a consultant for Sanofi Inc. M. R. reports being an employee of Sanofi US, Inc. T. D. reports being an employee and stock/shareholder of Sanofi US, Inc. J. C. reports being an employee of Xinyi, Inc., which is under contract with Sanofi US, Inc. J. W. reports past participation in advisory boards for Novo Nordisk, Inc. and Sanofi Inc. J. L. reports past participation in advisory boards for Eli Lilly and Company, Novo Nordisk, Inc., and Sanofi Inc., and being a member of the speakers bureau for Novo Nordisk, Inc.
Author contributions
J. M. T., M. R., T. D., J. W. and J. L. co‐developed the concept of the study, interpreted the data, critically reviewed the manuscript drafts, and provided final approval of the version of the manuscript to be submitted. J. C. collected the data, performed the analyses and provided the data tables, interpreted the data, critically reviewed the manuscript drafts, and provided final approval of the version of the manuscript to be submitted. J. M. T. is the guarantor of this work and, as such, had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Trujillo JM, Roberts M, Dex T, Chao J, White J, LaSalle J. Low incidence of gastrointestinal adverse events over time with a fixed‐ratio combination of insulin glargine and lixisenatide versus lixisenatide alone. Diabetes Obes Metab. 2018;20:2690–2694. 10.1111/dom.13444
Funding information Sanofi US Inc. Grant/Award Number: Writing/editing assistance. The authors received writing/editorial support in the preparation of this manuscript provided by PatriciaFonseca,PhD, of Excerpta Medica, funded by Sanofi US, Inc.
Please click on this https://players.brightcove.net/656326989001/default_default/index.html?videoId=5824269862001 to hear more about this post hoc evaluation of gastrointestinal adverse events in the LixiLan trials.
REFERENCES
- 1. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2017 executive summary. Endocr Pract. 2017;23:207‐238. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Standards of medical care in diabetes – 2018. Diabetes Care. 2018;41(suppl 1):S1‐S159.29222369 [Google Scholar]
- 3. Fineman MS, Cirincione BB, Maggs D, Diamant M. GLP‐1 based therapies: differential effects on fasting and postprandial glucose. Diabetes Obes Metab. 2012;14:675‐688. [DOI] [PubMed] [Google Scholar]
- 4. Balena R, Hensley IE, Miller S, Barnett AH. Combination therapy with GLP‐1 receptor agonists and basal insulin: a systematic review of the literature. Diabetes Obes Metab. 2013;15:485‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled with oral agents: the LixiLan‐O randomized trial. Diabetes Care. 2016;39:2026‐2035. [DOI] [PubMed] [Google Scholar]
- 6. Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan‐L randomized trial. Diabetes Care. 2016;39:1972‐1980. [DOI] [PubMed] [Google Scholar]
- 7. Riddle MC, Aronson R, Home P, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin. A 24‐week, randomized, placebo‐controlled comparison (GetGoal‐L). Diabetes Care. 2013;36:2489‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riddle MC, Forst T, Aronson R, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24‐week, randomized, placebo‐controlled study (GetGoal‐Duo 1). Diabetes Care. 2013;36:2497‐2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice‐daily exenatide in basal insulin‐treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154:103‐112. [DOI] [PubMed] [Google Scholar]
- 10. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal‐plus or basal‐bolus in type 2 diabetes: the GetGoal Duo‐2 trial. Diabetes Care. 2016;39:1318‐1328. [DOI] [PubMed] [Google Scholar]
- 11. Mathieu C, Rodbard HW, Cariou B, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD‐ON). Diabetes Obes Metab. 2014;16:636‐644. [DOI] [PubMed] [Google Scholar]
- 12. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP‐1 receptor agonist, versus thrice‐daily prandial insulin lispro. Diabetes Care. 2014;37:2317‐2325. [DOI] [PubMed] [Google Scholar]
- 13.Soliqua 100/33. Prescribing Information. http://products.sanofi.us/soliqua100-33/soliqua100-33.pdf. Accessed July 10, 2017.
- 14. Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed‐ratio combination of insulin degludec and liraglutide (iDegLira) compared with its components given alone: results of a phase 3, open‐label, randomised, 26‐week, treat‐to‐target trial in insulin‐naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885‐893. [DOI] [PubMed] [Google Scholar]
- 15. Trujillo JM, Nuffer W, Ellis SL. GLP‐1 receptor agonists: a review of head‐to‐head clinical studies. Ther Adv Endocrinol Metab. 2015;6:19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buysman EK, Liu F, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32:341‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McAdam‐Marx C, Bellows BK, Unni S, et al. Determinants of glycaemic control in a practice setting: the role of weight loss and treatment adherence (The DELTA Study). Int J Clin Pract. 2014;68:1309‐1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu M, Xie J, Fernandez Lando L, Kabul S, Swindle RW. Liraglutide versus exenatide once weekly: persistence, adherence, and early discontinuation. Clin Ther. 2016;38:149‐160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1.
Table S1. Baseline characteristics for patients experiencing nausea, vomiting or diarrhea vs. patients not reporting nausea, vomiting or diarrhea in the Lixilan‐L and Lixilan‐O clinical trials.
Table S2. Descriptive data on duration of nausea, vomiting or diarrhea in the Lixilan‐L and Lixilan‐O clinical trials.
Table S3. Total daily doses of insulin glargine and lixisenatide for patients experiencing nausea, vomiting, or diarrhea vs patients not reporting nausea, vomiting, or diarrhea in the Lixilan‐L and Lixilan‐O clinical trials.


