Abstract
Objectives
The single‐tablet regimen rilpivirine, emtricitabine and tenofovir alafenamide (RPV/FTC/TAF) for treatment of HIV‐1‐infected adults was approved based on bioequivalence. We assessed the clinical efficacy, safety and tolerability of switching to RPV/FTC/TAF from either RPV/FTC/tenofovir disoproxil fumarate (TDF) or efavirenz (EFV)/FTC/TDF.
Methods
We conducted two distinct randomized, double‐blind, active‐controlled, noninferiority trials in participants taking RPV/FTC/TDF (Study 1216) and EFV/FTC/TDF (Study 1160). Each study randomized virologically suppressed (HIV‐1 RNA < 50 copies/mL) adults (1:1) to switch to RPV/FTC/TAF or continue their current regimen for 96 weeks. We evaluated efficacy as the proportion with HIV‐1 RNA < 50 copies/mL using the Food and Drug Administration snapshot algorithm and prespecified bone and renal endpoints at week 96.
Results
We randomized and treated 630 participants in Study 1216 (RPV/FTC/TAF, n = 316; RPV/FTC/TDF, n = 314) and 875 in Study 1160 (RPV/FTC/TAF, n = 438; EFV/FTC/TDF, n = 437). In both studies, the efficacy of switching to RPV/FTC/TAF was noninferior to that of continuing baseline therapy at week 96, with respective percentages of patients with HIV RNA < 50 copies/mL being 89.2% versus 88.5% in Study 1216 [difference 0.7%; 95% confidence interval (CI) −4.3 to +5.8%] and 85.2% versus 85.1% in Study 1160 (difference 0%; 95% CI −4.8 to +4.8%). No participant on RPV/FTC/TAF developed treatment‐emergent resistance versus two on EFV/FTC/TDF and one on RPV/FTC/TDF. Compared with continuing baseline therapy, significant improvements in bone mineral density and renal tubular markers were observed in the RPV/FTC/TAF groups (P < 0.001).
Conclusions
Switching to RPV/FTC/TAF from RPV/FTC/TDF or EFV/FTC/TDF was safe and effective and improved bone mineral density and renal biomarkers up to 96 weeks with no cases of treatment‐emergent resistance.
Keywords: bone mineral density, HIV, renal disease, rilpivirine, tenofovir alafenamide
Introduction
Regimens in which a nonnucleoside reverse transcriptase inhibitor (NNRTI) is combined with two nucleos(t)ide reverse transcriptase inhibitors (NRTIs) remain among the most commonly prescribed regimens for the treatment of HIV‐1 infection worldwide. The NNRTIs efavirenz (EFV) and rilpivirine (RPV) are each coformulated with the NRTIs emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF) as complete single‐tablet regimens (STRs): EFV/FTC/TDF and RPV/FTC/TDF, respectively. Where EFV/FTC/TDF was the first once‐daily single‐tablet regimen, the association of EFV with central nervous system (CNS)‐related side effects has curtailed its use as better tolerated options, including RPV, have become available 1, 2, 3. Subsequently, it has been found that regimens containing tenofovir alafenamide (TAF) have improved bone and renal safety compared with TDF‐containing regimens, while maintaining excellent efficacy 4, 5.
Rilpivirine/FTC/TAF is a preferred or alternative recommended treatment regimen for initial antiretroviral therapy in European and US treatment guidelines for patients with a CD4 cell count > 200 cells/μL and HIV RNA < 100 000 HIV‐1 RNA copies/mL 6, 7. Whether to switch from an effective regimen remains of clinical interest.
We investigated the clinical efficacy, safety and tolerability of switching to RPV/FTC/TAF from a stable regimen of either RPV/FTC/TDF or EFV/FTC/TDF in HIV‐1‐infected, virally suppressed adults in two distinct but similarly designed randomized controlled trials. The primary 48‐week endpoints were previously reported 5, 8. Herein, we present efficacy, safety and tolerability outcomes up to week 96.
Methods
Study design and participants
Details of the design, inclusion criteria and methodology of the trials have been previously reported 8, 9. Briefly, we conducted two randomized, double‐blind, active‐controlled, 96‐week, phase 3b clinical studies in virologically suppressed (HIV‐1 RNA < 50 copies/mL), HIV‐1‐infected adults with creatinine clearance (CrCl) > 50 mL/min as calculated by the Cockcroft–Gault equation 10, taking either RPV/FTC/TDF (25/200/300 mg) in Study 1216 or EFV/FTC/TDF (600/200/300 mg) in Study 1160 for at least 6 months. Each study randomized participants (1:1) to switch to RPV/FTC/TAF (25/200/25 mg) or to continue their current regimen for 96 weeks. Participants received placebo tablets matching the alternative treatment. The studies were undertaken in accordance with the Declaration of Helsinki and approved by central or site‐specific review boards and ethics committees.
Statistical analyses
We assessed efficacy by examining the proportion in each group with plasma HIV‐1 RNA < 50 copies/mL at week 96 [US Food and Drug Administration (FDA)‐defined snapshot algorithm 11] and evaluated noninferiority with a two‐sided 95% confidence interval (CI) for the difference in response rates (RPV/FTC/TAF minus either RPV/FTC/TDF or EFV/FTC/TDF) with a prespecified noninferiority margin of 8%. Safety endpoints included percentage change from baseline in hip and spine bone mineral density (BMD) and percentage change or change from baseline in renal parameters [CrCl, urine albumin to creatinine ratio (UACR), retinol‐binding protein to creatinine ratio (RBP:Cr) and β2‐microglobulin to creatinine ratio (β2M:Cr)] at week 96. We measured fasting lipids [total cholesterol, low‐density lipoprotein (LDL) cholesterol, high‐density lipoprotein (HDL) cholesterol and triglycerides] at baseline and every 24 weeks up to week 96. We used the Wilcoxon rank sum test to compare differences between treatment groups for continuous laboratory test results (sas version 9.4; SAS Institute, Cary, NC). We coded adverse events with the Medical Dictionary for Regulatory Activities (MedDRA® version, 20.0 http://www.meddra.org).
Results
Between 26 January and 27 August 2015, we randomized and initiated study treatments in 630 participants in Study 1216 (RPV/FTC/TAF, n = 316; RPV/FTC/TDF, n = 314) and 875 in Study 1160 (RPV/FTC/TAF, n = 438; EFV/FTC/TDF, n = 437). Baseline characteristics were similar between the RPV/FTC/TAF groups and either the RPV/FTC/TDF or EFV/FTC/TDF group, with the exception of sex in Study 1216, which enrolled more women in the RPV/FTC/TAF group than in the RPV/FTC/TDF group (Table 1). Median time on the regimen prior to randomization was 2.4 years [interquartile range (IQR) 1.6–3.2 years] in Study 1216 and 6.5 years (IQR 4.2–8.5 years) in Study 1160. At the time of analysis, 87% of participants in Study 1216 and 84% of those in Study 1160 remained on their randomly assigned treatment. The reasons for discontinuation were similar across treatment arms in each study (Figure 1).
Table 1.
Baseline characteristics
| Randomized treatment | Study 1216 (baseline regimen RPV/FTC/TDF) | Study 1160 (baseline regimen EFV/FTC/TDF) | ||
|---|---|---|---|---|
| RPV/FTC/TAF (n = 316) | RPV/FTC/TDF (n = 314) | RPV/FTC/TAF (n = 438) | EFV/FTC/TDF (n = 437) | |
| Age (years) [median (IQR)] | 46 (37, 53) | 44 (36, 51) | 49 (42, 55) | 48 (41, 54) |
| Male [n (%)] | 275 (87) | 289 (92) | 373 (85) | 390 (89) |
| Race/ethnicity [n (%)] | ||||
| White | 238 (75) | 235 (75) | 291 (66) | 292 (67) |
| Black or African descent | 65 (21) | 54 (17) | 118 (27) | 120 (27) |
| Asian | 7 (2) | 17 (5) | 9 (2) | 8 (2) |
| Latino/Hispanic | 40 (13) | 53 (17) | 79 (18) | 78 (18) |
| Region [n (%)] | ||||
| USA | 222 (70) | 226 (72) | 351 (80) | 345 (79) |
| Non‐USAa | 94 (30) | 88 (28) | 87 (20) | 92 (21) |
| CD4 count (cells/μL) [median (IQR)] | 673 (521, 877) | 668 (525, 817) | 673 (507, 887) | 666 (505, 820) |
| Duration of baseline regimen at enrolment (years) [median (IQR)]b | 2.3 (1.5, 3.3) | 2.5 (1.6, 3.2) | 6.5 (4.5, 8.2) | 6.6 (4.1, 8.7) |
| CrCl (mL/min) [median (IQR)] | 104 (89, 120) | 100 (87, 120) | 110 (91, 132) | 108 (92, 133) |
| Proteinuria grade [n (%)] | ||||
| 1 | 31 (10) | 28 (9) | 26 (6) | 36 (8) |
| 2 | 0 | 1 (< 1) | 2 (< 1) | 1 (< 1) |
| 3 | 0 | 0 | 0 | 0 |
| Diabetes mellitus [n (%)] | 5 (2) | 10 (3) | 26 (6) | 24 (5) |
| Hypertension [n (%)] | 66 (21) | 55 (18) | 118 (27) | 122 (28) |
CrCl, creatinine clearance; EFV, efavirenz; FTC, emtricitabine; IQR, interquartile range; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Includes Canada and Europe.
A single‐tablet regimen of RPV/FTC/TDF for Study 1216 and a single‐tablet regimen of EFV/FTC/TDF for Study 1160.
Figure 1.
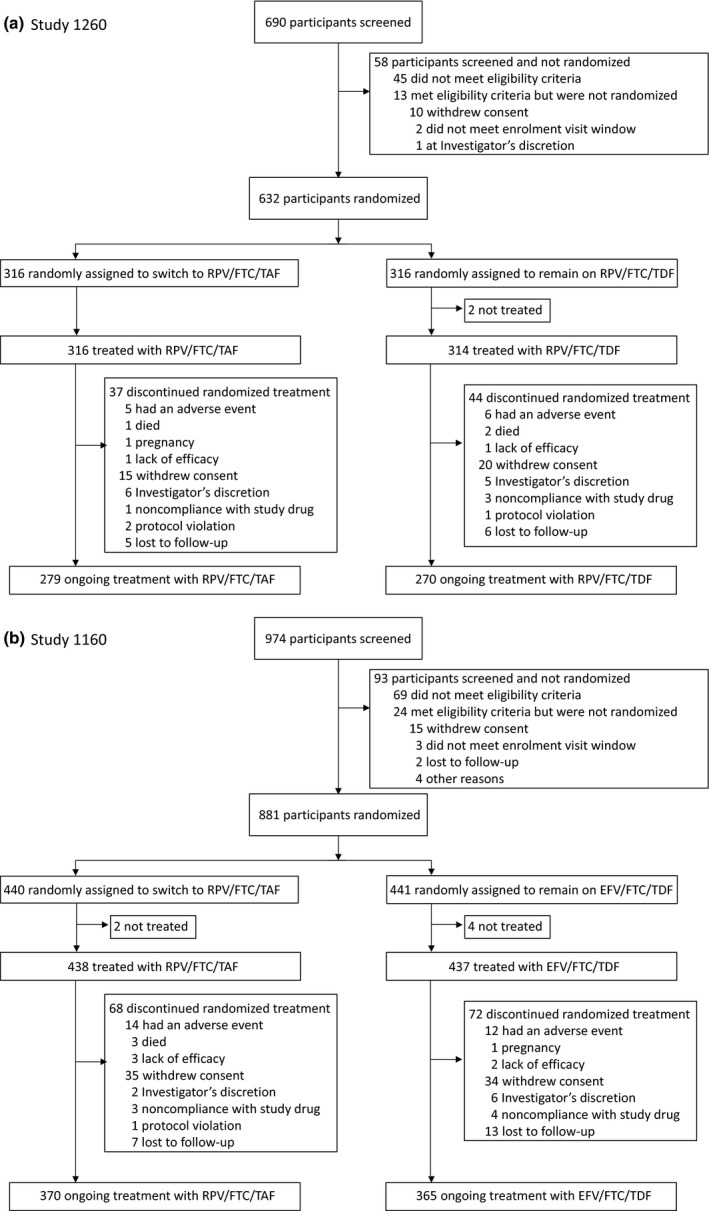
Disposition of study participants at week 96. (a) Study 1216: Switch to rilpivirine, emtricitabine and tenofovir alafenamide (RPV/FTC/TAF) from RPV/FTC/tenofovir disoproxil fumarate (TDF). (b) Study 1160: switch to RPV/FTC/TAF from efavirenz (EFV)/FTC/TDF.
In both studies, the efficacy of switching to RPV/FTC/TAF was noninferior to that of continuing baseline therapy at week 96. In Study 1216, 89.2% of participants on RPV/FTC/TAF versus 88.5% of those on RPV/FTC/TDF had HIV‐1 RNA < 50 copies/mL by FDA snapshot [difference in percentages 0.7%; 95% confidence interval (CI) −4.3 to +5.8%] at week 96 (Table 2). High rates of virological suppression were also seen at week 96 in Study 1160, with 85.2% of participants on RPV/FTC/TAF versus 85.1% of those on EFV/FTC/TDF having HIV‐1 RNA < 50 copies/mL (difference in percentages 0%; 95% CI −4.8 to +4.8%) at week 96.
Table 2.
Virological outcomes at week 96
| Study 1216 | Study 1160 | |||
|---|---|---|---|---|
| RPV/FTC/TAF (n = 316) | RPV/FTC/TDF (n = 313) | RPV/FTC/TAF (n = 438) | EFV/FTC/TDF (n = 437) | |
| HIV‐1 RNA < 50 copies/mL | 282 (89.2) | 277 (88.5) | 373 (85.2) | 372 (85.1) |
| Difference in percentages < 50 copies/mL (95% CI) | 0.7% (−4.3 to 5.8%)b (P = 0.80)a | 0.0% (−4.8 to 4.8%)b (P = 1.00)a | ||
| HIV‐1 RNA ≥ 50 copies/mL | 2 (0.6) | 3 (1.0) | 3 (0.7) | 4 (0.9) |
| Difference in percentages ≥ 50 copies/mL (95% CI) | −0.3% (−2.2 to 1.5%)b (P = 0.69)a | −0.2% (−1.7 to 1.2%)b (P = 0.73)a | ||
| HIV‐1 RNA ≥ 50 copies/mL in week 96 window | 1 (0.3) | 1 (0.3) | 0 | 0 |
| Discontinued study drug because of lack of efficacy | 1 (0.3) | 0 | 3 (0.7) | 2 (0.5) |
| Discontinued study drug because of AE/death and last available HIV‐1 RNA ≥ 50 copies/mL | 0 | 0 | 0 | 0 |
| Discontinued study drug for other reasonsc and last available HIV‐1 RNA ≥ 50 copies/mL | 0 | 2 (0.6) | 0 | 2 (0.5) |
| No virological data in week 96 window | 32 (10.1) | 33 (10.5) | 62 (14.2) | 61 (14.0) |
| Discontinued study drug because of AE/death and last available HIV‐1 RNA < 50 copies/mL | 5 (1.6) | 8 (2.6) | 16 (3.7) | 11 (2.5) |
| Discontinued study drug for other reasonsc and last available HIV‐1 RNA < 50 copies/mL | 24 (7.6) | 25 (8.0) | 45 (10.3) | 50 (11.4) |
| Missing data during window but on study drug | 3 (0.9) | 0 | 1 (0.2) | 0 |
The week 96 window was between days 631 and 714 (inclusive). Results are n (%) unless stated otherwise.
AE, adverse event; CI, confidence interval; EFV, efavirenz; FTC, emtricitabine; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
P‐values for the superiority test comparing the percentages of participants with HIV‐1 RNA < 50 or ≥ 50 copies/mL were from the Fisher exact test.
Differences in percentages of participants with HIV‐1 RNA < 50 or ≥ 50 copies/mL between treatment groups and their 95% CIs were calculated based on an unconditional exact method using two inverted one‐sided tests.
Discontinuation for other reasons includes participants who discontinued the study drug for the following reasons: investigator's discretion, withdrew consent, lost to follow‐up, noncompliance with study drug, protocol violation, pregnancy, and study terminated by sponsor.
The percentages of participants with HIV‐1 RNA ≥ 50 copies/mL at week 96 were low in both studies: in Study 1216, percentages were 0.6% for RPV/FTC/TAF versus 1.0% for RPV/FTC/TDF (difference in percentages −0.3%; 95% CI −2.2 to +1.5%), and in Study 1160 they were 0.7% for RPV/FTC/TAF versus 0.9% for EFV/FTC/TDF (difference in percentages −0.2%; 95% CI −1.7 to +1.2%). Efficacy was also similar in a prespecified analysis using the lower threshold for virological suppression of HIV‐1 RNA < 20 copies/mL (Study 1216: 85.8% for RPV/FTC/TAF versus 86.3% for RPV/FTC/TDF; difference −0.5%; 95% CI −6.0 to +5.0%; Study 1160: 83.1% for RPV/FTC/TAF versus 83.5% for EFV/FTC/TDF; difference in percentages −0.4%; 95% CI −5.5 to +4.6%).
Subgroup analyses comparing rates of virological suppression at week 96 within prespecified subgroups (age, sex, race, region, and study drug adherence) showed no differences between treatments in either study (Fig. S1). CD4 cell counts were maintained across all treatments up to week 96. The mean (standard deviation) changes from baseline in CD4 cell counts at week 96 in Study 1216 were +12 (180.6) cells/μL for the RPV/FTC/TAF group and +16 (171.9) cells/μL for the RPV/FTC/TDF group (P = 0.77), and in Study 1160 they were +12 (199.8) cells/μL for the RPV/FTC/TAF group and +6 (153.2) cells/μL for the EFV/FTC/TDF group (P = 0.64).
Up to 96 weeks, eight participants with protocol‐defined virological failure met the criteria for resistance analysis in Study 1216: three (0.9%) in the RPV/FTC/TAF group and five (1.6%) in the RPV/FTC/TDF group. No participant on RPV/FTC/TAF had treatment‐emergent resistance and two of the three participants analysed resuppressed to HIV RNA < 50 copies/mL while continuing RPV/FTC/TAF. The third had mutations detected at the time of virological failure; these mutations were pre‐existing at study enrolment, as shown by retrospective proviral DNA genotyping of the baseline sample (M41L, E44D, D67N, V118I, L210W and T215Y) as previously described 9. Two participants on RPV/FTC/TDF resuppressed to HIV RNA < 50 copies/mL without treatment‐emergent resistance while continuing RPV/FTC/TDF. One participant on RPV/FTC/TDF had treatment‐emergent resistance detected at week 84 with reverse transcriptase mutations K65R, M184V, K219K/E, K103N and Y181C conferring phenotypic resistance to FTC and RPV. Retrospective proviral DNA genotyping of the baseline sample showed that M184V and K103N were pre‐existing at baseline. One participant on RPV/FTC/TDF had a mutation detected at week 84 that was retrospectively found to be pre‐existing in the baseline sample (K103N) and subsequently discontinued study medications. The final RPV/FTC/TDF participant had wild‐type virus at their last visit on study medications.
Ten participants with virological failure met the criteria for resistance analysis in Study 1160: eight (1.8%) in the RPV/FTC/TAF group and two (0.5%) in the EFV/FTC/TDF group. No participant on RPV/FTC/TAF had treatment‐emergent resistance and seven of the eight participants subsequently resuppressed to HIV‐1 RNA < 50 copies/mL while continuing RPV/FTC/TAF. Two participants on RPV/FTC/TAF had detectable resistance mutations at virological failure that were pre‐existing at study entry as shown by retrospective proviral DNA genotyping of the baseline sample as previously described: one with K219K/E and K103N who resuppressed on continued RPV/FTC/TAF and one with V90V/I, K103N and E138E/A 8. Two participants on EFV/FTC/TDF had treatment‐emergent resistance. One developed resistance‐associated mutations M184V, V106I/L and Y188L as previously described and the other developed resistance‐associated mutations K101K/E, K103N and P225P/H.
All treatments were well tolerated and most adverse events were mild or moderate in severity and not related to study drugs. Adverse events led to discontinuation of the study drug for 11 participants in Study 1216; five (1.6%) on RPV/FTC/TAF and six (1.9%) on RPV/FTC/TDF. Only one (drug hypersensitivity in the RPV/FTC/TDF group) was considered related to the study drug by the investigator. Four of these discontinuations occurred after week 48, including one in a participant in the RPV/FTC/TAF group who developed a solitary cutaneous Kaposi's sarcoma lesion and was switched to a nonstudy regimen at the discretion of the investigator. Adverse events led to discontinuation of the study drugs for 26 participants in Study 1160; 14 (3.2%) on RPV/FTC/TAF and 12 (2.7%) on EFV/FTC/TDF. Seven of these discontinuations occurred after week 48, including one for Fanconi syndrome in a participant on EFV/FTC/TDF, which was considered related to the study medication. This diagnosis was preceded by several days of nausea and vomiting from ureterolithiasis, and concomitant with acute prerenal failure and metabolic acidosis, which were unrelated to study drugs. The study drug was discontinued, ureterolithiasis was surgically removed and the adverse events resolved. Three deaths occurred in Study 1216: from suicide, carbon monoxide poisoning and methamphetamine overdose. Four deaths occurred in Study 1160: from hypertensive cardiovascular disease, sepsis, pneumonia attributable to lung cancer and cocaine and methamphetamine overdose. No death was considered related to the study drugs.
In each study, increases from baseline in the mean per cent change of hip and spine BMD were observed at week 48 following switch to RPV/FTC/TAF 8, 9. Improvements continued up to week 96 and were significant compared with minimal changes in BMD with continuation of the baseline regimen (Figure 2; P < 0.001). Fractures were infrequent, being reported in similar numbers in the different treatment groups in both studies [Study 1216: nine (2.8%) for RPV/FTC/TAF and seven (2.2%) for RPV/FTC/TDF (P = 0.80); Study 1160: 14 (3.2%) for RPV/FTC/TAF and six (1.4%) for EFV/FTC/TDF (P = 0.11)]. Most were the result of an acute trauma, with the exception of two participants in Study 1160; one on RPV/FTC/TAF with pathological fractures attributable to bone metastases from prostate cancer, and one on EFV/FTC/TDF with a nontraumatic foot fracture.
Figure 2.
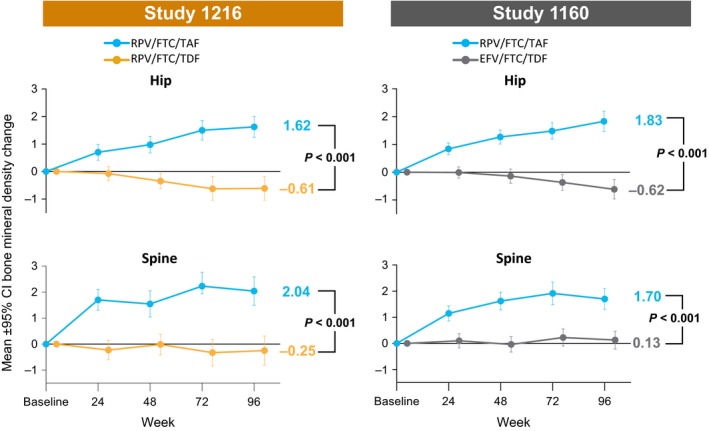
Changes in bone mineral density (BMD) at the hip and spine from baseline to week 96. BMD, bone mineral density; CI, confidence interval. P‐values are from the analysis of variance (ANOVA) model including treatment as a fixed effect.
There were no cases of proximal renal tubular disorders in Study 1216. One participant who received EFV/FTC/TDF in Study 1160 acquired Fanconi syndrome at week 70 which was considered related to the study drugs by the investigator. In Study 1216, participants who switched to RPV/FTC/TAF had an increase in CrCl at week 96 compared with those who continued RPV/FTC/TDF (Table 3). In Study 1160, there was a larger median decrease in CrCl for participants who switched to RPV/FTC/TAF than in those who continued EFV/FTC/TDF. Participants who switched to RPV/FTC/TAF had little change from baseline in UACR and RBP:Cr and a decrease from baseline in the β2M:Cr ratio at week 96, compared with increases from baseline in these measures for those who remained on treatment with RPV/FTC/TDF or EFV/FTC/TDF.
Table 3.
Changes in creatinine clearance (CrCl) and renal biomarkers from baseline to week 96
| Renal assessments | Study 1216 | Study 1160 | ||||
|---|---|---|---|---|---|---|
| RPV/FTC/TAF (n = 316) | RPV/FTC/TDF (n = 314) | P‐value | RPV/FTC/TAF (n = 438) | EFV/FTC/TDF (n = 437) | P‐value | |
| CrCl by Cockroft–Gault (mL/min) | ||||||
| Baseline | 103.5 (88.5, 119.8) | 99.7 (87.3, 119.8) | 0.47 | 110.4 (91.4, 132.0) | 107.6 (91.6, 132.7) | 0.63 |
| Change at week 96 | 5.9 (−3.0, 13.3) | −0.2 (−8.8, 8.1) | < 0.001 | −4.6 (−13.6, 5.4) | −1.1 (−10.3, 6.6) | 0.004 |
| UACR | ||||||
| Baseline | 5.5 (3.7, 10.0) | 5.4 (3.8, 9.2) | 0.98 | 6.9 (4.2, 13.6) | 6.4 (4.3, 11.8) | 0.40 |
| Percentage change at week 96 | 9.3 (−29.0, 57.9) | 32.9 (−12.5, 102.7) | < 0.001 | −1.0 (−40.4, 57.1) | 39.6 (−4.7, 136.8) | < 0.001 |
| RBP to creatinine ratio | ||||||
| Baseline | 101.2 (70.5, 157.6) | 111.1 (75.8, 196.9) | 0.12 | 115.8 (75.0, 254.4) | 131.8 (82.7, 265.7) | 0.14 |
| Percentage change at week 96 | 6.5 (−31.7, 57.8) | 55.8 (2.5, 137.9) | < 0.001 | −7.3 (−49.8, 42.2) | 87.1 (13.4, 202.5) | < 0.001 |
| β2‐microglobulin to creatinine ratio | ||||||
| Baseline | 111.6 (67.0, 260.0) | 116.1 (61.7, 326.1) | 0.72 | 130.1 (69.0, 498.6) | 153.6 (78.8, 480.8) | 0.13 |
| Percentage change at week 96 | −15.5 (−60.7, 29.2) | 43.7 (−24.5, 182.3) | < 0.001 | −31.7 (−76.7, 16.7) | 68.4 (−19.1, 203.2) | < 0.001 |
Values are median (IQR). P‐values were from the two‐sided Wilcoxon rank sum test to compare the two treatment groups.
EFV, efavirenz; FTC, emtricitabine; IQR, interquartile range; RBP, retinol‐binding protein; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; UACR, urine albumin to creatinine ratio.
In Study 1216, fasting total cholesterol, direct LDL cholesterol, HDL cholesterol and triglycerides had modest increases in participants who switched to RPV/FTC/TAF and remained stable in those who continued RPV/FTC/TDF (Table 4). There was a difference in the median change in total to HDL cholesterol ratio measured for participants who switched to RPV/FTC/TAF compared with those who continued RPV/FTC/TDF (Table 4). Median total to HDL cholesterol ratios were not significantly different between the treatment groups at week 96 (3.7 for RPV/FTC/TAF and 3.6 for RPV/FTC/TDF; P = 0.46). While on the study, a larger proportion of participants in the RPV/FTC/TAF group (8%) began lipid‐lowering drugs than in the RPV/FTC/TDF group (3%; P = 0.002). In Study 1160, switching to RPV/FTC/TAF was associated with decreases in fasting total and HDL cholesterol at week 96; lipids remained stable in the EFV/FTC/TDF group (Table 4). Neither the change from baseline nor the median total to HDL cholesterol ratio was significantly different at week 96 (median at week 96, 3.6 for RPV/FTC/TAF and 3.5 for EFV/FTC/TDF; P = 0.25). Similar proportions of participants in the RPV/FTC/TAF (5%) and EFV/FTC/TDF (6%) groups began lipid‐lowering drugs (P = 0.56) over the course of the study.
Table 4.
Changes in fasting lipids from baseline to week 96
| Metabolic assessment | Study 1216 | Study 1160 | ||||
|---|---|---|---|---|---|---|
| RPV/FTC/TAF (n = 316) | RPV/FTC/TDF (n = 314) | P‐value | RPV/FTC/TAF (n = 438) | EFV/FTC/TDF (n = 437) | P‐value | |
| Total cholesterol (mg/dL) | ||||||
| Baseline | 173 (152, 196) | 167 (152, 188) | 0.07 | 191 (170, 211) | 192 (167, 215) | 0.88 |
| Change at week 96 | 19 (4, 34) | 3 (−12, 18) | < 0.001 | −13 (−32, 10) | −3 (−19, 15) | < 0.001 |
| Direct LDL cholesterol (mg/dL) | ||||||
| Baseline | 109 (89, 129) | 105 (89, 123) | 0.12 | 115 (97, 135) | 117 (97, 139) | 0.46 |
| Change at week 96 | 15 (2, 28) | 3 (−9, 15) | < 0.001 | −2 (−20, 14) | 0 (−13, 13) | 0.22 |
| HDL cholesterol (mg/dL) | ||||||
| Baseline | 47 (39, 56) | 46 (38, 55) | 0.34 | 52 (43, 65) | 53 (44, 63) | 0.99 |
| Change at week 96 | 2 (−2, 8) | 0 (−5, 6) | 0.006 | −4 (−11, 1) | 0 (−6, 6) | < 0.001 |
| Triglycerides (mg/dL) | ||||||
| Baseline | 101 (74, 147) | 100 (72, 148) | 0.47 | 116 (83, 175) | 116 (79, 170) | 0.36 |
| Change at week 96 | 16 (−14, 53) | 3 (−27, 35) | < 0.001 | 1 (−34, 24) | 4 (−25, 35) | 0.14 |
| Total to HDL cholesterol ratio | ||||||
| Baseline | 3.6 (2.9, 4.5) | 3.5 (2.9, 4.5) | 0.60 | 3.6 (2.9, 4.3) | 3.5 (2.9, 4.4) | 0.95 |
| Change at week 96 | 0.2 (−0.3, 0.5) | 0 (−0.4, 0.5) | 0.03 | 0.1 (−0.4, 0.5) | 0 (−0.5, 0.4) | 0.06 |
Values are median (IQR). P‐values are from the two‐sided Wilcoxon rank sum test to compare the two treatment groups.
HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein; EFV, efavirenz; FTC, emtricitabine; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Discussion
These two phase 3 studies showed that switching to coformulated RPV/FTC/TAF from either RPV/FTC/TDF or EFV/FTC/TDF was noninferior for maintaining virological suppression, and was durable for at least 96 weeks compared with continuing either baseline regimen. Virological failure was rare in all treatment groups (≤ 1%). The majority of participants not included as successes in the FDA snapshot algorithm discontinued treatment prior to week 96 for nonvirological reasons with last on‐treatment HIV‐1 RNA < 50 copies/mL. No treatment‐emergent resistance was detected in participants who switched to RPV/FTC/TAF.
Improvements in BMD and urine biomarkers of renal safety were seen in the RPV/FTC/TAF groups over those whose treatment contained TDF. Bone density continued to improve to at least 96 weeks. Also, the improvements in measures of quantitative and tubular proteinuria in the TAF‐treated compared with the TDF‐treated groups were consistent with prior studies, and these benefits were evident for the duration of follow‐up 4, 5.
No renal tubular disorders were reported for any participant taking RPV/FTC/TAF, while one case of Fanconi syndrome occurred in a participant on EFV/FTC/TDF. This event was precipitated by prerenal acute kidney injury, which can cause elevated plasma concentrations of tenofovir as renal clearance of this TDF metabolite declines. TAF use results in 91% lower plasma concentrations of tenofovir than TDF and thus renal toxicities have rarely been seen in patients taking TAF 12, 13. Participants switching from EFV/FTC/TDF to RPV/FTC/TAF in Study 1160 did have an immediate small increase in serum creatinine consistent with inhibition by RPV of creatinine secretion in the renal tubule 14. The median change in serum creatinine at week 4 was 0.05 mg/dL for RPV/FTC/TAF versus 0.02 mg/dL for EFV/FTC/TDF (P < 0.001) and remained stable from week 12 to week 96.
In Study 1216, there were small increases from baseline in fasting total cholesterol, direct LDL cholesterol, HDL cholesterol, and triglycerides in participants who substituted TAF for TDF. Treatment with TDF‐containing regimens has consistently been associated with lower lipids compared with non‐TDF‐containing regimens, an effect thought to be a dose‐related effect of plasma tenofovir on reducing select lipid parameters 15, 16, 17, 18. The changes in fasting lipids seen in Study 1216 are consistent with differences seen in treatment‐naïve patients as well as in other studies evaluating the switch to TAF from TDF while maintaining other antiretroviral regimen components 4, 5.
Although there were modest increases in lipids, their clinical significance is unclear. There were no clinically meaningful differences in the total cholesterol to HDL cholesterol ratio, which is incorporated into cardiovascular clinical risk predictors for HIV‐infected and uninfected individuals 19, 20. Lipid differences of similar magnitude between TAF‐ and TDF‐treated participants have resulted in no differences in atherosclerotic cardiovascular disease (ASCVD) estimated cardiovascular risk, or eligibility for statins 21.
In comparison, participants in Study 1160 switched two component drugs of their antiretroviral regimen, both from EFV to RPV and from TDF to TAF. In aggregate, eliminating the lipid‐elevating influence of EFV 22 in favour of the more lipid‐neutral RPV 23 outweighed the modest effect of switching from TDF to TAF.
These studies have important limitations. Each was powered for a primary efficacy endpoint and may be unable to detect rare clinical safety events. For example, EFV is associated with short‐ and long‐term neuropsychiatric adverse effects and with an increase in suicidality in some reports 1, 22, 24, yet adverse event profiles and discontinuations were similar between the treatment groups of Study 1160. As neuropsychiatric symptoms may emerge early in treatment and lead to EFV discontinuation 24, 25, compared with the general population, our study population may be enriched for participants who tolerate EFV, as they were taking it for a median of 6.5 years prior to study enrolment. This may further limit the study's ability to detect a safety difference. Other limitations include study participants being predominantly male and relatively healthy with only a small proportion having advanced HIV disease.
Overall, virologically suppressed HIV‐1‐infected adults who switched to RPV/FTC/TAF from either RPV/FTC/TDF or EFV/FTC/TDF maintained virological suppression over 96 weeks with low rates of virological failure. There was no treatment‐emergent resistance to study medications in participants treated with RPV/FTC/TAF. The regimen of RPV/FTC/TAF had improved bone and renal safety profiles as well as excellent tolerability. These findings reinforce guidelines recommending use of RPV/FTC/TAF for treatment of HIV‐1 infection and support switching virologically suppressed patients on current NNRTI‐containing regimens to RPV/FTC/TAF for long‐term patient safety.
Supporting information
Fig. S1. Forest plot of treatment difference in HIV‐1 RNA < 50 copies/mL at week 96 (snapshot algorithm) by subgroup.
Acknowledgements
The authors extend their thanks to the participants, their partners and their families. The authors thank all principal investigators (Appendix), the complete GS‐US‐366‐1216 and US‐GS‐366‐1160 study teams, and Anna Kido (Gilead) for providing editorial assistance.
Parts of this work were previously presented at the Conference on Retroviruses and Opportunistic Infections (CROI), 4–7 March 2018, Boston, MA (poster 2168).
References
- 1. Mollan KR, Smurzynski M, Eron JJ et al Association between efavirenz as initial therapy for HIV‐1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med 2014; 161: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maggiolo F. Efavirenz: a decade of clinical experience in the treatment of HIV. J Antimicrob Chemother 2009; 64: 910–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shubber Z, Calmy A, Andrieux‐Meyer I et al Adverse events associated with nevirapine and efavirenz‐based first‐line antiretroviral therapy: a systematic review and meta‐analysis. AIDS 2013; 27: 1403–1412. [DOI] [PubMed] [Google Scholar]
- 4. Arribas JR, Thompson M, Sax PE et al Brief report: randomized, double‐blind comparison of tenofovir alafenamide (TAF) vs tenofovir disoproxil fumarate (TDF), each coformulated with elvitegravir, cobicistat, and emtricitabine (E/C/F) for initial HIV‐1 treatment: week 144 results. J Acquir Immune Defic Syndr 2017; 75: 211–218. [DOI] [PubMed] [Google Scholar]
- 5. Raffi F, Orkin C, Clarke A et al Brief report: long‐term (96‐Week) efficacy and safety after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in HIV‐infected, virologically suppressed adults. J Acquir Immune Defic Syndr 2017; 75: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Society EAC . European AIDS Clinical Society (EACS) Guidelines Version 9.0. 2017: 102.
- 7. The Department of Health and Human Services (DHHS) Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. (accessed 17 October 2017). [Google Scholar]
- 8. DeJesus E, Ramgopal M, Crofoot G et al Switching from efavirenz, emtricitabine, and tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV‐1 infection: a randomised, double‐blind, multicentre, phase 3b, non‐inferiority study. Lancet HIV 2017; 4: e205–e213. [DOI] [PubMed] [Google Scholar]
- 9. Orkin C, DeJesus E, Ramgopal M et al Switching from tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV‐1 infection: a randomised, double‐blind, multicentre, phase 3b, non‐inferiority study. Lancet HIV 2017; 4: e195–e204. [DOI] [PubMed] [Google Scholar]
- 10. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 11. U S Department of Health and Human Services FaDAF, Center for Drug Evaluation and Research (CDER) . Human immunodeficiency virus‐1 infection: developing antiretroviral drugs for treatment. Guidance for Industry. November, 2015.
- 12. Sax PE, Wohl D, Yin MT et al Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV‐1 infection: two randomised, double‐blind, phase 3, non‐inferiority trials. Lancet 2015; 385: 2606–2615. [DOI] [PubMed] [Google Scholar]
- 13. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir‐associated kidney toxicity in HIV‐infected patients: a review of the evidence. Am J Kidney Dis 2011; 57: 773–780. [DOI] [PubMed] [Google Scholar]
- 14. Gutierrez F, Fulladosa X, Barril G, Domingo P. Renal tubular transporter‐mediated interactions of HIV drugs: implications for patient management. AIDS Rev 2014; 16: 199–212. [PubMed] [Google Scholar]
- 15. Behrens G, Maserati R, Rieger A et al Switching to tenofovir/emtricitabine from abacavir/lamivudine in HIV‐infected adults with raised cholesterol: effect on lipid profiles. Antivir Ther 2012; 17: 1011–1020. [DOI] [PubMed] [Google Scholar]
- 16. Mulligan K, Glidden DV, Anderson PL et al Decreases in cholesterol in HIV‐seronegative men using emtricitabine/tenofovir pre‐exposure prophylaxis: lipid results of iPrEx [Abstract 005]. 15th International Workshop on Co‐morbidities and Adverse Drug Reactions in HIV Brussels, Belgium, 2013. [Google Scholar]
- 17. Santos JR, Saumoy M, Curran A et al The lipid‐lowering effect of tenofovir/emtricitabine: a randomized, crossover, double‐blind, placebo‐controlled trial. Clin Infect Dis 2015; 61: 403–408. [DOI] [PubMed] [Google Scholar]
- 18. Tungsiripat M, Kitch D, Glesby MJ et al A pilot study to determine the impact on dyslipidemia of adding tenofovir to stable background antiretroviral therapy: ACTG 5206. AIDS 2010; 24: 1781–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goff DC Jr, Lloyd‐Jones DM, Bennett G et al 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: S49–S73. [DOI] [PubMed] [Google Scholar]
- 20. Friis‐Moller N, Ryom L, Smith C et al An updated prediction model of the global risk of cardiovascular disease in HIV‐positive persons: the Data‐collection on Adverse Effects of Anti‐HIV Drugs (D:A:D) study. Eur J Prev Cardiol 2016; 23: 214–223. [DOI] [PubMed] [Google Scholar]
- 21. Huhn G, Rijnders B, Thompson M et al Switching from TDF to TAF in Patients with High Risk for CKD [Presentation]. Boston, MA, ASM Microbe, 2016. [Google Scholar]
- 22. SUSTIVA® and Bristol‐Myers Squibb Company . SUSTIVA® (efavirenz) capsules and tablets for oral use. US Prescribing Information. Princeton, NJ: Revised January, 2017. [Google Scholar]
- 23. Thamrongwonglert P, Chetchotisakd P, Anunnatsiri S, Mootsikapun P. Improvement of lipid profiles when switching from efavirenz to rilpivirine in HIV‐infected patients with dyslipidemia. HIV Clin Trials 2016; 17: 12–16. [DOI] [PubMed] [Google Scholar]
- 24. Ford N, Shubber Z, Pozniak A et al Comparative safety and neuropsychiatric adverse events associated with efavirenz use in first‐line antiretroviral therapy: a systematic review and meta‐analysis of randomized trials. J Acquir Immune Defic Syndr 2015; 69: 422–429. [DOI] [PubMed] [Google Scholar]
- 25. Lochet P, Peyriere H, Lotthe A, Mauboussin JM, Delmas B, Reynes J. Long‐term assessment of neuropsychiatric adverse reactions associated with efavirenz. HIV Med 2003; 4: 62–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Forest plot of treatment difference in HIV‐1 RNA < 50 copies/mL at week 96 (snapshot algorithm) by subgroup.


