Abstract
Few studies have investigated non‐target effects of neonicotinoid insecticides on mammalian physiology. This is largely due to the widespread perception that their weak affinity for nicotinic acetylcholine receptor subtypes in vertebrates makes mammalian exposures unlikely to pose health risks. To the best of our knowledge, we describe the first investigation evaluating the interaction of seven principal neonicotinoid insecticides (thiamethoxam, imidacloprid, clothianidin, flupyradifurone, dinotefuran, nitenpyram, thiacloprid) with oestrogen and thyroid hormone receptors, as well as their adipogenic effects, in mammalian cell culture assay systems. An E‐Screen with MCF‐7 and T‐Screen with GH3 cells respectively showed a lack of oestrogen and thyroid hormone receptor agonist effects for any of the neonicotinoids tested. Adipogenicity was assessed by the ability to stimulate lipid accumulation in adipocyte differentiated 3T3‐L1 cells, with only imidacloprid scoring positive in this assay causing triglyceride accumulation from a concentration of 50 mg l−1. Data mining of ToxCast high‐throughput screening assays revealed that this adipogenic effect of imidacloprid is probably mediated via the pregnane X receptor.
Keywords: neonicotinoid, endocrine disruptor, insecticide, lipid accumulation, obesogen
Short abstract
The oestrogenic, thyroidogenic and adipogenic potential of seven neonicotinoid insecticides (thiamethoxam, imidacloprid, clothianidin, flupyradifurone, dinotefuran, nitenpyram, thiacloprid) were studied in mammalian cell lines. None of the neonicotinoids tested displayed any oestrogen and thyroid hormone agonist properties in receptor‐mediated cell proliferation assays. Only imidacloprid caused triglyceride accumulation in the 3T3‐L1 adipogenicity cell assay. Data mining of ToxCast high‐throughput screening assays revealed that this adipogenic effect of imidacloprid is probably mediated via the pregnane X receptor.
1. INTRODUCTION
Neonicotinoids are synthetic insecticides targeting nicotinic acetylcholine receptors in the central nervous system of insects. Their intensive use in agriculture has been associated to a wide range of toxic effects on non‐target organisms, including colony collapse in social insects such as honeybees and bumblebees (Henry et al., 2012; Whitehorn, O'Connor, Wackers, & Goulson, 2012). Although their environmental effects have received a great deal of attention, few studies have investigated the possible effect of neonicotinoids on mammalian physiology. This is due to the widespread perception that the general weak affinity of neonicotinoids for nicotinic acetylcholine receptor subtypes in vertebrates makes mammalian exposures unlikely to pose any significant health risks (Sheets et al., 2016). However, further investigation of health risks is needed as some studies of human populations have found an association between exposure to neonicotinoids and adverse neurodevelopmental effects including teratogenicity and autism spectrum disorder (Cimino, Boyles, Thayer, & Perry, 2017).
Biomonitoring reports of neonicotinoid insecticides in human populations are very limited. Nevertheless, a survey of urine from 373 healthy individuals in Japan, detected neonicotinoids in more than half of the samples with an average excretion of 0.51 μg day−1 for clothianidin, 3.29 μg day−1 for dinotefuran, 0.07 μg day−1 for imidacloprid, 0.07 μg day−1 for nitenpyram and 0.18 μg day−1 for thiamethoxam (Harada et al., 2016). The European Food Safety Authority has estimated that each European Union citizen consumes a maximum of 0.19 μg kg−1 body weight (bw) day−1 of clothianidin, 0.354 μg kg−1 bw day−1 of imidacloprid, 0.19 μg kg−1 bw day−1 of thiacloprid and 0.24 μg kg−1 bw day−1 of thiamethoxam (European Food Safety Authority, 2017). However, these estimates were derived from long‐term dietary exposure assessments made using pesticide residue concentrations in the most commonly consumed food commodities with the PRIMo tool, and may thus not reflect the actual body burden of neonicotinoids.
Recent advances in endocrinology have linked exposure to pesticides acting as endocrine disruptors to a wide range of serious, negative health effects in humans (Mostafalou & Abdollahi, 2017). As early investigations concentrated mostly on reproduction problems in wild animals, endocrine disruptive effects of pesticides on the oestrogen and androgen hormone systems have been the most studied (Colborn, vom Saal, & Soto, 1993). More recently, it has been found that pesticides can provoke neurodevelopmental and neurobehavioural effects by interfering with the action of thyroid hormone (Fini et al., 2017). In addition, pesticides can disturb general metabolism, which can cause diabetes and obesity (Heindel et al., 2017). Pesticides can also act as obesogens by disturbing the activity of retinoid X receptors and peroxisome proliferator‐activated receptor gamma (Grun & Blumberg, 2006). Despite widespread concerns regarding non‐target effects of neonicotinoids, no study has described the potential hormone‐mimetic properties of this class of compounds.
To address the knowledge gap of the endocrine disrupting capability of neonicotinoids, to the best of our knowledge, we report here the first investigation of the effects of seven principal neonicotinoid insecticides (clothianidin, dinotefuran, flupyradifurone, imidacloprid, nitenpyram, thiacloprid, thiamethoxam) (Figure 1) on the oestrogen and thyroid hormone receptor (TR), as well as assessing their adipogenic capability in mammalian cell lines. Our results reveal that none of the neonicotinoids tested displayed either an oestrogenic or thyroidogenic activity but that imidacloprid is a potential obesogen.
Figure 1.
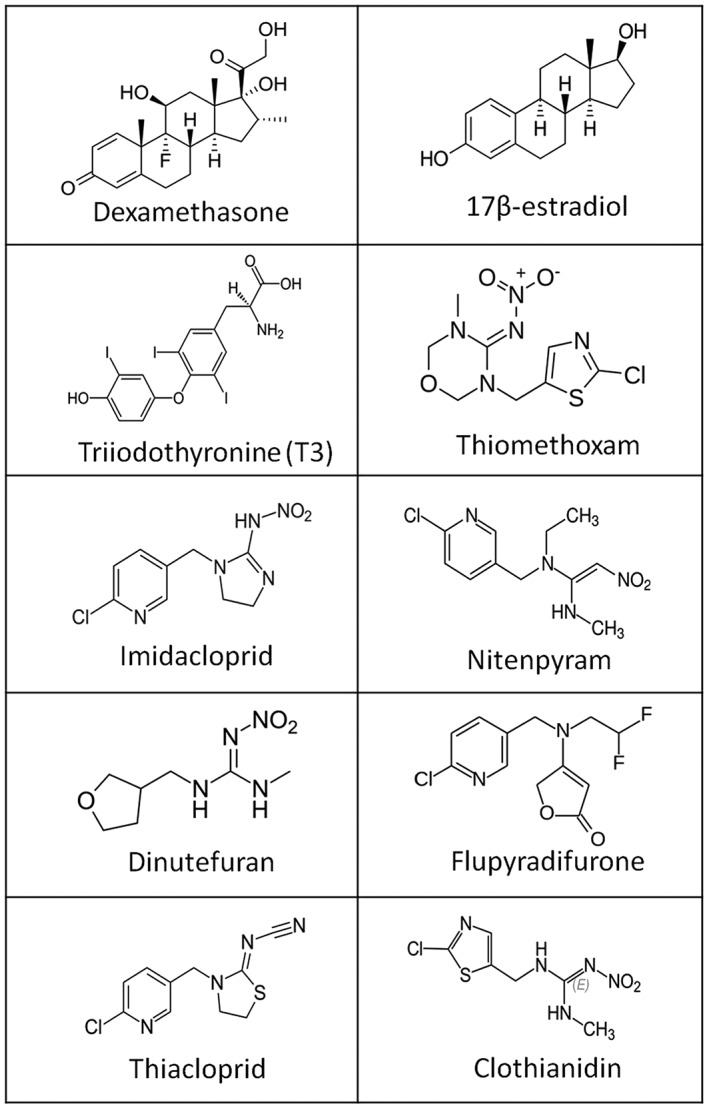
Molecular structures of the different neonicotinoid insecticides tested in this study
2. MATERIALS AND METHODS
2.1. Reagents
All reagents and chemicals, unless otherwise specified, were of analytical grade and were purchased from Sigma‐Aldrich Ltd (Gillingham, Dorset, UK). The seven neonicotinoid insecticides tested were thiamethoxam (CAS no. 153719–23‐4, purity ≥98%; Santa Cruz Biotechnology, Heidelberg, Germany), imidacloprid (CAS no. 138261–41‐3, purity ≥99.9%; Yorlab, York, UK), clothianidin (CAS no. 210880–92‐5, purity ≥98%; Santa Cruz Biotechnology), flupyradifurone (CAS no. 951659–408‐8, purity ≥99.9%; Yorlab), dinotefuran (CAS no. 165252–70‐0, purity >98.8%; Yorlab), nitenpyram (CAS no. 150824–47‐8; purity ≥99%; Santa Cruz Biotechnology) and thiacloprid (CAS no. 111988–49‐9, purity ≥99.9%; Santa Cruz Biotechnology).
2.2. Cell culture
The hormone‐dependent, human breast cancer cell line MCF‐7 was a kind gift from Prof. Joy Burchell (Research Oncology Department, King's College London). It was originally obtained from the Michigan Cancer Foundation (Detroit, MI, USA) and authenticated by LGF Standards in 2009 using short‐tandem repeat profiling of 16 loci. The rat pituitary GH3 cell line was obtained from the American Type Culture Collection (Manassas, VA, USA; CCL‐82.1™). These two cell lines were grown at 37°C (5% CO2) in 75 cm2 flasks (Corning, Tewksbury, MA, USA) in a medium composed of phenol red‐free Dulbecco minimal Eagle's medium (DMEM; Life Technologies, Warrington, Cheshire, UK), 10% fetal bovine serum (FBS; GE Healthcare Life Sciences, Little Chalfont, Bucks, UK), 2 mm glutamine (GE Healthcare Life Sciences) and 10 μg ml−1 penicillin/streptomycin (Life Technologies). The murine fibroblast 3T3‐L1 cell line was purchased from ZenBio (Cambridge Bioscience, Cambridge, UK). Undifferentiated 3T3‐L1 cells were grown in a similar medium except that FBS was replaced by newborn calf serum [New Zealand origin; Thermo Fisher Scientific (Life Technologies)] to avoid spontaneous differentiation to adipocytes. Cells were released from the flask substratum using 0.05% trypsin–EDTA (Life Technologies) and counted using a haemocytometer before re‐seeding. A 24 hour recovery period was allowed for cell adherence before cultures were subjected to the desired treatments. Stock solutions of neonicotinoids and the natural hormones dexamethasone, oestradiol and triiodothyronine (T3) were prepared in dimethyl sulphoxide (DMSO). Dilutions of these stock solutions administered to cell cultures always resulted in a DMSO concentration below 0.5%.
2.3. T‐ and E‐Screen assays
While the E‐Screen allows the determination of oestrogenic effects by determining oestrogen receptor‐mediated cell proliferation of MCF‐7 cells, the T‐Screen assay allows determination of TR agonist effects by monitoring TR‐mediated cell proliferation of GH3 cells. The T‐ and E‐Screen tests were conducted in a comparable manner as previously described (Soto et al., 1995) except that the treatments were terminated using an MTT assay (Mosmann, 1983). Briefly, cells were seeded into 48‐well plates (Dutscher Scientific, Brentwood, Essex, UK) at a density of 20 000 cells per well in 250 μl maintenance medium. The test medium for the E‐Screen consisted of phenol red‐free DMEM supplemented with 5% charcoal stripped FBS (Life Technologies), 2 mm glutamine (GE Healthcare Life Sciences) and 10 μg ml−1 penicillin/streptomycin (Life Technologies). For the T‐Screen assay, PCM medium was employed (Sirbasku, Pakala, Sato, & Eby, 1991). PCM medium is composed of DMEM‐F12 (Life Technologies) supplemented with 2 mm glutamine, 10 μg ml−1 penicillin/streptomycin, 10 μg ml−1 insulin from bovine pancreas, 10 μm ethanolamine, 10 ng ml−1 sodium selenite, 500 μg ml−1 bovine serum albumin and 10 μg human apotransferrin. Following a 24 hour incubation to allow cell attachment and clearance of steroid hormone residues, medium was changed to that containing the desired compounds.
The test medium for both the T‐ and E‐Screen assays was refreshed after 3 days. Following another 3 day period of incubation, an MTT assay was performed as follows. The test medium was removed and the cells were incubated with 250 μl of MTT solution (1 mg ml−1) for 2 hours and then lysed by the addition of 250 μl of DMSO. Optical density was measured at 570 nm using the SPECTROstar Nano plate reader (BMG Labtech, Aylesbury, Bucks, UK). Oestradiol and T3 were used as positive controls for oestrogen and TR agonist measurements respectively.
2.4. Adipogenesis assay
For differentiation of 3T3‐L1 cultures to adipocytes, cells were seeded into 96‐well plates (Dutscher Scientific) at a density of 20 000 cells per well in 100 μl maintenance medium. Following a 2 day stabilization period, they were switched to differentiation medium consisting of DMEM supplemented with 2 mm glutamine, 10 μg ml−1 penicillin/streptomycin, 10% fetal bovine calf serum (GE Healthcare Life Sciences), 500 μm 3isobutyl1methylxanthine (Sigma‐Aldrich Ltd) and 100 nm insulin from bovine pancreas (Sigma‐Aldrich Ltd). After a further 2 day incubation, the medium was refreshed but lacked insulin and contained various concentrations of the test neonicotinoids. Treatment with dexamethasone acted as a positive control. Media were replenished every 2 days for a further 6 days. Lipid accumulation on day 8 following induction of differentiation was visualized using fluorescent Nile Red staining in accordance with the manufacturer's instructions (AdipoRed™ Assay Reagent; Lonza, Walkersville, MD, USA) and quantified using a microplate reader (GloMax® Multi Microplate Multimode Reader; Promega, Madison, WI, USA).
2.5. Intracellular lipid staining
3T3‐L1 cells were seeded into 96‐well clear bottom black tissue culture‐treated plates (Corning) and differentiated as described above for 8 days with the indicated treatments. Medium was removed and cells were fixed by the addition of 100 μl of 4% paraformaldehyde (Thermo Fisher Scientific). Cells were then stained for intracellular lipid accumulation by adding 50 μl of 1 μg ml−1 Nile Red (Yorlab) and 1 μg ml−1 DAPI (Santa Cruz Biotechnology) in 0.2% Triton X‐100 phosphate‐buffered saline for 15 minutes in the dark (Boucher, Boudreau, Ahmed, & Atlas, 2015). Nile Red staining for lipid droplets and DAPI staining for cell nuclei were imaged at 530 and 405 nm respectively, using fluorescence imaging on a Nikon Eclipse Ts2 microscope (40× objective) (Kingston Upon Thames, UK).
2.6. ToxCast data mining
Publically available data from the Toxicity Forecaster (ToxCast) program were scrutinized using the iCSS ToxCast Dashboard (http://actor.epa.gov/dayashboard;lastaccessed1 December 2017) to search for the imidacloprid mode of action. The ToxCast program was launched by the US Environmental Protection Agency (EPA) to test about 10 000 chemical compounds using high‐throughput robotic screening systems. It includes in vitro assays allowing the detection of endocrine disrupting mechanisms such as gene reporter transactivation assays (Richard et al., 2016). Imidacloprid scored positive in the pregnane X receptor (PXR) assays. Of the three assays measuring the activity of the PXR, two were performed with imidacloprid. These tests conducted by Attagene Inc. (Morrisville, NC, USA) consisted of reporter gene assays in the human hepatocyte HepG2 cell line (ATG_PXR_TRANS_up and ATG_PXRE_CIS_up) and measurements performed 24 hours after exposure to imidacloprid. In the ToxCast database, the concentration giving 50% activation in an assay (AC50 value) was used as a quantitative measure to reflect the potency of imidacloprid.
2.7. Statistical analysis
Results were expressed as the mean ± standard deviations of the percentage of the control cell culture receiving no treatment. The different assays were performed three times in triplicate (n = 3).
3. RESULTS
The aim of this investigation was to test the endocrine disrupting effects of seven principal neonicotinoid insecticides compared to natural hormones. First, we used an oestrogen‐mediated proliferation assay using MCF‐7 cells (E‐Screen) to evaluate oestrogenic effects. The E‐Screen assay was highly sensitive to the natural hormone as oestradiol provoked a maximum 850% increase in MCF‐7 cell numbers. Contrastingly, no proliferative effect was observed with the seven neonicotinoids tested (Figure 2). Although they are negative for oestrogenic effects, these results were nevertheless informative regarding the differential toxicity of these insecticides as the MTT assay used to terminate the E‐Screen is also a well‐established determinant of cytotoxicity. Although some of the neonicotinoids, namely thiamethoxam, dinotefuran and nitenpyram produced only a small decrease in cell viability at concentrations up to 300 mg l−1, others such as thiacloprid, clothianidin and flupyradifurone displayed a greater degree of cytotoxicity starting at a concentration of 50 mg l−1.
Figure 2.
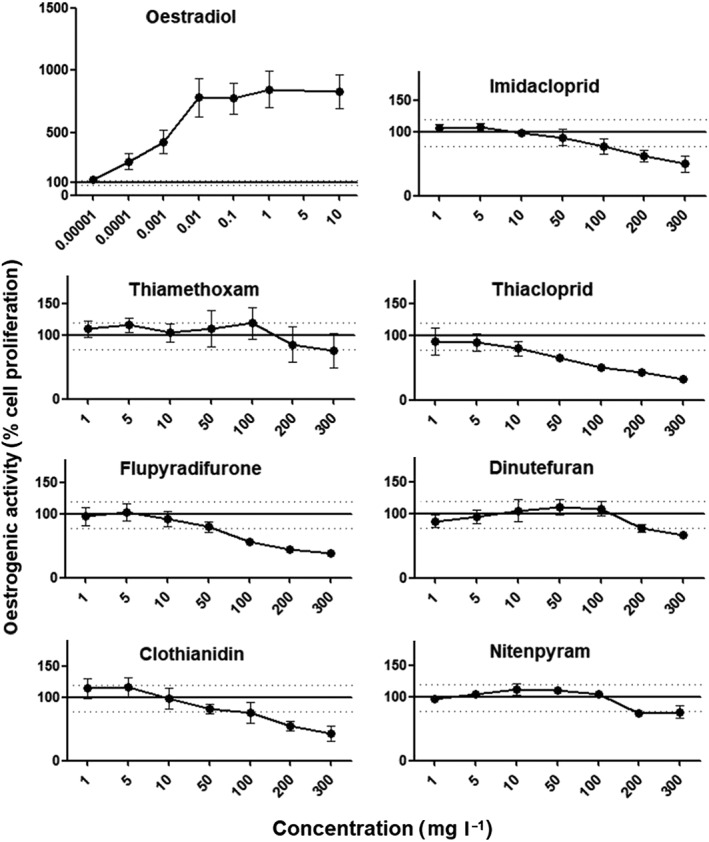
Evaluation of oestrogenic potential of neonicotinoid insecticides in an E‐Screen bioassay. MCF‐7 cells were starved of steroid hormones for 24 h and then cultured in the presence of the test compounds for a further 6 days. Cell proliferation was then assessed by an MTT assay. Results are expressed as a percentage of the proliferative effect observed in cultures of cells under hormone‐free conditions. Measurements are the mean ± SE of three independent experiments, each one performed in triplicate
We next investigated the thyroid‐hormone disrupting effects of the neonicotinoids by undertaking a T3‐mediated cell proliferation assay (T‐Screen). As expected, treatment with T3 resulted in a pronounced cell proliferation of 360% of GH3 cells. This demonstrates a high degree of sensitivity of the T‐Screen assay to this natural hormone. Unlike T3, none of the seven different neonicotinoids tested gave rise to GH3 cell proliferation thus showing no thyroidogenic effect in this assay (Figure 3). Cytotoxic effects were also observed with thiacloprid and clothianidin at concentrations starting from 50 mg l−1. Overall, differential cytotoxicity profiles were similar to those of the E‐Screen assay (Figure 3).
Figure 3.
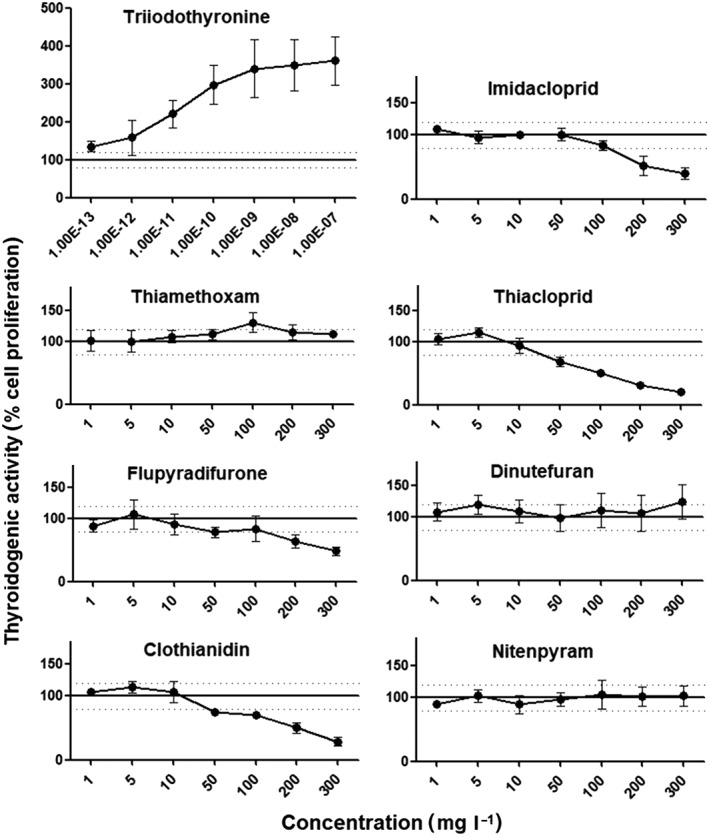
Assessing thyroidogenic potential of neonicotinoid insecticides in a T‐Screen bioassay. Following a steroid, including the thyroid hormone withdrawal period of 24 h, GH3 cells were cultured for a further 6 days in the presence of triiodothyronine and the test neonicotinoids as indicated. Cell proliferation was measured by an MTT assay. Results are expressed as a percentage of the proliferation observed in control cell cultures grown under hormone‐free conditions. Measurements are the mean ± SE of three independent experiments, each one performed in triplicate
Next, we conducted an adipogenesis assay to assess if the seven neonicotinoids tested in this study were able to stimulate lipid accumulation in 3T3‐L1 cells, which had undergone differentiation to adipocytes. The natural hormone dexamethasone, acting as a positive control, induced a marked 13‐fold increase in lipid accumulation (Figure 4). Our results showed that of the seven neonicotinoids tested, only imidacloprid gave a clear positive score in this assay causing up to a fivefold increase in lipid accumulation starting from a concentration of 50 mg l−1, and reaching a maximal effect at between 100 and 300 mg l−1 (Figure 4). Additionally, we noticed that neonicotinoids did not inhibit basal lipid accumulation caused by residual hormones in the culture medium (Figure 4).
Figure 4.
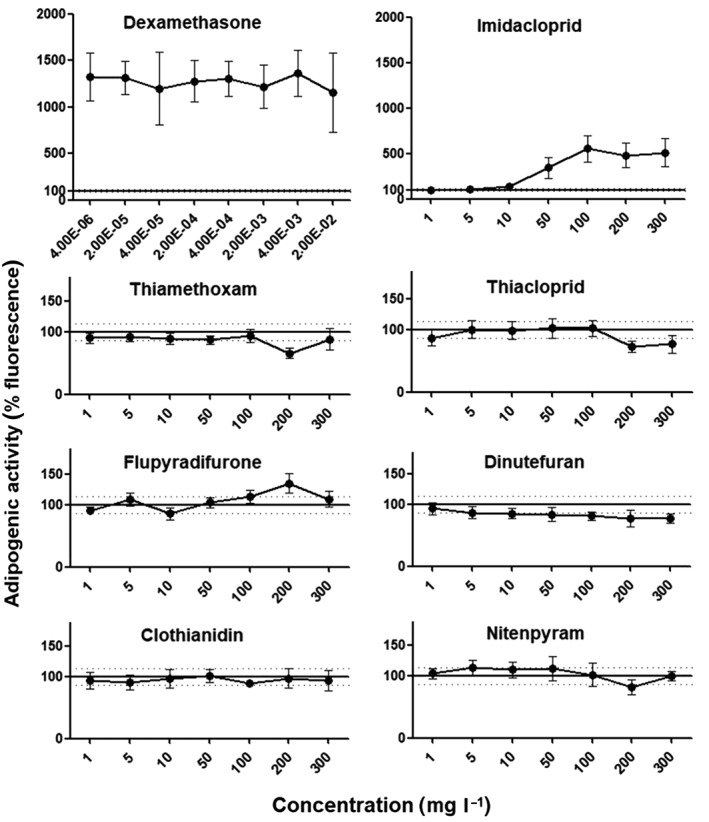
Determination of adipogenicity of neonicotinoid insecticides in 3T3‐L1 adipocyte cell cultures reveals the adipogenic potential of imidacloprid. Following induced differentiation of 3T3‐L1 cells to adipocytes, cells were cultured for a further 6 days in the presence of the indicated test compounds. Intracellular lipid accumulation was assessed by fluorescence staining with Nile Red and quantified using a microplate reader. Results are expressed as the percentage of the fluorescence intensity obtained with treatment‐free control cultures. Measurements are the mean ± SE of at least three independent experiments, each one performed in triplicate
The adipogenic effect of imidacloprid was confirmed by staining cells for intracellular lipid droplets with Nile Red and visualization by fluorescence microscopy. Differentiated 3T3‐L1 cells treated with 50 mg l−1 imidacloprid for 8 days showed a similar increase in lipid accumulation when compared to the cells treated with the positive control dexamethasone (0.01 nm) (Figure 5).
Figure 5.
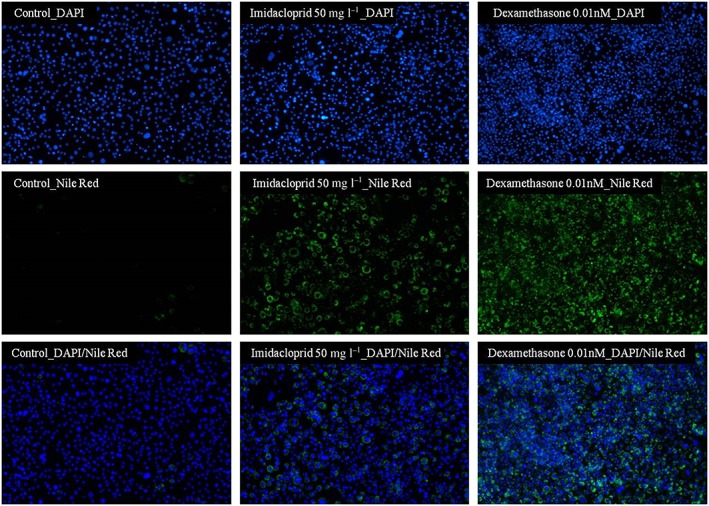
Confirmation of the adipogenic activity of imidacloprid by fluorescence microscopic imaging. Cultures of 3T3‐L1 cells were differentiated to adipocytes and then cultured for a further 6 days with either treatment‐free control medium (control), or in the presence of 50 mg l−1 imidacloprid or 0.01 nm dexamethasone. Lipid accumulation was visualized by fluorescence microscopy following staining with Nile Red and using a Nikon Eclipse Ts2 microscope and 40× objective
We then investigated the US EPA ToxCast program database to see if the results of the 1080 assays performed with imidacloprid could indicate possible mechanisms responsible for its adipogenic properties. This scrutiny of the ToxCast database revealed that imidacloprid could be an agonist of the PXR. In the PXRE_CIS assay measuring the activation of PXR responsive elements in a reporter gene assay, the AC50 was 26.7 μm for imidacloprid, compared to 0.713 μm for rifampicin, which is the reference compound used in this assay. In a transactivation assay for PXR (ATG_PXR_TRANS), the AC50 for imidacloprid was 81.8 μm compared to 0.317 μm for rifampicin (Figure 6).
Figure 6.
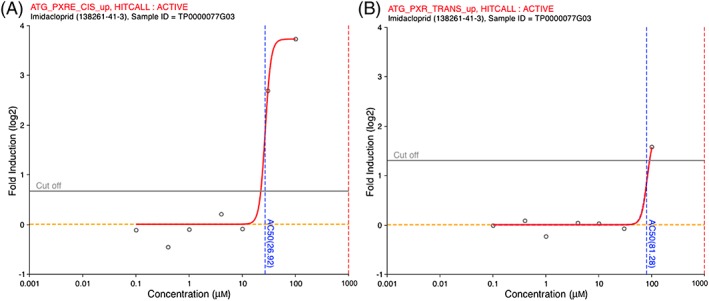
Imidacloprid activates PXR in reporter gene assays. Publically available data from the ToxCast program were scrutinized and the effects of imidacloprid on PXR in the Attagene cis‐ and trans‐FactorialTM assays ATG_PXRE_CIS_up (A) and ATG_PXR_TRANS_up (B) were extracted using the iCSS ToxCast Dashboard (http://actor.epa.gov/dayashboard; last accessed 1 December 2017). Dose–response of imidacloprid (0.1–100 μm) in these assays is presented and expressed as fluorescence fold induction. PXR, pregnane X receptor [Colour figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
To the best of our knowledge, the study we present here constitutes the first investigation of the oestrogenic, thyroidogenic and adipogenic potential of seven frequently used neonicotinoid insecticides. Our study shows that imidacloprid could be a potential obesogen as it promoted lipid accumulation in 3T3‐L1 cells that had undergone differentiation to adipocytes (Figures 4 and 5). This confirmed observations made in a previous study showing that imidacloprid treatment stimulated lipid accumulation in 3T3‐L1 adipocytes (Park et al., 2013). In an in vivo study, imidacloprid was found to promote adiposity in female C57BL/6 J mice (Park et al., 2013). Although most neonicotinoids have a comparable mode of action in insects, it is remarkable that despite their structural similarities (Figure 1), the other six neonicotinoids tested in this study failed to induce adipogenesis in 3T3‐L1 cells (Figure 4). Data mining of the ToxCast high‐throughput screening assay database revealed that the adipogenic effect of imidacloprid could occur via activation of the PXR (Figure 6). However, we note that the lowest active concentration of imidacloprid in this study showing an adipogenic effect (50 mg l−1) is probably several orders of magnitude higher than environmentally relevant concentrations (0.354 μg kg−1 bw day−1) in regard to standard conversion values (European Food Safety Authority, 2012). Thus, the relevance of our results to human health remains uncertain and requires further investigation in animal model systems.
The hormone‐dependent proliferation assays (T‐ and E‐Screen) showed that the seven neonicotinoids we tested are unlikely to activate oestrogen receptors and TRs (Figures 2 and 3). It has been reported that imidacloprid can have TR agonist effects in a GH3 luciferase reporter gene assay (Xiang et al., 2017). This observation would appear to contradict the findings we present here; however, upon close scrutiny of the data presented by Xiang and colleagues, the response to imidacloprid in their reporter gene assay was very weak (less than twofold of the control) (Xiang et al., 2017). Thus, this poor efficiency of interaction with the TR may be insufficient to provoke a physiological response such as cell proliferation in a T‐Screen as we present.
Another investigation concluded that imidacloprid disrupted the pituitary–thyroid axis of the wildlife avian species, red munia, at an environmentally relevant concentration (Pandey & Mohanty, 2015). However, the authors did not test imidacloprid but a commercial formulation containing multiple ingredients such as 1‐methyl‐2‐pyrrolidinone. It is thus not possible to attribute definitely the toxic effects observed to imidacloprid, particularly as 1‐methyl‐2‐pyrrolidinone is a known developmental toxicant, which can cause malformations such as incomplete ossification of the skull in rats (Saillenfait, Gallissot, Langonne, & Sabate, 2002). On a related note, the commercial pesticide formulation Confidor is at least 10 times more toxic than imidacloprid alone to placental, embryonic and hepatic human cell lines (Mesnage, Defarge, Spiroux de Vendomois, & Seralini, 2014). Similarly, thiacloprid has been found to cause alterations in free T3 and free thyroxine serum levels in rats following gavage administration of an acute dose of the commercial formulation of these pesticides (Sekeroglu, Sekeroglu, & Demirhan, 2014). The effects cannot be unequivocally attributed to the neonicotinoid component in these two cases (Saillenfait et al., 2002; Sekeroglu et al., 2014), as commercial formulations of pesticides contain co‐formulants, which have been demonstrated to be toxic in their own right. Hence, commercial pesticide formulations have almost invariably been found to be more toxic than the stated active ingredient alone (Mesnage et al., 2014). For example, in the case of the herbicide glyphosate, the toxicity of six (of nine tested) glyphosate‐based herbicides could be attributed to their co‐formulants as cytotoxic effects were linearly correlated to the concentrations of ethoxylated tallow amine adjuvants present in the formulations (Mesnage, Bernay, & Seralini, 2013). This highlights a general problem in the field of pesticide toxicology. Authors frequently fail to distinguish between the commercial formulations and their active ingredients, leading to confusion in the scientific literature and within regulatory circles, and misrepresentation of the safety profile of marketed pesticides (Mesnage & Antoniou, 2017).
A limitation of our study is that we do not directly assess the binding of the neonicotinoids we tested to hormone and other nuclear receptors. Rather, we measured cellular functional responses (cell proliferation, lipid accumulation) resulting from exposure to the neonicotinoids to assess their hormone mimetic properties. In addition, although the 3T3‐L1 and the GH3 cell lines have been validated as reliable models to assess hormone‐mimicking effects of chemical compounds, they do not harbour human receptors. Thus, it may not be possible to extrapolate fully the effects detected in this study to humans. In some cases, variation in ligand‐binding affinities between mammalian species can result in differences in biological effects such as for the aryl hydrocarbon receptor (Flaveny & Perdew, 2009). Further studies are needed to ascertain if neonicotinoids can cause disturbances in other hormone systems such as the monoamine, noradrenaline and serotonin pathways, which can be targets of endocrine disrupting compounds (Filer, Patisaul, Schug, Reif, & Thayer, 2014). Furthermore, for example, it would be particularly valuable to investigate neonicotinoids for non‐endocrine disruptive effects, which have been designed to disturb central nervous system function by binding to nicotinic acetylcholine receptors, although they present a weak affinity for nicotinic acetylcholine receptor subtypes in vertebrates and a poor penetration of the blood–brain barrier (Sheets et al., 2016). Recent studies have suggested that thiacloprid, thiamethoxam and imidacloprid can disturb the function of aromatase in feto‐placental co‐cultures, leading to an increased oestradiol and oestrone production (Caron‐Beaudoin, Denison, & Sanderson, 2016; Caron‐Beaudoin, Viau, Hudon‐Thibeault, Vaillancourt, & Sanderson, 2017). These three neonicotinoids have also been shown to induce the expression of CYP3A7, impeding the 16α‐hydroxylation of fetal dehydroepiandrosterone sulphate (Caron‐Beaudoin et al., 2017) suggesting an endocrine disruptive effect.
Although our results suggest a lack of short‐term effects on hormone receptor activation for the seven neonicotinoids tested here (except for imidacloprid and adipogenicity), it is important not to ignore the possibility of effects arising from long‐term exposure, which are the most typical scenario for endocrine disruptors. Assessment of outcomes from chronic exposure should be undertaken in laboratory animals in accordance with OECD guidelines, as there are no validated assays available to assess the long‐term effects of pollutants in cell lines in vitro. In addition, some chemical compounds have J‐ or inverted J‐shaped dose–responses, which become detectable with high confidence with a large number of replicates. This is the case of the hCAR inhibitor‐agonist effects of 4‐aminoazobenzene, which were detected after completion of 37 replicates (Bogen, 2017). In general, endocrine disrupting chemicals can have pleiotropic effects because they can bind to different receptors with different affinities causing non‐monotonic dose–responses (Vandenberg, 2014).
In conclusion, the seven neonicotinoid insecticides we tested were neither oestrogenic nor thyroidogenic in cell proliferation assays. On the other hand, we observed indications of adipogenic effects mediated by the activation of PXR by imidacloprid. Our data confirm that at least one neonicotinoid insecticide can have effects on mammalian physiology. Thus, evaluation of their non‐target effects should move beyond the assumption that their only potential targets are nicotinic acetylcholine receptors.
CONFLICT OF INTEREST
The authors did not report any conflict of interest.
AUTHORS' CONTRIBUTIONS
RM and MB performed the cell culture experiments, analysed the results and drafted the manuscript. DG and LW participated in the cell culture experiments. MNA co‐ordinated the investigation and drafted the manuscript.
ACKNOWLEDGEMENTS
This work was funded by the Sustainable Food Alliance (USA) whose support is gratefully acknowledged. We thank Dr Christopher D. Kassotis for his invaluable help in optimizing the adipogenesis assay.
Mesnage R, Biserni M, Genkova D, Wesolowski L, Antoniou MN. Evaluation of neonicotinoid insecticides for oestrogenic, thyroidogenic and adipogenic activity reveals imidacloprid causes lipid accumulation. J Appl Toxicol. 2018;38:1483–1491. 10.1002/jat.3651
REFERENCES
- Bogen, K. T. (2017). Linear‐no‐threshold default assumptions are unwarranted for cytotoxic endpoints independently triggered by ultrasensitive molecular switches. Risk Analysis, 37, 1808–1816. [DOI] [PubMed] [Google Scholar]
- Boucher, J. G. , Boudreau, A. , Ahmed, S. , & Atlas, E. (2015). In vitro effects of bisphenol a β‐d‐glucuronide (BPA‐G) on adipogenesis in human and murine preadipocytes. Environmental Health Perspectives, 123, 1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron‐Beaudoin, E. , Denison, M. S. , & Sanderson, J. T. (2016). Effects of neonicotinoids on promoter‐specific expression and activity of aromatase (CYP19) in human adrenocortical carcinoma (H295R) and primary umbilical vein endothelial (HUVEC) cells. Toxicological Sciences, 149, 134–144. [DOI] [PubMed] [Google Scholar]
- Caron‐Beaudoin, E. , Viau, R. , Hudon‐Thibeault, A. A. , Vaillancourt, C. , & Sanderson, J. T. (2017). The use of a unique co‐culture model of fetoplacental steroidogenesis as a screening tool for endocrine disruptors: The effects of neonicotinoids on aromatase activity and hormone production. Toxicology and Applied Pharmacology, 332, 15–24. [DOI] [PubMed] [Google Scholar]
- Cimino, A. M. , Boyles, A. L. , Thayer, K. A. , & Perry, M. J. (2017). Effects of neonicotinoid pesticide exposure on human health: a systematic review. Environmental Health Perspectives, 125, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn, T. , vom Saal, F. S. , & Soto, A. M. (1993). Developmental effects of endocrine‐disrupting chemicals in wildlife and humans. Environmental Health Perspectives, 101, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (2012). Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal, 10, 2579. [Google Scholar]
- European Food Safety Authority (2017). National summary reports on pesticide residue analysis performed in 2015. EFSA Supporting Publications, 14, 1211E–n/a. [Google Scholar]
- Filer, D. , Patisaul, H. B. , Schug, T. , Reif, D. , & Thayer, K. (2014). Test driving ToxCast: endocrine profiling for 1858 chemicals included in phase II. Current Opinion in Pharmacology, 19, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini, J. B. , Mughal, B. B. , Le Mevel, S. , Leemans, M. , Lettmann, M. , Spirhanzlova, P. , … Demeneix, B. A. (2017). Human amniotic fluid contaminants alter thyroid hormone signalling and early brain development in Xenopus embryos. Scientific Reports, 7, 43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaveny, C. A. , & Perdew, G. H. (2009). Transgenic humanized AHR mouse reveals differences between human and mouse AHR ligand selectivity. Molecular and Cellular Pharmacology, 1, 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun, F. , & Blumberg, B. (2006). Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology, 147, S50–S55. [DOI] [PubMed] [Google Scholar]
- Harada, K. H. , Tanaka, K. , Sakamoto, H. , Imanaka, M. , Niisoe, T. , Hitomi, T. , … Koizumi, A. (2016). Biological monitoring of human exposure to neonicotinoids using urine samples, and neonicotinoid excretion kinetics. PLoS One, 11, e0146335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel, J. J. , Blumberg, B. , Cave, M. , Machtinger, R. , Mantovani, A. , Mendez, M. A. , … Vom Saal, F. (2017). Metabolism disrupting chemicals and metabolic disorders. Reproductive Toxicology, 68, 3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, M. , Beguin, M. , Requier, F. , Rollin, O. , Odoux, J. F. , Aupinel, P. , … Decourtye, A. (2012). A common pesticide decreases foraging success and survival in honey bees. Science, 336, 348–350. [DOI] [PubMed] [Google Scholar]
- Mesnage, R. , & Antoniou, M. N. (2017). Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Frontiers in Public Health, 5, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnage, R. , Bernay, B. , & Seralini, G. E. (2013). Ethoxylated adjuvants of glyphosate‐based herbicides are active principles of human cell toxicity. Toxicology, 313, 122–128. [DOI] [PubMed] [Google Scholar]
- Mesnage, R. , Defarge, N. , Spiroux de Vendomois, J. , & Seralini, G. E. (2014). Major pesticides are more toxic to human cells than their declared active principles. BioMed Research International, 2014, 179691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65, 55–63. [DOI] [PubMed] [Google Scholar]
- Mostafalou, S. , & Abdollahi, M. (2017). Pesticides: an update of human exposure and toxicity. Archives of Toxicology, 91, 549–599. [DOI] [PubMed] [Google Scholar]
- Pandey, S. P. , & Mohanty, B. (2015). The neonicotinoid pesticide imidacloprid and the dithiocarbamate fungicide mancozeb disrupt the pituitary‐thyroid axis of a wildlife bird. Chemosphere, 122, 227–234. [DOI] [PubMed] [Google Scholar]
- Park, Y. , Kim, Y. , Kim, J. , Yoon, K. S. , Clark, J. , Lee, J. , & Park, Y. (2013). Imidacloprid, a neonicotinoid insecticide, potentiates adipogenesis in 3T3‐L1 adipocytes. Journal of Agricultural and Food Chemistry, 61, 255–259. [DOI] [PubMed] [Google Scholar]
- Richard, A. M. , Judson, R. S. , Houck, K. A. , Grulke, C. M. , Volarath, P. , Thillainadarajah, I. , … Thomas, R. S. (2016). ToxCast Chemical Landscape: Paving the Road to 21st Century Toxicology. Chemical Research in Toxicology, 29, 1225–1251. [DOI] [PubMed] [Google Scholar]
- Saillenfait, A. M. , Gallissot, F. , Langonne, I. , & Sabate, J. P. (2002). Developmental toxicity of N‐methyl‐2‐pyrrolidone administered orally to rats. Food and Chemical Toxicology, 40, 1705–1712. [DOI] [PubMed] [Google Scholar]
- Sekeroglu, V. , Sekeroglu, Z. A. , & Demirhan, E. (2014). Effects of commercial formulations of deltamethrin and/or thiacloprid on thyroid hormone levels in rat serum. Toxicology and Industrial Health, 30, 40–46. [DOI] [PubMed] [Google Scholar]
- Sheets, L. P. , Li, A. A. , Minnema, D. J. , Collier, R. H. , Creek, M. R. , & Peffer, R. C. (2016). A critical review of neonicotinoid insecticides for developmental neurotoxicity. Critical Reviews in Toxicology, 46, 153–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbasku, D. A. , Pakala, R. , Sato, H. , & Eby, J. E. (1991). Thyroid hormone dependent pituitary tumor cell growth in serum‐free chemically defined culture. A new regulatory role for apotransferrin. Biochemistry, 30, 7466–7477. [DOI] [PubMed] [Google Scholar]
- Soto, A. M. , Sonnenschein, C. , Chung, K. L. , Fernandez, M. F. , Olea, N. , & Serrano, F. O. (1995). The E‐SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environmental Health Perspectives, 103(Suppl. 7), 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg, L. N. (2014). Non‐monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol a as a case study. Dose‐Response, 12, 259–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehorn, P. R. , O'Connor, S. , Wackers, F. L. , & Goulson, D. (2012). Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science, 336, 351–352. [DOI] [PubMed] [Google Scholar]
- Xiang, D. , Han, J. , Yao, T. , Wang, Q. , Zhou, B. , Mohamed, A. D. , & Zhu, G. (2017). Editor's Highlight: Structure‐based investigation on the binding and activation of typical pesticides with thyroid receptor. Toxicological Sciences, 160, 205–216. [DOI] [PubMed] [Google Scholar]


