Abstract
Glasdegib is a Hedgehog pathway inhibitor. This ongoing, open‐label, phase 2 study (NCT01546038) evaluated glasdegib plus cytarabine/daunorubicin in patients with untreated acute myeloid leukemia (AML) or high‐risk myelodysplastic syndromes (MDS). Patients received glasdegib 100 mg orally, once daily in continuous 28‐day cycles from day −3, with intravenous cytarabine 100 mg/m2 on days 1‐7 and daunorubicin 60 mg/m2 on days 1‐3. Patients in remission then received consolidation therapy (2‐4 cycles of cytarabine 1 g/m2 twice daily on days 1, 3, 5 of each cycle), followed by maintenance glasdegib (maximum 6 cycles). Primary endpoint was complete remission (CR) in patients aged ≥55 years. Secondary endpoints included overall survival (OS), safety and outcome by mutational status. Patients had a median (range) age of 64.0 (27‐75) years, 60.0% were male, and 84.5% were white. In 69 evaluable patients, 46.4% (80% confidence interval [CI]: 38.7‐54.1) achieved investigator‐reported CR. Among patients ≥55 years old (n = 60), 40.0% (80% CI 31.9‐48.1) achieved CR. Among all 69 patients, median OS was 14.9 (80% CI 13.4‐19.3) months, with 12‐month survival probability 66.6% (80% CI 58.5‐73.4). The most common treatment‐related adverse events (≥50% patients) were diarrhea and nausea. There were no significant associations between mutational status (12 genes) and clinical response, suggesting potential benefit across diverse molecular profiles. Glasdegib plus cytarabine/daunorubicin was well tolerated and associated with clinical activity in patients with untreated AML or high‐risk MDS. A randomized phase 3 trial of glasdegib in combination with chemotherapy (7 + 3 schedule) is ongoing.
1. INTRODUCTION
Acute myeloid leukemia (AML) is the most common acute leukemia in adults, and incidence correlates with age. Older (>60 years) patients with AML typically have poorer prognoses and outcomes compared with younger patients, with response rates ≤50% to conventional treatments frequently reported, and few patients survive >2 years.1, 2, 3, 4, 5, 6, 7, 8
The Hedgehog (Hh) signaling pathway is critical for embryogenesis, and is typically repressed after birth.9, 10, 11 In normal tissue, Hh activation is dependent on the transmembrane protein Smoothened (SMO).9, 10, 11 Hh ligand‐binding results in the Patched receptor releasing SMO, which then moves from an intracellular vesicle into the primary cilium.9, 10, 11, 12 Activated glioma (GLI)‐associated proteins then translocate to the nucleus and promote target gene transcription.9, 10, 11, 12 Aberrations in Hh signaling have been identified in a variety of human leukemias and particularly in leukemia stem cells.10, 11, 12, 13, 14 Upregulation of Hh pathway components is implicated in chemoresistant AML cell lines, and pharmacologic inhibition of the Hh pathway results in decreased multidrug resistance and P‐glycoprotein expression in these cells.10, 11, 12, 15, 16 Studies in transgenic mouse models of leukemia have also supported a role for Hh signaling in disease progression.17, 18, 19 In addition, overexpression of GLI1 is associated with relapse, drug resistance, poor remission and reduced overall survival (OS) in patients with AML.10, 11
Glasdegib (PF‐04449913) is an oral, small molecule inhibitor of the Hh pathway component SMO.20, 21, 22, 23, 24, 25 Glasdegib prevents the translocation of SMO into primary cilia and prevents SMO‐mediated activation of downstream Hh targets.12, 23, 26 Previous studies have reported glasdegib inhibition of SMO reduced the expression of key intracellular leukemia stem cell regulators (eg, GLI2) and enhanced cell cycle transit.23, 27 These results and preclinical evidence that Hh inhibition may sensitize cells to cytarabine or azacitidine10, 11, 14, 28 provided rationale for evaluating glasdegib in combination with chemotherapeutic agents to reduce resistance and leukemic persistence or progression.
In an open‐label, phase 1 study (http://clinicaltrials.gov, NCT00953758) of glasdegib in adult patients with myeloid malignancies who were refractory, resistant, or intolerant to previous agents, treatment was generally well tolerated and some preliminary clinical activity was observed.25 The most common treatment‐related adverse events (AEs) included dysgeusia (28%), decreased appetite (19%) and alopecia (15%).25 None of the 15 deaths reported were considered to be treatment‐related.25 In an open‐label, phase 1B study (NCT01546038), glasdegib was well tolerated in combination with low‐dose cytarabine (LDAC) or decitabine, or in combination with cytarabine/daunorubicin, in patients with AML or high‐risk myelodysplastic syndromes (MDS).29 The most common treatment‐related nonhematologic AEs were mostly grade 1‐2. The recommended phase 2 dose was established as 100 mg daily in combination with standard chemotherapy.29 In the phase 2 portion of the study (NCT01546038) in previously untreated patients with AML or high‐risk MDS, glasdegib plus LDAC improved OS compared with LDAC alone; the improvement was consistent among subgroups.26 Glasdegib plus LDAC was associated with an acceptable safety profile.26
The objectives of this phase 2 study were to evaluate the efficacy of glasdegib when administered in combination with standard cytarabine/daunorubicin induction (on a 7 + 3 schedule) and consolidation, followed by maintenance therapy, in patients with previously untreated AML or high‐risk MDS.
2. METHODS
2.1. Study design
This was an open‐label, phase 2, multicenter study of glasdegib in combination with cytarabine/daunorubicin in patients with previously untreated AML or high‐risk MDS. The study was conducted globally at 25 centers. The study was approved by an institutional review board or independent ethics committee at each study center, and was conducted in accordance with the study protocol, International Ethical Guidelines for Biomedical Research Involving Human Subjects (2002), Guidelines for Good Clinical Practice (1996), the Declaration of Helsinki (1996 and 2008), and applicable local regulatory requirements and laws. All patients provided written informed consent. The study is registered on http://clinicaltrials.gov (NCT01546038; Pfizer study number B1371003).
2.2. Patients
Patients ≥18 years old were registered using an Interactive Registration System. The study was designed specifically for older (≥55 years) patients; however, up to 10 patients aged <55 years were permitted to enter the study for exploratory purposes. After these 10 patients were enrolled, enrollment was restricted to patients aged ≥55 years. Patients had newly diagnosed or previously untreated AML (de novo AML, AML evolving from an antecedent hematologic disease or MDS, or AML secondary to previous cytotoxic or radiation therapy) or refractory anemia with excess blast 2 high‐risk MDS, according to the World Health Organization 2008 classification.
Patients with MDS, as well as those with AML arising from MDS or other antecedent hematologic disease, may have had one prior regimen with a commercially available agent(s) for the treatment of prior hematologic disease. Prior therapy for AML was not permitted. Patients had to have adequate organ function, with an Eastern Cooperative Oncology Group performance status <2 in order to receive intensive chemotherapy.
Patients were excluded if they had any of the following: t(9;22) cytogenetic translocation, acute promyelocytic leukemia, hyperleukocytosis (leukocytes ≥30 × 109/L) at screening (hydroxyurea or leukopheresis were allowed before and up to 1 week after first dose of glasdegib for control of rapidly progressing leukemia), or known active leukemia in the central nervous system. Other exclusion criteria included: serum creatinine >1.3 mg/dL, severe cardiac disease (eg, left‐ventricular ejection fraction <45% by multiple gated acquisition or echocardiography at screening), or a cumulative anthracycline dose equivalent of ≥250 mg/m2 of daunorubicin or ≥125 mg/m2 of idarubicin.
2.3. Study treatment
Patients received glasdegib 100 mg orally, once daily in continuous 28‐day cycles. Patients received induction with glasdegib from day −3 in combination with intravenous daunorubicin 60 mg/m2 (on days 1‐3) and continuous intravenous cytarabine 100 mg/m2 (on days 1‐7). If required, a second induction cycle could be initiated at the same doses as early as day 21 of cycle 1. Patients achieving complete remission (CR) were eligible to receive consolidation therapy with 2‐4 cycles of cytarabine 1 g/m2 twice daily on days 1, 3 and 5 of each cycle. Following consolidation, single‐agent glasdegib 100 mg administered daily continuously, as maintenance therapy, for a maximum of 6 cycles (1 cycle = 28 days) was permitted. Patients who completed the maximum number of treatment cycles and demonstrated clinical benefit were allowed to remain on therapy upon agreement between the investigator and sponsor.
Study treatment continued until the patient withdrew consent, developed unacceptable toxicity, or demonstrated either resistant disease during induction or disease progression/relapse after induction. Patients could proceed to allogeneic stem cell transplant; those who did were removed from study treatment and followed for survival. The 100 mg glasdegib daily dose could be temporarily interrupted or reduced to 50 mg to manage toxicity. After cycle 1, if a toxicity was attributed to the backbone chemotherapy and not to glasdegib, chemotherapeutics could be delayed or reduced, with continuation of glasdegib. If a patient had a dose reduction for a study drug‐related toxicity, the dose was not to be re‐escalated.
2.4. Assessments
2.4.1. Clinical activity
Efficacy endpoints were based on investigator assessment, using the modified International Working Group criteria.30, 31 Bone marrow evaluations were performed on day 21 of induction cycle 1, and thereafter at the investigator's discretion prior to the next cycle of chemotherapy. If a second cycle of induction therapy was required, a bone marrow evaluation was performed on day 21 of induction cycle 2. Patients underwent bone marrow evaluations on day 21 of the consolidation final cycle, day 1 of maintenance cycles 3 and 6. A bone marrow aspirate was required at end of treatment regardless of when the patient discontinued the trial, unless the end of treatment was within 14 days of a prior evaluation. All aspirate collections were mandatory unless deemed inappropriate by the investigator and agreed by the sponsor. For the purposes of calculating response duration, bone marrow collection dates were used as the start/stop dates. Patients still in CR/CR with incomplete blood count recovery (CRi)/morphologic leukemia‐free state (MLFS) at the most recent bone marrow assessment were censored at the end of treatment.
2.4.2. Safety
Safety evaluations included physical examinations, laboratory tests, 12‐lead electrocardiograms, and AEs, with monitoring graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
2.4.3. Pharmacokinetics
Patient blood samples were collected for pharmacokinetic (PK) analysis of glasdegib at protocol‐defined time points. Glasdegib plasma predose concentration (C trough) levels were estimated based on sparse PK sampling data. Additional details of PK analyses can be found in the Supporting Information Materials.
2.5. Mutational analyses
Baseline central laboratory assessments included analysis of 12 genes frequently mutated in patients with AML or MDS. Additional details can be found in the Supporting Information Materials.
2.6. Statistical analyses
The primary endpoint analyses for this study were based on patients who were ≥55 years old. The primary endpoint was CR, with the final analysis timing defined as deaths in at least 40 of 60 patients ≥55 years old. However, due to a lower than anticipated death rate, a decision was made to declare the primary completion date and data cut‐off date as January 3, 2017, wherein 38 deaths were reported.
A key secondary endpoint was OS. Other secondary endpoints included: disease‐specific efficacy endpoints; the type, incidence, severity, timing, seriousness and relatedness of AEs; corrected QT (QTc) interval; glasdegib PK; and mutational analyses. Exploratory endpoints included: CR in patients younger than 55 years and efficacy endpoints based on cytogenetic risk. For AML, cytogenetic risk was assessed using European Leukemia Net (ELN) Risk Criteria 2010.32 For MDS, the International Prognostic Scoring System (IPSS) was used.33
The planned sample size was approximately 70 patients. The focus of the primary analysis was patients aged ≥55 years, with 60 patients ≥55 years old providing ≥82% power to reject the null hypothesis of CR rate of 54% (cytarabine/daunorubicin alone) if the true CR rate of the combination with glasdegib is 68%. This allowed a one‐sided type I error rate of 10% under a binomial distribution and one futility analysis at 2 months after the enrollment of the 30th patient. Up to 10 patients <55 years old were to be enrolled for exploratory purposes.
The full analysis set included all enrolled patients who received at least one dose of study medication. The PK analysis population included all treated patients who had at least one PK parameter estimated. The baseline mutational analysis population included all treated patients evaluable for both baseline mutational status and response.
Descriptive statistics were used throughout the study unless otherwise stated. For the primary efficacy endpoint and binary efficacy endpoints, point estimates and 80% confidence intervals (CI) were provided; all were based on investigator assessment data. Time‐to‐event endpoints were summarized using the Kaplan‐Meier method. Median event times and two‐sided 80% CIs were provided. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
3. RESULTS
3.1. Patients
A total of 71 patients aged ≥55 years (n = 61) or <55 years (n = 10) were enrolled: 66 patients with AML and five patients with MDS; 69 patients (n = 60/9, aged ≥55/<55 years) were treated and included in the full analysis set; two patients were enrolled, but not treated. Of 69 patients who were treated, six (8.7%) completed treatment and 63 (91.3%) patients discontinued treatment (Supporting Information Figure S1). The main reasons for discontinuations from study treatments were: insufficient clinical response (n = 29; 42.0%) and “other” (n = 15; 21.7%), with the majority of “other” due to transplantation (n = 12/15); 11 (15.9%) patients permanently discontinued study treatments due to treatment‐related AEs, which included neutrophil count decreased and pneumonitis (related to daunorubicin and cytarabine); myocarditis (related to daunorubicin and glasdegib); intermittent elevated alanine aminotransferase (ALT) (related to daunorubicin, cytarabine and glasdegib); diarrhea (related to daunorubicin, cytarabine and glasdegib) and nausea in one patient; gastrointestinal bleeding and infectious enterocolitis in one patient; adenovirus infection; worsening muscle cramps; epigastric pain; atrial fibrillation; and elevated aspartate aminotransferase and ALT in one patient.
Most patients were male (n = 43; 60.6%) and white (n = 60; 84.5%), with a median age of 64.0 (range 27‐75) years. The majority of patients had Eastern Cooperative Oncology Group performance status 1 (n = 44; 62.0%) and good/intermediate cytogenetic risk (ie, favorable, intermediate‐I, or intermediate‐II risk; n = 50; 70.4%) (Supporting Information Table S1). Seven (9.9%) patients received prior hypomethylating agents (decitabine or azacitidine).
The number of patients starting each cycle was: glasdegib induction, n = 69 (100.0%); re‐induction cycle 2, n = 14 (20.3%); consolidation, n = 26 (37.7%); and maintenance, n = 15 (21.7%). Of the 15 patients who received at least 1 cycle of maintenance therapy, 6 completed 6 cycles, 6 discontinued due to cytogenetic or morphological relapse, 2 discontinued due to AEs (grade 1‐2 nausea/diarrhea; grade 3 muscle spasms) and one patient refused further treatment after receiving 11 cycles of maintenance. Following discontinuation of the study treatment, 72.5% (n = 50) of patients received follow‐up systemic therapies, with the majority of patients (n = 39) receiving chemotherapy (Supporting Information Table S2).
The median exposure to glasdegib was 48.0 (range 10‐501) days. The mean relative glasdegib dose intensity was 89.6% and the mean relative chemotherapy dose intensities were 99.2% and 99.1% for cytarabine and daunorubicin, respectively.
3.2. Clinical activity
Of the 69 patients, 46.4% (80% CI 38.7‐54.1) achieved CR (Table 1). For the primary analysis, CR (80% CI) was reported in 40.0% (31.9‐48.1) of patients aged ≥55 years and 88.9% (75.5‐100.0) in patients aged <55 years; 37 (53.6% [80% CI 45.9‐61.3]) patients overall, 35 (54.7% [80% CI 46.7‐62.7]) with AML and 2 (40.0% [80% CI 11.9‐68.1]) with MDS achieved CR/CRi (Supporting Information Tables S3 and S4). The median (range) duration of CR was 94 (1‐480) days in all patients and 103 (1‐480) and 50 (1‐268) days in patients aged ≥55 years and <55 years, respectively. The median (range) duration of CR, CRi, or MLFS across all patients was 53 (1‐480) days. The number of patients censored for CR duration was 16/24 (66.7%) and 5/8 (62.5%) in the groups aged ≥55 and <55 years, respectively.
Table 1.
Summary of proportions of patients with investigator‐reported complete remission (full analysis set), by age group
| Total | ≥55 years | <55 years | |
|---|---|---|---|
| Total | n = 69 | n = 60 | n = 9 |
| CR, n (%) | 32 (46.4) | 24 (40.0) | 8 (88.9) |
| 80% CIa | (38.7‐54.1) | (31.9‐48.1) | (75.5‐100.0) |
| Cytogenetic risk | |||
| Good/intermediate | n = 48 | n = 41 | n = 7 |
| CR, n (%) | 26 (54.2) | 20 (48.8) | 6 (85.7) |
| 80% exact CIa | (45.0‐63.4) | (38.8‐58.8) | (68.8‐100.0) |
| Poor | n = 19 | n = 17 | n = 2 |
| CR, n (%) | 5 (26.3) | 3 (17.6) | 2 (100.0) |
| 80% exact CIa | (13.4‐39.3) | (5.8‐29.5) | (100.0‐100.0) |
| Not evaluated | n = 2 | n = 2 | n = 0 |
| CR, n (%) | 1 (50.0) | 1 (50.0) | ‐ |
| 80% exact CIa | (4.7‐95.3) | (4.7‐95.3) | ‐ |
Abbreviations: AML, acute myeloid leukemia; CI, confidence interval; CR, complete remission; MDS, myelodysplastic syndromes.
For AML, good/intermediate cytogenetic risk = favorable, intermediate‐I and intermediate‐II risk groups; poor cytogenetic risk = adverse risk group.
For MDS, good/intermediate cytogenetic risk = good and intermediate risk groups; poor cytogenetic risk = poor risk group.
CR included both confirmed and unconfirmed responses for MDS patients.
Using normal approximation and CIs are expressed in percentages.
Median OS was 14.9 (80% CI 13.4‐19.3) months, with a 12‐month survival probability of 66.6% (80% CI 58.5‐73.4) (Figure 1). Median OS (80% CI) in patients aged ≥55 years and <55 years was 14.7 (13.1‐17.7) months and not estimable (NE) (11.0‐NE) months, respectively (Figure 1).
Figure 1.
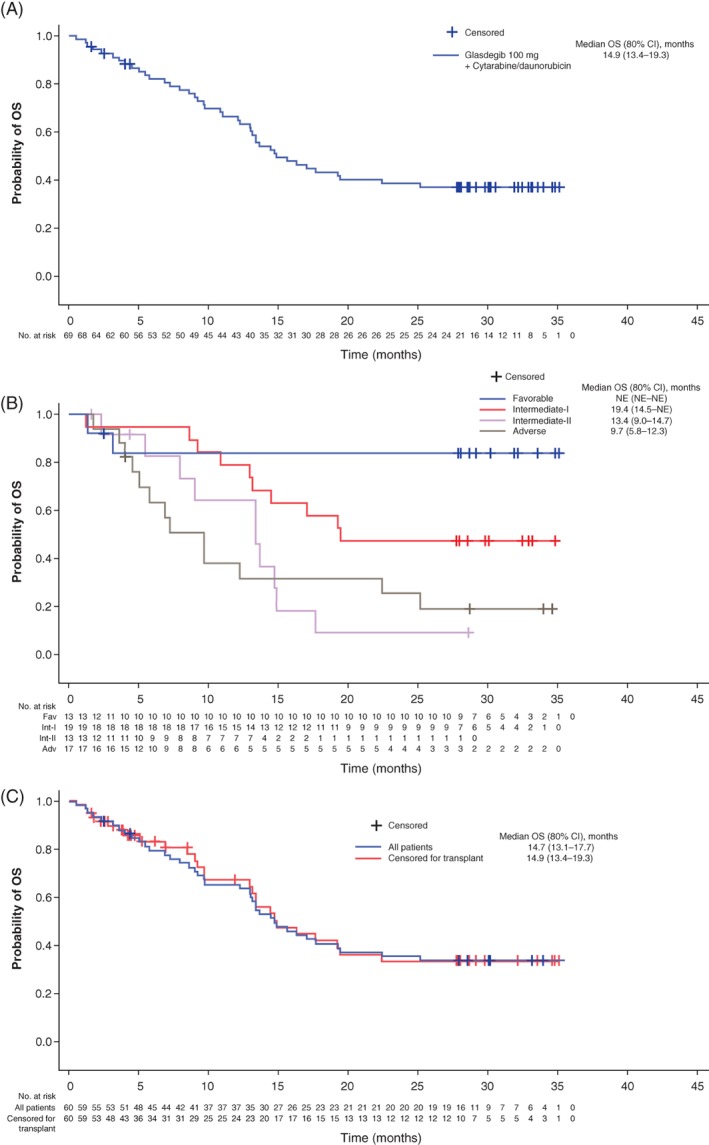
A, Kaplan‐Meier plot of OS in all patients; B, patients with AML by cytogenetic risk and C, patients aged ≥55 years with and without censoring for patients receiving a transplant. Adv, adverse; AML, acute myeloid leukemia; CI, confidence interval; NE, not evaluable; Fav, favorable; Int, intermediate; OS, overall survival [Color figure can be viewed at http://wileyonlinelibrary.com]
Twenty‐four (34.8%) patients received an allogeneic hematopoietic cell transplant: 18 aged ≥55 years and 6 aged <55 years. Twelve (17.4%) patients discontinued treatment in order to receive a transplant: 9 aged ≥55 years and 3 aged <55 years; 12 patients discontinued treatment for other reasons and later received a transplant. After censoring for transplant, median OS (80% CI) was 17.7 (14.5‐NE) months for all patients, 14.9 (13.4‐19.3) months in patients ≥55 years of age, and NE (NE‐NE) in patients <55 years of age (Figure 1).
In patients with AML vs. MDS, the estimated median OS (80% CI) was 16.3 (13.4‐19.4) months vs. 13.0 (11.0‐15.6) months (Supporting Information Table S5). In patients with AML who were aged ≥55 years, median OS (80% CI) was 14.7 (13.1‐19.3) months. Although patient numbers were small, OS in patients aged ≥55 years, as well as in patients aged >60 years, compared favorably with estimated OS according to ELN risk criteria32: median OS (80% CI) in patients with AML aged ≥55 years was NE (NE‐NE) in those with favorable risk, 19.3 (13.1‐NE) months in those with intermediate‐I risk, 13.4 (9.0‐14.7) months in those with intermediate‐II risk and 8.5 (5.8‐12.3) months in patients categorized with adverse risk (Table 2: Supportive Information Figure S2).
Table 2.
OS in patients with AML ≥55 years of age and aged >60 years, compared with historical controls
| Total | AML cytogenetic risk | |||||
|---|---|---|---|---|---|---|
| Favorable | Intermediate I | Intermediate II | Adverse | Not evaluated | ||
| Patients aged ≥55 years with AML | ||||||
| n | 58 | 12 | 15 | 13 | 16 | 2 |
| Deathsa, n (%) | 36 (62.1) | 2 (16.7) | 9 (60.0) | 10 (76.9) | 13 (81.3) | 2 (100) |
| Disease under study | 29 (50.0) | 1 (8.3) | 7 (46.7) | 10 (76.9) | 10 (62.5) | 1 (50.0) |
| Unknown | 4 (6.9) | 0 | 2 (13.3) | 0 | 1 (6.3) | 1 (50.0) |
| Other | 11 (19.0) | 1 (8.3) | 3 (20.0) | 2 (15.4) | 4 (25.0) | 1 (50.0) |
| Number censored, n (%) | 22 (37.9) | 10 (83.3) | 6 (40.0) | 3 (23.1) | 3 (18.8) | 0 |
| Probability of survival at month 12 (80% CI)b | 64.0 | 82.5 | 80.0 | 64.2 | 37.5 | 50.0 |
| (55.1‐71.6) | (62.0‐92.6) | (62.6‐89.9) | (42.8‐79.3) | (22.4‐52.6) | (7.7‐82.9) | |
| Median OS (80% CI)c, months | 14.7 | NE | 19.3 | 13.4 | 8.5 | 8.4 |
| (13.1‐19.3) | (NE‐NE) | (13.1‐NE) | (9.0‐14.7) | (5.8‐12.3) | (0.5‐16.3) | |
| Patients aged >60 years with AML | ||||||
| n | 44 | 9 | 12 | 11 | 10 | 2 |
| Deathsa, n (%) | 29 (65.9) | 1 (11.1) | 9 (75.0) | 9 (81.8) | 8 (80.0) | 2 (100) |
| Disease under study | 23 (52.3) | 0 | 7 (58.3) | 9 (81.8) | 6 (60.0) | 1 (50.0) |
| Unknown | 3 (6.8) | 0 | 2 (16.7) | 0 | 0 | 1 (50.0) |
| Other | 10 (22.7) | 1 (11.1) | 3 (25.0) | 2 (18.2) | 3 (30.0) | 1 (50.0) |
| Number censored, n (%) | 15 (34.1) | 8 (88.9) | 3 (25.0) | 2 (18.2) | 2 (20.0) | 0 |
| Probability of survival at month 12 (80% CI)b | 62.4 | 88.9 | 75.0 | 60.6 | 30.0 | 50.0 |
| (52.1‐71.1) | (65.4‐96.8) | (54.6‐87.2) | (38.3‐77.0) | (13.4‐48.7) | (7.7‐82.9) | |
| Median OS (80% CI)c, months | 14.5 | NE | 15.7 | 13.4 | 8.5 | 8.4 |
| (13.0‐17.7) | (NE‐NE) | (13.0‐19.4) | (7.9‐13.7) | (4.0‐9.7) | (0.5‐16.3) | |
| Historical controlsd (n = 710)32 | ||||||
| Median OS (95% CI), months | 8.7 | 14.6 | 9.5 | 9.2 | 4.8 | ‐ |
| (7.8‐9.7) | (11.7‐17.6) | (7.3‐11.7) | (7.1‐11.3) | (3.7‐5.9) | ||
Abbreviations: AML, acute myeloid leukemia; CI, confidence interval; NE, not estimable; OS, overall survival.
Patients may have multiple reasons for cause of death.
Calculated from the product‐limit method.
Based on the Brookmeyer and Crowley method.
Treatment with 7 + 3 schedule.
3.3. Safety
A total of 1824 AEs were reported among all 69 patients. The most common all‐causality AEs (in ≥50% of patients) were diarrhea, febrile neutropenia, nausea and hypokalemia (Table 3). The most frequently reported grade > 3 all‐causality AEs (in ≥30% of patients) were febrile neutropenia, anemia and thrombocytopenia; the most common nonhematologic grade > 3 all‐causality AEs were hypertension, pneumonia and sepsis (n = 7 [10.1%] each). The most common treatment‐related AEs (in ≥50% of patients) were diarrhea and nausea. The most frequently reported grade > 3 treatment‐related AEs (≥25% of patients) were febrile neutropenia and anemia. Median (range) investigator‐reported time to platelet recovery (≥100 000 μL) and neutrophil recovery (≥1000 μL) for patients achieving CR (n = 32) was 31.0 (20‐82) days and 32.0 (3‐95) days, respectively.
Table 3.
Treatment‐emergent all‐causality adverse events reported in ≥30% patients (safety analysis set)
| n (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|---|
| Any adverse event | 0 | 1 (1.4) | 11 (15.9) | 52 (75.4) | 5 (7.2) | 69 (100.0) |
| Diarrhea | 30 (43.5) | 18 (26.1) | 1 (1.4) | 0 | 0 | 49 (71.0) |
| Febrile neutropenia | 0 | 0 | 42 (60.9) | 2 (2.9) | 0 | 44 (63.8) |
| Nausea | 20 (29.0) | 18 (26.1) | 2 (2.9) | 0 | 0 | 40 (58.0) |
| Hypokalemia | 19 (27.5) | 9 (13.0) | 8 (11.6) | 1 (1.4) | 0 | 37 (53.6) |
| Pyrexia | 21 (30.4) | 9 (13.0) | 3 (4.3) | 1 (1.4) | 0 | 34 (49.3) |
| Constipation | 23 (33.3) | 9 (13.0) | 0 | 0 | 0 | 32 (46.4) |
| Anemia | 0 | 2 (2.9) | 26 (37.7) | 0 | 0 | 28 (40.6) |
| Decreased appetite | 15 (21.7) | 10 (14.5) | 1 (1.4) | 0 | 0 | 26 (37.7) |
| Fatigue | 9 (13.0) | 13 (18.8) | 3 (4.3) | 0 | 0 | 25 (36.2) |
| Vomiting | 20 (29.0) | 5 (7.2) | 0 | 0 | 0 | 25 (36.2) |
| Hyponatremia | 16 (23.2) | 0 | 8 (11.6) | 0 | 0 | 24 (34.8) |
| Thrombocytopenia | 0 | 1 (1.4) | 3 (4.3) | 19 (27.5) | 0 | 23 (33.3) |
| Abdominal pain | 11 (15.9) | 9 (13.0) | 2 (2.9) | 0 | 0 | 22 (31.9) |
| Headache | 11 (15.9) | 9 (13.0) | 2 (2.9) | 0 | 0 | 22 (31.9) |
| Hypocalcaemia | 5 (7.2) | 12 (17.4) | 5 (7.2) | 0 | 0 | 22 (31.9) |
| Edema peripheral | 18 (26.1) | 4 (5.8) | 0 | 0 | 0 | 22 (31.9) |
| ALT increased | 16 (23.2) | 1 (1.4) | 3 (4.3) | 1 (1.4) | 0 | 21 (30.4) |
| Chills | 13 (18.8) | 8 (11.6) | 0 | 0 | 0 | 21 (30.4) |
Safety analysis set included all enrolled patients who received at least one dose of any of the study medications.
Patients were counted only once per preferred term in each row. Each count was based on the maximum grade of events.
MedDRA (version 19.1) coding dictionary applied.
Adverse events were graded in accordance with National Cancer Institute CTCAE version 4.03.
Abbreviations: ALT, alanine aminotransferase; MedDRA, Medical Dictionary for Regulatory Activities; CTCAE, Common Terminology Criteria for Adverse Events.
Across all patients, 14 (20.3%) and 25 (36.2%) patients permanently and temporarily, respectively, discontinued study treatments (glasdegib and/or cytarabine/daunorubicin) due to AEs; 5 (7.2%) patients had dose reductions due to AEs and 35 (50.7%) patients reported serious AEs (SAEs). The most frequently reported SAEs (≥5% of patients) were febrile neutropenia, sepsis, and pneumonia, and all other SAEs were reported by no more than two patients.
Over the course of the study, 41 (59.4%) deaths were reported; 5 (7.2%) treatment‐emergent deaths occurred within 28 days of the last dose: disease progression (n = 2) and sepsis, pneumonia and septic shock (n = 1 each). One case of sepsis was considered by the investigator to be treatment‐related to combination glasdegib plus cytarabine/daunorubicin. One (1.4%) and four (5.8%) patients (all aged ≥55 years) died ≤30 and ≤60 days, respectively, from the first dose of study treatments. No patients with CR died while on active treatment. Thirty‐six (52.2%) deaths occurred during the follow‐up period (>28 days after the last dose); the main cause among all 69 patients was progression of the disease under study (n = 30; 43.5%).
AEs of special interests for glasdegib, including muscle spasms, dysgeusia, alopecia, acute kidney injury, or electrocardiogram prolonged QTc, were nonserious; the majority were grades 1 or 2, except for one case of serious acute kidney injury that was considered not treatment‐related. The grade 3 acute kidney injury (creatinine 2.47 mg/dL) occurred after a hypotensive episode that resolved without intervention and was considered to be related to analgesics and dehydration. On the same day, the patient was diagnosed with grade 3 acute myocardial infarction (MI); thus, the investigator considered the acute kidney injury to be related to the non‐ST‐elevation MI event together with severe hypotension. Muscle spasms led to permanent discontinuation of glasdegib in one patient. No clinically significant glasdegib‐related QTc prolongation was observed.
3.4. Pharmacokinetics
In all, 65 of 69 patients enrolled in the study provided glasdegib plasma concentration data. Forty‐two patients were considered to be dose compliant and did not receive cytochrome P450 (CYP)3A4 inhibitors around the time of PK sampling (which might have influenced glasdegib exposure), and provided steady‐state C trough parameter data on cycle 1 day 10. The observed glasdegib steady‐state C trough geometric mean value was 308.7 ng/mL (geometric % coefficient of variance, 74%).
3.5. Mutational analyses
Fifty patients were included in baseline mutational analyses of bone marrow and/or peripheral blood with relationship to response. No significant associations were evident between mutational status of any of the 12 genes analyzed and clinical response (Supporting Information Table S6).
4. DISCUSSION
This was a phase 2, open‐label, international, multicenter, safety and efficacy study wherein glasdegib was administered in combination with cytarabine/daunorubicin in previously untreated patients with AML or high‐risk MDS. Although the primary objective of demonstrating a ≥54% CR rate in patients aged ≥55 years was not achieved, the response rate of 46.4% (80% CI 38.7‐54.1) for all patients was within the range of those reported for other AML therapies (19‐76%, with most values ~40‐50%).2, 3, 32, 34, 35, 36, 37, 38, 39, 40, 41 This result included CR rates reported for patients with AML who received standard doses of cytarabine/daunorubicin on the 7 + 3 schedule.4, 42 However, comparisons between trials are limited due to differences in study design and patient populations, as well as the effect of age and AML risk category on CR rates.4
Glasdegib in combination with cytarabine/daunorubicin has the potential to demonstrate improved OS in patients with AML or high‐risk MDS. Median OS of 14.9 months was achieved with glasdegib 100 mg combined with cytarabine/daunorubicin in the overall patient population and was within the range for OS (6.5‐24.5 months) reported in the literature for patients treated with other AML treatment regimens, including a trial of high‐dose daunorubicin in older patients with AML.2, 3, 4, 5, 32, 34, 37, 38, 40, 41, 42, 43 Despite the limited sample sizes, in a post hoc analysis, the median OS compared favorably with historical controls across ELN risk groups.3, 32, 40, 41, 42
Around 24 months, the OS curve for patients 55 years or older began to plateau and extended to 36 months. These results suggest that patients who reached this time point (~40% of patients) had a low risk of death thereafter, which may indicate that a subpopulation of patients in particular derived a survival benefit from glasdegib treatment. The survival benefit does not appear to be influenced by transplantation, given that OS results with and without censoring for patients who underwent transplant were similar. Longer follow‐up and, particularly, randomized trials are required to better assess the possible effect glasdegib may have in improving OS. Responses were observed across diverse mutational profiles, suggesting the potential for broad efficacy of glasdegib in combination with cytarabine/daunorubicin.
The primary endpoint, CR rate 54%, was based on the CR rate of cytarabine/daunorubicin alone, adapted from Burnett et al.44 Given the unique mechanism of action of glasdegib as an inhibitor of SMO, traditional endpoints such as CR or CR/CRi rate may underestimate its clinical benefit. For example, preclinical studies have shown that SMO inhibitors have little direct cytotoxicity on bulk AML blasts and would therefore not be expected to have a large effect on CR rates.45, 46 If glasdegib eliminated AML stem cells, one would expect the effect to be primarily on relapse rate and OS. Indeed, despite the small patient numbers in this trial, there was encouraging evidence of potentially prolonged OS with glasdegib compared with historical data. Although the response duration was short in this analysis, it was impacted by patients who discontinued treatment due to transplant and, because disease status was not collected during survival follow‐up, it was also derived by censoring patients at the end of treatment (even those in CR, CRi, MLFS); therefore, the most conservative calculations were used.
Skin has been typically used to measure the pharmacodynamics of SMO inhibitors and glasdegib 100 mg once daily as monotherapy was previously associated with Hh pathway knockdown of >80% in skin.47 In the current study, assessment of the effects of glasdegib on Hh pathway‐dependent transcripts such as GLI1 and GLI2 in blood was attempted, but proved infeasible because baseline expression was undetectable in most patients (data not shown). Hence, a limitation of this study is the lack of specific data demonstrating modulation of Hh target genes in AML blasts or AML stem cells. Hh ligands are secreted proteins; therefore, any combination of autocrine and/or paracrine signaling between tumor and stroma would be susceptible to modulation by glasdegib.48 Recent reports have implicated Hh signaling in the bone marrow stem cell niche as a primary mediator of chemoresistance, suggesting an alternative mechanism of action for glasdegib in AML.49, 50
The combination of glasdegib with cytarabine/daunorubicin was well tolerated, with a safety profile consistent with those observed in patients with AML receiving intensive chemotherapy.1, 3, 5, 34, 36, 37, 40, 51, 52 Additionally, the AEs observed with glasdegib in combination cytarabine/daunorubicin were similar to those reported for glasdegib in combination with LDAC,26 as well as other SMO inhibitors, and the majority of muscle spasms, dysgeusia and alopecia AEs were grades 1 or 2.53, 54, 55 The most frequently reported grade > 3 treatment‐related AEs were febrile neutropenia and anemia, with the time to hematologic recovery in line with that previously reported in the literature for patients with AML who received cytarabine/daunorubicin on the 7 + 3 schedule.3 No new safety signals were detected.
Long‐term management of SMO inhibitor‐related toxicities has been evaluated with visomdegib and sonidegib in patients with advanced basal cell carcinoma.56, 57 The use of dose adjustments and interruptions to manage AEs have contributed to long treatment exposures and sustained clinical responses for these agents.56, 57 Therefore, it will be critical to evaluate the long‐term safety of glasdegib and potentially apply similar strategies for AE management in patients with AML or high‐risk MDS.
Although this phase 2 study is limited by small patient numbers and will require validation in a larger prospective trial, the results from this study are encouraging. Glasdegib in combination with cytarabine/daunorubicin was well tolerated and was associated with clinical activity in untreated patients with AML or high‐risk MDS, as reflected by potential prolongation of OS in the context of historical controls across all risk groups. CR rates matched historical controls; however, low rate of relapse and a suggestion of a favorable OS observed in the current study suggest that the antileukemia effect of glasdegib may be primarily mediated through the elimination of AML stem cells. To confirm this mechanism, future studies should evaluate the effect of glasdegib on MRD‐positive disease in the post‐remission setting. A randomized phase 3 trial of glasdegib in combination with chemotherapy on a 7 + 3 schedule is ongoing.
AUTHOR CONTRIBUTIONS
Conception or design of the study: Jorges E. Cortes, Weidong Wendy Ma, Mirjana Zeremski, M. Naveed Shaik, A. Douglas Laird, Ashleigh O'Connell and Geoffrey Chan. Acquisition of data: Jorges E. Cortes, Eunice S. Wang, Tadeusz Robak, Mark A. Schroeder, B. Douglas Smith, Akil Merchant, Vivian G. Oehler, Daniel J. DeAngelo, Daniel A. Pollyea and Mikkael A. Sekeres. All authors contributed equally to the analysis and interpretation of data. All authors contributed equally to the preparation and final approval of the manuscript.
CONFLICT OF INTERESTS
Jorge E. Cortes: I have research support and have worked as a paid consultant for Pfizer, Jazz Pharmaceuticals, Novartis, Astellas, Daiichi and Immunogen. B. Douglas Smith: Advisory Board and Consultant for Celgene, Jazz Pharmaceuticals, Novartis, Pfizer. Eunice S. Wang: I have served on advisory board and as a speaker for Pfizer. Akil Merchant: is an advisory board member for Agios, Pfizer and Takeda and has received research funding from Pfizer. Vivian G. Oehler: has served as an advisory board member for Pfizer. Martha Arellano: Received funding for research from Cephalon oncology. Daniel J. DeAngelo: is an advisory board member for Amgen, Pfizer, Celgene, Takeda, Shire, Jazz, Incyte and Novartis, and has received research funding from Abbvie. Daniel A. Pollyea: has served as an advisory board member for Pfizer, Servier, Takeda, Cusi, Celgene, Agios, AbbVie and Celyad, and has received research funding from Pfizer and Agios. Mikkael A. Sekeres: is an advisory board member for Celgene. Tadeusz Robak: research grant from Pfizer. Mark A. Schroeder has no conflicts of interest to disclose for this study. Weidong Wendy Ma, Mirjana Zeremski, M. Naveed Shaik, A. Douglas Laird, Ashleigh O'Connell and Geoffrey Chan are employees and shareholders of Pfizer Inc.
Supporting information
Appendix S1: Supplementary Materials
ACKNOWLEDGMENTS
The authors thank the patients and their families/caregivers, and the investigators, research nurses, study coordinators, and operations staff who contributed to this study. DNA sequencing data were generated by Mark Ozeck, Stephen Huang, Keith Ching and colleagues, at Pfizer, La Jolla, CA, USA. This study was sponsored by Pfizer Inc. Medical writing and editorial support were provided by Anne Marie McGonigal, PhD, of Engage Scientific Solutions and funded by Pfizer Inc.
Cortes JE, Douglas Smith B, Wang ES, et al. Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high‐risk MDS: Phase 2 study results. Am J Hematol. 2018;93 1301–1310. 10.1002/ajh.25238
Presented in abstract and poster form at the 22nd annual meeting of the European Hematology Association, Madrid, Spain, June 22‐25, 2017.
Funding information Pfizer Inc.
REFERENCES
- 1. Daver N, Kantarjian H, Ravandi F, et al. A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high‐risk myelodysplastic syndrome. Leukemia. 2016;30:268‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stone RM, Mazzola E, Neuberg D, et al. Phase III open‐label randomized study of cytarabine in combination with amonafide l‐malate or daunorubicin as induction therapy for patients with secondary acute myeloid leukemia. J Clin Oncol. 2015;33:1252‐1257. [DOI] [PubMed] [Google Scholar]
- 3. Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX‐351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123:3239‐3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Löwenberg B, Ossenkoppele GJ, van Putten W, et al. High‐dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361:1235‐1248. [DOI] [PubMed] [Google Scholar]
- 5. Seymour JF, Fenaux P, Silverman LR, et al. Effects of azacitidine compared with conventional care regimens in elderly (≥75 years) patients with higher‐risk myelodysplastic syndromes. Crit Rev Oncol Hematol. 2010;76:218‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Kouchkovsky I, Abdul‐Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127:53‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377:454‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059‐3087. [DOI] [PubMed] [Google Scholar]
- 10. Campbell V, Copland M. Hedgehog signaling in cancer stem cells: a focus on hematological cancers. Stem Cells Cloning. 2015;8:27‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aberger F, Hutterer E, Sternberg C, et al. Acute myeloid leukemia: strategies and challenges for targeting oncogenic Hedgehog/GLI signaling. Cell Commun Signal. 2017;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh M, Chaudhry P, Merchant AA. Primary cilia are present on human blood and bone marrow cells and mediate Hedgehog signaling. Exp Hematol. 2016;44:1181‐1187. e1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Irvine DA, Copland M. Targeting Hedgehog in hematologic malignancy. Blood. 2012;119:2196‐2204. [DOI] [PubMed] [Google Scholar]
- 14. Fukushima N, Minami Y, Kakiuchi S, et al. Small‐molecule Hedgehog inhibitor attenuates the leukemia‐initiation potential of acute myeloid leukemia cells. Cancer Sci. 2016;107:1422‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Queiroz KC, Ruela‐de‐Sousa RR, Fuhler GM, et al. Hedgehog signaling maintains chemoresistance in myeloid leukemic cells. Oncogene. 2010;29:6314‐6322. [DOI] [PubMed] [Google Scholar]
- 16. Zhou H, Ma H, Wei W, et al. B4GALT family mediates the multidrug resistance of human leukemia cells by regulating the Hedgehog pathway and the expression of p‐glycoprotein and multidrug resistance‐associated protein 1. Cell Death Dis. 2013;4:e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao C, Chen A, Jamieson CH, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dierks C, Beigi R, Guo GR, et al. Expansion of Bcr‐Abl‐positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238‐249. [DOI] [PubMed] [Google Scholar]
- 19. Lim Y, Gondek L, Li L, et al. Integration of Hedgehog and mutant FLT3 signaling in myeloid leukemia. Sci Transl Med. 2015;7:291ra296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munchhof MJ, Li Q, Shavnya A, et al. Discovery of PF‐04449913, a potent and orally bioavailable inhibitor of Smoothened. ACS Med Chem Lett. 2012;3:106‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackson‐Fisher AJ, McMahon MJ, Lam J, et al. PF‐04449913, a small molecule inhibitor of Hedgehog signaling, is effective in inhibiting tumor growth in preclinical models. Cancer Res. 2011;71:4504. [Google Scholar]
- 22. Schairer A, Shih A, Geron I, et al. Human blast crisis leukemia stem cell inhibition with a novel Smoothened antagonist. Blood. 2010;116:1223‐1223. [Google Scholar]
- 23. Jackson‐Fisher A, Whalen P, Elliott M, et al. Interrogating Hedgehog pathway and Smoothened inhibition by PF‐04449913 in patient‐derived acute myeloid leukemia models. Cancer Res. 2014;74:1958.24531750 [Google Scholar]
- 24. Minami Y, Fukushima N, Sadarangani A, Minami H, Jamieson C, Naoe T. Treatment with Hedgehog inhibitor PF‐913 attenuates leukemia‐initiation potential in acute myeloid leukemia cells. Cancer Res. 2014;74:1884‐1884. [Google Scholar]
- 25. Martinelli G, Oehler VG, Papayannidis C, et al. Treatment with PF‐04449913, an oral smoothened antagonist, in patients with myeloid malignancies: a phase 1 safety and pharmacokinetics study. Lancet Haematol. 2015;2:e339‐e346. [DOI] [PubMed] [Google Scholar]
- 26. Cortes JE, Heidel FH, Heuser M, et al. A phase 2 randomized study of low dose Ara‐C with or without glasdegib (PF‐04449913) in untreated patients with acute myeloid leukemia or high‐risk myelodysplastic syndrome. Blood. 2016;128:99‐99. [Google Scholar]
- 27. Sadarangani A, Pineda G, Lennon KM, et al. GLI2 inhibition abrogates human leukemia stem cell dormancy. J Transl Med. 2015;13:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tibes R, Al‐Kali A, Oliver GR, et al. The Hedgehog pathway as targetable vulnerability with 5‐azacytidine in myelodysplastic syndrome and acute myeloid leukemia. J Hematol Oncol. 2015;8:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Savona MR, Pollyea DA, Stock W, et al. Phase IB study of glasdegib, a Hedgehog pathway inhibitor, in combination with standard chemotherapy in patients with AML or high‐risk MDS. Clin Cancer Res. 2018;24:2294‐2303. [DOI] [PubMed] [Google Scholar]
- 30. Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the international working group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419‐425. [DOI] [PubMed] [Google Scholar]
- 31. Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642‐4649. [DOI] [PubMed] [Google Scholar]
- 32. Rollig C, Bornhauser M, Thiede C, et al. Long‐term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol. 2011;29:2758‐2765. [DOI] [PubMed] [Google Scholar]
- 33. Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079‐2088. [PubMed] [Google Scholar]
- 34. Bishop JF, Matthews JP, Young GA, et al. A randomized study of high‐dose cytarabine in induction in acute myeloid leukemia. Blood. 1996;87:1710‐1717. [PubMed] [Google Scholar]
- 35. Buchner T, Schlenk RF, Schaich M, et al. Acute myeloid leukemia (AML): different treatment strategies versus a common standard arm—combined prospective analysis by the German AML intergroup. J Clin Oncol. 2012;30:3604‐3610. [DOI] [PubMed] [Google Scholar]
- 36. Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794‐1803. [DOI] [PubMed] [Google Scholar]
- 37. Kantarjian H, Oki Y, Garcia‐Manero G, et al. Results of a randomized study of 3 schedules of low‐dose decitabine in higher‐risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52‐57. [DOI] [PubMed] [Google Scholar]
- 38. Kantarjian HM, O'Brien S, Huang X, et al. Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: comparison with historical experience. Cancer. 2007;109:1133‐1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kantarjian HM, O'Brien S, Shan J, et al. Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome. Cancer. 2007;109:265‐273. [DOI] [PubMed] [Google Scholar]
- 40. Thépot S, Itzykson R, Seegers V, et al. Azacitidine in untreated acute myeloid leukemia: a report on 149 patients. Am J Hematol. 2014;89:410‐416. [DOI] [PubMed] [Google Scholar]
- 41. Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fenaux P, Mufti GJ, Hellstrom‐Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562‐569. [DOI] [PubMed] [Google Scholar]
- 44. Burnett AK, Milligan D, Goldstone A, et al. The impact of dose escalation and resistance modulation in older patients with acute myeloid leukaemia and high risk myelodysplastic syndrome: the results of the LRF AML14 trial. Br J Haematol. 2009;145:318‐332. [DOI] [PubMed] [Google Scholar]
- 45. Boyd AL, Salci KR, Shapovalova Z, McIntyre BAS, Bhatia M. Nonhematopoietic cells represent a more rational target of in vivo Hedgehog signaling affecting normal or acute myeloid leukemia progenitors. Exp Hematol. 2013;41:858‐869. e854. [DOI] [PubMed] [Google Scholar]
- 46. Chaudhry P, Singh M, Triche TJ, Guzman M, Merchant AA. GLI3 repressor determines Hedgehog pathway activation and is required for response to SMO antagonist glasdegib in AML. Blood. 2017;129:3465‐3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Minami Y, Minami H, Miyamoto T, et al. Phase I study of glasdegib (PF‐04449913), an oral smoothened inhibitor, in Japanese patients with select hematologic malignancies. Cancer Sci. 2017;108:1628‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Merchant A, Matsui W. Targeting Hedgehog—a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130‐3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alonso S, Hernandez D, Chang YT, et al. Hedgehog and retinoid signaling alters multiple myeloma microenvironment and generates bortezomib resistance. J Clin Invest. 2016;126:4460‐4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alonso S, Jones RJ, Ghiaur G. Retinoic acid, CYP26, and drug resistance in the stem cell niche. Exp Hematol. 2017;54:17‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Krug U, Koschmieder A, Schwammbach D, et al. Feasibility of azacitidine added to standard chemotherapy in older patients with acute myeloid leukemia: a randomised SAL pilot study. PLoS One. 2012;7:e52695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Löwenberg B, Pabst T, Vellenga E, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364:1027‐1036. [DOI] [PubMed] [Google Scholar]
- 53. Jain S, Song R, Xie J. Sonidegib: mechanism of action, pharmacology, and clinical utility for advanced basal cell carcinomas. Onco Targets Ther. 2017;10:1645‐1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jacobsen AA, Aldahan AS, Hughes OB, Shah VV, Strasswimmer J. Hedgehog pathway inhibitor therapy for locally advanced and metastatic basal cell carcinoma: a systematic review and pooled analysis of interventional studies. JAMA Dermatol. 2016;152:816‐824. [DOI] [PubMed] [Google Scholar]
- 55. Ruiz‐Salas V, Alegre M, Lopez‐Ferrer A, et al. Vismodegib: a review. Acta Dermosifiliogr. 2014;105:744‐751. [DOI] [PubMed] [Google Scholar]
- 56. Sekulic A, Migden MR, Basset‐Seguin N, et al. Long‐term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lear JT, Migden MR, Lewis KD, et al. Long‐term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30‐month analysis of the randomized phase 2 BOLT study. J Eur Acad Dermatol Venereol. 2018;32:372‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Materials


