Abstract
Background
Fumaric acid esters (FAEs) are an established systemic treatment for moderate‐to‐severe psoriasis. However, the long‐term clinical safety and effectiveness of continuous FAE monotherapy and combination therapy have not been established.
Objective
To examine the long‐term safety and effectiveness of FAEs as monotherapy and in combination with phototherapy or methotrexate in patients with psoriasis treated at a single centre in Germany.
Methods
This monocentric, retrospective observational study, with a follow‐up period of up to 32.5 years, included 859 patients: 626 received FAE monotherapy, 123 received FAEs with concomitant phototherapy and 110 received FAEs with methotrexate.
Results
Approximately half of patients (49.0%) reported adverse events (566 total events), most of which involved the gastrointestinal tract. Serious adverse events were reported in 2.3% of patients, but none were deemed to have a causal relationship with any of the treatment regimens. Adverse events leading to treatment discontinuation were observed in 12.9% of patients. A median duration of 1 year was observed in all three treatment subcohorts (P = 0.70) from initiation of FAE treatment to a 50% response rate, where response was defined as achieving a cumulative static Physician's Global Assessment (PGA) score of ‘light’ and at least a 2‐point reduction in baseline PGA. A 50% response rate for the cumulative Psoriasis Area and Severity Index 75 was achieved in the FAE monotherapy subcohort after a median of 3 years of treatment, in the FAEs + phototherapy subcohort after 6.7 years and in the FAEs + methotrexate subcohort after 8.1 years (P = 0.001).
Conclusion
According to our data, FAEs as monotherapy or in combination with phototherapy or methotrexate are safe and beneficial for long‐term clinical use. However, multicentre, randomized controlled trials are required to establish the clinical value of monotherapy versus combination therapy and the optimal treatment duration.
Introduction
Psoriasis is a widely acknowledged Th1‐, Th17‐ and Th22‐mediated chronic inflammatory autoimmune disease of the skin and joints (psoriatic arthritis).1, 2 With a prevalence of 2–3% in industrialized western countries,3 psoriasis is considered the most frequent immune‐mediated disease in humans.4 In Germany, about 2 million people of all ages suffer from psoriasis.5 Many of these individuals are moderately to severely affected, with considerable impairment of quality of life.
Patients with moderate‐to‐severe psoriasis generally require long‐term or even lifelong systemic treatment.6, 7 For these patients, it is highly desirable to achieve adequate long‐term disease control with continued administration of safe and effective treatments.6 However, the available data on long‐term maintenance treatments remain scarce.8
Fumaric acid esters (FAEs) are recommended in European guidelines for the induction and long‐term treatment of adults (≥18 years of age) with moderate‐to‐severe psoriasis, unless topical treatment is adequate.9 The successful use of fumaric acid for the self‐treatment of psoriasis was first described in 1959 by the German chemist Schweckendiek.10 Since then, an FAE mixture has become increasingly standardized and has been commonly prescribed off‐licence for psoriasis, first in Germany and later in north‐western Europe.11 The reference product Fumaderm® is an enteric‐coated tablet containing a defined mixture of dimethyl fumarate (DMF) and the calcium, magnesium and zinc salts of monoethyl fumarate. The current evidence suggests that DMF acts only as an oral prodrug and that its metabolite monomethyl fumarate, which is rapidly formed by spontaneous or esterase‐mediated hydrolysis of DMF in the gut lumen or initial epithelial cell layer, is the pharmacologically active compound,12 with pleiotropic immunomodulating properties that inhibit particularly the Th17‐ and Th1‐dominated responses in patients with psoriasis.11, 13, 14, 15, 16 Fumaderm® received marketing approval in Germany in 1994.17 Since then, FAEs have become the most frequently used first‐line systemic treatment of psoriasis in Germany, accounting for more than 50% of all psoriasis‐related prescriptions,17 equivalent to approximately 20 000 patients with psoriasis receiving FAE therapy. Treatment is usually administered over several years, but to date only one small single‐centre, retrospective observational study has been reported.18 That study found no serious adverse events (SAEs) or malignancies in 12 patients with psoriasis treated continuously with FAEs for 10–14 years.
Despite contrary recommendations in the European guidelines,9, 19 long‐term FAE therapy in combination with phototherapy or with the classic systemic antipsoriatic agent methotrexate (MTX) is not uncommon in clinical practice,17, 20 especially where FAE monotherapy is insufficient to control skin symptoms or psoriatic arthritis. In a first, larger, prospective non‐interventional multicentre study of 363 adult patients, with an observation period of 12 months, FAEs in combination with phototherapy proved safe and effective.21 However, long‐term data on the safety and effectiveness of this regimen are lacking, and less evidence is available regarding FAEs in combination with MTX. Only one prospective study22 and two retrospective case series23, 24 are available in the literature; these studies reported beneficial combination treatment with FAEs and MTX, with no clinically significant drug–drug interactions in a total of 20 patients with psoriasis. However, because of the small number of reported cases, these results are only an initial indication that FAEs can be combined off‐label with MTX to safely and effectively treat therapy‐refractory psoriasis or psoriatic arthritis.
The aim of this monocentric, retrospective observational study was to evaluate the long‐term clinical safety and effectiveness of three treatment regimens (‘FAE monotherapy’, ‘FAEs + phototherapy’ and ‘FAEs + MTX’), with a follow‐up period of up to 32.5 years.
Methods
Study design
This investigator‐initiated, systematic, retrospective review of digital patient records included all patients with a clinical diagnosis of psoriasis of any subtype consecutively treated with FAEs (Fumaderm®), as monotherapy or in combination, at the Department of Dermatology, Venereology and Allergology, Ruhr University Bochum, Germany, between January 1975 (first prescription of FAEs in the department) and the data collection period (13 February 2012 to 31 October 2012).
Over the study period, diagnosis of psoriasis with final decision on FAE therapy initiation was made by the senior author of this manuscript (P.A.) or by medical staff specialized in dermatology under his supervision. Decisions on FAE therapy were individualized and agreed upon with each patient.
Patient selection
A total of 3332 digital records of psoriasis patients who received systemic therapy with FAEs were screened. Patients typically underwent monthly clinical assessment and quarterly monitoring of laboratory findings. Digital patient records were reviewed for the 879 patients who attended at least one routine follow‐up visit at our outpatient department. For further data analysis, patients were subdivided into three main treatment groups: ‘FAE monotherapy’ (626 patients), ‘FAEs + phototherapy’ (123 patients) and ‘FAEs + MTX’ (110 patients). Combination regimens with cyclosporine (n = 4), retinoids (n = 4), leflunomide (n = 2), glucocorticosteroids (n = 3) or biologics (n = 7) were disregarded because of the small number of cases. The final study population consisted of 859 patients.
Treatment modalities
Following negative screening tests, FAEs were initiated with a gradually increasing dosing regimen.25 Maintenance doses were individually adjusted according to therapeutic response and tolerability. FAEs were commonly administered as monotherapy (72.9% of the study population) or, less frequently, in combination with phototherapy (14.3%; median delay between FAE therapy initiation and addition of phototherapy, 14 days) or MTX (12.8%; median delay between FAE therapy initiation and addition of MTX, 441 days). Regular options for phototherapy were UVB (85 patients; broadband or narrowband), PUVA (37 patients; oral, bath or cream) or UVA (1 patient).19, 26 The starting MTX dosage was prescribed according to established guidelines; 15 mg/week was usually used and 10 mg/week was used in some cases.19, 26 Dosage adjustments during treatment were based on clinical improvement and ranged from 5 to 25 mg/week.27 MTX was only administered orally. All patients received folic acid supplementation 24 h after MTX at an equivalent dosage (i.e. 5–25 mg/week)27 to reduce side‐effects such as nausea and megaloblastic anaemia.28
Of note, all patients additionally received various topical antipsoriatic treatments (e.g. dithranol, glucocorticosteroids or vitamin D analogues) during treatment with the FAE regimens.
Variables
The following data were extracted from the hospital digital database and recorded on a predefined standardized paper case record form (CRF): patient identification number; date of record keeping; patient demographics; clinical type of psoriasis; comorbidities; antipsoriatic treatments prior to FAE therapy initiation; FAE treatment characteristics; clinical efficacy (static Physician's Global Assessment (sPGA) and Psoriasis Area and Severity Index (PASI)); safety laboratory values (relevant blood and serum parameters and urinalysis); adverse events (AEs); and SAEs.
For each patient, the start of the observation period (‘index date’) was defined as the date of first prescription for FAEs to treat psoriasis. Censoring (‘censorship date’) occurred either at the last visit for patients lost to follow‐up (who were thereafter seen by the referring dermatologist for continued therapy; relevant for 438/859 patients, 51.0%) or on the last date of data collection (31 October 2012; relevant for 137/859 patients, 15.9%), whichever occurred first. Reasons for FAE discontinuation (relevant for 284/859 patients, 33.1%) were as follows: clinical remission, lack of efficacy, AE, pregnancy or intention to become pregnant, patient choice, death or other reason (non‐compliance, no reimbursement, switch to an alternative systemic drug, etc.). AEs and SAEs documented throughout the observation period were classified using the Medical Dictionary for Regulatory Activities (MedDRA) version 20.1 September 2017. Any additional or emerging disease or clinically significant change in laboratory safety measurements in comparison with baseline findings was regarded as an AE or SAE.
Psoriasis severity was evaluated using two established tools, the sPGA and the PASI,29 of which the sPGA is more frequently employed in clinical practice and in retrospective studies.30 For the sPGA, the following 5‐point scale was used, according to Walker et al.:14 ‘light’ (comprising ‘clear’, ‘almost clear’ and ‘mild’),31 ‘moderate’, ‘moderate‐to‐severe’, ‘severe’ and ‘very severe’. PASI results range from 0.0 (no psoriasis) to 72.0 (most severe psoriasis).32 A PASI score of >10 and/or body surface area of >10 and Dermatology Life Quality Index of >10 are generally considered to indicate moderate‐to‐severe psoriasis.33 All average assessments of psoriatic lesions based on erythema, scale and induration (retrospectively classified according to the aforementioned sPGA) and PASI assessments were performed by P.A. at baseline and at irregular intervals during follow‐up visits. These assessments remained consistent over the whole study period. With one exception, sPGA was documented for all patients (n = 858, 99.9%); for this patient, only the PASI was documented. PASI values were available for only 681 patients (79.3%).
Data management
Primary data were collected and recorded on site on machine‐readable CRFs by an authorized member of the investigator's staff under the supervision of the first author (H.D.) as the principal investigator. Original CRFs were sent to INPADS GmbH, Bad Dürkheim, Germany, for data entry; copies remained on site. CRF data were stored in a relational database. Two‐person consistency checks were performed, and queries were issued for inconsistencies and implausibility.
The relational database was stored at the Institute of Medical Biometry and Informatics, University Hospital Heidelberg, Germany. All details of quality‐assured data analysis (performed by T.B.) were defined in a statistical analysis plan.
Statistical analysis
For descriptive purposes, we report categorical variables as absolute and relative frequencies and continuous variables as mean, standard deviation (SD), median and range (minimum, maximum). Statistical significance was evaluated with a chi‐square test, one‐sample t‐test, one‐way analysis of variance (ANOVA) or the Kruskal–Wallis test, depending on the underlying distribution of data. P‐values ≤ 0.05 were considered significant. However, all P‐values were purely descriptive and not confirmatory. Missing values were not considered in calculations.
Primary safety outcomes were defined as rates of discontinuation because of AEs in each treatment group. Coprimary effectiveness outcomes were defined as (i) the proportion of patients achieving an sPGA score of ‘light’ and at least a 2‐point reduction in baseline PGA score and (ii) the proportion of patients with ≥75% reduction in PASI (PASI 75; considered a less stringent reasonable therapeutic goal)34 versus baseline. Secondary effectiveness outcomes comprised PASI 50 (considered the minimum target)33 and PASI 90 [considered treatment success by the European Medicines Agency (EMA)] response rates.34 Additionally, we assessed absolute PASI (aPASI) values ≤10 (qualifying as PASI 50 response), ≤5 (qualifying as PASI 75 response) and ≤2 (qualifying as PASI 90 response), because of growing consensus that these values provide a better benchmark of therapeutic success, irrespective of the time of assessment and baseline PASI.34
We estimated the proportion of patients with psoriasis who reached a defined ‘event’ of effectiveness in their respective varying observation periods with the Kaplan–Meier method (Kaplan–Meier failure estimates).35 The single events were defined as follows: sPGA = ‘light’ and at least a 2‐point reduction in baseline PGA, PASI 50, PASI 75, PASI 90, aPASI ≤ 10, aPASI ≤ 5 and aPASI ≤ 2. The corresponding event dates were defined as the date of first occurrence during treatment. Censorship occurred at the last available follow‐up date at which the effectiveness event had failed to occur. All Kaplan–Meier failure curves of event by treatment group are depicted with the number of patients ‘at risk’ and 95% simultaneous confidence intervals (Hall–Wellner bands).36, 37, 38 ‘Patients at risk’ were defined as patients on therapy at a certain time point who had not yet reached the effectiveness event. We summarized Kaplan–Meier failure curve results by presenting the median event, which is the time point at which the cumulative response rate exceeds 50%.39 We used log‐rank tests to descriptively compare the event between treatment groups, taking the whole observation period into account.40 Because of the retrospective study design, no imputation of missing values was performed.
Data were analysed with SAS® statistical analysis software, version 9.4 (SAS Institute, Cary, NC, USA). This report of an observational study was written in close compliance with the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Initiative.41, 42
Ethical approval
This study was approved by the Ethics Committee of the Medical Faculty of the Ruhr University Bochum (registration no. 4203‐12; 6 February 2012) and performed in compliance with the principles of the Declaration of Helsinki (2008 revised version) and the German Data Protection Act (BDSG).
Role of the funding source
The sponsor was interested in evaluating the safety profile and effectiveness of continuous long‐term treatment with Fumaderm® in patients with psoriasis referred to our dermatological centre and provided funding for this observational study. H.D. designed the study in conjunction with the sponsor. No persons other than the authors were involved in the analysis and interpretation of data, the writing of the manuscript or the decision to submit for publication.
Results
Demographics and baseline clinical characteristics
Of the 859 psoriasis patients analysed, 528 (61.5%) were male. The mean patient age was 46.3 years (range 9–90 years). FAEs were used off‐label in children and adolescents; no patients younger than 18 years received MTX. Baseline demographic data of the total cohort and subcohorts are shown in Table 1. No significant differences were observed among the three treatment groups in any variable. Of note, numerous values for BMI, family history of psoriasis, smoking status and employment status were missing. Mean BMI of the study population with available data was 28.9 kg/m2, which is in the preobese range (equivalent to overweight). Approximately one‐third of patients with available data had a family history of psoriasis. About two‐thirds were smokers and about two‐thirds were employed.
Table 1.
Demographic and general characteristics of patients with psoriasis by treatment group prior to FAE therapy initiation; total number of patients = 859
| FAE monotherapy | FAEs + phototherapy | FAEs + MTX | All | P‐value | |
|---|---|---|---|---|---|
| Patients initiating FAE therapy, N (%) | 626 (72.9) | 123 (14.3) | 110 (12.8) | 859 (100.0) | |
| Sex | |||||
| Male, n (%) | 388 (62.0) | 77 (62.6) | 63 (57.3) | 528 (61.5) | 0.62* |
| Female, n (%) | 238 (38.0) | 46 (37.4) | 47 (42.7) | 331 (38.5) | |
| Age (years) | |||||
| Mean ± SD | 45.9 ± 14.6 | 48.3 ± 15.5 | 46.7 ± 14.2 | 46.3 ± 14.7 | 0.22** |
| Median (range) | 46.0 (9.0−89.0) | 47.0 (11.0−90.0) | 47.5 (18.0−89.0) | 46.0 (9.0−90.0) | |
| BMI (kg/m 2 ), n (% of N ) | 111 (17.7) | 55 (44.7) | 56 (50.9) | 222 (25.8) | |
| Mean ± SD | 28.6 ± 6.6 | 29.1 ± 6.2 | 29.4 ± 7.8 | 28.9 ± 6.8 | 0.75** |
| Median (range) | 27.3 (18.0−51.0) | 28.0 (19.7−52.0) | 27.5 (19.0−60.0) | 27.8 (18.0−60.0) | |
| Family history of psoriasis, n (% of N ) | 421 (67.3) | 85 (69.1) | 88 (80.0) | 594 (69.2) | |
| Positive, n (%) | 137 (32.5) | 26 (30.6) | 31 (35.2) | 194 (32.7) | 0.81* |
| Negative, n (%) | 284 (67.5) | 59 (69.4) | 57 (64.8) | 400 (67.3) | |
| Smoking status, n (% of N ) | 150 (24.0) | 58 (47.2) | 62 (56.4) | 270 (31.4) | |
| Positive/current, n (%) | 93 (62.0) | 33 (56.9) | 35 (56.5) | 161 (59.6) | 0.67* |
| Negative, n (%) | 57 (38.0) | 25 (43.1) | 27 (43.5) | 109 (40.4) | |
| Employment status, n (% of N ) | 203 (32.4) | 55 (44.7) | 56 (50.9) | 314 (36.6) | |
| Employed, n (%) | 149 (73.4) | 36 (65.5) | 38 (67.9) | 223 (71.0) | 0.44* |
| Unemployed/retired, n (%) | 54 (26.6) | 19 (34.5) | 18 (32.1) | 91 (29.0) | |
*P‐value derived from chi‐square test; **P‐value derived from one‐way analysis of variance (ANOVA).
FAEs, fumaric acid esters; phototherapy, ultraviolet A, ultraviolet B or psoralen + ultraviolet A; MTX, methotrexate; n, number of patients with available data; SD, standard deviation; BMI, body mass index.
Baseline clinical characteristics of the total cohort and subcohorts are shown in Table 2. Chronic plaque‐type psoriasis was the most frequent manifestation, followed by psoriasis capitis, nail psoriasis, psoriatic arthritis and inverse psoriasis. Patients suffering from psoriatic arthritis were proportionally overrepresented in the ‘FAEs + MTX’ treatment group, mainly because MTX is indicated for this condition.28
Table 2.
Disease characteristics of patients with psoriasis by treatment group prior to FAE therapy initiation
| FAE monotherapy | FAEs + phototherapy | FAEs + MTX | All | P‐value | |
|---|---|---|---|---|---|
| Patients initiating FAE therapy, N | 626 | 123 | 110 | 859 | |
| Clinical type of psoriasis ‡ , n (% of N ) | |||||
| Plaque psoriasis | 559 (89.3) | 102 (82.9) | 97 (88.2) | 758 (88.2) | <0.0001* |
| Psoriasis capitis | 133 (21.2) | 19 (15.4) | 16 (14.5) | 168 (19.6) | |
| Nail psoriasis | 97 (15.5) | 28 (22.8) | 34 (30.9) | 159 (18.5) | |
| Psoriatic arthritis | 50 (8.0) | 7 (5.7) | 43 (39.1) | 100 (11.6) | |
| Inverse psoriasis | 62 (9.9) | 19 (15.4) | 12 (10.9) | 93 (10.8) | |
| Guttate psoriasis | 17 (2.7) | 8 (6.5) | 4 (3.6) | 29 (3.4) | |
| Palmoplantar psoriasis | 18 (2.9) | 5 (4.1) | 3 (2.7) | 26 (3.0) | |
| Pustular psoriasis | 9 (1.4) | 3 (2.4) | 4 (3.6) | 16 (1.9) | |
| Erythrodermic psoriasis | 1 (0.2) | 0 | 0 | 1 (0.1) | |
| PGA score at baseline, n (% of N ) | 625 (99.8) | 123 (100) | 110 (100) | 858 (99.9) | |
| Mean ± SD | 3.6 ± 0.8 | 3.7 ± 0.8 | 3.9 ± 0.8 | 3.7 ± 0.8 | 0.01*** |
| Median (range) | 4 (1−5) | 4 (2−5) | 4 (2−5) | 4 (1−5) | |
| PASI score at baseline, n (% of N ) | 497 (79.4) | 100 (81.3) | 84 (76.4) | 681 (79.3) | |
| Mean ± SD | 22.3 ± 8.3 | 23.6 ± 7.9 | 24.9 ± 9.0 | 22.8 ± 8.4 | 0.02** |
| Median (range) | 22 (2.4−49.8) | 23.9 (2.4−42.0) | 25 (2.4−47.3) | 22.5 (2.4−49.8) | |
| Total patients with one or more comorbidities, n (% of N ) | 236 (37.7) | 73 (59.3) | 64 (58.2) | 373 (43.4) | <0.0001* |
| Comorbidity ‡ , n (% of N ) | |||||
| Hypertension | 99 (15.8) | 39 (31.7) | 20 (18.2) | 158 (18.4) | 0.86* |
| T2DM | 39 (6.2) | 14 (11.4) | 6 (5.5) | 59 (6.9) | |
| HLP | 36 (5.8) | 10 (8.1) | 6 (5.5) | 52 (6.1) | |
| CLD | 22 (3.5) | 11 (8.9) | 11 (10.0) | 44 (5.1) | |
| DJD | 24 (3.8) | 6 (4.9) | 9 (8.2) | 39 (4.5) | |
| Alcohol/drug abuse | 20 (3.2) | 7 (5.7) | 4 (3.6) | 31 (3.6) | |
| Mental illness | 17 (2.7) | 5 (4.1) | 4 (3.6) | 26 (3.0) | |
| CHD | 15 (2.4) | 8 (6.5) | 1 (0.9) | 24 (2.8) | |
| Other pulmonary disease | 10 (1.6) | 5 (4.1) | 6 (5.5) | 21 (2.4) | |
| Other chronic gastrointestinal disease | 10 (1.6) | 3 (2.4) | 7 (6.4) | 20 (2.3) | |
| Malignant neoplasm | 10 (1.6) | 3 (2.4) | 2 (1.8) | 15 (1.7) | |
| CKD | 9 (1.4) | 2 (1.6) | 3 (2.7) | 14 (1.6) | |
| HF | 8 (1.3) | 2 (1.6) | 2 (1.8) | 12 (1.4) | |
| COPD | 7 (1.1) | 2 (1.6) | 2 (1.8) | 11 (1.3) | |
| PUD | 6 (1.0) | 2 (1.6) | 1 (0.9) | 9 (1.0) | |
| CVD | 6 (1.0) | 0 | 2 (1.8) | 8 (0.9) | |
| Chronic viral infection | 3 (0.5) | 2 (1.6) | 1 (0.9) | 6 (0.7) | |
| Osteoporosis | 3 (0.5) | 1 (0.8) | 1 (0.9) | 5 (0.6) | |
| T1DM | 1 (0.2) | 0 | 0 | 1 (0.1) | |
| Other disease | 69 (11.0) | 17 (13.8) | 19 (17.3) | 105 (12.2) | |
*P‐value derived from chi‐square test; **P‐value derived from ANOVA; ***P‐value derived from Kruskal–Wallis test (nonparametric analysis of variance).
‡Multiple diagnoses possible.
FAEs, fumaric acid esters; phototherapy, ultraviolet A, ultraviolet B or psoralen + ultraviolet A; MTX, methotrexate; n, number of patients with available data; PASI, Psoriasis Area and Severity Index; PGA, Physician's Global Assessment; SD, standard deviation; T2DM, type 2 diabetes mellitus; HLP, hyperlipoproteinemia; CLD, chronic liver disease; DJD, degenerative joint disease; CHD, coronary heart disease; CKD, chronic kidney disease; HF, heart failure; COPD, chronic obstructive pulmonary disease; PUD, peptic ulcer disease; CVD, cerebrovascular disease; T1DM, type 1 diabetes mellitus. The PGA scale was defined as follows: 1 = light, 2 = moderate, 3 = moderate‐to‐severe, 4 = severe, 5 = very severe.
In the total cohort, mean PGA score was 3.7 (range 1–5), indicating ‘severe’ psoriasis. In the subgroup of patients with available data, mean PASI score was 22.8 (range 2.4–49.8), also reflecting severe psoriasis of the skin.17 Both scores were significantly higher in the ‘FAEs + MTX’ group than in the ‘FAE monotherapy’ group.
In 373 of the 859 patients (43.4%), at least one comorbidity was documented. Significantly fewer patients in the ‘FAE monotherapy’ group had comorbidities compared with the other two groups. However, comorbidity patterns were generally distributed equally among the treatment groups. The most frequent comorbidities were metabolic diseases, including hypertension, type 2 diabetes and hyperlipoproteinemia. A slightly greater proportion of patients had chronic liver disease or degenerative joint disease in the ‘FAEs + MTX’ group than in the other groups.
Treatment characteristics
All 859 psoriasis patients were treatment‐naïve for FAEs. The mean duration of psoriasis before FAE therapy initiation was 13.7 years (range 0–69.9 years). Almost all patients (99.8%) had received topical and physical (UVB, PUVA, UVA) pretreatment; only 11.2% had received systemic pretreatment, most frequently MTX (4.4%) or retinoids (4.4%). A significantly higher proportion of systemically pretreated patients was found in the ‘FAEs + MTX’ group than in other groups. In total, 763 patients (88.8%) received FAEs as their first systemic antipsoriatic therapy, many after a longer period of systemically untreated psoriasis. Full details of treatment characteristics over the whole observation period in the total cohort and in subcohorts are shown in Table 3.
Table 3.
Treatment characteristics of patients with psoriasis by treatment group
| FAE monotherapy | FAEs + phototherapy | FAEs + MTX | All | P‐value | |
|---|---|---|---|---|---|
| Patients initiating FAE therapy, N | 626 | 123 | 110 | 859 | |
| Prior topical and/or phototherapy, n (% of N ) | |||||
| Topical therapy | 325 (51.9) | 56 (45.5) | 56 (50.9) | 437 (50.9) | 0.50* |
| Topical therapy + phototherapy | 298 (47.6) | 66 (53.7) | 53 (48.2) | 417 (48.5) | |
| Phototherapy | 2 (0.3) | 1 (0.8) | 0 | 3 (0.4) | |
| None | 1 (0.2) | 0 | 1 (0.9) | 2 (0.2) | |
| Prior systemic therapy, n (% of N ) | |||||
| None | 567 (90.6) | 110 (89.4) | 86 (78.2) | 763 (88.8) | 0.0007* |
| MTX | 24 (3.8) | 3 (2.4) | 11 (10.0) | 38 (4.4) | |
| Acitretin | 22 (3.5) | 8 (6.5) | 8 (7.3) | 38 (4.4) | |
| Biologic | 6 (1.0) | 1 (0.8) | 3 (2.7) | 10 (1.2) | |
| Cyclosporine | 5 (0.8) | 0 | 1 (0.9) | 6 (0.7) | |
| GC | 2 (0.3) | 1 (0.8) | 1 (0.9) | 4 (0.5) | |
| Time between initial diagnosis and FAE therapy initiation [years] | |||||
| Mean ± SD | 13.6 ± 12.7 | 12.6 ± 13.3 | 15.2 ± 14.2 | 13.7 ± 13.0 | 0.32** |
| Median (range) | 10.1 (0−69.9) | 8.0 (0−68.4) | 10.8 (0−63.0) | 10.1 (0−69.9) | |
| Years on continuous therapy with FAEs | |||||
| Mean ± SD | 3.5 ± 4.2 | 3.2 ± 4.4 | 4.6 ± 4.6 | 3.6 ± 4.3 | 0.02** |
| Median (range) | 1.7 (0.1−32.5) | 1.5 (0.1−30.2) | 3.4 (0.2−28.1) | 1.8 (0.1−32.5)† | |
| Average DMF dose [mg/day] | |||||
| Mean ± SD | 373.7 ± 182.1 | 384.0 ± 200.3 | 443.6 ± 207.1 | 384.1 ± 189.3 | 0.0016** |
| Median (range) | 360 (32.6−960) | 411.4 (29.8−1030.6) | 409.7 (60−1008.1) | 367.6 (29.8−1030.6) | |
| Average Fumaderm ® dose [tablets/day] | |||||
| Mean ± SD | 3.3 ± 1.4 | 3.4 ± 1.5 | 3.8 ± 1.6 | 3.4 ± 1.5 | 0.0008** |
| Median (range) | 3.1 (1.0−8.0) | 3.5 (1.0−8.6) | 3.4 (1.4−8.4) | 3.1 (1.0−8.6) | |
| Years on continuous therapy with FAEs + phototherapy | |||||
| Mean ± SD | 1.8 ± 2.6 | ||||
| Median (range) | 0.7 (0.1−16.0) | ||||
| Years on continuous therapy with FAEs + MTX | |||||
| Mean ± SD | 2.2 ± 2.1 | ||||
| Median (range) | 1.7 (0−11.1) | ||||
| Average MTX dose [mg/week] | |||||
| Mean ± SD | 14.5 ± 3.3 | ||||
| Median (range) | 15.0 (5.0−25.0) | ||||
| Cumulative dose of MTX [mg] | |||||
| Mean ± SD | 1617.7 ± 1500.7 | ||||
| Median (range) | 1342.0 (20.0–9266.0) | ||||
*P‐value derived from chi‐square test; **P‐value derived from ANOVA.
†FAE treatment lasted >10 years in 9% of patients (n = 77); >20 years in 1.2% of patients (n = 10); and >30 years in 0.2% of patients (n = 2).
FAEs, fumaric acid esters; phototherapy, ultraviolet A, ultraviolet B or psoralen + ultraviolet A; MTX, methotrexate; GC, glucocorticoid; SD, standard deviation; DMF, dimethyl fumarate. Biologics included alefacept, efalizumab, etanercept and infliximab.
For the total cohort, the mean duration of continuous therapy with FAEs was 3.6 years (range 0.1–32.5 years), with a significantly longer mean duration of 4.6 years in the ‘FAEs + MTX’ group. The mean average daily maintenance dose was 384.1 mg DMF (range 29.8–1030.6 mg) or 3.4 Fumaderm® tablets (range 1–8.6 tablets). Some patients temporarily received up to nine Fumaderm® tablets per day, mainly because of lower than expected clinical improvement under the maximum recommended dose of six tablets daily (720 mg of DMF). In these patients, there was no significant increase in the number or severity of AEs. Differences among the treatment groups were observed, with significantly higher doses in the ‘FAEs + MTX’ subcohort.
Continuous combination therapy with phototherapy lasted on average 1.8 years (range 0.1–16 years); mean treatment duration of continuous combination therapy with MTX was 2.2 years (range 0–11.1 years). The mean average weekly dose of MTX was 14.5 mg (range 5–25 mg).
A significantly greater proportion of patients prematurely discontinued therapy in the ‘FAEs + MTX’ subcohort than in other groups (Table 4). In the ‘FAE monotherapy’ subcohort, the three main reasons for discontinuation of treatment were, in descending order: AEs, clinical remission and lack of efficacy. In the other subcohorts, the reasons were, in descending order: lack of efficacy, AEs and patients’ decisions.
Table 4.
Reasons for FAE treatment discontinuation according to treatment group
| FAE monotherapy | FAEs + phototherapy | FAEs + MTX | All | P‐valuea | |
|---|---|---|---|---|---|
| Patients initiating FAE therapy, N | 626 | 123 | 110 | 859 | |
| Total patients terminating therapy, n (% of N ) | 188 (30.0) | 48 (39.0) | 48 (43.6) | 284 (33.1) | 0.0063 |
| Reasons for discontinuation, n (% of N ) | |||||
| Adverse event | 81 (12.9) | 14 (11.4) | 15 (13.6) | 110 (12.8) | <0.0001 |
| Lack of efficacy (or lower than expected improvement) | 26 (4.2) | 16 (13.0) | 21 (19.1) | 63 (7.3) | |
| Clinical remission | 49 (7.8) | 4 (3.3) | 4 (3.6) | 57 (6.6) | |
| Patient choice | 11 (1.8) | 8 (6.5) | 5 (4.5) | 24 (2.8) | |
| Other reasons | 16 (2.6) | 4 (3.3) | 3 (2.7) | 23 (2.7) | |
| Pregnancy or intention to become pregnant | 4 (0.6) | 2 (1.6) | 0 | 6 (0.7) | |
| Death (from treatment‐unrelated causesb) | 1 (0.2) | 0 | 0 | 1 (0.1) | |
P‐value derived from chi‐square test.
Acute ischaemic stroke.
FAEs, fumaric acid esters; phototherapy, ultraviolet A, ultraviolet B or psoralen + ultraviolet A; MTX, methotrexate. Other reasons included not specified, non‐compliance, no reimbursement and switch to an alternative systemic drug.
Safety
A total of 421 patients (49.0%) experienced 566 AEs during their observation period (Table 5). Gastrointestinal complaints, flushing and blood and lymphatic system disorders were most frequently noted. However, only 111 patients (12.9%) ultimately stopped their respective therapy regimen because of an AE. Alterations in haematology, hepatobiliary disorders and renal disorders resulted relatively often in treatment discontinuation. Discontinuation rates resulting from AEs were similar in all treatment groups. The development of treatment‐related malignancies and opportunistic infections was not observed.
Table 5.
Adverse events (including serious adverse events) leading to FAE treatment discontinuation
| Patients initiating FAE therapy, N | FAE monotherapy 626 | FAEs + phototherapy 123 | FAEs + MTX 110 | All 859 | ||||
|---|---|---|---|---|---|---|---|---|
| At least one AE | FAE discontinuation | At least one AE | FAE discontinuation | At least one AE | FAE discontinuation | At least one AE | FAE discontinuation | |
| Total patients, n (% of N ) | 307 (49.0) | 82 (13.1) | 62 (50.4) | 14 (11.4) | 52 (47.3) | 15 (13.6) | 421 (49.0) | 111 (12.9) |
| AE, n (% of N ) | Total observeda | FAE discontinuation | Total observeda | FAE discontinuation | Total observeda | FAE discontinuation | Total observeda | FAE discontinuation |
| Gastrointestinal disorders | 187 (29.9) | 32 (5.1) | 46 (37.4) | 5 (4.1) | 36 (32.7) | 4 (3.6) | 269 (31.3) | 41 (4.8) |
| Flush/hot flushes | 86 (13.7) | 5 (0.8) | 7 (5.7) | 0 | 13 (11.8) | 1 (0.9) | 106 (12.3) | 6 (0.7) |
| Blood and lymphatic system disorders | 41 (6.5) | 27 (4.3) | 7 (5.7) | 6 (4.9) | 10 (9.1) | 7 (6.4) | 58 (6.8) | 40 (4.7) |
| Skin and subcutaneous tissue disorders | 35 (5.6) | 2 (0.3) | 8 (6.5) | 0 | 5 (4.5) | 0 | 48 (5.6) | 2 (0.2) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 13 (2.1) | 1 (0.2) | 2 (1.6) | 0 | 1 (0.9) | 0 | 16 (1.9) | 1 (0.1) |
| Hepatobiliary disorders | 9 (1.4) | 6 (1.0) | 3 (2.4) | 1 (0.8) | 3 (2.7) | 3 (2.7) | 15 (1.7) | 10 (1.2) |
| Renal and urinary disorders | 10 (1.6) | 4 (0.6) | 3 (2.4) | 1 (0.8) | 0 | 0 | 13 (1.5) | 5 (0.6) |
| Infections and infestations | 3 (0.5) | 0 | 0 | 0 | 4 (3.6) | 0 | 7 (0.8) | 0 |
| Metabolism and nutrition disorders | 1 (0.2) | 0 | 3 (2.4) | 1 (0.8) | 2 (1.8) | 0 | 6 (0.7) | 1 (0.1) |
| Others | 4 (0.6) | 2 (0.3) | 0 | 0 | 0 | 0 | 4 (0.5) | 2 (0.2) |
| Respiratory, thoracic and mediastinal disorders | 4 (0.6) | 1 (0.2) | 0 | 0 | 0 | 0 | 4 (0.5) | 1 (0.1) |
| Vascular disorders | 3 (0.5) | 0 | 0 | 0 | 1 (0.9) | 0 | 4 (0.5) | 0 |
| Nervous system disorders | 2 (0.3) | 1 (0.2) | 0 | 0 | 1 (0.9) | 0 | 3 (0.3) | 1 (0.1) |
| Psychiatric disorders | 2 (0.3) | 0 | 0 | 0 | 1 (0.9) | 0 | 3 (0.3) | 0 |
| Musculoskeletal and connective tissue disorders | 1 (0.2) | 0 | 1 (0.8) | 0 | 0 | 0 | 2 (0.2) | 0 |
| Cardiac disorders | 0 | 0 | 2 (1.6) | 0 | 0 | 0 | 2 (0.2) | 0 |
| Surgical and medical procedures | 2 (0.3) | 0 | 0 | 0 | 0 | 0 | 2 (0.2) | 0 |
| Reproductive system and breast disorders | 1 (0.2) | 1 (0.2) | 0 | 0 | 0 | 0 | 1 (0.1) | 1 (0.1) |
| Ear and labyrinth disorders | 0 | 0 | 1 (0.8) | 0 | 0 | 0 | 1 (0.1) | 0 |
| General disorders and administration site conditions | 1 (0.2) | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 0 |
| Injury, poisoning and procedural complications | 1 (0.2) | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 0 |
Multiple AEs possible. AE terminology based on MedDRA (Medical Dictionary for Regulatory Activities) system organ classification version 20.1 September 2017.
FAEs, fumaric acid esters; phototherapy, ultraviolet A, ultraviolet B or psoralen + ultraviolet A; MTX, methotrexate; AE, adverse event. Others included pruritus and vertigo.
Twenty of the total AEs (3.5%; 4.8% of patients with AEs), including one death, were classified as SAEs and were reported to the competent authorities concerned. An AE was classified as serious if it resulted in death or inpatient hospitalization or was of medical significance. However, all SAEs were determined to be unlikely to be related to the respective therapy regimen.
In the ‘FAE monotherapy’ subcohort, six SAEs resulted in treatment discontinuation: death caused by a massive acute ischaemic stroke in a 79‐year‐old woman after 1.5 years of therapy; non‐small cell bronchogenic carcinoma in a 56‐year‐old man after 1 year of therapy; prostate cancer in a 56‐year‐old man after 5 years of therapy; rectal carcinoma in a 71‐year‐old man after 0.5 years of therapy; acute pancreatitis in a 39‐year‐old woman after 5.5 years of therapy; and chronic kidney disease, stage 3, in a case of suspected hypertensive nephroangiosclerosis in a 78‐year‐old man after 1 year of therapy. In the same subcohort, 11 SAEs, including dizzy spell, initial ischaemic stroke, depression, basal cell carcinoma, mammary carcinoma, bladder cancer, small bowel resection, arm fracture, knee surgery, ulcera crurum and condylomata acuminata, did not lead to therapy discontinuation. The remaining three SAEs, which occurred in the ‘FAEs + MTX’ group, were lower respiratory tract infection, pneumonia and IgG kappa plasmacytoma; none resulted in treatment discontinuation. No SAE was reported in the ‘FAEs + phototherapy’ group.
Relevant laboratory abnormalities detected during therapy are shown in Table 6. All of these were transient and returned to normal after reducing the FAE dose or discontinuing FAE treatment. Nine patients (1.4%) in the ‘FAE monotherapy’ subcohort, five patients (4.1%) in the ‘FAEs + phototherapy’ subcohort and two patients (1.8%) in the ‘FAEs + MTX’ subcohort discontinued therapy because of leucopenia or grade 3 or 4 lymphopenia, according to the Common Terminology Criteria for Adverse Events (CTCAE v4.03). Eosinophilia (maximum measured level 2069 cells/μL) led to premature treatment discontinuation in three patients (0.5%) in the ‘FAE monotherapy’ subcohort and in one patient (0.8%) in the ‘FAEs + phototherapy’ subcohort. Thrombocytopenia did not necessitate interruption of treatment. Five patients (0.8%) in the ‘FAE monotherapy’ subcohort and three patients (2.7%) in the ‘FAEs + MTX’ subcohort discontinued therapy because of an increase in liver enzymes (SGOT, SGPT and/or GGT), classified as >3 times the normal upper limit.11 Two patients (0.3%) discontinued therapy in the ‘FAE monotherapy’ subcohort because of an increase in serum creatinine above the upper limit of the normal range. Therapy was interrupted in the same subcohort by one patient (0.2%) because of proteinuria.
Table 6.
Abnormal laboratory findings (following Mrowietz et al.64) among the different treatment groups at some point during treatment duration
| FAE monotherapy | FAEs + phototherapy | FAEs + MTX | All | P‐valuea | |
|---|---|---|---|---|---|
| Patients initiating FAE therapy, N | 626 | 123 | 110 | 859 | |
| Blood parameters, n (% of N ) | |||||
| Leucocytes <3000/μL | 29 (4.6) | 5 (4.1) | 3 (2.7) | 37 (4.3) | 0.66 |
| Lymphocytes <500/μL (CTCAE grade 3 and 4) | 105 (16.8) | 21 (17.1) | 14 (12.7) | 140 (16.3) | 0.55 |
| Eosinophils >450/μL (25% above normal upper limit) | 102 (16.3) | 20 (16.3) | 18 (16.4) | 140 (16.3) | 0.99 |
| Platelets <150 000/μL | 48 (7.7) | 12 (9.8) | 14 (12.7) | 74 (8.6) | 0.19 |
| Serum parameters, n (% of N ) | |||||
| SGOT >150 IU/L (3 times the normal upper limit) | 4 (0.6) | 0 | 0 | 4 (0.5) | 0.47 |
| SGPT >150 IU/L (3 times the normal upper limit) | 6 (1.0) | 3 (2.4) | 5 (4.5) | 14 (1.6) | 0.02 |
| GGT >213 IU/L (3 times the normal upper limit) | 19 (3.0) | 1 (0.8) | 4 (3.6) | 24 (2.8) | 0.33 |
| Creatinine >1.20 mg/dL | 75 (12.0) | 21 (17.1) | 21 (19.1) | 117 (13.6) | 0.06 |
| Urinalysis, n (% of N ) | |||||
| Proteinuria (dipstick urinalysis positive and 24‐hurine collection >0.14 g protein) | 79 (12.6) | 24 (19.5) | 12 (10.9) | 115 (13.4) | 0.09 |
P‐value derived from chi‐square test.
FAEs, fumaric acid esters; phototherapy, ultraviolet A, ultraviolet B or psoralen + ultraviolet A; MTX, methotrexate; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamate pyruvate transaminase; GGT, gamma‐glutamyltransferase; CTCAE, Common Terminology Criteria for Adverse Events (available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf; last accessed 30 December 2017). Normal ranges: leucocytes = 4600−9500/μL, lymphocytes = 1000−4050/μL, eosinophils = 40−360/μL, platelets = 150 000−400 000/μL, SGOT = 10−50 IU/L, SGPT = 10−50 IU/L, GGT = 10−71 IU/L, serum creatinine = 0.70−1.20 mg/dL, dipstick urinalysis = negative, 24‐h urine collection ≤0.14 g protein.
Changes in blood parameters measured within a 3‐year period to monitor the safety of the respective therapy regimens are displayed in Table 7. Of note, slightly over one‐third of blood counts at baseline (relevant for 308/859 patients) were missing because they were measured externally by referring physicians. Irrespective of treatment group, the mean lymphocyte count decreased by about 20% within the first 3 months of therapy; this decrease was highly statistically significant. Over the course of therapy, lymphocyte counts further decreased below 30% of baseline values. The reduction in the mean leucocyte count was comparable, but was less pronounced, with a total reduction approaching 20% over the course of therapy. Within the first 3 months of therapy, the mean eosinophil count showed its maximum increase from baseline values, but then decreased to standard values with ongoing therapy. Alterations in the mean platelet count were all within the normal range. Changes in the relevant serum parameters within a time frame of 3 years are displayed in Table 8 according to therapy regimen. One‐third of baseline serum levels (relevant for 287/859 patients) are missing because the referring physicians measured levels externally. All mean values during therapy were in the normal ranges.
Table 7.
Changes in safety laboratory values of relevant blood parameters in patients with psoriasis by treatment group within a period of 3 years; total number of patients with available data at baseline = 551
| Baseline | Month 3 | Month 6 | Month 12 | Month 24 | Month 36 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD [cells/μL] | Changea [%] | P‐valuea | Changea [%] | P‐valuea | Changea [%] | P‐valuea | Changea [%] | P‐valuea | Changea [%] | P‐valuea | |
| FAE monotherapy, n | 390 | 365 | 329 | 269 | 156 | 99 | |||||
| Leucocytes | 7314.0 ± 2207.3 | −5.4 | 0.006 | −13.0 | <0.0001 | −18.5 | <0.0001 | −16.4 | <0.0001 | −15.9 | <0.0001 |
| Lymphocytes | 2005.3 ± 693.6 | −19.4 | <0.0001 | −35.9 | <0.0001 | −40.3 | <0.0001 | −39.2 | <0.0001 | −42.8 | <0.0001 |
| Eosinophils | 203.1 ± 175.5 | 176.8 | <0.0001 | 33.4 | 0.0004 | 8.8 | 0.23 | 2.7 | 0.67 | 8.6 | 0.36 |
| Platelets | 261482.1 ± 79304.4 | −3.5 | 0.0002 | −4.6 | <0.0001 | −4.6 | <0.0001 | −4.4 | 0.002 | −6.8 | 0.0006 |
| FAEs + phototherapy, n | 83 | 72 | 62 | 55 | 32 | 11 | |||||
| Leucocytes | 7512.3 ± 2167.8 | −13.8 | <0.0001 | −17.9 | <0.0001 | −22.6 | <0.0001 | −21.6 | <0.0001 | −18.6 | 0.02 |
| Lymphocytes | 1887.5 ± 618.9 | −21.1 | <0.0001 | −35.9 | <0.0001 | −42.2 | <0.0001 | −36.2 | <0.0001 | −34.1 | 0.007 |
| Eosinophils | 194.9 ± 167.0 | 95.7 | 0.0006 | 16.1 | 0.24 | 3.8 | 0.77 | −1.3 | 0.88 | 30.5 | 0.27 |
| Platelets | 245867.5 ± 62151.2 | −2.6 | 0.10 | −5.8 | 0.0002 | −7.4 | 0.0002 | −6.2 | 0.02 | −9.5 | 0.06 |
| FAEs + MTX, n | 78 | 67 | 67 | 56 | 45 | 35 | |||||
| Leucocytes | 8187.1 ± 2429.0 | −14.0 | <0.0001 | −17.5 | <0.0001 | −18.7 | <0.0001 | −22.9 | <0.0001 | −19.9 | 0.0002 |
| Lymphocytes | 2056.4 ± 744.6 | −20.2 | 0.0002 | −32.8 | <0.0001 | −36.8 | <0.0001 | −32.9 | <0.0001 | −36.9 | <0.0001 |
| Eosinophils | 212.8 ± 156.1 | 103.2 | 0.006 | 24.5 | 0.15 | −4.5 | 0.67 | 1.6 | 0.88 | −15.6 | 0.05 |
| Platelets | 258730.8 ± 75548.3 | −4.4 | 0.02 | −7.4 | <0.0001 | −8.8 | <0.0001 | −7.8 | 0.0007 | −8.5 | 0.0007 |
FAEs, fumaric acid esters; phototherapy, ultraviolet A, ultraviolet B or psoralen + ultraviolet A; MTX, methotrexate; n, number of patients with available data; SD, standard deviation. Normal ranges: leucocytes = 4600−9500/μL, lymphocytes = 1000−4050/μL, eosinophils = 40−360/μL, platelets = 150 000−400 000/μL, SGOT = 10−50 IU/L, SGPT = 10−50 IU/L, GGT = 10−71 IU/L, serum creatinine = 0.70−1.20 mg/dL.
P‐value derived from one‐sample t‐test.
Mean percentage change from baseline
Table 8.
Changes in safety laboratory values of relevant serum parameters in patients with psoriasis by treatment group within a period of 3 years; total number of patients with available data at baseline = 572
| Baseline | Month 3 | Month 6 | Month 12 | Month 24 | Month 36 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Changeb [%] | P‐valuea | Changeb [%] | P‐valuea | Changeb [%] | P‐valuea | Changeb [%] | P‐valuea | Changeb [%] | P‐valuea | |
| FAE monotherapy, n | 403 | 375 | 340 | 278 | 162 | 103 | |||||
| SGOT [IU/L] | 23.2 ± 13.7 | 10.8 | <0.0001 | 9.3 | 0.01 | 8.6 | 0.004 | 44.0 | <0.0001 | 51.2 | <0.0001 |
| SGPT [IU/L] | 28.2 ± 21.6 | 25.1 | <0.0001 | 16.6 | <0.0001 | 19.0 | 0.0005 | 38.2 | 0.008 | 38.3 | 0.001 |
| GGT [IU/L] | 38.1 ± 47.4 | 14.7 | <0.0001 | 12.1 | <0.0001 | 17.0 | 0.04 | 41.4 | 0.001 | 44.5 | <0.0001 |
| Creatinine [mg/dL] | 0.92 ± 0.53 | −0.2 | 0.93 | −1.1 | 0.18 | 7.6 | 0.13 | 3.2 | 0.02 | 2.2 | 0.35 |
| FAEs + phototherapy, n | 89 | 75 | 66 | 55 | 33 | 13 | |||||
| SGOT [IU/L] | 24.2 ± 13.3 | 11.4 | 0.02 | 0.3 | 0.95 | 8.8 | 0.15 | 28.8 | 0.01 | 36.5 | 0.08 |
| SGPT [IU/L] | 28.3 ± 19.4 | 25.0 | 0.0005 | 3.8 | 0.46 | 16.5 | 0.12 | 21.3 | 0.03 | 38.1 | 0.19 |
| GGT [IU/L] | 36.4 ± 31.3 | 4.8 | 0.18 | 9.1 | 0.09 | 17.6 | 0.13 | 6.9 | 0.57 | 9.0 | 0.66 |
| Creatinine [mg/dL] | 0.91 ± 0.29 | 1.4 | 0.70 | 0.9 | 0.81 | 7.1 | 0.11 | 7.5 | 0.005 | 7.6 | 0.37 |
| FAEs + MTX, n | 80 | 70 | 68 | 57 | 46 | 36 | |||||
| SGOT [IU/L] | 25.5 ± 14.2 | 21.9 | 0.02 | 11.5 | 0.03 | 14.0 | 0.07 | 26.0 | 0.02 | 57.5 | 0.0007 |
| SGPT [IU/L] | 31.6 ± 19.3 | 54.0 | 0.003 | 28.0 | 0.002 | 22.3 | 0.05 | 27.6 | 0.03 | 63.8 | 0.002 |
| GGT [IU/L] | 42.6 ± 44.6 | 10.1 | 0.11 | 11.4 | 0.05 | 14.5 | 0.09 | 25.7 | 0.02 | 51.5 | 0.0007 |
| Creatinine [mg/dL] | 0.94 ± 0.19 | −2.8 | 0.19 | −3.2 | 0.05 | −2.0 | 0.34 | −1.1 | 0.64 | −1.5 | 0.57 |
P‐value derived from one‐sample t‐test.
Mean percentage change from baseline.
FAEs, fumaric acid esters; phototherapy, ultraviolet A, ultraviolet B or psoralen + ultraviolet A; MTX, methotrexate; n, number of patients with available data; SD, standard deviation; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamate pyruvate transaminase; GGT, gamma‐glutamyltransferase. Normal ranges: SGOT = 10−50 IU/L, SGPT = 10−50 IU/L, GGT = 10−71 IU/L, serum creatinine = 0.70−1.20 mg/dL.
Effectiveness
In general, sPGA and PASI assessments showed an improvement in the severity of psoriatic skin lesions with a sustained uptrend during treatment with FAEs, either as monotherapy or in combination therapy. In all treatment groups, the time from FAE therapy initiation to a cumulative sPGA response rate of 50% for achieving ‘light’ status and at least a 2‐point reduction in baseline PGA was 1 year (Fig. 1a–c). The sPGA results were not significantly different among treatment groups (P = 0.70).
Figure 1.
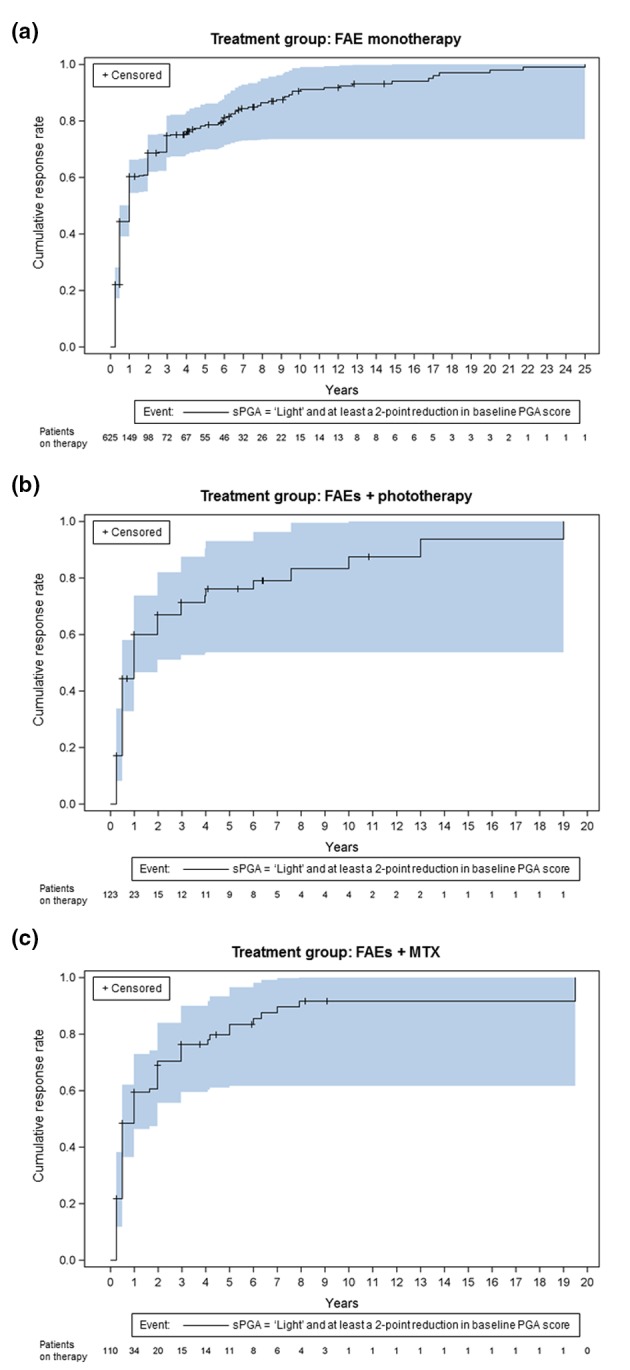
Cumulative improvement in static PGA response (event: sPGA = ‘light’ and at least a 2‐point reduction in baseline PGA score) by treatment group: (a) FAE monotherapy, (b) FAEs + phototherapy and (c) FAEs + MTX. Kaplan–Meier failure curves with the number of patients ‘at risk’ (on therapy) and 95% Hall–Wellner bands.
In the ‘FAE monotherapy’ subcohort, the time from FAE therapy initiation to a cumulative PASI 50/75/90 response rate of 50% was 2/3/7.9 years, respectively (Fig. 2a). In the ‘FAEs + phototherapy’ subcohort, the time from FAE therapy initiation to a cumulative PASI 50/75/90 response rate of 50% was 2/6.7/13.4 years (Fig. 2b). In the ‘FAEs + MTX’ subcohort, the time from FAE therapy initiation to a cumulative PASI 50/75/90 response rate of 50% was 3.2/8.1/10.4 years (Fig. 2c). Differences among treatment groups were statistically significant for PASI 50 (P = 0.007) and PASI 75 (P = 0.001) but not PASI 90 (P = 0.07).
Figure 2.
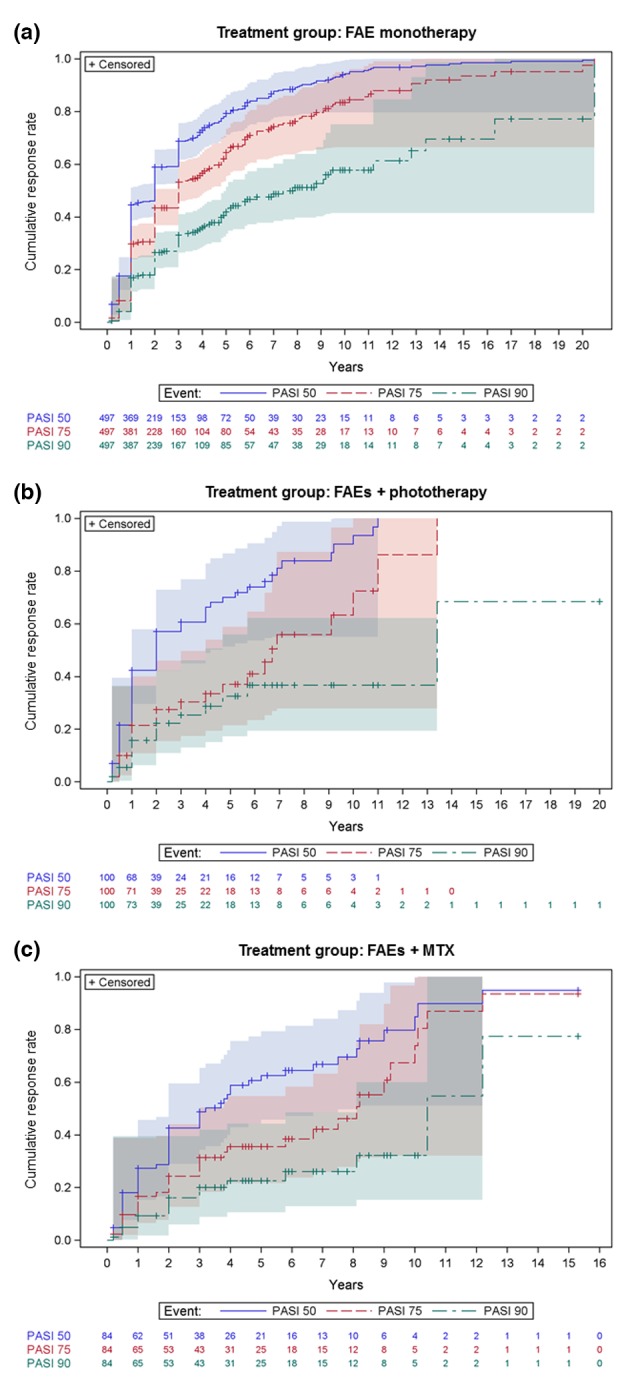
Cumulative improvement in PASI response (events: PASI 50, PASI 75 and PASI 90) by treatment group: (a) FAE monotherapy, (b) FAEs + phototherapy and (c) FAEs + MTX. Kaplan–Meier failure curves with the number of patients ‘at risk’ (on therapy) in each group and 95% Hall–Wellner bands.
In the ‘FAE monotherapy’ subcohort, the time from FAE therapy initiation to a cumulative aPASI ≤ 10/≤5/≤2 response rate of 50% was 2/3/5.8 years (Fig. 3a). In the ‘FAEs + phototherapy’ subcohort, these times were 2/6.7/(not reached) years (Fig. 3b), and in the ‘FAEs + MTX’ subcohort, these times were 3.9/8.2/12.2 years (Fig. 3c). Differences among the treatment groups were statistically significant for all three secondary outcomes: aPASI ≤ 10 (P = 0.002), aPASI ≤ 5 (P < 0.0001) and aPASI ≤ 2 (P = 0.003).
Figure 3.
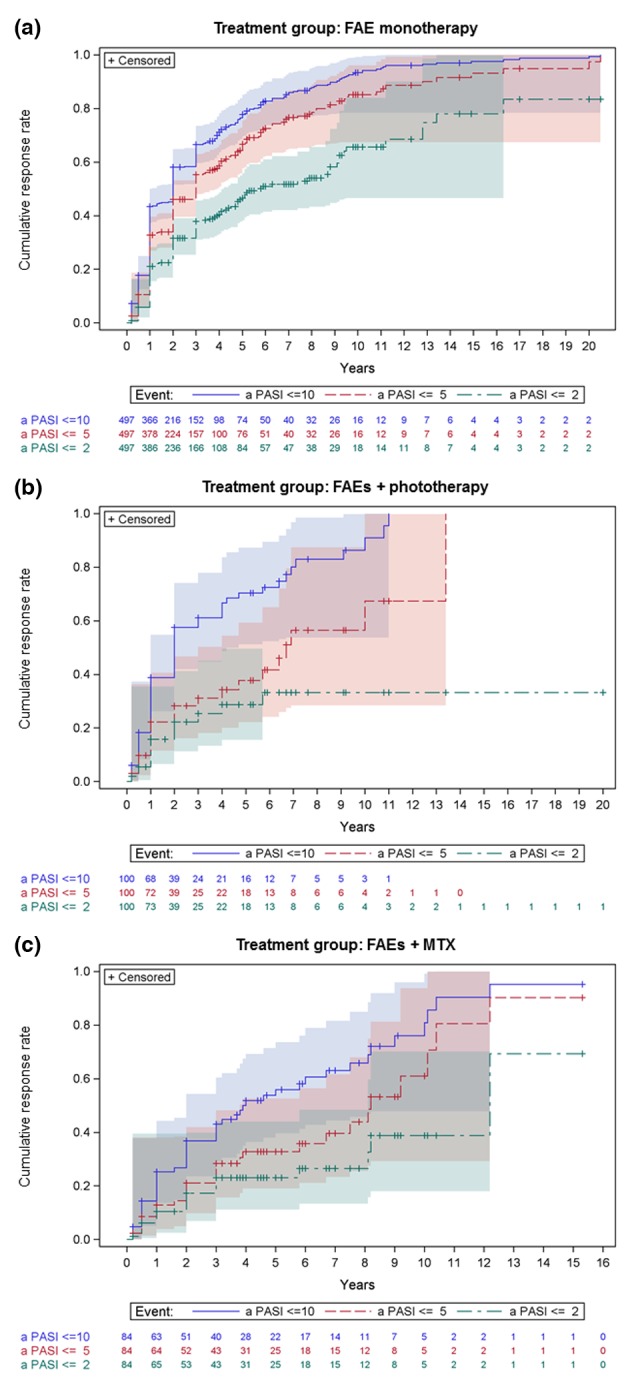
Cumulative improvement in absolute PASI response (events: aPASI ≤ 10, aPASI ≤ 5 and aPASI ≤ 2) by treatment group: (a) FAE monotherapy, (b) FAEs + phototherapy and (c) FAEs + MTX. Kaplan–Meier failure curves with the number of patients ‘at risk’ (on therapy) in each group and 95% Hall–Wellner bands.
Of note, in each of the three treatment groups, PASI 75 and aPASI ≤ 5 as well as PASI 90 and aPASI ≤ 2 correlated strongly (range of κ‐values 0.78–0.94; individual data not shown), whereas PASI 50 and aPASI ≤ 10 correlated only moderately (range of κ‐values 0.57–0.80; individual data not shown).43
Discussion
The results of this observational study describe, for the first time, the long‐term treatment of psoriasis with three different FAE therapy regimens (FAE monotherapy; FAEs + phototherapy; FAEs + MTX) in a real‐life setting. One strength of this study is that it is, to the best of our knowledge, the largest study of this kind to include a total study population continuously treated with FAEs for a mean of 3.6 years (43.9 months) and is therefore strongly representative of psoriasis patients treated with classic systemic therapies in everyday clinical practice. Each of the three therapy regimens was well characterized with a sufficiently large sample size and a relatively long observation period in many cases (Table 3); 27.1% of all 859 patients received FAEs in combination with phototherapy or MTX. To measure safety, we calculated AE rates and listed SAEs. To evaluate effectiveness, we measured reduction in sPGA and percentage reduction in PASI and aPASI as the outcomes of interest.44 In the long‐term treatment of patients with psoriasis, FAE monotherapy and combination therapies with phototherapy or MTX showed a favourable safety profile and satisfactory clinical effectiveness.
Safety aspects
In all treatment groups, the most frequent AEs were gastrointestinal symptoms, which were observed in approximately one‐third of treated patients (Table 5). In our experience, gastrointestinal symptoms typically occur within the first few weeks of FAE treatment and last for several minutes to half an hour after oral administration. Symptoms are generally mild to moderate and resolve with continued treatment. In this study, these symptoms were rarely so profound as to require treatment discontinuation. However, the comparatively low manifestation of gastrointestinal symptoms in our study defies explanation.45, 46 One can only speculate whether the great diversity in reports of treatment‐related gastrointestinal disorders is due in part to the wide heterogeneity in FAE dosage adjustments, which in real life are mainly based on the individual expertise of the treating physicians. The second most frequently reported AE was flushing of the skin (Table 5), which can range from a rapid sensation of heat to long‐lasting facial redness. This symptom was observed in up to 6–14% of patients, also typically in the earlier stages of treatment and with the tendency to resolve over time. This AE may be very unpleasant but is not serious and only very rarely represents a considerable burden for the patient, leading to treatment discontinuation. Improvement of this AE has been observed following treatment with acetylsalicylic acid.17, 19, 26 This treatment response has been later confirmed also by two randomized placebo‐controlled clinical trials (RCTs).47, 48 Haematologic disorders, primarily comprising leucopenia, lymphopenia and eosinophilia, were the third most reported AEs (Table 5). According to the literature, the proportion of patients who discontinue FAE treatment because of haematologic disorders ranges from 2.6% to 12%.5, 14, 30 Between 12% and 17% of patients in the three treatment groups developed severe lymphopenia or eosinophilia at some point during FAE therapy (Table 6). A retrospective case series reported that 26% of psoriasis patients (16/62) had a lymphocyte count <500/μL.49 Usually, these phenomena are transient and reversible14, 50, 51, 52 and may serve as a biomarker for drug efficacy of FAEs.53 The changes in leucocyte and lymphocyte counts observed in the present study (Table 7) compare well with those recently reported by Sondermann et al.54 In that study, which included a much smaller cohort of 105 patients with psoriasis, the median leucocyte count decreased by about 17.3% and the median lymphocyte count by about 35.8% in the first 6 months of FAE therapy. Over a maximum FAE treatment period of 112 months, that group observed severe lymphopenia (CTCAE grade 3 or 4) in 11.4% of patients. Although severe, persistent leucopenia and lymphopenia events were rare,49, 55 these AEs resulted relatively often in the physician's decision to discontinue treatment to decrease the risk of opportunistic infection.54 Although opportunistic infections are very rare, 19 cases of progressive multifocal leucoencephalopathy have been identified among patients treated with different FAE preparations to date;56, 57 14 of these occurred during therapy for psoriasis (11 patients used Fumaderm® and three patients used Psorinovo®, DMF, compounding pharmacy). All of these patients were lymphopenic and had been receiving FAE therapy for prolonged periods. Only patients with prolonged uncontrolled lymphopenia during FAE therapy seem to be at higher risk for this very rare but serious side‐effect of FAEs.7, 54 Additionally, fatal cases of West Nile encephalitis,58 generalized varicella zoster,59 and Kaposi sarcoma60 have been associated with FAEs.61 In our total cohort, we did not observe any severe or opportunistic infections leading to treatment discontinuation. Eosinophilia is frequently observed within the first 3 months of FAE therapy62, 63 and usually decreases to standard values with continued treatment,64 only rarely necessitating treatment discontinuation.7 Other frequent AEs among the three drug cohorts were hepatobiliary disorders (Table 5). A clinically relevant increase in liver enzymes was observed in up to 5% of patients (Table 6); increases were reversible after FAE dose reduction or treatment discontinuation. Mild‐to‐moderate elevations in liver enzymes (below twice the upper limit of normal) were reported in up to 40% of psoriasis patients taking FAEs but rarely necessitated discontinuation of therapy.18, 22, 50, 64 To date, no animal or human data have indicated any risk of permanent liver damage with FAE use. Although between 10% and 20% of patients in the three treatment groups showed increased serum creatinine levels and/or proteinuria (Table 6), only a few sporadic cases of disturbed renal function were documented as AEs in the ‘FAE monotherapy’ and ‘FAEs + phototherapy’ groups (Table 5). This result confirms again that treatment‐related renal disorders are reversible upon FAE dose reduction or treatment discontinuation.51, 65 According to our long‐term experience, increased serum creatinine levels and proteinuria very rarely lead to treatment discontinuation after FAE dose reduction. Other recorded AEs were comparatively rare or sporadic and were also reversible (Table 5). Given the above findings and the EMA's updated recommendations regarding lymphopenia during FAE therapy,66 patients receiving FAE therapy require regular and continuous clinical assessment and monitoring of all safety‐relevant laboratory parameters.54 Although we are currently adhering to the aforementioned EMA guidelines, which are based so far only on case‐report‐level evidence,49 we agree that their rigorous implementation should now be discussed on the basis of broader evidence.49, 54, 55
Our results support the reported safety profile of FAEs. In their systematic review, Balak et al.46 identified 37 observational studies between 1987 and 2015 with a total of 3457 patients. Eighteen studies analysed long‐term FAE treatment over a period of up to 14 years.18 No treatment‐related deaths or SAEs were reported. The most frequently reported AEs were gastrointestinal symptoms and flushing of the skin. Frequently reported laboratory abnormalities included lymphopenia, elevated liver enzymes and eosinophilia. Overall, 45% to 87% of patients experienced an AE. The proportion of patients discontinuing FAE treatment because of AEs ranged from 6% to 47%. The most frequent causes of early treatment discontinuation were intolerable gastrointestinal symptoms and, far less frequently, severe flushing symptoms. Only a few reported treatment discontinuations resulted from laboratory abnormalities.
The general goal of combining FAEs with phototherapy was to induce a faster and improved therapeutic response to FAEs.20, 67 In a first prospective non‐interventional multicentre study (‘FAST’ study), Weisenseel et al.21 investigated the combination of FAEs with various phototherapies in 363 patients over a shorter observation period of 12 months. Tolerability and safety of this combination were good. Only 7% of patients experienced AEs, all of which were within the spectrum of known FAE‐related adverse reactions. However, the final statement of the authors that a phototherapy duration >3 months may not be advisable, not least because of its oncogenic potential,21 is not supported by our long‐term safety data. Without doubt, uncontrolled long‐term use of phototherapy may result in UV damage and premature ageing of the skin.67 UV exposure increases the risk of non‐melanoma skin cancer, hence careful clinical monitoring of patients under phototherapy must be ensured. However, none of the patients in our ‘FAEs + phototherapy’ subcohort experienced these serious side‐effects, even in one case where continuous twice‐weekly phototherapy lasted for 16 years.
With an observed drug interaction rate of <5%,30 FAEs appear not only suitable for comorbid patients with psoriasis but also usable in combination with other systemic antipsoriatic agents. Off‐label combination of FAEs and MTX has a reasonably long tradition, not just in our dermatology clinic.22, 23, 24 Additional administration of MTX seems especially justified if FAE monotherapy lacks efficacy, or in cases with new onset of psoriatic arthritis during FAE monotherapy.23 In psoriatic arthritis, MTX remains the first drug of choice.28 In their meta‐analysis of RCTs involving adult patients with moderate‐to‐severe plaque psoriasis, Schmitt et al.68 found that FAEs were equally as efficacious as MTX, with similar rates of AEs and treatment discontinuations. It is therefore reasonable to assume that there might be an additive antipsoriatic effect of adding MTX, without any new or additional safety concerns.28 Transient and reversible hepatotoxicity was the most common limiting factor during combination therapy.7
Effectiveness aspects
The percentage of psoriasis patients assessed with the sPGA who improved considerably (≥2‐point reduction in baseline PGA score or assessment as ‘light’) was 50% after 1 year in all treatment groups (Fig. 1a–c). A previously published retrospective study (‘FUTURE’ study)5 of 984 psoriasis patients with a mean duration of 44 months of continuous FAE monotherapy reported greater improvement, as assessed with the dynamic PGA: 67% of patients were documented as improved or clear after 0.5 years, 78% after 2 years and 82% after 3 years of FAE therapy. However, the following parameters differed, preventing direct comparison: (i) different PGA scores were used and (ii) patients who stopped FAE monotherapy within the first 2 years because of lack of efficacy or insufficient tolerability were not recorded in the FUTURE study.69 In our study, all patients were included in data analysis, irrespective of treatment discontinuation.
Fifty per cent of psoriasis patients with available PASI values in the FAE monotherapy group achieved PASI 75 at 3 years; 50% response was seen at 6.7 years in the ‘FAEs + phototherapy’ group and at 8.1 years in the ‘FAEs + MTX’ group (Fig. 2a–c). Prima facie, the present study revealed no additional value of combining FAEs with phototherapy or MTX. However, the statistically significant differences among the three treatment groups must be considered in the context of differences in treatment group composition: more severe psoriasis cases with a therapy‐refractory course and more patients with comorbidities were included in the groups receiving combination therapy (Tables 2 and 3),70 mainly because of the non‐randomized study design.
Regarding the predefined coprimary effectiveness outcomes, the PGA scale used, which summarized ‘clear’, ‘almost clear’ and ‘mild’ under the single category of ‘light’, may have been too broad, and sPGA values may have overestimated clinical improvement. However, PASI tends to underestimate clinical improvement.71
Even given that FAEs typically have a long onset until full clinical efficacy and that clinical improvement continues during prolonged treatment without any signs of tachyphylaxis,17, 63, 67, 72 our FAE effectiveness results fall considerably short of expectations. We feel that our results once again highlight the gap between the efficacy found in RCTs that include only selected psoriasis patient cohorts and the real‐life setting of patients with moderate‐to‐severe psoriasis as documented in this observational study.73 Balak et al.46 found that mean reductions in PASI in observational studies ranged from 13% to 86% after 0.3 years of FAE treatment. Reported PASI‐75 responses ranged from 8% to 33%.
In the FAST study,21 FAEs in combination with phototherapy showed a substantially better efficacy than FAE monotherapy in the first 3 months of therapy only. Beyond this period (i.e. until the maximum observation period of 12 months), the difference in efficacy between the treatment groups disappeared. Neither the duration nor the type of phototherapy had an impact on efficacy. A comparable trend was observed in an unpublished pilot study with 15 patients.16
Effectiveness outcomes cannot be determined from the small number of currently reported psoriasis patients treated with FAEs in combination with MTX.22, 23, 24
Limitations
We acknowledge several potential limitations of this observational study. First, the study's retrospective design may have resulted in recall bias.65, 69 Incomplete data recording in the hospital digital database and patients lost to follow‐up resulted in missing data, particularly on continuation of FAE therapy regimens. Second, the inherent limitation of non‐randomization of concomitant phototherapy or MTX may have introduced selection bias.44, 74, 75 Although the demographic and general characteristics of the treatment groups were similar at baseline, the disease characteristics differed considerably among groups. Third, the absence of a control group is a limiting factor.65 Hence, the statements and comparisons made with respect to the data and subcohorts are purely descriptive. Clear evidence on the clinical value of administering FAEs in combination with phototherapy or MTX versus monotherapy, as well as on the optimal duration of these combination therapies, will require multicentre RCTs.21, 23, 76 Finally, the monocentric study setting may essentially limit the transferability of our findings.6 Due to our long‐term experiences with FAEs in the treatment of psoriasis,77, 78 unique local standards of practice have emerged: for instance, continuous phototherapy is not a common practice elsewhere,67 and the classic systemic antipsoriatic agent MTX is usually used as monotherapy.9
Conclusion
Although potentially influenced by many patient‐ and physician‐related factors, observational studies remain a valuable source of information regarding the long‐term safety and effectiveness of antipsoriatic therapy regimens.6 Our data indicate for the first time that the long‐term safety profile of FAE monotherapy as well as combination therapies with phototherapy or MTX can be judged as favourable, even in psoriasis patients with various comorbidities.30 Assuming compliance with safety laboratory values in all respects, there is currently no evidence supporting an increased risk of infection, malignancy or other SAEs in psoriasis patients treated with one of these FAE therapy regimens.18, 46, 79, 80 However, it is worth noting that for all three treatment options, achieving a satisfactory and sustained clinical response requires a long treatment duration.14
Despite the introduction of novel fast‐acting and highly efficacious biologics,81, 82 it is our opinion that the use of FAEs in the first‐line therapy of psoriasis remains fully justified, given (i) the possibility of timely and individualized dosage adjustments,83 (ii) a very low drug–drug interaction potential23, 24, 30 and (iii) a reasonable cost‐benefit‐risk ratio over long‐term treatment,5, 30 especially for patients moderately affected with psoriasis.
Finally, the findings presented here may offer insights for dermatologists and other physicians prescribing the new DMF monosubstance for moderate‐to‐severe psoriasis that was launched on 1 October 2017 in Germany and in the following months throughout Europe under the trade name Skilarence®.84 Of note, the clinical efficacy of the DMF monosubstance was not greater than that of Fumaderm® in the placebo‐controlled ‘BRIDGE’ registration trial.45 The prevalence of AEs in the DMF monosubstance group also appeared to be similar to that in the group receiving the marketed FAE mixture.
Acknowledgements
We are grateful to all the patients and the entire medical and non‐medical staff over the past decades who made this study possible. We thank Schapoor Hessam, MD, Melanie Riesenbeck, MD and Klara Bastek, MD, for their valuable assistance in data collection. We also thank Clare Cox, PhD, and Rebecca Tollefson, DVM, from Edanz Group (http://www.edanzediting.com/ac) for editing drafts of this manuscript.
Conflict of interests
H. Dickel has received travel grants for lecturing activities from Biogen GmbH; P. Altmeyer has received grants and/or honoraria as a consultant and/or speaker from Biogen GmbH; and T. Bruckner declares no conflict of interests.
Funding sources
Biogen GmbH, Ismaning, Germany, financially supported the collection, administration and analysis of study data.
References
- 1. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol 2017; 140: 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boehncke W‐H, Schön MP. Psoriasis. Lancet 2015; 386: 983–994. [DOI] [PubMed] [Google Scholar]
- 3. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 205–212. [DOI] [PubMed] [Google Scholar]
- 4. Diani M, Altomare G, Reali E. T cell responses in psoriasis and psoriatic arthritis. Autoimmun Rev 2015; 14: 286–292. [DOI] [PubMed] [Google Scholar]
- 5. Reich K, Thaci D, Mrowietz U et al Efficacy and safety of fumaric acid esters in the long‐term treatment of psoriasis – A retrospective study (FUTURE). J Dtsch Dermatol Ges 2009; 7: 603–611. [DOI] [PubMed] [Google Scholar]
- 6. Arnold T, Schaarschmidt M‐L, Herr R et al Drug survival rates and reasons for drug discontinuation in psoriasis. J Dtsch Dermatol Ges 2016; 14: 1089–1099. [DOI] [PubMed] [Google Scholar]
- 7. Volc S, Ghoreschi K. Pathophysiological basis of systemic treatments in psoriasis. J Dtsch Dermatol Ges 2016; 14: 557–571. [DOI] [PubMed] [Google Scholar]
- 8. Lucka TC, Pathirana D, Sammain A et al Efficacy of systemic therapies for moderate‐to‐severe psoriasis: a systematic review and meta‐analysis of long‐term treatment. J Eur Acad Dermatol Venereol 2012; 26: 1331–1344. [DOI] [PubMed] [Google Scholar]
- 9. Nast A, Gisondi P, Ormerod AD et al European S3‐Guidelines on the systemic treatment of psoriasis vulgaris – Update 2015 – Short version – EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol 2015; 29: 2277–2294. [DOI] [PubMed] [Google Scholar]
- 10. Schweckendiek W. Heilung von Psoriasis vulgaris. Med Monatsschr 1959; 13: 103–104. [PubMed] [Google Scholar]
- 11. Wakkee M, Thio HB. Drug evaluation: BG‐12, an immunomodulatory dimethylfumarate. Curr Opin Investig Drugs 2007; 8: 955–962. [PubMed] [Google Scholar]
- 12. Mrowietz U, Morrison PJ, Suhrkamp I et al The pharmacokinetics of fumaric acid esters reveal their in vivo effects. Trends Pharmacol Sci 2018; 39: 1–12. [DOI] [PubMed] [Google Scholar]
- 13. Eminel S, Jin N, Rostami M et al Dimethyl‐ and monomethylfumarate regulate indoleamine 2,3‐dioxygenase (IDO) activity in human immune cells. Exp Dermatol 2017; 26: 685–690. [DOI] [PubMed] [Google Scholar]
- 14. Walker F, Adamczyk A, Kellerer C et al Fumaderm® in daily practice for psoriasis: dosing, efficacy and quality of life. Br J Dermatol 2014; 171: 1197–1205. [DOI] [PubMed] [Google Scholar]
- 15. Ghoreschi K, Brück J, Kellerer C et al Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med 2011; 208: 2291–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mrowietz U, Adamczyk A, Augustin M et al Neue Erkenntnisse zu Fumarsäureestern (Fumaderm®): Ergebnisse eines experten‐workshops. J Dtsch Dermatol Ges 2011; 9(Suppl 4): 1–13. [DOI] [PubMed] [Google Scholar]
- 17. Mrowietz U, Rostami‐Yazdi M, Neureither M et al 15 Jahre Fumaderm®: Fumarsäureester für die systemische Behandlung der mittelschweren und schweren Psoriasis vulgaris. J Dtsch Dermatol Ges 2009; 7(Suppl 2): S3–S16. [DOI] [PubMed] [Google Scholar]
- 18. Hoefnagel JJ, Thio HB, Willemze R et al Long‐term safety aspects of systemic therapy with fumaric acid esters in severe psoriasis. Br J Dermatol 2003; 149: 363–369. [DOI] [PubMed] [Google Scholar]
- 19. Pathirana D, Ormerod AD, Saiag P et al European S3‐Guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol 2009; 23(Suppl 2): 1–70. [DOI] [PubMed] [Google Scholar]
- 20. Boehncke W‐H. “Evergreen” Psoriasis – Neues zu Genetik, Therapie, und überhaupt. J Dtsch Dermatol Ges 2017; 15: 111–112. [DOI] [PubMed] [Google Scholar]
- 21. Weisenseel P, Reich K, Griemberg W et al Efficacy and safety of fumaric acid esters in combination with phototherapy in patients with moderate‐to‐severe plaque psoriasis (FAST). J Dtsch Dermatol Ges 2017; 15: 180–186. [DOI] [PubMed] [Google Scholar]
- 22. Wain EM, Darling MI, Pleass RD et al Treatment of severe, recalcitrant, chronic plaque psoriasis with fumaric acid esters: a prospective study. Br J Dermatol 2010; 162: 427–434. [DOI] [PubMed] [Google Scholar]
- 23. Wilsmann‐Theis D, Frambach Y, Philipp S et al Systemic antipsoriatic combination therapy with fumaric acid esters for plaque‐type psoriasis: report on 17 cases. Dermatology 2015; 230: 119–127. [DOI] [PubMed] [Google Scholar]
- 24. Balasubramaniam P, Stevenson O, Berth‐Jones J. Fumaric acid esters in severe psoriasis, including experience of use in combination with other systemic modalities. Br J Dermatol 2004; 150: 741–746. [DOI] [PubMed] [Google Scholar]
- 25. Biogen Idec GmbH . FUMADERM® initial, FUMADERM® . Summary of Product Characteristics (Translation of original German version) 2013: 1–4.
- 26. Nast A, Kopp IB, Augustin M et al Evidence‐based (S3) guidelines for the treatment of psoriasis vulgaris. J Dtsch Dermatol Ges 2007; 5(Suppl 3): 1–119. [DOI] [PubMed] [Google Scholar]
- 27. Menting SP, Dekker PM, Limpens J et al Methotrexate dosing regimen for plaque‐type psoriasis: a systematic review of the use of test‐dose, start‐dose, dosing scheme, dose adjustments, maximum dose and folic acid supplementation. Acta Derm Venereol (Stockh) 2016; 96: 23–28. [DOI] [PubMed] [Google Scholar]
- 28. Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years’ experience with low‐dose long‐term treatment. J Eur Acad Dermatol Venereol 2000; 14: 382–388. [DOI] [PubMed] [Google Scholar]
- 29. Nast A, Schmitt J. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J Am Acad Dermatol 2013; 68: 1040–1041. [DOI] [PubMed] [Google Scholar]
- 30. Thaci D, Weisenseel P, Philipp S et al Efficacy and safety of fumaric acid esters in patients with psoriasis on medication for comorbid conditions – a retrospective evaluation (FACTS). J Dtsch Dermatol Ges 2013; 11: 429–435. [DOI] [PubMed] [Google Scholar]
- 31. Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician's global assessment. J Am Acad Dermatol 2004; 51: 563–569. [DOI] [PubMed] [Google Scholar]
- 32. Fredriksson T, Pettersson U. Severe psoriasis — oral therapy with a new retinoid. Dermatologica 1978; 157: 238–244. [DOI] [PubMed] [Google Scholar]
- 33. Mrowietz U, Kragballe K, Reich K et al Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res 2011; 303: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol 2015; 29: 645–648. [DOI] [PubMed] [Google Scholar]
- 35. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. JASA 1958; 53: 457–481. [Google Scholar]
- 36. Zwiener I, Blettner M, Hommel G. Überlebenszeitanalyse. Teil 15 der Serie zur Bewertung wissenschaftlicher Publikationen. Dtsch Arztebl 2011; 108: 163–169. [Google Scholar]
- 37. Pocock SJ, Clayton TC, Altman DG. Survival plots of time‐to‐event outcomes in clinical trials: good practice and pitfalls. Lancet 2002; 359: 1686–1689. [DOI] [PubMed] [Google Scholar]
- 38. Hall WJ, Wellner JA. Confidence bands for a survival curve from censored data. Biometrika 1980; 67: 133–143. [Google Scholar]
- 39. Stel VS, Dekker FW, Tripepi G et al Survival analysis I: the Kaplan‐Meier method. Nephron Clin Pract 2011; 119: c83–c88. [DOI] [PubMed] [Google Scholar]
- 40. Bland JM, Altman DG. The logrank test. BMJ 2004; 328: 1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vandenbroucke JP, von Elm E, Altman DG et al Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007; 18: 805–835. [DOI] [PubMed] [Google Scholar]
- 42. von Elm E, Altman DG, Egger M et al The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007; 18: 800–804. [DOI] [PubMed] [Google Scholar]
- 43. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012; 22: 276–282. [PMC free article] [PubMed] [Google Scholar]
- 44. Dávila‐Seijo P, Dauden E, Carretero G et al Survival of classic and biological systemic drugs in psoriasis: results of the BIOBADADERM registry and critical analysis. J Eur Acad Dermatol Venereol 2016; 30: 1942–1950. [DOI] [PubMed] [Google Scholar]
- 45. Mrowietz U, Szepietowski JC, Loewe R et al Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate‐to‐severe chronic plaque psoriasis: a randomized, double‐blind, Fumaderm(R) ‐ and placebo‐controlled trial (BRIDGE). Br J Dermatol 2017; 176: 615–623. [DOI] [PubMed] [Google Scholar]
- 46. Balak DMW, Fallah Arani S, Hajdarbegovic E et al Efficacy, effectiveness and safety of fumaric acid esters in the treatment of psoriasis: a systematic review of randomized and observational studies. Br J Dermatol 2016; 175: 250–262. [DOI] [PubMed] [Google Scholar]
- 47. O'Gorman J, Russell HK, Li J et al Effect of aspirin pretreatment or slow dose titration on flushing and gastrointestinal events in healthy volunteers receiving delayed‐release dimethyl fumarate. Clin Ther 2015; 37: e5. [DOI] [PubMed] [Google Scholar]
- 48. Sheikh SI, Nestorov I, Russell H et al Tolerability and pharmacokinetics of delayed‐release dimethyl fumarate administered with and without aspirin in healthy volunteers. Clin Ther 2013; 35: e9. [DOI] [PubMed] [Google Scholar]
- 49. Roche L, Lynch M, Ahmad K et al Lymphopenia and fumaric acid esters for psoriasis: a retrospective case series prompted by the European Medicines Agency's Pharmacovigilance Risk Assessment Committee (PRAC) recommendations. Clin Exp Dermatol 2018; 43: 72–75. [DOI] [PubMed] [Google Scholar]
- 50. Atwan A, Ingram JR, Abbott R et al Oral fumaric acid esters for psoriasis: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol 2016; 175: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ismail N, Collins P, Rogers S et al Drug survival of fumaric acid esters for psoriasis: a retrospective study. Br J Dermatol 2014; 171: 397–402. [DOI] [PubMed] [Google Scholar]
- 52. Nugteren‐Huying WM, van der Schroeff JG, Hermans J et al Fumaric acid therapy for psoriasis: a randomized, double‐blind, placebo‐controlled study. J Am Acad Dermatol 1990; 22: 311–312. [DOI] [PubMed] [Google Scholar]
- 53. Longbrake EE, Naismith RT, Parks BJ et al Dimethyl fumarate‐associated lymphopenia: Risk factors and clinical significance. Mult Scler J Exp Transl Clin 2015; 1: 1–8. 10.1177/2055217315596994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sondermann W, Rompoti N, Leister L et al Lymphopenia and CD4+/CD8+ cell reduction under fumaric acid esters. Dermatology 2017; 233: 295–302. [DOI] [PubMed] [Google Scholar]
- 55. Foley CC, Molloy OE, Lally A et al The implications of recent recommendations for managing patients with psoriasis treated with fumaric acid esters. Dermatology 2017; 233: 175–177. [DOI] [PubMed] [Google Scholar]
- 56. Gieselbach R‐J, Muller‐Hansma AH, Wijburg MT et al Progressive multifocal leukoencephalopathy in patients treated with fumaric acid esters: a review of 19 cases. J Neurol 2017; 264: 1155–1164. [DOI] [PubMed] [Google Scholar]
- 57. Gieselbach R‐J, Muller‐Hansma AH, Wijburg MT et al Erratum to: Progressive multifocal leukoencephalopathy in patients treated with fumaric acid esters: a review of 19 cases. J Neurol 2017; 264: 1833–1836. [DOI] [PubMed] [Google Scholar]
- 58. Berkovich R, Weiner LP. Effects of dimethyl fumarate on lymphocyte subsets. Mult Scler Relat Disord 2015; 4: 339–341. [DOI] [PubMed] [Google Scholar]
- 59. van Kester MS, Bouwes Bavinck JN, Quint KD. PML in patients treated with dimethyl fumarate. N Engl J Med 2015; 373: 583–584. [DOI] [PubMed] [Google Scholar]
- 60. Philipp S, Kokolakis G, Hund M et al Immunological changes in psoriasis patients under long‐term treatment with fumaric acid esters: risk of Kaposi sarcoma occurrence? Eur J Dermatol 2013; 23: 339–343. [DOI] [PubMed] [Google Scholar]
- 61. Longbrake EE, Ramsbottom MJ, Cantoni C et al Dimethyl fumarate selectively reduces memory T cells in multiple sclerosis patients. Mult Scler 2016; 22: 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mrowietz U, Christophers E, Altmeyer P et al Treatment of psoriasis with fumaric acid esters: results of a prospective multicentre study. Br J Dermatol 1998; 138: 456–460. [DOI] [PubMed] [Google Scholar]
- 63. Altmeyer P, Hartwig R, Matthes U. Das Wirkungs‐ und Sicherheitsprofil von Fumarsäureestern in der oralen Langzeittherapie bei schwerer therapieresistenter Psoriasis vulgaris. Hautarzt 1996; 47: 190–196. [DOI] [PubMed] [Google Scholar]
- 64. Mrowietz U, Christophers E, Altmeyer P. Treatment of severe psoriasis with fumaric acid esters: scientific background and guidelines for therapeutic use. The German Fumaric Acid Ester Consensus Conference. Br J Dermatol 1999; 141: 424–429. [DOI] [PubMed] [Google Scholar]
- 65. Menzies S, Ismail N, Abdalla A et al Renal dysfunction in patients taking fumaric acid esters – a retrospective cross‐sectional study. J Eur Acad Dermatol Venereol 2017; 31: 686–691. [DOI] [PubMed] [Google Scholar]
- 66. European Medicines Agency (EMA) . Updated recommendations to minimise the risk of the rare brain infection PML with Tecfidera: Related recommendations apply to other fumarate medicines. In: EMA/627077/2015 corr. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2015/10/WC500196017.pdf, 2015: 1–4.
- 67. Nast A, Boehncke W‐H, Mrowietz U et al S3‐Guidelines on the treatment of psoriasis vulgaris (English version). Update. J Dtsch Dermatol Ges 2012; 10(Suppl 2): S1–S95. [DOI] [PubMed] [Google Scholar]
- 68. Schmitt J, Rosumeck S, Thomaschewski G et al Efficacy and safety of systemic treatments for moderate‐to‐severe psoriasis: meta‐analysis of randomized controlled trials. Br J Dermatol 2014; 170: 274–303. [DOI] [PubMed] [Google Scholar]
- 69. Reich K, Hartl C, Gambichler T et al Retrospective data collection of psoriasis treatment with fumaric acid esters in children and adolescents in Germany (KIDS FUTURE study). J Dtsch Dermatol Ges 2016; 14: 50–58. [DOI] [PubMed] [Google Scholar]
- 70. Busard CI, Cohen AD, Wolf P et al Biologics combined with conventional systemic agents or phototherapy for the treatment of psoriasis: real‐life data from PSONET registries. J Eur Acad Dermatol Venereol 2017; 32: 245–253. [DOI] [PubMed] [Google Scholar]
- 71. Carlin CS, Feldman SR, Krueger JG et al A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol 2004; 50: 859–866. [DOI] [PubMed] [Google Scholar]
- 72. Balak DMW. Dimethyl fumarate finally coming of age. Br J Dermatol 2017; 176: 563–564. [DOI] [PubMed] [Google Scholar]
- 73. Maul J‐T, Djamei V, Kolios AGA et al Efficacy and survival of systemic psoriasis treatments: an analysis of the Swiss registry SDNTT. Dermatology 2016; 232: 640–647. [DOI] [PubMed] [Google Scholar]
- 74. Iskandar IYK, Ashcroft DM, Warren RB et al Patterns of biologic therapy use in the management of psoriasis: cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). Br J Dermatol 2017; 176: 1297–1307. [DOI] [PubMed] [Google Scholar]
- 75. Warren RB, Smith CH, Yiu ZZN et al Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol 2015; 135: 2632–2640. [DOI] [PubMed] [Google Scholar]
- 76. Lange S, Sauerland S, Lauterberg J et al The range and scientific value of randomized trials ‐ part 24 of a series on evaluation of scientific publications. Dtsch Arztebl Int 2017; 114: 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Altmeyer PJ, Matthes U, Pawlak F et al Antipsoriatic effect of fumaric acid derivatives: Results of a multicenter double‐blind study in 100 patients. J Am Acad Dermatol 1994; 30: 977–981. [DOI] [PubMed] [Google Scholar]
- 78. Pawlak FM, Matthes U, Bacharach‐Buhles M et al Behandlung einer therapieresistenten Psoriasis pustulosa generalisata mit Fumarsäureestern. Akt Dermatol 1993; 19: 6–8. [Google Scholar]
- 79. Balak DMW. Fumaric acid esters in the management of psoriasis. Psoriasis: Targets Therapy 2015; 5: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ormerod AD, Mrowietz U. Fumaric acid esters, their place in the treatment of psoriasis. Br J Dermatol 2004; 150: 630–632. [DOI] [PubMed] [Google Scholar]
- 81. Sticherling M, Mrowietz U, Augustin M et al Secukinumab is superior to fumaric acid esters in treating subjects with moderate to severe plaque psoriasis who are naïve to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol 2017; 177: 1024–1032. [DOI] [PubMed] [Google Scholar]
- 82. Balak DMW. First‐line systemic treatment of psoriasis: staying conventional or going biologic? Br J Dermatol 2017; 177: 897–898. [DOI] [PubMed] [Google Scholar]
- 83. Schaarschmidt M‐L, Kromer C, Herr R et al Treatment satisfaction of patients with psoriasis. Acta Derm Venereol (Stockh) 2015; 95: 572–578. [DOI] [PubMed] [Google Scholar]
- 84. Almirall SA. Skilarence® (Dimethyl fumarate). Summary of Product Characteristics 2017: 1–32.


