Abstract
Safety, immunogenicity, pharmacokinetics, and efficacy of the IgG‐degrading enzyme of Streptococcus pyogenes (IdeS [imlifidase]) were assessed in a single‐center, open‐label ascending‐dose study in highly sensitized patients with chronic kidney disease. Eight patients with cytotoxic PRAs (median cytotoxic PRAs of 64%) at enrollment received 1 or 2 intravenous infusions of IdeS on consecutive days (0.12 mg/kg body weight ×2 [n = 3]; 0.25 mg/kg ×1 [n = 3], or 0.25 mg/kg ×2 [n = 2]). IgG degradation was observed in all subjects after IdeS treatment, with <1% plasma IgG remaining within 48 hours and remaining low up to 7 days. Mean fluorescence intensity values of HLA class I and II reactivity were substantially reduced in all patients, and C1q binding to anti‐HLA was abolished. IdeS also cleaved the IgG‐type B cell receptor on CD19+ memory B cells. Anti‐IdeS antibodies developed 1 week after treatment, peaking at 2 weeks. A few hours after the second IdeS infusion, 1 patient received a deceased donor kidney offer. At enrollment, the patient had a positive serum crossmatch (HLA‐B7), detected by complement‐dependent cytotoxicity, flow cytometry, and multiplex bead assays. After IdeS infusion (0.12 mg/kg ×2) and when the HLA‐incompatible donor (HLA‐B7+) kidney was offered, the HLA antibody profile was negative. The kidney was transplanted successfully.
Keywords: clinical research/practice, clinical trial, crossmatch, desensitization, histocompatibility, kidney transplantation/nephrology, kidney transplantation: living donor, pharmacokinetics/pharmacodynamics
Short abstract
In highly sensitized patients with chronic kidney disease, the immunoglobulin G–degrading enzyme of Streptococcus pyogenes (imlifidase) degrades plasma IgG efficiently, reduces HLA antibodies substantially, and abolishes C1q binding to anti‐HLA, thus enabling HLA‐incompatible kidney transplantation.
Abbreviations
- ABMR
antibody‐mediated rejection
- AE
adverse event
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BCR
B cell receptor
- BW
body weight
- CDC
complement‐dependent cytotoxicity
- CKD
chronic kidney disease
- Cmax
maximum plasma concentration
- cPRA
calculated panel reactive antibody
- DSA
donor‐specific antibody
- eGFR
estimated glomerular filtration rate
- FCXM
flow cytometry crossmatch
- IdeS
IgG‐degrading enzyme of Streptococcus pyogenes
- IVIg
intravenous Ig
- IV
intravenous
- MFI
mean fluorescence intensity
- PBMC
peripheral blood mononuclear cell
- PD
pharmacodynamics
- PK
pharmacokinetics
- SAB
single antigen bead
- SAE
serious adverse event
- scIgG
single‐cleaved IgG
1. INTRODUCTION
The presence of anti‐HLA antibodies that are specific for ≥1 HLA alleles of the organ donor before transplant has been associated with very poor outcomes and, in many cases, graft loss through hyperacute and antibody‐mediated rejection (ABMR).1 In the past, the detection of donor‐specific antibodies (DSAs) pretransplant was considered a contraindication to transplant. However, because the number of highly sensitized patients on the transplant waiting lists has increased, desensitizing strategies have been developed to allow such patients to benefit from transplant with a kidney from a living HLA‐incompatible donor.2, 3, 4 These strategies use preconditioning with cycles of high‐dose intravenous Ig (IVIg), or plasmapheresis combined with low‐dose IVIg,5 but can include other agents such as rituximab.6, 7 Desensitization has increased transplant rates, reduced waiting times, and provided a significant survival benefit for patients with DSA compared with waiting for an HLA‐compatible transplant.8 Transplant of an HLA‐incompatible kidney after desensitization is, however, associated with an increased risk of ABMR, in both the short and longer term.4, 9
To date, there is no standard protocol for desensitization that is effective in every patient with an anti‐HLA antibody.10 Each patient requires careful monitoring using complement‐dependent cytotoxicity (CDC), flow cytometry crossmatch (FCXM), and/or multiplex single antigen bead (SAB) assays, before and after each round of treatment, to determine if the crossmatch has been rendered negative to enable transplant to proceed. The number of treatments required to reach a negative crossmatch depends on the characteristics of the anti‐HLA antibodies present; a patient with low levels of DSA detected only by SAB analysis and FCXM may be desensitized after 2 to 4 cycles of treatment, whereas a patient with a positive CDC crossmatch usually requires many more cycles and additional agents to convert to a negative crossmatch, if it is ever attained. Each cycle of treatment is a burden for the patient and expensive.11 The development of a more reliable, safe, and cost‐effective approach for desensitization would offer a major benefit.
The IgG‐degrading enzyme of Streptococcus pyogenes (IdeS [imlifidase]) is a 35‐kDa cysteine protease identified in group A streptococci12 where the enzyme inactivates opsonizing IgG antibodies bound to the bacterial surface.13 IdeS specifically cleaves IgG molecules at the lower hinge region of the heavy chain in a multistep process.12, 14, 15, 16, 17 Initially, IgG is degraded into single‐cleaved IgG (scIgG), where 1 of the 2 IgG heavy chains is cut. This is followed by cleavage of the second heavy chain in the molecule, producing 1 F(ab')2 and 1 homodimeric Fcfragment.
In a phase 1 clinical study, intravenous (IV) administration of IdeS (0.24 mg/kg body weight [BW]) to healthy human subjects was demonstrated to efficiently and rapidly cleave the whole pool of plasma IgG.18 Within minutes after dosing, plasma IgG was converted into scIgG, and within a few hours after IdeS treatment, plasma IgG was cleaved into F(ab')2 and Fcfragments with no intact IgG and only low levels of scIgG remaining. No reflux of extravascular IgG was observed after IdeS administration in these healthy volunteers, and newly synthesized IgG was detected 1 week after treatment.18 Based on these data, we hypothesized that treatment of sensitized patients with chronic kidney disease (CKD) with IdeS before transplant would eliminate IgG resulting in desensitization. Therefore, IdeS therapy may offer an alternative to the currently used strategies for desensitization of patients with a positive crossmatch to an HLA‐incompatible donor.
We report the findings from a phase 2 clinical study investigating the safety, immunogenicity, pharmacokinetics (PK), and efficacy of IV IdeS treatment in sensitized patients with CKD.
2. MATERIALS AND METHODS
2.1. Patients and study design
2.1.1. Eligibility
Patients with CKD stage V, in dialysis, and on the waiting list for a kidney transplant at the Department of Surgical Sciences, Section of Transplant Surgery at Uppsala University Hospital, were eligible for the study if they had ≥2 identified HLA antibodies of which ≥1 was >3000 MFI in single antigen bead analysis on ≥2 separate occasions. All eligible patients were prescreened and tested negative for the presence of IgE antibodies to IdeS.
2.1.2. Approvals
The study protocol was approved by the regional ethics committee in Uppsala, Sweden (approval number 2014/131). All patients provided written informed consent.
2.1.3. IdeS administration
IdeS was administered via IV infusion to patients enrolled in the study in ascending doses with an optional second dose within 48 hours. In group 1 (n = 3; patients 101, 102, and 103), patients received 0.12 mg/kg BW IV over 15 minutes. The investigator decided whether a patient should be given a second dose based on safety and efficacy. Safety evaluation included a review of safety laboratory results (clinical chemistry and hematology) and adverse events (AEs). All group 1 patients were given a second IdeS infusion (0.12 mg/kg BW). Corticosteroids (Solu‐Medrol 250 mg IV), antihistamine (10 mg loratadine), and antibiotics (875 mg/125 mg amoxicillin/clavulanic acid 3 times daily) were administered before each IdeS infusion. Dose escalation was based on safety and efficacy evaluation by a Data Monitoring Committee of previous dose groups. In group 2 (n = 2; patients 203 and 205), patients received 0.25 mg/kg via IV infusion on 2 occasions with safety assessment before administration of the second dose as just described. Administration of a second dose was started within 32 hours for all subjects in groups 1 and 2. In group 3 (n = 2; patients 201 and 204), patients received 0.25 mg/kg via IV infusion on 1 occasion.
One patient (202) was given 0.25 mg/kg BW but the infusion was halted after about 4 minutes due to suspected infusion reactions. The reaction resolved as soon as the infusion was interrupted, and the infusion was not restarted.
Peripheral blood was collected 28 days earlier, at the time of enrollment (day –1) and after administration of IdeS on days 1, 3, 4, 5, 7, 14, 28, and 64 for determination of clinical chemistry, coagulation, virology, total plasma IgG, IgG composition [ie, intact, single‐cleaved and F(ab')2/Fc], and HLA antibody reactivity by CDC, FCXM, and SAB assays.
2.2. Crossmatching, anti‐HLA antibody and total IgG analysis
At each time‐point, CDC PRA analysis was performed as previously described.19 The specificity and MFI of circulating HLA antibodies were determined by using SAB assays (One Lambda) on a Luminex platform. In selected cases, C1q binding assays were performed (One Lambda). The combined concentration of intact IgG and scIgG was investigated by using ELISA.18
2.3. Expression of membrane bound IgG B cell receptor by CD19+ B cells
Peripheral blood mononuclear cells (PBMCs) from all IdeS‐treated patients were double‐stained for CD19 and F(ab')2/Fcfragments. PBMCs were isolated from heparinized blood by using density gradient separation (Ficoll‐PaquePLUS), fixed in paraformaldehyde, and stored in PBS plus 0.5% bovine serum albumin until analysis. Cells were stained with a CaptureSelect Biotin Anti‐IgG‐CH1 conjugate (BAC; 10 μg/mL) for detection of the F(ab')2 part of IgG and with a biotinylated goat anti‐human Fc‐specific F(ab')2 fragment (Jackson) (0.5 μg/mL) for detection of the Fc part of IgG. Cells were double‐stained with PE‐conjugated anti‐CD19 (Immunotools) and streptavidin‐APC (Jackson) and analyzed with flow cytometry.
2.4. Pharmacokinetics and pharmacodynamics
Each subject underwent serial PK sampling for 3 weeks after IdeS infusion. Serum concentrations of IdeS were determined with a validated electrochemiluminescence immunoassay where IdeS was captured by a coated hen anti‐IdeS antibody. Bound IdeS was detected by using a biotinylated rabbit anti‐IdeS antibody followed by streptavidin‐Sulfo (assay range 100 ng/mL‐3000 ng/mL). All serum samples were diluted in dissociation buffer before analysis to avoid interactions between IdeS and anti‐IdeS antibodies.
PK evaluations were performed by using WinNonlin Professional (Pharsight Corporation, St Louis, MO).
Each subject underwent serial sampling for pharmacodynamics (PD) during the study period. PD was analyzed with the use of a validated quantitative ELISA measuring serum IgG captured on the F(ab')2 part and detected on the Fc part of the IgG molecule.18
2.5. Transplant
Transplant was not part of the study design but was allowed if a kidney was offered during the study period and the CDC and FCXM were negative. One patient in group 1 met these criteria (see Results).
2.6. Statistical analysis
CDC antibody reactivity was analyzed with use of the paired t‐test. Cleavage of anti‐HLA and C1q‐binding antibodies was analyzed with use of the Friedman test with Dunn multiple comparison, and expression of B cell receptors (BCRs) on circulating CD19+ B cells was analyzed with 1‐way ANOVA and Dunnett multiple comparison test.
3. RESULTS
3.1. Patient characteristics
The demographics and baseline characteristics of the 8 patients enrolled in the study are presented in Table 1.
Table 1.
Patient demographics and baseline characteristics
| All (N = 8) | Patient 102a | |
|---|---|---|
| Patient characteristics | ||
| Age (y), median (range), y | 48.5 (31‐69) | 60 |
| Sex, female:male, n | 5:3 | Male |
| Time in dialysis, median (range), y | 4.6 (1.9‐18.3) | 2.4 |
| Previous transplants, median (range), n | 0.5 (0‐3) | 1 |
| Immunologic characteristics | ||
| cPRAb, median (range), % | 93 (42‐100) | 42 |
| CDC‐PRA, median (range), % | 64 (0‐100) | 69 |
| Number of positivec HLA, median (range) | 31 (11‐130) | 11 |
| Average anti‐HLA level, median (range), MFI | 9200 (2700‐18 900) | 8000 |
| Donor characteristics | ||
| Deceased donor | — | Yes |
| DSA, antigen (MFI) | — | B7 (6000) |
| Cold ischemia time, h | — | 8 |
| Delayed graft function | — | No |
| Kidney function at 36 mo | ||
| Creatinine, μmol/L | — | 102 |
| eGFR, mL/min/1.73 m2 | — | 81 |
| Antibody‐mediated rejection, n | — | 0 |
cPRA, calculated panel reactive antibody; CDC, complement‐dependent cytotoxicity; MFI, mean fluorescence intensity; DSA, donor‐specific antibody; eGFR, estimated glomerular filtration rate.
Patient transplanted post‐IdeS treatment.
Acceptable MFI < 1100 (UNet based).
Defined as MFI > 1100 pre‐IdeS.
3.2. Pharmacokinetics
Maximum plasma concentrations (Cmax) of IdeS were observed at the end of the 15‐minute infusion or shortly thereafter. In patients who received a second dose, the second Cmax value was higher than the first value (see Figure 1). The plasma concentration curves showed a fast distribution phase with a mean half‐life of 5 hours and a slow elimination phase with a mean half‐life of 70 hours (range 50‐300 hours) and were well described by a 2‐compartment model. The volume of the central compartment of the model was 0.046 L/kg, while the distribution volume during the elimination phase was 0.090 L/kg. This may be interpreted as an initial distribution of IdeS in the plasma volume, from which IdeS is distributed into the extracellular compartment and then slowly eliminated. The distribution and elimination parameters of IdeS obtained in the kidney insufficient patients were similar to those observed in healthy subjects.18
Figure 1.
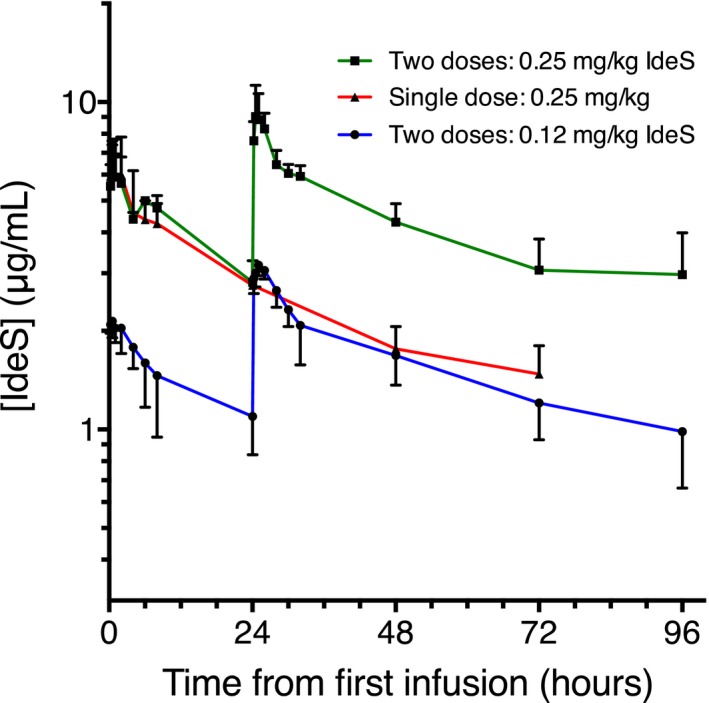
IdeS plasma concentration. Logarithm of mean IdeS plasma concentrations in patients given 2 doses of 0.12 mg/kg (n = 3), patients given a single dose of 0.25 mg/kg (n = 2), and patients given 2 doses of 0.25 mg/kg (n = 2). IdeS concentration measured using a validated assay after antibody dissociation (LLOQ: 0.1 μg/mL). Pharmacokinetics was evaluated using an open 2‐compartment model. Arithmetic mean values were reported for all parameters except for α and β half‐lives, where harmonic means were calculated. Cmax and Tmax were obtained directly from the experimental data. Nominal time is given on the x‐axis. Error bars = SD
3.3. IdeS degrades plasma IgG rapidly and efficiently
The efficacy of IdeS treatment on IgG was investigated by using serial sampling for Ig analysis by ELISA to determine the combined concentration of intact IgG and scIgG and visualized by SDS‐PAGE analysis. IdeS treatment of sensitized patients in group 1 (patients 101, 102, and 103; 0.12 mg/kg) resulted in a reduction of total IgG concentration, reflecting the cleavage of IgG into F(ab')2 and Fc fragments (Figure 2A). The mean IgG concentration was reduced from 11 g/L (predose) to 2.2 g/L after 6 hours and further to 0.61 g/L after 24 hours from dose 1. All 3 patients in group 1 received a second dose of IdeS (0.12 mg/kg), resulting in a further reduction in total IgG concentration to a mean of 0.021 g/L. The mean IgG concentration for patients 201 and 204 (group 3), who received 1 dose of 0.25 mg/kg IdeS, was reduced from 9.2 g/L (predose) to 0.096 g/L after 6 hours and further to 0.030 g/L after 24 hours (Figure 2B). In patients 203 and 205 (group 2), who received 2 doses with 0.25 mg/kg IdeS, the mean IgG concentration was reduced from 9.5 g/L (predose) to 0.17 g/L after 6 hours and further to 0.017 g/L after 24 hours from dose 1. After the second dose of IdeS (0.25 mg/kg), the mean IgG concentration dropped further to <0.01 g/L (Figure 2B). SDS‐PAGE analysis confirmed the ELISA data showing no detectable intact IgG and low levels of scIgG (Figure 2C).
Figure 2.
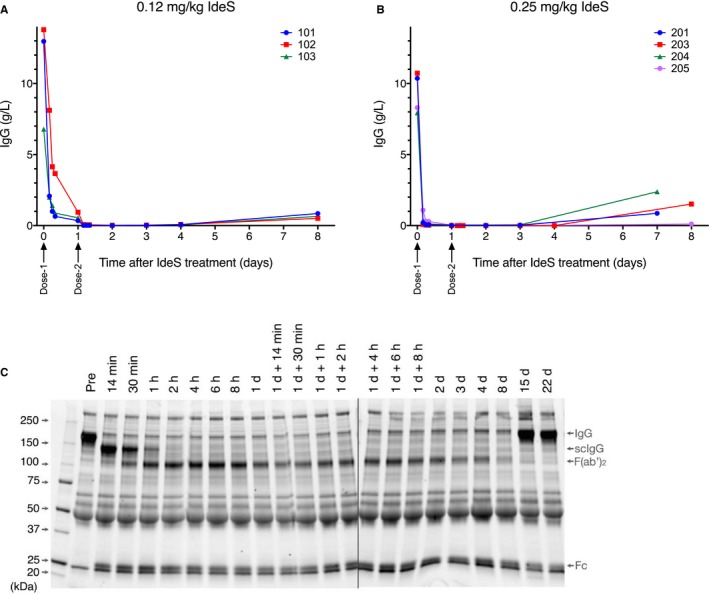
IdeS effectively cleaves IgG. A, B. IgG in serum samples collected before and at consecutive time‐points after IdeS treatment. C. Nonreducing SDS‐PAGE analysis of serum from patient 205 who received 2 doses of 0.25 mg/kg BW IdeS showing protein banding patterns at the indicated time‐points. Right. IgG, scIgG, F(ab')2, and Fc bands are indicated
3.4. IdeS significantly reduces CDC‐PRA and SAB HLA antibody reactivity
All patients treated with IdeS exhibited a significant reduction in predose PRAs detected by CDC crossmatch within 1 hour after IdeS treatment (data not shown). Within 24 hours after the first IdeS treatment, patients showed a large reduction in T cell and B cell PRAs (P = .0157 and 0.0031, respectively) with very low posttreatment PRAs (Figure 3). At 24 hours after completing IdeS treatment, all patients had a very low percentage of residual T cell (0‐7%) and B cell (0‐21%) PRAs. There was a large individual variation in the rate of PRA recovery, with the majority of patients starting to recover between days 7 and 14 (data not shown).
Figure 3.
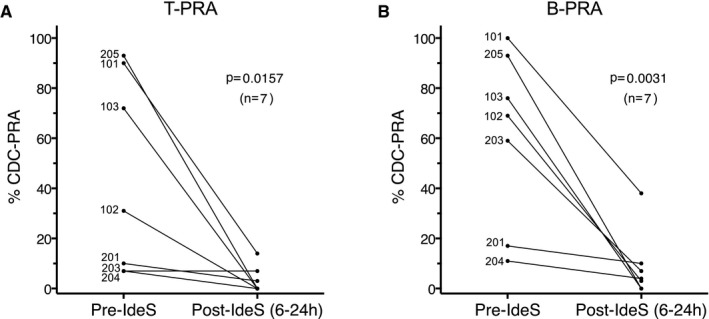
IdeS reduces CDC antibody reactivity. A. T‐cell non‐amplified CDC‐PRA 6‐24 hours after first treatment for patients dosed with 0.12 (patients 101, 102, and 103) or 0.25 (patients 201, 203, 204, and 205) mg/kg IdeS. B. B‐cell non‐amplified CDC‐PRA 6‐24 hours after first treatment. Paired t‐test
IdeS treatment of all patients resulted in reduction in the MFI signal in the SAB assays, reflecting reduced binding of antibodies to the HLA‐coated beads and complete elimination of C1q binding, exemplified by patients 101 and 205 (Figure 4). Patient 101 exhibited exceptionally high levels of antibodies with 75 class I or class II HLA antigens having an MFI >20 000 (above the quantitative range of the assay, Figure 4C). Despite this fact, IdeS treatment reduced HLA antibodies dramatically. The remaining signal did not consist of intact IgG but of scIgG, which has severely impaired FcR‐binding and complement‐binding properties (unpublished data). Thus, C1q activity was eliminated 1 hour after the initial dose (Figure 4D), illustrating that even in extreme cases, IdeS efficiently removes reactive HLA antibodies.
Figure 4.
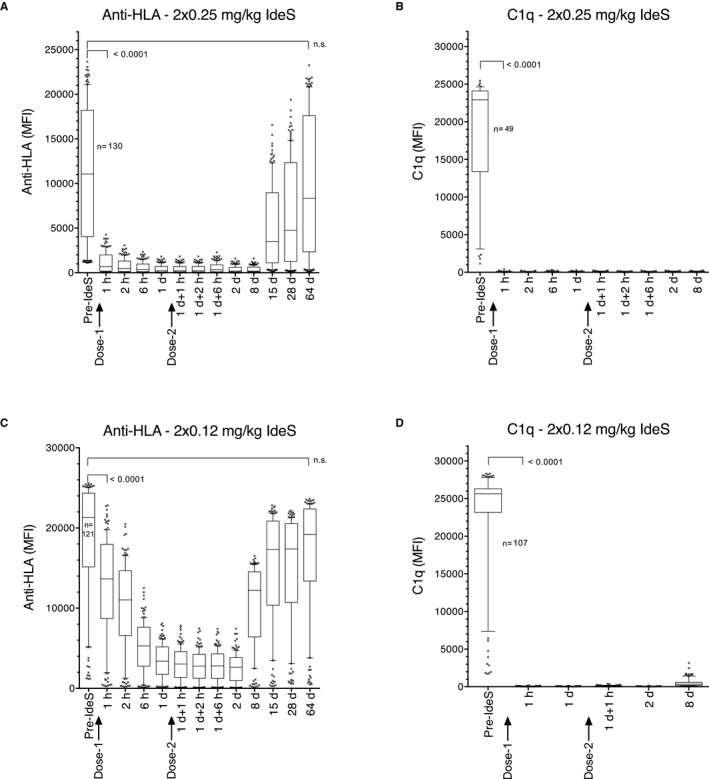
IdeS effectively cleaves anti‐HLA antibodies and eliminates C1q‐binding. Box‐plot of anti‐HLA antibodies having a pre‐dose MFI >1100 (cutoff in study) before and at consecutive time‐points following first and second IdeS treatment (0.12 or 0.25 mg/kg BW). A. Patient 205 LABScreen MFI. B. Patient 205 C1q‐Screen MFI. C. Patient 101 LABScreen MFI. D. Patient 101 C1q‐Screen MFI. Horizontal lines represent median MFI, boxes represent 25th and 75th percentile and vertical lines represent 10th and 90th percentile MFI. Dots are MFI of individual HLAs above or below the percentiles. The P‐values denote first significant difference between pre and post dose. N.S. is the time‐point when there is no longer any significance between pre and post dose. Statistics: Friedman test with Dunn multiple comparison test. The number (n) of HLAs having an MFI above the cutoff at 1100 is given in graph
The mean MFI of HLA antibodies with a predose MFI of >1100 in patients 101, 102, and 103 was reduced from 18 900, 8000, and 10 400 to 2500, 610, and 2100, respectively, after completion of treatment (Figure 5A). The mean MFI in patients 201, 203, 204, and 205 was reduced from 5600, 13 700, 2700, and 11 300 to 290, 850, 110, and 350, respectively (Figure 5B). All patients exhibited a reduction in MFI within a few hours after the first dose of IdeS. The effect of IdeS was stronger and more rapid in patients treated with 0.25 mg/kg compared with patients treated with 0.12 mg/kg. The higher IdeS dose resulted in a more complete conversion of scIgG into F(ab')2 and Fc (results not shown). For group 1 patients (0.12 mg/kg ×2), there was a clear effect of the second dose, which was not observed for group 2 patients (0.25 mg/kg ×2), who exhibited a more complete reduction of MFI after the first dose. The MFI values started to recover from day 7 or 8 after completion of IdeS infusions and were back to predose levels between day 14/15 and day 28 for all patients except for patient 102 who was transplanted with an HLA‐incompatible kidney during the study and received immunosuppression (see later). C1q binding to anti‐HLA antibodies was strongly reduced within 1 hour of the first treatment in both groups receiving 0.12 or 0.25 mg/kg IdeS (Figure 5C, D). There was no additive effect of the second dose.
Figure 5.
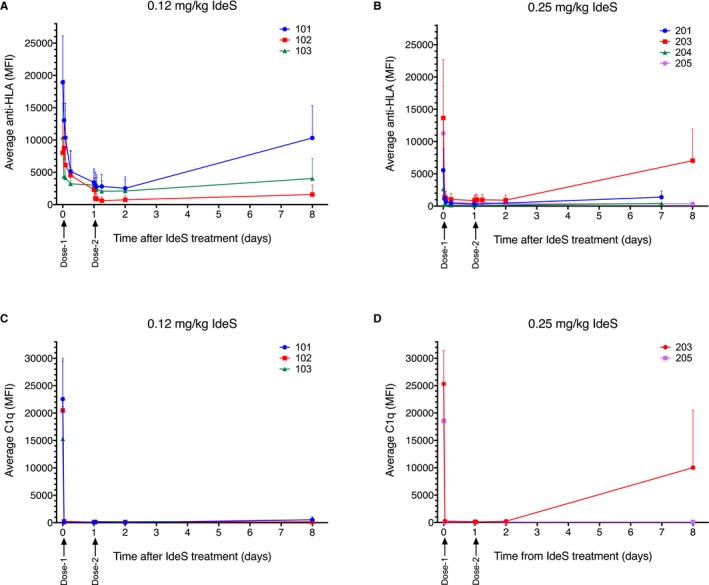
IdeS effectively cleaves anti‐HLA and C1q‐binding antibodies. A, B. Average (+SD) anti‐HLA antibodies (LABScreen) before and at consecutive time‐points after IdeS treatment in patients treated with 2 doses of 0.12 mg/kg IdeS (patients 101, 102, and 103), 1 dose of 0.25 mg/kg IdeS (patients 201 and 204), or 2 doses of 0.25 mg/kg IdeS (patients 203 and 205). C, D. Average (+SD) C1q‐binding antibodies (C1qScreen) before and at consecutive time‐points after IdeS treatment. Antigens having a predose MFI >1100 were selected for analyses
3.5. IdeS degrades BCR expressed by circulating CD19+ B cells
The integrity of membrane‐bound IgG‐BCR on circulating CD19+ B cells, predose and at different time‐points after IdeS administration, was investigated. The IgG‐type of BCR was found to be cleaved efficiently by IdeS in all study subjects, shown by a significant loss of the F(ab')2 staining on the surface of CD19+ cells 24 hours postdosing (Figure 6A), which remained low for several days. Fc staining of CD19+ cells was unaffected by IdeS treatment (Figure 6B). Together, these data imply that B cells with a cleaved IgG‐BCR [ie, having an Fc fragment on the surface but lacking the F(ab')2 part] were still circulating in the body at least 72 hours after the last dose of IdeS in all subjects. One week after IdeS infusions, cells with F(ab')2 (ie, intact IgG‐BCR) started to reappear in the circulation. These data demonstrate that the F(ab')2 part of the IgG‐type of BCR is efficiently cleaved by IdeS within 24 hours after treatment with a single dose of either 0.12 or 0.25 mg/kg BW.
Figure 6.
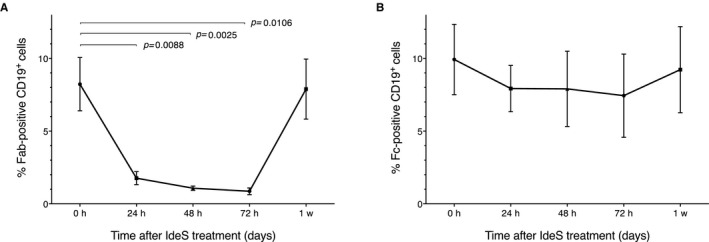
IdeS degrades B cell receptor expressed by circulating CD19+ B cells. Flow cytometry analysis of CD19+/IgG+ cells at different time‐points after IdeS treatment as a mean of 6 patients. A. The patients showed a significant reduction in percentage of Fab‐positive CD19+ cells. B. The patients showed no significant reduction in percentage of Fc‐positive CD19+ cells. Data presented as mean (±SEM). The x‐axis shows time post first dosing. Data from patient 102 were not included due to too few CD19+ cells in the majority of the sampling points, and samples from patient 202 were not collected for BCR evaluation as this patient did not receive a full dose of IdeS
3.6. Anti‐IdeS antibodies
At enrollment, none of the screened patients had detectable levels of IgE against IdeS. However, as previously reported from healthy individuals,18 all patients had detectable anti‐IdeS IgG levels with a median level of 11 mg/L (range 8.6‐19 mg/L, Figure 7). Immediately after dosing, the anti‐IdeS IgG levels dropped below the lower limit of quantification due to the cleavage of antibodies by IdeS. In patient 202, in whom the dosing was interrupted, no decrease in anti‐IdeS IgG was seen, consistent with the noneffective dose.
Figure 7.
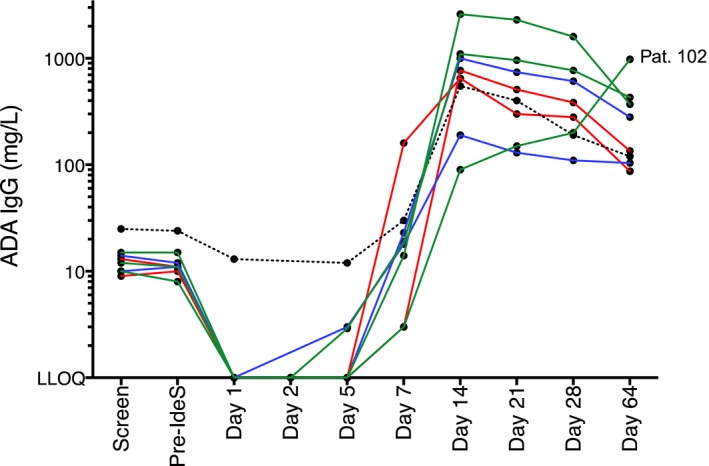
Anti‐IdeS IgG concentration in serum from all patients before and after IdeS treatment. The green lines represent group 1 (patients 101, 102, and 103) who received 2× 0.12 mg/kg BW IdeS, the red lines represent group 2 (patients 203 and 205) who received 2× 0.25 mg/kg BW IdeS, and the blue lines represent group 3 (patients 201 and 204) who received 1× 0.25 mg/kg BW IdeS. The dotted line is patient 202 who had dosing interrupted. Serum samples were analyzed using the IdeS‐ImmunoCAP (Thermo Fisher Scientific) on a Phadia 250 instrument. The cutoff (LLOQ) for IgG was 2 mg/L
An increase in anti‐IdeS IgG concentrations was detected at day 7 after treatment in all patients. The peak serum concentration occurred on day 14 (Figure 7), except for transplanted patient 102, who exhibited the highest anti‐IdeS IgG concentration at day 64. The reason for this delayed kinetic is unknown but may be related to the immunosuppressive medication administered after transplant. Substantial individual variation was observed for the magnitude of anti‐IdeS response, with a median peak concentration of 875 mg/L, ranging between 190 and 1000 mg/L. On day 64, the median concentration in serum had decreased to 120 mg/L (range 87‐280 mg/L). No notable difference in anti‐IdeS IgG concentration was detected between dose groups or between patients who received either 1 or 2 doses of IdeS.
3.7. AEs associated with IdeS infusion
The total number of AEs reported during the study was 76, of which 27 were classified as related (related AE is defined as possibly or probably related); 18 grade 1, 4 grade 2, and 5 grade 3 (Table 2).
Table 2.
Number of related AEs in patients receiving an effective IdeS dose
| System organ class (preferred term) | IdeS 0.12 mg/kg (n = 3) | IdeS 0.25 mg/kg (n = 4) |
|---|---|---|
| Vascular disorders | ||
| Flushing, n | 1 | |
| General disorders and administration site conditions | ||
| Infusion site pain, n | 2 | |
| Infections and infestations | ||
| Pneumonia, n | 1 | 1 |
| Upper respiratory tract infection, n | 1 | |
| Influenza, n | 1 | |
| Nervous system disorders | ||
| Headache, n | 1 | 2 |
| Dizziness postural, n | 2 | |
| Investigations | ||
| Alanine aminotransferase increased, n | 1 | 1 |
| Aspartate aminotransferase increased, n | 1 | 2 |
| Alkaline phosphatase, n | 1 | |
| Musculoskeletal and connective tissue disorders | ||
| Myalgia, n | 2 | |
There were 5 serious AEs (SAEs) reported. Four of these were reported as related. Three related SAEs were classified as infections and infestations: pneumonia (patients 102 and 205) and suspected upper respiratory infection (patient 204). Myalgia was reported in 2 patients, 1 SAE grade 2 (patient 204) and 1 SAE grade 3 (patient 203). Patient 204 recovered approximately 4 months after the IdeS infusion, and patient 203 was recovering at 12 months after corticosteroids had been tapered down.
One patient experienced a suspected infusion reaction and dosing was interrupted. All symptoms were grade 1 (flushing, hypertension, hot flashes, sinus tachycardia, dyspnea, scleral hemorrhage, visual impairment) and resolved 11 minutes after the infusion was stopped.
Two patients (102 and 201) had increased levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) reported as AEs, both of which were classified as related. These patients received prophylaxis with amoxicillin/clavulanic acid and 1 of the patients (patient 102; see later) also received tacrolimus. Both drugs can cause elevated liver enzyme levels. When amoxicillin/clavulanic acid was stopped and tacrolimus was adjusted, the liver enzyme levels decreased to normal levels. PK‐INR was normal throughout the follow‐up period. Albumin ranged between 25 and 30 g/L. Due to this finding, the amoxicillin/clavulanic acid was replaced with phenoxymethylpenicillin in subsequent patients, and no additional cases of elevated liver enzymes were observed.
3.8. Transplant of an HLA‐incompatible kidney after IdeS treatment
Transplant was allowed within the study if a kidney was offered during the study period and the CDC and FCXM were negative. One patient in group 1 met these criteria. The patient (102), a 60‐year‐old man with CKD (HLA‐A2, 3; ‐B8, 14; ‐DR4, 15; ‐DQ3, 6), had renal agenesis with progressive renal failure. The patient had received a first kidney transplant in 1997 with a functional graft until 2010. That kidney was lost due to chronic allograft nephropathy and the patient returned to peritoneal dialysis. At enrollment, HLA antibodies were detected by CDC with 69% PRAs (Table 1). A total of 13 circulating anti‐HLA antibodies of known specificity with an MFI >500 were defined using SAB assays; HLA‐A1, ‐A66, ‐B7, ‐B13, ‐B27, ‐B47, ‐B48, ‐B60, ‐B61, B73, ‐B81, ‐Cw4, and ‐Cw17.
A few hours after the second IdeS infusion, an AB0‐compatible HLA‐incompatible kidney (HLA‐B7) from a deceased donor was offered and crossmatch was performed on sera taken within the study. Before IdeS treatment, a 128/318 shift (T and B cells, respectively) in 3‐color FCXM with a positive nonamplified CDC crossmatch was demonstrated against the donor. Based on SAB assays, the patient was demonstrated to have an HLA‐B7 DSA with an MFI of 6000 with positive C1q binding (MFI 16 000). In serum taken 6 hours after completing IdeS treatment (0.12 mg/kg ×2), CDC and FCXM tests were negative and the DSA level was reduced to MFI 300. Total IgG detectable in plasma was reduced from a starting value of 13 g/L to 0.06 g/L at 6 hours after the second IdeS infusion.
Based on the negative crossmatch, the patient was successfully transplanted and received induction treatment with ATGAM, tacrolimus (target serum level 10‐12 ng/mL), mycophenolate mofetil (2 g per day), and glucocorticoids administered intraoperatively (500 mg Solu‐Medrol). Glucocorticoids were tapered during a 2‐week period (20 mg daily).20
DSA levels remained below MFI 1100 for 2 weeks after transplant. On day 64, plasma IgG levels had returned to the normal range (6.3 g/L) but not to predose levels. Anti‐HLA antibody (non‐DSA) degradation and recovery followed the same pattern as for total IgG. By 8 weeks, anti–HLA‐B7 antibody was detected with MFI 2700, and by 7 months, with MFI 500. Stable graft function has been maintained for >36 months with normal creatinine clearance (see Figure 8), with no proteinuria and no rejection episodes.
Figure 8.
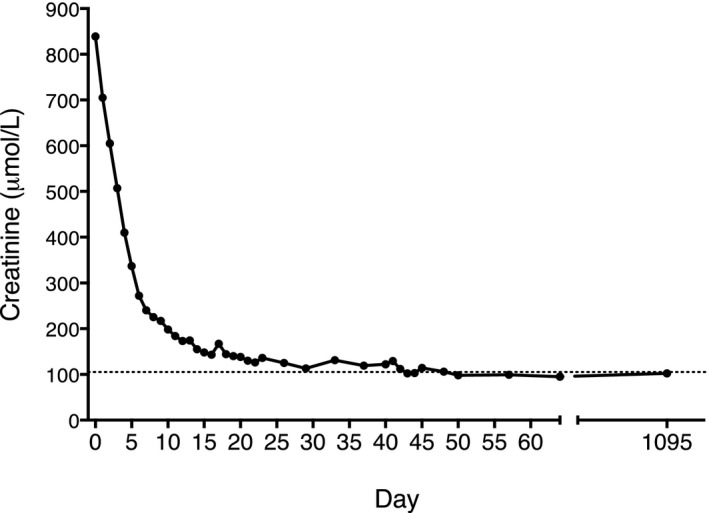
Normal creatinine levels in patient 102 after transplant. Plasma creatinine levels at different time‐points in an IdeS‐treated recipient transplanted with an HLA‐incompatible kidney from a deceased donor
Elevated levels of liver enzymes were found 7 days after transplant with a peak on day 12 (ALT 1186 U/L and AST 545 U/L on day 12). AST was normal on day 16, and ALT was normal on day 36. The patient's tacrolimus serum concentration was above the target level of 12 μg/L at the time of the liver enzyme elevation, which in combination with amoxicillin and clavulanic acid was the likely cause of the elevated liver enzyme levels, as previously mentioned. There were no other clinically significant changes in hematology, clinical chemistry or coagulation values.
Five weeks after transplant, the patient experienced a bacterial pneumonia, which was treated successfully with meropenem.
4. DISCUSSION
The development of a more reliable, safe, and cost‐effective approach for desensitization of sensitized patients on the transplant waiting list would offer a major benefit, enabling more patients to receive a kidney transplant from an HLA‐incompatible donor. The IgG‐degrading enzyme IdeS specifically cleaves IgG molecules at the lower hinge region of the heavy chain in a multistep process. Based on data from a phase 1 clinical study in healthy volunteers,18 we hypothesized that treatment with IdeS before transplant may offer an alternative to the currently used strategies for desensitizing patients with a positive crossmatch to an HLA‐incompatible living donor. We report the findings from a phase 2 clinical study investigating the safety, immunogenicity, PK, and efficacy of an open‐label dose‐ascending study of IdeS treatment in highly sensitized patients with stage V CKD.
Patients eligible for enrollment in the study received 1 or 2 doses of IdeS at 0.12 mg/kg or 0.25 mg/kg BW. IdeS treatment at either dose resulted in rapid and efficient degradation of plasma IgG within 24 hours of administration of the final dose. One dose of 0.25 mg/kg BW resulted in a 300‐fold reduction of the IgG concentration within 24 hours. These results were supported by SDS‐PAGE analysis, demonstrating that only scIgG remained 1 hour after a single dose of 0.25 mg/kg BW IdeS. Intact IgG started to return in plasma between days 7 and 14 after treatment. The rate of recovery was different in individual patients. For patient 102, who received an HLA‐incompatible kidney transplant during the study, recovery was affected by immunosuppressive drug therapy.
Cleavage of IgG‐BCR on CD19+ B cells was observed in all patients. We have previously shown that the BCR on CD19+/CD27+/IgG+ memory B cell population from healthy volunteers was cleaved by IdeS in vitro and that PBMCs treated with IdeS were unable to respond to antigen.21 In the current phase 2 study, IgG‐BCR was restored on CD19+ B cells at the 7‐day sampling point while plasma IgG levels were still low, demonstrating that the recovery of IgG‐BCR on the surface of CD19+ cells is faster than the recovery of plasma IgG levels.
CDC crossmatching against a panel of HLA‐typed T and B cells was performed for each patient before and after IdeS treatment. The percentage of both T cell and B cell PRAs was reduced in all patients within 1 hour after IdeS treatment. By 24 hours after completing IdeS treatment, all patients had a very low percentage of residual T cell and B cell PRAs. Deeper analysis examining the individual T and B cell CDC crossmatches performed between recipient and hypothetical kidney donors revealed that in the majority of patients, the CDC crossmatch was converted to a negative CDC crossmatch within 1 hour after a single dose of IdeS (0.12 or 0.25 mg/kg BW). The crossmatch remained negative for 1 week. Analysis of the specificity of the HLA antibodies present in patients treated with IdeS using SAB confirmed that all antibodies were degraded after IdeS treatment. This was also the case for patient 102 who had high levels of HLA‐B7 reactive antibodies before IdeS treatment and who received an offer of an HLA‐B7+‐incompatible kidney during the study. Crossmatching immediately before transplant confirmed the absence of HLA‐B7 reactivity, and the transplant was performed. The patient had a smooth posttransplant course with no evidence of rejection and retains good graft function 3 years later (creatinine 102 μmol/L and estimated glomerular filtration rate [eGFR] 81 mL/min). As part of open label phase 1/2 trials performed in Sweden and the United States, additional patients treated with IdeS have been transplanted successfully.20
IdeS originates from Streptococcus pyogenes and is consequently expected to be recognized as foreign to the human immune system. The fact that all patients had detectable anti‐IdeS IgG before dosing implies that the patients had been preexposed to IdeS, undoubtedly during previous streptococcal infections, and would therefore be expected to mount a memory response against IdeS. The finding that substantial levels of IdeS‐reactive IgG develop in patients treated with IdeS is consistent with a typical recall immune response. The implications of anti‐IdeS antibodies were not explored in this study as patients received only a single dose or a second dose on the next day. The level of anti‐IdeS IgG and a potentially neutralizing capacity to IdeS may preclude retreatment if a patient does not receive an offer of a transplant within 7 days of treatment. However, in the phase 1 study, it was demonstrated that after 6 to 12 months, anti‐IdeS antibodies had returned to normal range. Thus, retreatment after this period may be possible and requires further investigation.
The data obtained in this phase 2 clinical study demonstrate that IdeS treatment in sensitized patients with CKD is not only effective but also safe and well tolerated and has the potential to generate a window of 7 days after treatment when HLA antibodies are reduced below the threshold level, enabling the patient to be a candidate for transplant with an organ from an HLA‐incompatible donor.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Hansa Medical AB was the formal sponsor and funder of the clinical study. LW, SJ, KM, AR, YS, and CK are employed by Hansa Medical AB. GT, LBj, and KW are medical/scientific advisors to Hansa Medical. Hansa Medical AB holds IdeS patents on which LBj is listed as an inventor. TL, BME, LW, SJ, YS, AR, KM, LBj, LBä, EL, and CK are holders of shares or share warrants in Hansa Medical AB.
ACKNOWLEDGMENTS
We thank the research nurses Mia Elofsson, Maria Svennaeus Lundgren, Malin Dackborn, Åsa Aringskog, and Anna Högvall and patient coordinator Ingrid Skarp for invaluable professional help.
Lorant T, Bengtsson M, Eich T, et al. Safety, immunogenicity, pharmacokinetics, and efficacy of degradation of anti‐HLA antibodies by IdeS (imlifidase) in chronic kidney disease patients. Am J Transplant. 2018;18:2752–2762. 10.1111/ajt.14733
Funding information
Hansa Medical AB was the formal sponsor and funder of the clinical study. LW, SJ, KM, AR, YS, and CK are employed by Hansa Medical AB. Hansa Medical AB provided support in the form of salaries for authors LW, SJ, KM, AR, YS, and CK but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Gunnar Tufveson and Christian Kjellman contributed equally.
ClinicalTrials.gov identifier: NCT02224820.
REFERENCES
- 1. Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280(14):735‐739. [DOI] [PubMed] [Google Scholar]
- 2. Higgins RM, Bevan DJ, Carey BS, et al. Prevention of hyperacute rejection by removal of antibodies to HLA immediately before renal transplantation. The Lancet. 1996;348(9036):1208‐1211. [DOI] [PubMed] [Google Scholar]
- 3. Glotz D, Antoine C, Julia P, et al. Desensitization and subsequent kidney transplantation of patients using intravenous immunoglobulins (IVIg). Am J Transplant. 2002;2(8):758‐760. [DOI] [PubMed] [Google Scholar]
- 4. Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA‐Incompatible Kidney Recipients and Survival. N Engl J Med. 2011;365(4):318‐326. [DOI] [PubMed] [Google Scholar]
- 5. Stegall MD, Gloor J, Winters JL, Moore SB, DeGoey S. A comparison of plasmapheresis versus high‐dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006;6(2):346‐351. [DOI] [PubMed] [Google Scholar]
- 6. Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359(3):242‐251. [DOI] [PubMed] [Google Scholar]
- 7. Macklin PS, Morris PJ, Knight SR. A systematic review of the use of rituximab for desensitization in renal transplantation. Transplantation. 2014;98(8):794‐805. [DOI] [PubMed] [Google Scholar]
- 8. Orandi BJ, Luo X, Massie AB, et al. Survival benefit with kidney transplants from HLA‐incompatible live donors. N Engl J Med. 2016;374(10):940‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riella LV, Safa K, Yagan J, et al. Long‐term outcomes of kidney transplantation across a positive complement‐dependent cytotoxicity crossmatch. Transplantation. 2014;97(12):1247‐1252. [DOI] [PubMed] [Google Scholar]
- 10. Montgomery RA, Warren DS, Segev DL, Zachary AA. HLA incompatible renal transplantation. Curr Opin Organ Transplant. 2012;17:386‐392. [DOI] [PubMed] [Google Scholar]
- 11. Vo AA, Jordan SC. Benefits, efficacy, cost‐effectiveness and infectious complications in transplant patients desensitized with intravenous immunoglobulin and anti‐CD20 therapy. Clin Exp Immunol. 2014;178:48‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Pawel‐Rammingen U, Johansson BP, Björck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002;21(7):1607‐1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. von Pawel‐Rammingen U, Johansson BP, Tapper H, Bjorck L. Streptococcus pyogenes and phagocytic killing. Nat Med. 2002;8(10):1044‐1045. [DOI] [PubMed] [Google Scholar]
- 14. Vincents B, von Pawel‐Rammingen U, Björck L, Abrahamson M. Enzymatic characterization of the streptococcal endopeptidase, IdeS, reveals that it is a cysteine protease with strict specificity for IgG cleavage due to exosite binding. Biochemistry. 2004;43(49):15540‐15549. [DOI] [PubMed] [Google Scholar]
- 15. Wenig K, Chatwell L, von Pawel‐Rammingen U, Björck L, Huber R, Sondermann P. Structure of the streptococcal endopeptidase IdeS, a cysteine proteinase with strict specificity for IgG. Proc Natl Acad Sci USA. 2004;101(50):17371‐17376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johansson BP, Shannon O, Björck L. IdeS: a bacterial proteolytic enzyme with therapeutic potential. PLoS ONE. 2008;3(2):e1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan MH, Petrone D, Nemeth JF, Barnathan E, Björck L, Jordan RE. Proteolysis of purified IgGs by human and bacterial enzymes in vitro and the detection of specific proteolytic fragments of endogenous IgG in rheumatoid synovial fluid. Mol Immunol. 2008;45(7):1837‐1846. [DOI] [PubMed] [Google Scholar]
- 18. Winstedt L, Järnum S, Nordahl EA, et al. Complete removal of extracellular IgG antibodies in a randomized dose‐escalation phase I study with the bacterial enzyme IdeS – a novel therapeutic opportunity. PLoS ONE. 2015;10(7):e0132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wahlberg J, Bengtsson M, Bergstrom C, et al. Impact of flow cytometry cross‐matching results on the outcome of cadaveric kidney transplantation. Transpl Proc. 1994;26:1752‐1753. [PubMed] [Google Scholar]
- 20. Jordan SC, Lorant T, Choi J, et al. IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med. 2017;377(5):442‐453. [DOI] [PubMed] [Google Scholar]
- 21. Järnum S, Bockermann R, Runström A, Winstedt L, Kjellman C. The bacterial enzyme IdeS cleaves the IgG‐type of B cell receptor (BCR), abolishes BCR‐mediated cell signaling, and inhibits memory B cell activation. J Immunol. 2015;195(12):5592‐5601. [DOI] [PMC free article] [PubMed] [Google Scholar]


