Abstract
There is growing public concern about neurodegenerative changes (e.g., Chronic Traumatic Encephalopathy) that may occur chronically following clinically apparent and clinically silent (i.e., subconcussive blows) pediatric mild traumatic brain injury (pmTBI). However, there are currently no biomarkers that clinicians can use to objectively diagnose patients or predict those who may struggle to recover. Non-invasive neuroimaging, electrophysiological and neuromodulation biomarkers have promise for providing evidence of the so-called “invisible wounds” of pmTBI. Our systematic review, however, belies that notion, identifying a relative paucity of high-quality, clinically impactful, diagnostic or prognostic biomarker studies in the sub-acute injury phase (36 studies on unique samples in 28 years), with the majority focusing on adolescent pmTBI. Ultimately, well-powered longitudinal studies with appropriate control groups, as well as standardized and clearly-defined inclusion criteria (time post-injury, injury severity and past history) are needed to truly understand the complex pathophysiology that is hypothesized (i.e., still needs to be determined) to exist during the acute and sub-acute stages of pmTBI and may underlie post-concussive symptoms.
Keywords: concussion, mild traumatic brain injury, pediatric, neuroimaging, electrophysiology, neuromodulation
Introduction
Clinicians face multiple challenges when treating the 750,000 new cases of pediatric mild traumatic brain injury (pmTBI) that occur each year (Zemek et al., 2016a). Specifically, the etiology (e.g., diffuse brain injury vs. disrupted cerebral blood flow [CBF] vs. psychological factors) of post-concussive symptoms (PCS), the long-term impact of pmTBI on academic and social functioning, and the effect of age-at-injury on short-term and long-term clinical outcomes are largely unknown (Dennis et al., 2013; Taylor et al., 2010; Yeates et al., 2012). Animal and emerging human literature demonstrate both white (WM) and gray (GM) matter pathologies following injury, with each pathology exhibiting unique time-courses for recovery (Barkhoudarian et al., 2016; Mayer et al., 2011; Meier et al., 2015; Mondello et al., 2011). Computed tomography (CT) scans used for assessment of macroscopic intracranial hemorrhage are not sensitive to the most probable pathological features of pmTBI, including diffuse neural injuries, CBF disruptions and edema (Keightley et al., 2014b). Further, neither routine nor advanced magnetic resonance imaging (MRI) are currently endorsed by either the American Academy of Neurology (Giza et al., 2013) or the American Medical Society for Sports Medicine (Harmon et al., 2013) for m BI, primarily because of limited diagnostic/prognostic evidence, presumed low base-rates of positive findings, and high cost. In contrast, The Head Injury Institute of the American College of Radiology does recommend MRI in the context of persistent symptomatology following mTBI or in the presence of new or worsening symptoms (Wintermark et al., 2015).
Thus, no clear, objective neurobiological biomarkers exist upon which physicians can predict recovery from pmTBI despite the unique vulnerabilities of the developing brain. As a result, clinicians do not know when it is truly safe for children of different ages to return to play/learn. This potentially places children at risk for a second, temporally proximate injury resulting in increased symptoms, pathology and delayed recovery (Broglio et al., 2007; Gilbert and Johnson, 2011; Prins et al., 2010; Van Kampen et al., 2006). Conversely, the lack of objective markers may also result in unnecessary restriction of children from social, cognitive and physical activities for longer than is physiologically necessary. These issues have recently risen to the forefront, with growing concerns regarding the possible neurodegenerative sequelae (such as Chronic Traumatic Encephalopathy, CTE) of both clinically apparent and clinically silent injuries (i.e., subconcussive blows), and the increasingly recognized need to quantify the physiology of concussion (Mayer et al., 2017).
This systematic review addresses this gap by providing a critical review of the pmTBI biomarker (i.e., imaging, electrophysiology and neuromodulation) literature to date, as well as by discussing the major obstacles for performing research in this challenging population. In contrast to the adult literature, existing pmTBI biomarker reviews have predominantly focused on sport-related concussion (SRC; Chamard and Lichtenstein, 2018; Guenette et al., 2018) or chronic injury effects in mixed severity groups (Ashwal et al., 2014; Keightley et al., 2014b). The current review includes pmTBI from all injury mechanisms, and specifically focuses on biomarkers obtained during the acute and sub-acute injury phases. Importantly, we also include studies that assessed non-routine biomarkers as part of clinical care in the hospital setting.
General Background
The terms “concussion” and “mTBI” have either been used synonymously or to describe distinct diagnostic entities based on differing injury severity (mTBI > concussion); however, to date, no formal clinical criteria have been proffered to support the latter clinical distinction (Mayer et al., 2017). Thus, for the purpose of the current paper, these terms are used interchangeably. pmTBI represents a large public health concern due to the sheer volume of new cases (approximately 750,000) that occur each year (Zemek et al., 2016b). Importantly, the rates of reported high school concussions increased 4.2-fold between the mid-1990s and the mid-2000s, with similar increases in those seeking care in the emergency room (ER; Lincoln et al., 2011). Recent studies suggest that this may only be the tip of the iceberg, with pmTBI patients often seeking care with their primary care providers for concussion rather than in the ER setting (Arbogast et al., 2016). The highest incidence rates for all TBI severities occur in the first four years of life, with a secondary spike occurring between the ages of 15 and 19 years of age (Faul et al., 2010), suggesting that concussion is a particularly pertinent problem for children and adolescents. The increased incidence in adolescence is primarily driven by participation in organized sports and engagement in other risk-taking behaviors, including the initiation of driving.
It has been established that pmTBI patients experience cognitive, neurosensory and emotional symptoms during the acute to sub-acute injury phase (Master et al., 2016; Mayer et al., 2018; Yeates et al., 2009), with a recent ER-based study indicating that up to 30% of pmTBI patients remain symptomatic at 4 weeks post-injury (Zemek et al., 2016a). According to the International ollaboration on Mild Traumatic Brain Injury Prognosis, literature on long-term outcomes is sparse, with few well-designed prospective studies (Hung et al., 2014; Keightley et al., 2014b). Of particular concern are reports of increased incidences of neuropsychiatric conditions, serious academic delays, and decreases in overall quality of life following pmTBI (Bijur et al., 1990; McKinlay et al., 2009). In one large prospective study, pmTBI patients exhibited increased somatic and cognitive symptoms relative to orthopedic injury (OI) controls for up to 12 months post-injury (Yeates et al., 2012). Deficits in attention, memory, and processing speed are also commonly observed in the sub-acute phase of pmTBI (Babikian et al., 2011; Catroppa et al., 2007), with long-term executive dysfunction also being reported 12 months post-injury (Sesma et al., 2008).
The Role of Neurodevelopment
Childhood trauma is unique in that both the immediate and longitudinal effects of injury are superimposed on a rapidly changing brain. Neurodevelopmental trajectory varies as a function of age and biological sex (Gogtay et al., 2004; Lebel et al., 2008), suggesting that findings relevant for one group of children (e.g., male adolescents, the typical sample in most pmTBI studies) may not directly translate to other developmental phases or biologic sex. In general, females develop faster than males both in terms of behavior and cognition, a trend that is accelerated by differential entry points into puberty (Gogtay et al., 2004; Lebel et al., 2008). Classic theories espoused by Piaget and Erikson (reviewed in Berk, 2014; Berk and Meyers, 2016) separate childhood into distinct developmental stages including infancy/very young childhood (≤ 5 y.o., spanning multiple developmental stages), middle childhood (6 or 7 to 11 or 12 y.o.; single stage) and adolescence (≥ 12 y.o.; single stage), with each stage primarily distinguished based on emotional and cognitive capabilities. The National Institutes of Health defines four separate periods of growth and development corresponding to infancy (0–3 y.o.), preschool (3–6 y.o.), middle childhood (6–12 y.o.) and adolescence (12–18 y.o.).
However, other literature suggests more distinct neurodevelopmental stages occurring within middle childhood. For example, a single factor is derived across tests of executive functioning in 6–7 y.o., whereas children aged 8–13 tend to demonstrate the 3-factor structure commonly observed in adult models (Lehto et al., 2003). Similarly, Anderson (2002) outlined a steep development trajectory between the ages of 7 and 9 years for cognitions frequently impaired in brain injury (e.g., cognitive flexibility, goal setting, and information processing), with full maturation by 12 years. For the purposes of the current review, we divide childhood into the following 6 developmental stages: infancy (0–3 y.o.), preschool (3–6 y.o.), early childhood (6–8 y.o.), middle childhood (9–12 y.o.) and adolescence (13–19 y.o.).
Accounting for the variance associated with neurodevelopment and biological sex is especially relevant for imaging studies (see Guenette et al., 2018 for review). Sensorimotor development is typically complete by ages 6–7 years, but the development of association areas involved in top-down control/executive functioning occurs later (Casey et al., 2005). Neurodevelopmental trajectories differ markedly between middle childhood and adolescence, with linear and quadratic effects reported in WM volumes (Lenroot et al., 2007). Although WM tracts are developed by age 11 or 12 years, fractional anisotropy (FA) increases in association and projection fibers through age 20 years (Lebel et al., 2008), driven primarily by changes in axial diffusivity (Qiu et al., 2008; Snook et al., 2005).
Cortical thickness, subcortical volumes and functional connectivity (fcMRI) also vary with age (Lenroot et al., 2007; Paus, 2007; Power et al., 2012), with significant differences in GM organization (Khundrakpam et al., 2013) and cerebral blood flow (CBF; Satterthwaite et al., 2014a) arising between middle childhood and adolescence. Finally, research suggests reductions in the amplitude of delta and theta band oscillations, more prominent alpha and beta band oscillations, and increased short distance beta band coherence during adolescence (Thatcher et al., 2008; Uhlhaas and Singer, 2011). Developmental trajectories also differ between sexes, with a notable divergence at the onset of puberty (females=10–14 y.o.; males=12–16 y.o.) in subcortical brain development (Goddings et al., 2014; Satterthwaite et al., 2014b), cortical GM (Mutlu et al., 2013), WM (Giedd et al., 2012) and fcMRI (Satterthwaite et al., 2015). Testosterone levels further predict differential effects in males (Herve et al., 2009). Thus, large samples, relatively homogenous age groups, and appropriate control groups are necessary to overcome individual variability when trying to disambiguate pmTBI from typical brain development, especially when one considers that developmental trajectories may be fundamentally affected by injury.
It has been suggested that younger pmTBI patients are less likely to accurately self-report symptoms and may underestimate the risks involved in continued sports participation, frequently resulting in the under-diagnosis of pmTBI and premature decisions about return to physical activity (Broglio et al., 2007; Gilbert and Johnson, 2011; Van Kampen et al., 2006). Additional distinctions between adult versus childhood injuries include mechanisms of injury (fall/sports-related concussion versus motor vehicle crash), tissue mechanics (skull thickness, water content and ongoing myelination), musculoskeletal development (head to body weight ratio, decreased neck strength), incidence of cerebral edema, incidence of auto-dysregulation, and immaturity of excitatory neurotransmitter systems (Giza et al., 2007; Kochanek, 2006). Finally, both animal (Bayly et al., 2006; Huh et al., 2008) and human (Hessen et al., 2007) studies have shown that the developing brain is more susceptible to mild diffuse brain injury, providing a potential neuroimaging biomarker for the field to investigate moving forward.
Age also interacts with recovery in animal models as a result of differences in plasticity (early vulnerability vs. early plasticity) or due to critical periods (Kolb et al., 2011; Kolb and Teskey, 2012). However, empirical evidence on the influence of age-at-injury on pmTBI outcomes in the human literature is currently mixed as a result of heterogeneous sampling strategies that frequently conflate injury severity (i.e., mild through severe TBI) and overlapping neurodevelopmental periods (Ewing-Cobbs et al., 2016; Ryan et al., 2015a; Ryan et al., 2015b). There is some evidence that injury during middle childhood represents a critical period for developing cognitive deficits later in life (Alosco et al., 2017; Anderson et al., 2009; Crowe et al., 2012; Ryan et al., 2015a). Other studies examining age-at-injury effects on brain microstructure report different trajectories of brain damage and recovery in adolescents versus middle childhood (Ewing-Cobbs et al., 2016; Ryan et al., 2015a; Ryan et al., 2015b). For example, mixed-severity TBI patients injured during middle childhood exhibited more WM microstructure damage, yet showed the greatest recovery over time, suggestive of plasticity (Ewing-Cobbs et al., 2016). In contrast, initial changes in WM microstructure post-injury were smaller for adolescent patients relative to controls yet persisted over time (Ewing-Cobbs et al., 2016). These findings have been interpreted to suggest that middle childhood TBI is associated with continued neurodevelopment whereas this process is slowed in adolescents, leading to more detrimental outcomes.
Rationale and Additional Issues for Biomarker Research in pmTBI
There has recently been a proliferation of interest in developing non-invasive biomarkers of injury using advanced imaging (Eierud et al., 2014), electrophysiology (Haneef et al., 2013; Huang et al., 2009) and biofluids (Mondello et al., 2011; Yuh et al., 2013) to provide objective evidence of so-called “invisible wounds”. For any biomarker to be clinically useful in diagnosis, prognosis and treatment, it must be 1) sensitive and specific, 2) provide biologically meaningful information about underlying mechanisms of injury, and 3) provide information/evidence about the course of recovery or non-recovery. As reviewed in the sections below, far fewer biomarker studies exist in children, especially in younger age ranges. The following sections review the most pertinent issues to consider when conducting imaging, electrophysiological and neuromodulation biomarker studies following pmTBI.
Potentially the biggest concern for conducting imaging and electrophysiological studies in pediatric cohorts is the increased risk of head motion (Power et al., 2012; Wehner et al., 2008). Head motion degrades quality on basic MRI structural scans, increasing the challenge of quantifying different lesions for radiological common data elements. The impact of head motion on more advanced imaging is even more deleterious, altering fundamental properties in functional connectivity (i.e., altering short versus long distance connectivity relationships) and diffusion (scalar values) metrics (Ling et al., 2012; Power et al., 2012), and further decreasing already low signal- or contrast-to-noise ratios. Head motion also affects both electro- (EEG) and magnetoencephalography (MEG) estimates in children by “smearing” sources, confounding localization and affecting evoked waveforms (Wehner et al., 2008). Critically, cranial (e.g., scalp lacerations) or musculoskeletal pain associated with trauma also increases the likelihood of excess head motion in pmTBI patients, even relative to their own uninjured state. Total acquisition time is therefore an important factor for younger patients. Recent advances such as simultaneous multi-slice technology has increased clinical feasibility of obtaining more advanced imaging data in children, as well as increased quality, due to reduced motion.
Second, work in both adults (Kamins et al., 2017; Mayer et al., 2011) and children (Mayer et al., 2012) suggests that physiological recovery may lag behind resolution of behavioral and cognitive symptoms, further emphasizing the need for objective biomarkers of injury. Multiple imaging modalities are also necessary for capturing the complex and multifaceted pathologies associated with mTBI in human (Bigler and Maxwell, 2012) and animal studies (Barkhoudarian et al., 2016). Similarly, animal models indicate a dynamic and temporally complex pattern in terms of how individual biomarkers are affected by injury which varies over the course of minutes to days to weeks (Barkhoudarian et al., 2016). Thus, the field of pmTBI must recognize that “recovery” does not represent a unitary concept as currently posited in the majority of research studies and clinical practice, but is likely multidimensional and fluid. Finally, known variability in the type, location, and time-course of various pathologies should influence analytic considerations in biomarker-based research. For example, assuming that each individual injury (e.g., head rotation in a motor vehicle crash, an intentional blow to the left temple, or a fall with occipital strike) results in a homogeneous pattern of “lesions” within the brain is undoubtedly flawed (Mayer et al., 2015b).
The preceding section detailed a complex neurodevelopmental trajectory that, even in normal/uninjured children, adds additional levels of variability for biological assays relative to “more” homogenous adult populations, implicitly highlighting the need for longitudinal designs. Another critical consideration is the choice of appropriate control groups. The utilization of OI patients has been posited to be a superior strategy to control for non-specific symptoms (pain, increased fatigue, disruptions to normal routine, etc.) that may differentiate pmTBI patients from typically developing children. However, research with large adult cohorts has indicated no clear advantage for utilization of OI in terms of injury factors, PCS, psychosocial outcomes or confounding variables (Mathias et al., 2013). Moreover, the impact of non-specific symptomatology is likely greater on functional biomarkers (i.e., studies of active cognition or emotion) relative to structural studies (e.g., diffusion or spectroscopy), suggesting that the optimal control group may also depend on the biomarker being examined. Finally, most samples of SRC have utilized either contact or non-contact athletes to control for other confounding effects such as the effect of cardiovascular fitness on autonomic regulation.
There are also growing concerns that concussions, and potentially even participation in collision sports, may detrimentally affect brain functioning over both short- and long-term periods of time (Gardner and Yaffe, 2015; McKee et al., 2016; Montenigro et al., 2016). Several studies have implicated both “diagnosable” mTBI (i.e., an injury that meets existing diagnostic criteria), as well as exposure to repeated, sub-clinical blows (also known as sub-concussive or non-concussive injury), as contributing to neurobehavioral sequelae and the potential development of neuropathology (Gardner and Yaffe, 2015; McKee et al., 2016; Montenigro et al., 2016; Nordstrom et al., 2014; Smith et al., 2013; Stein et al., 2015; Stewart et al., 2017). A history of previous concussion not only increases the risk of future concussions, but also increases pre-injury symptoms and long-term cognitive and psychiatric dysregulation in athletes (Harmon et al., 2013) and ER samples (Dams-O’Connor et al., 2013).
Pertinent to the current systematic review, recent studies have demonstrated progressive WM changes as a function of participation in a single season of contact sports (Bazarian et al., 2014; Koerte et al., 2012; McAllister et al., 2014) and heading the ball in soccer players (Lipton et al., 2013). Other studies have correlated microstructural changes with independent measures of head impact (Bazarian et al., 2014; Davenport et al., 2014). Exposure history has been variably estimated by examining dose, frequency and duration of concussive, as well as sub-concussive, brain trauma (Erlanger, 2015; Manley et al., 2017). The quantification of sub-concussive blows remains a controversial topic, and currently there are no diagnostic criteria available (Mayer et al., 2017). However, there is a considerable amount of ongoing biomarker work in this area with several recently published reviews (Bailes et al., 2013; Manley et al., 2017; Tarnutzer et al., 2017). Although the majority of sub-concussive studies focus on more chronic effects, there is the potential for overlap of participants in terms of single acute injury (Talavage et al., 2014). Therefore, although sub-concussive terms were included as part of the overall search strategy, studies predominantly focused on the long-term effects of exposure/subconcussive blows were excluded from the current review.
Criteria for Literature Review
The systematic literature search was developed in consultation with an expert medical librarian (E.S.) and was conducted in Ovid/Medline, Scopus, Cochrane Library, and Web of Science databases. The MeSH terms are shown in Appendix, and the review was registered in the international prospective register of systematic reviews (PROSPERO). A sensitive filter developed by the Cochrane Child Health Field for identifying pediatric studies was used to maximize the number of reviewed articles (Boluyt et al., 2008). Articles were included if they met the following criteria: (1) peer-reviewed original research articles (i.e., no case studies or reviews) published or e-published in English between January 1990 and December 31 2017; (2) focused on patients with TBI; (3) included participants only ≤ 19 years of age; (4) pertinent to only pmTBI or concussion; and (5) included at least one advanced biomarker assessment on average within 4 months after injury. Studies with heterogeneous injury severities (i.e., mild to severe traumatic brain injury), mixed age ranges (i.e., adolescents through early adults) or those solely focused on intentional trauma were excluded. The four-month cut-off was chosen based on 1) current clinical conceptualizations of prolonged post-concussive syndrome (Quinn et al., 2017) and 2) to minimize the likelihood of obtaining a biased sample for studies that enrolled patients at later post-injury times.
A graphical representation of the entire review process is provided in Figure 1. The search results across the multiple databases were first collated (N= 2480 articles) and checked for duplicates (N= 1025 articles). The title and abstract of all articles (N= 1455 articles) were next reviewed by two coauthors (either A.M., M.K., E.S., or T.M.) in an independent and blinded fashion using the Rayyan platform (Ouzzani etal., 2016) as the first step for determining eligibility. Disagreements between the reviewers were reconciled by consensus. The review process was then repeated for all screened articles that appeared to meet eligibility criteria (N= 311 articles) by two independent reviewers (either A.M., M.K., or T.M.), using the full text to make more granular decisions regarding inclusion. The selected articles were then reviewed to determine whether the study primarily focused on the effects of sub-concussive blows (22 articles, subsequently excluded), or included the assessment of acute to subacute pmTBI within the specified 4 month window (N= 56 articles). Exclusion criteria for the remaining articles are detailed in Figure 1, whereas Figure 2 depicts the frequency of all 56 articles as a function of year. Data extraction focused on diagnostic inclusion criteria, cohort type, sample size, sample age and primary biomarkers utilized in the study. Cohorts were classified based on predominant sample type, point-of-care and presence absence of lesions, recognizing that these categories are not mutually exclusive (e.g., ER samples typically include SRC and motor vehicle crashes).
Figure 1: Review Process.
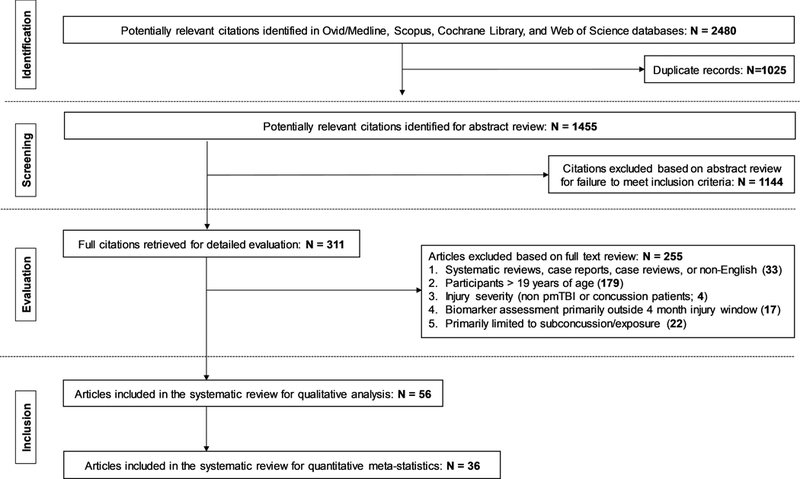
Figure 1 presents the PRISMA flow diagram detailing the identification, screening, evaluation, and inclusion and exclusion of articles at each stage of the current systematic review.
Figure 2: Number of Articles per Year.
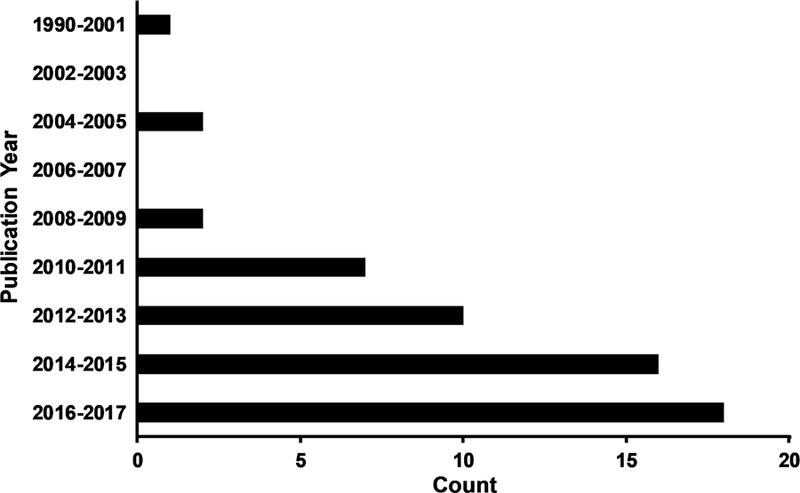
Figure 2 presents the total number of peer-reviewed biomarker studies (N=56) that were published on acute to semi-acute pediatric mild traumatic brain injury during the period under review (1990–2017). With the exception of the first bin (1990–2001), years are grouped into 2 year intervals.
Finally, to avoid erroneous inflation for quantitative meta-statistics, we determined which studies utilized either completely unique (N= 26 articles) or overlapping samples (N= 30 articles). Overlap was determined based on comparison of previous publications from the same group or through direct contact with the corresponding author. All articles presented in the review were verified in this fashion. In the case of multiple published studies with overlapping samples, the single study having a longitudinal design or with the highest pmTBI sample size was considered to be the “primary” article. Quantification for meta-statistics was therefore based on 26 unique and 10 primary articles. The extracted information from articles meeting all inclusion criteria is presented in Table 1. All studies were evaluated according to the Newcastle-Ottawa Quality Assessment Scale to determine bias (Wells et al., 2016) as well as Strength of Recommendation Taxonomy (SORT) criteria for determining levels of evidence (Ebell et al., 2004).
Table 1:
Summary of reviewed biomarker literature during acute and sub-acute stages of pmTBI.
| pmTBI Characteristics | HC Characteristics |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author & Year (Related publications) |
Biomarkers | Design (Referral/ Lesion) |
Dx Criter ia |
N (% BM) |
Age | 1st Visit | Additional Visits |
Type (% BM) |
Age | SOR T |
NO S |
| Yeates 1999 | T1/T2/T2*/PD | PC (ER/±) |
Other | 26 (92%) |
10.85±2. 22 (8-15) |
(0-7) | ~3 months | 8 HC (0%) |
12.38±2.1 3 (8-15) |
3 | 6.5 |
|
Yeates 2009 (Maillard-Wermelinger 2009; Fay 2010; Taylor 2010/2015; Yeates 2012) |
T1/T2/PD/T2*/ FLAIR/DWI |
PC (ER/±) |
Other | 186 (97.8% ) |
11.96±2. 22 (8-15) |
11.35±3.42 (≤21) |
~1 month*; ~3 months*; ~1 year* |
99 OI (0%) |
11.76±2.2 3 (8-15) |
1 | 6.5 |
| Chern 2014 | MRI | RC (ER/±) |
NS | 937 (2.35% ) |
5.53±5.3 9 (0-19.5) |
(Acute)* | 48 | 3 | 3 | ||
|
Max 2015 (Max 2013a/b) |
T1/FLAIR | CE (ER/±) |
Other | 87 (84%) |
10.02±2. 99 (5-14) |
(1-14)* | ~3 months; (6-12 months)*; ~24 months* |
2 | 3 | ||
| Morgan 2015 | MRI | CE (CC/±) |
Other | 52 (37%) |
15 (4-18) |
(>14) | 2 | 3 | |||
| Bonow 2017 | T1/T2/FLAIR/ SWI/DWI |
CE (CC/±) |
Berlin | 3338 (12.8% ) |
14 | 32: [21-45] |
3 | 3 | |||
| Rose 2017 | MRI | CE (CC/±) |
Berlin | 1953 (6.86% ) |
14.1±2.1 (10-19) |
39.4±40.1 (1-201) |
3 | 3 | |||
| Wendling-Keim 2017 | MRI/ Sonography |
RC (ER/±) |
Other | 267 (18.72 %) |
4.1 (0-16) |
(Acute) | (3-8 months)* | 3 | 3 | ||
|
Wu 2010 (Wilde 2008; Chu 2010; Yallampalli 2013) |
dMRI/T1/T2/T2* | CS (ER/±) |
Other | 12 (100%) |
15.3±1.2 (14-17) |
2.92 (1-6) |
11 HC (100%) |
15.8±1.8 (14-19) |
3 | 9 | |
|
Mayer 2012 (Mayer 2015b; Yang 2012) |
dMRI/T1/ SWI/T2 |
PC (ER/-) |
ACR M |
15 (100%) |
13.47±2. 2 (10-17) |
15.87±4.93 (7-21) |
127.82±14.60 (3-5 months) |
15 HC (100%) |
13.4±1.84 (11-17) |
3 | 8 |
|
Virji-Babul 2013 (Borich 2013/2015) |
dMRI/T1 | CS (CA/NS) |
NS | 12 (100%) |
15.5±1.2 (14-17) |
35.6±15.0 (17-61) |
10 UCA (100%) |
15.7±0.9 (14-17) |
3 | 6.5 | |
|
Van Beek 2015c (Van Beek 2015a/b) |
dMRI | PC (ER/NS) |
ACR M |
20 (80%) |
10.8±1.6 (7.0- 13.1) |
12.85±7.52 (2-26)* |
20.25±7.35 (6- 30); 201.23±21.55 (170-248) |
20 HC (80%) |
10.9±1.5 (7.6-12.8) |
2 | 6.5 |
|
Yuan 2015 (Babcock 2015) |
dMRI/T1/T2/S WI |
CS (ER/-) |
ACR M |
23 (100%) |
13.7±1.8 (11.0- 16.7) |
1.89±0.73 (≤4) |
20 OI (100%) |
13.2±1.4 (11.1- 16.6) |
3 | 8 | |
| Wu 2017 | dMRI/T1 | PC (ER/-) |
ACR M |
10 (100%) |
(12-17) | (0.92-4.83) | (84-143) | 12 HC/ 12 OI (100%) |
(12-18) | 2 | 8.5 |
| Yuan 2017 | dMRI/T1 | PC (Mixed/-) |
ACR M |
22 (100%) |
15.45±1. 72 |
55.37±23.9 8 (4-16 weeks) |
(9-13 weeks later) | 20 HC (100%) |
16.28±1. 38 |
2 | 8.5 |
| Goodrich- Hunsaker 2017 |
dMRI/T1 | CS (NS/NS) |
NS | 94 (100%) |
12.68±0. 45 (8-17) |
(0-10) | 59 OI (100%) |
12.46±0. 50 |
3 | 4 | |
| Friedman 2017 | MRS/T1/SWI/ dMRI/fMRI/fc MRI |
CS (CC/- ) |
Berlin | 11 (91%) |
15.2±1.2 | 12±8 (2-28)* |
30.4±6.1 (23-44) |
11 OI (91%) |
15.26±1. 2 |
3 | 8.5 |
| Agrawal 2005 | SPECT | PC (ER/-) |
ACR M |
14 (100%) |
(2-18) | (0-3) | ~3 months | 16 Other (100%) |
(2-18) | 2 | 7.5 |
| Maugans 2012 | ASL/dMRI/MR S/ SWI/T1 |
PC (Mixed/-) |
Berlin | 12 (100%) |
13.4±1.2 (11-15) |
1.9±0.8 (0.7-2.9) |
14; 54.9±8.7 (41- 70) |
12 HC (100%) |
13.4±1.2 (11-15) |
2 | 7.5 |
| Auerbach 2015 | Cranial accelerometry |
PC (CA/NS) |
NS | 13 (100%) |
15.4 (13-18) |
(Pre- season) |
2.2 (0-6); (Post-season) |
69 UCA (100%) |
15.2 (13-17) |
3 | 7 |
|
Barlow 2017ǂ (see Seeger 2017) |
ASL/T1 | 3 | 8 | ||||||||
| Povzun 2017 | Sonography | PC (ER/±) |
Other | 256 (100%) |
8.10±5.5 6 (0-18) |
(Acute) | (Acute to sub- acute) |
3 | 3 | ||
| Stephens 2017 | ASL/T1 | PC (CC/NS) |
Other | 15 (80%) |
15.6±1.2 (13-17) |
8.8±3.4 | 43.2±12.5 | 15 NCA (100%) |
15.2±1.7 (13-17) |
2 | 9 |
| Krivitzky 2011 | fMRI/MRI | CS (CC/-) |
Other | 13 (100%) |
13.3±3.1 | 29±22 (8-82) |
13 HC (100%) |
12.2±3.5 | 3 | 9 | |
|
Yang 2012ǂ (see Mayer 2012) |
fMRI/T1/SWI/T 2 |
3 | 8 | ||||||||
| Hammeke 2013 | fMRI/T1 | PC (CA/NS) |
AAN | 12 (100%) |
16.5±0.5 2 |
0.54±0.15 (0.5-2) |
49±6.8 (35-63) |
12 UCA (100%) |
16.5±0.5 2 |
3 | 7.5 |
|
Keightley 2014a (Saluja 2015) |
fMRI/T1 | CS (CC/- ) |
WHO | 15 (100%) |
14.47±2. 29 (10-17) |
41.13±24.5 0 (9-90) |
15 HC (100%) |
14±2.3 (10-17) |
3 | 8.5 | |
| Ho 2017 | fMRI/T1 | CS (Mixed/N S) |
NS | 30 (100%) |
13.8±2.6 (10-17) |
5.9±8.0* | 8.2±8.1 | 3 | 3 | ||
|
Borich 2015ǂ (see Virji-Babul 2013) |
fcMRI/T1 | 3 | 7 | ||||||||
| Newsome 2016 | fcMRI/T1 | CS (CC/NS) |
Berlin | 13 (100%) |
16.0±1.1 (13-19) |
33.1±1.7 (<37) |
13 OI (100%) |
16.4±1.3 (13-19) |
3 | 7 | |
| Dona 2017 | fcMRI/T1 | CS (NS/NS) |
NS | 15 (100%) |
13.4±2.3 | 33.0±43.8 | 56 HC (100%) |
13.7±7.8 | 3 | 1 | |
| Manning 2017 | fcMRI/dMRI/ MRS/T1/T2/FL AIR |
PC (CC/NS) |
NS | 17 (88%) |
13.3±0.6 (11-14) |
(1-3) | ~3 months | 26 UCA (69%) |
13.0±1.0 | 3 | 5 |
| Semenova 2016 | NIR | CS (ER/±) |
NS | 95 (100%) |
9.1±4.6 (0.58-17) |
(0-3) | 3 | 3 | |||
| Korinthenberg 2004 | EEG | PC (NS/±) |
Other | 100 (98%) |
(3-13) | (Acute) | (4-6 weeks) | 2 | 5 | ||
| Oster 2010 | EEG/MRI/ Sonography |
CE (NS/-) |
ACR M |
150 (79%) |
4.3±3.6 (0-16) |
(0-6) | 3 | 3.5 | |||
|
Balkan 2015 (Virji-Babul 2014) |
EEG | CS (CA/NS) |
NS | 21 (100%) |
16.5 | (<3 months) |
33 UCA (100%) |
16.0±0.9 | 3 | 2 | |
|
Van Beek 2015aǂ (see Van Beek 2015c) |
EEG | 3 | 8 | ||||||||
| Broglio 2016 | EEG | PC (CC/NS) |
NS | 24 (100%) |
16.3±2.2 | 6.2±2.4 | 26.2±43.8; 49.2±60.9; 79.5±60.1 |
21 UCA (100%) |
17.1±2.9 | 2 | 8.5 |
| Broglio 2017 | EEG | PC (CA/NS) |
NS | 8 (100%) |
16.6±0.5 | (Pre- season) |
(0-3); (Asymptomatic) ; (Post-season); |
8 UCA (100%) |
16.6±0.5 | 3 | 7.5 |
|
Seeger 2017 (Barlow 2017) |
TMS | CS (ER/NS) |
Other | 62 (92%) |
14.1±2.4 (8-18) |
39.7 | 28 HC (100%) |
14.31±3. 14 (8-18) |
3 | 8 | |
Notes: Research groups who published multiple articles in the same modality but with overlapping samples are noted in parenthesis next to the primary article. In addition, ǂ indicates a research group who published a separate article on a distinct biomarker but utilized a shared sample of patients as the primary article. Summary statistics are only presented for primary articles to avoid inflation of meta-statistics. Age and post-injury visit are given as mean ± standard deviation (SD) or by median: [interquartile range] depending on the reported measure of central tendency. single point value denotes that the authors only reported the mean or median. Ranges of age and post-injury assessment time are also included in parenthesis when reported. Years and days are the default units for age and assessment time, respectively. An * denotes that biomarkers were not collected at that assessment visit.
Abbreviations: Biomarkers: ASL = arterial spin labeling, EEG = electroencephalography, dMRI = diffusion magnetic resonance imaging, DWI = diffusion weighted imaging, fcMRI = functional connectivity magnetic resonance imaging, FLAIR = fluid attenuated inversion recovery, fMRI = functional magnetic resonance imaging, MRI = unspecified structural magnetic resonance imaging sequence, MRS = magnetic resonance spectroscopy, NIR = near infrared, PD = proton density, SPECT = single-photon emission computed tomography, SWI = susceptibility weighted imaging, TMS = transcranial magnetic stimulation. Study design: CE = case series, CS = cross-sectional, PC = prospective cohort, RC = retrospective cohort. Referral source: CA = concussed community athlete, CC = concussion clinic, ER = emergency room, Mixed = heterogeneous source, NS = not specified. Lesion status: - = patients with negative imaging findings only, ± = patients with both positive and negative imaging findings. Diagnostic (Dx) criteria: AAN = American Academy of Neurology, ACRM = American Congress of Rehabilitation Medicine, Berlin = Berlin Consensus Statement, WHO = World Health Organization, Other = criteria defined but not matching a formal system. N = sample size. % BM = percentage of sample for which biomarker data were collected. Control type: HC = typically developing healthy control, NCA = non-contact athlete, OI = orthopedically injured control, UCA = uninjured contact athlete. NOS = Newcastle-Ottawa Quality Assessment Scale (average score from two raters). SORT = Strength of Recommendation Taxonomy.
Literature Review Results
Structural MRI (43 Studies)
Standard clinical neuroimaging methods (e.g., computed tomography scans; T1− and T2− weighted images) represent a long-standing biomarker that has been used for diagnostic and prognostic purposes and to denote the presence of lesions in pmTBI (i.e., complicated pmTBI). These first generation scans are typically negative for the majority (75–90%) of concussed patients (Hughes et al., 2004; Iverson, 2006), a finding that initially helped propagate the view that mTBI does not lead to frank neuronal pathology. Second generation MRI sequences, such as fluid-attenuated inversion recovery (FLAIR) and gradient recalled echo (T2*)/susceptibility weighted imaging (SWI), were developed in mid-1990s and early 2000s, and demonstrated increased sensitivity for detecting lesions across a wide-spectrum of neurologically injured patients (Bigler and Maxwell, 2012; Gardner and Yaffe, 2015; Haacke et al., 2009; McKee et al., 2016; Mittal et al., 2009; Montenigro et al., 2016; Yuh et al., 2013). T2* and SWI are particularly sensitive to the by-products of hemorrhage (e.g., deoxyhemoglobin and hemosiderin) that have been observed both in-vivo and at autopsy following mTBI (Bigler and Maxwell, 2012; Huang et al., 2015). Structural MRIs can be rated according to radiological common data elements (Broglio et al., 2018; Haacke et al., 2010) or can be quantified to measure changes in cortical thickness or volume occurring either as a function of disease progression (i.e., atrophy; Beauchamp et al., 2011) or through typical neurodevelopment (Giedd et al., 1999).
Although structural MRI is rarely prescribed following pmTBI as part of clinical care, several studies (typically retrospective in nature) have evaluated its utility across various clinical settings. Importantly, the types of imaging sequences utilized (e.g., T1 vs. FLAIR) are rarely reported in these studies, and are thus subsequently assigned the generic label of “MRI” in Table 1. One study (Chern et al., 2014) reported that surveillance imaging (including structural MRI) performed as part of clinical care was not associated with additional operative procedures. Morgan and colleagues (Morgan et al., 2015) observed that structural MRI in pmTBI with chronic PCS (>2 weeks post injury) resulted in little additional diagnostic yield (1 new finding on 19 MRIs) and therefore was not cost-effective. Another study (Rose et al., 2017) reported that CT scans were acquired sooner on average than structural MRI following SRC, and that ordering either scan was associated with the presence of prolonged symptoms. Patients were more likely to receive an MRI if they had a prior concussion, were still participating in the activity that caused the concussion or had cephalic or emotional symptoms on the day they visited the clinic (Rose et al., 2017). Bonow and colleagues (Bonow et al., 2017) reported that only 2 out of 427 SRC patients (0.5%) who received a structural MRI (T1-weighted, T2-weighted, FLAIR, SWI, diffusion weighted imaging) as part of clinical care (total sample of 3338 CT-negative patients) exhibited findings potentially related to trauma (petechial microhemorrhage). Finally, a retrospective survey (Wendling-Keim et al., 2017) reported that ambulatory pmTBI patients were less likely to seek follow-up care after discharge and were less likely to have positive imaging findings (including but not limited to MRI and sonography) relative to a hospitalized sample.
Structural MRI is most frequently used qualitatively to denote the presence (i.e., complex pmTBI) or absence of lesions. In the first study of this nature, Yeates and colleagues (Yeates et al., 1999) observed trauma-related lesions on structural scans (T1-weighted,T2-weighted, PD, T2*-weighted) for 1/23 pmTBI patients in an ER-based sample. A series of articles by a multisite consortium (Fay et al., 2010; Maillard-Wermelinger et al., 2009; Taylor et al., 2010; Taylor et al., 2015; Yeates et al., 2009) reported that 32/182 pmTBI exhibited intracranial abnormalities on MRI, and that these abnormalities were associated with loss of consciousness, increased PCS, impaired cognitive performance and worse outcomes in younger children. The multisite team also reported that pmTBI patients were slower to return to preinjury levels than OI controls, and exhibited 4 different longitudinal trajectories in terms of PCS recovery. nother multisite study focused on the development of new psychiatric disorders in ER patients (Max et al., 2013a; Max et al., 2013b; Max et al., 2015) reported lesions in 38/73 (52%) of pmTBI patients (T1–weighted and FLAIR). Several pre-injury factors and the presence of frontal WM lesions were associated with the development of novel psychiatric disorders in 17/54 participants at the 6 month and 2 year follow-up periods.
Evidence of trauma-related pathologies have been observed in some research studies that used multiple structural MRI sequences (T1-weighted, T2-weighted, FLAIR and T2*-weighted: Wilde et al., 2008), but not all multi-sequence studies demonstrate this finding (T1-weighted, T2-weighted and SWI: Babcock et al., 2015; T1-weighted and SWI: Maugans et al., 2012; Mayer et al., 2012; Yuan et al., 2015). Independent studies by several groups using only a T1-weighted scan (Friedman et al., 2017; Keightley et al., 2014a; Wu et al., 2017) did not observe any lesions in pmTBI patients, which is not surprising given the known high negative rate previously reported in the literature with first generation sequences. Finally, several of the research studies reviewed herein did not specify whether MRI structural scans were read by a radiologist or whether the presence or absence of lesions was determined based on the results from CT or MRI scans.
Lastly, several studies have quantified changes in volume or cortical thickness using structural MRI. In the first quantitative study (T1-weighted, T2-weighted, PD, T2*-weighted), Yeates and colleagues (Yeates et al., 1999) observed no differences in GM or CSF volume at 3 months post-injury in an ER-based cohort of pmTBI patients relative to sibling controls. However, WM volumes significantly varied between pmTBI with increased PCS (smaller volume) relative to patients with lower PCS (larger volume). The New Mexico group (Mayer et al., 2015b) next reported decreased cortical thickness in an overlapping pmTBI cohort at 4 months post-injury based on a quantitative analyses performed with Freesurfer. In contrast, no volumetric differences on quantified MRI structural data were noted by another group between SRC and OI/typically developing control groups over a three month period (Wu et al., 2017).
In summary, evidence from both routine-care and research-based studies suggest that the incidence of lesions on structural MRI scans is relatively low following pmTBI. However, the presence of lesions is more likely to be detected on certain sequences (SWI, T2*-weighted, FLAIR > T1-weighted and T2-weighted) and in certain pmTBI samples (ER > concussed athletes recruited from field). Importantly, even though the presence of positive findings is relatively rare, current evidence suggests that MRI-identified lesions may be associated with increased adverse events in the long- (e.g., increased PCS, new psychiatric diseases) rather than short-term (e.g., new surgical intervention or required hospitalizations).
Diffusion MRI (19 Studies)
Accumulating evidence from both animal (Budde et al., 2011; Mac Donald et al., 2007; Spain et al., 2010) and human (Dodd et al., 2014; Shenton et al., 2012) studies suggests that subtle abnormalities following trauma are better captured by diffusion magnetic resonance imaging (dMRI) relative to the conventional MRI sequences reviewed in the preceding section. It is now well established that the rate (CSF > GM > WM) and anisotropy (WM > GM > CSF) of diffusion differs in tissue types as a function of microstructural properties (Basser and Jones, 2002). Non-Gaussian diffusion is restricted or hindered (i.e., membranes and organelles), with at least two differential rates (i.e., intraaxonal ≈ 0.07 µm2/ms and extraaxonal ≈ 0.85 μm2/ms) based on compartment type (Fieremans et al., 2011; Maier et al., 2004; Mulkern et al., 2000). While any trauma-related change to parenchymal microstructure will alter the rate of diffusion, the most frequently cited pathologies include structural damage (e.g., changes in axonal membranes or myelin), alterations in the net concentration of intraaxonal and extraaxonal water (cytotoxic or vasogenic edema) and inflammatory processes (Bazarian et al., 2007; Mayer et al., 2010; Wilde et al., 2008). Although animal studies (Budde et al., 2011; Zhuo et al., 2012) indicate that diffusion sequences are capable of capturing microstructural changes (reactive gliosis) in GM, certain dMRI scalars (i.e., FA) approach the noise floor in GM and thus may be less reliable (Holleran et al., 2017).
In the first of a series of dMRI articles on pmTBI, the Baylor group (Chu et al., 2010; Wilde et al., 2008; Wu et al., 2010; Yallampalli et al., 2013) reported increased FA/reduced mean diffusivity (MD) in the corpus callosum, fornix and cingulum in the sub-acute stage of pmTBI patients recruited from the ER. dMRI scalars were also associated with severity of PCS and deficits in word recall. The New Mexico group conducted the first prospective dMRI study in pmTBI, reporting increased FA in conjunction with reduced radial diffusivity across several WM tracts (Mayer et al., 2012) as well as within deep and cortical GM (Mayer et al., 2015b) in an ER-based cohort. These abnormal diffusion metrics remained elevated at 4 months post-injury in a subset of patients and were able to classify pmTBI from HC with 90% accuracy.
The British Columbia group (Borich et al., 2013; Virji-Babul et al., 2013) next reported increased FA and decreased MD in anterior WM/motor tracts (ROI and voxel-wise analyses) in a cohort of SRC relative to uninjured contact athletes. These diffusion abnormalities were associated with worse performance on neurocognitive testing. In a series of articles on 20 pmTBI patients, a group from Belgium (van Beek et al., 2015b; van Beek et al., 2015c) observed increased FA in conjunction with mathematical task difficulties in the sub-acute phase relative to HC (van Beek et al., 2015b), findings which largely resolved in the early chronic phase (van Beek et al., 2015c). In contrast, pmTBI patients continued to demonstrate both working memory deficits and developmental abnormalities within the corpus callosum across the 6–8 month follow-up period.
The Cincinnati group (Babcock et al., 2015; Yuan et al., 2015) reported that pmTBI patients assessed within 96 hours of injury exhibited several abnormalities in graph theory metrics (i.e., higher small-worldness, higher normalized clustering coefficients, higher normalized characteristic path length, higher modularity and lower global efficiency), increased FA and reduced MD in diffusion data relative to OI controls, with PCS associated with nodal degree in the superior and middle frontal gyrus. PCS was also associated with decreased radial diffusivity observed in the corpus callosum and medial frontal gyrus. Yuan and colleagues (Yuan et al., 2017) reported that pmTBI with prolonged PCS exhibited a number of abnormal graph metrics (e.g., higher small-worldness, higher normalized clustering coefficient, and lower global efficiency) relative to controls. Following either aerobic or stretching regimens, there was a significant increase in global efficiency and a decrease in normalized characteristic path length in the aerobic training group (Yuan et al., 2017).
Manning (Manning et al., 2017) observed significant group differences across several WM tracts across various diffusion metrics in a cohort of SRC and uninjured athlete controls prospectively studied up to 3 months following injury. Wu and colleagues (Wu et al., 2017) reported that FA and apparent diffusion coefficient (ADC) did not significantly differ between SRC and OI/typically developing control groups in any ROI at 96-hours post injury. At 3 months post-injury, FA was significantly reduced and ADC elevated for SRC relative to healthy controls across several different WM tracts. In a series of within-group analyses, Wu further demonstrated that FA was decreased over visits only for SRC, whereas ADC increased over visits in SRC and decreased in OI.
In contrast to these positive findings, there have also been reports of null findings in the literature. Goodrich-Hunsaker and colleagues (Goodrich-Hunsaker et al., 2018) observed no differences in diffusion metrics between pmTBI and OI across multiple automated methods (TBSS, AFQ, TRACULA). Maugans and colleagues (Maugans et al., 2012) reported no significant differences across several dMRI metrics in a sample of SRC. Similarly, Friedman and colleagues (Friedman et al., 2017) did not observe any differences between SRC and a matched cohort on various diffusion metrics (FA and MD).
In summary, in contrast to widespread opinion (Hulkower et al., 2013), the majority of studies reporting abnormalities have observed increased, rather than decreased, FA during sub-acute pmTBI (Dodd et al., 2014). To date, all diffusion studies in pmTBI have utilized a single b-value and linear modelling (i.e., diffusion tensor imaging). The use of multiple b-values and more advanced modeling to potentially understand such findings as compartmental water fractions (Fieremans et al., 2011; Zhang et al., 2012) is of particular interest for future studies given reports of increased edema in children relative to adults for more severe forms of trauma (Adelson and Kochanek, 1998).
Metabolic Imaging (3 Studies)
Positron emission tomography (PET) and magnetic resonance spectroscopy (MRS) have been used to assess metabolic changes following severe TBI in children (Ashwal et al., 2014; Munson et al., 2006). PET assays a wide variety of underlying pathophysiology (e.g., glucose metabolism, tracers for different neurotransmitters) and has reasonable spatial resolution (Munson et al., 2006). However, exposure to radioactive tracers (radiopharmaceuticals) renders PET a less desirable modality for children and adolescents. In contrast, MRS measures the concentration of various metabolites in the brain, with each metabolite potentially sensitive to different pathophysiology. For example, N-acetylaspartate is a marker for neuronal loss/dysfunction, choline for demyelination or cell membrane synthesis/repair and glutamate/glutamine for excitotoxicity. MRS studies have typically reported decreased N-acetylaspartate, increased choline and glutamate/glutamine, and the existence of lactate and lipids after more severe forms of pediatric TBI (Ashwal et al., 2004; Ashwal et al., 2014).
To date, no studies have used PET imaging in pmTBI. In the first MRS study, Maugans (Maugans et al., 2012) did not observe any group differences (SRC vs. controls) or longitudinal changes (acutely and approximately 2 months post-injury) in concentrations of N-acetylaspartate or N-acetylaspartate/creatine ratio across several ROI. In another multimodal study which also employed dMRI and functional connectivity (fcMRI), Manning (Manning et al., 2017) demonstrated that choline was significantly reduced in a SRC group at 3 months relative to uninjured athlete controls. In the most recent study to date, Friedman and colleagues (Friedman et al., 2017) reported an increased frontal lobe gamma-aminobutyric acid/creatine ratio in SRC patients relative to OI in conjunction with null findings for other metabolites (N-acetylaspartate and glutamate). Moreover, a positive correlation existed between gamma-aminobutyric acid/creatine levels and evoked blood oxygen level dependent (BOLD) data during a working memory task in the frontal cortex for OI, a finding which was absent in SRC.
Static Measures of Hemodynamics (8 Studies)
Similar to axonal pathology measured with dMRI, the structural integrity of the microvasculature is also directly affected by trauma. educed CBF is purportedly the longest lasting sign of injury in animal models (Giza and Hovda, 2014). Animal models indicate a sub-acute reduction in capillary number and diameter both at the injury site and distally (Park et al., 2009), with other research suggesting a similar reduction in cerebral vascular reactivity (CVR; Metting et al., 2009). More severe forms of TBI have been shown to directly affect CBF transit time, as well as cerebral perfusion (Soustiel and Sviri, 2007). Clinical researchers have examined TBI-related changes in CBF through the use of single photon emission computed tomography (SPECT), arterial spin labeling (ASL) and ultrasonography. During ASL, the spin magnetization history of arterial blood water is inverted through the application of a radiofrequency pulse (i.e., a “tagged” proton), which is then contrasted with “untagged” protons to quantify CBF (Alsop et al., 2015). In contrast, CVR is typically either measured directly through carbon dioxide gas challenges (Lu et al., 2014) or via more endogenous techniques such as the breath-hold, both of which are based on BOLD contrast.
In the first study to examine CBF deficits following pmTBI, Agrawal and colleagues (Agrawal et al., 2005) reported that 14/30 pmTBI patients exhibited hypoperfusion in the medial temporal lobe when scanned within 72 hours of injury using SPECT imaging. These perfusion deficits persisted for 3 months in 13/14 patients, and no additional children developed perfusion deficits between acute and follow-up visits. Twelve of the 14 patients with perfusion deficits exhibited prolonged PCS at 3 months relative to 2/16 patients without perfusion deficits (Agrawal et al., 2005). Maugans and colleagues (Maugans et al., 2012) reported decreased CBF in SRC relative to uninjured controls, with perfusion deficits persisting through the final study visit (approximately 2 months post-injury). However, the CBF abnormalities were not associated with scores on neurocognitive testing. Auerbach (Auerbach et al., 2015) assessed CBF pulsatility using a novel cranial accelerometry approach. They reported higher harmonics of cardiac-induced pulsations of the skull following concussion, which was 77% sensitive and 87% specific at a diagnostic level. The time course of CBF abnormalities began hours to days after concussion and appeared to outlast the duration of clinical symptomatology.
In an ER cohort with overlapping patients (see Neuromodulation section), the Calgary group reported higher global CBF in symptomatic pmTBI and lower global CBF in asymptomatic pmTBI relative to HC (Barlow et al., 2017). Povzun (Povzun et al., 2017) found a 7.2% incidence of structural intracranial changes in an ER-based sample, and clinical ultrasonography was both sensitive (90%) and specific (97%) for identifying these intracranial changes. Finally, Stephens and colleagues (Stephens et al., 2018) used ASL to assess relative CBF in SRC at 2 weeks and 6 weeks post-injury compared to uninjured control athletes (scanned only once). Results indicated increased CBF in the left insula and dorsal anterior cingulate cortex of SRC patients at 2 weeks post-injury, which remained elevated in the left dorsal anteriorcingulate cortex at 6 weeks post-injury. CBF was also increased in SRC patients with higher physical symptoms relative to those with a lower symptom burden.
In summary, within this limited body of research there is yet little consensus, with increased CBF, decreased CBF and varying levels of CBF that depend upon PCS reported across only a small number of pmTBI studies to date. There have been no published studies on CVR in this cohort.
Functional MRI (fMRI)/Near Infrared (NIR; 12 Studies)
Neurovascular coupling is frequently disrupted following trauma (Jang et al., 2017) as part of a complex process that can directly involve neurons, astrocytes, the vasculature or any combination of the above. Following excitatory neurotransmission, excess glutamate must be rapidly removed from the synaptic cleft by astrocytes and converted to glutamine (Attwell et al., 2010; Logothetis, 2008). Oxidative metabolism and CBF become decoupled as a result of vasodilation, ultimately culminating in an excess of oxygenated blood and an associated decrease in the ratio of deoxyhemoglobin relative to oxyhemoglobin. Differences in the magnetic or refractive properties of these two forms of hemoglobin form the primary physical basis of fMRI or NIR signals, respectively. Thus, these techniques measure disruptions in neurovascular coupling after TBI, but are unable to distinguish whether the underlying cause is neuronal or hemodynamic in nature (Mayer et al., 2015a). Importantly, in contrast to previously discussed imaging modalities, both techniques also provide the opportunity to examine the brain “in action” during challenging tasks, when patients frequently complain of increased symptom burden.
In the first fMRI study to examine the impact of pmTBI on neurovascular coupling, Krivitzky and colleagues reported no differences in brain activation between pmTBI patients (ER-based sample) and HC on a working memory task. However, behavioral deficits and hyperactivation in the posterior cerebellum were observed during an inhibitory control task for pmTBI patients, with imaging findings in the cerebellum negatively correlating with PCS (Krivitzky et al., 2011). The New Mexico group (Yang et al., 2012) similarly reported inhibitory deficits during an auditory spatial orienting task as well as hypoactivation within deep GM structures (primarily cingulate gyrus, thalamus and cerebellum) in ER patients during the sub-acute injury phase (see dMRI and quantitative structural imaging findings presented in Mayer et al., 2012; Mayer et al., 2015b). In the first prospective study, Hammeke and colleagues (Hammeke et al., 2013) compared activity during a Sternberg working memory task at approximately 13 hours and 7 weeks post-injury in a SRC sample relative to uninjured contact athlete controls. SRC patients demonstrated worse task performance and hypoactivation in a right lateralized network consisting of prefrontal, parietal and occipital regions. Conversely, activation was greater for SRC relative to controls in these same regions at 13 weeks post-injury with no differences in task performance.
The Montreal group reported hypoactivation (HC > pmTBI) within several frontal and parietal regions during a working memory task in a sample of concussion clinic patients, with dorsolateral prefrontal cortex activity positively associated with task performance (Keightley et al., 2014a). In an overlapping sample, Saluja and colleagues (Saluja et al., 2015) observed both hyper- and hypoactivation across a variety of regions in patients during a navigational memory task, some of which correlated with PCS. Finally, Ho and colleagues (Ho et al., 2017) observed that engaging in inhibitory control processes resulted in fewer areas of brain activity in comparison to simple, automated tasks in adolescent pmTBI patients within a week of injury (no control group studied). Sub-group analyses indicated that patients with elevated levels of depressive symptoms engaged more frontal lobe regions during the task than did patients with typical depressive symptomatology.
BOLD contrast can also be used to indirectly measure intrinsic neural activity that occurs synchronously over spatially distributed networks, which accounts for approximately 60–80% of the brain’s overall metabolic load (Raichle and Mintun, 2006). Critical for pediatric studies, these resting state fcMRI studies also reduce several confounds (inability to perform complex tasks, lack of effort, differences in behavioral performance, effects of pain, effects of fatigue, etc.) that influence evoked BOLD signals (Mayer et al., 2015a). For similar reasons, fcMRI also permits the comparison of results across the entire (e.g., mildest injury to minimally conscious patients) TBI spectrum (Sharp et al., 2014), and can be used to assay the neuronal integrity of multiple sensory, motor and cognitive networks in a relatively short period of time (Mayer et al., 2015c; Smith et al., 2009) without the complicated equipment necessary for evoked fMRI studies.
The first fcMRI study in pmTBI was performed by Borich and colleagues (Borich et al., 2015) using a similar SRC cohort as previously described (Borich et al., 2013; Virji-Babul et al., 2013). The authors reported both increased and decreased fcMRI within the default-mode, executive, right frontal pole and left frontal operculum networks using independent component analyses. Newsome and colleagues (Newsome et al., 2016) found that asymptomatic pmTBI patients recruited from a concussion clinic demonstrated increased connectivity relative to OI between the posterior cingulate cortex and the ventral lateral prefrontal cortex, as well as between the right lateral parietal cortex and lateral temporal cortex. These findings were not associated with differences in verbal learning and memory at 30 days post injury.
Using a cohort of SRC patients and uninjured athlete controls, Manning and colleagues (Manning et al., 2017) reported that pmTBI had significant increases in resting state connectivity at 3 months post-injury within visual and cerebellar resting state networks (see DTI and MRS findings in same cohort). Dona and colleagues (Dona et al., 2017) calculated fractal dimension as a measure of complexity in rsfMRI data, reporting that it was reduced throughout GM in pmTBI patients relative to HC. Somewhat paradoxically, higher fractal dimension (i.e., greater time series complexity) in GM was positively associated with post-concussive symptoms. However, the results from this study should be interpreted with caution, as the authors utilized a subject-specific analyses method (i.e., uncorrected z-score transformations) that is known to be associated with bias in imaging data (Dodd et al., 2018; Mayer et al., 2014). In contrast to these positive findings, Friedman (Friedman et al., 2017) did not observe any fcMRI differences in a cohort of SRC that also involved several other imaging modalities.
In summary, the majority of evoked BOLD studies report hypoactivation following a variety of attention, inhibition or working memory tasks, with deficits in neurovascular coupling correlating with the degree of PCS. clear pattern of fcMRI deficits has not been observed in this population. To date, no studies have examined alternative connectivity metrics in pmTBI including dynamic connectivity, regional homogeneity or global connectivity. To our knowledge, there was only a single study that utilized NIR technology at the time of this systematic review, with no studies examining the functional NIR signal in the acute or sub-acute phases of pmTBI. Specifically, Semenova and colleagues (Semenova et al., 2016) examined the effectiveness of a NIR scanner for detecting intracranial hematomas relative to CT scans following pmTBI in an ER-based cohort. Results from the two modalities coincided in 39 cases, with both identifying intracranial hematomas in eight patients. NIR resulted in additional false-positives in three patients.
Electroencephalography (EEG) and Magnetoencephalography (MEG; 7 Studies)
EEG was the first clinical neurodiagnostic assessment to reveal compromised brain function following TBI (Glaser and Sjaardema, 1940; Jasper et al., 1940), and has continued to be a useful clinical tool for such routine procedures as the evaluation of post-traumatic epilepsy (for a reveiw see Arciniegas, 2011). Unlike the multiple signal sources that underlie fMRI and NIR, EEG and MEG directly measure neural electrical currents at millisecond-level temporal resolutions during both rest and cognitively active states (Jackson and Bolger, 2014; Lewine and Orrison, 1995). Using these techniques, investigators are able to quantify the dynamics/coherence of neuronal function, measuring the amplitude of event-related potentials/fields, or the absolute or relative amplitude/power within different frequency bands, localized to either an electrode/channel or brain area (magnetic source imaging; co-registration with structural MRI). Slow wave abnormalities in delta and theta bands and changes in long-range connectivity are thought to be generated by injured neuronal tissue in adult mTBI (Dunkley et al., 2015; Huang et al., 2009) and coherence/disconnectivity between cortical regions have been reported across the TBI spectrum (Amyot et al., 2015; Dunkley et al., 2015).
The first published EEG study on pmTBI (Korinthenberg et al., 2004) reported that 64/98 patients (ER based cohort) exhibited abnormal findings within 24 hours of injury, and that acute EEG abnormalities correlated with somatic PCS. Although 23 patients remained symptomatic at 4–6 weeks post-injury, EEG was qualitatively deemed to be normal in 73 cases. Moreover, there was no correlation between PCS on the second visit and either the acute or follow-up EEG. Oster and colleagues (Oster et al., 2010) observed that EEG ordered within 48 hours of admission as part of clinical care (N=118) was normal for the majority of pmTBI in an ER-based setting. Of the 11 children (9.3%) with pathologic EEG, none of them exhibited abnormal imaging (CT or MRI), and the presence of abnormal EEG was not associated with either persistent symptoms or adverse clinical outcomes.
In a cross-sectional study utilizing resting-state EEG, the British Columbia group (Balkan et al., 2015; Virji-Babul et al., 2014) reported increased beta, reduced theta, and reduced delta power across several frontal sources for SRC patients relative to uninjured control athletes, as well as abnormal connectivity metrics. In a study with overlapping samples, the Belgium group (van Beek et al., 2015a) utilized EEG to demonstrate that pmTBI patients exhibited lower amplitude in a late positivity component (posited to reflect attentional failure) during cognitive tasks, but were similar across more basic early sensory components. Finally, Broglio and colleagues (Broglio et al., 2016) prospectively (symptomatic, self-report asymptomatic, return to play, and one-month post asymptomatic) examined an SRC sample and matched uninjured athlete controls for changes in Brain Network Activation, an EEG measure of interconnectedness, during auditory oddball and go/no go tasks. Although several significant differences were observed on clinical measures, EEG findings were unable to differentiate SRC and control groups. In another study with the same EEG metric, Broglio (Broglio et al., 2017) reported that both SRC and control group’s data changed in a similar fashion across pre- and post-injury visits. Moreover, EEG interconnectedness failed to improve diagnostics beyond traditional clinical measures. The authors therefore concluded that EEG metrics of interconnectedness did not have any clinical utility for pmTBI (Broglio et al., 2017).
To our knowledge, MEG studies have not been performed in this population.
Neuromodulation (1 Study)
Unlike previously reviewed biomarkers which only measure brain functioning, transcranial magnetic stimulation (TMS) has the potential to both measure and alter neuronal activity. TMS is based on the principle of electromagnetic induction, delivering a magnetic pulse to a targeted brain region (e.g., primary motor cortex) leading to induction of secondary ionic current that produces neuronal depolarization (Kobayashi and Pascual-Leone, 2003). Depending on the types of pulses delivered, TMS can assess cortical excitation (e.g., motor threshold, central motor conduction time) or inhibition (e.g., cortical silent period, intracortical inhibition) mediated by a variety of underlying receptors (Lefebvre et al., 2015; Major et al., 2015). Both excitation and inhibition can be altered following the initial TBI as well as the secondary neurometabolic/chemical cascade (Barkhoudarian et al., 2016). Studies reviewing TMS findings after mTBI in adults most often report changes in intracortical inhibition (Lefebvre et al., 2015; Major et al., 2015), with additional findings of increased stimulation threshold and reduced conduction time.
TMS has only recently been used to examine cortical excitation and inhibition following pmTBI. Seeger and colleagues (Seeger et al., 2017) examined multiple TMS parameters at approximately 1 month post-injury in symptomatic pmTBI, asymptomatic pmTBI and HC. Results indicated that the TMS procedures were safe and well-tolerated. Although the cortical silent period was similar across groups (primary analyses), significant differences were observed between HC and symptomatic pmTBI patients for long-interval intracortical inhibition, suggestive of inhibitory deficits.
Meta-Statistics Summary
The meta-statistics for the 36 articles (26 unique + 10 primary) are presented in Figures 3–7. Our review suggests that a total of 1987 pmTBI patients were assessed with biomarkers during the acute and sub-acute injury phase either as part of clinical care (16.7%) or during research protocols (83.3%) over the past 28 years. To place this number in context, it represents approximately 0.26% of the 750,000 new cases of pmTBI that occur each year alone (Zemek et al., 2016a) and 0.009% of the cases that have occurred over the 28 year period spanning the review. The majority of the study designs (Figure 3A) were either cross-sectional (36.1%) or prospective cohort (44.4%) in nature, with case series (13.9%) and retrospective cohort (5.6%) studies typically conducted on convenience samples derived from clinical care. Among all of the studies, 33.3% used mixed samples consisting of patients with and without evidence of lesions, whereas 38.9% did not specify lesion status. The majority of studies were derived from ER (38.9%) and concussion clinic (27.8%) cohorts (Figure 3B). Somewhat surprisingly, a substantial portion of studies used mixed samples or did not explicitly state how their samples were derived (19.4%). Similarly, 61.1% of studies did not specifically identify which formal diagnostic criteria (e.g., ACRM, AAN, Berlin) were used for establishing patient inclusion into the study (Figure 3C). The problem of multiple diagnostic criteria from different organizations is potentially the largest barrier facing the field of mTBI (Mayer et al., 2017), and appears to be compounded by the myriad of different criteria applied in each individual study.
Figure 3: Pie Charts of Primary Study Parameters.
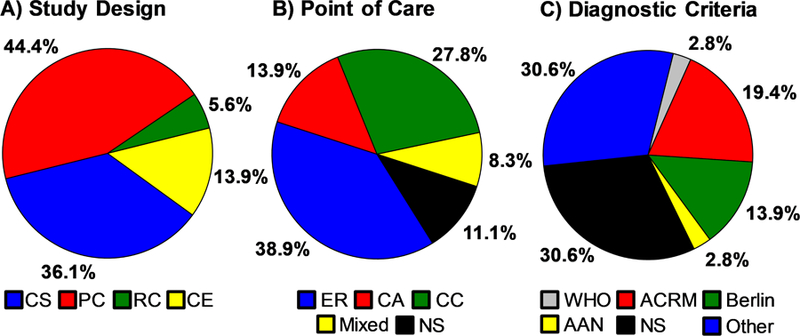
Panel A displays the percent age of the studies that utilized each study design (CS cross-sectional; PC = prospective cohort; RC = retrospective cohort; CE = case series). Panel B indicates the source for patient recruitment/point-of-care(ER=Emergency Room; CA = community athletes with concussion; CC = concussion clinic; Mixed = multiple recruitment sources; NS = Not Specified). Note that R, CC and Mixed samples likely contain athletes with concussions as well. Finally, Panel C presents the different diagnostic criteria (WHO = World Health Organization; ACRM = American ongress of Rehabilitation Medicine; Berlin = Berlin Consensus Statement; AAN = American Academy of Neurology; Other = criteria defined but not matching a formal system; NS = Not Specified) used in these same studies. The denominator for percentages is represented by unique plus primary studies (N=36).
Figure 7: Utilization of Individual Biomarkers.
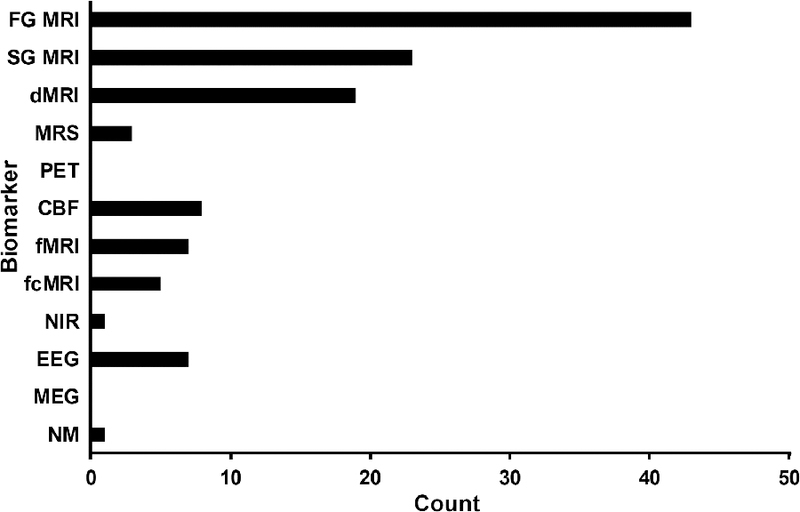
Figure 7 depicts the number of studies that utilized biomarkers as part of clinical care or through experimental research. Biomarkers were stratified into first generation (FG) MRI structural sequences (T-weighted,-weighted,proton density), second generation (SG) MRI structural sequences (fluid attention inversion recovery, susceptibility weighted imaging, 2*-weighted/gradient-recalled echo), diffusion MRI (dMRI), magnetic resonance spectroscopy (MRS), positron emission tomography (PET), hemodynamics (CBF; arterial spin labeling, single photon emission computerized tomography, sonography, and cranial accelerometry), task-based functional MRI (fMRI), functional connectivity MRI (fcMRI), near infrared (NIR), electroencephalography (EEG), magnetoencephalography (MEG), and neuromodulation (NM). The utilization of each modality was summed within and across all 56 articles. Therefore, the total sum is greater than the total number of articles (e.g., an article that utilized fMRI, MRS and dMRI was assigned a value of 1 for each biomarker category).
Due to the preponderance of cross-sectional and convenience samples, the SORT level of evidence was deemed to be relatively weak (i.e., score of 3) in the majority (72.5%) of studies. The evidence of bias (Newcastle Ottawa Scale) across studies was more variably distributed (see Table 1), with increased risk for bias associated with studies that were conducted during clinical care. Not surprisingly, biological sex was disproportionally represented by males (60.6% of pmTBI patients), a rate that is similar to the overall incidence rate reported in the literature (Meehan, III and Mannix, 2010). However, more studies are clearly required to identify any potentially moderating effects of biological sex on injury. Large gaps in the literature also exist for younger patients, with the majority of pmTBI studies focused on late childhood through adolescence (Figure 4). Among ER studies, 38.5% used typically developing youth as a control sample whereas 15.4% utilized OI. Studies on SRC more typically used uninjured athletes (57.1%), rather than typically developing youth (7.1%), as controls. Figure 5 demonstrates that the majority of studies were conducted in the first days to week post-injury, with others studies looking at a larger range of days post-injury falling across the entire acute to sub-acute phase. Importantly, as evidenced by the preponderance of off-diagonal elements in Figure 6, the majority of studies did not use similarly powered control groups, a critical factor for understanding the impact of injury on neurodevelopment.
Figure 4: Patient Age Across Studies.
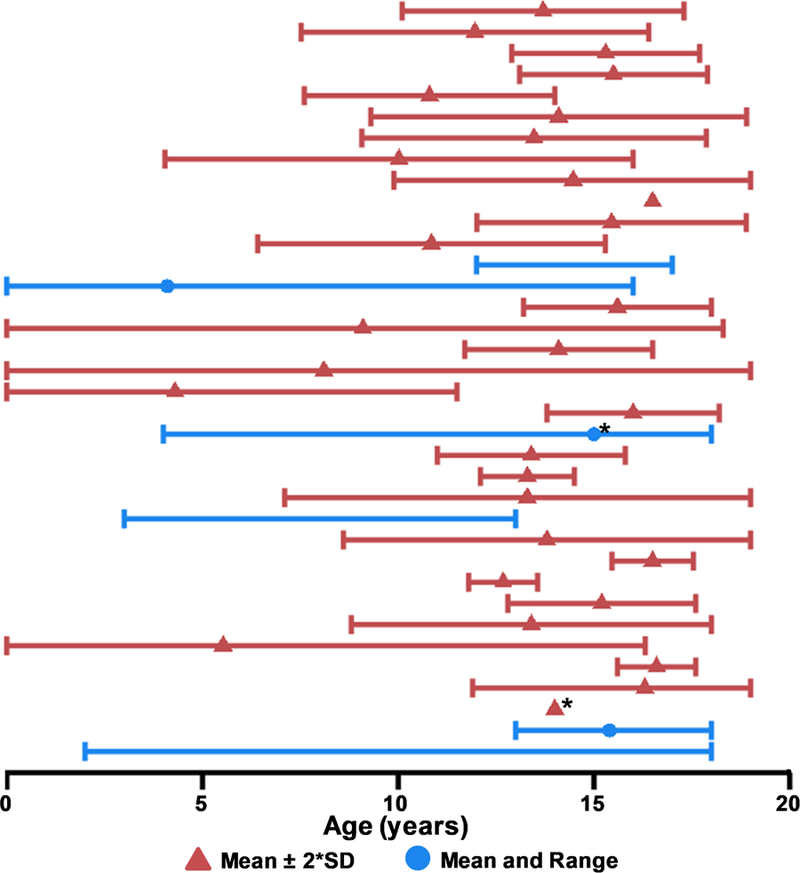
The mean age (triangle) and either two times the standard deviation (SD; red) or age range (minimum to maximum; blue) of included patients with pediatric mild traumatic brain injury (pmTBI) are presented in Figure 4. Asterisks (*) denote studies reporting the median rather than mean as a measure of central tendency. Studies which did not report an explicit age statistic are not graphed, and those that did not list SD or range are depicted by a single point. All SDs were artificially capped at 0 and 19 for data display purposes as studies exceeding these bounds were not considered for review. Only data from unique or primary studies (N=36) are presented in Figure 4.
Figure 5: Day Post-Injury at First Biomarker Assessment.
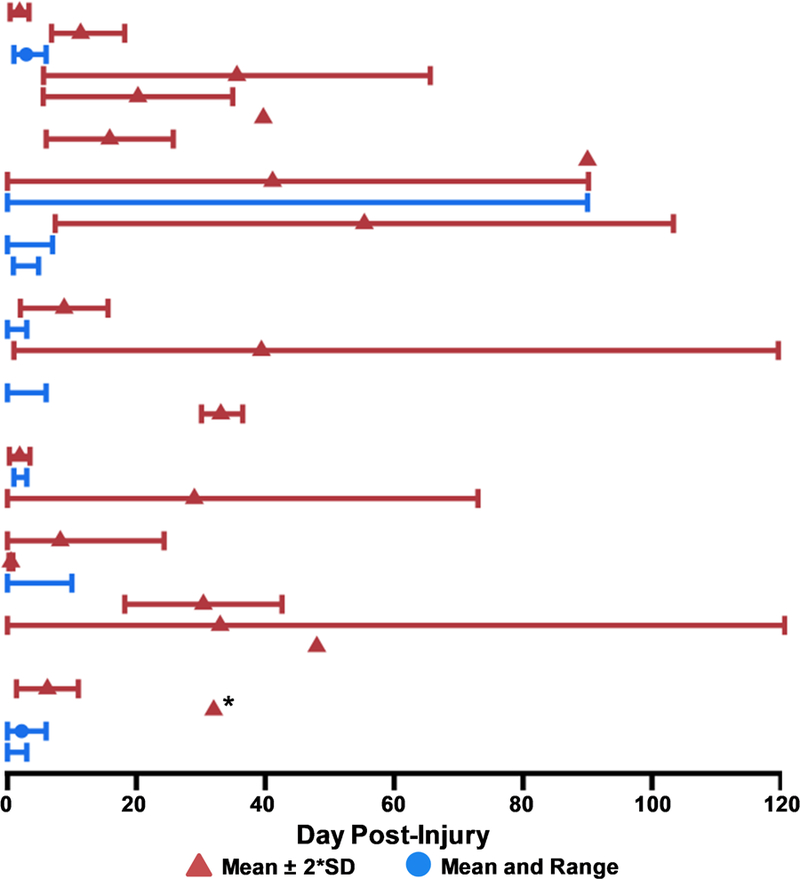
The mean day post-injury (triangle) and either two times the standard deviation (SD; red) or day post-injury range (minimum to maximum; blue) of patients with pediatric mild traumatic brain injury (pmTBI) is presented in Figure 5. Asterisks (*) denote studies reporting only a median rather than mean as a measure of central tendency. Studies that did not report explicit day post-injury statistics are not graphed, and those that did not list SD or range are depicted by a single point. All SDs were artificially capped at 0 and 120 days for data display purposes. Only data from unique or primary studies (N=36) are presented in Figure 5.
Figure 6: Sample Sizes.
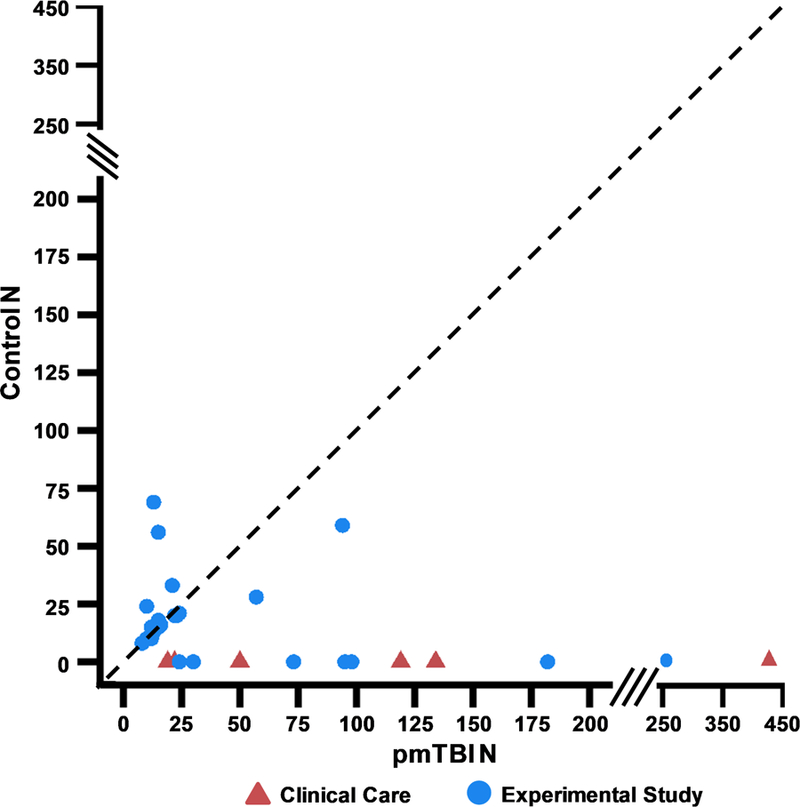
Figure 6 plots the number (N) of patients with pediatric mild traumatic brain injury (pmTBI; X-axis) relative to the number of controls (Y-axis) utilized in the unique and primary studies (N=36). Only the number of participants who were characterized with biomarker data are plotted for studies that obtained clinical data on larger cohorts. To represent the data with higher fidelity, the X and Y axes are both split (see solid slanted lines) between N=0–200 (interval=25) and N=250–450 (interval=100) range. Red triangles indicate that the study was conducted as part of clinical care whereas blue circles indicate that the study design was experimental in nature. The dashed line represents an ideal study design in which equal numbers of pmTBI and controls are included. The majority of studies exhibit a rightward deviation from the dashed line, which was much more pronounced for clinical care studies and suggests increased risk of bias.
Assessment with standard structural scans (variable use of T1, T2, T2*, FLAIR, PD and SWI sequences) represents the most common biomarker utilized following pmTBI (Figure 7), several of which were conducted as part of clinical care. dMRI studies represent the most commonly used advanced imaging modality (33.9% of total 56 studies), potentially a result of the field’s focus on WM injury in both pre-clinical and clinical injury models (Bigler and Maxwell, 2012). Unlike these structural biomarkers, fMRI, EEG, MEG and functional NIR offer great promise for directly correlating the neurobehavioral sequelae (e.g., poor attention) of acute/sub-acute pmTBI with perturbed physiology (Mayer et al., 2015a; McDonald et al., 2012). fMRI (7 evoked and 5 fcMRI studies) is relatively unique in the ability to probe both superficial cortical and deep gray matter structures at similar signal-to-noise ratios, which represents a powerful advantage since both modeling and animal studies suggest that shear stresses are more likely to accumulate in these regions (Zhang et al., 2004).
However, the BOLD signal (fMRI/NIR) is temporally sluggish and represents a complex measure of neurovascular coupling. Thus, separate indices of CBF and CVR are necessary to disambiguate hemodynamic/perfusion (i.e., vascular injury) from true neuronal abnormalities following injury. To date, 8 studies have examined CBF in pmTBI with mixed results, and no studies have examined CVR in sub-acute pmTBI or employed multiple hemodynamic measures in the same cohort of patients. In contrast to hemodynamic measures, EEG and MEG offer exquisite temporal resolution and the ability to directly measure neuronal dysfunction related to trauma. Although EEG has been deployed in both clinical care and experimental research settings, MEG has been underutilized in the study of pmTBI. Similarly, neuromodulation (e.g., TMS) offers the unique confluence of diagnosis, prognosis, and treatment, but has only been used in 1 sub-acute pmTBI study. Ultimately, studies that combine information across various neuroimaging modalities are necessary to capture the multi-faceted pathology that characterizes mTBI in animal models (Barkhoudarian et al., 2016).
Conclusions
In summary, results from current and previous (Hung et al., 2014; Keightley et al., 2014b) systematic reviews identify relatively few diagnostic or prognostic biomarker studies in pmTBI to date. Importantly, only 1/36 pmTBI studies (see Yeates et al., 2009 and associated studies) received a SORT score of 1, indicating the lack of high quality, clinically impactful research in the field. Results from structural MRI studies highlight the potential importance of point-of-care and cohort-type for advanced biomarkers, with several ER samples (e.g., 52% in Max et al., 2015; 17.6% in Yeates et al., 2009) reporting much higher incidence rates of positive MRI findings relative to athlete only samples (e.g., 0.5% in Bonow et al., 2017). Although there is a general paucity of research for most biomarkers outside of structural and dMRI, specifically identified gaps in the literature include studies in MEG, functional NIR, PET, CVR and neuromodulation across any pediatric age group. Reports of increased FA during the sub-acute stage of pmTBI represents the only relatively consistent finding observed across multiple dMRI studies in pmTBI (Dodd et al., 2014). There are almost no published biomarker studies for infancy (0–3 y.o.) and preschool-aged children (3–6 y.o.), with relatively few studies in middle childhood (6–12 y.o.) as well.
The results of this review reinforce the assertion that there is considerable “heterogeneity” and “chaos” inherently associated with mTBI research (Rosenbaum and Lipton, 2012). However, several simple steps such as 1) specifying and using formal diagnostic criteria for patient inclusion, 2) specifying the point-of-care from which the patient sample was derived, and 3) reducing known sources of bias (e.g., no or limited control groups, convenience sampling, disproportionate numbers of older children/males) would greatly advance the field. Ultimately, well-powered longitudinal studies with appropriate control groups, as well as standardized and clearly-defined inclusion criteria (time post-injury, injury severity and past history) are needed to truly understand the complex pathophysiology which may (i.e., still needs to be determined by biomarkers) exist following pmTBI. Well-designed studies represent an essential first step towards both distinguishing typical from atypical recovery and for determining when it is safe to return children to typical activities.
Highlights.
Only 1987 pmTBI have been examined over 28 years, or 0.26% of the annual pmTBI burden
Only one of the reviewed studies meets criteria for high level of scientific evidence
Very few of the reviewed studies included infants, early or middle childhood
Incidence of structural MRI findings depends on sampling strategy and point-of-care
Acknowledgements:
This research was supported by grants from the National Institutes of Health [https://www.nih.gov; grant numbers NIH 01 R01 NS098494–01A1 and −03S1A1 and 01 P20 GM109089–01] to Andrew Mayer. The NIH had no role in study review, data collection and analysis, decision to publish, or preparation of the manuscript.
Appendix A:
List of search terms used. Pediatric and Brain Injury Search Terms were used for all searches.
Pediatric Search Terms:
exp Infant/ or Infant*.mp. or infancy.mp. or Newborn*.mp. or Baby*.mp. or Babies.mp. or Neonat*.mp. or Preterm*.mp. or Prematur*.mp. or Postmatur*.mp. or exp Child/ or Child*.mp. or Schoolchild*.mp. or School age*.mp. or Preschool*.mp. or Kid.mp. or kids.mp. or Toddler*.mp. or exp Adolescent/ or Adoles*.mp. or Teen*.mp. or Boy*.mp. or Girl*.mp. or exp Minors/ or Minors*.mp. or exp Puberty/ or Pubert*.mp. or Pubescen*.mp. or Prepubescen*.mp. or exp Pediatrics/ or Paediatric*.mp. or Paediatric*.mp. or Peadiatric*.mp. or exp Schools/ or Nursery school*.mp. or Kindergar*.mp. or Primary school*.mp. or Secondary school*.mp. or Elementary school*.mp. or High school*.mp. or Highschool*.mp.
Brain Injury Search Terms:
Exp Brain Concussion/ or mild traumatic brain injury.mp. or mtbi.mp or concuss*.mp. or sub-concuss*.mp. or subconcuss*.mp.
Advanced Imaging Search Terms:
exp Magnetic Resonance Imaging/ or mri.mp. or fluid-attenuated inversion recovery.mp. or FLAIR.mp. or magnetization transfer.mp. or susceptibility weighted imaging.mp. or swi.mp. or cortical thick*.mp. or exp atrophy/ or atroph*.mp. or morphometr*.mp. or volumetr*.mp. or dmri.mp. or exp Diffusion Tensor Imaging/ or Diffusion Tractography.mp. or dti.mp. or diffusion tensor imaging.mp. or exp Magnetic Resonance Spectroscopy/ or Magnetic Resonance Spectroscopy.mp. or Spectroscop*.mp. or Magnetic Resonance.mp. or fmri.mp. or Functional Magnetic Resonance Imaging.mp. or exp hemodynamics/ or hemodynamic*.mp. or connectivit*.mp. or resting state*.mp. or functional near infrared spectroscopy.mp. or fnirs.mp. or exp optical imaging/ or fluorescence Imaging.mp. or autofluorescence Imaging.mp. or exp Electroencephalography/ or eeg.mp. or Electroencephalogra*.mp. or exp magnetoencephalography/ or magnetoencephalo*.mp. or exp Transcranial Magnetic Stimulation/ or Transcranial Magnetic Stimulation*.mp. or TMS.mp. or exp Ultrasonography, Doppler, Transcranial/ or (transcranial and (doppler or sonograph*)).mp. or neurosonolog*.mp. or exp perfusion imaging/ or perfusion*.mp. or cerebrovascular reactivit*.mp. or vascular reactivit*.mp. or CVR.mp. or exp Cerebrovascular Circulation/ or cerebral circulation*.mp. or cerebral blood flow*.mp. or Cerebrovascular circulation*.mp. or CBF.mp. or arterial spin labeling.mp. or asl.mp. or tcd.mp. or tomograph*.mp. or Positron-Emission.mp. or PET.mp. or exp Tomography, Emission-Computed/ or SPECT.mp. or Single Photon Emission.mp. or proton*.mp.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Adelson PD, Kochanek PM, 1998. Head injury in children. J Child Neurol 13, 2–15. [DOI] [PubMed] [Google Scholar]
- Agrawal D, Gowda NK, Bal CS, Pant M, Mahapatra AK, 2005. Is medial temporal injury responsible for pediatric post concussion syndrome? A prospective controlled study with single-photon emission computerized tomography. J. Neurosurg 102, 167–171. [DOI] [PubMed] [Google Scholar]
- Alosco ML, Kasimis AB, Stamm JM, Chua AS, Baugh CM, Daneshvar DH, Robbins CA, Mariani M, Hayden J, Conneely S, Au R, Torres A, McClean MD, McKee AC, Cantu RC, Mez J, Nowinski CJ, Martin BM, Chaisson CE, Tripodis Y, Stern RA, 2017. Age of first exposure to American football and long-term neuropsychiatric and cognitive outcomes. Transl. Psychiatry 7, e1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ,Parkes LM,Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G, 2015. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson. Med 73, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyot F, Arciniegas DB, Brazaitis MP, Curley KC, Diaz-Arrastia R, Gandjbakhche A, Herscovitch P, Hinds SR, Manley GT, Pacifico A, Razumovsky A, Riley J, Salzer W, Shih R, Smirniotopoulos JG, Stocker D, 2015. A Review of the Effectiveness of Neuroimaging Modalities for the Detection of Traumatic Brain Injury. J Neurotrauma 32, 1693–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, 2002. Assessment and development of executive function (EF) during childhood. Child Neuropsychol 8, 71–82. [DOI] [PubMed] [Google Scholar]
- Anderson V, Spencer-Smith M, Leventer R, Coleman L, Anderson P, Williams J, Greenham M, Jacobs R, 2009. Childhood brain insult: can age at insult help us predict outcome? Brain 132, 45–56. [DOI] [PubMed] [Google Scholar]
- Arbogast KB, Curry AE, Pfeiffer MR, Zonfrillo MR, Haarbauer-Krupa J, Breiding MJ, Coronado VG, Master CL, 2016. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr 170, e160294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciniegas DB, 2011. Clinical electrophysiologic assessments and mild traumatic brain injury: state-of-the-science and implications for clinical practice. Int. J Psychophysiol 82, 41–52. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Holshouser B, Tong K, Serna T, Osterdock R, Gross M, Kido D, 2004. Proton MR spectroscopy detected glutamate/glutamine is increased in children with traumatic brain injury. J. Neurotrauma 21, 1539–1552. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Tong KA, Ghosh N, Bartnik-Olson B, Holshouser BA, 2014. Application of advanced neuroimaging modalities in pediatric traumatic brain injury. J Child Neurol 29, 1704–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA, 2010. Glial and neuronal control of brain blood flow. Nature 468, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach PS, Baine JG, Schott ML, Greenhaw A, Acharya MG, Smith WS, 2015. Detection of concussion using cranial accelerometry. Clin. J. Sport Med 25, 126–132. [DOI] [PubMed] [Google Scholar]
- Babcock L, Yuan W, Leach J, Nash T, Wade S, 2015. White matter alterations in youth with acute mild traumatic brain injury. J. Pediatr. Rehabil. Med 8, 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babikian T, Satz P, Zaucha K, Light R, Lewis RS, Asarnow RF, 2011. The UCLA longitudinal study of neurocognitive outcomes following mild pediatric traumatic brain injury. J Int Neuropsychol Soc 17, 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailes JE, Petraglia AL, Omalu BI, Nauman E, Talavage T, 2013. ole of subconcussion in repetitive mild traumatic brain injury. J. Neurosurg 119, 1235–1245. [DOI] [PubMed] [Google Scholar]
- Balkan O, Virji-Babul N, Miyakoshi M, Makeig S, Garudadri H, 2015. Source-domain spectral EEG analysis of sports-related concussion via Measure Projection Analysis. Conf. Proc. IEEE Eng Med. Biol. Soc 2015, 4053–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhoudarian G,Hovda DA,Giza CC, 2016. The Molecular Pathophysiology of Concussive Brain Injury-an Update. Phys. Med. Rehabil. Clin. N. Am 27, 373–393. [DOI] [PubMed] [Google Scholar]
- Barlow KM, Marcil LD, Dewey D, Carlson HL, MacMaster FP, Brooks BL, Lebel RM, 2017. Cerebral Perfusion Changes in Post-Concussion Syndrome: A Prospective Controlled Cohort Study. J. Neurotrauma 34, 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Jones DK, 2002. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed 15, 456–467. [DOI] [PubMed] [Google Scholar]
- Bayly PV, Dikranian KT, Black EE, Young C, Qin YQ, Labruyere J, Olney JW, 2006. Spatiotemporal evolution of apoptotic neurodegeneration following traumatic injury to the developing rat brain. Brain Res 1107, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D, 2007. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma 24, 1447–1459. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Zhu T, Zhong J, Janigro D, Rozen E, Roberts A, Javien H, Merchant-Borna K, Abar B, Blackman EG, 2014. Persistent,long-termcerebralwhitematter changes after sports-related repetitive head impacts. PLoS. ONE 9, e94734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Ditchfield M, Maller JJ, Catroppa C, Godfrey C, Rosenfeld JV, Kean MJ, Anderson VA, 2011. Hippocampus, amygdala and global brain changes 10 years after childhood traumatic brain injury. Int. J. Dev. Neurosci 29, 137–143. [DOI] [PubMed] [Google Scholar]
- Berk L, 2014. Development through the lifespan. Pearson, Boston [Google Scholar]
- Berk LE, Meyers AB, 2016. Infants, children, and adolescents. Pearson, Boston [Google Scholar]
- Bigler ED,Maxwell WL,2012. Neuropathology of mild traumatic brain injury: Relationship to neuroimaging findings. Brain Imaging Behav 6, 108–136. [DOI] [PubMed] [Google Scholar]
- Bijur PE, Haslum M, Golding J, 1990. Cognitive and behavioral sequelae of mild head injury in children. Pediatrics 86, 337–344. [PubMed] [Google Scholar]
- Boluyt N, Tjosvold L, Lefebvre C, Klassen TP, Offringa M, 2008. Usefulness of systematic review search strategies in finding child health systematic reviews in MEDLINE. rch. Pediatr. Adolesc. Med 162, 111–116. [DOI] [PubMed] [Google Scholar]
- Bonow RH, Friedman SD, Perez FA, Ellenbogen RG, Browd SR, Mac Donald CL, Vavilala MS, Rivara FP, 2017. Prevalence of Abnormal Magnetic Resonance Imaging Findings in Children with Persistent Symptoms after Pediatric Sports-Related Concussion. J. Neurotrauma 34, 2706–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borich M, Babul AN, Yuan PH, Boyd L, Virji-Babul N, 2015. Alterations in resting-state brain networks in concussed adolescent athletes. J Neurotrauma 32, 265–271. [DOI] [PubMed] [Google Scholar]
- Borich M, Makan N, Boyd L, Virji-Babul N, 2013. Combining whole-brainvoxel-wise analysis with in vivo tractography of diffusion behavior after sports-related concussion in adolescents: A preliminary report. J. Neurotrauma 30, 1243–1249. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Kontos AP, Levin H, Schneider K, Wilde EA, Cantu RC, Feddermann-Demont N, Fuller G, Gagnon I, Gioia G, Giza CC, Griesbach GS, Leddy JJ, Lipton ML, Mayer A, McAllister T, McCrea M, McKenzie L, Putukian M, Signoretti S, Suskauer SJ, Tamburro R, Turner M, Yeates KO, Zemek R, Ala’i S, Esterlitz J, Gay K, Bellgowan PSF, Joseph K, 2018. The National Institute of NeurologicalDisordersandStrokeand Department of Defense Sport-Related Concussion Common Data Elements Version 1.0 Recommendations. J Neurotrauma [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio SP, Macciocchi SN, Ferrara MS, 2007. Sensitivity of the concussion assessment battery. Neurosurgery 60, 1050–1057. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Rettmann A, Greer J, Brimacombe S, Moore B, Narisetty N, He X, Eckner J, 2016. Investigating a Novel Measure of Brain Networking Following Sports Concussion. Int. J. Sports Med 37, 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio SP, Williams R, Lapointe A, Rettmann A, Moore B, Meehan SK, Eckner JT, 2017. Brain Network Activation Technology Does Not Assist with Concussion Diagnosis and Return to Play in Football Athletes. Front Neurol 8, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Janes L, Gold E, Turtzo LC, Frank JA, 2011. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: Validation in the rat using Fourier analysis of stained tissue sections. Brain 134, 2248–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S, 2005. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci 9, 104–110. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson VA, Morse SA, Haritou F, Rosenfeld JV, 2007. Children’s attentional skills 5 years post-TBI. J. Pediatr. Psychol 32, 354–369. [DOI] [PubMed] [Google Scholar]
- Chamard E, Lichtenstein JD, 2018. A systematic review of neuroimaging findings in children And adolescents with sports-related concussion. Brain Inj 1–16. [DOI] [PubMed] [Google Scholar]
- Chern JJ, Sarda S, Howard BM, Jea A, Tubbs RS, Brahma B, Wrubel DM, Reisner A, Boydston W, 2014. Utility of surveillance imaging after minor blunt head trauma. J. Neurosurg. Pediatr 14, 306–310. [DOI] [PubMed] [Google Scholar]
- Chu Z, Wilde EA, Hunter JV, McCauley SR, Bigler ED, Troyanskaya M, Yallampalli R, hia JM, Levin HS, 2010. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am. J. Neuroradiol 31, 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe LM, Catroppa C, Babl FE, Rosenfeld JV, Anderson V, 2012. Timing of traumatic brain injury in childhood and intellectual outcome. J. Pediatr. Psychol 37, 745–754. [DOI] [PubMed] [Google Scholar]
- Dams-O’Connor K, Spielman L, Singh A, Gordon WA, Lingsma HF, Maas AI, Manley GT, Mukherjee P, Okonkwo DO, Puccio AM, Schnyer DM, Valadka AB, Yue JK, Yuh EL, 2013. The impact of previous traumatic brain injury on health and functioning: a TRACK-TBI study. J Neurotrauma 30, 2014–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport EM, Whitlow CT, Urban JE, Espeland MA, Jung Y, Rosenbaum DA, Gioia GA, Powers AK, Stitzel JD, Maldjian JA, 2014. Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. J. Neurotrauma 31, 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Spiegler BJ, Juranek JJ, Bigler ED, Snead OC, Fletcher JM, 2013. Age, plasticity, and homeostasis in childhood brain disorders. eurosci. Biobehav. Rev 37, 2760–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AB, Epstein K, Ling JM, Mayer AR, 2014. Diffusion tensor imaging findings in semi-acutemildtraumaticbraininjury. J. Neurotrauma 31, 1235–1248. [DOI] [PubMed] [Google Scholar]
- Dodd AB, Ling JM, Bedrick EJ, Meier TB, Mayer AR, 2018. Spatial distribution bias in subject-specific abnormalites analyses. Brain Imaging Behav [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dona O, Noseworthy MD, DeMatteo C, Connolly JF, 2017. Fractal Analysis of Brain Blood Oxygenation Level Dependent (BOLD) Signals from Children with Mild Traumatic Brain Injury (mTBI). PLoS One 12, e0169647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley BT, da CL, Bethune A, Jetly R, Pang EW, Taylor MJ, Doesburg SM, 2015. Low-frequency connectivity is associated with mild traumatic brain injury. Neuroimage. Clin 7, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, Bowman M, 2004. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Am. Board Fam. Pract 17, 59–67. [DOI] [PubMed] [Google Scholar]
- Eierud C, Craddock RC, Fletcher S, Aulakh M, King-Casas B, Kuehl D, LaConte SM, 2014. Neuroimaging after mild traumatic brain injury: Review and meta-analysis. Neuroimage. Clin 4, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger DM, 2015. Exposure to sub-concussive head injury in boxing and other sports. Brain Inj 29, 171–174. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Johnson CP, Juranek J, DeMaster D, Prasad M, Duque G, Kramer L, Cox CS, Swank PR, 2016. Longitudinal diffusion tensor imaging after pediatric traumatic brain injury: Impact of age at injury and time since injury on pathway integrity. Hum. Brain Mapp 37, 3929–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. 2010. Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths Atlanta (GA): Centers for Disease Control and revention, National Center for Injury Prevention and Control. [Google Scholar]
- Fay TB, Yeates KO, Taylor HG, Bangert B, Dietrich A, Nuss KE, Rusin J, Wright M, 2010. Cognitive reserve as a moderator of postconcussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. J. Int. Neuropsychol. Soc 16, 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieremans E, Jensen JH, Helpern JA, 2011. White matter characterization with diffusional kurtosis imaging. Neuroimage 58, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SD, Poliakov AV, Budech C, Shaw DWW, Breiger D, Jinguji T, Krabak B, Coppel D, Lewis TM, Browd S, Ojemann JG, 2017. GABA alterations in pediatric sport concussion. Neurology 89, 2151–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Yaffe K, 2015. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell Neurosci 66,75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL, 1999. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 2, 861–863. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK, 2012. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol. Sex Differ 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert F, Johnson LS, 2011TheImpact of American Tackle Football-Related Concussion in Youth Athletes. AJOB Neuroscience 2, 48–59. [Google Scholar]
- Giza CC, Hovda DA, 2014. The new neurometabolic cascade of concussion. Neurosurgery 75, S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TS, Gioia GA, Gronseth GS, Guskiewicz K, Mandel S, Manley G, McKeag DB, Thurman DJ, Zafonte R, 2013. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 80, 2250–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza CC, Mink RB, Madikians A, 2007. Pediatric traumatic brain injury: not just little adults. Curr. Opin. Crit Care 13, 143–152. [DOI] [PubMed] [Google Scholar]
- Glaser MA, Sjaardema H, 1940. The value of the electroencephalograph in craniocerebral injuries. West J Surg 48, 689–696. [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ, 2014. The influence of puberty on subcortical brain development. Neuroimage 88, 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM, 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci U. S 101, 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Abildskov TJ, Black G, Bigler ED, Cohen DM, Mihalov LK, Bangert BA, Taylor HG, Yeates KO, 2018. Age- and sex-related effects in children with mild traumatic brain injury on diffusion magnetic resonance imaging properties: A comparison of voxelwise and tractography methods. J. Neurosci. Res 96, 626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenette JP, Shenton ME, Koerte IK, 2018. Imaging of Concussion in Young Athletes. Neuroimaging Clin. N. Am 28, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC, 2009. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol 30, 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Duhaime AC, Gean AD, Riedy G, Wintermark M, Mukherjee P, Brody DL, DeGraba T, Duncan TD, Elovic E, Hurley R, Latour L, Smirniotopoulos JG, Smith DH, 2010. Common data elements in radiologic imaging of traumatic brain injury. J. Magn Reson. Imaging 32, 516–543. [DOI] [PubMed] [Google Scholar]
- Hammeke TA, McCrea M, Coats SM, Verber MD, Durgerian S, Flora K, Olsen GS, Leo PD, Gennarelli TA, Rao SM, 2013. Acute and subacute changes in neural activation during the recovery from sport-related concussion. J. Int. Neuropsychol. Soc 19, 863–872. [DOI] [PubMed] [Google Scholar]
- Haneef Z, Levin HS, Frost JD Jr., Mizrahi EM, 2013. Electroencephalography and quantitative electroencephalography in mild traumatic brain injury. J Neurotrauma 30, 653–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon KG, Drezner JA, Gammons M, Guskiewicz K, Halstead M, Herring SA, Kutcher JS,Pana A,Putukian M, Roberts WO, 2013. American Medical Society for Sports Medicine position statement: Concussion in sport. Br J Sports Med 47, 15–26. [DOI] [PubMed] [Google Scholar]
- Herve PY, Leonard G, erron M, Pike B, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T, 2009. Handedness, motor skills and maturation of the corticospinal tract in the adolescent brain. Hum. Brain Mapp 30, 3151–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessen E, Nestvold K, Anderson V, 2007. Neuropsychological function 23 years after mild traumatic brain injury: a comparison of outcome after paediatric and adult head injuries. Brain Inj 21, 963–979. [DOI] [PubMed] [Google Scholar]
- Ho RA, Hall GB, Noseworthy MD, DeMatteo C, 2017. An Emotional Go/No-Go fMRI study in adolescents with depressive symptoms following concussion. Int. J. Psychophysiol [DOI] [PubMed] [Google Scholar]
- Holleran L, Kim JH, Gangolli M, Stein T, Alvarez V, McKee A, Brody DL, 2017. xonal disruption in white matter underlying cortical sulcus tau pathology in chronic traumatic encephalopathy. Acta Neuropathol 133,367–380. [DOI] [PubMed] [Google Scholar]
- Huang M, Theilmann RJ, Robb A, Angeles A, Nichols S, Drake A, Dandrea J, Levy M, Holland M, Song T, Ge S, Hwang E, Yoo K, Cui L, Baker DG, Trauner D, Coimbra R, Lee RR, 2009. Integrated imaging approach with MEG and DTI to Detect Mild Traumatic Brain Injury in Military and Civilian Patients. J. Neurotrauma 26, 1213–1226. [DOI] [PubMed] [Google Scholar]
- Huang YL, Kuo YS, Tseng YC, Chen DY, Chiu WT, Chen CJ, 2015. Susceptibility-weighted MRI in mild traumatic brain injury. Neurology 84, 580–585. [DOI] [PubMed] [Google Scholar]
- Hughes DG, Jackson A, Mason L, Berry E, Hollis S, Yates DW, 2004. Abnormalities on magnetic resonance imaging seen acutely following mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology 46, 550–558. [DOI] [PubMed] [Google Scholar]
- Huh JW, Widing G, Raghupathi R, 2008. Midline brain injury in the immature rat induces sustained cognitive deficits, bihemispheric axonal injury and neurodegeneration. Exp Neurol 213, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML, 2013. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am. J. Neuroradiol 34, 2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung R, Carroll LJ, Cancelliere C, Cote P,Rumney P,Keightley M,Donovan J, Stalnacke BM, Cassidy JD, 2014. Systematic review of the clinical course, natural history, and prognosis for pediatric mild traumatic brain injury: results of the nternational Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil 95, S174–S191. [DOI] [PubMed] [Google Scholar]
- Iverson GL, 2006. Complicated vs uncomplicated mild traumatic brain injury: acute neuropsychological outcome. Brain Inj 20, 1335–1344. [DOI] [PubMed] [Google Scholar]
- Jackson AF, Bolger DJ, 2014. The neurophysiological bases of EEG and EEG measurement: a review for the rest of us. Psychophysiology 51, 1061–1071. [DOI] [PubMed] [Google Scholar]
- Jang H, Huang S, Hammer DX, Wang L, Rafi H, Ye M, Welle CG, Fisher JAN, 2017. Alterations in neurovascular coupling following acute traumatic brain injury. Neurophotonics 4, 045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper HH, Kershman J, Elvidge AR, 1940. Electroencephalographic studies of injury to the head. Archives of Neurology & Psychiatry 44, 328–350. [Google Scholar]
- Kamins J, Bigler E, Covassin T, Henry L, Kemp S, Leddy JJ, Mayer A, McCrea M, Prins M, Schneider KJ, 2017. What is the physiological time to recovery after concussion? A systematic review. Br J Sports Med 51, 935–940. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Singh SR, Chen JK, Gagnon I, Leonard G, Petrides M, Ptito A, 2014a. A functional magnetic resonance imaging study of working memory in youth after sports-related concussion: is it still working? J. Neurotrauma 31, 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley ML, Sinopoli KJ, Davis KD, Mikulis DJ, Wennberg R, Tartaglia MC, Chen JK, Tator CH, 2014b. Is there evidence for neurodegenerative change following traumatic brain injury in children and youth? A scoping review. Front Hum. Neurosci 8, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khundrakpam BS, Reid A, Brauer J, Carbonell F, Lewis J, Ameis S, Karama S, Lee J, Chen Z, Das S, Evans AC, 2013. Developmental changes in organization of structural brain networks. Cereb. Cortex 23, 2072–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A, 2003. Transcranial magnetic stimulation in neurology. Lancet Neurol 2, 145–156. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, 2006. Pediatrictraumatic brain injury: quo vadis? Dev. Neurosci 28, 244–255. [DOI] [PubMed] [Google Scholar]
- Koerte IK, Kaufmann D, Hartl E, Bouix S, Pasternak O, Kubicki M, Rauscher A, Li DK, Dadachanji SB, Taunton JA, Forwell LA, Johnson AM, Echlin PS, Shenton ME, 2012. A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4. Neurosurg. Focus 33, E3–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Mychasiuk R, Williams P, Gibb R, 2011. Brain plasticity and recovery from early cortical injury. Dev. Med Child Neurol 53 Suppl 4, 4–8. [DOI] [PubMed] [Google Scholar]
- Kolb B, Teskey GC, 2012. Age, experience, injury, and the changing brain. Dev. Psychobiol 54, 311–325. [DOI] [PubMed] [Google Scholar]
- Korinthenberg R, Schreck J, Weser J, Lehmkuhl G, 2004. Post-traumatic syndrome after minor head injury cannot be predicted by neurological investigations. Brain Dev 26, 113–117 [DOI] [PubMed] [Google Scholar]
- Krivitzky LS, Roebuck-Spencer TM, Roth RM, Blackstone K, Johnson CP, Gioia G, 2011. Functional magnetic resonance imaging of working memory and response inhibition in children with mild traumatic brain injury. J Int Neuropsychol oc 17, 1143–1152. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C, 2008. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40, 1044–1055. [DOI] [PubMed] [Google Scholar]
- Lefebvre G, Tremblay S, Theoret H, 2015. Probing the effects of mild traumatic brain injury 117. With transcranial magnetic stimulation of the primary motor cortex. Brain Inj 29, 1032–1043. [DOI] [PubMed] [Google Scholar]
- Lehto J, Puujarvi P, Kooistra L, Pulkkinen L (2003). Dimensions of executive funcitoning: Evidence from children. British Journal of Developmental Psychology 21, 59–80. [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN, 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewine JD, Orrison WW, 1995. Magnetoencephalography and magnetic source imaging. Functional brain imaging 369–417. [Google Scholar]
- Lincoln AE, Caswell SV, Almquist JL, Dunn RE, Norris JB, Hinton RY, 2011. Trends in concussion incidence in high school sports: a prospective 11-year study. Am. J. Sports Med 39, 958–963. [DOI] [PubMed] [Google Scholar]
- Ling J, Merideth F, Caprihan A, Pena A, Teshiba T, Mayer AR, 2012. Head injury or head motion? Assessment and quantification of motion artifacts in diffusion tensor imaging studies. Hum. Brain Mapp 33, 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton ML, Kim N, Zimmerman ME, Kim M, Stewart WF, Branch CA, Lipton RB, 2013. Soccer heading isassociated with white matter microstructural and cognitive abnormalities. Radiology 268, 850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, 2008. What we can do and what we cannot do with fMRI. Nature 453, 869–878. [DOI] [PubMed] [Google Scholar]
- Lu H, Liu P, Yezhuvath U, Cheng Y, Marshall O, Ge Y, 2014. MRI mapping of cerebrovascular reactivity via gas inhalation challenges. JVis.Exp 94,e52306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CL, Dikranian K, Song SK, Bayly PV,Holtzman DM, Brody DL, 2007. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp. Neurol 205, 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SE, Vajapeyam S, Mamata H, Westin CF, Jolesz FA, Mulkern RV, 2004. Biexponential diffusion tensor analysis of human brain diffusion data. Magn Reson. Med 51, 321–330. [DOI] [PubMed] [Google Scholar]
- Maillard-Wermelinger A, Yeates KO, Gerry TH, Rusin J, Bangert B, Dietrich A, Nuss K, Wright M, 2009. Mild traumatic brain injury and executive functions in school-aged children. Dev. Neurorehabil 12, 330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major BP, Rogers MA, Pearce AJ, 2015. Using transcranial magnetic stimulation to quantify electrophysiological changes following concussive brain injury: a systematic review. Clin. Exp. Pharmacol. Physiol 42, 394–405. [DOI] [PubMed] [Google Scholar]
- Manley G, Gardner AJ, Schneider KJ, Guskiewicz KM, Bailes J, Cantu RC, Castellani RJ, Turner M, Jordan BD, Randolph C, Dvorak J, Hayden KA, Tator CH, McCrory P, Iverson GL, 2017. A systematic review of potential long-term effects of sport-relatedconcussion. Br.J.Sports Med 51, 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning KY, Schranz A, Bartha R, Dekaban GA, Barreira C, Brown A, Fischer L, Asem K, Doherty TJ, Fraser DD, Holmes J, Menon RS, 2017. Multiparametric MRI changes persist beyond recovery in concussed adolescent hockey players. Neurology 89, 2157–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master CL, Scheiman M, Gallaway M, Goodman A, Robinson RL, Master SR, Grady MF, 2016. Vision Diagnoses Are Common After Concussion in Adolescents. Clin. Pediatr. (Phila) 55, 260–267. [DOI] [PubMed] [Google Scholar]
- Mathias JL, Dennington V, Bowden SC, Bigler ED, 2013. Community versus orthopaedic controls in traumatic brain injury research: how comparable are they? Brain Inj 27, 887–895. [DOI] [PubMed] [Google Scholar]
- Maugans TA, Farley C, Altaye M, Leach J, Cecil KM, 2012. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics 129, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max JE, Friedman K, Wilde EA, Bigler ED, Hanten G, Schachar RJ, Saunders AE, Dennis M, Ewing-Cobbs L, Chapman SB, Yang TT, Levin HS, 2015. Psychiatric disorders in children and adolescents 24 months after mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci 27, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max JE, Pardo D, Hanten G, Schachar RJ, Saunders AE, Ewing-Cobbs L, Chapman SB, Dennis M, Wilde EA, Bigler ED, Thompson WK, Yang TT, Levin HS, 2013a. Psychiatric disorders in children and adolescents six-to-twelve months after mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 25, 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max JE, Schachar RJ, Landis J, Bigler ED, Wilde EA, Saunders AE, Ewing-Cobbs L, Chapman SB, Dennis M, Hanten G, Levin HS, 2013b. Psychiatric disorders in children and adolescents in the first six months after mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci 25, 187–197. [DOI] [PubMed] [Google Scholar]
- Mayer A, Wertz C, Ryman S, Storey E, Park G, Phillips J, Dodd AB, Oglesbee S, Campbell R, Yeo R, Wasserott B, Shaff N, Leddy JJ, Mannix R, Arbogast KB, Meier T, Grady M, Master C, 2018. Neurosensory Deficits Vary as a Function of Point of Care in Pediatric Mild Traumatic Brain Injury. J Neurotrauma [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Bedrick EJ, Ling JM, Toulouse T, Dodd A, 2014. Methods for identifying subject-specific abnormalities in neuroimaging data. Hum. Brain Mapp 35, 5457–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Bellgowan PS, Hanlon FM, 2015a. Functional magnetic resonance imaging of mild traumatic brain injury. Neurosci. Biobehav. Rev 49, 8–18. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Hanlon FM, Ling J, 2015bGraymatterabnormalitiesinpediatricmild traumatic brain injury. J. Neurotrauma 32, 723–730. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Ling J, Allen EA, Klimaj S, Yeo R, Hanlon FM, 2015c. Static and dynamic intrinsic connectivity following mild traumatic brain injury. J. Neurotrauma 32, 1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, Reichard R, Yeo RA, 2010. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 74, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Ling JM, Yang Z, Pena A, Yeo RA, Klimaj S, 2012. Diffusion abnormalities in pediatric mild traumatic brain injury. J. Neurosci 32, 17961–17969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA, 2011. Functional connectivity in mild traumatic brain injury. Hum. Brain Mapp 32, 1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Quinn DK, Master CL, 2017. The Spectrum of Mild Traumatic Brain Injury: A Review. Neurology [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Ford JC, Flashman LA, Maerlender A, Greenwald RM, Beckwith JG, Bolander RP, Tosteson TD, Turco JH, Raman R, Jain S, 2014. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology 82, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Saykin AJ, McAllister TW, 2012. Functional MRI of mild traumatic brain injury (mTBI): progress and perspectives from the first decade of studies. Brain Imaging Behav 6, 193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, Perl DP, Stein TD, Vonsattel JP, Stewart W, Tripodis Y, Crary JF, Bieniek KF, Dams-O’Connor K, Alvarez VE, Gordon WA, 2016. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 131, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay A, Grace R, Horwood J, Fergusson D, MacFarlane M, 2009. Adolescent psychiatric symptoms following preschool childhood mild traumatic brain injury: evidence. From a birth cohort. J Head Trauma Rehabil 24, 221–227. [DOI] [PubMed] [Google Scholar]
- Meehan WP III, Mannix R, 2010. Pediatric concussions in United States emergency departments in the years 2002 to 2006. J Pediatr 157, 889–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Bellgowan PS, Bergamino M, Ling JM, Mayer AR, 2015. Thinner cortex in collegiate football players with, but not without, a self-reported history of concussion. J. Neurotrauma 33, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metting Z, Rodiger LA, Stewart RE, Oudkerk M, De KJ, van der NJ, 2009. Perfusion computed tomography in the acute phase of mild head injury: regional dysfunction and prognostic value. Ann. Neurol 66, 809–816. [DOI] [PubMed] [Google Scholar]
- Mittal S, Wu Z, Neelavalli J, Haacke EM, 2009. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am. J. Neuroradiol 30, 232–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello S, Muller U, Jeromin A, Streeter J, Hayes RL, Wang KK, 2011. Blood-based diagnostics of traumatic brain injuries. Expert. Rev. Mol. Diagn 11, 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenigro PH, Alosco ML, Martin B, Daneshvar DH, Mez J, Chaisson C, Nowinski CJ, Au R, McKee AC, Cantu RC, McClean MD, Stern RA, Tripodis Y, 2016. Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J. Neurotrauma Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CD, Zuckerman SL, King LE, Beaird SE, Sills AK, Solomon GS, 2015. Post-concussion syndrome (PCS) in a youth population: defining the diagnostic value and cost-utility of brain imaging. Childs Nerv. Syst 31, 2305–2309. [DOI] [PubMed] [Google Scholar]
- Mulkern RV,Zengingonul HP,Robertson RL, Bogner P, Zou KH, Gudbjartsson H, Guttmann CR, Holtzman D, Kyriakos W, Jolesz FA, Maier SE, 2000. Multi-component apparent diffusion coefficients in human brain: relationship to spin-lattice relaxation. Magn Reson. Med 44, 292–300. [DOI] [PubMed] [Google Scholar]
- Munson S, Schroth E, Ernst M, 2006. The role of functional neuroimaging in pediatric brain injury. Pediatrics 117, 1372–1381. [DOI] [PubMed] [Google Scholar]
- Mutlu AK, Schneider M, Debbane M, Badoud D, Eliez S, Schaer M, 2013. Sex differences in thickness, and folding developments throughout the cortex. Neuroimage 82, 200–207. [DOI] [PubMed] [Google Scholar]
- Newsome MR, Li X, Lin X, Wilde EA, Ott S, Biekman B, Hunter JV, Dash PK, Taylor BA, Levin HS, 2016. Functional Connectivity Is Altered in Concussed Adolescent Athletes Despite Medical Clearance to Return to Play: A Preliminary Report. Front Neurol 7, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom P, Michaelsson K, Gustafson Y,Nordstrom A,2014Traumaticbraininjuryand young onset dementia: a nationwide cohort study. Ann. Neurol 75, 374–381. [DOI] [PubMed] [Google Scholar]
- Oster I, Shamdeen GM, Gottschling S, Gortner L, Meyer S, 2010. Electroencephalogram in children with minor traumatic brain injury. J. Paediatr. Child Health 46, 373–377. [DOI] [PubMed] [Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A, 2016. Rayyan-a web and mobile app for systematic reviews. Syst. Rev 5, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Bell JD, Siddiq IP, Baker AJ, 2009. An analysis of regional microvascular loss And recovery following two grades of fluid percussion trauma: a role for hypoxia-inducible factors in traumatic brain injury. J Cereb Blood Flow Metab 29, 575–584. [DOI] [PubMed] [Google Scholar]
- Paus T, 2007. Maturation of Structural and Functional Connectivity in the Human Brain, in: Jirsa V, McIntosh AR (Eds.), Handbook of Brain Connectivity. Springer Berlin Heidelberg, pp. 463–475. [Google Scholar]
- Povzun AA, Shchugareva LM, Iova AS, Kruchina MK, Shulgina MA, 2017. Clinical and ultrasonographic evaluation of the neurological status of children with mild brain injury in acute phase. Pediatric Traumatology, Orthopaedics and Reconstructive Surgery 5, 36–42. [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Hales A, Reger M, Giza C., Hovda DA, 2010. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev. Neurosci 32, 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL, 2008. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage 41, 223–232. [DOI] [PubMed] [Google Scholar]
- Quinn DK, Mayer AR, Master CL, Fann J, 2017. Prolonged Postconcussive Symptoms. American Journal of Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME,Mintun MA,2006. Brain work and brain imaging. Annu Rev Neurosci 29, 449–476. [DOI] [PubMed] [Google Scholar]
- Rose SC, Schaffer CE, Young JA, McNally KA, Fischer AN, Heyer GL, 2017. Utilization of conventional neuroimaging following youth concussion. Brain Inj 31, 260–266. [DOI] [PubMed] [Google Scholar]
- Rosenbaum SB, Lipton ML, 2012. Embracing chaos: The scope and importance of clinical and pathological heterogeneity in mTBI. Brain Imaging Behav 6, 255–282. [DOI] [PubMed] [Google Scholar]
- Ryan NP, Catroppa C, Beare R, Coleman L, Ditchfield M, Crossley L, Beauchamp MH, Anderson VA, 2015a. Predictors of longitudinal outcome and recovery of pragmatic language and its relation to externalizing behaviour after pediatric traumatic brain injury. Brain Lang 142, 86–95. [DOI] [PubMed] [Google Scholar]
- Ryan NP, Catroppa C, Cooper JM, Beare R, Ditchfield M, Coleman L, Silk T, Crossley L, Beauchamp MH, Anderson VA, 2015b. The emergence of age-dependent social cognitive deficits after generalized insult to the developing brain: a longitudinal prospective analysis using susceptibility-weighted imaging. Hum. Brain Mapp 36, 1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluja RS, Chen JK, Gagnon IJ, Keightley M, Ptito A, 2015. Navigational memory functional magnetic resonance imaging: a test for concussion in children. J. Neurotrauma 32, 712–722. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliott MA, Vandekar SN, Ruparel K, Calkins ME, Roalf DR, Gennatas ED, Jackson C, Erus G, Prabhakaran K, Davatzikos C, Detre JA, Hakonarson H, Gur RC, Gur RE, 2014a. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc. Natl. Acad. Sci. U. S. A 111, 8643–8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Vandekar S, Wolf DH, Ruparel K, Roalf DR, Jackson C, Elliott MA, Bilker WB, Calkins ME, Prabhakaran K, Davatzikos C, Hakonarson H, Gur RE, Gur RC, 2014b. Sex differences in the effect of puberty on hippocampal morphology. J. Am. Acad. Child Adolesc. Psychiatry 53, 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, Gennatas ED, Elliott MA, Smith A, Hakonarson H, Verma R, Davatzikos C, Gur RE, Gur RC, 2015. Linked Sex Differences in Cognition and Functional Connectivity in Youth. Cereb. Cortex 25, 2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger TA, Kirton A, Esser MJ, Gallagher C,Dunn J,Zewdie E,Damji O,Ciechanski P, Barlow KM, 2017. Cortical excitability after pediatric mild traumatic brain injury. Brain Stimul 10, 305–314. [DOI] [PubMed] [Google Scholar]
- Semenova Z, Marshintsev AV, Melnikov AV, Meshcheryakov SV, Adayev AR, Lukyanov VI, 2016. Infrascanner in the diagnosis of intracranial lesions in children with traumatic brain injuries. Brain Inj 30, 18–22. [DOI] [PubMed] [Google Scholar]
- Sesma HW, Slomine BS, Ding R, McCarthy ML, 2008. Executive functioning in the first year after pediatric traumatic brain injury. Pediatrics 121, e1686–e1695. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott G, Leech R, 2014. Network dysfunction after traumatic brain injury. Nat. Rev. Neurol 10,156–166. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, Vu MA, Purohit M, Helmer K, Koerte I, Lin AP, Westin CF, Kikinis R, Kubicki M, Stern R ., Zafonte R, 2012. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 6, 137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, Stewart W, 2013. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol 9, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF, 2009. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 106, 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C, 2005. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage 26, 1164–1173. [DOI] [PubMed] [Google Scholar]
- Soustiel JF, Sviri GE, 2007. Monitoring of cerebral metabolism: non-ischemic impairment of oxidative metabolism following severe traumatic brain injury. Neurol Res 29, 654–660. [DOI] [PubMed] [Google Scholar]
- Spain A, Daumas S, Lifshitz J, Rhodes J, Andrews J, Horsburgh K, Fowler JH, 2010. Mild fluid percussion injury in mice produces evolving selective axonal pathology and cognitive deficits relevant to human brain injury. J. Neurotrauma 27, 1429–1438. [DOI] [PubMed] [Google Scholar]
- Stein TD, Alvarez VE, McKee AC, 2015. Concussion in chronic traumatic encephalopathy. Curr. Pain Headache Rep 19, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JA, Liu P, Lu H, Suskauer SJ, 2018. Cerebral Blood Flow after Mild Traumatic Brain Injury: Associations between Symptoms and Post-Injury Perfusion. J. Neurotrauma 35, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Kim N, Ifrah S, Lipton RB, Bachrach TA, Zimmerman ME, Kim M, Lipton ML, 2017. Symptoms from repeated intentional and unintentional head impact in soccer players. Neurology 88, 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavage TM, Nauman E, Breedlove EL, Yoruk U, Dye AE, Morigaki K, Feuer H, Leverenz LJ, 2014. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J. Neurotrauma 31, 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnutzer AA, Straumann D, Brugger P, Feddermann-Demont N, 2017. Persistent effects of playing football and associated (subconcussive) head trauma on brain structure and function: a systematic review of the literature. Br. J. Sports Med 51, 1592–1604. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Dietrich A, Nuss K, Wright M, Rusin J, Bangert B, Minich N, Yeates KO, 2010. Post-concussive symptoms in children with mild traumatic brain injury. Neuropsychology 24, 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Orchinik LJ, Minich N, Dietrich A, uss K, Wright M, Bangert B, Rusin J, Yeates KO, 2015. Symptoms of Persistent Behavior Problems in Children With Mild Traumatic Brain Injury. J. Head Trauma Rehabil 30, 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher RW, North DM, Biver CJ, 2008. Development of cortical connections as measured by EEG coherence and phase delays. Hum Brain Mapp 29, 1400–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W, 2011. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr. Bull 37, 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek L, Ghesquiere P, De SB, Lagae L, 2015a. Arithmetic difficulties in children with mild traumatic brain injury at the subacute stage of recovery. Dev. Med. Child Neurol 57, 1042–1048. [DOI] [PubMed] [Google Scholar]
- van Beek L, Ghesquiere P, Lagae L, De SB, 2015b. Mathematical Difficulties and White Matter Abnormalities in Subacute Pediatric Mild Traumatic Brain Injury. J. Neurotrauma 32, 1567–1578. [DOI] [PubMed] [Google Scholar]
- van Beek L, Vanderauwera J, Ghesquiere P, Lagae L, De SB, 2015c. Longitudinal changes in mathematical abilities and white matter following paediatric mild traumatic brain injury. Brain Inj 29, 1701–1710. [DOI] [PubMed] [Google Scholar]
- Van Kampen DA, Lovell MR, Pardini JE, Collins MW, Fu FH, 2006. The “value added” of neurocognitive testing after sports-related concussion. Am. J. Sports Med 34, 1630–1635. [DOI] [PubMed] [Google Scholar]
- Virji-Babul N, Borich MR, Makan N, Moore T, Frew K, Emery CA, Boyd LA, 2013. Diffusion tensor imaging of sports-related concussion in adolescents. Pediatr. Neurol 48, 24–29. [DOI] [PubMed] [Google Scholar]
- Virji-Babul N,Hilderman CG,Makan N, Liu A, Smith-Forrester J, Franks C, Wang ZJ, 2014. Changes in functional brain networks following sports-related concussion in adolescents. J. Neurotrauma 31, 1914–1919. [DOI] [PubMed] [Google Scholar]
- Wehner DT, Hamalainen MS, Mody M, Ahlfors SP, 2008. Head movements of children in MEG: quantification, effects on source estimation, and compensation. Neuroimage 40, 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GA, Shea B, OΓÇÖconnell D, Peterson J, Welch V, Losos M, Tugwell P, 2016. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Ottawa (ON: ): Ottawa Hospital Research Institute; 2009. Available in March. [Google Scholar]
- Wendling-Keim DS, Konig A, Dietz HG, Lehner M, 2017. Ambulatory or inpatient management of mild TBI in children: a post-concussion analysis. Pediatr. Surg. Int 33, 249–261. [DOI] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, Hanten GR, Troyanskaya M, Yallampalli R, Li X, Chia J, Levin HS, 2008. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 70, 948–955. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Sanelli PC, Anzai Y, Tsiouris AJ, Whitlow CT, 2015. Imaging evidence and recommendations for traumatic brain injury: advanced neuro- and neurovascular imaging techniques. AJNR Am. J Neuroradiol 36, E1–E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Merkley TL, Wilde EA, Barnes A, Li X, Chu ZD, McCauley SR, Hunter JV, Levin HS, 2017. A preliminary report of cerebral white matter microstructural changes associated with adolescent sports concussion acutely and subacutely using diffusion tensor imaging. BrainImagingBehav 01–12. [DOI] [PubMed] [Google Scholar]
- Wu TC, Wilde EA, Bigler ED, Yallampalli R, McCauley SR, Troyanskaya M, Chu Z, Li X, Hanten G, Hunter JV, Levin HS, 2010. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J. Neurotrauma 27, 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallampalli R, Wilde EA, Bigler ED, McCauley SR, Hanten G, Troyanskaya M, Hunter JV, Chu Z, Li X, Levin HS, 2013. Acute white matter differences in the fornix following mild traumatic brain injury using diffusion tensor imaging. J. Neuroimaging 23, 224–227. [DOI] [PubMed] [Google Scholar]
- Yang Z, Yeo R, Pena A, Ling J, Klimaj S, Campbell R, Doezema D, Mayer A, 2012. A fMRI study of auditory orienting and inhibition of return in pediatric mild traumatic brain injury. J. Neurotrauma 26, 2124–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Kaizar E, Rusin J, Bangert B, Dietrich A, Nuss K, Wright M, Taylor HG, 2012. Reliable change in postconcussive symptoms and its functional consequences among children with mild traumatic brain injury. Arch. Pediatr. Adolesc. Med 166, 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Luria J, Bartkowski H, Rusin J, Martin L, Bigler ED, 1999. Postconcussive symptoms in children with mild closed head injuries. J. Head Trauma Rehabil 14, 337–350. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Rusin J, Bangert B, Dietrich A, Nuss K, Wright M, Nagin DS, Jones BL, 2009. Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics 123, 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Wade SL, Babcock L, 2015. Structural connectivity abnormality in children with acute mild traumatic brain injury using graph theoretical analysis. Hum. Brain Mapp 36, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Wade SL, Quatman-Yates C, Hugentobler JA, Gubanich PJ, Kurowski BG, 2017. Structural Connectivity Related to Persistent Symptoms After Mild TBI in Adolescents and Response to Aerobic Training: Preliminary Investigation. J. Head Trauma Rehabil 32, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuh EL, Mukherjee P, Lingsma HF, Yue JK, Ferguson AR, Gordon WA, Valadka AB, Schnyer DM, Okonkwo DO, Maas AI, Manley GT, 2013. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann. Neurol 73, 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemek R, Barrowman N, Freedman SB, Gravel J, Gagnon I, McGahern C, Aglipay M, Sangha G, Boutis K, Beer D, Craig W, Burns E, Farion KJ, Mikrogianakis A, Barlow K, Dubrovsky AS, Meeuwisse W,Gioia G,Meehan WP III,Beauchamp MH, Kamil Y, Grool AM, Hoshizaki B, Anderson P, Brooks BL, Yeates KO, Vassilyadi M, Klassen T, Keightley M, Richer L, DeMatteo C, Osmond MH, 2016a. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA 315, 1014–1025. [DOI] [PubMed] [Google Scholar]
- Zemek RL, Grool AM, Rodriguez DD, DeMatteo C, Rothman L, Benchimol EI, Guttmann A, Macpherson AK, 2016b. Annual and Seasonal Trends in Ambulatory Visits for Pediatric Concussion in Ontario between 2003 and 2013. J. Pediatr [DOI] [PubMed] [Google Scholar]
- Zhang H,Schneider T,Wheeler-Kingshott CA, Alexander DC, 2012. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61, 1000–1016. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yang KH, King I, 2004. A proposed injury threshold for mild traumatic brain injury. J. Biomech. Eng 126, 226–236. [DOI] [PubMed] [Google Scholar]
- Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, Gullapalli RP, 2012. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage 59, 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]


