Abstract
Background & Aims:
Bile acid transporters maintain bile acid homeostasis. Little is known about the functions of some transporters in cholestasis or their regulatory mechanism. We investigated the hepatic expression of solute carrier organic anion transporter family member 3A1 (SLCO3A1, also called OATP3A1) and assessed its functions during development of cholestasis.
Methods:
We measured levels of OATP3A1 protein and mRNA, and localized the protein, in liver tissues from 22 patients with cholestasis and 21 patients without cholestasis, using real-time quantitative PCR, immunoblot, and immunofluorescence analyses. We performed experiments with Slco3a1-knockout and C57BL/6J (control) mice. Mice and Sprague-Dawley rats underwent bile-duct ligation (BDL) or a sham operation. Some mice were placed on a 1%-CA diet, to induce cholestasis, or a control diet. Serum and liver tissues were collected and analyzed; hepatic levels of bile acids and 7-alpha-C4 were measured using liquid chromatography mass spectrometry. Human primary hepatocytes and hepatoma (PLC/PRF/5) cell lines were used to study mechanisms that regulate OATP3A1 expression and transport.
Results:
Hepatic levels of OATP3A1 mRNA and protein were significantly increased in liver tissues from patients with cholestasis and from rodents with BDL or 1%CA diet-induced cholestasis. Levels of fibroblast growth factor 19 (FGF19, FGF15 in rodents) were also increased in liver tissues from patients and rodents with cholestasis. FGF19 signaling activated the Sp1 transcription factor (SP1) and nuclear factor-kappa B (NF-κB) to increase expression of OATP3A1 in hepatocytes; we found binding sites for these factors in the SLCO3A1 promoter. Slco3a1-knockout mice had shorter survival times, increased hepatic levels of bile acid, and developed more liver injury following the 1%-CA diet or BDL than control mice. In hepatoma cell lines, we found OATP3A1 to take prostaglandin E2 and thyroxine into cells and efflux bile acids.
Conclusions:
We found levels of OATP3A1 to be increased in cholestatic liver tissues from patients and rodents, compared to healthy liver tissues. We show that OATP3A1 functions as bile acid efflux transporter that is upregulated as an adaptive response to cholestasis.
Keywords: Organic anion transporting polypeptides (OATPs), bile acid transporters, fibroblast growth factor (FGF) 19, extracellular signal related kinase (ERK)
Graphic abstract
Introduction
Bile acid transporters play a pivotal role in maintaining bile acid homeostasis [1]. When bile formation is impaired, bile acids accumulate in the blood and liver, which causes cholestatic liver injury [1]. During cholestasis, an adaptive response has evolved to protect the liver and attenuate this injury, including reduction of de novo bile acid synthesis and alteration of bile acid transporters expression [1]. Of note, the hepatic expression of bile acid uptake transporters are down regulated (e.g. NTCP, OATP1B1 and OATP1B3), whereas the expression of efflux transporters are up-regulated (e.g. MRP3, MRP4 and OSTα/β) [1]. In an effort to study the expression regulation of these transporters in cholestatic human livers, we sought to determine whether there could be any additional membrane transporters whose expression is altered. We analyzed the mRNA expression of 11 members of the solute carrier organic anion transporter (SLCO/OATP) family. As expected, the hepatic expression of OATP1B1 and OATP1B3 were down-regulated in the cholestatic livers [1]. To our surprise, OATP3A1 mRNA expression, in contrast, was up-regulated in these same livers. However, the functional role of OATP3A1 was unknown.
OATP3A1 (also called SLCO3A1, SLC21A11 and OATP-D) is poorly characterized in liver health and disease. It has been reported that OATP3A1 is highly expressed in the brain, vasculature, breast, and reproductive systems, but is expressed at very low levels in the human liver under normal conditions [2–5]. It is mainly located at the plasma membrane of choroid plexus epithelial cells in the brain and lactiferous ducts in the breast [4, 5]. Since it is a member of the OATP family, it was characterized as an uptake transporter with substrate specificity for prostaglandins and thyroxine, but not for bile acids or methotrexate [2–4]. This separates OATP3A1 from its homologs OATP1B1 and OATP1B3 [3–6]. Recently, Wei and colleagues reported that the rs207959 (T) allele of SLCO3A1 (OATP3A1) was associated with Crohn’s disease and intestinal perforation in a small cohort of patients from Taiwan [7]. However, the regulatory mechanisms of OATP3A1 expression are unknown. Moreover, the expression, regulation, and functional role of OATP3A1 in liver health and disease have yet to be evaluated.
Fibroblast growth factor (FGF) 19 is an endocrine hormone that plays a very important role in maintaining bile acid homeostasis [1, 8]. In rodents, it is mainly secreted by the ileum, but it is also expressed in the liver and gallbladder of humans [9]. Elevated circulating FGF19 levels were found in certain types of cholestatic patients, and positively correlate with the severity of the disease [9–11]. Interestingly, FGF19 mimetics can alleviate cholestatic liver injury, even in patients with primary biliary cholangitis (PBC) unresponsive to ursodeoxycholic acid (UDCA) treatment [12, 13]. This therapeutic effect is at least in part due to its repression of the bile acid-synthetic enzyme CYP7A1 and bile acid pool sizes in cholestasis [12, 13]. However, the detailed mechanism underlying these alterations remains unknown. Recently, studies have reported that FGF19 suppresses CYP7A1 expression along with extracellular signal related kinase (ERK) activation [14, 15]. Moreover, up-regulated bile acid efflux transporters (e.g. MRP3, MRP4 and OSTα/β), as an adaptive response, play an important role in eliminating excessive levels of intrahepatic bile acids in cholestatic conditions [1, 16]. Therefore, whether and how elevated FGF19 expression in human cholestasis regulates bile acid efflux transporters, and if FGF19-ERK or other signaling pathways mediate the expression of the bile acid-synthetic enzyme CYP7A1, needs to be elucidated.
In the current study, we aimed to determine the expression and regulation of OATP3A1/Oatp3a1 in the liver under cholestatic conditions, and to delineate its functional role in cholestasis. The results gained through this study may advance our understanding of the OATP3A1 gene, and help in the development of novel treatments for cholestasis.
Materials and Methods
Patients and liver sample collection
This study was carried out in accordance with the Declaration of Helsinki (2008) of the World Medical Association. The study protocol was reviewed and approved by the Institutional Ethics Review Board at the Southwest Hospital Chongqing, China. Patients were recruited from the Institute of Hepatobiliary Surgery, Southwest Hospital. Corresponding written informed consent was obtained from all patients prior to the study. Patients were scheduled to undergo pancreatoduodenectomy (PD) resection with curative intent. Cholestatic liver samples (n=22) were obtained from patients suspected to have a pancreatic or periampullary malignancy. All patients had severe symptoms of obstructive cholestasis and jaundice caused by periampullary tumor growth at initial presentation, and underwent surgery within 1 week without preoperative biliary drainage. Control liver tissues were also obtained from patients undergoing resection of liver metastases without cholestasis (n=21; three neuroendocrine tumor, seven colorectal, seven colonic, and four rectal metastases). In addition, liver biopsy samples were obtained from patients with PBC. The liver samples were immediately cut into small pieces and fixed in 4% paraformaldehyde or stored in liquid nitrogen. The biochemical characteristics of the patients were described in Table S1.
Generation and verification of Slco3a1-knockout mice
The Slco3a1-knockout (KO) mouse model was developed by Shanghai Model Organisms Center Inc. (Shanghai, China). In brief, Cas9 mRNA was transcribed in vitro with mMESSAGE mMACHINE T7 Ultra Kit (Ambion, TX, USA) according to the manufacturer’s instructions. The sgRNAs targeting exon2 of the Slco3a1 gene were designed using the online tool (http://crispr.mit.edu/) (Suppl.Fig.1A). The 127bp deletion in exon2 resulted in a frameshift mutation of Slco3a1 and inactivated the Slco3a1 gene. The generation of homozygous Slco3a1-KO mice was identified by polymerase chain reaction (PCR) using the primer set P1(5’-CTAGCAGGGCTACAGTGCTTACAA-3’)/P2(5’-CCCATTGGTGG CACAGACATCG-3’), and had a 970bp PCR product (Suppl.Fig.1B). Finally, Slco3a1-KO mice were further confirmed using western blotting and immunofluorescent (IF) analysis (Suppl.Figs.1C&D). Additional information can be found in the supplementary materials.
Experimental animals
All rodent experiments were performed according to the guidelines of the animal care and use committees at the medical research center, after the study protocol was approved by the Institutional Animal Care and Use Committee of the Southwest Hospital affiliated to the Third Military Medical University (Chongqing, China). All animals were housed with a 12 hr dark/light cycle, and with ad libitum access to food and water. Male C57BL/6J mice at age 8 weeks of age and Sprague-Dawley rats weighting 200–230 g were purchased from the Center of Laboratory Animals of Third Military Medical University (Chongqing, China). (1) For bile duct ligation (BDL) experiments, rats were randomly divided into four groups, i.e. sham operation (7 days or 14 days, n=5 each) and BDL (7 days or 14 days, n=9 each); wild-type (WT) and Slco3a1-KO mice were divided into four groups, i.e. sham operation or BDL (n=3–9 in each group, performed at 3 or 9 days post-operation). (2) In the 14-day feeding experiment, WT mice and Slco3a1-KO mice were each divided into two groups, i.e. control diet (n=5, for each genotype) and 1%-CA-feeding (n=9 for WT group, n=12 for Slco3a1-KO group). The animals were fasted overnight before they were sacrificed. Serum was collected and immediately stored at −80°C until analyses were performed. Serum biochemistry was assayed by the Clinical Laboratory at the Southwest Hospital affiliated to the Third Military Medical University (Chongqing, China). The liver tissues were quickly perfused with phosphate-buffered saline (PBS) to flush out blood, and immediately cut into small pieces and rapidly frozen in liquid nitrogen until analyses were performed.
LC-MS/MS analysis of bile acids and 7-alpha-C4 in mouse liver tissues extracts
The composition of bile acids from mouse liver tissue was analyzed by the Dalian Institute of Chemical Physics, Chinese Academy of Sciences (Dalian, Liaoning, China), using the LC-MS/MS method. Hepatic 7-alpha-C4 (7alpha-hydroxy-4-cholesten-3-one) levels in mice were determined by Shanghai Omicspro Biotech Company (Shanghai, China) as described previously [17]. Detailed procedures are described in the supplementary materials.
Primary human hepatocytes
Primary human hepatocytes were obtained through the Liver Tissue Cell Distribution System (University of Pittsburgh, Pennsylvania, USA), funded by NIH Contract #N01-DK-7–0004 / HHSN267200700004C. The cells were maintained as previously described [18].
OATP3A1-farnesoid X receptor (FXR/NR1H4)-ileal bile acid-binding protein (I-BABP) luciferase reporter assay for bile salt transport, bile acid uptake, and trans-cellular transport of [3H] taurocholate assays
To investigate the functional role of OATP3A1 in hepatocytes, we initially selected four stably transfected PLC/PRF/5 (or MDCK) cell lines (-CTR, -ASBT, -OATP3A1, and -ASBT plus OATP3A1) in which the protein level of ASBT or OATP3A1 in single-plasmid cells was similar to the protein level in ASBT and OATP3A1 in double-plasmid cells (Suppl.Figs.2A&B and data not shown). The phRL-CMV, pCMX-human FXR (farnesoid X receptor), pCMX-human RXRα (retinoid X receptor alpha), and pGL3-human I-BABP (ileal bile acid-binding protein) constructs were used for the OATP3A1-FXR-I-BABP luciferase reporter assay for bile salt transport in the above four stably transfected PLC/PRF/5 cell lines as described previously [19]. Uptake or trans-cellular transport of [3H]-taurocholate (TCA), [3H]-prostaglandin E2 (PGE2), or [125I]-labelled thyroxine (T4) in the stably transfected MDCK or PLC/PRF/5 cell lines (-CTR, -ASBT, -OATP3A1 and –ASBT plus OATP3A1) were performed as reported previously [3, 4, 20]. Although human OATP3A1 has two isoforms (OATP3A1_v1 and v2), both exerted similar functions (Suppl.Fig.3E). Therefore, the long variant OATP3A1_v2 expression construct, namely OATP3A1, was used in this study. Detailed procedures were described in the supplementary materials.
Statistical analysis
All data were analyzed using the independent-samples Student’s t-test (two-tailed) and were expressed as the mean ± standard deviation (SD), using GraphPad Prism 6.01 (GraphPad Software Inc., San Diego, CA). A value of P<0.05 was considered statistically significant.
Results
Hepatic OATP3A1 mRNA and protein levels were markedly increased in cholestatic patients and in rodent models of cholestasis
Hepatic OATP3A1 expression was significantly greater in cholestatic patients with 3.6-fold and 4.8-fold increases in the OATP3A1 mRNA and protein levels, respectively (P<0.05; Figs.1A&B). IF and IHC examinations of OATP3A1 and IF double-labeling analysis of OATP3A1 and MRP2 in human liver sections further confirmed these findings, and revealed its localization at the basolateral membrane of the cholestatic hepatocytes (Figs.1C&D and Suppl.Fig.4A). These observations were further confirmed in the liver biopsy samples from patients with PBC (Suppl.Fig.4B). Interestingly, we also detected a markedly increased expression of OATP3A1 in cholestatic bile duct epithelial cells when compared to controls (Arrows, Figs. 1C&D).
Figure 1.
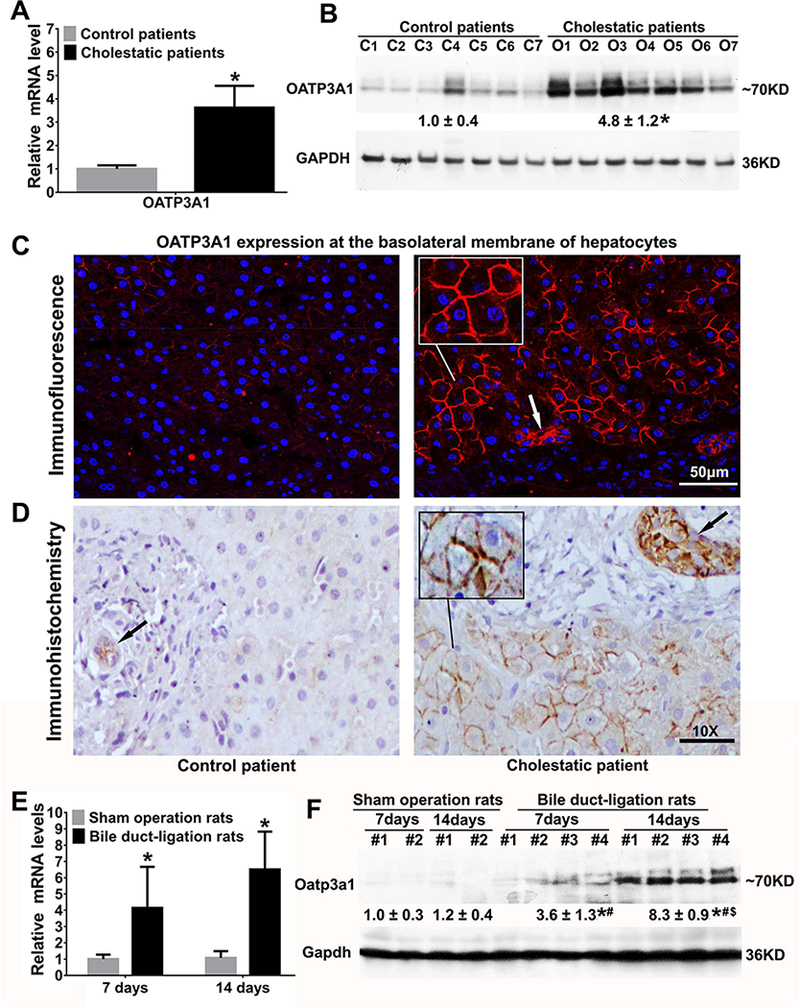
Hepatic OATP3A1/Oatp3a1 expression was markedly increased in obstructive cholestatic patients and rats. (A) The mRNA expression of OATP3A1 in human livers (relative to control group, n=21 for control group, n=22 for obstructive cholestatic group). *P<0.05 vs. controls. (B) Representative western blots for OATP3A1 and their corresponding densitometry values (relative to control patient group, n=21 for the control group, n=22 for the obstructive cholestatic patient group); C1–7, controls; O1–7, obstructive cholestasis livers. *P<0.05 vs. controls. (C) Immunofluorescence (IF) labeling of OATP3A1 protein (red) in the liver of a non-cholestatic control patient and a patient with obstructive cholestasis. The nuclei were stained with 4′−6-Diamidino-2-phenylindole (DAPI) (blue). (D) Immunohistochemistry (IHC) labeling of OATP3A1 in the liver of a control patient and a patient with obstructive cholestasis. Increased expression of OATP3A1 in cholestatic bile duct epithelial cells was observed (Arrows). (E) Liver mRNA expression of OATP3A1 in cholestatic rats (relative to control group, n=5 for each sham operation group, n=9 for each bile duct-ligation (BDL) group). (F) Representative western blots for Oatp3a1 in cholestatic rat liver and their corresponding densitometry. *P<0.05 vs. sham 7-day group; #P<0.05 vs. sham 14-day group; $P<0.05 vs. BDL 7-day group.
We were intrigued by the findings in the human subjects and subsequently used cholestatic rodent models for experimental validation and further in-depth investigation. Similar to that observed in the human-derived samples, hepatic OATP3A1 mRNA and protein levels were significantly increased in a time-dependent manner (7 and 14 days) in BDL rats compared to sham operation rats (Figs.1E&F). Furthermore, a similar up-regulation of gene expression was also observed in the liver samples of BDL mice (3 and 9 days post-operation) and 1%CA-fed mice (14 days) (Suppl.Figs.5A-D). These findings offered additional direct evidence that cholestasis led to a marked induction of OATP3A1 expression.
Slco3a1 deficiency aggravated cholestatic liver injury with a dramatic increase of liver bile acid levels in cholestatic mice
To gain insight into the potential function of OATP3A1 in cholestasis, we generated Slco3a1-KO mice and first treated them with 1%CA to conduct additional experiments. As shown in Table 1, the survival rate of mice in the Slco3a1-KO group was lower than the WT group (58% vs 100%) when fed with 1%CA. The bile acid level in the livers of Slco3a1-KO mice was markedly greater compared to WT mice after CA-feeding (P<0.05; Table.1). Further LC-MS/MS analysis of the liver extracts revealed that the conjugated bile acids taurocholic acid, taurodeoxycholate acid, and tauromuricholate acid in livers of 1%CA-fed Slco3a1-KO mice were significantly increased by 1.51-, 9.04-, and 1.92-fold compared with the 1%CA-fed WT controls (P<0.05; Table 1). Furthermore, levels of hepatic 7-alpha-C4 were also markedly reduced in 1%CA-fed Slco3a1-KO mice (P<0.05; Table.1). These data suggested that Oatp3a1 deficiency directly resulted in further accumulation of bile acids in the cholestatic mouse livers. Moreover, serum biochemistry tests revealed that the levels of alanine transaminase (ALT), aspartate transaminase (AST), and total bile acids (TBA) in 1%CA-fed Slco3a1-KO mice were significantly higher compared to the 1%CA-fed WT control group, whereas there was no significant difference in serum levels of alkaline phosphatase (ALP), direct bilirubin, and total bilirubin between these two groups (Table. 1).
Table 1.
Survival rates, serum biochemistry, and liver tissue bile acid and C4 levels in mice fed with 1% CA diet for 14 days
| Chow diet 14 d |
1% CA diet 14 d |
|||
|---|---|---|---|---|
| WT | Slco3a1 KO | WT | Slco3a1 KO | |
| Survival/total mice (%) | 5 / 5 (100%) | 5 / 5 (100%) | 9/9(100%) | 7/12 (58%) |
| Serum ALT (IU/L) | 29.54±5.22 | 26.65±2.80 | 151.44±62.73*,¶ | 291.39±119.27*,¶,§ |
| Serum AST (IU/L) | 99.32±4.88 | 99.22±30.88 | 224.12±73.01*,¶ | 431.35±173.01*,¶,§ |
| Serum ALP (IU/L) | 89.0±43.92 | 92.69±42.56 | 157.04±29.38*,¶ | 165.71±31.98*,¶ |
| Serum TBA (μmol/L) | 1.59±0.69 | 2.16±1.02 | 239.66±142.01*,¶ | 433.19±161.09*,¶,§ |
| Serum TBIL (μmol/L) | 2.53±2.50 | 1.73±0.82 | 12.12±6.17 | 18.86±11.87*,¶ |
| Serum DBIL (μmol/L) | 1.08±1.50 | 0.13±0.23 | 6.86±3.04 | 10.23±10.89¶ |
| Liver tissue BAs (μmol /kg of liver) | 166.47±40.51 | 189.6±65.89 | 721.81±l98.24*,¶ | 1193.75±412.52*,¶,§ |
| Liver TCA (relative MS response) | Not detected | Not detected | 5230570±730880 | 7894977±2078538§ |
| Liver TDCA (relative MS response) | Not detected | Not detected | 36434±442353 | 3292990±3734785§ |
| Liver TmCA (relative MS response) | Not detected | Not detected | 227707±85375 | 436803±207875§ |
| Liver 7-alpha-C4 (relative MS response) | 6478±2825 | 4074±2817 | 1222±227*,¶ | 625±323*,¶,§ |
Values are means±SD.
P < 0.05 VS chow diet WT mice
P < 0.05 VS chow diet Slco3a1 KO mice
P < 0.05 VS 1% CA diet WT mice.
Abbreviations: CA, cholic acid; KO, knock out; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; TBA, total bile salts; TBIL, total bilirubin; DBIL, direct bilirubin; BAs, Bile salts; TmCA, tauromuricholate acid; TDCA, taurodeoxycholate acid; TCA, taurocholic acid; 7-alpha-C4, 7alpha-hydroxycholest-4-en-3-one.
To further examine the role of Oatp3a1 in cholestasis, we performed BDL in WT and Slco3a1-KO mice for 3 and 9 days in an additional cholestatic mouse model (Table.S2). As expected, significantly higher levels of serum ALT and AST were observed in Slco3a1-KO compared to WT mice after BDL for 9 days (Table.S2B). There was no significant difference between Slco3a1-KO and WT mice after BDL for 3 days (Table.S2A). To understand the reasons for this difference in 9-day but not in 3-day BDL groups between WT and Slco3a1-KO, we analyzed Oatp3a1 mRNA and protein expression in WT BDL livers. Interestingly, we found that Oatp3a1 expression in 9-day WT BDL livers was higher than in 3-day WT BDL livers (Suppl.Fig.5A). Together, these findings suggest that OATP3A1/Oatp3a1 plays an important role in the adaptive response and maintenance of bile acid homeostasis during cholestasis, and that depletion of OATP3A1/Oatp3a1 could aggravate cholestatic liver injury.
Compromised hepatic adaptive response to cholestasis in 1% CA-fed Slco3a1-KO mice
To develop insight into how Oatp3a1 deficiency could worsen cholestatic liver injury, we assessed the expression of genes involved in the regulation of bile acid homeostasis and cholestatic liver injury in 1%CA-fed mice. To our surprise, mRNA and protein levels of hepatic bile acid efflux transporters Mrp2, Mrp3, Mrp4, and Ostα/β were significantly greater in the 1%CA-fed Slco3a1-KO group compared to the 1%CA-fed WT group (Figs.2A&C), whereas hepatic mRNA levels of Asbt, Mdr1a, and Mdr2 were not different between these two groups (Fig.2A). In contrast, the expression of the bile acid synthesis enzymes, Cyp7a1 and Cyp8b1, was significantly lower in the 1%CA-fed Slco3a1-KO group compared to the 1%CA-fed WT group, while the nuclear receptor Shp was significantly increased (Figs.2B&C). These finding suggested that Slco3a1 ablation contributes to the further accumulation of intrahepatic bile acids in cholestasis, despite resulting in further upregulation in the expression of other genes involved in maintaining bile acid homeostasis.
Figure 3.
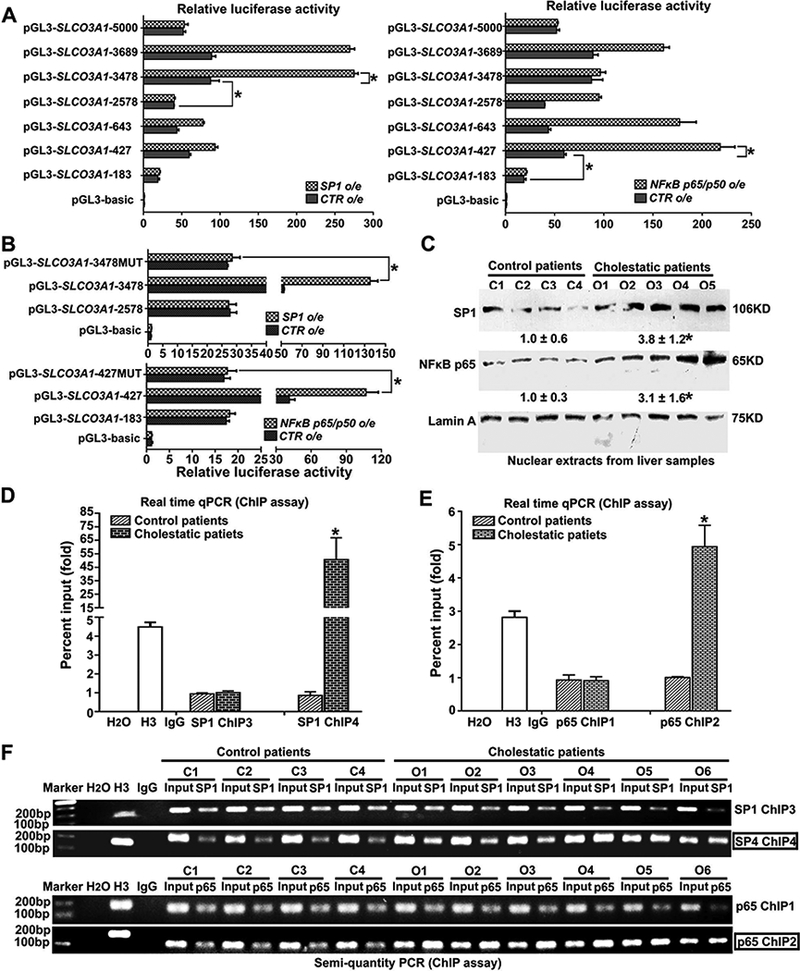
Transcriptional regulation of OATP3A1 was mediated through the transcription factors SP1 and NFkB p65 in human hepatocytes and in livers of patients with cholestasis. (A) Seven different lengths of human SLCO3A1 promoter-luciferase constructs were transiently transfected into PLC/PRF/5 cells with or without co-transfected SP1 or NFkB p65/p50 to identify the sites of transcriptional regulation. The cells were harvested after 24 hours, and relative luciferase activity was determined using the dual-luciferase reporter assay system. P< 0.001, n=3. (B) The SLCO3A1 promoter-Luc constructs (−3478 to +25) (pGL3-SLCO3A1-3478), which contains potential SP1 binding sites, and its mutant (pGL3-SLCO3A1-3478MUT) were used to transfect PLC/PRF/5 cells with or without SP1 co-transfection (Suppl.Fig.7A). Meanwhile, pGL3-SLCO3A1-427, which contains potential NFkB p65 binding sites, and its mutant pGL3-SLCO3A1-427MUT were also transfected into PLC/PRF/5 cells with or without NFkB p65/p50 co-transfection (Suppl.Fig.7A). The pGL3-SLCO3A1-2578, pGL3-SLCO3A1-183, and pGL3-basic constructs were set as the relative controls, respectively. After 24 hours, the transfected cells were lysed for the dual-luciferase reporter assay. P<0.001, n=3. (C) Representative western blotting and corresponding densitometry of SP1 and NFκB p65 from nuclear extracts of human liver samples (n=21 for control group; n=22 for cholestatic group). *P<0.05 vs. controls. (D&E) According to the above luciferase reporter results, we analyzed the potential binding sites on the SLCO3A1 promoter, and designed PCR primers (SP1 ChIP1–6 and NFkB ChIP1–2) for ChIP-real time qPCR assays in human livers (n=12 for control patients; n=15 for obstructive cholestatic patients) (Suppl.Figs.7B&C and Table.S6); *P<0.05 vs. controls. The left data can be found in Suppl. Fig.7C. (F) Validation of the binding activities of SP1 and NFkB p65 to SLCO3A1 promoter in the liver of obstructive cholestatic patients were further confirmed by ChIP-semi-quantitative PCR.
OATP3A1 was identified as a novel transporter to facilitate bile acid efflux in the liver
A previous study reported that OATP3A1 mediated neither bile acid nor methotrexate uptake, two preferable substrates for the OATP family [2–4]. We observed that depletion of Oatp3a1 in cholestatic mouse liver worsened the accumulation of intrahepatic bile acids. Therefore, we asked whether induction of OATP3A1 in response to cholestasis could aid in removing excess bile acids, and what was the mechanism by which it could restore bile acid homeostasis? As shown in Fig.2D, dual-luciferase assays indicated that FXR/RXR activation in the presence of 25μM conjugated bile acids glycochenodexycholate acid (GCDCA) and glycocholate acid (GCA) was significantly lower in PLC/PRF/5-ASBT plus OATP3A1 than in PLC/PRF/5-ASBT, suggesting that OATP3A1 was able to eliminate but not take up conjugated bile acids in hepatocytes. Furthermore, we also treated all four stably transfected cell lines with the unconjugated bile acid chenodeoxycholic acid (CDCA) (100μM), and the conjugated bile acids glycocholate acid (GCA) or taurocholic acid (TCA) (100μM), and cell lysates were sent for bile acid detection. Similar to the luciferase reporter results, our data clearly demonstrated that intracellular levels of the conjugated bile acids GCA and TCA were significantly lower in PLC/PRF/5-ASBT plus OATP3A1 than in PLC/PRF/5-ASBT (P<0.05), supporting our previous hypothesis (Fig.2E). Trans-cellular transport of [3H]-taurocholate assay further confirmed that the MDCK-ASBT plus OATP3A1 cell line mediated significant taurocholate trans-cellular transport, whereas the MDCK-CTR, -ASBT, or -OATP3A1 cell lines exhibited only background levels of apical to basolateral taurocholate trans-cellular transport (Fig.2F). In contrast, the cell lines stably transfected with the OATP3A1 construct all showed significantly higher uptake activity for the typical OATP substrates PGE2 and T4, as measured by the uptake of [3H]-PGE2 and [125I]-T4, in agreement with previous functional studies carried out in Xenopus oocytes or CHO cells [3–5] (Suppl.Figs.3A-D). The above findings provided solid evidence in support of the novel role of OATP3A1 as a transporter for the efflux of excessive bile acids in hepatocytes.
Transcription factors SP1 and NFkB p65 directly induced OATP3A1 expression in human hepatocytes and in livers of obstructive cholestatic patients
Next, OATP3A1 induction under cholestatic conditions and the mechanisms of OATP3A1 regulation during cholestasis were investigated. First, the promoter region of SLCO3A1 (−5.0kB) was analyzed (http://jaspar.genereg.net), and no putative FXR/RXR response element was found. Instead, numerous putative SP1 and NFκB response elements were identified (Suppl.Fig.6A). Furthermore, conjugate bile acids, known as FXR agonists, did not increase OATP3A1 mRNA expression (Suppl.Fig.8A) or FXR/RXR-SLCO3A1 promoter activity (Suppl.Fig.6B) in PLC/PRF/5-ASBT cell lines. To verify whether these response elements were functional, different lengths of the promoter region (−5000, −3689, −3478, −2578, −643, −427, and −183 to +25) were inserted into a gene reporter vector, pGL3-basic. A dual luciferase reporter assay demonstrated that multiple effective elements located at −3478 to −2578 and −427 to −183 contributed to the activity of the SLCO3A1 promoter (black bar, Fig.3A). Furthermore, when co-transfected with SP1 or NFκB p65/p50 in PLC/PRF/5 cells, the activity of the SLCO3A1 promoters (pGL3-−3478/+25 or −427/+25) showed the highest fold change, indicating that the −3478 to −2578 or −427 to −183 region of promoter had crucial SP1 or NFκB response elements, respectively (Fig.3A). When key motifs of these potential response elements were mutated (Suppl.Fig.7A), the increased luciferase activity of the SLCO3A1 promoters (pGL3-−3478/+25 or −427/+25) by SP1 or NFκB p65/p50 co-transfection was completely abolished (Fig.3B). These results demonstrated that SP1 and NFκB p65 directly regulated OATP3A1 expression. To determine whether these two transcription factors had a role in the up-regulation of OATP3A1 in cholestatic patients, their expression was first detected. As shown in Fig.3C, the nuclear protein levels of SP1 and NFκB p65 in the liver samples of patients with obstructive cholestasis were markedly increased to 3.8-fold and 3.1-fold, respectively. ChIP-real time qPCR assays demonstrated that the binding activities of SP1 and NFkB p65 to the SLCO3A1 promoter (SP1 ChIP4 site located −3478 to −2578, and NFκB p65 ChIP2 site located −427 to −183) were markedly increased in the livers of cholestatic patients, when compared to control patients (Figs.3D&E and Suppl.Figs.7B&C). These results were further confirmed by additional ChIP assays (semi-quantitative PCR) (Fig.3F). Therefore, these data clearly showed that SP1 and NFkB p65 directly induced OATP3A1 expression in cholestatic patients.
Up-regulation of OATP3A1 was mediated through activation of FGF19-ERK/NFkB-SP1/p65 signaling pathways in human hepatocytes
To gain further insight into the regulation of hepatic OATP3A1 expression in cholestasis, we asked whether conjugated bile acids or cytokines that were elevated in cholestatic patients stimulated OATP3A1 expression in hepatocytes. Interestingly, FGF19 and TNFα but not conjugated bile acids significantly induced OATP3A1 mRNA expression (3.3-fold and 1.6-fold, respectively) in hepatoma PLC/PRF/5 cells with or without the stably transfected bile acid up-take transporter ASBT (Suppl. Fig.8A). Because both SP1 and NFkB p65 regulated OATP3A1 expression, we tested if FGF19 could stimulate OATP3A1 expression via these two transcription factors. In primary human hepatocytes, FGF19 significantly increased mRNA levels of OATP3A1, NFkB1, and SP1, but markedly decreased CYP7A1 (Fig.4A). Corresponding to these changes, cell surface expression of OATP3A1 protein and nuclear expression of SP1 and NFkB p65 protein were increased in a dose- and time-dependent manner in PLC5/PRF/5 cells treated with FGF19 (Fig.4B and Suppl.Fig.8B). A dual luciferase reporter assay demonstrated that the FGF19-induced SLCO3A1 promoter activity was markedly increased when co-transfected with SP1 or NFkB p65/p50 in PLC5/PRF/5 cells, but was abolished by mutations in their binding site (Figs.4C&D). ChIP assays (both real-time qPCR and semi-quantitative PCR) further confirmed that the binding activities of SP1 and NFkB p65 to the SLCO3A1 promoter (SP1 ChIP4 and NFkB p65 ChIP2) were induced by FGF19 in a dose- and time-dependent manner (Figs.4E&F and Suppl.Fig.8C). Taken together, the above results indicated that FGF19 stimulated OATP3A1 expression via up-regulation of the SP1 and NFkB p65 transcription factors in human hepatocytes.
Figure 4.
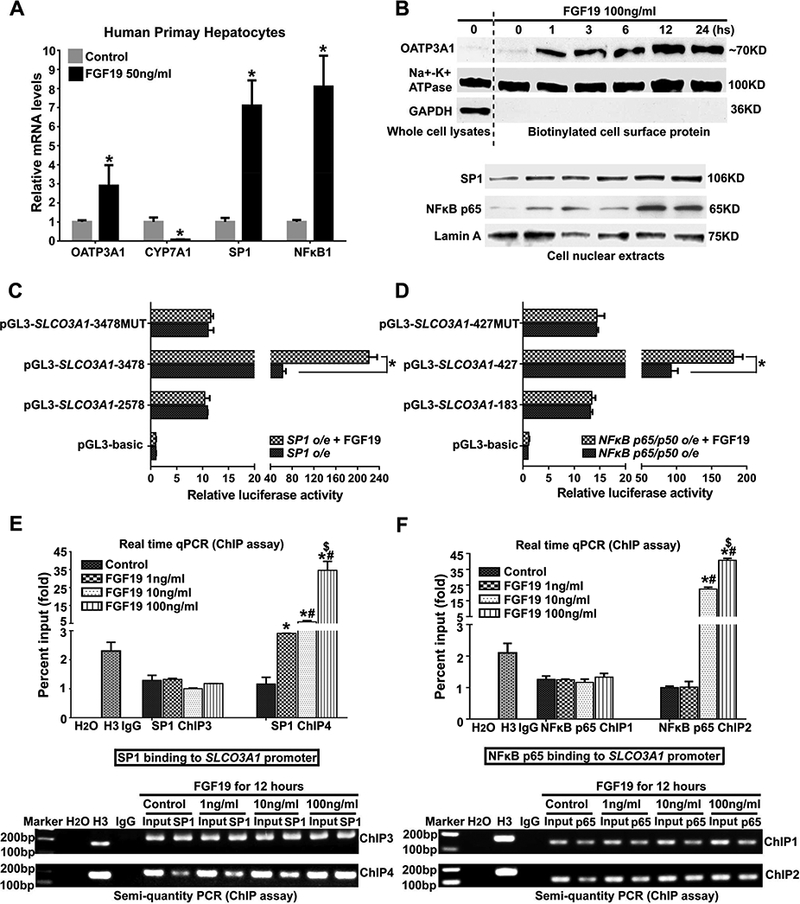
Transcription factors SP1 and NFkB p65 mediated FGF19 induction of OATP3A1 expression and binding activity to the SLCO3A1 promoter in human hepatocytes. (A) FGF19 induced OATP3A1, SP1, and NFkB1 expression at mRNA levels, and reduced CYP7A1 mRNA expression in primary human hepatocytes. (B) Cell surface protein expression of OATP3A1 and nuclei protein expression of SP1 and NFkB p65 were increased in a time-dependent manner in PLC/PRF/5 cells with FGF19 treatment (100ng/mL). (C&D) The induced SLCO3A1 promoter activity by SP1 or NFkB p65/p50 construct co-transfection was dramatically increased in the presence of FGF19 (100ng/mL). However, these alterations were diminished if SP1 and NFkB p65 response elements were mutated in the SLCO3A1 promoter. *P<0.001, n=3. (E&F) ChIP assay results (upper, real-time qPCR; lower, semi-quantity PCR) demonstrated that FGF19 increased SP1 or NFkB p65 binding activities to their response elements (SP1 ChIP4 and NFkB p65 ChIP2) in the SLCO3A1 promoter in a dose-dependent manner in PLC/PRF/5 cells. *P<0.05 vs. control group; #P<0.05 vs. FGF19 (1ng/mL) group; $P<0.05 vs. FGF19 (10ng/mL) group.
A previous study has demonstrated that FGF19 activated ERK signaling in HepG2 cells [14], and similar results were observed in PLC/PRF/5 cells (Fig.5A). Furthermore, we first reported here that FGF19 induced not only the phosphorylation but also total expression of NFkB p65 in a time-dependent manner (Fig.5A). When PLC/PRF/5 cells were pretreated with PD98059 (25μM), an inhibitor of ERK signaling, and BAY 11–7082 (10μM), a selective and irreversible inhibitor of NFkB signaling, the FGF19-induced cell surface expression of the OATP3A1 protein and nuclear expression of SP1 and NFkB p65 protein were abolished (Fig.5B). Particularly, our data implied that ERK signaling mainly mediated FGF19-induced SP1 expression while NFkB signaling mainly regulates FGF19-induced p65 expression, although there is cross-talk between ERK and NFkB signaling pathways (Fig.5B). These results were further confirmed by ChIP assays (real-time qPCR and semi-quantitative PCR). The FGF19-induced activity of SP1 binding to the SLCO3A1 promoter (SP1 ChIP4) was diminished in the presence of both PD98059 and BAY 11–7082 (Fig.5C). However, the FGF19-induced activity of NFkB p65 binding to the SLCO3A1 promoter (NFkB p65 ChIP2) was abolished only in the presence of BAY 11–7082, not PD98059 (Fig.5D). These data indicated that both FGF19-ERK/NFκB-SP1 and FGF19-NFkB-p65 signaling mediated OATP3A1 expression in hepatocytes. In addition, our data showed that the FGF19-mediated SHP/CYP7A1 expression was abolished in the presence of PD98059 and BAY 11–7082, indicating that FGF19-ERK/NFκB signaling was involved in the regulation of bile acid synthesis (Fig.5E).
Figure 5.
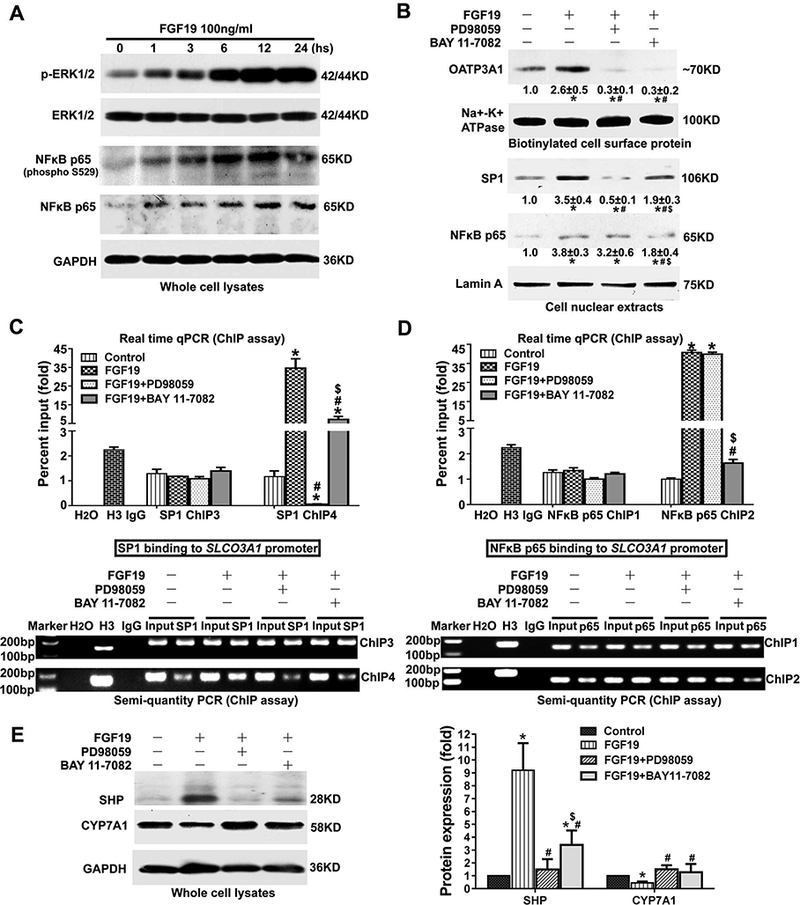
FGF19-ERK/NFkB-SP1/p65 or SHP pathway regulated OATP3A1 or CYP7A1 expression in human hepatocytes. (A) FGF19 increased phosphorylation levels of ERK1/2 and NFkB p65 in a time-dependent manner in PLC/PRF/5 cells. FGF19 also induced the expression of total NFkB p65 protein but not total ERK1/2 protein. (B) When PLC/PRF/5 cells were pretreated with ERK and NFkB signaling inhibitors (25μM PD98059 and 10μM BAY 11–7082), the FGF19-induced cell surface expression of OATP3A1 protein and nuclear expression of SP1 and NFkB p65 protein were diminished. (C&D) ChIP assay results revealed that PD98059 and BAY 11–7082 abolished the increased SP1 and NFkB p65 binding activities to their response elements in the SLCO3A1 promoter (SP1 ChIP4 and NFkB p65 ChIP2) in PLC/PRF/5 cells when treated with FGF19 (100ng/mL). (E) The FGF19-mediated SHP/CYP7A1 expression was abolished in the presence of the PD98059 and BAY 11–7082 inhibitors in PLC/PRF/5 cells. *P<0.05 vs. control group; #P<0.05 vs. FGF19 group; $P<0.05 vs. FGF19 plus PD98059 group, n=3.
The FGF19-ERK/NFKB-SP1/p65 signaling was also activated in patients with obstructive cholestasis
Following the previous finding with human hepatocytes and liver samples (Figs.3–5), it was tested whether the FGF19-ERK/NFkB-SP1/p65 signaling pathways could be similarly activated in patients with cholestasis. As shown in Figs.6A-C, the elevated FGF19 levels in the serum (3.3-fold) and liver (8.4-fold at mRNA level, 4.7-fold at protein level) of obstructive cholestatic patients were observed. Hepatic expression of fibroblast growth factor receptor (FGFR) 4 protein was also increased (3.4-fold) in cholestatic patients compared to control patients (Fig.6C). However, β-Klotho expression at mRNA and protein levels was unchanged (Figs.6B&C). Western blotting analysis demonstrated that phosphorylated ERK was significantly increased (4.8-fold), whereas total ERK protein was unaltered in the liver of cholestatic patients (Fig.6D). In contrast, as was observed in FGF19-treated human primary hepatocytes, both the phosphorylation and total levels of NFκB p65 were markedly increased in cholestatic patients (Fig.6D). Together, these results showed that FGF19-ERK/NFκB signaling was activated in human cholestasis.
Figure 6.
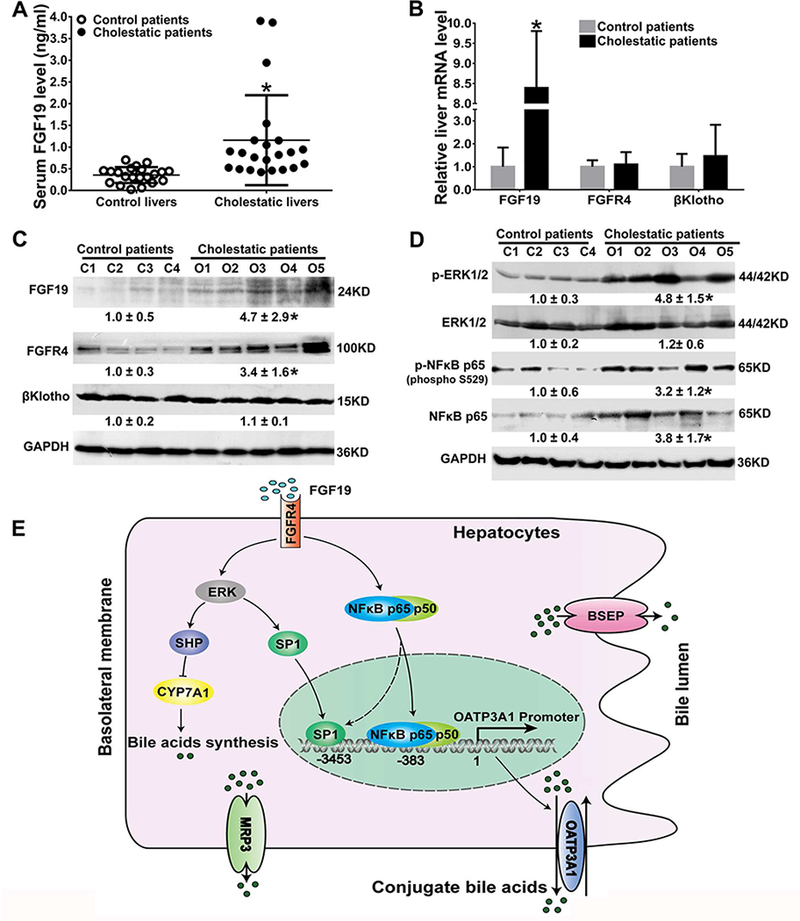
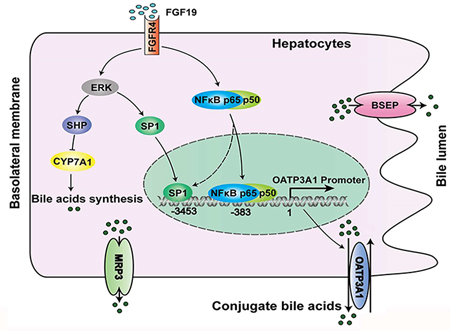
FGF19-ERK/NFkB signaling was activated in human cholestatic livers. (A) Serum level of FGF19 in control and cholestatic patients; (B) mRNA expression of FGF19, FGFR4, and β-Klotho in patient livers. (C) Representative western blots and corresponding densitometry of liver FGF19, FGFR4, and β-Klotho in patients. (D) Representative western blots and corresponding densitometry of phosphorylated and total ERK1/2 and NFkB p65 in patients (relative to the control group, n=21 for control group, n=22 for obstructive cholestatic group). *P<0.05 vs. control patients. (E) A proposed model for hepatic OATP3A1 expression regulation in human cholestasis. Increased plasma FGF19 activates ERK and NFkB signaling, which leads to the increased expression of the transcription factors SP1 and NFkB p65 and their binding activities to the SLCO3A1 promoter. The increased OATP3A1 expression eliminates conjugated bile acids in cholestatic hepatocytes. On the other hand, FGF19 also represses bile acid synthesis through the ERK/NFkB-SHP/CYP7A1 pathway. This dual safety mechanism would be effective to eliminate bile acids in cholestatic hepatocytes and thereby reduce liver injury.
Discussion
This study on the role of OATP3A1 in the liver under cholestasis has four major novel findings. (1) Hepatic OATP3A1 mRNA and protein levels were markedly elevated in both humans and rodents under cholestatic conditions (Fig.1 and Suppl.Figs.4&5). (2) Hepatic OATP3A1 was established as a novel bile acid efflux transporter to assist in eliminating excessive bile acids accumulation in response to cholestasis, as demonstrated by several lines of evidence, including initial assays for bile acid transport in hepatocytes (Fig.2), and a functional study with Slco3a1-KO mice (Suppl.Fig.1, Tables.1&S2). (3) Investigation into the regulatory mechanisms of OATP3A1 expression in human hepatocytes indicated that induction of OATP3A1 during cholestasis was mediated by activation of the FGF19-ERK/NFkB-SP1/p65 signaling pathway (Figs.3, 4&5), as further confirmed by analysis of human liver tissues from patients with obstructive cholestasis (Fig.6). (4) Genetic ablation of Slco3a1 led to increased accumulation of bile acids in the liver in cholestasis and aggravated liver injury (Suppl.Fig.1, Fig.2, and Tables.1&S2), indicating a critical role for this OATP in the protective adaptive response to cholestasis, as the liver attempts to restore bile acid homeostasis (Fig. 6E).
In recent years, it has been increasingly clear that OATPs play a key role in transporting multiple endogenous substances such as bile acids, bilirubin, hormone conjugates, and anti-cancer drugs, and that a majority of OATPs function as organic solute exchangers [2–6]. In human cholestasis, expression of the liver-specific proteins OATP1B1 (SLCO1B1 and OATP-C) and OATP1B3 (SLCO1B3 and OATP-8) were significantly decreased, which was thought to be an adaptive response to reduce bile acid accumulation in cholestatic hepatocytes since these transporters mediate bile acid uptake in hepatocytes [1, 16]. In contrast, we first report here that hepatic OATP3A1 expression is dramatically increased in cholestasis (Fig. 1), whereas it has a very low level of expression in the normal liver [3]. Adachi and colleagues previously demonstrated that OATP3A1 mediated neither taurocholate nor methotrexate uptake, two preferable substrates for OATP family members [3], indicating its distinct functional role in hepatocytes. In our present study, we demonstrated a markedly increased level of bile acids in liver tissues from Slco3a1-deficient mice compared to WT mice under cholestasis. LC-MS/MS analysis of these mouse liver samples further confirmed that conjugated bile acids (e.g. tauromuricholate acid, taurodeoxycholate acid, and taurocholic acid) were significantly increased in Slco3a1-KO mice, compared to WT mice under cholestasis (Table.1). Therefore, it was logical to speculate that OATP3A1 would be a novel bile acid efflux transporter. As expected, our data from bile acid uptake and trans-cellular transport of [3H]-taurocholate assays clearly supported the conclusion that OATP3A1 can eliminate conjugated bile acids from hepatocytes. Further studies demonstrated that the well-known up-regulated expression of bile acid efflux transporters Mrp2–4 and Ostα/β during cholestasis were insufficient to compensate for the loss of Oatp3a1 under cholestatic conditions (Table.1 and Figs.2A&C). These data imply that OATP3A1 exerts a crucial protective function in the cholestatic liver by accelerating the efflux of bile acids out of the liver into the blood. In addition, OATP3A1 is most abundantly expressed in the brain, heart and testis where the levels of bile acids are normally very low under normal conditions [2–5]. However, in cholestasis, bile acid levels are elevated not only in the liver but also in the blood and potentially other tissues. Therefore, it is possible that OATP3A1 could play a protective role in other tissues as well by facilitating the elimination of bile acids. In this study, we have demonstrated that OATP3A1 functions to efflux bile acids. Considering that OATPs are bidirectional transporters working as electroneutral exchangers [2, 6], it remains to be determined if there are endogenous hepatic uptake substrates for OATP3A1 in the cholestatic liver.
Since levels of serum total bile acids were elevated in 1%CA-fed Slco3a1-KO mice, one might argue that this contradicts the proposed function of OATP3A1/Oatp3a1 as a hepatic efflux bile acid transporter. If fewer hepatic bile acids re-enter the blood, one would expect lower serum bile acid levels in these mice. However: 1) the cholestatic liver injury was more severe in Slco3a1-KO mice with some animals dying prior to study (Table.1), for which bile acids had highly accumulated in the liver, and subsequently in the blood. Indeed, we observed higher levels of bile acids in the liver of these mice (Table.1); and 2) we found that OATP3A1 was also localized on the apical membrane of colonic epithelial cells (Suppl.Fig.9), supporting the hypothesis that intestinal Oatp3a1 may increase elimination of bile acids in the feces. Moreover, H&E staining and IHC labeling of CK19 in the 9-day Slco3a1-KO-BDL group demonstrated that bile duct injury, proliferation and inflammation were all increased when compared to the 9-day WT-BDL group (arrows, Suppl.Figs.10A&B), suggesting that OATP3A1 might also play a role in modulating cholangiocyte injury in cholestasis. However, future studies are needed to evaluate this hypothesis.
Serum FGF19 levels are elevated in some forms of human cholestasis [10, 11], and we also observed increased FGF19 levels in our cholestatic patients and mice (Figs.6A-C and Suppl.Fig.5E). Most recently, FGF19 has emerged as a potential target for treating cholestasis by repressing CYP7A1 expression and the size of the bile acid pools [12, 13]. However, mechanistic details are poorly understood. A previous study has shown that FGF19 suppresses CYP7A1 expression along with activation of ERK signaling [14]. Here, we identified a novel mechanism where ERK/NFκB signaling contributed to FGF19-mediated SHP and CYP7A1 expression. It is intriguing that FGF19-ERK/NFκB-SP1/p65 pathway induced the expression of the novel bile acid efflux transporter OATP3A1 in hepatocytes. Oatp3a1 deficiency increased cholestatic liver injury that is likely due to excessive intrahepatic bile acid accumulation (Table.1). Therefore, the increased expression of OATP3A1 may be viewed as an adaptive response to cholestasis, to restore bile acid homeostasis. In our previous study, we found that expression of the basolateral adaptive overflow transporter MRP3 was also significantly increased in the liver of cholestatic patients [21]. Indeed, we demonstrated that FGF19 stimulated MRP3 expression in primary human hepatocytes and PLC/PRF/5 hepatoma cells (data not shown). Together, elevated serum FGF19 in cholestasis not only suppresses bile acids synthesis by repressing the expression of the rate limiting enzyme CYP7A1, but also enhances bile acid elimination by inducing bile acid efflux transporter expression, resulting in the decrease of bile acid accumulation in cholestatic hepatocytes. Together, our findings have provided potential mechanisms for the SLCO3A1 gene regulation and aided in understating the therapeutic effects of FGF19 in cholestasis.
In conclusion, we have found that OATP3A1 functions as a bile acid efflux transporter, and that its expression is up-regulated in cholestatic liver through the FGF19-ERK/NFkB-SP1/p65 signaling pathways, suggesting the functional significance of facilitating the removal of excessive intracellular bile acids and protecting the liver from cholestasis. Thus, we speculate that over-expression of OATP3A1 could be a new therapeutic approach to prevent or ameliorate cholestasis-associated liver injury and other serious liver disease.
Supplementary Material
Figure 2.
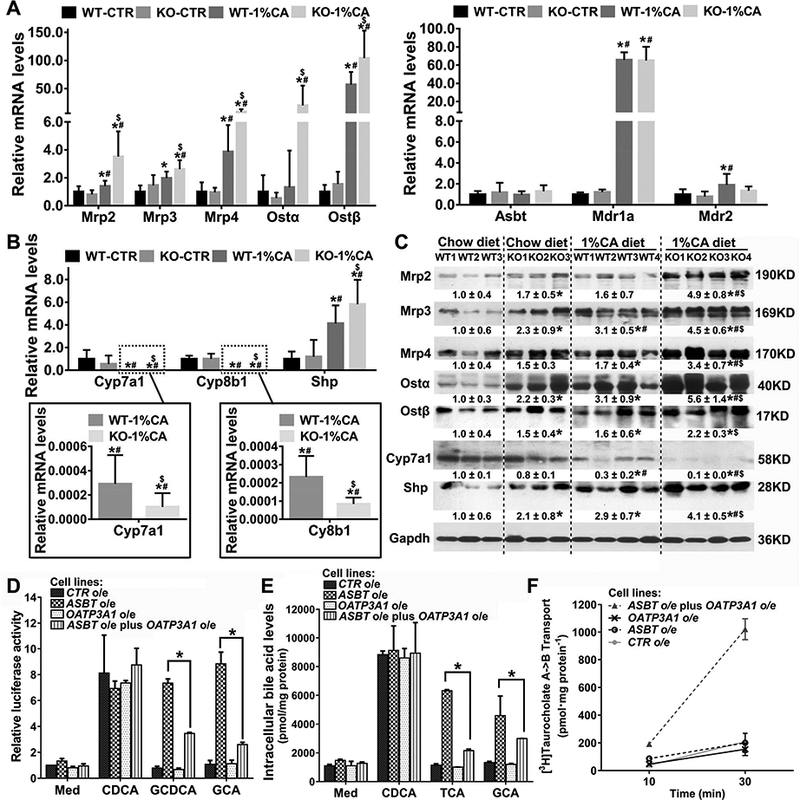
OATP3A1 was identified as a bile acid efflux transporter in hepatocytes. Hepatic mRNA levels (A) Mrp2, Mrp3, Mrp4, Ostα/β, Asbt, Mdr1a, and Mdr2; and(B) Cyp7a1, Cyp8b1 and Shp. (C) Representative western blots for Mrp2, Mrp3, Mrp4, Ostα/β, Cyp7a1, and Shp protein expression. WT-CTR, chow diet wild-type group (n=5); KO-CTR, chow diet Slco3a1-KO group (n=5); WT-1%CA, 1%CA-fed wild-type group (n=9); KO-1%CA, 1%CA-fed Slco3a1-KO group (n=7). *P<0.05 VS chow diet WT mice; #P<0.05 VS chow diet Slco3a1-KO mice; $P<0.05 VS 1%CA diet WT mice. (D) OATP3A1-FXR-I-BABP luciferase reporter assay for bile salt transport. The phRL-CMV, human FXR, human RXRα, and pGL3-human I-BABP constructs were transiently transfected into four stably transfected PLC/PRF/5 cell lines (-CTR, -ASBT, -OATP3A1, and -ASBT plus OATP3A1). After 24 hours, these cell lines were treated with the unconjugated bile acid chenodeoxycholic acid (CDCA) and conjugated bile acids glycochenodexycholate acid (GCDCA), and glycocholate acid (GCA) (25μM) for 12 hours. CDCA treatment was used as a positive control. The cells were lysed for the dual-luciferase reporter assay. *P<0.05, n=4. (E) The above four stably transfected cell lines were cultured in a T-75 cell culture flask. After the cell density reached ~90%, cells were treated with the unconjugated bile acid CDCA and conjugated bile acids taurocholic acid (TCA) and glycocholate acid (GCA) (100μM) for 12 hours. CDCA treatment was used as a positive control. The cells were quickly washed with cold PBS three times, and collected for ultrasonication. The cell lysates were sent to determine bile acid concentration. *P<0.05, n=4. (F) Trans-cellular transport of [3H] taurocholate in stably transfected MDCK cell lines (-CTR, -ASBT, -OATP3A1 and -ASBT plus OATP3A1) were performed as reported previously [20]. Values are presented as mean± SD, n=4.
Acknowledgements
We thank Prof. Cheng Qian, Dr. Limei Liu (Southwest Hospital, China), Ms Wenjing Yu, Dr. Xinchan Feng, Dr. Haiwei Feng, Dr. Tingting Wu (Cholestatic Liver Diseases Center, Southwest Hospital), Dr. Ruilin Sun (Shanghai Model Organisms Center, Inc., China), and Lina Zhou (Dalian Institute of Chemical Physics, Chinese Academy of Sciences, China) for technical assistance. We thank the Gastroenterology’s Science Editor Dr. Kristine Novak for editing of the abstract and title. We also thank Dr. Xinshou Ouyang (Department of Internal Medicine, Yale University School of Medicine) for gifting NFκB p65 and p50 constructs.
Grants Support:
This work was supported by National Natural Science Foundation of China (81470880, 81770583, 81672901, and 81570576), Natural Science Foundation of Chongqing (cstc2016jcyjA0149), Southwest Hospital (SWH2016YQFY-01) and Third Military Medical University (2017YQRC-01) Science Foundation of Outstanding Youth, and USPHS R37 DK 025636. S-YC and JLB were supported by NIH grants DK34989 and DK25636 (Yale Liver Center).
Abbreviations:
- OATP
organic anion-transporting polypeptide
- FGF19
fibroblast growth factor 19
- MRP3
multidrug resistance protein 3
- CYP7A1
cholesterol 7-alpha hydroxy-lase
- IF
immunofluorescence
- IHC
immunohistochemistry
- UDCA
ursodeoxycholic acid
- Slco3a1-KO mice
Slco3a1 knockout mice
- ChIP
chromatin Immunoprecipitation
Footnotes
Conflict of Interests
The authors disclose no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wagner M, Trauner M Recent advances in understanding and managing cholestasis.F1000Res 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol 2012;165(5):1260–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi H, Suzuki T, Abe M, et al. Molecular characterization of human and rat organic anion transporter OATP-D. Am J Physiol Renal Physiol 2003;285:F1188–1197. [DOI] [PubMed] [Google Scholar]
- 4.Huber RD, Gao B, Sidler Pfändler MA, et al. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am J. Physiol Cell Physiol 2007;292:C795–806. [DOI] [PubMed] [Google Scholar]
- 5.Kindla J, Rau TT, Jung R, et al. Expression and localization of the uptake transporters OATP2B1, OATP3A1 and OATP5A1 in non-malignant and malignant breast tissue. Cancer Biol Ther 2011;11:584–591. [DOI] [PubMed] [Google Scholar]
- 6.Mahagita C, Grassl SM, Piyachaturawat P, et al. Human organic anion transporter 1B1 and 1B3 function as bidirectional carriers and do not mediate GSH-bile acid cotransport. Am J Physiol Gastrointest Liver Physiol 2007;293:G271–278. [DOI] [PubMed] [Google Scholar]
- 7.Wei SC, Tan YY, Weng MT, et al. SLCO3A1, A novel crohn’s disease-associated gene, regulates nf-kappaB activity and associates with intestinal perforation. PLoS One 2014;9:e100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2005;2:217–225. [DOI] [PubMed] [Google Scholar]
- 9.Zweers SJ, Booij KA, Komuta M, et al. The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in theenterobiliary tract. Hepatology 2012;55:575–83. [DOI] [PubMed] [Google Scholar]
- 10.Schaap FG, van der Gaag NA, Gouma DJ, et al. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 2009;49:1228–1235. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Lin B, Lin G, et al. Circulating FGF19 closely correlates with bile acid synthesis and cholestasis in patients with primary biliary cirrhosis. PLoS One 2017;12:e0178580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou M, Learned RM, Rossi SJ, et al. Engineered fibroblast growth factor19 reduces liver injury and resolves sclerosing cholangitis in Mdr2-deficientmice. Hepatology 2016;63:914–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo J, Ko B, Elliott M, et al. A nontumorigenic variant of FGF19 treatscholestatic liver diseases. Sci Transl Med 2014;6:247ra100. [DOI] [PubMed] [Google Scholar]
- 14.Kir S, Beddow SA, Samuel VT, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 2011;331:1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng Y, Zhao H, Gao L, et al. FGF19 Protects Hepatocellular Carcinoma Cells against Endoplasmic Reticulum Stress via Activation of FGFR4-GSK3beta-Nrf2 Signaling. Cancer Res 2017;77:6215–6225. [DOI] [PubMed] [Google Scholar]
- 16.Boyer JL. Nuclear receptor ligands: rational and effective therapy for chronic cholestatic liver disease? Gastroenterology 2005;129:735–740. [DOI] [PubMed] [Google Scholar]
- 17.Galman C, Arvidsson I, Angelin B, et al. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. J lipid Res 2003;44(4):859–866. [DOI] [PubMed] [Google Scholar]
- 18.Cai SY, Ouyang X, Chen Y, et al. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight 2017;2:e90780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai SY, He H, Nguyen T, et al. Retinoic acid represses CYP7A1 expression in human hepatocytes and HepG2 cells by FXR/RXR-dependent and independent mechanisms. J Lipid Res 2010;51:2265–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawson PA, Hubbert M, Haywood J, et al. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem 2005;280:6960–6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chai J, He Y, Cai SY, et al. Elevated hepatic multidrug resistance-associated protein 3/ATP-binding cassette subfamily C 3 expression in human obstructive cholestasis is mediated through tumor necrosis factor alpha and c-Jun NH2-terminal kinase/stress-activated protein kinase-signaling pathway. Hepatology 2012;55:1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


