Abstract.
Heartland virus (HRTV) is a North American phlebovirus suspected to be transmitted by the lone star tick Amblyomma americanum. White-tailed deer (WTD) have been shown to develop HRTV-neutralizing antibodies following experimental infection. To further define the geographic distribution of HRTV through retrospective sampling of WTD, sera from the WTD herd health serum archive at the Southeastern Cooperative Wildlife Disease Study between 2001 and 2015 were analyzed using serum neutralization. Of 783 serum samples tested, 57 (7.3%) were positive for HRTV-neutralizing antibodies. Deer with moderate to heavy tick burdens were more likely seropositive. Seropositive samples were obtained from deer originating from states with documented human cases of HRTV-associated disease. Seropositive samples were identified from years before the recognition of the first human case in 2009. Overall, this study indicates that WTD in the southeastern United States have been exposed to HRTV as early as 2001 and that the presence of seropositive animals corresponds roughly with reported human HRTV-associated disease.
Heartland virus (HRTV; family Phenuiviridae, genus Phlebovirus) was first recognized in 2009 as a cause of febrile disease with thrombocytopenia and leukopenia that has since been documented in 30 people, including at least one fatal case. Human disease has been reported in nine states, mainly in the Midwestern and southern United States.1–4 The virus is most likely transmitted by the lone star tick Amblyomma americanum and has been isolated from wild-caught ticks in the vicinity of the first reported human cases,5,6 as well as experimentally transmitted by co-feeding between A. americanum ticks.7 Amblyomma americanum has a vast and expanding geographical range including 39 states and the District of Columbia, predominantly in the southeastern states (as far west as Texas), and extending north through the mid-Atlantic and New England regions.8
Heartland virus–reactive antibodies have been detected in serum samples of free-ranging white-tailed deer (WTD) (Odocoileus virginianus), in the central and eastern United States.9,10 White-tailed deer have recently been experimentally infected, and although they did not become viremic or shed virus, they did develop neutralizing antibody titers or increases in existing titers.11 White-tailed deer are also known to be a host for A. americanum12 and could act as an important host for maintaining the tick vector where virus can be transmitted between arthropods by co-feeding.
Although current evidence indicates that HRTV is likely maintained within the tick population without involvement of a mammalian host,7 seropositive animals may be useful as sentinel indicators of the presence of HRTV in an area.9,10 The Southeastern Cooperative Wildlife Disease Study (University of Georgia) has maintained an archive of free-ranging WTD serum collected during herd health evaluations from approximately 150 sampling sites throughout the southeastern United States. While previous retrospective serosurveys for HRTV-reactive antibodies have investigated serum samples from as far back as 2009,9 the present study evaluates older archived samples from 2001 to 2015. The objectives of the present study were to 1) determine whether there are HRTV-seropositive WTD in the southeastern United States, 2) determine when and where neutralizing antibodies first appeared relative to the recognition of human disease, and 3) associate the presence of neutralizing antibodies to the presence of ticks.
Between 1 and 10 individual WTD serum samples were available from each sampling location and year, which was recorded by county or parish, state, and date. Serum samples were thawed and diluted 1:10 in virus media comprising Minimum Essential Medium™ (MEM; Sigma-Aldrich, Darmstadt, Germany) with 3% bovine serum albumin, and 5% antibiotics and antimycotics (virus media). The samples were then heat-inactivated at 56°C for 45 minutes, then challenged with 100 tissue culture infectious dose (TCID50) of HRTV suspension for 1 hour at 37°C to screen at a 1:20 dilution. Wells were then seeded with Vero E6 cells and incubated at 37°C for 7 days. The samples were considered positive if there was > 50% neutralization. Samples testing positive for neutralizing antibodies at 1:20 were titrated in seven 2-fold serial dilutions, beginning with a 1:4 dilution and challenged with virus as described previously. Serum from an HRTV-experimentally infected fawn was used as a positive control. Samples with titers ≥ 16 were considered seropositive based on previous experimental infections.11
In an effort to determine the specificity of this assay, a subset of 15 HRTV-neutralizing antibody–positive samples and 15 negative samples were also evaluated for neutralizing antibody against MP-12, a live attenuated vaccine strain of the Rift Valley fever virus (family Phenuiviridae, genus Phlebovirus).13 Serial 2-fold dilutions of selected samples were made, and each well was challenged with 100 TCID50 mutagenesis passage (MP)-12 as described previously. None of the samples tested had evidence of MP-12–neutralizing antibodies. A previous study has evaluated the cross-reactivity of HRTV antibodies with other known phleboviruses in the United States, including Sunday Canyon virus, Rio Grande virus, and Lone Star virus, and no significant cross-reactivity was reported.9
A total of 783 serum samples were available for evaluation. Between 29 and 83 samples were available from each year, with an average of 52 (standard deviation [SD] = 18) (Supplemental Table 1, Figure 1). Gender, age, body condition, and tick burdens of all deer screened were compared with those of deer that had HRTV-neutralizing antibodies (Table 1). Fifty-seven (7.3%) serum samples were positive for HRTV-neutralizing antibodies (Table 2). Of all collected sera from males, 6.6% were seropositive and 7.7% of all collected sera from females were seropositive. The average age of a seropositive animal was 2.86 years (SD = 1.46), and the average age of seronegative deer was 2.70 years (SD = 1.55) (no significant difference). The 2.6–3.5 age group had the highest percentage of seropositive deer (11.6%). Of all deer with poor body condition, 10.3% were seropositive, compared with 6.7% of deer in fair condition, 6.3% of deer in good condition, and 6.9% of deer in excellent condition. Seropositive deer were 6.25 times more likely to have a heavy tick burden than negative deer (95% confidence interval [CI]: 2.38–17.14, P = 0.0004) and 7.09 times more likely to have a moderate tick burden than negative deer (95% CI: 3.75–13.4, P < 0.0001). Tick species were not noted in most of the reports assessed, but given the geographic areas and the season of sampling (July through October), A. americanum is most likely.
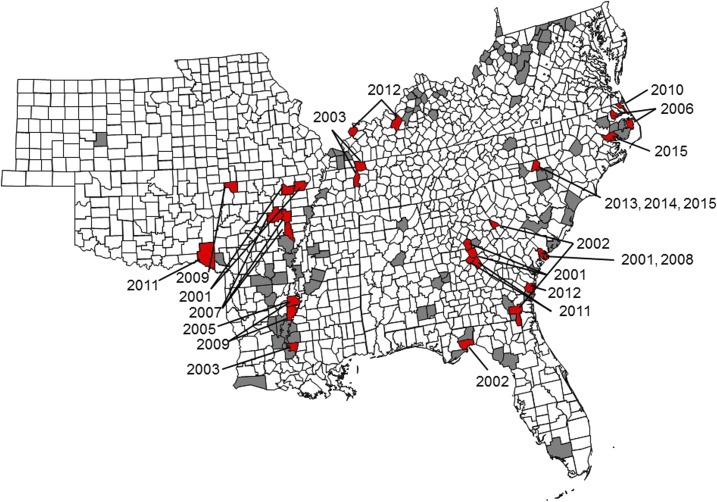
Figure 1.
County-level distribution of where white-tailed deer serum samples were collected and where positive samples were found. Gray counties had serum samples collected but none were positive. Red counties had at least one positive sample, with the year that positive sample was collected indicated. White counties were not tested. This figure appears in color at www.ajtmh.org.
Table 1.
Summary of signalment data, body condition, and tick burdens from all deer tested via serum neutralization and deer with Heartland virus–neutralizing antibodies
| No. tested (%) | No. seropositive (%) | |
|---|---|---|
| Total | 783 | 57 |
| Gender | ||
| Male | 165 (21.1) | 11 (19.3) |
| Female | 601 (76.8) | 46 (80.7) |
| Unknown/Unreported | 17 (2.1) | 0 (0) |
| Age, year | ||
| ≤ 0.5 | 8 (1.0) | 0 (0) |
| 0.6–1.5 | 246 (31.4) | 13 (22.8) |
| 1.6–2.5 | 180 (23.0) | 13 (22.8) |
| 2.6–3.5 | 147 (18.8) | 17 (29.8) |
| 3.6–4.5 | 90 (11.5) | 8 (14) |
| 4.6–5.5 | 42 (5.4) | 1 (1.8) |
| ≥ 5.6 | 50 (6.4) | 5 (8.8) |
| Unknown/Unreported | 20 (2.5) | 0 (0) |
| Body condition | ||
| Poor | 87 (11.1) | 9 (15.8) |
| Fair | 462 (59.0) | 31 (54.4) |
| Good | 159 (20.3) | 10 (17.5) |
| Excellent | 29 (3.7) | 2 (3.5) |
| Unknown/Unreported | 46 (5.9) | 5 (8.8) |
| Tick burden | ||
| No ticks | 349 (44.6) | 2 (3.5) |
| Light | 332 (42.4) | 31 (54.4) |
| Moderate | 61 (7.8) | 18 (31.6) |
| Heavy | 19 (2.4) | 6 (10.5) |
| Unknown/Unreported | 22 (2.8) | 0 (0) |
Table 2.
Counties with Heartland virus–neutralizing antibody–positive samples, including the year samples were collected and the total number of samples tested for each time point
| County or parish, state | Year | No. tested | No. (%) seropositive |
|---|---|---|---|
| Greene, AR | 2001 | 5 | 2 (40) |
| Lawrence, AR | 2001 | 6 | 1 (17) |
| Woodruff/Monroe, AR | 2007 | 11 | 2 (18) |
| White, AR | 2007 | 5 | 1 (20) |
| Carroll, AR | 2009 | 5 | 1 (20) |
| Wakulla, FL | 2002 | 10 | 4 (40) |
| Jones/Jasper, GA | 2001 | 5 | 2 (40) |
| Lincoln, GA | 2002 | 5 | 1 (20) |
| Charleton, GA | 2002 | 5 | 1 (20) |
| Bibb/Twiggs, GA | 2011 | 5 | 3 (60) |
| McIntosh, GA | 2012 | 6 | 1 (17) |
| Glynn, GA | 2012 | 6 | 2 (33) |
| Union, KY | 2012 | 6 | 3 (50) |
| Harden, KY | 2012 | 5 | 2 (40) |
| West Feliciana, LA | 2003 | 5 | 1 (20) |
| Madison, LA | 2005 | 10 | 2 (20) |
| Madison/Tensas, LA | 2009 | 10 | 3 (30) |
| Dare, NC | 2006 | 5 | 3 (60) |
| Perquimans, NC | 2006 | 5 | 1 (20) |
| Currituck, NC | 2010 | 11 | 3 (27) |
| Stanly, NC | 2013 | 10 | 3 (30) |
| 2014 | 3 | 1 (33) | |
| 2015 | 5 | 2 (40) | |
| Beaufort, NC | 2015 | 5 | 3 (60) |
| McCurtain, OK | 2011 | 5 | 2 (40) |
| Beaufort, SC | 2001 | 5 | 1 (20) |
| 2008 | 5 | 2 (40) | |
| Benton, TN | 2003 | 5 | 2 (40) |
| Stewart, TN | 2003 | 5 | 2 (40) |
Seropositive samples were collected every year except 2004. The year with the highest percentage (19.5%) and largest number (8) of positive samples collected was in 2012. Titers ranged from 16 to 128. Positive samples were from counties or parishes in nine states (Figure 1). Thirty-two positive samples were collected between 2001 and 2009. Positive counties or parishes often had clustering of 40–60% positive out of deer sampled and neighboring counties or parishes frequently had seropositive samples (Figure 1). Although the interval between sampling was variable, 33 counties were sampled in more than 1 year (Supplemental Table 2).
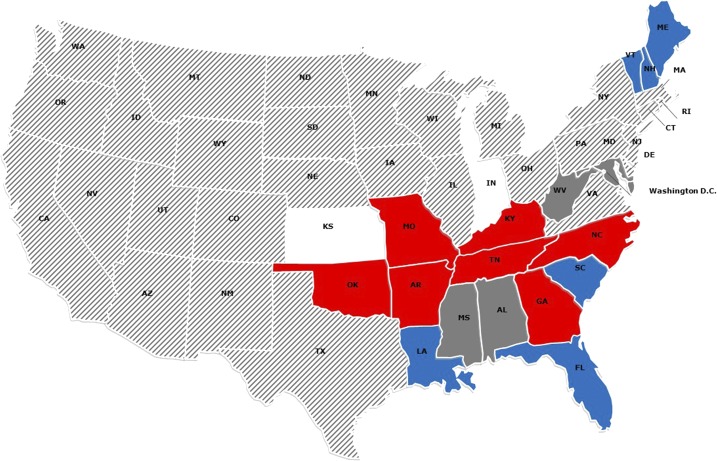
According to the Centers for Disease Control and Prevention,4 human cases of HRTV infection and associated disease have occurred in Arkansas, Georgia, Indiana, Kansas, Kentucky, Missouri, North Carolina, Oklahoma, and Tennessee (Figure 2). Indiana and Missouri were not represented in our sample set, and only five samples from 1 year were available from Kansas. All other states with reported human HRTV infection had at least one seropositive animal. There were three states with seropositive deer and no currently reported human cases of HRTV infection (Louisiana, Florida, and South Carolina). A previous serosurvey similarly found that HRTV-neutralizing antibody–positive animals were present in states as of yet without human cases.9 It is likely, and predicted,4 that there are more cases of human exposure that go unrecognized, due in part to the nonspecific symptoms of disease in humans.
Figure 2.
Correlation between detected Heartland virus (HRTV)–neutralizing antibody seropositive deer in the present study and reported literature8,9 and reported human cases of human HRTV infections.4 Red states have reported human cases of Heartland infection with seropositive deer. Blue states have no reported human cases but do have reported seropositive deer. White states have reported human cases but no reported seropositive deer. Dark gray states have no reported human cases and no reported seropositive deer. Hash-marked states have not reported human cases and have not been tested for seropositive deer. This figure appears in color at www.ajtmh.org.
Given the sporadic geographic and temporal method of sampling and the retrospective nature of this study, there are some limitations in interpreting the dynamics of HRTV spread in the southeastern United States. There is evidence, however, of exposure to HRTV or a serologically cross-reactive virus in WTD as much as 8 years before the first recognized human case in 2009. The lack of cross-reactivity seen in the related Phlebovirus (MP-12) challenge, as well as previous cross-reactivity assessments,9 suggests that serum neutralization is a specific test for HRTV. Ecological factors that affect tick populations, such as temperature, humidity, foliage types, and other mammalian hosts, may influence where WTD are likely to be exposed to HRTV, leading to clustering of positive samples. Biases may also be present in which deer were sampled because there were significantly more females than males represented and more deer over 1 year of age. More consistent sampling of WTD for HRTV-neutralizing antibodies may elucidate other factors of viral ecology and transmission and may better characterize the magnitude and duration of the humoral response in WTD.
There was a significant association with moderate and heavy tick burdens and HRTV seropositivity, which is consistent with other evidence of A. americanum being the primary vector for HRTV.5–7 In addition, HRTV-exposed deer have been detected in most of the states where human cases of HRTV-associated disease have been documented. The presence of seropositive and tick-laden WTD in an area may be another useful indicator for the presence or increased circulation of HRTV in a given area, alerting human health providers and specialists to the potential of human HRTV infections.
Supplementary Material
Acknowledgments:
We thank the SCWDS submitters for providing case materials, and the SCWDS diagnosticians and students for providing necropsy reports. We also thank David Stallknecht for his advice, and John Wlodkowski and Michelle Willis for laboratory assistance.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1.McMullan LK, et al. 2012. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med 367: 834–841. [DOI] [PubMed] [Google Scholar]
- 2.Pastula DM, Turabelidze G, Yates KF, Jones TF, Lambert AJ, Panella AJ, Kosoy OI, Velez JO, Fischer M, Staples JE; Centers for Disease Control and Prevention (CDC) , 2014. Notes from the field: Heartland virus disease-United States 2012–2013. MMWR Morb Mortal Wkly Rep 63: 270–271. [PMC free article] [PubMed] [Google Scholar]
- 3.Muehlenbachs A, et al. 2014. Heartland virus-associated death in Tennessee. Clin Infect Dis 59: 845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control , 2017. Heartland Virus Available at: https://www.cdc.gov/heartland-virus/index.html. Accessed September 6, 2017.
- 5.Savage HM, Godsey MS, Jr., Lambert A, Panella NA, Burkhalter KL, Harmon JR, Lash RR, Ashley DC, Nicholson WL, 2013. First detection of Heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg 89: 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savage HM, Godsey MS, Jr., Panella NA, Burkhalter KL, Ashley DC, Lash RR, Ramsay B, Patterson T, Nicholson WL, 2016. Surveillance for Heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae). J Med Entomol 53: 607–612. [DOI] [PubMed] [Google Scholar]
- 7.Godsey MS, Jr., Savage HM, Burkhalter KL, Bosco-Lauth AM, Delorey MJ, 2016. Transmission of Heartland virus (Bunyaviridae: Phlebovirus) by experimentally infected Amblyomma americanum (Acari: Ixodidae). J Med Entomol 52: 1226–1233. [DOI] [PubMed] [Google Scholar]
- 8.Springer YP, Eisen L, Beati L, James AM, Eisen RJ, 2014. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol 51: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riemersma KK, Komar N, 2015. Heartland virus neutralizing antibodies in vertebrate wildlife, United States, 2009–2014. Emerg Infect Dis 21: 1830–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosco-Lauth AM, et al. 2015. Serological investigation of Heartland virus (Bunyaviridae: Phlebovirus) exposure in wild and domestic animal adjacent to human case sites in Missouri 2012–2013. Am J Trop Med Hyg 92: 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke LL, Ruder MG, Mead D, Howerth EW, 2018. Experimental infection of white-tailed deer (Odocoileus virginanus) with Heartland virus. Am J Trop Med Hyg 98: 1194–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kollars TM, Durden LA, Masters EJ, Oliver JH, 1997. Some factors affecting infestation of white-tailed deer by blacklegged ticks and winter ticks (Acari: Ixodidae) in southeastern Missouri. J Med Entomol 34: 372–375. [DOI] [PubMed] [Google Scholar]
- 13.Ikegami T, Hill TE, Smith JK, Zhang L, Juelich TL, Gong B, Slack OAL, Ly HJ, Lokugamage N, Freiberg AN, 2015. Rift valley fever virus MP-12 vaccine is fully attenuated by a combination of partial attenuations in the S, M, and L segments. J Virol 89: 7262–7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.