Abstract.
Fasciola hepatica is the most widely distributed trematode-affecting humans. The Andes Mountains are highly endemic for fascioliasis. We report results of a cross-sectional study evaluating the epidemiology of Fasciola among children in 26 agricultural communities in the Cusco region of Peru. Children 3 to 16 years old were enrolled in preschools and schools. Blood from participants was tested for complete blood counts, transaminases, and Fasciola antibodies. Stool samples were tested for Fasciola and other parasites. A total of 2,515 children were included in the analysis and the mean age was 9.6 years (±3.6). Ten percent (253) of the children had at least one positive test for Fasciola, 6% had chronic infection, and 0.4% acute infection. The rest of the subjects had only antibodies against Fasciola. The prevalence of infection varied from 0% to 20% between communities. Children with evidence of Fasciola exposure were older, lived at higher altitudes, and had a lower socioeconomic status than children without infection. The logistic regression analysis showed that children from Ancahuasi district, older children, and children with higher measures of poverty were more likely to have Fasciola exposure. Fascioliasis is common in the Cusco region and associated with poverty. However, the distribution varies markedly between communities.
INTRODUCTION
Fascioliasis is a zoonotic parasitic infection of humans caused by the trematodes Fasciola hepatica and Fasciola gigantica.1 These parasites have complex clinical presentations reflecting the different stages in the parasite’s life cycle. After the ingestion of the infectious metacercariae on water plants or in water, juvenile flukes are released, cross the intestine, migrate through the abdominal cavity, and then burrow through the liver parenchyma into the biliary tract. The migratory stage of the parasite causes acute fascioliasis characterized by fever, right upper quadrant pain, and eosinophilia. Juvenile flukes reach sexual maturity and become adults only after reaching the biliary tree. Adult flukes in the biliary tree cause obstructive symptoms such as biliary colic.2 Most infections in the community remain undiagnosed. However, complications such as anemia and undernutrition may arise even in subjects with subclinical fascioliasis with devastating consequences for infected children.3–5 Large community-based studies evaluating the epidemiology, symptoms, and health consequences of fascioliasis are required for an accurate estimation of the burden of disease in endemic areas.
Fasciola hepatica is reported from more than 81 countries.6,7 The burden of Fasciola is increasing, especially in Southeast Asia. However, half of the human infections occur in the Andean countries of South America.8,9 The highest recorded human prevalence (70%) and intensity of infection (5,000 eggs/gram of stool) were reported at very high altitudes in the northern Bolivian altiplano.9 Studies from the northern Peruvian Andes have shown a correlation between the prevalence of Fasciola infection among children and the altitude of their communities.10 Other environmental conditions including man-made modifications can have an impact on the prevalence of fascioliasis. Esteban et al.11 described a high prevalence of Fasciola infection among children living in an area where an artificial irrigation system caused flooding and oversaturation of agricultural land. Demographic and socioeconomic factors tend to be influenced by the environment and are intimately associated with fascioliasis in developing countries. El-Sanh showed that children from mothers with Fasciola were three times more likely to be infected than children from negative mothers.12 Cabada et al.13 reported that socioeconomic markers such as number of siblings and years of education of the father were associated with a higher prevalence of Fasciola infection among children in the Cusco region. Even in highly endemic areas, the distribution can vary markedly between adjacent communities as documented in a few large community studies.9,14 Estimates of the burden of disease based on small surveys may be misleading.3 To better characterize the epidemiology of Fasciola infection, we conducted a large community-based study evaluating the epidemiology of fascioliasis among preschool and school-age children in agricultural communities in the highlands of the Cusco region in Peru.
MATERIALS AND METHODS
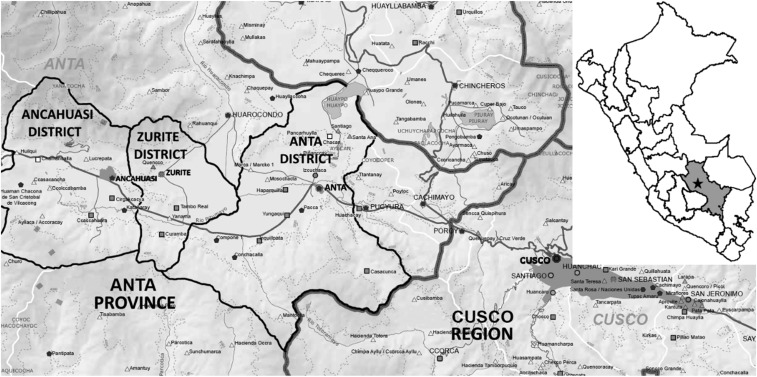
We conducted a cross-sectional study among children in 26 communities of the Anta Province in the Cusco region of Peru (Figure 1). Participants were from rural agricultural communities located on a plateau comprising the Ancahuasi, Anta, and Zurite districts. The elevation of the plateau is around 3,350 m and the rainfall exceeds 77 mm from November to March and is less than 49 mm from April to October. Most of the population in the Anta Province (65%) lives in rural areas. The literacy rate is 80% and between 27% and 34% live under the poverty line.15,16 Twelve health posts in the area provide basic health services to a population of 56,300. Infectious and parasitic diseases are among the main causes of morbidity and mortality.17 School-based soil-transmitted helminths and anemia programs provide preemptive treatment to children in the region.
Figure 1.
Map of the Anta Province showing the three districts and major preschool and schools where the children were enrolled. Modified from Direccion Regional de Educacion del Cusco—Escale (http://escale.minedu.gob.pe/documents/10156/1367931/DRE_Cusco.pdf).
All children between ages 3 and 16 years attending preschools and schools in the three districts were contacted between August of 2013 and August of 2017. Children were enrolled if their parents provided signed consent for their participation and the child provided assent. Children and their parents were interviewed at the preschool or school on enrollment and at home within a month to collect demographic, socioeconomic, past medical history, nutrition, and environmental information. The socioeconomic status was evaluated using the Simple Poverty Scorecard (Microfinance Risk Management LLC) validated for Peru,18 which measures the probability of living under different poverty line for individuals in a given household. The height for age z-scores (HAZ) were calculated following WHO recommendations. One blood sample and three stool samples were requested from each participant. Blood samples were tested for complete blood count, transaminases, and Fas2 enzyme-linked immunosorbent assay (ELISA) (Bionoma SRL, Lima, Peru) for F. hepatica antibodies. Containers and instructions for stool sample collection were provided to each child or parent to collect three specimens on consecutive days. Portions of each stool sample were aliquoted without preservation solution and in 10% formalin. Aliquots without preservation solution were tested by the Kato–Katz test within 24–48 hours of collection. The Lumbreras rapid sedimentation test was performed in formalin-preserved stool samples within a week. All positive results were corroborated by a second observer. Mock positive and negative stool samples were included in the laboratory routine for quality control. All blood and stool samples were processed and tested at the laboratories of the Cusco Branch of Alexander von Humboldt Tropical Medicine Institute of Universidad Peruana Cayetano Heredia.
Statistical analysis was performed using the Statistical Package for the Social Sciences version 23.0 (SPSS IBM Corp., New York, NY). Fasciola exposure was defined as having one or more microscopic or serologic tests positive for Fasciola. Acute fascioliasis was defined as having a positive Fas2 ELISA with eosinophilia greater than 500 cell/μL and abnormally elevated aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) enzymes. Chronic fascioliasis was defined as having any stool test positive for the presence of F. hepatica eggs, regardless of serology. The arithmetic mean of the Kato–Katz test of all stool samples tested for each subject was considered as the number of eggs per grams of stool or intensity of infection. Frequencies, means (±standard deviation [SD]), and medians with interquartile range (IQR) were used to describe the distribution of the variables. The t-test or χ2 test as appropriate was used to compare variables associated with Fasciola exposure. For multivariate analysis, Fasciola exposure was used as the dependent variable and relevant variables found to have a P value ≤ 0.1 in t-test or χ2 test were included as independent variables in backward logistic regression analysis. Independent variables entered in the model included district, age, gender, history of treatment of malnutrition, years of education for the father, years of education for the mother, > 34.4% probability of living in poverty, construction material of the home, stunting, anemia, elevation of the house, and drinking water from irrigation channel. The tests were considered statistically significant if the P value was less than 0.05.
This study was reviewed by the Institutional Ethics Committee of Universidad Peruana Cayetano Heredia and the University of Texas Medical Branch. Informed consent was obtained from the children’s guardians. The consent process was performed in Quechua or Spanish according to subject’s preference. Children older than 6 years were included only after they had provided verbal assent.
RESULTS
The total child population in the 26 communities enrolled in preschool and schools at the time of the study was 4,424 and consent and assent for enrollment was obtained from 2,577 (58.2%). Sixty-two children were excluded because of failure to meet the inclusion criteria, missing all stool, or blood samples; 2,515 children were included in the analysis. The mean age of the participants was 9.6 years (±3.6), and 49.9% (1,254/2,515) were female. Almost half of the participants were enrolled in Ancahuasi district (45.5%, 1,145/2,515) and in elementary schools (51.4%, 1,288/2,505) (Table 1). Most houses were constructed with adobe (94.4%, 2,321/2,459) and had running water supplied, at least a few hours a day, by a community municipal reservoir piped into homes (98.6%, 2,416/2,451). Homes were often connected to the municipal sewage system (37.6%, 928/2,471), but a number of subjects defecated in open fields (21.1%, 522/2,471) or simple pits near the home (17.8%, 441/2,471).
Table 1.
Demographic and socioeconomic characteristics of the study participants
| Characteristic | Category | n | % |
|---|---|---|---|
| Gender | Female | 1,254 | 49.9 |
| Male | 1,261 | 50.1 | |
| Age (years) | Mean (±SD) | 9.6 (±3.6) | |
| District | Ancahuasi | 1,145 | 45.5 |
| Anta | 1,064 | 42.3 | |
| Zurite | 306 | 12.2 | |
| School grade | Preschool | 559 | 22.3 |
| Elementary | 1,288 | 51.4 | |
| High school | 658 | 26.3 | |
| Construction material of the house | Adobe | 2,321 | 94.4 |
| Brick | 134 | 5.4 | |
| Other | 4 | 0.2 | |
| Reported previous treatment of anemia | Yes | 154 | 6.4 |
| No | 2,242 | 93.6 | |
| Reported previous treatment of malnutrition | Yes | 105 | 4.4 |
| No | 2,289 | 95.6 | |
| Reported previous treatment of parasites | Yes | 338 | 14.2 |
| No | 2,050 | 85.8 | |
| Father’s years of education | Median (IQR) | 8 (IQR 6–11) | |
| Mother’s years of education | Median (IQR) | 6 (IQR 3–10) | |
IQR = interquartile range.
The median household monthly income was US$100 (IQR: 66.6–200). Most of the households (58.8%, 1,204/2,045) had a probability of living with less than US$3.25 a day of 34.4% according to the Simple Poverty Scorecard score. One of the parents was missing in 14.7% (362/2,458) of the households. The mean age of the mothers was 37.7 years (±8) and their median years of education was 6 (IQR: 3–10). The mean age of the fathers was 40.9 years (±8.4) and the mean years of education was 7.7 (±3.4). The median number of diarrhea episodes reported per year was 1 (IQR: 0–2) and the median number of episodes of upper respiratory infections reported per year was 1 (IQR: 1–2). Six percent (6.4%, 154/2,396) of the children had a history of anemia treated in the previous 12 months, 4.4% (105/2,394) had been treated for malnutrition, and 14.2% (338/2,388) had received antiparasitic treatment in addition to the school-based mass chemotherapy. The median height-for-age Z-score (HAZ) was −1.45 (IQR: −2.07 to −0.81) and the mean body-mass index (BMI) was 17.7 (±2.75).
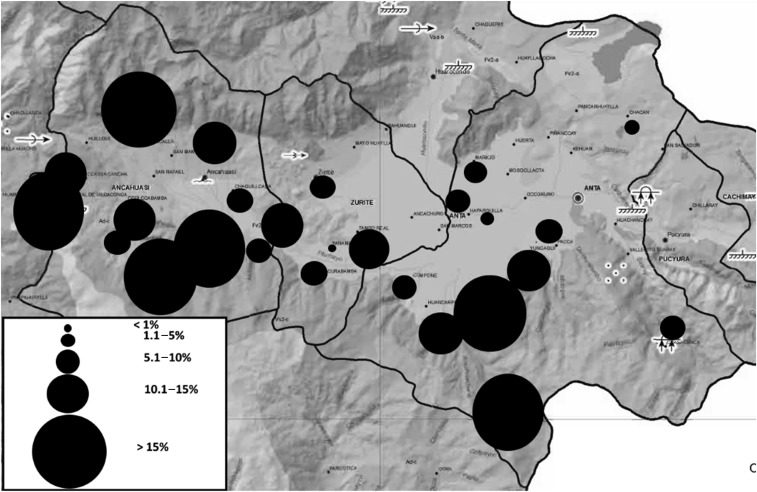
Protozoans were the most common parasites identified, with Giardia intestinalis infecting 23.5% (592/2,515) and Blastocystis hominis 37.1% (932/2,515) of children. The prevalence and egg burden of helminth infections are detailed in Table 2. Ten percent (253/2,513) of participants had one or more positive tests for F. hepatica. Six percent (154/2,515) had at least one positive microscopic test and 8.4% (211/2,513) had detectable Fasciola antibodies by Fas2 ELISA. Thus, the overall prevalence of Fasciola exposure was 10.1% (253/2,513), including chronic infection in 6.1% (154/2,515) and acute infection in 0.4% (11/2,512). The median eosinophil count of those with acute infection was 1,350 cells/μL. There was a significant variation in the prevalence of Fasciola exposure between communities, ranging from 0% to 20.4% (P < 0.01) (Figure 2). Exposed children lived in homes at a higher altitude (3,486 m (±200) versus 3,453 (±194), P = 0.01) than unexposed ones. There were nonsignificant differences in the prevalence of Fasciola exposure by district (Ancahuasi (11.3%), Anta (9.6%), and Zurite (7.2%), P = 0.07), and by history of drinking water from irrigation channels (yes (27.3%) versus no (10.1%), P = 0.06). A comparison of demographic characteristics between those exposed and not exposed to Fasciola is reported in Table 3. We did not find any differences in the prevalence of Fasciola exposure among children with anemia compared with children without anemia (9.8% versus 10.1%, P = 0.83). There was also no difference in Fasciola exposure between children with stunting and those without stunting (10.9% versus 9.7%, P = 0.35). The backward logistic regression analysis showed that age, district, and probability of living in poverty were independently associated with Fasciola exposure (Table 4).
Table 2.
Prevalence of helminthic infections and distribution of the mean Kato–Katz egg counts (N = 2,515)
| Parasite | Prevalence % (n) | Mean (±SD) | Geometric mean | Range |
|---|---|---|---|---|
| Eggs/gram of stool | ||||
| Fasciola hepatica | 6.1 (154) | 57.0 (±76.6) | 39.2 | 6.6–820 |
| Hymenolepis nana | 17.1 (431) | 543.9 (±1,079.6) | 138.9 | 6.6–8,213.3 |
| Ascaris lumbricoides | 5.7 (143) | 372.2 (±667.9) | 155.3 | 6.6–4,333.3 |
| Strongyloides stercoralis | 1.4 (34) | NA | NA | NA |
| Trichuris trichura | 0.8 (21) | 27.4 (±21.4) | 20.3 | 6.6–66.6 |
| Hookworm | 0.7 (18) | 10.0 (±4.7) | 9.4 | 6.6–13.3 |
Figure 2.
Prevalence of Fasciola hepatica in 26 communities of the Anta Province (Adapted from sdot.pcm.gob.pe/wp-content/uploads/2016/06/EDZ_ANTA_comp.pdf).
Table 3.
Comparison of demographic and epidemiologic variables between subjects with any positive test for Fasciola and subjects with negative tests*
| Fasciola (+) n (%) | Fasciola (−) n (%) | P | ||
|---|---|---|---|---|
| Age (years) | Mean (±SD) | 10.7 (±3.4) | 9.5 (±3.6) | < 0.01 |
| School grade | Preschool | 34 (6.1%) | 524 (93.9%) | < 0.01 |
| Elementary | 131 (10.2%) | 1,157 (89.8%) | ||
| High school | 87 (13.2%) | 571 (86.9%) | ||
| Probability of living in poverty† | Greater than 34.4% | 147 (12.2) | 1,062 (87.8) | < 0.01 |
| Under 34.4% | 70 (8.4) | 765 (91.6) | ||
| Blastocystis hominis infection | No | 181 (11.4) | 1,400 (88.6) | < 0.01 |
| Yes | 72 (7.7) | 860 (92.3) | ||
| Mother’s years of education | Mean (±SD) | 5.4 (±3.5) | 6.3 (±3.6) | < 0.01 |
| Father’s years of education | Mean (±SD) | 7.2 (±3.4) | 7.8 (±3.3) | 0.02 |
| Construction material of the house | Adobe | 241 (10.4) | 2,079 (89.6) | 0.04 |
| Brick and other | 7 (5.1) | 131 (94.9) | ||
| Giardia intestinalis infection | No | 206 (10.7) | 1,716 (89.3) | 0.05 |
| Yes | 47 (8.0) | 544 (92.0) | ||
| Reported prior treatment of malnutrition | No | 225 (9.8) | 2,063 (90.2) | 0.07 |
| Yes | 16 (15.2) | 89 (84.8) | ||
| District | Ancahuasi | 129 (11.3) | 1,014 (88.7) | 0.08 |
| Anta | 102 (9.6) | 962 (90.4) | ||
| Zurite | 22 (7.2) | 284 (92.8) | ||
| Reported previous treatment of parasites | No | 202 (9.9) | 1,847 (90.1) | 0.34 |
| Yes | 39 (11.5) | 299 (88.5) | ||
| Reported prior treatment of anemia | No | 223 (10) | 2,018 (90) | 0.48 |
| Yes | 18 (11.7) | 136 (88.3) |
The sum of the values reported in this table may not add to the total number of children enrolled because of missing information.
Simple poverty scorecard probabilities to live with less than US$3.25 a month.
Table 4.
Backward logistic regression analysis of factors associated with Fasciola hepatica exposure
| Characteristic | Odds ratio | 95% Confidence interval | P value | |
|---|---|---|---|---|
| District | Ancahuasi | Reference | ||
| Anta | 2.34 | 0.99–5.51 | 0.051 | |
| Zurite | 2.43 | 1.03–5.74 | 0.043 | |
| Age (years) | 1.05 | 1.01–1.10 | 0.014 | |
| > 34.4% Probability of living in poverty* | 1.63 | 1.14–2.31 | 0.006 |
Independent variables entered in the model: district, age, gender, history of treatment of malnutrition, years of education for the father, years of education for the mother, > 34.4% probability of living in poverty, construction material of the home, stunting, anemia, elevation of the house, and drinking water from irrigation channel. Wald = 68.29 P < 0.001. Hosmer and Lemeshow test: χ2 = 10.4, df: 8, P = 0.23.
According to the simple poverty scorecard probabilities of living with less than US$3.25 a day.
DISCUSSION
Fasciola hepatica is a common health problem among children in the highlands of Peru. In this study, we found an overall prevalence of Fasciola exposure of 10% in preschool and school-aged children in the Anta Province of the Cusco region. The prevalence ranged from 0% to 20% according to the community of residence. The prevalence of infection also varied by age of the children and by multiple socioeconomic factors. However, we did not find an association between anemia or stunting and Fasciola exposure among children in the Anta Province.
Within a relatively small geographic area, the prevalence of fascioliasis ranged from nonendemic to hyperendemic. These data add to a prior body of evidence that suggests a patchy distribution of fascioliasis.14,19 A similar study in the Bolivian Altiplano by Parkinson et al.14 demonstrated prevalence variations from less than 10% to greater than 40% among 36 communities around Lake Titicaca. Mas-Coma et al.20 showed variations between 0% and 72% among school-age children in 38 communities of northern Bolivia. In that study, the prevalence of fascioliasis was associated with the presence of large water bodies and large Lymnaeid snail populations in the community.
Heydarian et al.21 reported a higher prevalence of Fasciola antibodies in groups of subjects consuming untreated surface water. We described similar findings in a smaller study showing a higher prevalence of fascioliasis among children drinking untreated water in the Paucartambo Province of Cusco.4 Although the difference in Fasciola exposure in those who drank water from irrigation channels as compared with those who did not was not statistically significant, three times more subjects had fascioliasis in the group that drank water from irrigation channels. This suggests contamination of superficial water bodies with metacercariae and stresses the need to improve access to clean water to prevent fascioliasis and other helminth infections in children.
Children with Fasciola exposure were more likely to be older than those without it. This is consistent with results reported by Esteban et al.9 in Bolivia, who found a higher prevalence of Fasciola exposure in schoolchildren aged 9–19 years compared with younger children. Although, the reason for this difference is unclear, it is possible that prolonged stays in endemic areas with low to moderate transmission increase the cumulative risk of infection. The lack of routine screening in combination with subclinical infections may allow children to harbor the parasite for many years without treatment. This contrasts with our previous report among children in the Paucartambo Province; a region of Cusco at higher altitude. In that study, we found a higher Fasciola prevalence, but did not find a significant difference in the prevalence by age, which suggests that infections occurred at an earlier age.4 Similarly, Gonzalez et al.10 reported no overall differences in Fasciola prevalence by age in the northern highlands of Peru. In this study, the communities with the highest prevalence did not have a difference in prevalence by age. By contrast, the communities with a low-moderate prevalence (like in our study population) had an increased prevalence in older children.
Children who had a positive test for Fasciola were more likely to live at higher altitude (Figure 2). Similar findings have been reported in Cajamarca, where altitude of the communities correlated with the prevalence of infection.10 The Lymnaeid snail is a more effective secondary host at higher altitudes. Studies comparing snails from lowland European populations and snails from the Peruvian and Bolivian highlands have shown that high-altitude snails produce more cercariae, for a longer period, and live longer after infection than low altitude European snails.22,23 The adaptation of F. hepatica and its intermediate hosts to high altitude likely accounts for the wide distribution of fascioliasis in the Andes and the high intensities of infection reported.
The results of this study show a strong association between Fasciola and poverty, showing that several indicators of low socioeconomic status were more common among infected children. These children were at higher risk of living in poverty according to the Simple Poverty Scorecard score, their parents were less educated, and their dwellings were constructed of adobe, a traditional material that is being replaced by brick in areas with higher income. In Peru, poverty and fascioliasis overlap in the Andes highlands. However, even within the same poor communities, subjects with the lowest socioeconomic status were at higher risk for infection. Poor families have less access to hygiene and safe water, likely resulting in higher environmental contamination around their homes.24–27 In addition, fewer years of education in mothers has been shown to be associated with higher rates of parasitic infection in rural populations in Mexico and in Iran.22,24 A shorter period of education can impact health practices in the home and increase the risk of exposure to parasites and other infections.
In contrast to prior studies,4 we did not find a higher prevalence of anemia in children with a positive test for Fasciola. It is possible that our studied population had a lower intensity of Fasciola exposure that was not enough to cause anemia.28,29 Alternatively, several interventions by the Peruvian Ministry of Health were ongoing at the same time with our study. Programs targeting anemia and malnutrition among school-age children may have decreased the prevalence of anemia and prevented us from finding an association. Another aspect to consider is the improvement in living conditions in Peru. Between 1990 and 2015, the gross national income has more than doubled and the human development index increased from 0.613 to 0.74.30 These may have improved access to better nutrition despite the disparities that still exist.
There are several limitations to this study. The cross-sectional design did not allow stronger conclusion regarding factors associated with fascioliasis. The Anta Province had a lower than expected Fasciola prevalence and intensity of infection, which likely decreased our power to detect associated factors. Ministry of Health interventions to improve the health conditions among children likely hampered our ability to measure the impact of Fasciola in our population. Participation in the study was high, but about 40% of eligible children were not enrolled. Also, some participants had positive Fas2 ELISA without other evidence in stool or blood of Fasciola infection. This group could represent patients who were infected and then cleared the infection, patients with true infection, with egg burdens in stool below the limits of detection, or false-positive Fas2 ELISA tests.
In conclusion, one in 10 school-age children in the studied area of the Peruvian highlands had evidence of F. hepatica infection. The infections were highly variable between communities. The poorest children in these communities and those living at higher altitudes were at higher risk of infection. Interventions aiming to improve living conditions and reduce poverty will likely also decrease the risk of Fasciola transmission among children in the Anta Province of Cusco.
Disclaimer: The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute for Allergy and Infectious Diseases.
REFERENCES
- 1.WHO , 2017. Foodborne Trematode Infections: Fascioliasis. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Moazeni M, Ahmadi A, 2016. Controversial aspects of the life cycle of Fasciola hepatica. Exp Parasitol 169: 81–89. [DOI] [PubMed] [Google Scholar]
- 3.Cabada MM, White AC, Jr., 2012. New developments in epidemiology, diagnosis, and treatment of fascioliasis. Curr Opin Infect Dis 25: 518–522. [DOI] [PubMed] [Google Scholar]
- 4.Lopez M, White AC, Jr., Cabada MM, 2012. Burden of Fasciola hepatica infection among children from Paucartambo in Cusco, Peru. Am J Trop Med Hyg 86: 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karahocagil MK, Akdeniz H, Sunnetcioglu M, Cicek M, Mete R, Akman N, Ceylan E, Karsen H, Yapici K, 2011. A familial outbreak of fascioliasis in eastern Anatolia: a report with review of literature. Acta Trop 118: 177–183. [DOI] [PubMed] [Google Scholar]
- 6.McDaniel CJ, Cardwell DM, Moeller RB, Jr., Gray GC, 2014. Humans and cattle: a review of bovine zoonoses. Vector Borne Zoonotic Dis 14: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashrafi K, Bargues MD, O’Neill S, Mas-Coma S, 2014. Fascioliasis: a worldwide parasitic disease of importance in travel medicine. Travel Med Infect Dis 12 (6 Pt A): 636–649. [DOI] [PubMed] [Google Scholar]
- 8.Furst T, Keiser J, Utzinger J, 2012. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis 12: 210–221. [DOI] [PubMed] [Google Scholar]
- 9.Esteban JG, Flores A, Angles R, Mas-Coma S, 1999. High endemicity of human fascioliasis between Lake Titicaca and La Paz valley, Bolivia. Trans R Soc Trop Med Hyg 93: 151–156. [DOI] [PubMed] [Google Scholar]
- 10.González LC, Esteban JG, Bargues MD, Valero MA, Ortiz P, Náquira C, Mas-Coma S, 2011. Hyperendemic human fascioliasis in Andean valleys: an altitudinal transect analysis in children of Cajamarca province, Peru. Acta Trop 120: 119–129. [DOI] [PubMed] [Google Scholar]
- 11.Esteban JG, González C, Bargues MD, Angles R, Sánchez C, Náquira C, Mas-Coma S, 2002. High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Trop Med Int Health 7: 339–348. [DOI] [PubMed] [Google Scholar]
- 12.El-Sahn F, Farghaly A, El-Masry A, Mandil A, Gad A, El-Morshedy H, 1995. Human fascioliasis in an Egyptian village: prevalence and some epidemiological determinants. J Egypt Public Health Assoc 70: 541–557. [PubMed] [Google Scholar]
- 13.Cabada MM, Goodrich MR, Graham B, Villanueva-Meyer PG, Lopez M, Argue E, White AC, Jr, 2014. Fascioliasis and eosinophilia in the highlands of Cuzco, Peru and their association with water and socioeconomic factors. Am J Trop Med Hyg 91: 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkinson M, O’Neill SM, Dalton JP, 2007. Endemic human fasciolosis in the Bolivian Altiplano. Epidemiol Infect 135: 669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MEF , 2007. Tasa de Analfabetismo Segun Departamento, Provincial, y Distrito 2007 Available at: http://www.mef.gob.pe/contenidos/estadisticas/pol_econ/cuadro60.xls. Accessed July 10, 2018.
- 16.INEI , 2013. Mapa de Pobreza Provincial y Distrital 2013 Instituto Nacional de Estadistica e Informatica, Lima, Peru Available at: https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1261/Libro.pdf. Accessed July 10, 2018.
- 17.DIRESA-Cusco , 2016. Boletin Estadistico Año 1 Número 1, 2016 Dirección Regional de Salud del Cusco, Cusco, Peru Available at: http://www.diresacusco.gob.pe/estaditica/monitoreo/Boletin%20estadistico2015.pdf. Accessed July 10, 2018.
- 18.Schreiner M, 2012. A Simple Poverty Scorecard for Peru Available at: http://microfinance.com/English/Papers/Scoring_Poverty_Peru_2010_EN.pdf. Accessed July 10, 2018.
- 19.Mas-Coma MS, Esteban JG, Bargues MD, 1999. Epidemiology of human fascioliasis: a review and proposed new classification. Bull World Health Organ 77: 340–346. [PMC free article] [PubMed] [Google Scholar]
- 20.Mas-Coma S, Anglés R, Esteban JG, Bargues MD, Buchon P, Franken M, Strauss W, 1999. The northern Bolivian Altiplano: a region highly endemic for human fascioliasis. Trop Med Int Health 4: 454–467. [DOI] [PubMed] [Google Scholar]
- 21.Heydarian P, Ashrafi K, Mohebali M, Kia EB, Aryaeipour M, Chegeni Sharafi A, Mokhayeri H, Bozorgomid A, Rokni MB, 2017. Seroprevalence of human fasciolosis in Lorestan Province, western Iran, in 2015–16. Iran J Parasitol 12: 389–397. [PMC free article] [PubMed] [Google Scholar]
- 22.Mas-Coma S, Funatsu IR, Bargues MD, 2001. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology 123 (Suppl): S115–S127. [DOI] [PubMed] [Google Scholar]
- 23.Vignoles P, Favennec L, Dreyfuss G, Rondelaud D, 2002. Highland populations of Lymnaea truncatula infected with Fasciola hepatica survive longer under experimental conditions than lowland ones. Parasitol Res 88: 386–388. [DOI] [PubMed] [Google Scholar]
- 24.Nematian J, Nematian E, Gholamrezanezhad A, Asgari AA, 2004. Prevalence of intestinal parasitic infections and their relation with socio-economic factors and hygienic habits in Tehran primary school students. Acta Trop 92: 179–186. [DOI] [PubMed] [Google Scholar]
- 25.Quihui L, Valencia ME, Crompton DWT, Phillips S, Hagan P, Morales G, Díaz-Camacho SP, 2006. Role of the employment status and education of mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. BMC Public Health 6: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yentur Doni N, Gurses G, Simsek Z, Yildiz Zeyrek F, 2015. Prevalence and associated risk factors of intestinal parasites among children of farm workers in the southeastern Anatolian region of Turkey. Ann Agric Environ Med 22: 438–442. [DOI] [PubMed] [Google Scholar]
- 27.Ngui R, Ishak S, Chuen CS, Mahmud R, Lim YA, 2011. Prevalence and risk factors of intestinal parasitism in rural and remote west Malaysia. PLoS Negl Trop Dis 5: e974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Shazly AM, El-Nahas HA, Abdel-Mageed AA, El Beshbishi SN, Azab MS, Abou El Hasan M, Arafa WA, Morsy TA, 2005. Human fascioliasis and anaemia in Dakahlia Governorate, Egypt. J Egypt Soc Parasitol 35: 421–432. [PubMed] [Google Scholar]
- 29.Shang Y, Tang LH, Zhou SS, Chen YD, Yang YC, Lin SX, 2010. Stunting and soil-transmitted-helminth infections among school-age pupils in rural areas of southern China. Parasit Vectors 3: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Human Development Reports United Nations Development Programme , 2018. Available at: http://hdr.undp.org/en/countries/profiles/PER. Accessed July 10, 2018.