Abstract
Quorum sensing (QS) and nucleotide-based second messengers are vital signaling systems that regulate bacterial physiology in response to changing environments. Disrupting bacterial signal transduction is a promising direction to combat infectious diseases, and QS and the second messengers are undoubtedly potential targets. In Vibrio cholerae, both QS and the second messenger 3’, 5’—cyclic diguanylate (c-di-GMP) play a central role in controlling motility, motile-to-sessile life transition, and virulence. In this study, we found that water-soluble extract from the North American cranberry could significantly inhibit V. cholerae biofilm formation during the development/maturation stage by reducing the biofilm matrix production and secretion. The anti-biofilm effect by water-soluble cranberry extract was possibly through modulating the intracellular c-di-GMP level and was independent of QS and the QS master regulator HapR. Our results suggest an opportunity to explore more functional foods to fight stubborn infections through interference with the bacterial signaling systems.
Introduction
Quorum sensing (QS) and the nucleotide-based second messengers, especially the cyclic dinucleotides, are two central signaling systems utilized by many bacteria to regulate their physiological functions in response to changing environmental conditions or during the developmental process. Due to their decisive roles in bacterial physiology, QS and the second messengers have been considered as potential targets for new drug development to tackle the increasingly grim situation of antibiotic resistance. By blocking the signaling transduction rather than targeting the essential genes, placing selective pressure on resistant strains of bacteria is avoided. In the past twenty years, natural QS inhibitors (QSIs) have been identified from a number of organisms, and a list of synthetic QSIs have also been developed in research labs [1, 2]. In contrast, finding inhibitors of the cyclic dinucleotide-based signaling pathways has progressed slowly. To date, only a very limited number of compounds have been characterized as cyclic dinucleotide signaling inhibitors [3].
In the Gram-negative bacterial pathogen Vibrio cholerae, both QS and the cyclic dinucleotides play a fundamental role in controlling cell motility, biofilm formation, and virulence gene expression. V. cholerae is the causative agent of a frequently fatal disease called cholera. Since the first cholera pandemic occurred ~ 200 years ago, the disease has affected millions of people. With a better knowledge of the disease control and improved water and sanitation facilities, the disease transmission has been eliminated in the developed countries, yet cholera remains a threat in many parts of the developing world. An essential component to this pathogen’s success and persistence in the environment is its ability to attach to both biotic and abiotic surfaces via biofilm formation [4]. Biofilms not only aid in surface attachment, they also provide a barrier that protects and enhances survival. In human infection, when V. cholerae enters the body, it must first survive the acidic environment of the stomach and then proceed to attach to the intestinal wall. Biofilms provide cells resistance to high acidity and therefore are critical for the transmission and infectivity of V. cholerae [5, 6]. The major component of V. cholerae biofilm, Vibrio polysaccharide, is synthesized by enzymes encoded in the two vps (Vibrio polysaccharide synthesis) gene operons (vps-I and vps-II) [7, 8]. Additionally, genes from the intergenic region of the two operons (rbmA, rbmB, and rbmC) as well as bap1, which is located downstream of vps-II, are involved in the production of matrix proteins and maintenance of the biofilm structure. RbmA provides cell-cell adhesion, RbmC and Bap1 form the envelopes to encase the cell clusters, and RbmB, whose function is yet to be characterized, is proposed to help with the detachment of bacteria from mature biofilms [9–11]. The two vps operons are positively regulated by two major transcriptional regulators, VpsR and VpsT [12, 13], and rbmA, rbmB, rbmC, and bap1 are positively regulated by VpsR [11, 14]. Expression of vpsR and vpsT is regulated by the cell density through the upstream QS pathway, and by the intracellular concentration of 3’, 5’—cyclic diguanylate (c-di-GMP), an important second messenger identified in a wide variety of bacteria [15–17].
Unlike many other pathogenic bacteria that cause persistent infections in which QS typically activates biofilm formation and virulence at high cell density, in V. cholerae, genes involved in biofilm formation and virulence are maximally expressed at low cell density and are turned off at high cell density. This unique system is thought to aid in V. cholerae’s life cycle during acute infection, allowing it to colonize in the intestine and promoting infection at low cell density, and helping the bacteria detach and transition back to its natural environment at high cell density [5, 18, 19]. V. cholerae responds to at least two QS signaling molecules (called autoinducers), CAI-1 and AI-2, through the response regulator LuxO. At low cell density, LuxO is in the phosphorylated form and activates expression of a set of small regulatory RNAs, which in turn inhibits expression of the major QS regulator HapR, allowing expression of vpsR, vpsT, as well as virulence genes. At high cell density, LuxO is dephosphorylated; thus HapR is de-repressed and inhibits biofilm and virulence genes.
In V. cholerae, increasing the intracellular c-di-GMP level promotes the transition from motile to sessile lifestyle [20–22]. C-di-GMP has been shown to regulate both the initial attachment and biofilm matrix formation. Binding of c-di-GMP to the ATPase MshE promotes the polymerization of the MshA subunits to form the pili, which are critical for initial surface attachment [23]. Both the transcription activators VpsR and VpsT can sense c-di-GMP and directly bind to it, leading to activation of biofilm genes [24, 25]. C-di-GMP is synthesized by the diguanylate cyclases (DGCs) containing a GGDEF domain and degraded by the phosphodiesterases (PDEs) containing either an EAL or HD-GYP domain [26]. The V. cholerae genome encodes 31 proteins with the GGDEF domain, 12 proteins with the EAL domain, nine proteins with the HD-GYP domain, and ten proteins with both GGDEF and EAL domains [27, 28]. Prior research has identified a list of DGCs and PDEs involved in regulating V. cholerae c-di-GMP level and biofilm formation [21, 29, 30]. Many DGCs and PDEs have N-terminal sensory input domain, indicating their activities could be modulated by various environmental signals, which in turn affects the intracellular c-di-GMP level [28].
Previously, we reported that supplementation of water-soluble cranberry extract standardized to 4.0% proanthocyanidins (WCESP) to Caenorhabditis elegans, a nematode model, could significantly enhance the host’s resistance to infection by various bacterial pathogens including Vibrio cholerae. Mechanistic studies showed that the WCESP-mediated protection was mainly through promoting the host’s innate immunity. Under our experimental condition, WCESP treatment did not affect the growth of V. cholerae or expression of the major bacterial virulence genes, but a slight reduction of bacterial colonization within C. elegans intestine was noticed [31]. In this study, we tested WCESP’s ability to inhibit V. cholerea biofilm formation and further investigated the inhibition mechanism. We found that low concentration of WCESP could significantly impede V. cholerae biofilm formation during the development/maturation stage; this inhibition is independent of the QS pathway but is possibly through modulating the intracellular c-di-GMP level. This study suggests that cranberry contains certain active constituents that can interfere with V. cholerae c-di-GMP signal transduction and provides a potential means to control V. cholerae biofilm.
Material and methods
Plasmids, bacterial strains, and growth media
The plasmid pBAD33 was obtained from Coli Genetic Stock center (CGSC), and pAT1662 (pBAD33::VCA0956-His6) was obtained from Dr. Andrew Camilli (Tufts University) [32]. The V. cholerae strains (except the MO10 strain) used in this study were all derived from the wild-type C6706 strain (O1 serotype El Tor isolated from Peru) [33]. The C6706 with PhapR-lacZ fusion, luxO- (luxO deletion), hapR- (hapR deletion), luxOC (constitutively active LuxO), cqsA- (cqsA deletion), luxS- (luxS deletion), cqsA-/luxS- (cqsA and luxS double deletion) strains were obtained from Dr. Jay Zhu (University of Pennsylvania) [5, 19, 34]. The MO10 strain (serotype O139) was isolated during the cholera outbreak in India and Bangladesh in 1992 [35, 36], also obtained from Dr. Jay Zhu. Other bacterial strains used in this study were Staphylococcus aureus (S. aureus, ATCC#25923), Pseudomonas aeruginosa (P. aeruginosa, ATCC#27853), Enterococcus faecalis (E. faecalis, ATCC#47077), Salmonella typhimurium (S. typhimurium, ATCC#14028), E. coli O157:H7 (ATCC#700927), Listeria monocytogenes (L. monocytogenes) ScottA (serotype 4b), and L. monocytogenes Mac (serotype 1/2a). The E. coli, V. cholerae, P. aeruginosa, and S. typhimurium strains were cultured in Lysogeny broth (LB). The S. aureus, E. faecalis, and L. monocytogenes strains were cultured in brain-heart infusion (BHI) medium.
Preparation of water-soluble cranberry extract
The cranberry extract used in this study was obtained from Naturex-DBS, LLC (Sagamore, MA, USA) as described in previously published studies [31]. A stock solution of WCESP was freshly prepared by dissolving the powder in distilled water to a concentration of 50 mg/ml immediately before use.
Biofilm growth and quantification
Overnight bacterial cultures were inoculated (1:100 dilution) into 1 ml of LB or BHI medium in the presence or absence of 2 mg/ml of WCESP and incubated statically in polystyrene tubes for 24 hours to allow biofilm development. The temperatures used to develop biofilms were 25°C for V. cholerae, and 37°C for other bacterial strains. Quantifications of sessile/biofilm cells were conducted by the crystal violet staining method and the direct enumeration of live cells. For the staining method, the planktonic bacterial cells were carefully removed from each tube, and the tube was washed twice with PBS. The sessile bacterial cells were stained with 1% crystal violet for 30 minutes, followed by distilled water wash for five times. To enumerate biofilm bacterial cells, planktonic cells were carefully removed from underneath the biofilm pellicle. The pellicle and surface-attached cells were washed twice with PBS and resuspended in 1 ml of PBS. The cells were vortexed with glass beads (ϕ = 1mm) for two minutes to disrupt the biofilm and then plated onto LB agar plates with proper dilutions. Colonies were counted on the second day. In a parallel set of experiments, total bacterial cells (both planktonic and biofilm cells) were measured by first vortexing the cells with glass beads and then plating onto LB agar plates with proper dilutions. Data shown are the average results from three independent experiments, and triplicate samples were tested each time. The data were pooled and analyzed using unpaired Student’s t-test. A p-value < 0.05 was accepted as statistically significant.
Biofilm inhibition in pre-established V. cholerae culture
The overnight culture of V. cholerae was inoculated into 1ml of LB in polystyrene test tubes (1:100 dilution) and incubated statically at 25°C for 4 hours. Then, 40 μl of WCESP stock solution (50 mg/ml) was added to the test tubes to reach a final concentration of 2 mg/ml. In the control tubes, 40 μl of sterile ddH2O was added. The tubes were incubated at 25°C under static condition for the remaining 20 hours. Biofilm cells were measured using the standard plate count method as described above. Data shown are the average results from three independent experiments, and triplicate samples were tested each time. The data were pooled and analyzed using unpaired Student’s t-test. A p-value < 0.05 was accepted as statistically significant.
Motility assay
Overnight cultures of V. cholerae were inoculated into 1 ml of LB with or without 2 mg/ml WCESP in polystyrene test tubes (1:100 dilution). Cultures were incubated with shaking at 150 rpm at 25°C until reaching mid-log phase. Three microliters of the culture were dropped onto motility assay plates (LB with 0.3% agar). The plates were incubated at 25°C. The diameters of the motility zones were measured after 4 and 24 hours, respectively.
Cell-surface hydrophobicity measurement
The cell-surface hydrophobicity was measured based on the method described by Prabu et al. [37]. Overnight V. cholerae C6706 culture was inoculated (1:100 dilution) into LB with or without 2 mg/ml of WCESP and incubated at 25°C with gentle shaking. Cell samples were collected at 4 and 24 hours respectively and washed twice with 0.85% NaCl. Samples were then transferred to glass test tubes and resuspended in 3 ml of 0.85% NaCl. Spectrophotometer reading was then taken from each sample at 600nm and recorded as the OD Initial. Next, 0.25 ml of toluene was added to each sample and vortexed for 2 minutes. Samples were then allowed to equilibrate at room temperature for 30 minutes. Following the complete separation of the toluene phase from the aqueous phase, OD of the aqueous phase of each sample was measured at 600nm and recorded as the OD Final. The Hydrophobicity Index (HPBI) was then calculated using the following formula: HPBI = (OD Initial—OD Final) / OD Initial x 100%. Data shown are the average results from three independent experiments, and triplicate samples were tested each time. The data were pooled and analyzed using unpaired Student’s t-test. A p-value < 0.05 was accepted as statistically significant.
β-galactosidase assay for PhapR-lacZ
The overnight culture of V. cholerae C6706/PhapR-lacZ was inoculated into LB medium (1:100 dilution) with or without 2 mg/ml WCESP. Cultures were incubated statically at 25°C, and 1 ml of planktonic bacterial sample was collected at 4, 8, 16, and 24 hours, respectively. The β-galactosidase activity of each sample was measured according to the method developed by Miller [38]. Data shown are the average results from three independent experiments, and triplicate samples were tested each time. The data were pooled and analyzed using unpaired Student’s t-test. A p-value < 0.05 was accepted as statistically significant.
Gene expression analysis by quantitative real-time PCR (qRT-PCR)
Overnight V. cholerae C6706 culture was inoculated (1:100 dilution) into 1 ml of LB with or without 2 mg/ml WCESP. Cultures were grown statically at 25°C. Planktonic bacterial samples were harvested after 4 and 24 hours of incubation and washed once with PBS. Total RNA was prepared using RNAzol RT reagent (Molecular Research Center, INC.) and stored at -80°C. Complementary DNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad). qRT-PCR was performed using SensiFAST SYBR No-Rox Kit (Bioline) and the CFX96 real-time PCR detection system according to the manufacturer’s suggested protocol (Bio-Rad). The qRT-PCR conditions were: 95°C for 2 minutes, followed by 40 cycles of 5 seconds at 95°C, 10 seconds at 50°C, and 15 seconds at 72°C. 16S rRNA was used as the endogenous control to normalize the expression levels of target transcripts [31]. Relative fold-changes for transcripts were calculated using the comparative CT (2−ΔΔCT) method [39]. Cycle thresholds of amplification were determined by Light Cycler software (Bio-Rad). Each qRT-PCR experiment was repeated three times using independent RNA preparations. The data were pooled and analyzed using unpaired Student’s t-test, and a p-value < 0.05 was accepted as statistically significant. The primers used in this study are listed in Table 1.
Table 1. Oligos used in qRT-PCR.
| Oligo | Sequence |
|---|---|
| 16S rRNA-F | 5'-GGAAACGATGGCTAATACCG-3' |
| 16S rRNA-R | 5'-GCCCTTACCTCACCAACTAG-3' |
| bap1-F | 5'-CGCTGGCACACTAAACAA G-3' |
| bap1-R | 5'-CCATACATTCATACCCAAGAGC-3' |
| epsE-F | 5'-CTAACCCAAGTCTATCAG-3' |
| epsE-R | 5'-AATCTTCGTTTTGAGGC-3' |
| flaA-F | 5'-GGATTAAAGATACGGATTTTG-3' |
| flaA-R | 5'-CGAGATTGCAGAGTTTG-3' |
| hapR-F | 5'-CGATTGTCACTGGCTCAAAG-3' |
| hapR-R | 5'-GCAGTTGGTTAGTTCGGTTG-3' |
| luxO-F | 5'-GCGAAAGTGGTACAGGTAAAG-3' |
| luxO-R | 5'-CCCTTTGACGTGACCAAAC-3' |
| mshA-F | 5'-CATTGCCCATAAGTTTG-3' |
| mshA-R | 5'-GTTCCTGTAGACGATTG-3' |
| pomB-F | 5'-CTCTTGCTCTCGTTTTC-3' |
| pomB-R | 5'-AATACTGGTCCCTTTGG-3' |
| rbmA-F | 5'-TGGGTTCCAGAGTATATG-3' |
| rbmA-R | 5'-GAGTTCAGGTAGGCTATT-3' |
| rbmB-F | 5'-CAGCAGGAACAGAAATG-3' |
| rbmB-R | 5'-CCTTAGCTCCTCTAGTATC-3' |
| rbmC-F | 5'-CGAGCAATAAGAAAGTGG-3' |
| rbmC-R | 5'-GCCTTCAACTAACCAAC-3' |
| vpsD-F | 5'-CATCCAAGAGCAACTGAAAG-3' |
| vpsD-R | 5'-GCAAGGTCAACACATTACGAG-3' |
| vpsL-F | 5'-TTCTTTACATACGGCATTC-3' |
| vpsL-R | 5'-GCCAATAAAAGAACCGAC-3' |
| vpsR-F | 5'-GGCCATGTATTGGTATTGTGG-3' |
| vpsR-R | 5'-GGCAAATGGTATCTGAACTGAG-3' |
| vpsT-F | 5'-GTCCGCAGGATATTGAGCAT-3' |
| vpsT-R | 5'-GCCTTTGATCAGGGTATCCA-3' |
c-di-GMP quantification assay
The thiazole orange (TO)-based fluorescent detection method was used to quantify the intracellular c-di-GMP level [40]. In brief, overnight V. cholerae C6706 culture was inoculated (1:100 dilution) into LB medium and incubated statically at 25°C. The planktonic cells from the static cultures were collected at 4h, 8h, 16h, and 24h time-points to represent the key growth phases, and the total cell number in each sample was determined using the plate count method. Bacterial cells were pelleted by centrifugation and resuspended in 1 ml of 10 mM Tris-HCl (pH 8.0) buffer containing 100 mM NaCl. The cells were lysed by sonication. Trichloroacetic acid was added to the cell lysate to a final concentration of 12% to precipitate cellular macromolecules. The suspension was incubated on ice for 10 minutes and neutralized by 3 M KOH containing 0.4 M Tris and 2 M KCl, followed by centrifugation at full speed for 10 minutes. The supernatant was filtered through a 0.2 μm filter and then 1:10 diluted into the reaction buffer (10 mM Tris-HCl (pH 8.0) containing 1M NaCl), incubated at 95°C for 5 minutes, and cooled to room temp. TO was then added to each sample at a working concentration of 30 μM and incubated at 4°C for 12h until fluorescence readings were taken at excitation and emission wavelengths at 508nm and 557nm, respectively. The concentration of c-di-GMP was calculated using a calibration curve and normalized to 1 x 109 bacterial cells in 1 ml.
Results
WCESP at non-lethal concentration specifically inhibits V. cholerae biofilm formation
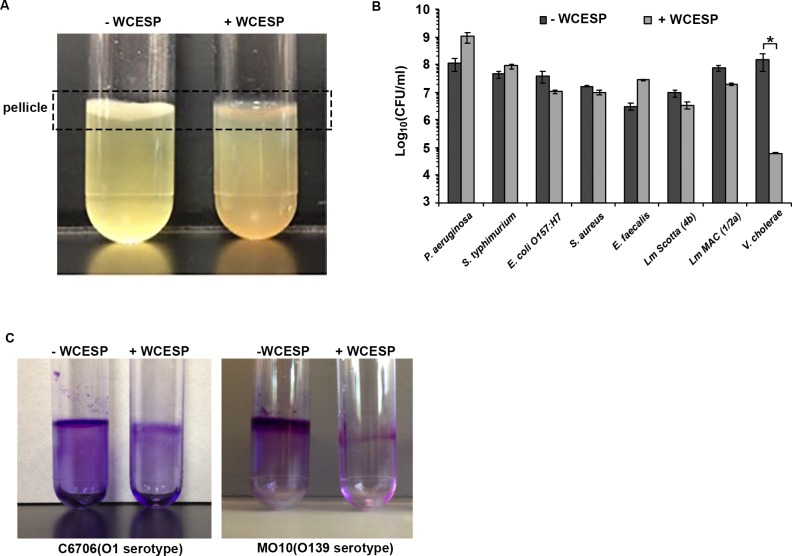
To test whether WCESP could affect V. cholerae biofilm formation, we grew V. cholerae wild-type C6706 strain statically in LB medium at 25°C, a temperature that more closely resembles the natural condition. WCESP was added into the growth medium at the same time during inoculation to reach a final concentration of 2 mg/ml. After incubation for 24 hours, the outcome of adding WCESP was palpable. As shown in Fig 1A, a thick pellicle was formed at the air-liquid interface in the control tube but not in the tube with WCESP. The bacterial culture in the WCESP tube also showed higher turbidity than that in the control tube, indicating that most of the bacterial cells remained in the planktonic form when WCESP was added into the medium. A standard plate count method was used to enumerate the bacterial cells in the pellicles and those attached to the wall of the culture tubes from both tubes, i.e., biofilm cells. An apparent reduction of cell numbers was observed in the WCESP tube (Fig 1B). In a parallel set of tubes, we quantified the total bacterial cells (both planktonic and biofilm cells) in the presence or absence of 2 mg/ml of WCESP by plate counting during the 24-hour time course. No visible difference in cell numbers was detected, indicating the WCESP concentration used in the biofilm inhibition assays did not cause any growth defects, which is consistent with our previous results (S1 Table) [31]. Considering that V. cholerea is a human pathogen, we also tested WCESP’s ability to inhibit biofilm at 37°C. A similar inhibition effect was observed as well (S1 Fig).
Fig 1. WCESP inhibits V. cholerae biofilm formation.
The bacterial cultures were incubated for 24 hours without shaking in the absence and presence of 2 mg/ml of WCESP. (A) Biofilm pellicle formed at the air-water interface in the absence of WCESP, but not in the presence of WCESP. (B) Quantification of the pellicle and surface-attached bacterial cells of V. cholerae C6706 and other pathogenic bacteria in the presence and absence of WCESP. Results shown are the average of three independent experiments, and triplicate samples were tested each time; error bars are standard error of the mean. (* indicates p < 0.05). (C) Crystal violet staining results of surface-attached V. cholerae bacterial cells.
To test whether the biofilm inhibition by WCESP is a general feature towards a broad spectrum of bacterial pathogens, we examined seven other bacterial species including both Gram-positive and Gram-negative bacteria from our lab collections. No inhibition of biofilm formation by 2 mg/ml of WCESP was observed for any of the seven tested strains (Fig 1B). The wild-type V. cholerae strain used in our experiments is a clinical isolate belonging to the O1 serotype El Tor biotype. We further tested the WCESP’s impact on a V. cholerae MO10 strain, which belongs to the O139 serotype by a quick crystal violet staining method. Strong inhibition of biofilm formation by WCESP was detected (Fig 1C). Thus, our results revealed that at non-lethal concentration, WCESP specifically inhibits V. cholerae biofilm formation.
WCESP impedes mainly the development/maturation stage of V. cholerae biofilm formation
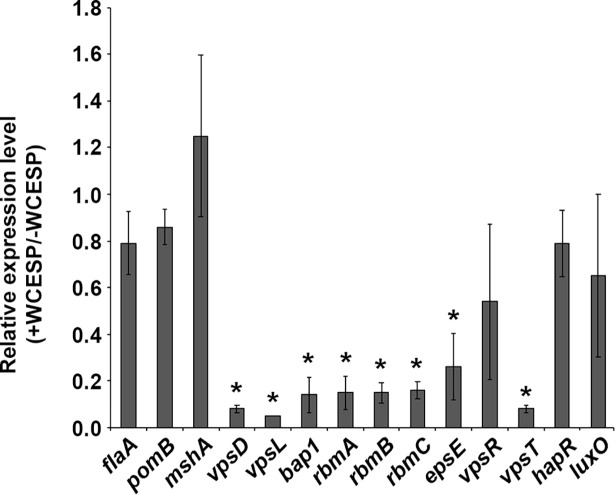
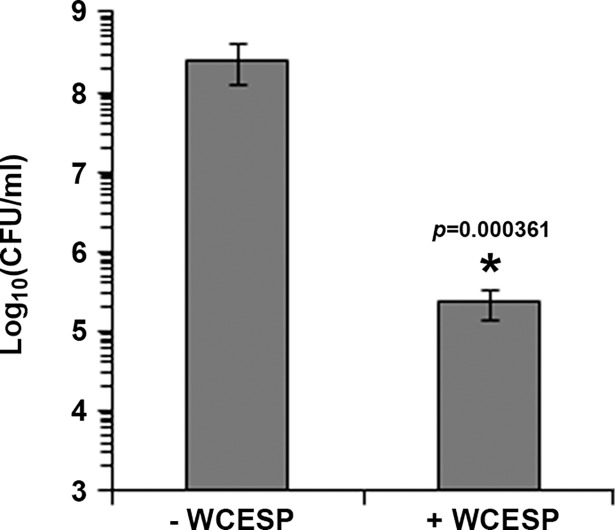
Biofilm formation is generally divided into initiation, development/maturation, and dispersion stages. Flagellar motility and the mannose-sensitive haemagglutinin type IV pilus (MSHA) are crucial for the initial attachment to abiotic surfaces, and the flagellum also mediates the spread across the surface area [41]. Mass production of exopolysaccharide (EPS) and matrix proteins is associated with the development of the three-dimensional structures during the maturation stage. To test which stage or stages were possibly affected by the addition of WCESP, we first performed a motility assay. The wild-type V. cholerae C6706 was grown to mid-log phase with shaking in the presence or absence of WCESP, and then 3 μl of the bacterial culture were dropped onto the motility agar plate and incubated at room temperature. The diameters of the motility zones were measured after 4 and 24 hours. As shown in Table 2, adding WCESP to the growth medium did not hinder flagellar motility. We further compared the expression of flaA (encoding the core flagellin), pomB (encoding the flagellar motor protein), and mshA (encoding the type IV pilus) in the presence or absence of WCESP using qRT-PCR. No noticeable changes were detected after either 4 hours (S2 Fig) or 24 hours of WCESP treatment (Fig 2). These results suggested that WCESP does not interfere with the initial attachment stage. We reasoned that if the initiation stage is not affected, we would see the same degree of inhibition if WCESP were added into a static culture that has already passed the first stage (we named it “pre-established culture”). As expected, the inhibition of a 4 hour-pre-established culture was evident and comparable to the result obtained when WCESP was added at inoculation (Fig 3). The same level of inhibition was also achieved on a 6 hour-pre-established culture. Together, these data indicated that WCESP impedes mainly the development/maturation stage of V. cholerae biofilm formation.
Table 2. The diameters of the motility zones.
| T = 4 hr | T = 24 hr | |
|---|---|---|
| no WCESP | 0.30 ± 0.00 cm | 16.17 ± 0.29 cm |
| WCESP (2mg/ml) in the brotha | 0.32 ± 0.03 cm | 15.83 ± 0.29 cm |
| WCESP (2mg/ml) in the agarb | 0.30 ± 0.00 cm | 16.67 ± 0.29 cm |
Note: The data shown are the average results from three repeats.
a V. cholerae was cultured in the presence of WCESP until mid-log phase then dropped onto the motility plate.
b V. cholerae was cultured in the absence of WCESP until mid-log phase then dropped onto the motility plate which contains WCESP.
Fig 2. qRT-PCR analysis of V. cholerae biofilm-related genes in response to 2 mg/ml WCESP (normalized to 16S rRNA).
Results are the average of three independent experiments, and error bars are standard error of the mean. (* indicates p < 0.05).
Fig 3. WCESP impedes mainly the development stage of V. cholerae biofilm formation.
Inhibition of 4-hr pre-established biofilm formation by 2 mg/ml WCESP. Results shown are the average of three independent experiments, and triplicate samples were tested each time; error bars are standard error of the mean. (* indicates p < 0.05).
WCESP reduces V. cholerae cell-surface hydrophobicity, VPS, and major matrix proteins production and secretion
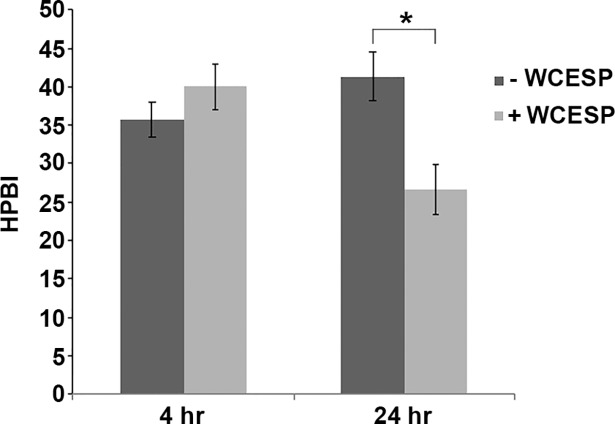
Bacterial cell-surface hydrophobicity (CSH) depicts the tendency of a bacterial cell to aggregate with cells of similar hydrophobicity as opposed to water, which is mainly affected by the bacterial cell surface composition and structure [42]. CSH also influences bacterial surface adhesion and biofilm development. Early study by Dahlbäck et al. showed that the hydrophobic interactions among bacteria played an important role in their initial adhesion at the air-water interface [43]. In V. cholerae, the biofilm matrix protein Bap1 is the crucial determinant of pellicle mechanical strength and hydrophobicity, allowing the pellicle to spread and remain at the air-liquid interface [44]. To test whether WCESP would change CSH, we incubated V. cholerae C6706 with and without 2 mg/ml WCESP and collected bacterial samples after 4 and 24 hours for the CSH measurement. As shown in Fig 4, there was no obvious difference of the hydrophobicity index (HPBI) between the 4-hour samples. But after 24 hours, the WCESP treated cells had a significant decrease of CSH (HPBI = 26.6 ± 3.3 vs. 41.3 ± 3.2 of untreated cells).
Fig 4. WCESP (2 mg/ml) decreases V. cholerae cell-surface hydrophobicity.
The Hydrophobicity Index (HPBI) was calculated using the following formula: HPBI = (OD Initial—OD Final) / OD Initial x 100%. Data shown are the average results from three independent experiments, and triplicate samples were tested each time; error bars are standard error of the mean. (* indicates p < 0.05).
Using qRT-PCR, the expression of genes that are responsible for the production of VPS and matrix proteins was compared in the presence and absence of WCESP at 4 and 24 hours. No significant difference was detected at 4 hr. At 24 hr, downregulation of the vpsD (the fifth gene of the vps-I operon), vpsL (the first gene of the vps-II operon), as well as genes encoding the matrix proteins bap1, rbmA, rbmB, and rbmC, was evident in the presence of WCESP (Fig 2). Expression of the two transcriptional activators of the vps and rbm operons, VpsT and VpsR, was also measured. While vpsT expression showed a 12.5-fold decrease, vpsR transcription only slightly declined (1.9-fold). The extracellular protein secretion (eps) system plays a vital role in V. cholerae pathogenesis and environmental survival; it is involved in exporting toxins and VPS across the outer membrane [45]. Expression of epsE (the third gene of the eps operon) was measured to denote the change of the eps operon when exposed to WCESP. As shown in Fig 2, a 3.8-fold of decrease was observed when WCESP was added to the growth medium. These results suggested WCESP reduces the production and secretion of VPS and major matrix proteins.
WCESP-mediated biofilm inhibition is independent of HapR and the quorum-sensing pathway
QS pathway plays a central role in the regulation of V. cholerae biofilm, and cranberry constituents have been reported to attenuate QS-mediated cell-cell signaling in V. harveyi and P. aeruginosa [46–48]. Thus, it is natural to link WCESP’s inhibition of V. cholerae biofilm to the effects on QS system. In V. cholerae, high cell density (when CAI-1 and AI-2 concentrations are high) results in downregulation of biofilm formation through AI-sensing, LuxO dephosphorylation, and activation the master QS regulator HapR expression. HapR then inhibits vpsR, vpsT, and the vps-I, vps-II, and rbm genes. We reasoned that if the inhibition is through QS, then (1) WCESP would enhance the expression of HapR, and (2) in QS-deficient mutants (such as hapR deletion or cqsA, luxS deletions), WCESP would no longer inhibit biofilm formation. To test this, we first examined the hapR transcription using a strain carrying a chromosomal hapR-lacZ fusion [5]. As shown in Fig 5A, hapR transcription was slightly lower (less than 2-fold) in the presence of WCESP at T = 4, 8, and 16 hr, but at T = 24 hr, hapR transcription was marginally higher (1.3-fold). qRT-PCR of hapR from 24 hr WCESP-treated bacterial culture showed no distinct change (Fig 2). Results from the two experiments indicated WCESP does not enhance HapR expression. Next, we evaluated biofilm formation in the presence and absence of WCESP using the plate count method in the following QS-deficient mutant strains: hapR-, luxOc (in which LuxO is locked into the active form), cqsA- (no CAI-1), luxS-(no AI-2), and a cqsA- / luxS- double deletion. The luxO- mutant was included to serve as a biofilm-negative control, as HapR is overexpressed in luxO- and strongly inhibits biofilm formation. The results are shown in Fig 5B, in which strong inhibition of biofilm formation by WCESP was observed in all tested QS-deficient strains. Taken together, we conclude that WCESP-mediated biofilm inhibition is independent of HapR and QS pathway.
Fig 5. WCESP-mediated biofilm inhibition is independent of HapR and the quorum-sensing pathway.
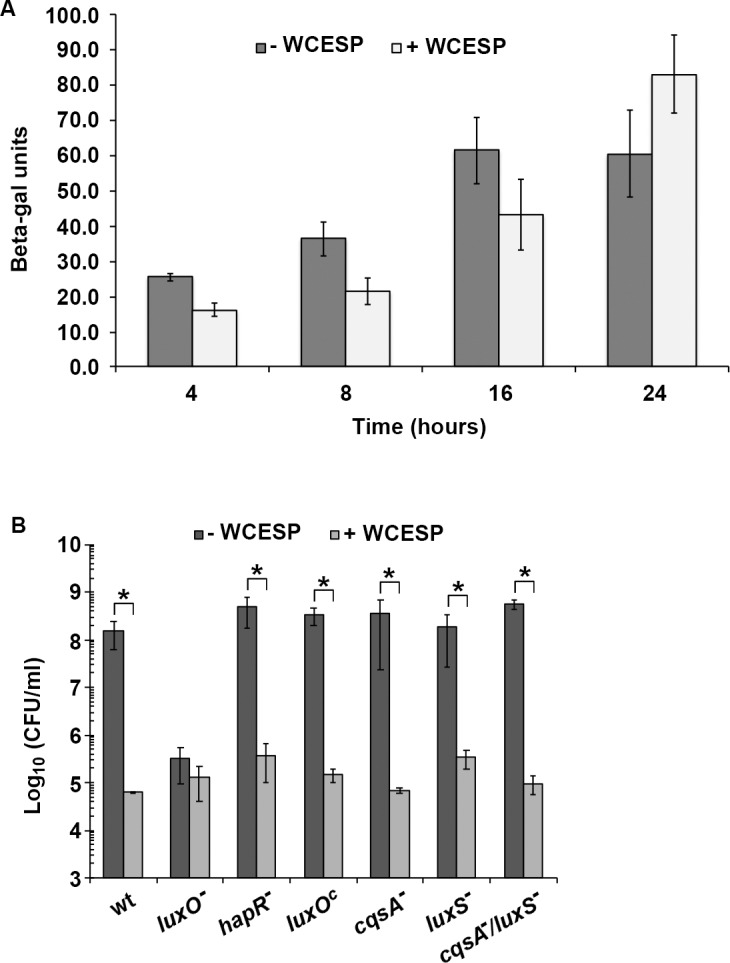
(A) β-galactosidase assay of hapR transcription using a V. cholerae C6706 strain carrying a chromosomal hapR-lacZ fusion. (B) Biofilm formation in the presence and absence of 2 mg/ml WCESP using the plate count method in V. cholerae C6706 derived mutant strains. Data shown are the average results from three independent experiments, and triplicate samples were tested each time; error bars are standard error of the mean. (* indicates p < 0.05).
WCESP modulates the intracellular concentration of the second messenger 3’,5’ -c-di-GMP
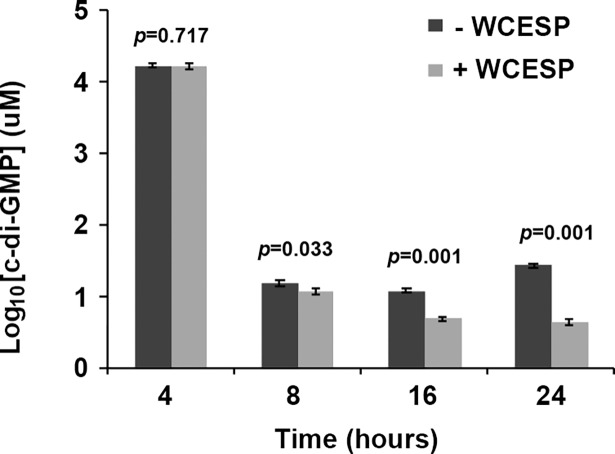
The bacterial second messenger 3’, 5’—cyclic diguanylate (c-di-GMP) is another vital signaling system in V. cholerae that regulates the planktonic to biofilm transition and the biofilm matrix production. To examine WCESP’s influence on c-di-GMP, we sought to directly measure the intracellular c-di-GMP concentration using the thiazole orange (TO)-based fluorescent detection method [40]. To do this, V. cholerae C6706 was grown statically at 25°C in the presence and absence of 2 mg/ml WCESP. The planktonic portion of the static cultures was collected at 4, 8, 16, and 24 hours, respectively. As the bacterial cell number changes during the growth phase and also is affected by the presence of WCESP, we normalized the final c-di-GMP concentration to 1 x 109 bacterial cells in 1 ml. As shown in Fig 6, no difference was found between the two 4-hr samples (which represent the initiation stage). At T = 8 hr, a slight decrease was noticeable in the WCESP-treated cells (11.8 ± 1.2 μM) as compared to the control (15.5 ± 1.5 μM). At T = 16 and 24 hr, the difference was remarkable. Results from this experiment suggest that the inhibition of V. cholerae biofilm formation by WCESP is possibly through reducing the intracellular c-di-GMP level during the development/maturation stage of the biofilm formation. If this holds true, then increasing the c-di-GMP level would at least partially counteract WCESP’s anti-biofilm effect. To test this, pAT1662 (a plasmid that contains the diguanylate cyclase VCA0956 gene under the control of the arabinose-inducible araBAD promoter) [32] was introduced into V. cholerae C6706 by electroporation. Biofilm formation of the resulting strain was examined when 0.2% of L-arabinose and 2 mg/ml of WCESP were added to the growth medium simultaneously. As shown in Fig 7, a partial recovery of the biofilm pellicle was observed in tube 8 (upper panel). Crystal violet staining also revealed more surface-attached cells in tube 8 (bottom panel) as compared to tube 6.
Fig 6. Quantification of intracellular c-di-GMP concentration in the presence and absence of 2 mg/ml WCESP using the thiazole orange (TO)-based fluorescent detection method.
The concentration of c-di-GMP was calculated using a calibration curve and normalized to the concentration of a bacterial culture with 1 x 109 cells/ml. Data shown are the average results from three independent experiments, and error bars are standard error of the mean.
Fig 7. Induction of c-di-GMP synthesis partially restored biofilm formation in the presence of WCESP.
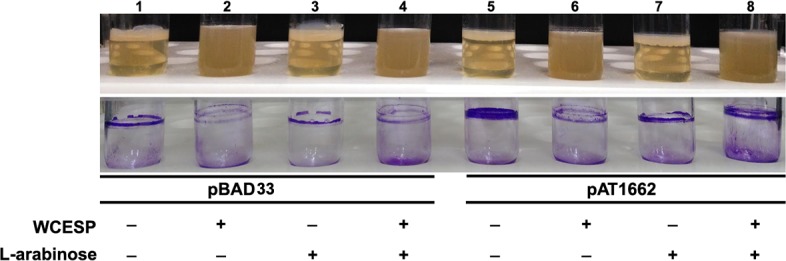
The overnight bacterial culture was 1:100 diluted into 5 ml of fresh LB medium and incubated at 37°C with shaking until OD600 reached ~ 0.25. The culture was then aliquoted into four tubes with 1 ml each. WCESP and L-arabinose were added to the corresponding tubes with a final concentration of 2 mg/ml and 0.2%, respectively. All the tubes were subsequently incubated at 37°C for 24 hours without shaking. Tube 1–4, VC6706 with the empty vector pBAD33 to serve as controls. Tube 5–8, VC6706 with pAT1662 (pBAD33::VCA0956-His6).
Discussion
Due to the selective pressure of antibiotics, bacterial resistance to antibiotics is becoming an increasing health threat worldwide. A growing body of evidence demonstrated that plant extracts offer considerable antimicrobial and anti-biofilm potentials and do not come with the significant risk of antibiotic resistance. A vast number of phytochemicals have been recognized as a valuable alternative and complementary medicine to treat bacterial infections [49, 50]. The North American cranberries (Vaccinium macrocarpon) are rich in polyphenols, including anthocyanins, proanthocyanidins (PACs), flavonoids, and phenolic acid derivatives [51–53]. The antimicrobial activity of cranberry and cranberry products has been well acknowledged, such as preventing urinary tract disorders [54–56], dental decay [57–60], as well as stomach ulcers and cancers [61]. There are only a limited number of reports on cranberry’s anti-biofilm ability; most of these studied the effects on the periodontal pathogenic bacteria [57–59, 62–65] or the urinary tract infections (UTIs) associated pathogens [66–68]. Cranberry extracts have also been shown to reduce Staphylococcus epidermidis biofilm formed on soft contact lenses [69], and Pseudomonas aeruginosa biofilm [70]. Despite all the potential benefits, there is not enough research on cranberry’s anti-biofilm mechanism. In the present study, we investigated the effects of WCESP on V. cholerae biofilm formation. Our results indicated that sub-lethal level of WCESP could inhibit V. cholerae biofilm formation during the development/maturation stage by reducing the production and excretion of VPS and biofilm matrix proteins. We further investigated the involvement of the two key signaling systems and found that the inhibition is independent of QS and the QS master regulator HapR. On the other hand, the intracellular c-di-GMP level was decreased in the presence of WCESP. Our results imply WCESP’s anti-biofilm effect is possibly through modulating c-di-GMP signaling, though we could not rule out the possibility that other regulatory pathways might be involved.
In most bacterial pathogens, biofilm formation is activated at high cell density. Thus, inhibition of QS would result in the reduction of biofilm. But in V. cholerae, this is opposite; inhibiting QS (like the hapR deletion or the luxOC strain) would enhance biofilm formation. Initially, we hypothesized that cranberry extract might promote QS signaling, lead to the activation of HapR, which inhibits biofilm, but our results didn’t support this idea. Meanwhile, cranberry constituents have been reported to attenuate QS-mediated cell-cell signaling in V. harveyi and P. aeruginosa. In V. harveyi, the nondialyzable material (NDM) of cranberry competes with the autoinducer for binding to its receptor [46]. In P. aeruginosa, cranberry-derived PACs (contains > 95% PACs) inhibits autoinducer production and also antagonizes the activation of QS transcriptional regulators [48]. These studies showed that the NDM or PACs is the major component in cranberry that functions as QS inhibitor. The cranberry extract used in our study is water-soluble and contain only 4% PACs; this probably explains why we did not see any impacts on QS signaling but also indicates the aqueous cranberry extract possesses anti-biofilm activity in a QS-independent mode.
The second messenger c-di-GMP has been identified in almost all major bacterial phyla, and its role as a signaling molecule involved in a variety of physiological processes has been well documented [71]. In V. cholerae, c-di-GMP controls motile to sessile transition at both the initial attachment and the matrix production/development stages. Jones et al. reported that c-di-GMP promotes the polymerization of MshA subunits to form the MshA pili which are indispensable for the initial attachment to abiotic surfaces [23]. C-di-GMP also promotes the biofilm matrix production through interaction with the two key transcriptional activators, VpsR and VpsT [24, 25]. Since the V. cholerae genome encodes numerous proteins containing the functional domains for c-di-GMP metabolism, instead of measuring the expression of individual genes, we directly measured the change of intracellular c-di-GMP concentration when WCESP is added to the growth medium. The decrease of c-di-GMP was detected in the 8, 16, and 24 hr samples, but not in the 4 hr samples. Consistent with the results from the motility, qRT-PCR, and pre-established biofilm inhibition assays, this result suggested that the soluble constituents from WCESP impede the development/maturation stage of biofilm formation through modulating the c-di-GMP level. It’s worth mentioning that we didn’t observe enhanced motility at 24 hr, when c-di-GMP level was reduced by WCESP. High concentration of c-di-GMP is known to repress V. cholerea motility [20], thus we should expect to see enhanced motility if c-di-GMP level is decreased. This discrepancy might reflect the signaling specificity among different c-di-GMP receptors or binding proteins. We propose multiple mechanisms might be involved in moderating the c-di-GMP concentration by WCESP. The active ingredients from WCESP may affect c-di-GMP synthesis and degradation through downregulating the diguanylate cyclases or upregulating the phosphodiesterases at the expression level or changing their activities; the active ingredients may also function as a c-di-GMP sequestrator to compete with the c-di-GMP receptors. In addition, WCESP may affect the communication between c-di-GMP and its receptor through binding to the receptor protein or changing its conformation or activity. Studies are underway to elucidate the molecular mechanisms. Characterization of the active ingredients from WCESP is another focus of our research.
In this study, we also tested WCESP’s anti-biofilm activity on other pathogenic bacteria, including four Gram-positive strains (S. aureus, E. faecalis, and two L. monocytogenes strains) and three Gram-negative bacteria (P. aeruginosa, S. typhimurium, and E. coli O157:H7). No inhibition was observed in all these strains. In contrast to V. cholerae, QS (high cell density) promotes biofilm formation in these strains. Because the content of PACs or NDM (the potential QSI) is quite low in our WCESP sample, there was probably no impact on QS signaling in these strains. In the three Gram-negative bacteria, c-di-GMP signaling has also been characterized to regulate biofilm formation, yet we didn’t see biofilm inhibition effect. Maybe higher concentrations of WCESP are required, or more importantly, each bacterium has a unique c-di-GMP signaling network.
Conclusions
Targeting QS or the nucleotide-based second messengers is considered the new hope to address the growing problem of antibiotic resistance. While various natural and synthetic compounds have been characterized as QSIs, inhibitors of the cyclic dinucleotide signaling are still lacking. In this study, we revealed soluble constituents in cranberry extract could inhibit V. cholerae biofilm formation possibly through modulating the c-di-GMP level. Although there are more questions related to the inhibition mechanisms and the active ingredients to be answered, this work suggests that cranberry, as well as many functional foods, may contain potent inhibitors of the bacterial nucleotide-based signaling pathway and leads to a new direction of the functional food study and the antimicrobial and anti-biofilm drug development.
Supporting information
The bacterial cultures were incubated for 24, 48, and 72 hours without shaking in the absence and presence of 2 mg/ml of WCESP.
(TIF)
Results are the average of three independent experiments, and error bars are standard error of the mean.
(TIF)
The same experiment was repeated three times. The data shown are the representative results from one of the experiments.
(DOCX)
Acknowledgments
We would like to thank Dr. Jay (Jun) Zhu for generously providing all the V. cholerae strains, Dr. Andrew Camilli for providing the pAT1662 plasmid, and Reza Ghaedian (Decas) for providing the cranberry extract. We thank Joseph Angeloni, Ojas Natarajan, and Zane Williams for insightful discussion. We appreciate Lauren V. Blakely for initial trials on the experimental conditions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31(2):224–45. Epub 2012/11/09. 10.1016/j.biotechadv.2012.10.004 . [DOI] [PubMed] [Google Scholar]
- 2.Rampioni G, Leoni L, Williams P. The art of antibacterial warfare: Deception through interference with quorum sensing-mediated communication. Bioorg Chem. 2014;55:60–8. Epub 2014/04/21. 10.1016/j.bioorg.2014.04.005 . [DOI] [PubMed] [Google Scholar]
- 3.Opoku-Temeng C, Zhou J, Zheng Y, Su J, Sintim HO. Cyclic dinucleotide (c-di-GMP, c-di-AMP, and cGAMP) signalings have come of age to be inhibited by small molecules. Chem Commun (Camb). 2016;52(60):9327–42. Epub 2016/06/24. 10.1039/c6cc03439j . [DOI] [PubMed] [Google Scholar]
- 4.Lutz C, Erken M, Noorian P, Sun S, McDougald D. Environmental reservoirs and mechanisms of persistence of Vibrio cholerae. Front Microbiol. 2013;4:375 Epub 2013/12/16. 10.3389/fmicb.2013.00375 ; PubMed Central PMCID: PMCPMC3863721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev Cell. 2003;5(4):647–56. . [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Stirling FR, Zhu J. Temporal quorum-sensing induction regulates Vibrio cholerae biofilm architecture. Infect Immun. 2007;75(1):122–6. Epub 2006/10/30. 10.1128/IAI.01190-06 ; PubMed Central PMCID: PMCPMC1828391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yildiz FH, Schoolnik GK. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc Natl Acad Sci U S A. 1999;96(7):4028–33. ; PubMed Central PMCID: PMCPMC22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong JC, Syed KA, Klose KE, Yildiz FH. Role of Vibrio polysaccharide (vps) genes in VPS production, biofilm formation and Vibrio cholerae pathogenesis. Microbiology. 2010;156(Pt 9):2757–69. Epub 2010/05/13. 10.1099/mic.0.040196-0 ; PubMed Central PMCID: PMCPMC3068689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong JC, Yildiz FH. The rbmBCDEF gene cluster modulates development of rugose colony morphology and biofilm formation in Vibrio cholerae. J Bacteriol. 2007;189(6):2319–30. Epub 2007/01/12. 10.1128/JB.01569-06 ; PubMed Central PMCID: PMCPMC1899372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berk V, Fong JC, Dempsey GT, Develioglu ON, Zhuang X, Liphardt J, et al. Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science. 2012;337(6091):236–9. 10.1126/science.1222981 ; PubMed Central PMCID: PMCPMC3513368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong JC, Karplus K, Schoolnik GK, Yildiz FH. Identification and characterization of RbmA, a novel protein required for the development of rugose colony morphology and biofilm structure in Vibrio cholerae. J Bacteriol. 2006;188(3):1049–59. 10.1128/JB.188.3.1049-1059.2006 ; PubMed Central PMCID: PMCPMC1347326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yildiz FH, Dolganov NA, Schoolnik GK. VpsR, a Member of the Response Regulators of the Two-Component Regulatory Systems, Is Required for Expression of vps Biosynthesis Genes and EPS(ETr)-Associated Phenotypes in Vibrio cholerae O1 El Tor. J Bacteriol. 2001;183(5):1716–26. 10.1128/JB.183.5.1716-1726.2001 ; PubMed Central PMCID: PMCPMC95057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casper-Lindley C, Yildiz FH. VpsT is a transcriptional regulator required for expression of vps biosynthesis genes and the development of rugose colonial morphology in Vibrio cholerae O1 El Tor. J Bacteriol. 2004;186(5):1574–8. 10.1128/JB.186.5.1574-1578.2004 ; PubMed Central PMCID: PMCPMC344397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yildiz FH, Liu XS, Heydorn A, Schoolnik GK. Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol Microbiol. 2004;53(2):497–515. 10.1111/j.1365-2958.2004.04154.x . [DOI] [PubMed] [Google Scholar]
- 15.Jenal U, Malone J. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu Rev Genet. 2006;40:385–407. 10.1146/annurev.genet.40.110405.090423 . [DOI] [PubMed] [Google Scholar]
- 16.Boyd CD, O'Toole GA. Second messenger regulation of biofilm formation: breakthroughs in understanding c-di-GMP effector systems. Annu Rev Cell Dev Biol. 2012;28:439–62. 10.1146/annurev-cellbio-101011-155705 ; PubMed Central PMCID: PMCPMC4936406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Römling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77(1):1–52. 10.1128/MMBR.00043-12 ; PubMed Central PMCID: PMCPMC3591986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer BK, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol. 2003;50(1):101–4. . [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A. 2002;99(5):3129–34. Epub 2002/02/19. 10.1073/pnas.052694299 ; PubMed Central PMCID: PMCPMC122484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava D, Hsieh ML, Khataokar A, Neiditch MB, Waters CM. Cyclic di-GMP inhibits Vibrio cholerae motility by repressing induction of transcription and inducing extracellular polysaccharide production. Mol Microbiol. 2013;90(6):1262–76. Epub 2013/11/08. 10.1111/mmi.12432 ; PubMed Central PMCID: PMCPMC3881292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim B, Beyhan S, Meir J, Yildiz FH. Cyclic-diGMP signal transduction systems in Vibrio cholerae: modulation of rugosity and biofilm formation. Mol Microbiol. 2006;60(2):331–48. 10.1111/j.1365-2958.2006.05106.x . [DOI] [PubMed] [Google Scholar]
- 22.Beyhan S, Tischler AD, Camilli A, Yildiz FH. Transcriptome and phenotypic responses of Vibrio cholerae to increased cyclic di-GMP level. J Bacteriol. 2006;188(10):3600–13. 10.1128/JB.188.10.3600-3613.2006 ; PubMed Central PMCID: PMCPMC1482859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones CJ, Utada A, Davis KR, Thongsomboon W, Zamorano Sanchez D, Banakar V, et al. C-di-GMP Regulates Motile to Sessile Transition by Modulating MshA Pili Biogenesis and Near-Surface Motility Behavior in Vibrio cholerae. PLoS Pathog. 2015;11(10):e1005068 Epub 2015/10/27. 10.1371/journal.ppat.1005068 ; PubMed Central PMCID: PMCPMC4624765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava D, Harris RC, Waters CM. Integration of cyclic di-GMP and quorum sensing in the control of vpsT and aphA in Vibrio cholerae. J Bacteriol. 2011;193(22):6331–41. Epub 2011/09/16. 10.1128/JB.05167-11 ; PubMed Central PMCID: PMCPMC3209240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krasteva PV, Fong JC, Shikuma NJ, Beyhan S, Navarro MV, Yildiz FH, et al. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science. 2010;327(5967):866–8. 10.1126/science.1181185 ; PubMed Central PMCID: PMCPMC2828054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J Bacteriol. 2005;187(5):1792–8. 10.1128/JB.187.5.1792-1798.2005 ; PubMed Central PMCID: PMCPMC1064016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galperin MY, Nikolskaya AN, Koonin EV. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol Lett. 2001;203(1):11–21. . [DOI] [PubMed] [Google Scholar]
- 28.Conner JG, Zamorano-Sánchez D, Park JH, Sondermann H, Yildiz FH. The ins and outs of cyclic di-GMP signaling in Vibrio cholerae. Curr Opin Microbiol. 2017;36:20–9. Epub 2017/02/05. 10.1016/j.mib.2017.01.002 ; PubMed Central PMCID: PMCPMC5534393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beyhan S, Yildiz FH. Smooth to rugose phase variation in Vibrio cholerae can be mediated by a single nucleotide change that targets c-di-GMP signalling pathway. Mol Microbiol. 2007;63(4):995–1007. 10.1111/j.1365-2958.2006.05568.x . [DOI] [PubMed] [Google Scholar]
- 30.Shikuma NJ, Fong JC, Yildiz FH. Cellular levels and binding of c-di-GMP control subcellular localization and activity of the Vibrio cholerae transcriptional regulator VpsT. PLoS Pathog. 2012;8(5):e1002719 Epub 2012/05/24. 10.1371/journal.ppat.1002719 ; PubMed Central PMCID: PMCPMC3359988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dinh J, Angeloni JT, Pederson DB, Wang X, Cao M, Dong Y. Cranberry extract standardized for proanthocyanidins promotes the immune response of Caenorhabditis elegans to Vibrio cholerae through the p38 MAPK pathway and HSF-1. PLoS One. 2014;9(7):e103290 Epub 2014/07/25. 10.1371/journal.pone.0103290 ; PubMed Central PMCID: PMCPMC4111578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tischler AD, Camilli A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol Microbiol. 2004;53(3):857–69. 10.1111/j.1365-2958.2004.04155.x ; PubMed Central PMCID: PMCPMC2790424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thelin KH, Taylor RK. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun. 1996;64(7):2853–6. ; PubMed Central PMCID: PMCPMC174155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vance RE, Zhu J, Mekalanos JJ. A constitutively active variant of the quorum-sensing regulator LuxO affects protease production and biofilm formation in Vibrio cholerae. Infect Immun. 2003;71(5):2571–6. 10.1128/IAI.71.5.2571-2576.2003 ; PubMed Central PMCID: PMCPMC153284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albert MJ, Siddique AK, Islam MS, Faruque AS, Ansaruzzaman M, Faruque SM, et al. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet. 1993;341(8846):704 . [DOI] [PubMed] [Google Scholar]
- 36.Ramamurthy T, Garg S, Sharma R, Bhattacharya SK, Nair GB, Shimada T, et al. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet. 1993;341(8846):703–4. . [DOI] [PubMed] [Google Scholar]
- 37.Prabu GR, Gnanamani A, Sadulla S. Guaijaverin—a plant flavonoid as potential antiplaque agent against Streptococcus mutans. J Appl Microbiol. 2006;101(2):487–95. 10.1111/j.1365-2672.2006.02912.x . [DOI] [PubMed] [Google Scholar]
- 38.Miller J. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor laboratory Press.; 1972. [Google Scholar]
- 39.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. . [DOI] [PubMed] [Google Scholar]
- 40.Nakayama S, Kelsey I, Wang J, Roelofs K, Stefane B, Luo Y, et al. Thiazole orange-induced c-di-GMP quadruplex formation facilitates a simple fluorescent detection of this ubiquitous biofilm regulating molecule. J Am Chem Soc. 2011;133(13):4856–64. Epub 2011/03/08. 10.1021/ja1091062 . [DOI] [PubMed] [Google Scholar]
- 41.Watnick PI, Kolter R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol Microbiol. 1999;34(3):586–95. ; PubMed Central PMCID: PMCPMC2860543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Mei HC, Roseberg M, Busscher HJ. Microbial Cell Surface Analysis: Structural and Physicochemical Methods: VCH; 1991. [Google Scholar]
- 43.Dahlbäck B, Hermansson M, Kjelleberg S, Norkrans B. The hydrophobicity of bacteria—an important factor in their initial adhesion at the air-water interface. Arch Microbiol. 1981;128(3):267–70. . [DOI] [PubMed] [Google Scholar]
- 44.Hollenbeck EC, Fong JC, Lim JY, Yildiz FH, Fuller GG, Cegelski L. Molecular determinants of mechanical properties of V. cholerae biofilms at the air-liquid interface. Biophys J. 2014;107(10):2245–52. 10.1016/j.bpj.2014.10.015 ; PubMed Central PMCID: PMCPMC4241461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ali A, Johnson JA, Franco AA, Metzger DJ, Connell TD, Morris JG, et al. Mutations in the extracellular protein secretion pathway genes (eps) interfere with rugose polysaccharide production in and motility of Vibrio cholerae. Infect Immun. 2000;68(4):1967–74. ; PubMed Central PMCID: PMCPMC97374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldman M, Weiss EI, Ofek I, Steinberg D. Interference of cranberry constituents in cell-cell signaling system of Vibrio harveyi. Curr Microbiol. 2009;59(4):469–74. Epub 2009/08/11. 10.1007/s00284-009-9462-3 . [DOI] [PubMed] [Google Scholar]
- 47.Harjai K, Gupta RK, Sehgal H. Attenuation of quorum sensing controlled virulence of Pseudomonas aeruginosa by cranberry. Indian J Med Res. 2014;139(3):446–53. ; PubMed Central PMCID: PMCPMC4069740. [PMC free article] [PubMed] [Google Scholar]
- 48.Maisuria VB, Los Santos YL, Tufenkji N, Déziel E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci Rep. 2016;6:30169 Epub 2016/08/09. 10.1038/srep30169 ; PubMed Central PMCID: PMCPMC4977528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 2012;23(2):174–81. Epub 2011/09/16. 10.1016/j.copbio.2011.08.007 . [DOI] [PubMed] [Google Scholar]
- 50.Slobodníková L, Fialová S, Rendeková K, Kováč J, Mučaji P. Antibiofilm Activity of Plant Polyphenols. Molecules. 2016;21(12). Epub 2016/12/13. 10.3390/molecules21121717 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pappas E, Schaich KM. Phytochemicals of cranberries and cranberry products: characterization, potential health effects, and processing stability. Crit Rev Food Sci Nutr. 2009;49(9):741–81. 10.1080/10408390802145377 . [DOI] [PubMed] [Google Scholar]
- 52.Côté J, Caillet S, Doyon G, Sylvain JF, Lacroix M. Analyzing cranberry bioactive compounds. Crit Rev Food Sci Nutr. 2010;50(9):872–88. 10.1080/10408390903042069 . [DOI] [PubMed] [Google Scholar]
- 53.Côté J, Caillet S, Doyon G, Sylvain JF, Lacroix M. Bioactive compounds in cranberries and their biological properties. Crit Rev Food Sci Nutr. 2010;50(7):666–79. 10.1080/10408390903044107 . [DOI] [PubMed] [Google Scholar]
- 54.Walker EB, Barney DP, Mickelsen JN, Walton RJ, Mickelsen RA. Cranberry concentrate: UTI prophylaxis. J Fam Pract. 1997;45(2):167–8. . [PubMed] [Google Scholar]
- 55.Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66(18):2281–91. 10.1016/j.phytochem.2005.05.022 . [DOI] [PubMed] [Google Scholar]
- 56.Howell AB. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol Nutr Food Res. 2007;51(6):732–7. 10.1002/mnfr.200700038 . [DOI] [PubMed] [Google Scholar]
- 57.Steinberg D, Feldman M, Ofek I, Weiss EI. Effect of a high-molecular-weight component of cranberry on constituents of dental biofilm. J Antimicrob Chemother. 2004;54(1):86–9. Epub 2004/05/28. 10.1093/jac/dkh254 [pii]. . [DOI] [PubMed] [Google Scholar]
- 58.Yamanaka A, Kimizuka R, Kato T, Okuda K. Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation. Oral Microbiol Immunol. 2004;19(3):150–4. 10.1111/j.0902-0055.2004.00130.x . [DOI] [PubMed] [Google Scholar]
- 59.Yamanaka A, Kouchi T, Kasai K, Kato T, Ishihara K, Okuda K. Inhibitory effect of cranberry polyphenol on biofilm formation and cysteine proteases of Porphyromonas gingivalis. J Periodontal Res. 2007;42(6):589–92. 10.1111/j.1600-0765.2007.00982.x . [DOI] [PubMed] [Google Scholar]
- 60.Bodet C, Grenier D, Chandad F, Ofek I, Steinberg D, Weiss EI. Potential oral health benefits of cranberry. Crit Rev Food Sci Nutr. 2008;48(7):672–80. 10.1080/10408390701636211 . [DOI] [PubMed] [Google Scholar]
- 61.Burger O, Weiss E, Sharon N, Tabak M, Neeman I, Ofek I. Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular-weight constituent of cranberry juice. Crit Rev Food Sci Nutr. 2002;42(3 Suppl):279–84. Epub 2002/06/13. 10.1080/10408390209351916 . [DOI] [PubMed] [Google Scholar]
- 62.Koo H, Nino de Guzman P, Schobel BD, Vacca Smith AV, Bowen WH. Influence of cranberry juice on glucan-mediated processes involved in Streptococcus mutans biofilm development. Caries Res. 2006;40(1):20–7. 10.1159/000088901 . [DOI] [PubMed] [Google Scholar]
- 63.Labrecque J, Bodet C, Chandad F, Grenier D. Effects of a high-molecular-weight cranberry fraction on growth, biofilm formation and adherence of Porphyromonas gingivalis. J Antimicrob Chemother. 2006;58(2):439–43. Epub 2006/05/30. 10.1093/jac/dkl220 . [DOI] [PubMed] [Google Scholar]
- 64.Babu J, Blair C, Jacob S, Itzhak O. Inhibition of Streptococcus gordonii metabolic activity in biofilm by cranberry juice high-molecular-weight component. J Biomed Biotechnol. 2012;2012:590384 Epub 2012/01/18. 10.1155/2012/590384 ; PubMed Central PMCID: PMCPMC3270421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim D, Hwang G, Liu Y, Wang Y, Singh AP, Vorsa N, et al. Cranberry Flavonoids Modulate Cariogenic Properties of Mixed-Species Biofilm through Exopolysaccharides-Matrix Disruption. PLoS One. 2015;10(12):e0145844 Epub 2015/12/29. 10.1371/journal.pone.0145844 ; PubMed Central PMCID: PMCPMC4699891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tapiainen T, Jauhiainen H, Jaakola L, Salo J, Sevander J, Ikäheimo I, et al. Biofilm formation and virulence of uropathogenic Escherichia coli in urine after consumption of cranberry-lingonberry juice. Eur J Clin Microbiol Infect Dis. 2012;31(5):655–62. Epub 2011/08/07. 10.1007/s10096-011-1355-2 . [DOI] [PubMed] [Google Scholar]
- 67.Rane HS, Bernardo SM, Howell AB, Lee SA. Cranberry-derived proanthocyanidins prevent formation of Candida albicans biofilms in artificial urine through biofilm- and adherence-specific mechanisms. J Antimicrob Chemother. 2014;69(2):428–36. Epub 2013/10/10. 10.1093/jac/dkt398 ; PubMed Central PMCID: PMCPMC3937597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wojnicz D, Tichaczek-Goska D, Korzekwa K, Kicia M, Hendrich AB. Study of the impact of cranberry extract on the virulence factors and biofilm formation by Enterococcus faecalis strains isolated from urinary tract infections. Int J Food Sci Nutr. 2016;67(8):1005–16. Epub 2016/07/26. 10.1080/09637486.2016.1211996 . [DOI] [PubMed] [Google Scholar]
- 69.Leshem R, Maharshak I, Ben Jacob E, Ofek I, Kremer I. The effect of nondialyzable material (NDM) cranberry extract on formation of contact lens biofilm by Staphylococcus epidermidis. Invest Ophthalmol Vis Sci. 2011;52(7):4929–34. Epub 2011/07/01. 10.1167/iovs.10-5335 . [DOI] [PubMed] [Google Scholar]
- 70.Ulrey RK, Barksdale SM, Zhou W, van Hoek ML. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement Altern Med. 2014;14:499 Epub 2014/12/16. 10.1186/1472-6882-14-499 ; PubMed Central PMCID: PMCPMC4320558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jenal U, Reinders A, Lori C. Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol. 2017;15(5):271–84. Epub 2017/02/06. 10.1038/nrmicro.2016.190 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The bacterial cultures were incubated for 24, 48, and 72 hours without shaking in the absence and presence of 2 mg/ml of WCESP.
(TIF)
Results are the average of three independent experiments, and error bars are standard error of the mean.
(TIF)
The same experiment was repeated three times. The data shown are the representative results from one of the experiments.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.