Supplemental Digital Content is available in the text.
Keywords: aortic valve stenosis, magnetic resonance imaging, mortality, myocardium
Abstract
Background:
Aortic valve replacement (AVR) for aortic stenosis is timed primarily on the development of symptoms, but late surgery can result in irreversible myocardial dysfunction and additional risk. The aim of this study was to determine whether the presence of focal myocardial scar preoperatively was associated with long-term mortality.
Methods:
In a longitudinal observational outcome study, survival analysis was performed in patients with severe aortic stenosis listed for valve intervention at 6 UK cardiothoracic centers. Patients underwent preprocedural echocardiography (for valve severity assessment) and cardiovascular magnetic resonance for ventricular volumes, function and scar quantification between January 2003 and May 2015. Myocardial scar was categorized into 3 patterns (none, infarct, or noninfarct patterns) and quantified with the full width at half-maximum method as percentage of the left ventricle. All-cause mortality and cardiovascular mortality were tracked for a minimum of 2 years.
Results:
Six hundred seventy-four patients with severe aortic stenosis (age, 75±14 years; 63% male; aortic valve area, 0.38±0.14 cm2/m2; mean gradient, 46±18 mm Hg; left ventricular ejection fraction, 61.0±16.7%) were included. Scar was present in 51% (18% infarct pattern, 33% noninfarct). Management was surgical AVR (n=399) or transcatheter AVR (n=275). During follow-up (median, 3.6 years), 145 patients (21.5%) died (52 after surgical AVR, 93 after transcatheter AVR). In multivariable analysis, the factors independently associated with all-cause mortality were age (hazard ratio [HR], 1.50; 95% CI, 1.11–2.04; P=0.009, scaled by epochs of 10 years), Society of Thoracic Surgeons score (HR, 1.12; 95% CI, 1.03–1.22; P=0.007), and scar presence (HR, 2.39; 95% CI, 1.40–4.05; P=0.001). Scar independently predicted all-cause (26.4% versus 12.9%; P<0.001) and cardiovascular (15.0% versus 4.8%; P<0.001) mortality, regardless of intervention (transcatheter AVR, P=0.002; surgical AVR, P=0.026 [all-cause mortality]). Every 1% increase in left ventricular myocardial scar burden was associated with 11% higher all-cause mortality hazard (HR, 1.11; 95% CI, 1.05–1.17; P<0.001) and 8% higher cardiovascular mortality hazard (HR, 1.08; 95% CI, 1.01–1.17; P<0.001).
Conclusions:
In patients with severe aortic stenosis, late gadolinium enhancement on cardiovascular magnetic resonance was independently associated with mortality; its presence was associated with a 2-fold higher late mortality.
Clinical Perspective.
What Is New?
In patients with severe aortic stenosis, focal myocardial fibrosis (scar) determined by cardiovascular magnetic resonance was present in >50% of patients and was associated with a 2-fold higher late mortality.
Focal scar (both infarct and noninfarct patterns) was independently associated with all-cause and cardiovascular mortality after both surgical and transcatheter aortic valve replacement.
What Are the Clinical Implications?
In severe aortic stenosis, late gadolinium enhancement appears to be a useful biomarker of left ventricular remodeling, and its presence is associated with worse long-term outcomes after aortic valve intervention.
This raises the hypothesis that, for some patients, timing of aortic valve intervention may be too late once scar has developed and that randomized trials of earlier intervention are now required.
Editorial, see p 1948
Aortic stenosis (AS) is the most common valvular heart disease.1 It is characterized by progressive narrowing of the aortic valve and by hypertrophic remodeling of the left ventricular (LV) myocardium.2 This process maintains wall stress and cardiac performance for many years, but ultimately the LV decompensates, heralding the transition to heart failure, symptom development, and death.3 The treatment for AS is valve replacement, with the goal of reducing both symptoms and mortality.
Current guidelines recommend aortic valve intervention by surgical aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR) in symptomatic severe AS or for asymptomatic severe AS in the presence of LV dysfunction or exercise-invoked symptoms.4 However, symptoms can be difficult to interpret, especially in the elderly, who may be less active or have multiple comorbidities, and reduction in ejection fraction is often irreversible and associated with increased risk of heart failure and death.5
Although the primary insult is valve stenosis, the cardiac response to this may be equally important. Therefore, there is growing interest in objective and early markers of cardiac decompensation. Histological and imaging studies have suggested that focal myocardial fibrosis is a key driver in the transition from hypertrophy to heart failure.6–10 Myocardial replacement fibrosis (scar) can be detected by cardiovascular magnetic resonance (CMR) with the late gadolinium enhancement (LGE) technique. From single-center studies, focal fibrosis has been associated with increased levels of myocardial injury, diastolic and systolic dysfunction, electrocardiographic changes, and adverse clinical outcomes.7–10 Focal scar by LGE is irreversible at 9 and 12 months after SAVR.5,11 CMR-detected myocardial fibrosis therefore appears to be a useful and objective biomarker of LV decompensation in AS.
Prior studies have been too small to evaluate the independent association of imaging biomarkers and demographic factors with total and cardiovascular mortality in patients with severe AS.7–10 We established a UK consortium to determine which preoperative factors were most strongly associated with long-term postoperative mortality in patients with severe AS on conventional management pathways, which could potentially be used to time surgery better in the future. We hypothesized that myocardial scarring detected by LGE-CMR would be independently associated with mortality in patients with severe AS undergoing aortic valve intervention.
Methods
The data, analytical methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. The data are available from the corresponding author on reasonable request.
Patients and Study Design
A longitudinal, observational outcome study in patients with severe AS referred to 6 UK cardiothoracic surgical centers and listed for valve intervention (Brompton Hospital and Barts Heart Center in London; Edinburgh Heart Center; Glenfield Hospital in Leicester; Leeds Teaching Hospitals National Health Service Trust; John Radcliffe Hospital in Oxford). Between January 2003 and May 2015, patients were prospectively recruited after evaluation by the multidisciplinary heart team. The study was approved by the UK National Research Ethics Service (13/NW/0832) and conformed to the principles of the Declaration of Helsinki, and all patients gave written informed consent. The primary end point was all-cause mortality. The secondary end point was cardiovascular disease–related mortality as defined by diagnosis on the UK death certificate. Patients >18 years of age with severe AS (1 of the following: aortic valve area <1 cm2, peak pressure gradient >64 mm Hg, mean pressure gradient >40 mm Hg, or peak velocity >4 m/s) who had undergone CMR imaging for research purposes were included.
Image Acquisition
Echocardiographic parameters were acquired as part of the clinical workup following the guidelines for assessment of AS severity recommended by the American and European societies of echocardiography.12 Global hemodynamic load was measured by calculating the valvulo-arterial impedance index, defined as the ratio of the estimated LV systolic pressure (sum of systolic arterial pressure and mean pressure gradient) to the stroke volume indexed for body surface area. CMR was performed on 1.5- and 3-T scanners using standardized protocols. In brief, cine images were acquired in long-axis planes and contiguous short-axis slices for ventricular volumes, mass, and function. Phase-contrast velocity-encoded images were acquired for valve hemodynamics, and the LGE technique was used to identify myocardial scar, as previously described.13 All participating centers have previously published single-center mechanistic data in AS in which image quality and specific CMR pulse sequence parameters can be reviewed.10,14–17
Data Management and Outcomes
Anonymized clinical and imaging data were collected and managed with REDCap (Research Electronic Data Capture) software18 hosted at Barts Heart Center/University College London. All deaths were identified through the UK National Health Service National Spine Database. Cardiovascular mortality was established in all deceased individuals from the official death certificates, which in the United Kingdom list up to 3 causes of death and were adjudicated by 2 readers (P.B., J.P.G.) blinded to all clinical data. Cardiovascular mortality was defined as death attributable to myocardial ischemia and infarction, heart failure, cardiac arrest resulting from arrhythmia or unknown cause, or cerebrovascular accident.
Data Analysis
All CMR scans were centralized and rereported in core laboratory fashion by experienced readers blinded to clinical parameters using CVI42 software (Circle Calgary). Each center analyzed a single component of the CMR scan for the entire study population, according to a prespecified standard operating procedure (online-only Data Supplement) and after a period of training and reproducibility evaluation. LV volume and mass analysis was performed by manual contouring of the endocardial and epicardial borders at end diastole and end systole.19 Left atrial area and length at end systole were measured in the horizontal (4-chamber) and vertical long-axis (2-chamber) views for calculation of left atrial volumes by the biplane area length method and indexed.19 Aortic flow for regurgitant volume and fraction was quantified from phase-contrast velocity-encoded images.20 LGE was categorized by 2 observers into 3 patterns (none, infarct, or noninfarct patterns) and quantified with the full width at half-maximum method as percentage of the LV.13 Examples of typical echocardiographic and CMR images are shown in Figure 1. Further technical details of the image analysis can be found in the online-only Data Supplement.
Figure 1.
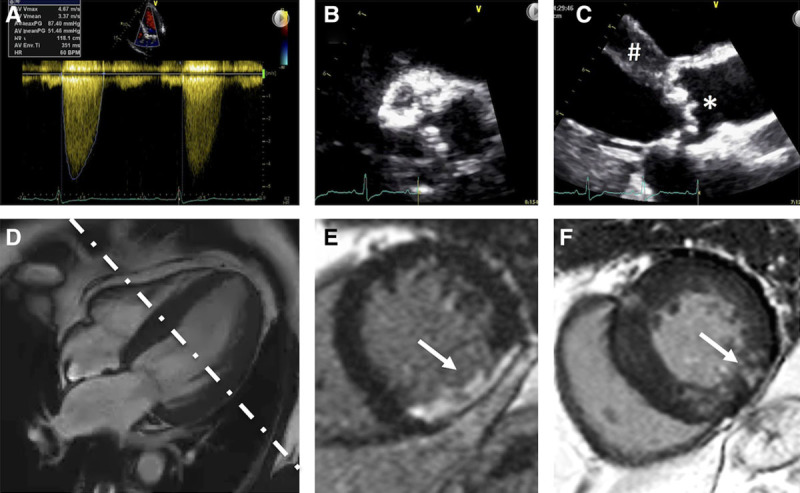
Multi modality assessment of aortic stenosis (AS). Assessment of AS by transthoracic echocardiography (TTE; A–C) and cardiovascular magnetic resonance (D–F). A, Continuous Doppler trace across the aortic valve in the apical 5-chamber demonstrating hemodynamic parameters consistent with severe AS (peak velocity, 4.67 m/s; peak gradient, 87 mm Hg; mean gradient, 51 mm Hg). B, Short-axis TTE image of a severely calcified aortic valve. C, Parasternal long-axis image demonstrating left ventricular hypertrophy (#) and a calcified aortic valve (*). D, Four-chamber balanced steady-state free precession cine image demonstrating left ventricular hypertrophy; white dotted line demonstrates the axis of acquisition of the short axis (E and F). E, Late gadolinium enhancement (LGE) image in a midventricular short axis showing transmural LGE of a full-thickness myocardial infarct (arrow). F, LGE image in a midventricular short axis showing patchy nonischemia LGE in the mid inferolateral segment (arrow) and more subtle LGE in the inferoseptum and right ventricular insertion points.
Statistics
Statistical analysis was performed in R (version 3.0.1; The R Foundation for Statistical Computing). Distribution of data was assessed on histograms and with the Shapiro-Wilk test. Continuous variables are expressed as mean±SD or as median and interquartile range; categorical variables, as counts and percent. Baseline characteristics of participants were compared with the unpaired Student t test, Mann-Whitney-Wilcoxon test, χ2 test, or Fisher exact test as appropriate. The primary end point was all-cause mortality. The secondary end point was cardiovascular disease–related mortality. In addition, we computed early postintervention (TAVR/SAVR) mortality (defined as 30-day or in-hospital mortality). Survival in patients with and without LGE was evaluated with the Kaplan-Meier method and compared among groups with the log-rank test. The index date was the date of CMR. Hazard ratios (HRs) were expressed as mean±95% CIs.
All clinical parameters were proposed for inclusion in a univariate Cox proportional hazards model. The most predictive candidate variable was selected from each of 3 domains if applicable (clinical, echocardiography, CMR) to avoid colinearity and then entered into the final model. Unique, clinically relevant predictor variables with a value of P<0.10 in univariate analysis were entered into final multivariable models; a forward stepwise procedure was used. The incremental value between steps was measured by the χ2 method. The proportional hazards assumption was tested with the use of log-log plots and examination of Schoenfeld residuals. All tests were 2-sided; values of P<0.05 were considered significant.
Role of the Funding Source
No additional funding was obtained for this consortium study beyond that of the original single-center research funding. Funders provided financial support for the original data collection but had no role in the consortium study design, data collection, data analysis, data interpretation, or writing of the report. All authors had access to the primary data and have final responsibility for publication.
Results
Baseline Characteristics
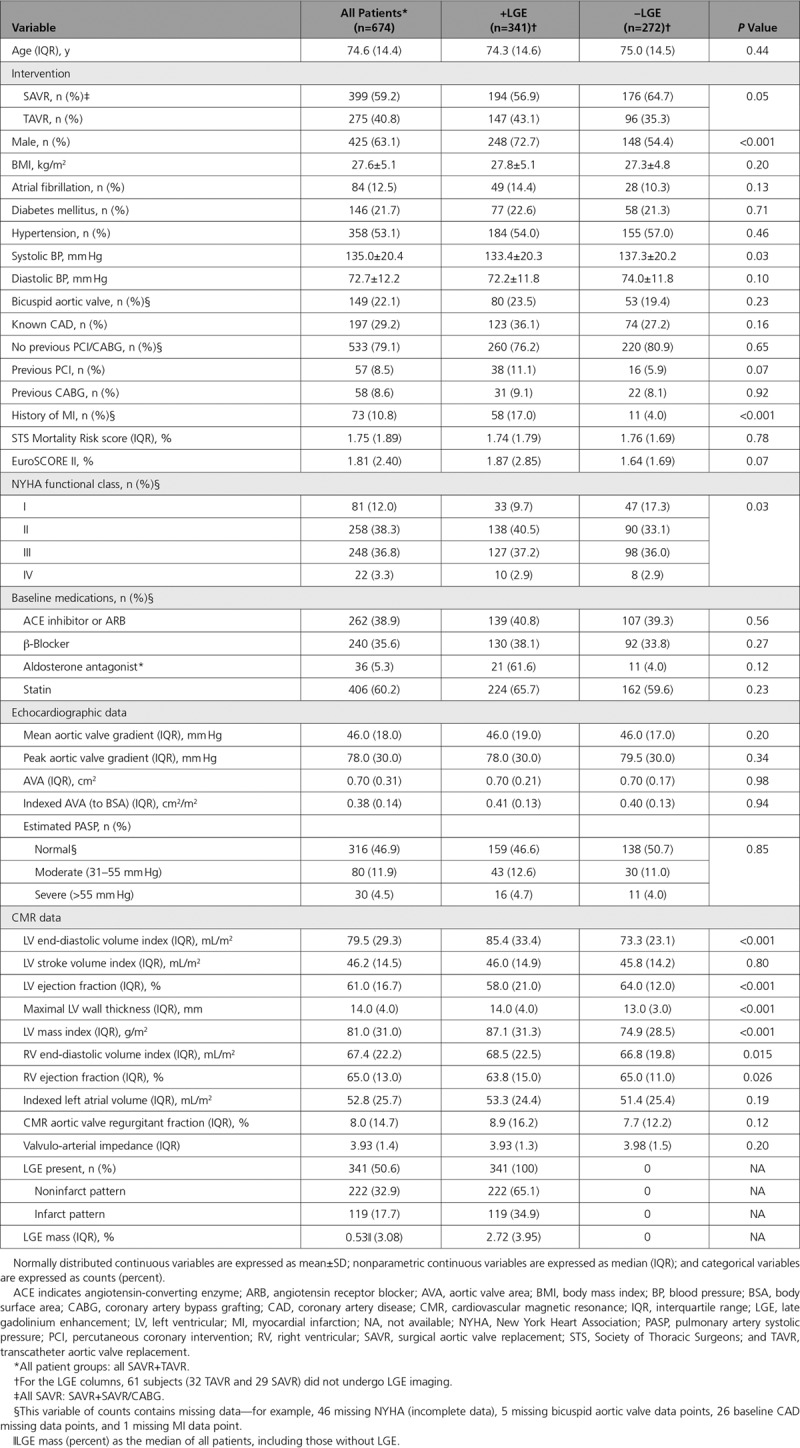
Baseline characteristics of the 674 patients included are shown in Table 1 and Figure I in the online-only Data Supplement (study flowchart). Mean age was 75±14 years (63% male) with a mean aortic valve area of 0.38±0.14 cm2/m2 and mean gradient of 46±18 mm Hg. Median aortic valve regurgitant fraction was 8.0% (interquartile range, 2.7%–17.3%); 16% of patients had at least moderately elevated pulmonary arterial systolic pressure (defined as 30–55 mm Hg by echocardiography). LV myocardial scar, as assessed by LGE, was present in 51% of patients in a 2:1 ratio between noninfarct (33%) and infarct (18%) pattern scar.
Table 1.
Baseline Characteristics
Management by Surgical Replacement Versus Transcatheter Replacement
Management was SAVR (n=399) or TAVR (n=275). Median time from CMR to SAVR was 44 days (interquartile range, 11–103 days) and to TAVR was 13 days (interquartile range, 1–61 days). Compared with SAVR, patients managed with TAVR were older (79.2±7.8 versus 68.6±10.3 years; P<0.001) and more likely female (48% versus 29%; P<0.001) with more atrial fibrillation (21.1% versus 6.5%; P<0.001), more coronary artery disease (39.3% versus 19.5%; P<0.001), less hypertension (42.6% versus 59.5%; P<0.001), and fewer bicuspid aortic valves (5.5% versus 33.8%; P<0.001). Patients undergoing TAVR had higher peak aortic valve gradients and smaller aortic valve area. Furthermore, patients undergoing TAVR had larger LV volumes and lower LV ejection fraction and had more severe symptoms; LV mass and LGE prevalence were not different between groups, although infarct pattern scar was more prevalent in the TAVR group and noninfarct scar was more prevalent in the SAVR group.
Patient Characteristics According to LGE Status
LGE positive patients were more likely to be male (72.7 versus 54.4%; P<0.001) and to have had a previous myocardial infarct (17.0% versus 4.0%; P<0.001) and had larger indexed LV end-diastolic volume, higher indexed LV mass, and lower LV ejection fraction (all P<0.001) than LGE negative patients (Table 1). In the SAVR cohort only, men also had higher New York Heart Association functional class (P=0.006) and higher systolic blood pressure (138.5±20.5 versus 134.0±17.8 mm Hg; P=0.036).
Outcome
During a median 3.6 years of follow-up (interquartile range, 2.6–5.9 years), 145 patients (21.5%) died (52 after SAVR and 93 after TAVR). This equated to 52 deaths per 1000 patient-years (27 and 104 for SAVR and TAVR groups, respectively). A cardiovascular cause of death was ascribed to 70 patients (10.4% of whole cohort; 19 after SAVR [4.8%], 51 after TAVR [18.5%]). At 30 days after the intervention, overall mortality was 1.8% (n=12), with 1.3% (n=5) for SAVR and 2.5% (n=7) for TAVR. At 1 year, overall mortality was 6.2% (n=42), with 3.0% (n=12) for SAVR and 10.9% (n=30) for TAVR (Table I in the online-only Data Supplement).
Predictors of Outcome
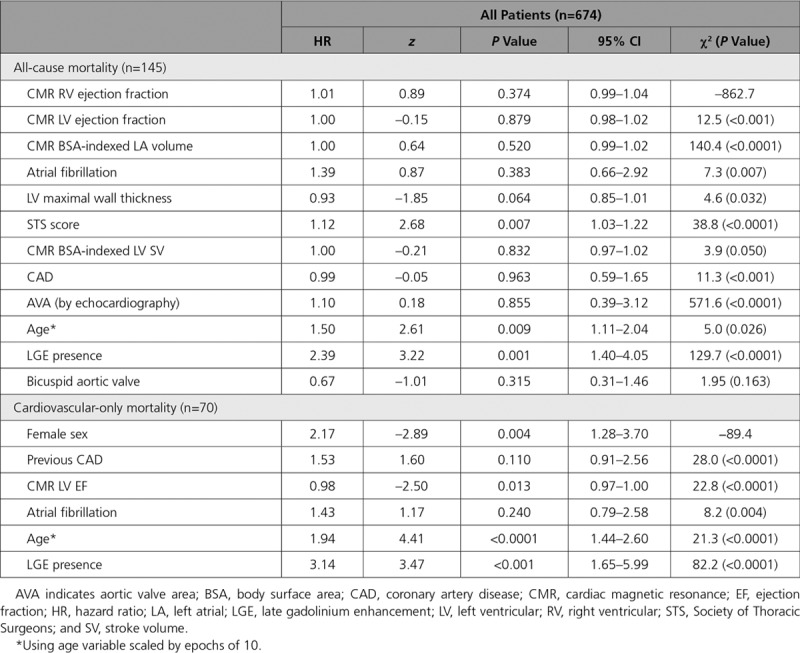
Fifty-two variables were compared with outcome (including demographic, comorbidities, therapies, Society of Thoracic Surgeons score, and imaging [echocardiography/CMR] parameters). In univariate analysis (Table 2 and Tables II and III in the online-only Data Supplement for all, SAVR, and TAVR, respectively), 28 of them were associated with outcome. In multivariable analysis (Table 3 and Tables IV and V in the online-only Data Supplement for all, SAVR, and TAVR, respectively), the factors independently associated with all-cause mortality were age (HR, 1.50; 95% CI, 1.11–2.04; P=0.009, scaled by epochs of 10 years), Society of Thoracic Surgeons score (HR, 1.12; 95% CI, 1.03–1.22; P=0.007), and scar presence (HR, 2.39; 95% CI, 1.40–4.05; P=0.001). The incremental effect of adding age, Society of Thoracic Surgeons score, and LGE presence to the risk stratification model is demonstrated in Figure II in the online-only Data Supplement; global Wald χ2 is shown for separate Cox regression models predicting all-cause death. For cardiovascular mortality, the factors independently associated with all-cause mortality were age (HR, 1.94; 95% CI, 1.44–2.60; P<0.0001, scaled by epochs of 10 years), female sex (HR, 2.17; 95% CI, 1.28–3.70; P<0.001), LGE presence (HR, 3.14; 95% CI, 1.65–5.99; P<0.001), and reduced LV ejection fraction (HR, 0.98; 95% CI, 0.96–1.00; P=0.013). Pulmonary artery systolic pressure was not included in the main model because data were available in only 63.3% (SAVR, 82.7%; TAVR, 49.5%), but when included, the presence of severely elevated pulmonary arterial systolic pressure (>55 mm Hg) was an independent predictor of all-cause mortality (HR, 2.73; 95% CI, 1.21–6.17; P=0.016; Table VI in the online-only Data Supplement). Neither coronary artery disease nor previous coronary revascularization (percutaneous coronary intervention or coronary artery bypass graft surgery) was an independent predictor of mortality (Table VII in the online-only Data Supplement). Furthermore, no echocardiographic or CMR markers of aortic valve stenosis severity were independently predictive of mortality.
Table 2.
Univariate Parameters
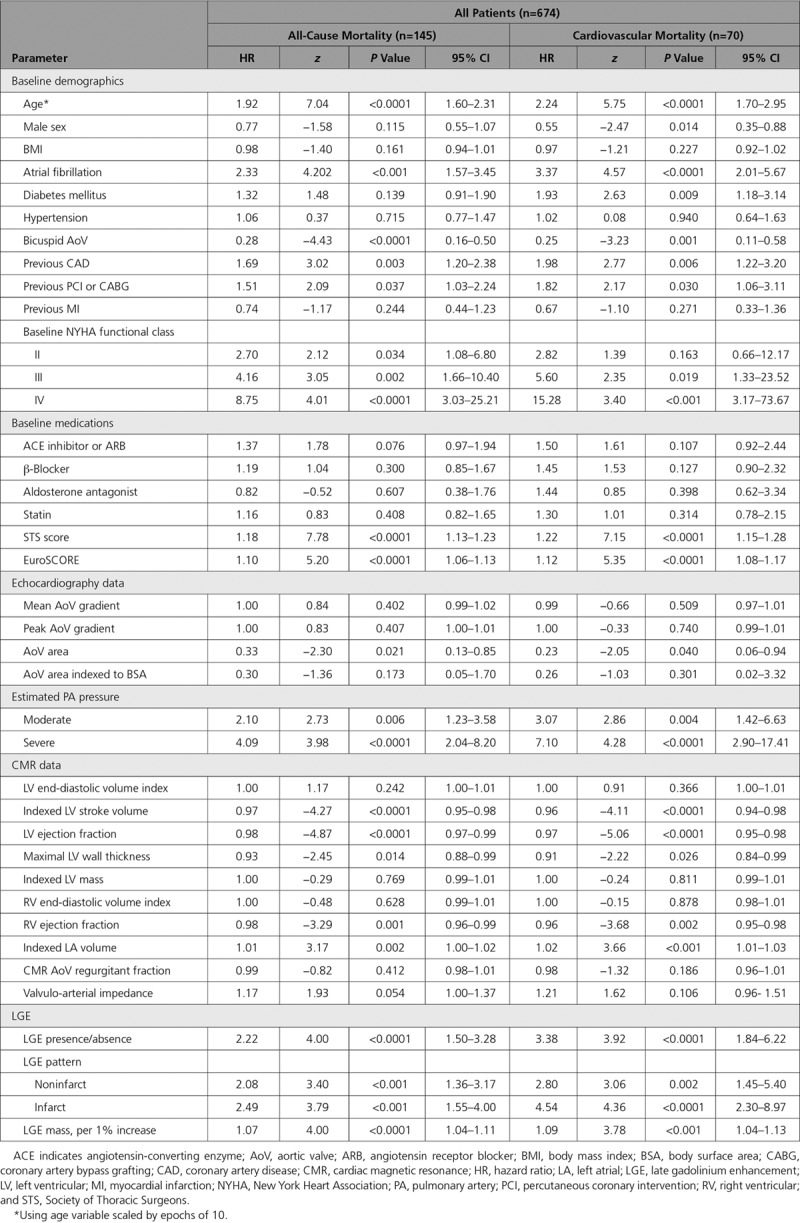
Table 3.
Multivariable Model: All-Cause and Cardiovascular Mortality
Patients with myocardial scar had higher (double) all-cause mortality (26.4% versus 12.9%; P<0.001) and 3 times the cardiovascular mortality (15.0% versus 4.8%; P<0.001), regardless of valve intervention type (TAVR, P=0.002; SAVR, P=0.026; Figure 2) and scar type, with both infarct and noninfarct scar being associated with similarly adverse outcomes (P<0.001 for both; Figure 3)—for example, all-cause mortality, 25.2% noninfarct pattern LGE, 28.6% infarct pattern, and 12.9% no LGE. Quantitatively, every 1% increase in LV myocardial scar burden was associated with an 11% higher all-cause mortality hazard (HR, 1.11; 95% CI, 1.05–1.17; P<0.001) and an 8% higher cardiovascular mortality hazard (HR, 1.08; 95% CI, 1.01–1.17; P<0.001; Table VIII in the online-only Data Supplement). There was no significant change in results when events within 30 days of intervention were excluded or the index date was changed from time of CMR to time of intervention (Tables IX and X in the online-only Data Supplement).
Figure 2.
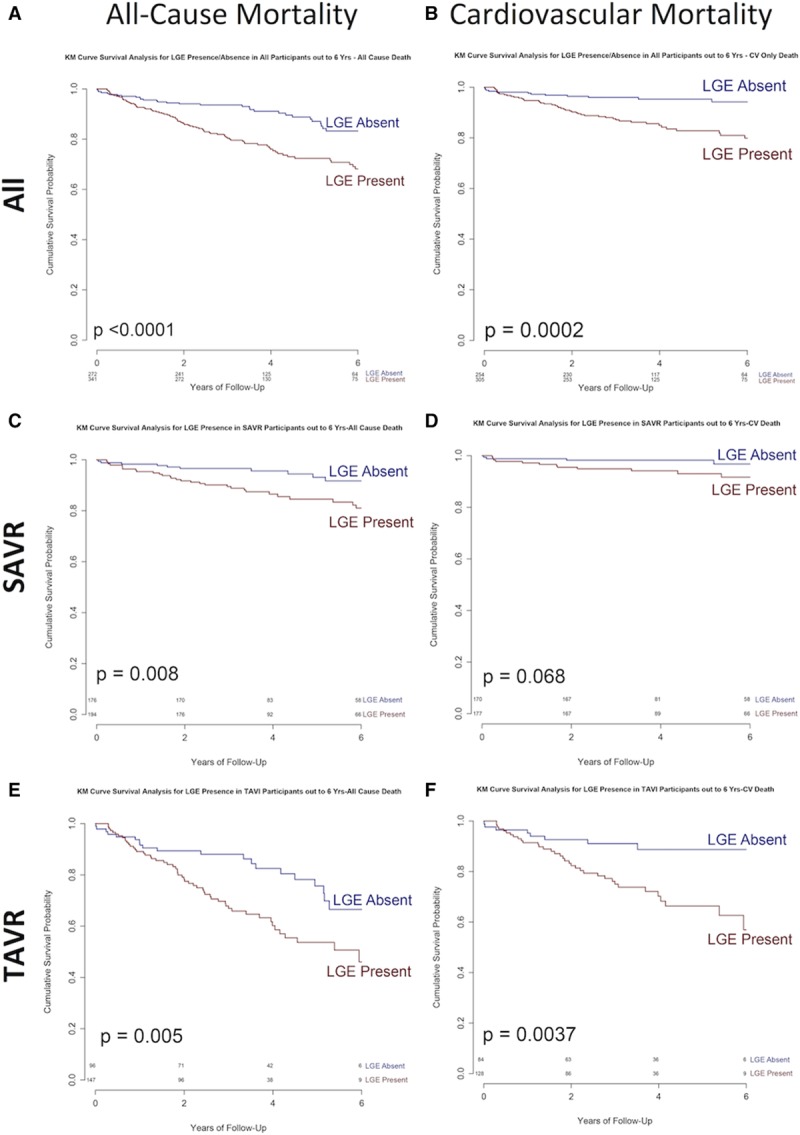
All-cause and cardiovascular mortality in severe aortic stenosis by late gadolinium enhancement (LGE) status. Kaplan-Meier (KM) survival plots showing all-cause (left) and cardiovascular (CV; right) mortality in all patients (A and B; n=674), patients treated with surgical aortic valve replacement (SAVR; C and D; n=399), and patients treated with transcatheter aortic valve replacement (TAVR; E and F; n=275), according to the presence or absence of LGE preoperatively. TAVI indicates transcatheter aortic valve implantation.
Figure 3.

All-cause mortality in severe aortic stenosis (AS) by late gadolinium enhancement (LGE) pattern. Kaplan-Meier (KM) survival plot showing all-cause mortality in all patients with severe AS (n=674) by pattern of late gadolinium enhancement (no LGE, infarct LGE, noninfarct LGE; both P<0.001). The plot summarizes 6-year follow-up data.
Discussion
In patients with severe AS, in terms of disease-based parameters, we have shown that myocardial fibrosis (scar) is independently associated with mortality. This was the case for all-cause and cardiovascular mortality, after both surgical and transcatheter intervention, and for both infarct and noninfarct scar patterns. Specifically, every 1% increase in scar burden increased mortality hazard by 11% and cardiovascular mortality hazard by 8%. Given that most of this scar is AS-related and that scar was present in half of the patients, we postulate that, for many patients, AS surgery is potentially occurring too late and leaving patients with residual risk.
AS is important, because it is the most common valvular heart disease in the developed world (>3% of those >75 years of age), and the advent of TAVR now offers a treatment option for many of those with significant comorbidities who were previously deemed inoperable. Current guidelines recommend valve intervention to improve survival and symptom status when AS is severe and ventricular decompensation is present, suggested by the onset of symptoms or a reduction in LV ejection fraction.4 We have highlighted in this study an additional component of this risk-benefit analysis that has been underrecognized: silent irreversible scar is very common and is associated with increased mortality. Moreover, the greater the scar burden, the higher the mortality. Previous studies have suggested that operating earlier may be beneficial for patients, but identifying which patients are likely to benefit is difficult given that many will remain asymptomatic for years. Our findings suggest that scar burden might be used to optimize the timing of surgical intervention, with half of patients demonstrating irreversible scar and a consequent doubling of postoperative medium-term mortality. Noninfarct pattern scar was twice as prevalent as infarct scar, and both predicted worse outcome, as previously suggested.8,9 In asymptomatic severe AS, the risks of early surgery (1%–2% mortality) and prolonged risk of prosthesis-associated complications (eg, endocarditis, pacemaker dependency, bleeding, thrombosis, valve degeneration) need to be balanced against the “silent” risk of sudden cardiac death (1.5%/y) and increased risk of intervention and long-term outcome after symptoms have developed.21 Our results may therefore provide a mechanism for better selection of appropriate patients for early surgery, but this remains to be tested.
Potential Pathophysiology of Scar Formation
The ventricle in AS initially responds to pressure loading by LV hypertrophy resulting in adaptive LV remodeling to maintain wall stress and cardiac performance. Despite compensatory capillary vasodilatation, over time, myocardial oxygen demand outstrips supply, leading to subendocardial ischemia and eventually LV decompensation.22–24 The transition to LV decompensation occurs by fibrosis and myocyte degeneration with irreversible cell loss, mainly by autophagy and oncosis.6 This process is driven by subendocardial ischemia and preceded by 2 phenomena: perfusion defects and troponin elevation (indicating myocardial cell death).25,26 Replacement fibrosis ensues, which starts in the subendocardial layers first, then over time affects deeper myocardial layers,17 and in turn contributes significantly to the progression of LV systolic dysfunction.6 Diffuse myocardial fibrosis, with increased collagen I and III deposition around cardiomyocytes and bundles, occurs predominantly in the midmyocardium.17 Patchy foci of fibrosis on LGE imaging can be indicative of widespread diffuse fibrosis. Diffuse fibrosis can be assessed by CMR T1 mapping17,27 but was not investigated in this study because it has become available only more recently.
Focal fibrosis identified by LGE is associated with adverse outcome across a wide range of myocardial pathologies28 and has been shown in small single-center studies to be associated with outcome in AS.7–10 The presented data place LGE-detected scar firmly as a key outcome predictor in AS and suggest that current timing of valve intervention (TAVR or SAVR), based on a combination of valve severity and symptoms, may be too late for optimal long-term outcomes. This was highlighted in a recently completed multicenter observational study in asymptomatic patients with moderate to severe AS (the PRIMID-AS study [Microvascular Dysfunction in Aortic Stenosis]; NCT01658345) showing that the presence of scar on LGE did not predict symptom onset.14
Earlier intervention, for example, in asymptomatic severe AS, may therefore warrant investigation. Despite numerous observational studies to assess risk prediction in asymptomatic AS, there have been no randomized trials of early intervention to improve outcome. Patients at risk of myocardial decompensation resulting from scar or myocardium in the process of developing scar can be identified early through the use of high-sensitivity troponin, perfusion defects, or CMR LGE techniques.15 One study that will go some way in addressing this issue is the EVOLVED-AS trial (Early Valve Replacement Guided by Biomarkers of LV Decompensation in Asymptomatic Patients With Severe AS; NCT03094143), a parallel-group, multicenter, prospective randomized (open-label blinded end point) trial of early aortic valve intervention in asymptomatic patients with severe AS and evidence of LV decompensation, as evidenced by noninfarct pattern LGE. In the absence of prospective randomized trials, only registry data suggest the likely impact of early surgery.29,30
Stratifying intervention on the basis of the presence of LGE may be too late because even the small amount of scar detected in our cohort is associated with residual increased risk of all-cause and cardiovascular mortality, but until EVOLVED-AS and further studies report results, the role of, timing of, and intervals for CMR to guide decision making in patients with moderate to severe AS remain unclear.
Our study has limitations. This was an observational study of patients at surgical centers with an interest in CMR and echocardiography for clinical and research indications, potentially introducing selection bias. As a result of the contraindications for contrast-enhanced CMR, patients with severe renal impairment and preoperative pacemaker/defibrillators were not represented. Sixty-one patients did not undergo LGE imaging. There were no reported invasive measures of hemodynamics (during angiography), hematocrit, brain natriuretic peptides, or blood troponin; per clinical routine, renal function was checked before LGE CMR but was not systematically captured or easily retrieved for this analysis. Furthermore, no routine imaging follow-up was performed. Although studies of other populations have shown that unrecognized infarct scar increases with age31 and can be found in up to 10% of subjects, this would account for only a minority of the scar burden found in our population. Both TAVR and SAVR have been associated with de novo LGE, which may be associated with further myocardial decompensation.32,33 Because of the lack of follow-up CMR data, the possibility of further periprocedural damage could not be excluded. Finally, multivariate analysis was not controlled for the type of intervention. This may have been important in TAVR in particular, for which the learning curve and patient selection have changed over the years.
Conclusions
In patients with severe AS, preoperative focal myocardial scar is independently associated with mortality, its presence being associated with a 2-fold higher late mortality.
Acknowledgments
Prof Greenwood was the principal investigator of this study. Dr Dweck, Prof Moon, Prof Myerson, Prof McCann, and Dr Prasad were coinvestigators at each site. Dr Treibel wrote the manuscript. Dr Captur performed the statistical analysis. Drs Captur and Law provided statistical oversight. Drs Greenwood, Musa, and Bijsterveld obtained ethics approval and coordinated the study. P. Bijsterveld and Dr Greenwood adjudicated the outcomes. Dr Captur set up and maintained the REDCap database. Drs Musa, Treibel, Vassiliou, Singh, Chin, and Rigolli performed the data collection, anonymization, and upload. Drs Musa, Foley, and Dobson performed the CMR LGE analysis. Drs Vassiliou and Malley performed the atrial volume analysis. Drs Singh and Foley performed the aortic flow analysis. Drs Treibel, Chin, Pica, Loudon, and Rigolli performed the left and right ventricular volume and function analyses. All authors have read and approved the manuscript.
Sources of Funding
This study was in part supported by the British Heart Foundation (University of Leeds, Prof Greenwood [PG/11/126/29321]; University of Leicester, Prof McCann [PG/07/068/2334]; University of Oxford, Prof Myerson [FS/10/015/28104]; University of Edinburgh, Dr Dweck [FS/10/026]; University College London, Prof Moon [FS/08/028/24767]), and the National Institute for Health Research (University College London, Dr Treibel [DRF-2013-06-102]), including via its Biomedical Research Center and Clinical Research Facility programs, as well as Rosetrees Trust. The views expressed are those of the authors and not necessarily those of the UK National Health Service, National Institute for Health Research, or Department of Health.
Disclosures
None.
Supplementary Material
Footnotes
Drs Musa and Treibel contributed equally.
Sources of Funding, see page 1946
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.117.032839.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–1863. doi: 10.1016/j.jacc.2012.02.093. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 3.Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373:956–966. doi: 10.1016/S0140-6736(09)60211-7. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL ESC Scientific Document Group. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 5.Weidemann F, Herrmann S, Störk S, Niemann M, Frantz S, Lange V, Beer M, Gattenlöhner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 6.Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, Bauer EP, Klövekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 7.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 8.Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S, Prasad NA, Wage R, Quarto C, Angeloni E, Refice S, Sheppard M, Cook SA, Kilner PJ, Pennell DJ, Newby DE, Mohiaddin RH, Pepper J, Prasad SK. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–1279. doi: 10.1016/j.jacc.2011.03.064. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 9.Barone-Rochette G, Piérard S, De Meester de Ravenstein C, Seldrum S, Melchior J, Maes F, Pouleur AC, Vancraeynest D, Pasquet A, Vanoverschelde JL, Gerber BL. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol. 2014;64:144–154. doi: 10.1016/j.jacc.2014.02.612. doi: 10.1016/j.jacc.2014.02.612. [DOI] [PubMed] [Google Scholar]
- 10.Vassiliou VS, Perperoglou A, Raphael CE, Joshi S, Malley T, Everett R, Halliday B, Pennell DJ, Dweck MR, Prasad SK. Midwall fibrosis and 5-year outcome in moderate and severe aortic stenosis. J Am Coll Cardiol. 2017;69:1755–1756. doi: 10.1016/j.jacc.2017.01.034. doi: 10.1016/j.jacc.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Treibel TA, Kozor R, Schofield R, Benedetti G, Fontana M, Bhuva AN, Sheikh A, López B, González A, Manisty C, Lloyd G, Kellman P, Díez J, Moon JC. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J Am Coll Cardiol. 2018;71:860–871. doi: 10.1016/j.jacc.2017.12.035. doi: 10.1016/j.jacc.2017.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M EAE/ASE. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. doi: 10.1093/ejechocard/jen303. doi: 10.1093/ejechocard/jen303. [DOI] [PubMed] [Google Scholar]
- 13.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh A, Greenwood JP, Berry C, Dawson DK, Hogrefe K, Kelly DJ, Dhakshinamurthy V, Lang CC, Khoo JP, Sprigings D, Steeds RP, Jerosch-Herold M, Neubauer S, Prendergast B, Williams B, Zhang R, Hudson I, Squire IB, Ford I, Samani NJ, McCann GP. Comparison of exercise testing and CMR measured myocardial perfusion reserve for predicting outcome in asymptomatic aortic stenosis: the PRognostic Importance of MIcrovascular Dysfunction in Aortic Stenosis (PRIMID AS) Study. Eur Heart J. 2017;38:1222–1229. doi: 10.1093/eurheartj/ehx001. doi: 10.1093/eurheartj/ehx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chin CW, Messika-Zeitoun D, Shah AS, Lefevre G, Bailleul S, Yeung EN, Koo M, Mirsadraee S, Mathieu T, Semple SI, Mills NL, Vahanian A, Newby DE, Dweck MR. A clinical risk score of myocardial fibrosis predicts adverse outcomes in aortic stenosis. Eur Heart J. 2016;37:713–723. doi: 10.1093/eurheartj/ehv525. doi: 10.1093/eurheartj/ehv525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairbairn TA, Steadman CD, Mather AN, Motwani M, Blackman DJ, Plein S, McCann GP, Greenwood JP. Assessment of valve haemodynamics, reverse ventricular remodelling and myocardial fibrosis following transcatheter aortic valve implantation compared to surgical aortic valve replacement: a cardiovascular magnetic resonance study. Heart. 2013;99:1185–1191. doi: 10.1136/heartjnl-2013-303927. doi: 10.1136/heartjnl-2013-303927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Treibel TA, López B, González A, Menacho K, Schofield RS, Ravassa S, Fontana M, White SK, DiSalvo C, Roberts N, Ashworth MT, Díez J, Moon JC. Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non-invasive study in 133 patients. Eur Heart J. 2018;39:699–709. doi: 10.1093/eurheartj/ehx353. doi: 10.1093/eurheartj/ehx353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelfand EV, Hughes S, Hauser TH, Yeon SB, Goepfert L, Kissinger KV, Rofsky NM, Manning WJ. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: optimizing correlation with Doppler echocardiography. J Cardiovasc Magn Reson. 2006;8:503–507. doi: 10.1080/10976640600604856. [DOI] [PubMed] [Google Scholar]
- 21.Lund O, Nielsen TT, Emmertsen K, Flø C, Rasmussen B, Jensen FT, Pilegaard HK, Kristensen LH, Hansen OK. Mortality and worsening of prognostic profile during waiting time for valve replacement in aortic stenosis. Thorac Cardiovasc Surg. 1996;44:289–295. doi: 10.1055/s-2007-1012039. doi: 10.1055/s-2007-1012039. [DOI] [PubMed] [Google Scholar]
- 22.Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 1989;79:744–755. doi: 10.1161/01.cir.79.4.744. [DOI] [PubMed] [Google Scholar]
- 23.Fielitz J, Hein S, Mitrovic V, Pregla R, Zurbrügg HR, Warnecke C, Schaper J, Fleck E, Regitz-Zagrosek V. Activation of the cardiac renin-angiotensin system and increased myocardial collagen expression in human aortic valve disease. J Am Coll Cardiol. 2001;37:1443–1449. doi: 10.1016/s0735-1097(01)01170-6. [DOI] [PubMed] [Google Scholar]
- 24.Garcia D, Camici PG, Durand LG, Rajappan K, Gaillard E, Rimoldi OE, Pibarot P. Impairment of coronary flow reserve in aortic stenosis. J Appl Physiol (1985) 2009;106:113–121. doi: 10.1152/japplphysiol.00049.2008. doi: 10.1152/japplphysiol.00049.2008. [DOI] [PubMed] [Google Scholar]
- 25.Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, Strachan FE, Hunter AL, Maria Choy A, Lang CC, Walker S, Boon NA, Newby DE, Mills NL, Dweck MR. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–2321. doi: 10.1093/eurheartj/ehu189. doi: 10.1093/eurheartj/ehu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn JH, Kim SM, Park SJ, Jeong DS, Woo MA, Jung SH, Lee SC, Park SW, Choe YH, Park PW, Oh JK. Coronary microvascular dysfunction as a mechanism of angina in severe AS: prospective adenosine-stress CMR study. J Am Coll Cardiol. 2016;67:1412–1422. doi: 10.1016/j.jacc.2016.01.013. doi: 10.1016/j.jacc.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G, Jenkins W, Koo M, Mirsadraee S, White AC, Japp AG, Prasad SK, Semple S, Newby DE, Dweck MR. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging. 2017;10:1320–1333. doi: 10.1016/j.jcmg.2016.10.007. doi: 10.1016/j.jcmg.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheong BY, Muthupillai R, Wilson JM, Sung A, Huber S, Amin S, Elayda MA, Lee VV, Flamm SD. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120:2069–2076. doi: 10.1161/CIRCULATIONAHA.109.852517. doi: 10.1161/CIRCULATIONAHA.109.852517. [DOI] [PubMed] [Google Scholar]
- 29.Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kawase Y, Izumi C, Miyake M, Mitsuoka H, Kato M, Hirano Y, Matsuda S, Nagao K, Inada T, Murakami T, Takeuchi Y, Yamane K, Toyofuku M, Ishii M, Minamino-Muta E, Kato T, Inoko M, Ikeda T, Komasa A, Ishii K, Hotta K, Higashitani N, Kato Y, Inuzuka Y, Maeda C, Jinnai T, Morikami Y, Sakata R, Kimura T CURRENT AS Registry Investigators. Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2015;66:2827–2838. doi: 10.1016/j.jacc.2015.10.001. doi: 10.1016/j.jacc.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Généreux P, Stone GW, O’Gara PT, Marquis-Gravel G, Redfors B, Giustino G, Pibarot P, Bax JJ, Bonow RO, Leon MB. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2016;67:2263–2288. doi: 10.1016/j.jacc.2016.02.057. doi: 10.1016/j.jacc.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 31.Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, Gudnason V, Harris TB, Arai AE. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890–896. doi: 10.1001/2012.jama.11089. doi: 10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim WK, Rolf A, Liebetrau C, Van Linden A, Blumenstein J, Kempfert J, Bachmann G, Nef H, Hamm C, Walther T, Möllmann H. Detection of myocardial injury by CMR after transcatheter aortic valve replacement. J Am Coll Cardiol. 2014;64:349–357. doi: 10.1016/j.jacc.2014.03.052. doi: 10.1016/j.jacc.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 33.Dobson LE, Musa TA, Uddin A, Fairbairn TA, Swoboda PP, Ripley DP, Garg P, Evans B, Malkin CJ, Blackman DJ, Plein S, Greenwood JP. Post-procedural myocardial infarction following surgical aortic valve replacement and transcatheter aortic valve implantation. EuroIntervention. 2017;13:e153–e160. doi: 10.4244/EIJ-D-16-00558. doi: 10.4244/EIJ-D-16-00558. [DOI] [PubMed] [Google Scholar]


